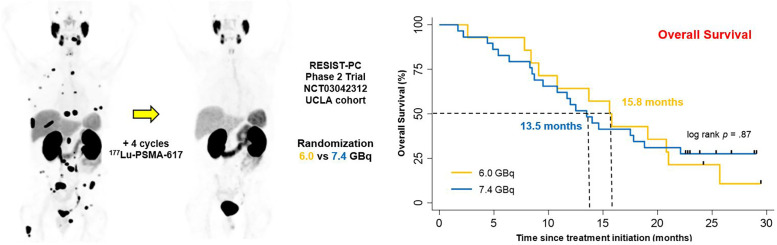
Visual Abstract
Keywords: metastatic castration-resistant prostate cancer, radionuclide therapy, molecular radiotherapy, prostate-specific membrane antigen, 177Lu, RESIST-PC, prospective randomized phase 2 trial, theranostics
Abstract
The objective of this study was to determine prospectively the efficacy profile of 2 activity regimens of 177Lu-PSMA therapy in patients with progressive metastatic castrate-resistant prostate cancer (mCRPC): 6.0 vs. 7.4 GBq. Methods: RESIST-PC (NCT03042312) was a prospective multicenter phase 2 trial. Patients with progressive mCRPC after ≥ 1 novel androgen-axis drug, either chemotherapy naïve or postchemotherapy, with sufficient bone marrow reserve, normal kidney function, and sufficient PSMA expression by PSMA PET were eligible. Patients were randomized (1:1) into 2 activity groups (6.0 or 7.4 GBq) and received up to 4 cycles every 8 wk. The primary endpoint was the efficacy of 177Lu-PSMA measured by the prostate-specific antigen (PSA) response rate (RR) after 2 cycles (≥50% decline from baseline). Secondary endpoints included the PSA RR (≥50% decline) at any time (best response), and overall survival (OS). Results: The study was closed at enrollment of 71/200 planned patients because of sponsorship transfer. We report here the efficacy of the University of California Los Angeles cohort results only (n = 43). The PSA RRs after 2 cycles and at any time were 11/40 (28%, 95% CI 15–44), 6/13 (46%, 95% CI 19–75), and 5/27 (19%, 95% CI 6–38), and 16/43 (37%, 95% CI 23–53), 7/14 (50%, 95% CI 23–77), and 9/29 (31%, 95% CI 15–51) in the whole cohort, the 6.0-GBq group, and the 7.4-GBq group, respectively (P = 0.12 and P = 0.31). The median OS was 14.0 mo (95% CI 10.1–17.9), 15.8 (95% CI 11.8–19.4), and 13.5 (95% CI 10.0–17.0) in the whole cohort, the 6.0-GBq group, and the 7.4 GBq group, respectively (P = 0.87). OS was longer in patients who experienced a PSA decline ≥ 50% at any time than in those who did not: median, 20.8 versus 10.8 mo (P = 0.005). Conclusion: In this prospective phase 2 trial of 177Lu-PSMA for mCRPC, the median OS was 14 mo. Despite the heterogeneous study population and the premature study termination, the efficacy profile of 177Lu-PSMA appeared to be favorable and comparable with both activity regimens (6.0 vs. 7.4 GBq). Results justify confirmation with real-world data matched-pair analysis and further clinical trials to refine and optimize the 177Lu-PSMA therapy administration scheme to improve tumor radiation dose delivery and efficacy.
See an invited perspective on this article on page 1438.
The prostate-specific membrane antigen (PSMA) is highly expressed by prostate cancer (PCa) cells and is a relevant target for PCa imaging and therapy. 177Lu PSMA-617 (177Lu-PSMA) therapy is an emerging therapeutic option in men with metastatic castrate-resistant PCa (mCRPC). Retrospective studies (1–3) and recent prospective trials from Australia (single-arm LuPSMA trial (4,5), randomized TheraP trial (6)) reported the efficacy and safety of 177Lu-PSMA in men with mCRPC.
Here we present the first U.S. prospective results of 177Lu-PSMA (RESIST-PC, NCT03042312). This multicenter prospective phase 2 study investigated the efficacy and safety of 177Lu-PSMA in patients who were randomized between 2 commonly used activity regimens: 6.0 GBq and 7.4 GBq. We hypothesized that the 2 activities result in comparable antitumor effects and safety profile. This study is the first attempt to compare prospectively 2 activity regimens of 177Lu-PSMA therapy.
The study was investigator-initiated and self-funded, but the development rights of PSMA-617 were acquired by Endocyte Inc. during the enrollment phase and the study was closed before reaching the target population. Therefore, data acquisition and analysis as initially planned was not possible. The safety results of both study sites were used for regulatory approval and will be reported separately. We report here the efficacy results of the University of California Los Angeles (UCLA) single study-site cohort with more than 2 y of follow-up after end of therapy.
