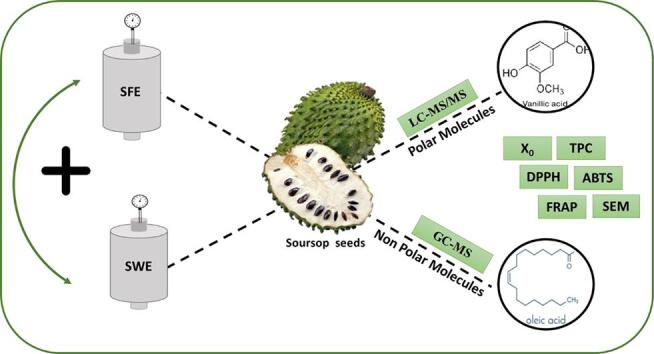
Graphical abstract
Keywords: Annona muricata seeds, Supercritical fluid extraction, Subcritical water extraction, Phenolic compounds, Antioxidant activity, Principal component analysis
Highlights
-
•
SFE and SWE in single or combined mode allow extraction of value-added compounds.
-
•
SFE modifies the cell wall, and the oil fraction is rich in fatty acids.
-
•
PCA is used to correlate phenolic compounds with extraction methods.
-
•
Vanillic acid is the major phenolic compound quantified in all extracts.
-
•
First report of detection of 29 new phenolic compounds from soursop seed extracts.
Abstract
Soursop (Annona muricata L.) seeds, which is a residue obtained from juice agro-industries, were subjected to supercritical fluid extraction (SFE) and subcritical water extraction (SWE) in single or combined mode to extract the potential value-added compounds. Different extraction methods were evaluated in terms of the extraction yield, phenolics content, antioxidant activity (DPPH, ABTS, and FRAP), and Maillard reaction products. The extracts were analyzed using SEM, GC-MS, and LC-MS/MS techniques. The temperature and a combination of high-pressure techniques positively affected the overall results (SFE + SWE), affording nonpolar and polar extracts rich in phenolics and antioxidant compounds. SEM analysis showed that the use of SFE caused modifications in the cell wall, and the oil fraction was rich in fatty acids. Twenty-nine compounds associated with soursop seed extracts were detected for the first time using LC-MS/MS, showing the potential of the raw material as well as promoting resource re-utilization in circular economy.
Introduction
Tropical fruit production, trade, and consumption have increased significantly in domestic and international markets because of the attractive sensory properties of these fruits and growing recognition of their nutritional constituents such as minerals, fibers, vitamins, secondary phytochemical compounds, and therapeutic values (da Silva et al., 2020). Agribusiness is one of Brazil’s most important commercial activities, and the country is the third-highest fruit producer worldwide, behind China and India (Vidal, 2019). Among the various fruit species, the soursop tree (Annona muricata L.), a plant native to South America, is widely distributed in the tropical and subtropical regions of the world. It belongs to the Annonaceae family and has high economic potential, mainly because its fruit has a pleasant taste and aroma (Oliveira et al., 2016). Soursop fruit is primarily consumed as fresh fruit, and processed products comprising soursop fruit include juice, nectar, puree, jellies, yogurt, syrups, sweets, ice cream, and other foods (Oliveira et al., 2016). However, the residues generated during the processing steps, which correspond to 33% of the whole fruit, are commonly not used or discarded, producing a significant amount of waste that causes environmental contamination (Aguilar-Hernández et al., 2019). Once food security is ensured, this waste can be valorized in an integrated manner during the industrial application of downstream processes, transforming waste into secondary raw materials and allowing the extraction of value-added compounds via sustainable and green methodologies (Campos et al., 2020). These approaches are related to circular economy concepts including the recovery and valorization of waste materials, which allows their reusage and input back into the supply chain, affording economic growth from environmental losses (Campos et al., 2020). Many bioactive and phytochemical compounds have been identified from the organic and aqueous extracts of primary and secondary raw materials of soursop (fruits, bark, leaves, roots, and seeds), with acetogenins being the predominant class of compounds, followed by alkaloids, phenols, flavonoids, carbohydrates, cardiac glycosides, saponins, tannins, phytosterols, terpenoids, and proteins (Orak et al., 2019). Accordingly, the integration of the valorization concept allows the conversion of fruit waste into high-value products with potential applications for human consumption, such as the extraction of specific molecules and production of extracts with antioxidant activities (Campos et al., 2020). Conventional solvent extraction techniques, such as maceration and Soxhlet (SOX) extraction, are used to obtain the bioactive compounds of inedible soursop parts such as seeds, leaves, and bark (Nam et al., 2017, Orak et al., 2019). Novel extraction methods have also attracted the attention of researchers because of their advantages in comparison to the conventional extraction techniques, including faster speed of extraction and reduction in solvent consumption, as well as the possibility of combination with other processes in the biorefinery, thereby reducing or eliminating process residues (Herrero et al., 2015). Among these new methods, supercritical fluid extraction (SFE) and subcritical water extraction (SWE) are efficient tools to extract bioactive components from different natural sources (Rodrigues, Mazzutti, Vitali, Micke, & Ferreira, 2019). SFE combines liquid-like and gas-like properties, affording high density, solubilization, and solvent diffusivity, thereby increasing the mass transport (Pereira & Meireles, 2010). SWE is an attractive technique because of it affords an enhancement in the yield and decrease in the extraction time, using water in liquid state below its critical point (Tc = 374 °C and Pc = 22.064 MPa) instead of traditional solvents to recover important phenolic compounds from various agricultural and food by-products (Gonçalves Rodrigues et al., 2019, Plaza and Turner, 2015, Rodrigues et al., 2020). This study aims to evaluate the chemical composition and antioxidant activity (AA) of the soursop seed extracts obtained using different techniques. An extraction method combining SFE in the first step and SWE in a second step was used to obtain biologically active extracts from the soursop seeds. In addition, the results of single-step SWE extraction and SOX technique were compared. Therefore, this study provides information to obtain value-added products from soursop by-products, promoting resource re-utilization and circular economy.
Material and methods
Raw material and sample preparation
Soursop seeds, which are the by-products of fruit pulp processing, were provided by the Tropicássia Polpa de Fruta company, Fortaleza, Brazil. The seeds collected in 2018 were placed in plastic bags, frozen at −18 °C, and transported to the Laboratory of Thermodynamics and Supercritical Technology (LATESC) of the Federal University of Santa Catarina (UFSC). Upon arrival, the raw material was thawed and dried in an air-circulated oven (DeLeo, Porto Alegre/RS, Brazil) for 10 h at 50 °C. The dried material was crushed in a knife mill (DeLeo, Porto Alegre/RS, Brazil) and stored in polyethylene packaging at − 18 °C until use. The raw material presented a mean particle size of 0.53 mm, and moisture content of 4.98 ± 0.15% (w/w), determined according to the method reported by Gomide (1983) and the AOAC method 925.09 (AOAC, 2005), respectively.
Extraction procedures
SFe
The SFE unit and extraction procedure have been previously described in a report by Mazzutti, Rodrigues, Mezzomo, Venturi, and Ferreira (2018). Pure CO2 (99.9%) delivered at a pressure of up to 0.6 MPa (White Martins, Brazil) was used for SFE. Briefly, 30 g of raw material (dried and milled samples) was placed inside a stainless-steel extraction vessel (internal diameter of 20 mm and height of 440 mm, volume of 138.2 mL), which the empty space was filled with glass beads and cotton to form a fixed bed. Kinetic evaluation of the overall extraction curve (OEC) for the soursop seeds was performed to determine the extraction time. This assay was performed using supercritical CO2 at 20 MPa, solvent flow rate of 0.7 kg∙h−1, and temperature of 50 °C, where the extract samples were collected at pre-established time intervals. Based on the OEC, the extraction time was fixed at 3.5 h for the SFE assays (as presented in Supplementary material Fig. S1A). The SFE experiments were carried out in duplicate at temperatures of 40, 50, and 55 °C, pressures of 15, 20, 25, and 30 MPa (CO2 density 0.653, 0.784, 0.834, and 0.909 g cm−3, respectively), and a constant solvent flow rate of 0.7 kg CO2 h−1. The kinetic and the SFE experiments conditions were established based on prior experience of the group (Mazzutti et al., 2018). After each experiment, the obtained extracts were collected in amber flasks, weighed, and stored in a domestic freezer at −18 °C.
SWe
The SWE assays were performed in a customized unit following the experimental procedure described by Rodrigues et al. (2019), at least in duplicate. The SWE period was defined based on a kinetic assay performed to obtain the OEC by collecting the extract samples at pre-established period intervals at 10 MPa, 110 °C, and using a solvent flow rate of 4 mL∙min−1. These conditions were established based on previous experience of the group (Gonçalves Rodrigues et al., 2019, Rodrigues et al., 2020). The extraction time of SWE was established (five minutes) in dynamic mode, the assays were interrupted, and the system was drained to examine the OEC for recovering most of the soluble material, as presented in Fig. S1B of the Supplementary material.
Soursop seeds were subjected to extraction in a fixed extractor vessel in two modes: (i) combined-mode (CM), which involved SFE in the first step followed by SWE in the second step (SFE + SWE); (ii) single-mode (SM), where SWE was performed for comparison with the CM. In CM, soursop seeds were first subjected to SFE at 30 MPa and 40 °C (first step), and the residue of SFE was then subjected to SWE (second step). Briefly, the extraction procedure consisted of placing 5 g of soursop seeds (raw material soursop seeds or residue of SFE step), followed by mixing with 64 g of glass spheres to form a fixed bed of particles inside the AISI 316 stainless-steel extraction vessel (internal diameter of 25 mm and height of 180 mm, volume of 90 mL).
