Abstract
Licochalcone H (LCH) is a phenolic compound synthetically derived from licochalcone C (LCC) that exerts anticancer activity. In this study, we investigated the anticancer activity of LCH in human skin cancer A375 and A431 cells. The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay was used to evaluate the antiproliferative activity of LCH. Cell cycle distribution and the induction of apoptosis were analyzed by flow cytometry. Western blotting assays were performed to detect the levels of proteins involved in cell cycle progression, apoptosis, and the JAK2/STAT3 signaling pathway. LCH inhibited the growth of cells in dose- and time-dependent manners. The annexin V/propidium iodide double staining assay revealed that LCH induced apoptosis, and the LCH-induced apoptosis was accompanied by cell cycle arrest in the G1 phase. Western blot analysis showed that the phosphorylation of JAK2 and STAT3 was decreased by treatment with LCH. The inhibition of the JAK2/STAT3 signaling pathway by pharmacological inhibitors against JAK2/STAT3 (cryptotanshinone (CTS) and S3I-201) simulated the antiproliferative effect of LCH suggesting that LCH induced apoptosis by modulating JAK2/STAT3 signaling.
Keywords: Licochalcone H, JAK2, STAT3, Apoptosis, Human skin cancer
INTRODUCTION
Cancer can initiate from mutations in specific genes, which induce constant cell proliferation, uncontrolled growth, tumor formation, and metastasis or recurrence (Bertram, 2000; Gibbs, 2003). Although diverse approaches have been used in attempts to unravel the complex mechanisms of oncogenesis and treat cancers, complete prevention or a perfect remedy remains unattainable (Baldwin, 2001).
Skin cancer is the most commonly diagnosed cancer in the United States, accounting for a third of all cancer diagnoses (Rager et al., 2005; Lomas et al., 2012). Skin cancer is defined as the malignant transformation of tumors that develop on the skin. The term encompasses all skin cancers such as basal cell carcinomas (BCC), squamous cell carcinomas (SCC), melanomas, Kaposi’s sarcoma, Paget’s disease, and mycosis fungoides. BCC and SCC are classified as non-melanoma skin cancer. BCC is the most common type of skin cancer, and the majority of cases occur on sun-exposed head and neck (Raasch et al., 2006). Likewise, SCC is most commonly found in fair-skinned individuals with high sun exposure (Diepgen and Mahler, 2002). Melanoma is a malignant neoplasm of the melanocytes (Dong et al., 2010; Liu and Sheikh, 2014), less common than BCC and SCC, but with a fatal outcome (Torpy et al., 2004). Even though multimodal therapeutic approaches including surgery, radiation therapy, and chemotherapy are administered to patients with skin cancer, the incidence of the disease has continued to increase over the last three decades (Diepgen and Mahler, 2002; Apalla et al., 2017).
Natural products of diverse origin are widely used in the treatment of cancer (Demain and Vaishnav, 2011; Rajesh et al., 2015). Licochalcones are natural phenolic agents used as flavoring products and for the treatment of bronchial asthma, gastric ulcer, and inflammation (Huang et al., 2010; Kim et al., 2014). In the family of licochalcones, Licochalcone C (LCC) isolated from Glycyrrhiza inflata has been evaluated as a chemotherapeutic agent against various cancer cells including lung, melanoma, cervical, prostate, bladder, lymphoma, and colon cancers (Zi and Simoneau, 2005; Kim et al., 2014). Although LCC has excellent efficacy (Franceschelli et al., 2017), very low extraction yields are fatal disadvantage. Therefore, to overcome the limited supply of LCC from natural resources, a synthetic approach was developed to produce licochalcone H (LCH), an isomer of LCC (Wang et al., 2013). LCH is a synthetic regioisomer of LCC. However, the biological activity of LCH has not been widely studied. A recent study reported the anticancer activity of LCH in human skin melanoma A375 and squamous cell carcinoma A431 cells (Kang et al., 2017b).
The JAK/STAT signaling pathway is involved in diverse biological processes such as embryonic development, stem cell maintenance, hematopoiesis, and inflammatory responses (Lopez-Onieva et al., 2008; Bollrath and Greten, 2009). JAK/STAT signaling is initiated by the stimulation of cytokine receptors by various cytokines (Buettner et al., 2002; Yu et al., 2009). The stimulation of Janus kinase (JAK) proteins by various cytokines induces phosphorylation and constant stimulation leads to the dimerization of signal transducer and activator of transcription (STAT). Then, the STAT dimer translocates into the cell nucleus to induce the transcription of specific genes (Darnell, 1997). STAT proteins, highly expressed in cancer cells (Bromberg et al., 1999) are major transcription factors involved in cell differentiation, apoptosis, and survival (Buettner et al., 2002). Aberrant activation of the JAK2/STAT3 signaling pathway can trigger oncogenesis, and the JAK2/STAT3 signaling pathway plays an important role in tumorigenesis and cancer progression (Wang and Sun, 2014). In contrast, the inhibition of JAK2/STAT3 induced apoptosis in diverse cancer cells and reduced the growth of cancer cells (Shan et al., 2010). Therefore, JAK2 or STAT3 proteins represent effective signal targets for various anticancer drugs (Johnston and Grandis, 2011; Du et al., 2012; Siveen et al., 2014). The apoptotic effect of LCH in human skin cancer cells via modulation of the JAK2/STAT3 signaling pathway has not been reported yet. We investigated whether LCH could induce the apoptosis of human skin cancer and the effect of LCH on JAK2/STAT3 signaling.