The CM was performed at 10 MPa using a water flow rate of 4 mL∙min−1 and temperatures of 70, 90, 110, and 130 °C. In contrast, SM SWE (raw material soursop seeds) was performed at 10 MPa using a water flow rate of 4 mL∙min−1, and temperatures of 70 and 130 °C, at least in duplicate, for comparison with the CM. All experiments were performed using sonicated distilled water pumped directly into the extraction cell packed with dried samples using an HPLC pump. The extracts were collected in glass flasks, rapidly cooled using a cooling fan, and stored in a refrigerator without light for solvent removal by freeze-drying for 24 h (Liotop, model LD101, São Paulo, Brazil).
Conventional extraction
Conventional atmosphere-pressure extraction was performed using the SOX technique with hexane (Hex) and ethanol (EtOH) as solvents, following the AOAC method 920.39C (AOAC, 2005), with assays performed at least in duplicate. The procedure employed 150 mL of solvent recycling over 5 g of crushed dried soursop seeds in a SOX apparatus for six hours at the solvent boiling temperature with an average of 10–15 solvent refluxes. The obtained extracts were stored in amber flasks at −18 °C before analysis. The results, expressed in extraction yield (X0), represent the mean values ± standard deviation of triplicate experiments.
Global extraction yield (X0)
The global extraction yield (X0) was calculated as the percentage of dried mass extracted () relative to the total mass of the raw material on a wet basis (), according to Eq. (1):
| (1) |
Total phenolic content (TPC)
TPC of the soursop seed extracts was determined using the Folin–Ciocalteu method (Koşar et al., 2005). Briefly, a 10 μL aliquot of the extract water solution (10 mg∙mL−1) and 600 μL of water were mixed with 50 μL of undiluted Folin–Ciocalteu reagent (Sigma-Aldrich, USA), a solution of hexavalent phosphomolybdic and phosphotungstic acid complexes. After allowing the solution to stand and stirring it for one minute, 150 μL of 20% Na2CO3 (w/v) was added, and the volume was increased to 1 mL using water. The samples were incubated for two hours at 25 °C in the dark. The absorbance was measured at 760 nm using the standard calibration curve of gallic acid. The results were expressed in milligrams of gallic acid equivalent (GAE) per gram of the dry extract, based on triplicate measurements.
DPPH free radical scavenging assay
The free radical scavenging capability of the soursop seed extracts was evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH; Sigma-Aldrich, USA) method (Mensor et al., 2001). Briefly, different extract concentrations were tested (five concentrations for each extract) by mixing 0.3 mM DPPH solution (710 μL) and 290 μL of the extract solution, providing the reaction medium (1 mL). The absorbance at 517 nm was monitored after 30 min in darkness at room temperature and converted into antioxidant activity percentage (AA%). As the mean value of triplicate assays, the results are expressed as EC50 values (test concentration required to decrease 50% absorbance compared to the blank solution) in μg∙mL−1 units. The EC50 values were calculated based on the linear regression of the AA% curves obtained for all extract concentrations. The AAs of the extracts are expressed as antiradical power (ARP), the inverse of EC50, which is used to define the AA of an antioxidant as a reciprocal of EC50.
TEAC-ABTS assay
The ABTS assay of the soursop seed extracts was performed according to the method described by Re et al. (1999) with some modifications. Synthetic vitamin E, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid; Sigma-Aldrich, USA), was used as an antioxidant standard. First, 7 mM ABTS solution and 2.45 mM potassium persulfate solution were reacted at room temperature for 16 h in darkness to produce the radical ABTS+ (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)) diammonium salt. The ABTS+ solution was then diluted with 5 mM sodium phosphate (pH 7.4) until an absorbance of 0.70 (±0.05) was achieved. Thirty microliters of the extract (five concentrations) was mixed with 970 µL of ABTS•+ and incubated in the dark for 45 min, followed by measurement of absorbance at 734 nm, providing the standard curve (0.25–2 mM). The final data are expressed in micromoles of Trolox equivalent per gram of the dry extract (μmol TE∙g−1). The results are expressed as mean ± standard deviation (average of triplicate assays).
FRAP assay
The FRAP assay of soursop seeds extracts was performed according to the method described by Benzie and Strain (1996). Briefly, 10 μL (0.1–0.5 mg∙mL−1) of the solubilized extract was placed together with 290 μL of the FRAP reagent (0.3 M, pH 3.6 acetate buffer, 10 mM TPTZ (2,4,6-tripyridyl-s-triazine), and 20 mM ferric chloride; Sigma-Aldrich, USA) in a microplate. This solution was kept in the dark at room temperature for 30 min, and the absorbance was measured at 593 nm using a microplate reader (Tecan Infinite M200). The analyses were performed in quintuplicate using a blank for each sample (10 μL of solvent + 290 μL of FRAP). Trolox (Merck, Germany) was used as a reference, and the values were calculated from the standard curve (50–500 μM). The results are expressed as micromoles of Trolox equivalent per gram of the dry extract (μmol TE∙g−1).
Analysis of the final Maillard reaction products (MRPs)
The products of the Maillard reaction (melanoidin formation) were estimated by the darkening intensity of the soursop seed extracts obtained by SWE extraction (SM and CM) according to the methodologies reported by Samaras et al., 2005, Plaza et al., 2010, with some modifications. The extracts obtained by SWE under different conditions were diluted, starting with a concentration of 1 mg∙mL−1 in water and DMSO (70:30, v/v) solution, which was filtered with a hydrophobic PTFE syringe filter (25-mm diameter and 0.45-μm pores). Absorbance at 420 nm was measured using a cuvette with a light path of 10 mm to determine the Maillard reaction degree. When necessary, the samples were diluted to obtain an absorbance reading of < 1.5 arbitrary units. The analysis was performed in duplicate, and the results are expressed as absorbance ± standard deviation.
Volatile compound analysis by gas chromatography-mass spectrometry (GC-MS)
The extract samples obtained by SFE at 30 MPa and 40 °C (based on the high yields and AA; Sections 3.2 and 3.3) and SOX extraction with hexane were selected for GC-MS analysis. The samples were subjected to methylation fractionation reaction (FAME) to assist the analysis of the compounds by GC-MS (O’Fallon, Busboom, Nelson, & Gaskins, 2007). The identification and relative quantification of the volatile compounds in the soursop seed extract were performed using a gas chromatography system equipped with a mass spectrophotometer (GC-MS, model 7890 A, mass detector 5975C, Agilent Technologies, USA), attached to an HP-5MS column (30 m × 0.32 mm (internal diameter) with a film thickness of 0.25 µm, Agilent Technologies, USA), following the method described by Mazzutti et al. (2018). Helium was used as a carrier gas with a flow rate of 4 mL∙min−1, split ratio of 5:1, injector temperature of 250 °C, and Thermal Aux 2 (MSD Transfer Line). The column temperature was increased from 60 °C to 230 °C at a rate of 3 °C∙min−1, in a total time of 55.56 min, and a quadrupole detector temperature of 150 °C was used. The major components of the selected extracts were identified by comparing the mass spectra and retention times of the compounds to those available in the NIST 11 Mass Spectral Library.
Identification and quantification of phenolic compounds by LC-ESI-MS/MS
Sample preparation
The samples were prepared according to the protocol described by Schulz et al. (2015), with some modifications. Briefly, defatted soursop seed extracts were subjected to acid hydrolysis at 85 °C using 5 mL of methanol and 5 mL of hydrochloric acid for 30 min. The solution pH was adjusted to 2 using 6 mol∙L−1 sodium hydroxide solution. Next, the acidified solution was partitioned with 10 mL of diethyl ether by centrifugation at 3000 × g for 10 min. This process was repeated twice for each sample. The supernatants were then combined in a round-bottom flask. The solvent was removed using a rotary evaporator at 40 °C until dryness. The dried sample was then resuspended in 1 mL of chromatographic grade methanol and diluted 10 times with methanol:water (30:70, v/v) mixture for injection into the LC-ESI-MS/MS system.
LC-ESI-MS/MS analysis
The identification and quantification of 41 phenolic compounds were performed using a high-performance liquid chromatography (HPLC) system (1200 Series, Agilent Technologies, Waldbronn-BW, Germany), following the methodology described by Schulz et al. (2015). A Synergi column (4.0 μm, 2.0 × 150 mm d.i.; Phenomenex, Torrance-CA, USA) was used for HPLC separation by employing gradient elution conditions. The mobile phases were composed of methanol:water (95:5 %, v/v)– A and an aqueous formic acid solution (0.1 %, v/v) – B. The separation was carried out at 30 °C using a segmented elution gradient of 10% A for 0–5 min, 10–90% A for 5–7 min, 90% A for 7–10 min, and 10% A for 10–17 min. The column was conditioned for five minutes between the analyses using the mobile phase employed at the beginning of the separation. The flow rate was 250 μL∙min−1, and the injection sample volume was 10 μL.
The LC system was coupled to a hybrid triple quadrupole/linear ion trap mass spectrometer (Q Trap 3200 Applied Biosystems/MDS Sciex, Concord-ON, Canada). The mass spectrometer was operated in negative electrospray ionization mode (TurboIonSpray Applied Biosystems/MDS Sciex, Concord-ON, Canada). The MS/MS parameters were as follows: capillary needle maintained at 4500 V; curtain gas pressure of 7 × 10-2 MPa; temperature of 400 °C; gas 1 and gas 2 pressure of 3 × 10-1 MPa; CAD gas: medium. Analyst software (version 1.6.2) was used to control the LC-ESI-MS/MS system and for data analysis. The mass spectrometer parameters of each phenolic compound were obtained based on the data reported by Schulz et al. (2015). Quantitative data for phenolic compounds were obtained from calibration curves constructed using the standards.
Scanning electron microscopy (SEM) analysis
SEM analysis (JEOL JSM 6390 L V, Musashino, Akishima, Japan) was performed at the Central Laboratory of Electronic Microscopy (Federal University of Santa Catarina, Florianópolis, SC, Brazil), with operation at 5 kV and × 300 magnification. Two samples were analyzed: (A) raw material soursop seeds before the SFE procedure and (B) solid residue of soursop seeds after SFE at 30 MPa and 40 °C. Before scanning, the samples were coated with a thin gold layer using a sputter coater.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) based on triplicate measurements. One-way analysis of variance (ANOVA) for the extraction yield, TPC, AA, and MRP assays was conducted using the Statistica software (Statsoft Inc., USA), while Tukey test was used to evaluate significant differences (p < 0.05). Principal component analysis (PCA) was performed according to the method described by Zielinski et al. (2014) using the Origin Lab Software. Before PCA and HCA analysis, all variables were auto-scaled (transformation into z-scores) to standardize the statistical importance of all responses. Then, the PCA data indicated the patterns in the dataset that showed the interrelationships between the recovery of phenolic compounds and SM and CM extraction parameters through a projection in a bidimensional scatter plot.
Results and discussion
Extraction kinetics
A kinetic study of SFE and SWE was performed to determine the process extraction time. Fig. S1A of the supplementary material shows the overall extraction curves (OEC) obtained by the SFE of raw material soursop seeds with pure CO2 at 20 MPa, 50 °C, and 0.7 kg∙h−1 and SWE of the residual material of SFE with distilled water at 110 °C, 10 MPa, and 4 mL∙min−1. The extraction curves indicate different periods: constant extraction rate (CER) period, controlled by convection and characterized by the extraction of easily accessible solute; falling extraction rate (FER) period, combining convection and diffusion mechanisms due to the partial exhaustion of surface solute; diffusion-controlled rate (DIF) period, where the solute from the particle surface is depleted (Pereira & Meireles, 2010). Then, for the SFE considering the OEC, the extraction time was fixed at 210 min, allowing the recovery of > 88% of the extractable material up to the DIF period. An analogous extraction curve was obtained for SWE, where > 62% of the total extracted mass was accumulated in the first five minutes of the process (fixed time for SWE). According to Viganó and Martinez (2015), the pressurized liquid extraction (PLE)/SWE process can be divided into two stages. The first stage corresponds to the extraction controlled by solubility (CER), and the diffusion-controlled solutes into the solvent (DIF) represent the second stage, as shown in Fig. S1B of the supplementary material. As previously reported by our research group (Gonçalves Rodrigues et al., 2019, Rodrigues et al., 2020), after an established time for SWE (five minutes), representing the first linear part of the OEC, the process is controlled by the convection mechanism because of the recovery of the easily accessible extract on the particle surface (Viganó & Martinez, 2015). The assays were interrupted, and the system was drained employing a dynamic model. This drain step allowed the recovery of soluble compounds inside the vessel and particles, combining CER and DIF fractions, representing the convection and diffusion mass transfer mechanisms, respectively (Viganó et al., 2016). This SWE methodology decreased the solvent use and process time, affording a high yield (Viganó et al., 2016).
Global extraction yield and TPC
Table 1 shows the data for global extraction yield (X0, w.b.), TPC values obtained by different methods (SFE, SWE, and SOX), and process settings for soursop seeds extraction. The SFE data in Table 1 are affected by variations in pressure and temperature, with X0 ranging from 3.6% to 16.4%. This increase can be explained by changes in the CO2 density and solvation power of the solvent (Pereira & Meireles, 2010).
Table 1.
Global extraction yield (X0), total phenolics content (TPC), and antioxidant activity, evaluated by DPPH, ARP, ABTS, and FRAP assays, of soursop seed extracts obtained by different extraction techniques in single-mode (SM) and combined mode (CM).
| Extraction method | X0 (%) | TPC (mg GAE∙g−1) | DPPH EC50 (µg∙mL−1) | ARP1 | ABTS (µmol TE∙g−1 extract) | FRAP (µmol TE∙g−1 extract) | |
|---|---|---|---|---|---|---|---|
| SM | SFE 15 MPa/55 °C | 3.6 ± 0.4i | 6.0 ± 0.5 g | >2500 | <4.0x10-4 | 2.5 ± 0.9 g | 2.3 ± 0.5 g |
| SFE 20 MPa/50 °C | 13.9 ± 0.2d,e | 13.5 ± 0.3e,f | >2500 | <4.0x10-4 | 3.3 ± 1.5 g | 7.8 ± 0.5f | |
| SFE 25 MPa/50 °C | 15.3 ± 0.2c,d | 15.4 ± 0.5e | >2500 | <4.0x10-4 | 3.9 ± 0.8 g | 8.4 ± 0.2f | |
| SFE 30 MPa/40 °C | 16.4 ± 0.0c | 20.9 ± 0.5d | >2500 | <4.0x10-4 | 4.6 ± 1.1 g | 8.6 ± 0.4f | |
| SOX EtOH | 21.2 ± 0.3b | 15.6 ± 0.9e | >2500 | <4.0x10-4 | 3.8 ± 0.5 g | 5.5 ± 1.4f,g | |
| SOX Hex | 23.5 ± 1.4a | 21.9 ± 1.8d | >2500 | <4.0x10-4 | 2.0 ± 0.1 g | 5.2 ± 0.0f,g | |
| SWE 70 °C | 11.6 ± 0.0 h | 11.4 ± 0.7f | >2500 | <4.0x10-4 | 246.5 ± 5.2e | 41.3 ± 0.3e | |
| SWE 130 °C | 11.8 ± 0.1 h | 72.2 ± 0.3b | 2337 ± 69b | 4.3x10-4 | 523.2 ± 3.9b | 152.5 ± 4.6b | |
| CM* | SWE 70 °C | 12.1 ± 0.1 h | 11.8 ± 0.5f | >2500 | <4.0x10-4 | 148.7 ± 3.9f | 30.2 ± 0.3d |
| SWE 90 °C | 12.2 ± 0.5 h | 13.8 ± 0.2e,f | >2500 | <4.0x10-4 | 282.6 ± 4.0d | 44.2 ± 0.8e | |
| SWE 110 °C | 12.3 ± 0.3f,g | 33.4 ± 2.0c | >2500 | <4.0x10-4 | 323.9 ± 2.3c | 59.6 ± 0.8c | |
| SWE 130 °C | 13.6 ± 0.2e,f | 77.3 ± 0.4a | 1264 ± 31a | 7.9x10-4 | 587.3 ± 8.8a | 162.5 ± 2.9a | |
| BHT | nd | nd | 67 ± 0.3** | 149x10-4** | 391.9 ± 0.6** | 215 ± 2** |
* SFE 30 MPa/40 °C (first step) + SWE (second step); SFE: supercritical fluid extraction; SWE: subcritical water extraction; SOX: Soxhlet extraction; EtOH: ethanol; HEX: hexane; GAE: gallic acid. TE: Trolox equivalent. BHT: butyl hydroxytoluene (synthetic antioxidant). **(Battistella Lasta et al., 2019); (1) antiradical power (ARP) inverse of EC50. Superscript letters indicate the groups that are statistically different (p < 0.05) in each column.
Corroborating these results, Santos et al., 2018, Dorado et al., 2016 reported the extraction yields of 6.9% and 12.9%, respectively, for soursop seeds using SFE under different conditions (20 MPa, 40 °C, 0.12 kg CO2∙h−1, and 145 min and 38.1 MPa, 49.8 °C, 1.8Kg CO2∙h−1 and 150 min, respectively).
In SWE, constant pressure of 10 MPa was employed because the pressure had a negligible effect in comparison to temperature on the solvent characteristics, and therefore, on process selectivity and efficiency (Gonçalves Rodrigues et al., 2019). This is because water is relatively incompressible at temperatures below 300 °C. No significant effect of pressure on the physical properties of the liquid state was observed (Plaza & Turner, 2015).
In this study, a combined extraction procedure was performed, in which the residual soursop seeds treated with SFE were further subjected to SWE (second step) to afford extracts with polar characteristics and more abundant phenolic compounds. Therefore, SFE at 30 MPa and 40 °C was selected to perform the combination experiments with soursop seeds, as the highest values of X0 and TPC were obtained under these conditions (Table 1). In SFE + SWE CM, different compound classes from the same raw material could be obtained, with nonpolar compounds extracted primarily by SFE (30 MPa/40 °C), followed by use of water under pressure to obtain extracts with polar characteristics. According to Pereira & Meireles (2010), fractionation can be used in the extraction and/or separation steps to increase the selectivity and recovery of different extracts of the same raw material.