MATERIALS AND METHODS
Materials
Dulbecco’s Modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate buffered saline (PBS), penicillin and streptomycin (P/S) and 0.5% trypsin-EDTA were obtained from Thermo Fisher Scientific (Rockford, IL, USA). The primary antibodies against phospho (p)-STAT3 (Tyr 705), STAT3, Bcl-xl, Bcl-2, myeloid cell leukemia-1 (Mcl-1), Survivin, Cyclin-D1, p21, p27, p53, Bad, Bax, Bid and β-Actin, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against p-JAK2 (Tyr 1007/1008), JAK2, Caspase 3 and Poly ADP-ribose Polymerase (PARP), cleaved-Caspase 3, cleaved-PARP were obtained from Cell Signaling Inc (Danvers, MA, USA). 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), 4’-6-diamidino-2-phenylindole (DAPI) and cryptotanshinone (CTS), and S3I-201 were purchased from Sigma-Aldrich, Inc (St. Louis, MO, USA). LCH was synthesized and purified based on the synthetic method described elsewhere (Wang et al., 2013).
Cell culture
Human melanoma A375 cells and human epidermoid carcinoma A431 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). These human skin cancer cells were cultured in DMEM containing 10% heat-inactivated FBS, and 100 U/mL each of penicillin and streptomycin at 37˚C in a humidified air with 5% CO2.
The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay
To confirm the viability of human skin cancer cells, 4.0×103 of A375 and 5.0×103 of A431 cells were seeded in 96-well microtiter plate. After a day of incubation, cells were treated with vehicle (for control), 5, 10, 20, and 30 µM of LCH. Cells were re-incubated for 24 and 48 h. After entire incubation, cell viabilities were measured with the MTS reagent (Abcam, Cambridge, MA, USA). Briefly, the dehydrogenase enzyme substrate MTS reagent was added to the respective wells and the plates were incubated at 37˚C with 5% CO2 for 2 h. The absorbance was measured at 490 nm using a microplate reader (Biotek, Winooski, VT, USA). The relative cell viability was calculated compared to the negative controls (0 µM LCH in DMSO). The data represent mean values obtained from three independent experiments.
Propidium iodide staining
A375 and A431 cells were seeded and treated with LCH (0, 10, 20, and 30 μM) for 48 h. After incubation, cells were harvested and fixed with 70% ethanol at –20°C for 2h. The cells were washed with 1×PBS, and stained with RNase A and propidium iodide (PI; BD Biosciences, Piscataway, NJ, USA). Finally, samples were incubated at 37˚C for 30 min in the dark. After reaction with reagent, they were analyzed using a fluorescence-activated cell sorting (FACS; BD Biosciences) according to the manufacturer’s instructions.
Western blot analysis
LCH (0-30 µM for 48 h)-treated A375 and A431 cells were harvested and washed with PBS. Proteins were then extracted using RIPA buffer (Thermo Fisher Scientific) containing protease inhibitor cocktail (Roche, Basel, Switzerland). Quantification of protein extracts was conducted with Pierce® BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of protein samples were resolved by 8~15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the resolved proteins were transferred to polyvinylidene-difluoride membranes. Membranes were blocked for 1 h at room temperature with 5% non-fat dried milk in TBS containing 0.1% Tween 20 (TBST), and incubated overnight at 4˚C with specific antibodies, followed by washing with TBST for 30 min and incubation with horseradish peroxidase-conjugated secondary antibody. The targeted protein bands were reacted using an ECL Plus Western Blotting Detection system from Santa Cruz Biotechnology and detected using ImageQuant LAS-4000 Mini (GE Healthcare Life Sciences, Buckinghamshire, UK) according to the manufacturer’s instructions.
Annexin-V/PI staining
Cells were treated with LCH (0, 10, 20, and 30 μM) for 48 h. Detached A375 and A431 cells were collected by centrifugation and combined with adherent cells. The cells were washed with cold 1×PBS, and stained with the FITC-Annexin-V Apoptosis Detection Kit (BD Biosciences) followed by analysis using a fluorescence activated cell sorter (FACS; BD Biosciences).
Statistical analysis
Results were presented as mean ± SD of at least three independent experiments performed in triplicate. Data were analyzed for statistical significance using one-way analysis of variance. p values of less than 0.05 were considered statistically significant.
RESULTS
LCH suppresses the proliferation of skin cancer cells
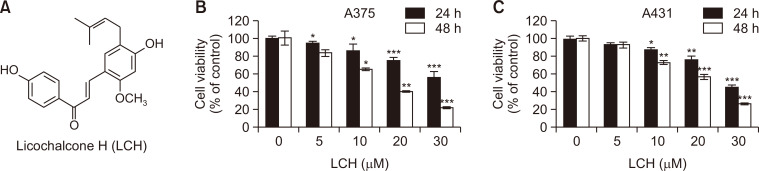
First, we determined if LCH (Fig. 1A) had an anti-proliferative effect in skin cancer cells, as the anticancer effect of LCH in skin cancer cells has not been studied yet. The MTS cell viability assay showed significant decreases in the viability of A375 and A431 cells in concentration- (0, 5, 10, 20, and 30 μM) and time- (24 and 48 h) dependent manners (Fig. 1B, 1C). The viability of A375 cells after treatment with 5, 10, 20, and 30 μM LCH for 24 h was 94%, 86%, 75%, and 56%, respectively, compared to 0 μM LCH. For 48 h treatment, the viability of A375 cells was 83%, 65%, 40%, and 22% in the 5, 10, 20, and 30 μM LCH-treated groups, respectively, compared to the controls. Likewise, we could observe the anti-proliferative effect of LCH in A431 cells in the similar manner. The viability of A431 cells after treatment with 5, 10, 20, and 30 μM LCH for 24 h was 94%, 88%, 74%, and 46%, respectively, compared to 0 μM LCH, and the viability after 48 h treatment went down to 93%, 74%, 57%, and 27% in the 5, 10, 20, and 30 μM LCH-treated groups, respectively, compared to the controls.