The data presented in Table 1 show a gradual increase in the yield with an increase in the extraction temperature from 70 to 130 °C in the CM (SFE + SWE) and SM extraction, affording a maximum value of 13.6%, which results in a cumulative global yield of 30% (SFE 30 MPa/40 °C + SWE 130 °C). According to Herrero et al. (2015), the water temperature affects the extraction efficiency and selectivity in SWE. An increase in temperature results in increased diffusion rate, reduced viscosity, surface tension, and water polarity. Thus, moderately polar and nonpolar materials can be recovered in an aqueous medium at high temperatures (Herrero et al., 2015).
Additionally, the hexane and ethanol SOX extraction method yielded the highest yields of 23.5% and 21.2%, respectively. This result could be related to the longer extraction time and greater contact of the plant matrix with the solvent. Vegetable seed oils are traditionally obtained through extraction with nonpolar solvents, typically hexane, and other solvents with boiling points of up to 70 °C (Belwal et al., 2018). This process generally affords high yields, but a late-stage for solvent elimination after extraction is required, demanding high energy; additionally, the solvents are toxic to humans and dangerous to the environment. The novelty is evident because no SWE SM or CM yields are available for comparison purposes to the best of our knowledge.
Furthermore, Table 1 shows the TPC values obtained for soursop seeds using different extraction techniques, ranging from 6.0 (SFE 15 MPa/55 °C) to 77.3 mg GAE∙g−1 of dry extract (SFE 30 MPa/40 °C + SWE 130 °C). SFE at 30 MPa and 40 °C afforded the highest TPC value among the SFE experiments, and it was selected for the CM process. The TPC values obtained in the CM showed a significant increase of approximately six times when the temperature was increased from 70 to 130 °C (Table 1). Interestingly, the SWE SM and CM processes were compared at 130 °C, and a significant difference was observed in the TPC values. This indicates that the combination of extraction processes is a promising method for the efficient separation of polar and apolar fractions of soursop seeds owing to the differences in the solubilization of the plant matrix components, which improves the usage and value-addition of this raw material through the application of sustainable and green methodologies.
For comparison, conventional SOX extraction using ethanol and hexane as polar and nonpolar solvents, respectively, was performed to afford results for TPC (Table 1). These results were in agreement with the data reported in the literature for the TPC of soursop seeds obtained by conventional extraction techniques at room temperature and different organic solvents (8.2–78.5 mg GAE∙g−1 extract; Menezes et al., 2019, Moreno and Jorge, 2012). New extraction techniques that can allow energy conservation and reduce the usage of organic solvents can allow the recovery of bioactive compounds from soursop seeds.
Antioxidant activity
A mixture of different antioxidants with different action mechanisms determines the antioxidant capacity of foods; therefore, the AAs of food products must be assessed using various methods to evaluate different mechanisms (da Silva et al., 2020). Table 1 shows the AAs of soursop seeds obtained by other techniques, including the DPPH, ABTS, and FRAP methods. As expected, the SFE extracts exhibited low AAs. The goal was to remove nonpolar compounds and evaluate the extracts for AA. The SFE extracts afforded high EC50 values or low AA capacities (ARP values) in the DPPH/ARP assay (Table 1). Pinto et al. (2018) also reported that the Annona muricata seed oil showed a low AA in vitro. These results could be explained by the nonpolar characteristics of carbon dioxide that did not favor the solubilization of phenolic compounds of intermediate to high polarities (Mazzutti et al., 2018).
Furthermore, an increase in the AA was observed during analysis by all methods when the extraction temperature was increased for the extracts obtained by SWE, and the best values were obtained at the highest temperature (130 °C). Accordingly, the CM technique at 130 °C afforded the best AA value (significant results or relatively close to those for the synthetic antioxidant, BHT), probably because of the degreasing step used for the raw material sample, which allowed greater access to the compounds that were protected by the lipid layer in the soursop seeds. In the SWE technique, an increase in the extraction temperature changed the physical properties of water, allowing the recovery of different compounds, increasing the AAs of the compounds present in the extracts, or forming new compounds, for example, through the Maillard reaction. Plaza et al. (2010) reported that the compounds formed by Maillard reaction, caramelization, and thermo-oxidation could increase the AAs of the extracts obtained with SWE at temperatures above 100 °C.
The AA values for the extracts obtained by SFE and SOX with hexane were not significantly different (p > 0.05) in the TEAC-ABTS assay, which was slightly more reactive than the extracts obtained by SWE. The best results were afforded for the extracts obtained by SWE SM and CM at 130 °C (523.2 and 587.3 μmol TE∙g−1 of dry extract, respectively), and a significant difference (p > 0.05) was observed.
Based on the comparison of the methods used for determining the AAs, Table 1 shows that the best AA values (closer or greater than those for BHT) are obtained by the ABTS method, which is generally used to evaluate the AAs of hydrophilic compounds. All AA methods have been validated in the literature; however, the global values are not always similar, owing to the affinity of the antioxidant compounds and the reaction rate of each method (Gonçalves Rodrigues et al., 2019).
According to the literature, considerable variations and differences in the AA data of Annonaceae seeds are observed using different methods. For the DPPH method, the EC50 values vary from 40 to 724.1 μg∙mL−1, while those for the ABTS and FRAP methods are 0.8 –905 μmol TE∙g−1 of dry extract and 194.9 µmol TE∙g−1 of dry extract, respectively, for different conventional extraction techniques employed at atmospheric pressure using organic solvents (Benites et al., 2015, Orak et al., 2019). The results obtained using the SWE method were superior to those reported in the literature for the AAs of the soursop seeds. For example, Pinto et al. (2018) obtained extracts using methanol and chloroform as solvents with a value of 40.2 μmol TE∙g−1 of dry extract for the ABTS assay. In a cold extraction study using chloroform, methanol, and water in a 2:1:0.8 (v/v/v) ratio, da Silva and Jorge (2014) reported a value of 0.8 μmol TE∙g−1 of dry extract for the ABTS assay. Therefore, the SM and CM SWE can be used as an alternative to SOX with hexane or ethanol, providing superior or similar extracts in a significantly shorter period. Additionally, as shown in Table 1, the highest ARP values are obtained by SM SWE at 130 °C (4.3 × 10-4) and CM SWE at 130 °C (7.9 × 10-4) in comparison to other conditions for soursop seeds.
Thus, the AA of soursop seed extracts is dependent on several conditions related to the origin of the raw material, differences in cultivation, variety of species, as well as the extraction technique, solvent characteristics, and extraction time and temperature that affect the selectivities of the extracted compounds (da Silva et al., 2020).
Analysis of the final MRPs of SWE extracts
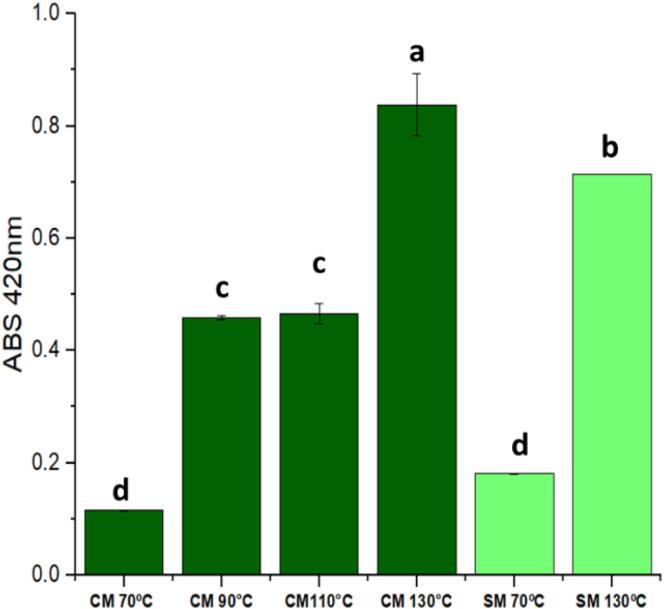
Maillard reaction is a non-enzymatic reaction employed in food thermal processing of reducing sugars and amino acids, peptides, or proteins, which affords a complex matrix of compounds called the MRPs (Plaza et al., 2010). The soursop seed extracts obtained by SWE were analyzed spectrophotometrically at 420 nm to determine the thermal effect during extraction and monitor the progress of the Maillard reaction (Fig. 1).
Fig. 1.
Effect of subcritical water extraction (SWE) temperature in single-mode (SM) and combined mode (CM) on the amounts of Maillard reaction products of soursop seed extracts. Same letters indicate no significant difference at 5 % (p < 0.05).
As reported in prior literature, temperature is an important factor affecting the Maillard reaction, which is performed at high temperatures and typically used in SWE (Gonçalves Rodrigues et al., 2019). The MRPs increased with an increase in the temperature to 130 °C in the SM and CM SWE, affording the highest yields of the extracts with significant values (p < 0.05). Therefore, an increase in temperature increased the reactivity between the sugars and amino groups in the plant matrix, indicating the progression of the Maillard reaction, which led to new antioxidant compounds (Plaza & Turner, 2015).
SEm
Fig. 2 shows the SEM data of the dried material soursop seeds material and SFE extraction residue (30 MPa/40 °C). The raw material sample shows tiny oil droplets on its surface (Fig. 2A), whereas the residue material obtained by SFE shows the decreased amount of droplets and an opening in the vegetable matrix surface, allowing high recovery of polar bioactive compounds (Fig. 2B). The SEM images assist in interpreting the results for global extraction yield, TPC, and AA described in previous sections and further confirm that the combined process can allow the recovery of compounds with different phytochemical properties. The pressure applied during SFE causes a rupture of the matrix structure. In addition, the best SWE data obtained for SFE residues are related to the structural modifications caused in the raw material by the first extraction.
Fig. 2.
Scanning electron microscopy (SEM) images of soursop seeds with a magnification of 1000×: (A) raw material; (B) residue material after supercritical fluid extraction (SFE) at 30 MPa and 40 °C.
Chromatographic analysis
GC-MS analysis of volatile compounds of soursop seeds extract
SFE using CO2 allows the extraction of easily oxidizable or thermosensitive compounds by operating at low temperatures using a non-oxidizing medium. Furthermore, in this process, the compounds are not exposed to light. It’s important, due CO2 is ideal for lipid, greasy, and non-polar substances (such as carotenoids, aromas, volatile compounds) and limited in its affinity for polar solutes (Herrero et al., 2015). For this reason, the chemical profile of the volatile fraction was determined to allow the identification of the main compounds present in the soursop seed extracts obtained by SFE at 30 MPa and 40 °C comparing to SOX using hexane. Soursop seed oil extracted by SFE contained 27.6% saturated fatty acids and 70.0% unsaturated fatty acids (Table 2). Similar fatty acid profiles have also been reported in the literature, with unsaturated fatty acid percentages of 64–75% (Dorado et al., 2016, Pinto et al., 2018). The major compounds identified in terms of the relative area percentage and/or relevance in the extracts were the unsaturated fatty acids, oleic acid (ω9), 10,13-octadecadienoic acid (PUFA), linoleic acid (ω6), palmitoleic acid (ω7), and elaidic acid (a trans geometric isomer of oleic acid), as well as saturated fatty acids, palmitic acid, and stearic acid.
Table 2.
Major compounds determined by gas chromatography-mass spectrometry (GC-MS) in the volatile fraction of soursop seeds obtained by supercritical fluid extraction (SFE) at 30 MPa and 40 °C and Soxhlet (SOX) technique using hexane as a solvent.
| Compounds | Relative peak area (%) | |
|---|---|---|
| SFE 30 MPa/40 °C | SOX Hexane | |
| Miristic acid | 0.1 | 0.1 |
| Palmitoleic acid | 1.5 | 1.8 |
| Palmitic acid | 21.7 | 22.6 |
| Linolieic acid | 12.3 | 19.1 |
| 10,13-Octadecadienoico acid | 24.4 | 10.9 |
| Elaidic acid | – | 38.3 |
| Oleic acid | 32.1 | – |
| Stearic acid | 5.9 | 5.6 |
| Methylpalmitic acid | 0.7 | 0.6 |
* SFE 30 MPa/40 °C (first step); SFE: Supercritical fluid extraction.
The unsaturated fatty acids were predominant in the soursop seeds obtained by SFE, mainly including oleic and linoleic acid as well as essential fatty acids, which regulate various functions including blood pressure, blood clotting, blood lipid levels, immune response, and anti-inflammatory properties, and protect the cardiovascular system (Bhardwaj et al., 2014). These also aid in maintaining integrity and nutrition, strengthening the lipid barrier, hydrating the skin, and other vital functions of the human body (Dorado et al., 2016).
The SOX method, employing hexane as a solvent for extraction, is one of the most common methods for preparing raw material soursop seeds oil (Orak et al., 2019). However, using temperatures close to that of the hexane boiling point (69 °C) during extraction can affect the quality of the extracted oil (Belwal et al., 2018). During SOX extraction, the isomerization of oleic acid can occur as it is a thermally induced process (Cheng et al., 2018), resulting in the formation of elaidic acid, which is a trans-isomer of oleic acid. Trans fatty acids are correlated to coronary diseases and arteriosclerosis (Debbabi et al., 2017). In contrast, the oil obtained by low-temperature SFE has a significant amount of oleic acid, and the formation of trans isomers does not occur, affording a better-quality extract.
Only a few efforts have been directed toward the industrial exploitation of soursop seeds commonly discarded during the processing of commercially harvested fruit juices and pulps (Pinto et al., 2018). Thus, based on the composition of fatty acids, soursop seeds contain essential compounds that can be applied in food industry. Therefore, seeds are a potential source of oil, which should be further investigated to determine their properties; oil extraction from these can be an alternative to the use of commercial fruit seeds waste, which can contribute toward a sustainable and circular economy (Menezes et al., 2019, Campos et al., 2020).
LC-MS/MS analysis
Table 3 shows the phenolic compounds identified and quantified by LC-ESI-MS/MS in soursop seed extracts obtained by different methods. Of the 41 phenolic compounds tested (standards), all 41 were detected, and 23 were quantified in the SWE and SOX ethanol extracts; the concentrations ranged from 0.0465 to 0.2656 mg g−1 of extract. Among these, the process that afforded the highest amounts of phenolic compounds was SM SWE at 130 °C (21 compounds quantified), and that which provided the lowest amounts was SOX (11 compounds quantified). The phenolic profiles of soursop seed extracts were predominantly composed of phenolic acids and flavonoids, but other phenolic compound classes were also detected. The major phenolic compounds found in soursop seed extracts were vanillic acid (17), p-coumaric acid (12), 3,4 dihydroxybenzoic acid (1), ellagic acid (7), vanillin (38), kaempferol (26), 4-aminobenzoic acid (2), 4-hydroxymethylbenzoic acid (3), caffeic acid (4), and ferulic acid (8). The extract also contained smaller amounts of quercetin (30), mandelic acid (10), salicylic acid (14), myricetin (27), gallic acid (9), sinapic acid (15), syringaldehyde (37), coniferaldehyde (35), umbelliferone (40), syringic acid (16), epicatechin (21), taxifolin (32), rosmarinic acid (13), and other compounds below the limit of quantification (LOQ).
Table 3.
Antioxidant compounds in soursop seeds extract obtained by single-mode (SM) and combined-mode (CM) subcritical water extraction (SWE) and Soxhlet (SOX) technique using ethanol (EtOH) as a solvent.
| Phenolic Compound | SWE SM 70 °C (mg∙g−1) | SWE SM 130 °C (mg∙g−1) | SWE CM 70 °C (mg∙g−1) | SWE CM 130 °C (mg∙g−1) | SOX EtOH (mg∙g−1) | |
|---|---|---|---|---|---|---|
| Phenolic acid | ||||||
| 1 | 3,4 Dihydroxybenzoic acid | 0.0021 ± 0.0 | 0.0374 ± 0.0025 | 0.0033 ± 0.0003 | 0.0474 ± 0.0051 | 0.0032 ± 0.0007 |
| 2 | 4- Aminobenzoic acid* | < LOQ | 0.0194 ± 0.0026 | < LOQ | 0.0220 ± 0.0058 | <LOQ |
| 3 | 4-Hydroxymethylbenzoic acid* | < LOQ | 0.0135 ± 0.015 | < LOQ | 0.0267 ± 0.0028 | 0.0005 ± 0.0003 |
| 4 | Caffeic acid | 0.0015 ± 0.0001 | 0.0152 ± 0.0010 | 0.0017 ± 0.0003 | 0.0109 ± 0.0015 | 0.0011 ± 0.0 |
| 5 | Cinnamic acid | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| 6 | Chlorogenic acid | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| 7 | Ellagic acid* | 0.0043 ± 0.0005 | 0.0382 ± 0.0024 | 0.0018 ± 0.0 | 0.0080 ± 0.0014 | 0.0044 ± 0.0010 |
| 8 | Ferulic acid* | 0.0052 ± 0.0018 | 0.0073 ± 0.0011 | 0.0038 ± 0.0005 | 0.0074 ± 0.0010 | 0.0031 ± 0.0003 |
| 9 | Gallic acid | < LOQ | 0.0014 ± 0.0002 | < LOQ | 0.0016 ± 0.0001 | <LOQ |
| 10 | Mandelic acid* | 0.0012 ± 0.0002 | 0.0039 ± 0.0005 | 0.0014 ± 0.0 | 0.0056 ± 0.0001 | 0.0013 ± 0.0002 |
| 11 | p-Anisic acid* | <LOQ | < LOQ | <LOQ | <LOQ | <LOQ |
| 12 | p-Coumaric acid | 0.0182 ± 0.0036 | 0.0189 ± 0.0028 | 0.0166 ± 0.0017 | 0.0243 ± 0.0046 | 0.0270 ± 0.0058 |
| 13 | Rosmarinic acid* | <LOQ | <LOQ | <LOQ | 0.0001 ± 0.0 | <LOQ |
| 14 | Salicylic acid* | 0.0024 ± 0.0 | 0.0028 ± 0.0002 | 0.0001 ± 0.0 | 0.0033 ± 0.0004 | <LOQ |
| 15 | Sinapic acid* | nd | 0.0004 ± 0.0001 | 0.0006 ± 0.0002 | 0.0014 ± 0.0 | <LOQ |
| 16 | Syringic acid | <LOQ | 0.0017 ± 0.0 | < LOQ | <LOQ | <LOQ |
| 17 | Vanillic acid* | 0.0060 ± 0.0010a | 0.0400 ± 0.0047 | 0.0086 ± 0.0006 | 0.0635 ± 0.0068 | 0.0141 ± 0.0019 |
| Flavonoid | ||||||
| 18 | Apeginin* | <LOQ | nd | <LOQ | <LOQ | <LOQ |
| 19 | Catechin | <LOQ | nd | nd | nd | <LOQ |
| 20 | Chrysin* | <LOQ | <LOQ | nd | <LOQ | <LOQ |
| 21 | Epicatechin* | <LOQ | 0.0005 ± 0.0 | 0.0001 ± 0.0 | <LOQ | <LOQ |
| 22 | Eriodictyol* | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| 23 | Fustin* | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 24 | Galagngina* | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 25 | Hispidulin* | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 26 | Kaempferol* | 0.0411 ± 0.0065 | <LOQ | 0.0025 ± 0.0007 | <LOQ | <LOQ |
| 27 | Myricetin* | 0.0008 ± 0.0 | 0.0011 ± 0.0 | 0.0009 ± 0.0 | 0.0026 ± 0.0001 | 0.0012 ± 0.0 |
| 28 | Naringenin* | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 29 | Pinocembrin* | <LOQ | <LOQ | < LOQ | nd | nd |
| 30 | Quercetin | 0.0117 ± 0.0011 | 0.0006 ± 0.0001 | 0.0051 ± 0.0007 | 0.0028 ± 0.0002 | 0.0039 ± 0.0006 |
| 31 | Rutin | <LOQ | <LOQ | < LOQ | <LOQ | <LOQ |
| 32 | Taxifolin* | <LOQ | 0.0004 ± 0.0001 | < LOQ | <LOQ | <LOQ |
| 33 | Vitexina* | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Stilbene | ||||||
| 34 | Resveratrol | nd | nd | nd | <LOQ | nd |
| Phenolic Aldehyde | ||||||
| 35 | Coniferaldehyde* | <LOQ | 0.0005 ± 0.0001 | < LOQ | 0.0014 ± 0.0002 | <LOQ |
| 36 | Sinapaldehyde* | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 37 | Syringaldehyde* | <LOQ | 0.0022 ± 0.0008 | <LOQ | <LOQ | <LOQ |
| 38 | Vanillin | 0.0003 ± 0.0001 | 0.0152 ± 0.0025 | < LOQ | 0.0357 ± 0.0071 | 0.0008 ± 0.0004 |
| Coumarin | ||||||
| 39 | Scopoletin* | <LOQ | nd | < LOQ | nd | <LOQ |
| 40 | Umbelliferone* | <LOQ | 0.0008 ± 0.0001 | < LOQ | 0.0009 ± 0.0003 | <LOQ |
| Phenolic Diterpene | ||||||
| 41 | Carnosol* | <LOQ | <LOQ | < LOQ | <LOQ | <LOQ |
| Total Phenolic Content (mg g−1) | 0.0948 | 0.2214 | 0.0465 | 0.2656 | 0.0606 | |
< LOQ, not quantifiable.