Fig. 1.
Growth inhibition of human skin cancer cells by LCH. (A) The chemical structure of licochalcone H (LCH). (B, C) The viability of skin cancer cell lines A375 and A431 treated with 0, 5, 10, 20, and 30 μM of LCH for 24 and 48 h was measured using the MTS reagent. The data represent mean percentages ± SD (n=3; *p<0.05, **p<0.01, ***p<0.001 compared to the control group).
LCH induces apoptosis in skin cancer cells
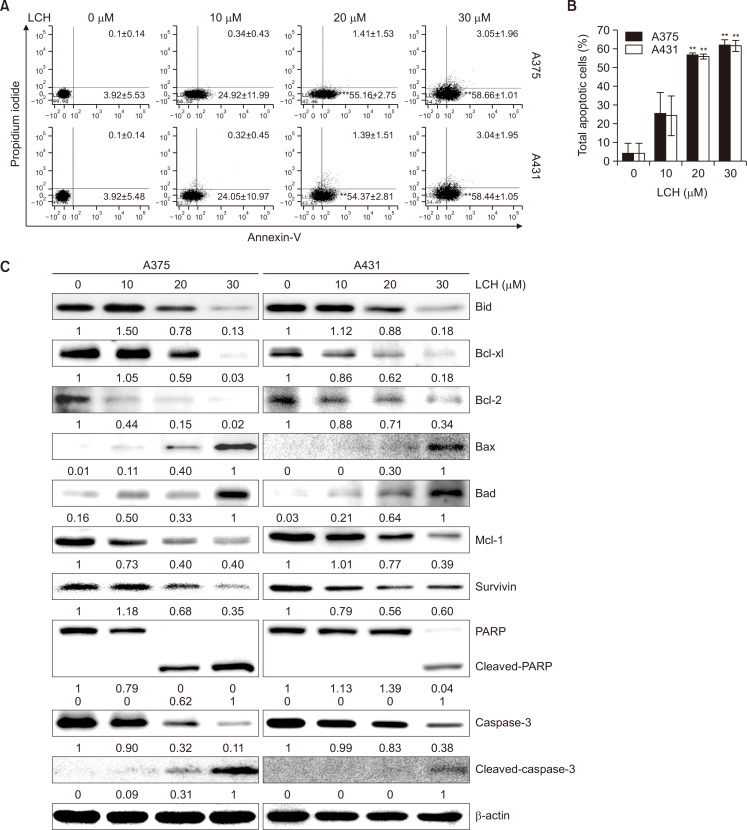
To investigate whether LCH induced apoptosis in skin cancer cells, the annexin V apoptosis assay with annexin V/PI double staining was used. The percentage of apoptotic cells (Fig. 2A, annexin V+/PI- cells and annexin V+/PI+, on the right side) after treatment with 0, 10, 20, and 30 μM LCH for 48 h was 4.02%, 25.25%, 56.57%, and 61.70%, respectively in A375 cells. Similarly, the percentage of apoptotic cells in A431 cells increased from 4.02% in the control group (0 μM LCH) to 24.37%, 55.76%, and 61.48% in the 10, 20, and 30 μM LCH-treated groups, respectively (Fig. 2A, 2B). These results suggested that skin cancer cell apoptosis was induced by LCH. To determine if LCH regulated the mitochondrial apoptosis pathway, we analyzed the protein expression levels of Bcl-2 family members by Western blots. When skin cancer cells were incubated with LCH, the expression of Bid, Bcl-xl, Bcl-2, and Mcl-1 decreased in a dose-dependent manner, whereas the levels of Bax and Bad increased. Treatment with LCH resulted in a decrease in survivin and an increase in the levels of cleaved caspase 3 and PARP in A375 and A431 cells (Fig. 2C).
Fig. 2.
Effect of LCH on apoptosis induction in human skin cancer cells. (A, B) Quantitative detection of annexin-V-FITC and PI-positive cells via FACS analysis. A375 and A431 cells were treated with 0, 10, 20, and 30 μM of LCH, and apoptosis was analyzed after annexin-V-FITC and PI double staining. The data represent mean percentages ± SD (n=3; **p<0.01 compared to the control group). (C) A375 and A431 cells treated with 0, 10, 20, and 30 μM LCH for 48 h were harvested to prepare cell extracts. The cell extracts were subjected to SDS-PAGE and Western blot analysis to detect the levels of Bid, Bcl-xl, Bcl-2, Bax, Bad, Mcl-1, survivin, PARP, and caspase-3. β-Actin was used as the denominator to quantify relative protein expression levels.