nd: not detected; * reported for the first time.
In both SM and CM SWE, a high temperature (130 °C) favored extracting the highest amounts of total phenolic compounds (Table 3). For example, at this temperature, vanillic acid (17) was the main component of the phenolic content of soursop seeds extracts, and the highest amounts of phenolics were detected for the first time in the soursop seeds (Annona muricata; 0.0060–0.0635 mg∙g−1) with SM and CM SWE. These findings corroborate the TPC and AA data (Table 1, Table 3, respectively). The profiles of the phenolic compounds obtained for the SOX samples showed the presence of 39 compounds, of which 11 were quantified; p-coumaric and vanillic acid were the most abundant compounds with amounts of 0.0270 and 0.0141 mg∙g−1, respectively. The low quantity of compounds detected in SOX extracts compared to those in the extracts obtained by SWE could be attributed to the long process time of 360 min for the SOX method compared to five minutes for SWE. Furthermore, these differences in the quantification of phenolic compounds found (Table 3) may also be due to the solvent and its solubility characteristic of the desired analyte, and their diffusivity in the solvent. In subcritical water medium, the temperature increase resulted in an increase in the diffusion rate and higher solubility of phenolic compounds. According to Munir et al. (2018), depending on temperature, polar to medium polar compounds can be extracted with high solubility using SWE. Besides that, the effect of the high pressure of the system (at SFE and SWE processes) gives rise to a phenomenon called penetration, increasing the interaction between the matrix and solvent (Chaves et al., 2020). It is noteworthy that the SWE process, if integrated with the SFE, allows the SFE to weaken cell walls from the solid phase, changing the characteristics of the plant matrix, enabling greater solubility of the compounds (Ferro et al., 2019).
Ideally, an extract of high purity and high selectivity should be achieved, which implies that the analyte of interest should have high solubility in the solvent while other compounds should have no or minimal solubility, and when extracting analytes at low concentrations, the rate of extraction is not affected by the analyte concentration but rather by the rate of mass transfer (Mustafa & Turner, 2011). However, to measure the solubility of molecularly complex and very polar solutes in hot pressurized water is very difficult, due to their high cost and low thermo-stability at high temperatures and pressures (Srinivas et al., 2009). Therefore, this result shows the advantages of the SWE technique, including faster and greener characteristics compared to that of the SOX method (Gonçalves Rodrigues et al., 2019).
Vanillic acid was also found in high concentrations in the methanol/water (80:20, v/v) extracts of the peel and seeds of Annona cherimola cultivars, ‘Campa’ and ‘Fino de Jete,’ as reported in a study describing the identification and quantification of phenolics and other polar compounds in the edible part of Annona cherimola and its byproducts by HPLC-DAD-ESI-QTOF-MS (García-Salas et al., 2015). Vanillic acid content was 0.0812 and 0.3813 mg∙g−1 in ‘Campa’ and ‘Fino de Jete’, respectively, and it was also higher in ‘Fino de Jete’ peel than in ‘Campa’ peel. The concentration of vanillic acid was higher in ‘Campa’ seeds than in ‘Fino de Jete’ seeds, i.e., 0.132 and 1.0129 mg g−1, respectively.
Other phenolic compounds, such as 3,4 dihydroxybenzoic acids (1), caffeic acid (4), cinnamic acid (5), chlorogenic acid (6), ferulic acid (8), gallic acid (9), p-coumaric acid (12), syringic acid (16), catechin (19), epicatechin (21), kaempferol (26), naringenin (28), quercetin (30), rutin (31), taxifolin (32), and vanillin (38), are known to contribute to the AAs of the soursop seed, pulp, and leaf extracts obtained using different techniques and solvents (Aguilar-Hernández et al., 2019, Menezes et al., 2019). These were also detected in the soursop extracts obtained in the present study.
The identified compounds, caffeic acid (4), cinnamic acid (5), ferulic acid (8), and syringic acid (16), have been reported to have therapeutic applications in preventing diabetes, cancer, and cerebral ischemia, as well as possess antioxidant, antimicrobial, anti-inflammatory, antitumor, antiendotoxic, neuro and hepatoprotective activities (Srinivasulu et al., 2018, Nam et al., 2017, Peperidou et al., 2017). Several factors can affect the extraction characteristics, including the extraction and analytical method, time of harvest, maturity, variety, climate and soil conditions, sun exposure, location of the fruits on the plant, and post-harvest handling (Menezes et al., 2019, Nam et al., 2017). Additionally, as shown in Table 3, 29 phenolic compounds have been detected for the first time, 16 of which are quantified in the extracts obtained by SWE of the soursop seeds (Annona muricata) by LC-ESI-MS/MS analysis, while six compounds are quantified in the SOX ethanol extracts for the first time.
The scarcity of studies describing the recovery of phenolic compounds from soursop seeds by SWE makes the information presented herein important for value addition to the raw material. Additionally, it has not been extensively explored as an alternative greener technique compared to the traditional methods. Therefore, information regarding the chemical profile and AA is valuable for its applications in food or nutraceutical industries.
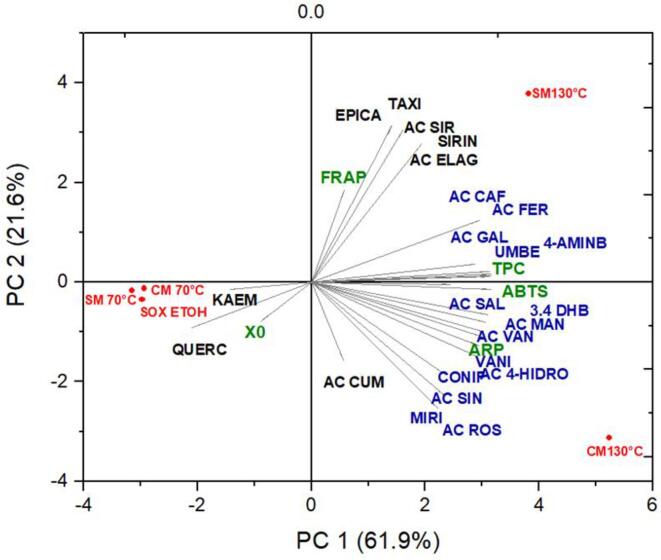
Principal component analysis (PCA)
PCA was applied to correlate the results obtained for X0, TPC, ABTS, ARP, FRAP, and the compounds identified by LC-MS/MS with the performance of the SWE (SM and CM) and SOX extraction methods. Multivariate treatment of the sample data allowed the reduction of the variables to two principal components. Fig. 3 shows a two-dimensional graph with 83.5% experimental data variability. Principal component 1 (PC1) accounted for up to 61.9% of the total variance, and PC2 for 21.6% of the explained variance determined by eigenvalues of > 1. As shown in Fig. 3, the extracts obtained by SWE at 130 °C in the SM or CM, TPC, ARP, ABTS, and FRAP data, and most phenolic compounds are positively correlated with the PC1 and PC2 axis. The SWE (SM and CM) extracts obtained at 70 °C, and quercetin and kaempferol contents are negatively correlated with the PC2 axis. In addition, SOX and X0 are negatively correlated.