LCH triggers G1 phase cell cycle arrest and affects cell cycle regulatory proteins
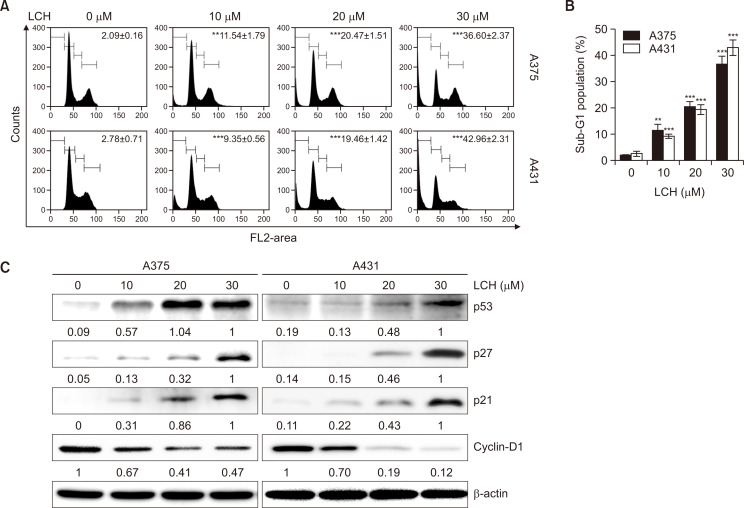
To investigate if the suppression of cell proliferation by LCH was related to cell cycle arrest, we performed cell cycle analysis using PI staining. Incubating skin cancer cells with different LCH concentrations for 48 h led to a dose-dependent increase in the sub-G1 phase in both A375 and A431 cells. In A375 cells, the sub-G1 phase cell cycle population was 2.09%, 11.54%, 20.47%, and 36.60% at 0, 10, 20, and 30 μM LCH, respectively (Fig. 3A, 3B). Likewise, the sub-G1 phase cell cycle population in A431 cells increased from 2.78% in the control group (0 μM LCH) to 9.35%, 19.46%, and 42.96% in the 10, 20, and 30 μM LCH-treated groups, respectively (Fig. 3A, 3B). These results suggested G1 cell cycle arrest in human skin cancer cells by LCH treatment. We then assessed the protein levels of cyclin D1, CDK2, CDK6, p21, and p27 to elucidate the mechanism of LCH-induced cell cycle arrest in A375 and A431 cells. Western blot analysis revealed that the expression of cyclin D1 was downregulated, and the expression of p21, p27, and p53 was upregulated (Fig. 3C). These results indicated that cell cycle arrest may be associated with the anti-proliferative effect of LCH on A375 and A431 cells.
Fig. 3.
Effects of LCH on human skin cancer cell cycle distribution. (A, B) A375 and A431 cells were treated with 0, 10, 20, and 30 μM LCH for 48 h, stained with PI, and analyzed for DNA content by FACS analysis. The data represent mean percentages ± SD (n=3; **p<0.01, ***p<0.001 compared to the control group). (C) A375 and A431 cells treated with 0, 10, 20, and 30 μM LCH for 48 h were harvested to prepare cell extracts. The cell extracts were subjected to SDS-PAGE and Western blot analysis to detect the levels of p53, p27, p21, and cyclin-D1. β-Actin was used as the loading control.
LCH inhibits the phosphorylation of JAK2 and STAT3 to exert antiproliferative activity
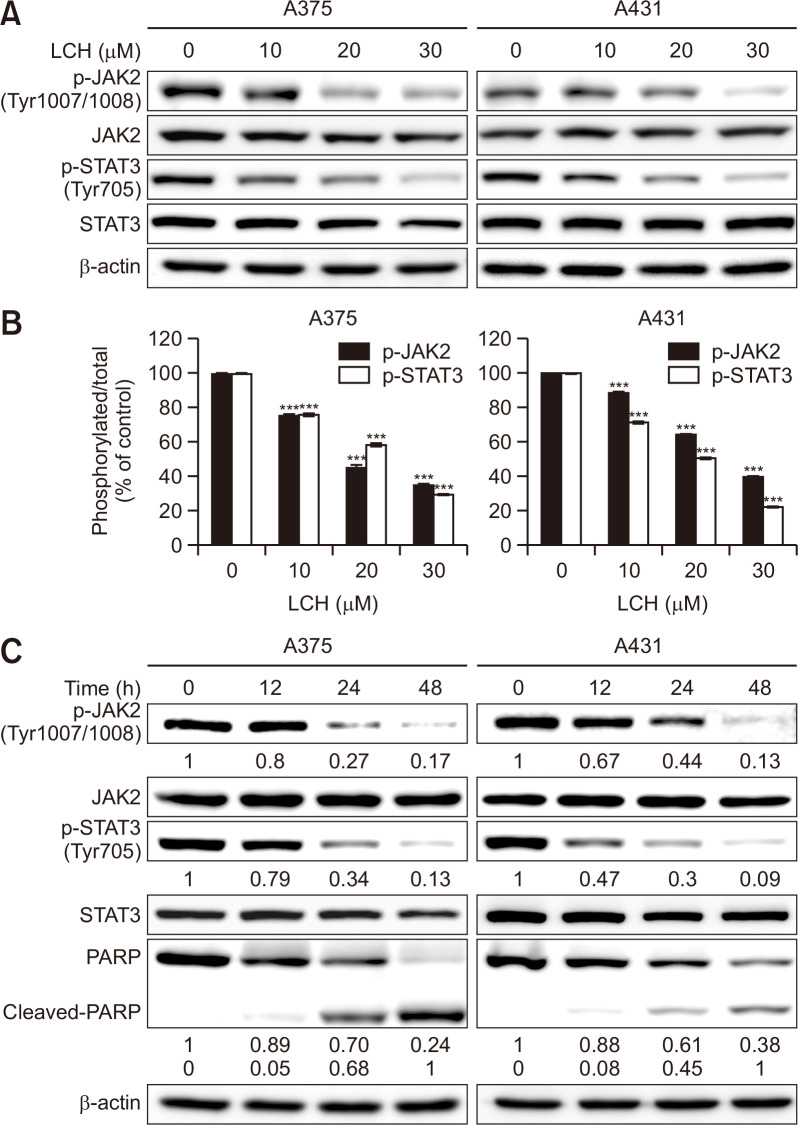
We performed Western blot assay to examine the effects of LCH on JAK2 and STAT3 signaling and the role of these pathways in the LCH-induced apoptosis of skin cancer cells, as the JAK2/STAT3 signaling controls the survival and proliferation of cancer cells (Bromberg et al., 1999; Yu et al., 2009). The level of JAK2 and STAT3 protein remained relatively unchanged. However, we observed that the phosphorylation of JAK2 and STAT3 decreased in human skin cancer cells in dose- (0, 10, 20, and 30 μM) and time- (0, 12, 24, and 48 h) dependent manners (Fig. 4). Interestingly, the decrease in the levels of phosphorylated JAK2 and STAT3 accompanied the cleavage of PARP in a time-dependent manner (Fig. 4C), indicating that the inhibition of JAK2 and STAT3 signaling was involved in LCH-induced apoptosis.
Fig. 4.
Effects of LCH on the modulation of JAK2/STAT3 signaling in human skin cancer cells. (A) A375 and A431 cells were treated with LCH (0, 10, 20, and 30 μM) for 48 h. Whole cell extracts were prepared, separated by SDS-PAGE, and subjected to Western blots using JAK2, p-JAK2, STAT3, and p-STAT3 antibodies. β-Actin was used as the loading control. (B) The percentage of p-JAK2 and p-STAT3 expression is presented. The data represent mean percentages ± SD (n=3; ***p<0.001). (C) Time-dependent effects of LCH on p-JAK2, p-STAT3, and cleaved-PARP were observed using A375 and A431 cells treated with LCH (30 μM) for 0, 12, 24, and 48 h.
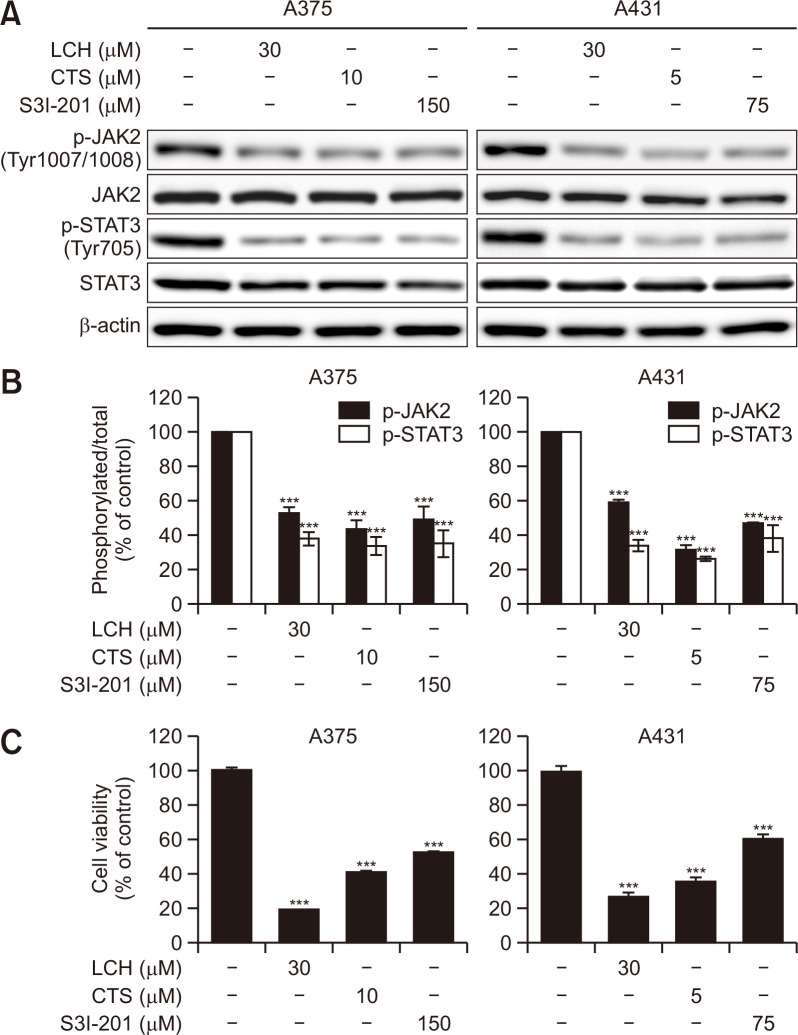
To further confirm the effects of LCH on the regulation of JAK2/STAT3 signaling, we compared the antiproliferative activity of LCH to those of pharmacological inhibitors of JAK2/STAT3 signaling (CTS and S3I-201). Expectedly, the levels of phosphorylated JAK2 and STAT3 assessed by Western blots were decreased in human skin cancer A375 and A431 cells treated with LCH, CTS, or S3I-201 for 48 h (Fig. 5A, 5B). Moreover, the MTS cell viability assay showed that decreases in JAK2 and STAT3 phosphorylation correlated with cell viability in both A375 and A431 cells, and the antiproliferative effects of CTS and S3I-201 were comparable to those of LCH (Fig. 5C).
Fig. 5.
Effects of inhibitors of JAK2/STAT3 signaling in human skin cancer cells. (A) A375 and A431 cells were treated with the indicated concentrations of LCH, cryptotanshinone (CTS), and S3I-201 for 48 h. Western blot analysis to detect the levels of JAK2, p-JAK2, STAT3, and p-STAT3. β-Actin was used as the loading control. (B) The percentages of p-JAK2 and p-STAT3 expression is shown. The data represent mean percentages ± SD (n=3; ***p<0.001). (C) Viability of A375 and A431 skin cancer cell lines treated with the indicated concentrations of LCH, CTS, and S3I-201 for 48 h measured using the MTS reagent. The data represent mean percentages ± SD (n=3; ***p<0.001).