Fig. 3.
Principal component analysis (PCA) of subcritical water extraction (SWE) in single-mode (SM) and combined mode (CM) at 70 °C and 130 °C and using the Soxhlet technique (SOX) with ethanol (EtOH) as a solvent, based on the global extraction yield (X0), total phenolics content (TPC), antiradical power (ARP), ABTS, FRAP assays, and phenolic compounds scores for the biplot of the first two PCs. Compounds: 3,4 DHB (3,4-dihydroxybenzoic acids); 4-AMINB (4-aminobenzoic acid); AC 4-HIDRO (4-hydroxymethylbenzoic acid); AC CAF (caffeic acid); AC ELAG (ellagic acid); AC FER (ferulic acid); AC GAL (gallic acid); AC MAN (mandelic acid); AC CUM (p-coumaric acid); AC ROS (rosmarinic acid); AC SAL (salicylic acid); AC SIN (sinapic acid); AC SIR (syringic acid); AC VAN (vanillic acid); EPICA (epicatechin); KAEM (kaempferol); MIRI (myricetin); QUERC (quercetin); TAXI (taxifolin); CONIF (coniferaldehyde); SIRIN (sinapaldehyde); VANI (vanillin); UMBE (umbelliferone).
Fig. 3 also shows a higher number of phenolic compounds on the right side of the graph, near SM and CM at 130 °C, forming a group with 15 phenolic compounds with higher TPC, ARP, and ABTS data, as well as a second group containing eight phenolic compounds, X0, and FRAP lower data, according to HCA analysis. The PCA revealed that SOX and SWE at 70 °C (SM and CM) showed a few phenolic compounds recovered on the left side of the graph but a high concentration of quercetin (0.0117 mg.g−1) and kaempferol (0.0411 mg.g−1). PCA Biplot shows more influence of CM extraction at 130 °C than the SM in recovery phenolic compounds. These data are consistent with the results of the previous studies describing the AA and phenolic compounds identified from soursop seed extracts (Table 1, Table 3). Therefore, PCA is a suitable technique to determine similarities among the phenolic compound compositions of the extracts obtained by SWE and SOX methods. The PCA scatter plot is significant once all samples are projected in a two-dimensional graph and comparisons between the samples are performed based on the response variables used in the study.
Conclusion
This study shows that a combination of green extraction techniques allows the collection of different fractions from soursop seeds. In the SWE method, X0, TPC, AA, and MRPs were positively correlated with temperature for a combination of high-pressure techniques, SFE + SWE. SEM analysis showed that the SFE step allowed lipid removal and caused the raw material cell wall rupture, which afforded a high recovery of polar bioactive compounds of the SFE residue when the CM SFE + SWE method was used. The soursop seeds oil extracted by SFE was mainly composed of unsaturated fatty acids, oleic and linoleic acids, and saturated fatty acids such as palmitic and stearic acids. Furthermore, the soursop seeds extract obtained from SWE, and SFE residue under different conditions showed the presence of vanillic acid, 3,4 dihydroxybenzoic acids, p-coumaric acid, vanillin, and caffeic acid as the most abundant phenolic compounds.
Moreover, 29 phenolic compounds were detected for the first time from soursop seed extracts. PCA analysis was a valuable tool for correlating phenolic compounds and AA with the extraction and technique temperature to obtain the soursop seeds extracts. Thus, nonpolar and polar extracts with phenolic and antioxidant compounds could be obtained using sequential high-pressure extraction steps, showing the potential valorization of the soursop industrial by-product from the cosmetic, pharmaceutical, and food industries. Then, the results found in this work are important information to the circular economy approach by recycling food waste derived from the agri-food production chain, using green-based extraction techniques to recover bioactive compounds, encouraging a perspective zero waste.
CRediT authorship contribution statement
Patricia C. Mesquita: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Luiz Gustavo G. Rodrigues: Supervision, Writing – original draft, Investigation, Visualization, Supervision. Simone Mazzutti: Conceptualization, Writing – review & editing. Mayara da Silva: Formal analysis, Writing – review & editing. Luciano Vitali: Formal analysis, Writing – review & editing. Marcelo Lanza: Investigation, Resources, Conceptualization, Validation, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the Tropicassia Polpa de Frutas-ME for raw material donation and the Brazilian funding agencies for the financial support and fellowship. We acknowledge the Coordination for the Improvement of Higher-Level Personnel (CAPES), Brazil projects, CAPES/DINTER UFSC-IFCE (Project 1955/2016), CAPES/PROEX (Project 1624/2018), and INCT-Catálise/FAPESC/CNPq/CAPES (FAPESC-2019TR0847, CNPq-444061/2018). We are also grateful to the Laboratório Multiusuário de Estudos em Biologia (LAMEB), Biological Sciences Center (CCB), and the Central Laboratory of Electron Microscopy (LCME/UFSC) of the Federal University of Santa Catarina (UFSC).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100164.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aguilar-Hernández G., García-Magaña M., Vivar-Vera M., Sáyago-Ayerdi S., Sánchez-Burgos J., Morales-Castro J.…Montalvo González E. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Annona muricata By-Products and Pulp. Molecules. 2019;24(5):904. doi: 10.3390/molecules24050904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC. (2005). Official Methods of Analysis of AOAC INTERNATIONAL (W. liam Horwitz & J. George W. Latimer (eds.); 18th ed., Issue February). AOAC International Suite 500 481 North Frederick Avenue, Gaithersburg, Maryland 20877-2417, USA.
- Battistella Lasta H.F., Lentz L., Gonçalves Rodrigues L.G., Mezzomo N., Vitali L., Salvador Ferreira S.R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatalysis and Agricultural Biotechnology. 2019;21(July):101353. doi: 10.1016/j.bcab.2019.101353. [DOI] [Google Scholar]
- Belwal T., Ezzat S.M., Rastrelli L., Bhatt I.D., Daglia M., Baldi A.…Atanasov A.G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC - Trends in Analytical Chemistry. 2018;100:82–102. doi: 10.1016/j.trac.2017.12.018. [DOI] [Google Scholar]
- Benites R., Formagio A., Argandoña E., Volobuff C., Trevizan L., Vieira M., Silva M. Contents of constituents and antioxidant activity of seed and pulp extracts of Annona coriacea and Annona sylvatica. Brazilian Journal of Biology. 2015;75(3):685–691. doi: 10.1590/1519-6984.21313. [DOI] [PubMed] [Google Scholar]
- Benzie I.F.F.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘“ Antioxidant Power ”’: The FRAP Assay. Analytical Biochemistry. 1996;239(0292):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhardwaj, A., Satpathy, G., & Gupta, R. K. (2014). Preliminary screening of nutraceutical potential of Annona squamosa , an underutilized exotic fruit of India and its use as a valuable source in functional foods. Journal of Pharmacognosy and Phytochemistry, 3(2), 172–180. https://doi.org/http://dx.doi.org/10.22271/phyto.