DISCUSSION
Cancer accounts for a very high percentage of overall mortality. It ranks number two in the causes of death in the United States (Siegel et al., 2016, 2019). In addition to surgery, chemotherapy and radiotherapy are widely used in treating cancer. However, these treatments are accompanied by unavoidable adverse effects (Haas et al., 2011; Kuchuk et al., 2013). Natural products could potentially be used to reduce these side effects (Chae et al., 2014; Kang et al., 2017a). Licorice products exhibit pharmacological effects such as anti-ulcer, detoxifying, anti-inflammatory, anti-viral and anti-carcinogenic activities. Additionally, polyphenols derived from licorices are known to induce apoptosis in a wide range of cancer cells. The anticancer activity of LCH in skin cancer has not been studied yet. In the present study, we investigated the potential cytotoxic effects of LCH in human skin cancer using the A375 and A431 cell lines (Fig. 1B, 1C). The cell viability of these cells decreased, indicating the inhibition of proliferation by LCH in dose- and time- dependent manners.
In addition, we detected LCH-mediated apoptosis in human skin cancer cells. The rate of apoptosis, determined by the annexin V apoptosis assay, increased significantly following LCH treatment. We observed a shift in the balance between pro-apoptotic and anti-apoptotic Bcl-2 proteins (Lee et al., 2019). Western blot analysis showed that the level of anti-apoptotic proteins (Bcl-xl, Bcl-2, and Mcl-1) was decreased by LCH treatment, whereas the level of Bax and Bad pro-apoptotic proteins increased (Fig. 2C). The level of pro-apoptotic Bid in full-length form was decreased by treatment with LCH, suggesting the activation by cleavage (Esposti, 2002). Mcl-1 is known to be overexpressed in many cancer types, protecting cells from apoptosis (Quinn et al., 2011). Further studies could elucidate the detailed mechanism of how LCH induces decreases in Mcl-1 levels. In addition to shifting the balance of Bcl-2 proteins, decreased levels of survivin and cleavage of procaspase 3 and PARP were observed. These results indicated that LCH-induced apoptosis was mediated through the intrinsic apoptotic pathway (Fig. 2C).
To further elucidate the antiproliferative effect of LCH, we analyzed cell cycle distribution. Treatment with LCH increased the sub-G1 cell population of A375 and A431 cells in a dose-dependent manner (Fig. 3A, 3B). To understand the molecular mechanism of cell cycle arrest induced by LCH, we monitored the level of cyclin D1. Cyclin D is involved in G1 phase progression (Narasimha et al., 2014), which is one of the main check points (Villanueva et al., 2007). The level of cyclin D1 decreased in response to LCH treatment in a dose-dependent manner (Fig. 3C). Moreover, decreases in cyclin D1 levels were accompanied by increases in the levels of p21 and p27, inhibitors of cyclin/CDK complexes, suggesting the downregulation cyclin D1 induced by LCH as well as the inhibition of cyclin D1 by p21 and p27. We also observed an increase in the level of p53. We suspect that p53 mediated the G1 arrest induced by LCH (Waldman et al., 1995).
Cancer cells survive by modulating several signaling pathways such as JAK/STAT, MAPK/ERK, and PI3K/Akt (Franceschelli et al., 2011; Wu et al., 2017). The uncontrolled activation of JAK2/STAT3 signaling regulates proliferation in various types of cells (Nam et al., 2012; Yun et al., 2018). JAK2/STAT3 signaling controls the survival and proliferation of cancer cells, and high levels of phosphorylated STAT3 have been reported in various cancer cells and tissues (Bromberg et al., 1999; Yu et al., 2009). STAT3 protein is known as a transcription factor and a key molecule in cell differentiation, proliferation, and apoptosis (Buettner et al., 2002; Park et al., 2021). STAT3 is phosphorylated by JAK proteins (Lopez-Onieva et al., 2008; Yu et al., 2009), followed by dimerization and translocation into the nucleus to promote the transcription of oncogenes. In this study, we investigated the inhibitory effect of LCH on the JAK2/STAT3 signaling pathway in human skin cancer cells. As shown in Fig. 5A and 5B, p, p-JAK2 and p-STAT3 protein levels were significantly down-regulated by LCH in dose- and time-dependent manners (Fig. 4). Cleaved-PARP levels were also increased in a time-dependent manner (Fig. 4C). Additionally, we showed that the inhibitory effect of LCH on JAK2/STAT3 could be simulated by pharmacological inhibitors S3I-201 (Gurbuz et al., 2014; Ball et al., 2016) and CTS (Ke et al., 2017), which have been used to inhibit STAT3 activity (Fig. 5A, 5B). Moreover, the inhibition of JAK2/STAT3 signaling by these inhibitors alone suppressed cell growth, suggesting that JAK2/STAT3 signaling was involved in the LCH-induced apoptosis in human skin cancer cells.
Based on our results, LCH induced cell cycle arrest and apoptosis in human skin cancer cells by modulating JAK2/STAT3 signaling. JAK2 and STAT3 phosphorylation was downregulated in A375 and A431 cells by treatment with LCH, and the inhibition of JAK2/STAT3 signaling resulted in cell cycle arrest and apoptosis. These findings suggest that LCH has the potential to treat human skin cancer.
ACKNOWLEDGMENTS
We greatly appreciated using the Convergence Research Laboratory (established by the MNU Innovation Support Project in 2019) to conduct this research. This research was funded by the Basic Science Research Program of National Research Foundation Korea, grant number 2019R1A2C1005899. This work was carried out with the support of Cooperative Core Technology Development Project for Environmental Diseases Prevention and Management (2021003310003), funded by the Korea Ministry of Environment (MOE).
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest.
REFERENCES
- Apalla Z., Lallas A., Sotiriou E., Lazaridou E., Ioannides D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball D. P., Lewis A. M., Williams D., Resetca D., Wilson D. J., Gunning P. T. Signal transducer and activator of transcription 3 (STAT3) inhibitor, S3I-201, acts as a potent and non-selective alkylating agent. Oncotarget. 2016;7:20669–20679. doi: 10.18632/oncotarget.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram J. S. The molecular biology of cancer. Mol. Aspects Med. 2000;21:167–223. doi: 10.1016/S0098-2997(00)00007-8. [DOI] [PubMed] [Google Scholar]
- Bollrath J., Greten F. R. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J. F., Wrzeszczynska M. H., Devgan G., Zhao Y., Pestell R. G., Albanese C., Darnell J. E., Jr. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Buettner R., Mora L. B., Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- Chae J. I., Jeon Y. J., Shim J. H. Anti-proliferative properties of kahweol in oral squamous cancer through the regulation specificity protein 1. Phytother. Res. 2014;28:1879–1886. doi: 10.1002/ptr.5217. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Vaishnav P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011;4:687–699. doi: 10.1111/j.1751-7915.2010.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepgen T. L., Mahler V. The epidemiology of skin cancer. Br. J. Dermatol. 2002;146 Suppl 61:1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- Dong Y., Lu B., Zhang X., Zhang J., Lai L., Li D., Wu Y., Song Y., Luo J., Pang X., Yi Z., Liu M. Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis. 2010;31:2097–2104. doi: 10.1093/carcin/bgq167. [DOI] [PubMed] [Google Scholar]
- Du W., Hong J., Wang Y. C., Zhang Y. J., Wang P., Su W. Y., Lin Y. W., Lu R., Zou W. P., Xiong H., Fang J. Y. Inhibition of JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via mitochondrial pathway. J. Cell. Mol. Med. 2012;16:1878–1888. doi: 10.1111/j.1582-4934.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposti M. D. The roles of Bid. Apoptosis. 2002;7:433–440. doi: 10.1023/A:1020035124855. [DOI] [PubMed] [Google Scholar]
- Franceschelli S., Pesce M., Ferrone A., Gatta D. M., Patruno A., Lutiis M. A., Quiles J. L., Grilli A., Felaco M., Speranza L. Biological effect of licochalcone C on the regulation of PI3K/Akt/eNOS and NF-kappaB/iNOS/NO signaling pathways in H9c2 cells in response to LPS stimulation. Int. J. Mol. Sci. 2017;18:690. doi: 10.3390/ijms18040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli S., Pesce M., Vinciguerra I., Ferrone A., Riccioni G., Patruno A., Grilli A., Felaco M., Speranza L. Licocalchone-C extracted from Glycyrrhiza glabra inhibits lipopolysaccharide-interferon-gamma inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression. Molecules. 2011;16:5720–5734. doi: 10.3390/molecules16075720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs W. W. Untangling the roots of cancer. Sci. Am. 2003;289:56–65. doi: 10.1038/scientificamerican0703-56. [DOI] [PubMed] [Google Scholar]
- Gurbuz V., Konac E., Varol N., Yilmaz A., Gurocak S., Menevse S., Sozen S. Effects of AG490 and S3I-201 on regulation of the JAK/STAT3 signaling pathway in relation to angiogenesis in TRAIL-resistant prostate cancer cells in vitro. Oncol. Lett. 2014;7:755–763. doi: 10.3892/ol.2014.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J. S., Liang S. Y., Hassett M. J., Shiboski S., Elkin E. B., Phillips K. A. Gene expression profile testing for breast cancer and the use of chemotherapy, serious adverse effects, and costs of care. Breast Cancer Res. Treat. 2011;130:619–626. doi: 10.1007/s10549-011-1628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. Y., Cai Y. Z., Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr. Cancer. 2010;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Grandis J. R. STAT3 signaling: anticancer strategies and challenges. Mol. Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T. H., Seo J. H., Oh H., Yoon G., Chae J. I., Shim J. H. Licochalcone A suppresses specificity protein 1 as a novel target in human breast cancer cells. J. Cell. Biochem. 2017a;118:4652–4663. doi: 10.1002/jcb.26131. [DOI] [PubMed] [Google Scholar]
- Kang T. H., Yoon G., Kang I. A., Oh H. N., Chae J. I., Shim J. H. Natural compound licochalcone B induced extrinsic and intrinsic apoptosis in human skin melanoma (A375) and squamous cell carcinoma (A431) cells. Phytother. Res. 2017b;31:1858–1867. doi: 10.1002/ptr.5928. [DOI] [PubMed] [Google Scholar]
- Ke F., Wang Z., Song X., Ma Q., Hu Y., Jiang L., Zhang Y., Liu Y., Zhang Y., Gong W. Cryptotanshinone induces cell cycle arrest and apoptosis through the JAK2/STAT3 and PI3K/Akt/NFkappaB pathways in cholangiocarcinoma cells. Drug Des. Devel. Ther. 2017;11:1753–1766. doi: 10.2147/DDDT.S132488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Park M. R., Lee S. Y., Kim D. K., Moon S. M., Kim C. S., Cho S. S., Yoon G., Im H. J., You J. S., Oh J. S., Kim S. G. Licochalcone A induces apoptosis in KB human oral cancer cells via a caspase-dependent FasL signaling pathway. Oncol. Rep. 2014;31:755–762. doi: 10.3892/or.2013.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchuk I., Bouganim N., Beusterien K., Grinspan J., Vandermeer L., Gertler S., Dent S. F., Song X., Segal R., Mazzarello S., Crawley F., Dranitsaris G., Clemons M. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res. Treat. 2013;142:101–107. doi: 10.1007/s10549-013-2727-3. [DOI] [PubMed] [Google Scholar]