- Campos, D. A., Gómez-García, R., Vilas-Boas, A. A., Madureira, A. R., & Pintado, M. M. (2020). Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules, 25(320), 1–22. https://doi.org/doi:10.3390/molecules25020320. [DOI] [PMC free article] [PubMed]
- Chaves J.O., de Souza M.C., da Silva L.C., Lachos-Perez D., Torres-Mayanga P.C., Machado A.P.d.F.…Rostagno M.A. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Frontiers Chemistry. 2020;8 doi: 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasulu C., Ramgopal M., Ramanjaneyulu G., Anuradha C.M., Suresh Kumar C. Syringic acid (SA) - A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomedicine & Pharmacotherapy. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Cheng, N., Zhang, J., Yin, J., & Li, S. (2018). Computational and experimental research on mechanism of cis / trans isomerization of oleic acid. Heliyon, April, e00768. https://doi.org/10.1016/j.heliyon.2018.e00768. [DOI] [PMC free article] [PubMed]
- Debbabi M., Zarrouk A., Bezine M., Meddeb W., Nury T., Badreddine A.…Lizard G. Comparison of the effects of major fatty acids present in the Mediterranean diet (oleic acid, docosahexaenoic acid) and in hydrogenated oils (elaidic acid) on 7-ketocholesterol-induced oxiapoptophagy in microglial BV-2 cells. Chemistry and Physics of Lipids. 2017;207:151–170. doi: 10.1016/j.chemphyslip.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Dorado D.J., Hurtado-Benavides A.M., Martínez-Correa H.A. Extracción con CO2 Supercrítico de aceite de semillas de guanábana (Annona muricata): Cinética, perfil de ácidos grasos y esteroles. Informacion Tecnologica. 2016;27(5):37–48. doi: 10.4067/S0718-07642016000500005. [DOI] [Google Scholar]
- Ferro D.M., Mazzutti S., Vitali L., Oliveira Müller C.M., Ferreira S.R.S. Integrated extraction approach to increase the recovery of antioxidant compounds from Sida rhombifolia leaves. The Journal of Supercritical Fluids. 2019;149:10–19. doi: 10.1016/j.supflu.2019.03.013. [DOI] [Google Scholar]
- García-Salas P., Gómez-Caravaca A.M., Morales-Soto A., Segura-Carretero A., Fernández-Gutiérrez A. Identification and quantification of phenolic and other polar compounds in the edible part of Annona cherimola and its by-products by HPLC-DAD-ESI-QTOF-MS. Food Research International. 2015;78(2015):246–257. doi: 10.1016/j.foodres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Gomide R. 1st ed. Camara Brasileira do Livro-SP; 1983. Operações Unitárias-OPERAÇÕES COM SISTEMAS SÓLIDOS GRANULARES. [Google Scholar]
- Gonçalves Rodrigues L.G., Mazzutti S., Vitali L., Micke G.A., Ferreira S.R.S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatalysis and Agricultural Biotechnology. 2019;22(August):101367. doi: 10.1016/j.bcab.2019.101367. [DOI] [Google Scholar]
- Herrero M., Sánchez-Camargo A.D.P., Cifuentes A., Ibáñez E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC - Trends in Analytical Chemistry. 2015;71:26–38. doi: 10.1016/j.trac.2015.01.018. [DOI] [Google Scholar]
- Koşar M., Dorman H.J.D., Hiltunen R. Effect of an acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae species. Food Chemistry. 2005;91(3):525–533. doi: 10.1016/j.foodchem.2004.06.029. [DOI] [Google Scholar]
- Mazzutti S., Rodrigues L.G.G., Mezzomo N., Venturi V., Ferreira S.R.S. Integrated green-based processes using supercritical CO 2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. The Journal of Supercritical Fluids. 2018;135:52–59. doi: 10.1016/j.supflu.2017.12.039. [DOI] [Google Scholar]
- Menezes E.G.T., Oliveira É.R., Carvalho G.R., Guimarães I.C., Queiroz F. Assessment of chemical , nutritional and bioactive properties of Annona crassiflora and Annona muricata wastes. Food Sci. Technol. 2019;39(Suppl 2):662–672. doi: 10.1590/fst.22918. [DOI] [Google Scholar]
- Mensor L.L., Menezes F.S., Leitão G.G., Reis A.S., Santos T.C.D., Coube C.S., Leitão S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytotherapy Research. 2001;15(2):127–130. doi: 10.1002/ptr.v15:210.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Moreno D.M., Jorge L.N. Soursop (Annona muricata L.) and sugar apple (Annona squamosa L.) Antioxidant activity, fatty acids profile and determination of tocopherols. Nutrition & Food Science. 2012;42(6):434–441. doi: 10.1108/00346651211277690. [DOI] [Google Scholar]
- Munir M.T., Kheirkhah H., Baroutian S., Quek S.Y., Young B.R. Subcritical water extraction of bioactive compounds from waste onion skin. Journal of Cleaner Production. 2018;183:487–494. doi: 10.1016/j.jclepro.2018.02.166. [DOI] [Google Scholar]
- Mustafa A., Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Analytica Chimica Acta. 2011;703(1):8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Nam J., Park S.-Y., Jang H.-L., Rhee Y.H. Phenolic compounds in different parts of young Annona muricata cultivated in Korea and their antioxidant activity. Applied Biological Chemistry. 2017;60(5):535–543. doi: 10.1007/s13765-017-0309-5. [DOI] [Google Scholar]
- O’Fallon J.V., Busboom J.R., Nelson M.L., Gaskins C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. Journal of Animal Science. 2007;85(6):1511–1521. doi: 10.2527/jas.2006-491. [DOI] [PubMed] [Google Scholar]
- Oliveira, E. N. A. de, Santos, D. da C., Santos, Y. M. G. dos, Buchweitz, P. R., & Gomes, J. P. (2016). Soursop liquor processing: Influence of the process variables on the physical and chemical characteristics. Rev. Caatinga, 29(1), 246–256. https://doi.org/http://dx.doi.org/10.1590/1983-21252016v29n129rc.
- Orak, H. H., Bahrisefit, I. S., & Sabudak, T. (2019). Antioxidant Activity of Extracts of Soursop (Annona muricata L .) Leaves , Fruit Pulps , Peels , and Seeds. Pol. J. Food Nutr. Sci, 69(4), 359–366. https://doi.org/10.31883/pjfns/112654.
- Peperidou A., Pontiki E., Hadjipavlou-Litina D., Voulgari E., Avgoustakis K. Multifunctional Cinnamic Acid Derivatives. Molecules. 2017;22(1247):1–17. doi: 10.3390/molecules22081247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C.G., Meireles M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food and Bioprocess Technology. 2010;3(3):340–372. doi: 10.1007/s11947-009-0263-2. [DOI] [Google Scholar]
- Pinto L.C., De Souza C.O., De Souza S.A., Da Silva H.B., Da Silva R.R., Cerqueira-Lima A.T.…Figueiredo C.A. Potential of <em>Annona muricata</em> L. seed oil: Phytochemical and nutritional characterization associated with non-toxicity. Grasas y Aceites. 2018;69(1):234. doi: 10.3989/gya.0777171. [DOI] [Google Scholar]
- Plaza M., Amigo-Benavent M., del Castillo M.D., Ibáñez E., Herrero M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Research International. 2010;43(10):2341–2348. doi: 10.1016/j.foodres.2010.07.036. [DOI] [Google Scholar]
- Plaza M., Turner C. Pressurized hot water extraction of bioactives. TrAC Trends in Analytical Chemistry. 2015;71:39–54. doi: 10.1016/j.trac.2015.02.022. [DOI] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues L.G.G., Mazzutti S., Siddique I., da Silva M., Vitali L., Ferreira S.R.S. Subcritical water extraction and microwave-assisted extraction applied for the recovery of bioactive components from Chaya (Cnidoscolus aconitifolius Mill.) Journal of Supercritical Fluids. 2020;165:104976. doi: 10.1016/j.supflu.2020.104976. [DOI] [Google Scholar]
- Samaras T.S., Camburn P.A., Chandra S.X., Gordon M.H., Ames J.M. Antioxidant properties of kilned and roasted malts. Journal of Agricultural and Food Chemistry. 2005;53(20):8068–8074. doi: 10.1021/jf051410f. [DOI] [PubMed] [Google Scholar]
- Santos M.F., Gomes J.J., Santos D., Cardozo-Filho L., de Jesus E. Parâmetros de transferência de massa da extração do óleo de sementes de mangaba (Hancornia speciosa Gomes) e de graviola (Annona muricata L.) utilizando dióxido de carbono supercrítico. Scientia Plena. 2018;14(6):1–7. doi: 10.14808/sci.plena.2018.064206. [DOI] [Google Scholar]
- Schulz M., Borges G.d.S.C., Gonzaga L.V., Seraglio S.K.T., Olivo I.S., Azevedo M.S.…Fett R. Chemical composition, bioactive compounds and antioxidant capacity of juçara fruit (Euterpe edulis Martius) during ripening. Food Research International. 2015;77:125–131. doi: 10.1016/j.foodres.2015.08.006. [DOI] [Google Scholar]
- da Silva A.C., Jorge N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Research International. 2014;66:493–500. doi: 10.1016/j.foodres.2014.10.025. [DOI] [Google Scholar]
- Silva, L. M. R. da, Sousa, P. H. M. de, Sabino, L. B. de S., Prado, G. M. do, Torres, L. B. V., Maia, G. A., Figueiredo, R. W. de, & Ricardo, N. M. P. S. (2020). Brazilian (North and Northeast) Fruit By-Products. In R. Campos‐Vega, B. D. Oomah, & H. A. Vergara‐Castañeda (Eds.), Food Wastes and By‐products: Nutraceutical and Health Potential (1st ed., pp. 127–158). John Wiley & Sons Ltd. https://doi.org/https://doi.org/10.1002/9781119534167.ch5.
- Srinivas K., King J.W., Monrad J.K., Howard L.R., Hansen C.M. Optimization of subcritical fluid extraction of bioactive compounds using hansen solubility parameters. Journal of Food Science. 2009;74(6):342–354. doi: 10.1111/j.1750-3841.2009.01251.x. [DOI] [PubMed] [Google Scholar]
- Vidal, M. D. F. (2019). Fruticultura na área de atuação do bnb: produção e mercado (No. 84; Caderno Setorial ETENE).
- Viganó J., Brumer I.Z., Braga P.A.d.C., da Silva J.K., Maróstica Júnior M.R., Reyes Reyes F.G., Martínez J. Pressurized liquids extraction as an alternative process to readily obtain bioactive compounds from passion fruit rinds. Food and Bioproducts Processing. 2016;100:382–390. doi: 10.1016/j.fbp.2016.08.011. [DOI] [Google Scholar]
- Viganó J., Martinez J. Trends for the Application of Passion Fruit Industrial By-products: A Review on the Chemical Composition and Extraction Techniques of Phytochemicals. Food and Public Health. 2015;5(5):164–173. doi: 10.5923/j.fph.20150505.03. [DOI] [Google Scholar]
- Zielinski A.A.F., Haminiuk C.W.I., Nunes C.A., Schnitzler E., van Ruth S.M., Granato D. Chemical Composition, Sensory Properties, Provenance, and Bioactivity of Fruit Juices as Assessed by Chemometrics: A Critical Review and Guideline. Comprehensive Reviews in Food Science and Food Safety. 2014;13(3):300–316. doi: 10.1111/crf3.2014.13.issue-310.1111/1541-4337.12060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.