- Lee Y. J., Kim W. I., Kim S. Y., Cho S. W., Nam H. S., Lee S. H., Cho M. K. Flavonoid morin inhibits proliferation and induces apoptosis of melanoma cells by regulating reactive oxygen species, Sp1 and Mcl-1. Arch. Pharm. Res. 2019;42:531–542. doi: 10.1007/s12272-019-01158-5. [DOI] [PubMed] [Google Scholar]
- Liu Y., Sheikh M. S. Melanoma: molecular pathogenesis and therapeutic management. Mol. Cell. Pharmacol. 2014;6:228. [PMC free article] [PubMed] [Google Scholar]
- Lomas A., Leonardi-Bee J., Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Onieva L., Fernandez-Minan A., Gonzalez-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–540. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- Nam S., Xie J., Perkins A., Ma Y., Yang F., Wu J., Wang Y., Xu R. Z., Huang W., Horne D. A., Jove R. Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells. Mol. Oncol. 2012;6:484–493. doi: 10.1016/j.molonc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimha A. M., Kaulich M., Shapiro G. S., Choi Y. J., Sicinski P., Dowdy S. F. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife. 2014;3:e02872. doi: 10.7554/eLife.02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. K., Kim D., Park S., Maharjan S., Kim J., Choi J. K., Akauliya M., Lee Y., Kwon H. J. Differential signaling and virus production in Calu-3 cells and Vero cells upon SARS-CoV-2 infection. Biomol. Ther. (Seoul) 2021;29:273–281. doi: 10.4062/biomolther.2020.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn B. A., Dash R., Azab B., Sarkar S., Das S. K., Kumar S., Oyesanya R. A., Dasgupta S., Dent P., Grant S., Rahmani M., Curiel D. T., Dmitriev I., Hedvat M., Wei J., Wu B., Stebbins J. L., Reed J. C., Pellecchia M., Sarkar D., Fisher P. B. Targeting Mcl-1 for the therapy of cancer. Expert Opin. Investig. Drugs. 2011;20:1397–1411. doi: 10.1517/13543784.2011.609167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasch B. A., Buettner P. G., Garbe C. Basal cell carcinoma: histological classification and body-site distribution. Br. J. Dermatol. 2006;155:401–407. doi: 10.1111/j.1365-2133.2006.07234.x. [DOI] [PubMed] [Google Scholar]
- Rager E. L., Bridgeford E. P., Ollila D. W. Cutaneous melanoma: update on prevention, screening, diagnosis, and treatment. Am. Fam. Physician. 2005;72:269–276. [PubMed] [Google Scholar]
- Rajesh E., Sankari L. S., Malathi L., Krupaa J. R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied Sci. 2015;7:S181–183. doi: 10.4103/0975-7406.155895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X. L., Zhou X. Y., Yang J., Wang Y. L., Deng Y. H., Zhang M. X. Inhibitory effect of cucurbitacin E on the proliferation of ovarian cancer cells and its mechanism. Chin. J. Cancer. 2010;29:20–24. doi: 10.5732/cjc.009.10223. [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Siveen K. S., Sikka S., Surana R., Dai X., Zhang J., Kumar A. P., Tan B. K., Sethi G., Bishayee A. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim. Biophys. Acta. 2014;1845:136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Torpy J., Lynm C., Glass R. M. JAMA patient page. Melanoma. JAMA. 2004;292:2800. doi: 10.1001/jama.292.22.2800. [DOI] [PubMed] [Google Scholar]
- Villanueva J., Yung Y., Walker J. L., Assoian R. K. ERK activity and G1 phase progression: identifying dispensable versus essential activities and primary versus secondary targets. Mol. Biol. Cell. 2007;18:1457–1463. doi: 10.1091/mbc.e06-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman T., Kinzler K. W., Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- Wang S. W., Sun Y. M. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (review) Int. J. Oncol. 2014;44:1032–1040. doi: 10.3892/ijo.2014.2259. [DOI] [PubMed] [Google Scholar]
- Wang Z., Cao Y., Paudel S., Yoon G., Cheon S. H. Concise synthesis of licochalcone C and its regioisomer, licochalcone H. Arch. Pharm. Res. 2013;36:1432–1436. doi: 10.1007/s12272-013-0222-3. [DOI] [PubMed] [Google Scholar]
- Wu K. J., Huang J. M., Zhong H. J., Dong Z. Z., Vellaisamy K., Lu J. J., Chen X. P., Chiu P., Kwong D. W. J., Han Q. B., Ma D. L., Leung C. H. A natural product-like JAK2/STAT3 inhibitor induces apoptosis of malignant melanoma cells. PLoS ONE. 2017;12:e0177123. doi: 10.1371/journal.pone.0177123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S., Yun C. W., Lee J. H., Kim S., Lee S. H. Cripto enhances proliferation and survival of mesenchymal stem cells by up-regulating JAK2/STAT3 pathway in a GRP78-dependent manner. Biomol. Ther. (Seoul) 2018;26:464–473. doi: 10.4062/biomolther.2017.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi X., Simoneau A. R. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005;65:3479–3486. doi: 10.1158/0008-5472.CAN-04-3803. [DOI] [PubMed] [Google Scholar]