MATERIALS AND METHODS
Study Design
RESIST-PC was a prospective, randomized, open-label, multicenter phase 2 study conducted at UCLA (Los Angeles, CA, USA) and Excel Diagnostics Nuclear Oncology Center (Houston, TX, USA). We aimed at assessing the efficacy and safety of 2 177Lu-PSMA activity regimens in patients with mCRPC. The study was investigator-initiated and conducted under a physician-sponsored investigational new drug (IND#133661) application. There was no external funding for this study. Patients were charged for the drug under Title 21 of the Code of Federal Regulation Section (CFR) 312.8. The UCLA institutional review board approved the study protocol (IRB#17-000330) provided in the supplemental materials (supplemental materials are available at http://jnm.snmjournals.org). The study was registered on ClinicalTrials.gov (NCT03042312). Endocyte Inc. licensed the rights to the study drug, initiated a prospective international multicenter trial (VISION; NCT03511664), and closed RESIST-PC at a total enrollment of 71 of the 200 planned patients at both sites (see the “Statistical Analysis” section for rationale of sample size). Here we report the efficacy results of the UCLA cohort only (n = 43). The corresponding author had complete data access and had final responsibility to submit for publication.
Patients
Patients ≥ 18 y, who had histologically confirmed PCa, castrate levels of serum testosterone (<0.5 ng/mL), progressive disease (biochemical, radiographic, or clinical), who had received abiraterone or enzalutamide, had an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 to 2, and had the ability to understand and sign the written informed consent form were eligible. We included patients without prior chemotherapy or with any number of prior chemotherapies if at least 6 wk passed since the last treatment cycle. Patients who had received PSMA-targeted radionuclide therapy were excluded. Pretreatment PSMA PET was required to document sufficient target expression (see the “Procedures” section). Additional inclusion criteria were a sufficient bone marrow reserve (hemoglobin ≥ 9.9 g/dL, platelet count ≥ 100 × 109/L, white blood cell count ≥ 2.5 × 109/L, and absolute neutrophil count ≥ 1.5 × 109/L). Patients with diffuse bone involvement by bone scintigraphy (superscan), impaired kidney function (glomerular filtration rate < 40 mL/min, serum creatinine > 1.5×upper limit of normal [ULN], urinary tract obstruction, or marked hydronephrosis), or impaired liver function (aspartate aminotransferase [AST] and alanine aminotransferase [ALT] > 5×ULN) were excluded. Informed written and verbal consent was obtained from all patients.
Procedures
All patients underwent a screening 68Ga-PSMA-11 PET/CT scan (≤3 mo before enrollment) to confirm PSMA expression assessed visually by the local investigators (tumor uptake above the liver background). Patients with PSMA-negative soft-tissue lesions seen on conventional scans (CT, MRI) were excluded (screening failure). Complete blood counts, kidney and liver function, and serum prostate-specific antigen (PSA) levels were measured within 2 wk of treatment initiation.
Patients were randomized (1:1 ratio) to receive either 6.0 or 7.4 GBq of 177Lu-PSMA. Randomization (1:1 ratio) was performed in accordance with Vickers et al. (7) We concealed allocation by creating a list of random allocations for patients 1 to 200 and stored it at the investigator’s site without modification. A clinical research coordinator who was not involved in clinical management assigned the randomized allocation. There was no masking of patients or physicians.
177Lu-PSMA-617 was radiolabeled with carrier-free 177Lu (RadioMedix, Inc.). The labeled product was produced, tested, released, and delivered under good-manufacturing-practice conditions as a sterile, ready-to-use solution for infusion.
177Lu-PSMA was intravenously applied at 8-wk intervals (±1 wk) up to a maximum of 4 cycles (cycle 02 at wk 08; cycle 03 at wk 16; cycle 04 at wk 24). Treatment cycles continued until disease progression, severe toxicity occurred, patients withdrew consent, or investigators decided to discontinue treatment.
We performed hematologic and serum assessments at baseline and in 2-wk intervals up to the 12-wk follow-up visit after the last study drug injection. We measured serum PSA levels at baseline and every 6 wk. Subsequent assessments continued at 3-mo intervals until follow-up concluded at 24 mo or on disease progression.
Bone pain intensity was assessed at each cycle using the pain intensity score, a component of the Brief Pain Inventory–Short Form (8): scores ranged from 0 to 10, with lower scores representing lower levels of pain intensity; a change of 2 was required to consider a change relevant (9).
Because of cost considerations (no follow-up imaging was built in the study budget), imaging follow-up was performed by patient and referring oncologist preference. Because of the lack of standardization, effective conclusions could not be assured. The imaging follow-up analysis (methods, radiographic progression-free survival, disease control rate by imaging) is provided in the supplemental materials.
Outcomes
The primary endpoint measure was the PSA response rate (RR) after 2 cycles defined as the proportion of patients with a ≥ 50% decline in serum PSA levels from baseline (10).
Secondary endpoints included the PSA RR (≥50% decline) at any time (best response), biochemical progression-free survival (PSA PFS), pain progression-free survival (pain PFS), and pain RR. A post hoc analysis assessed overall survival (OS). These parameters were defined as the time from first treatment cycle to PSA progression, pain progression, or death from any cause, respectively. We recorded new pain development as a 2-point increase on the pain intensity score without a decrease in opiate use. Patients were included in the pain analysis if they had available baseline assessments and at least 1 follow-up data point 4–6 wk after the last treatment cycle.
All endpoints were analyzed by the local investigators.
Statistical Analysis
On the basis of previous reports (1), we hypothesized that the PSA RR after 2 cycles would range between 38% and 65% for both treatment activities. On the basis of the design of a single-arm phase 2 study in mCRPC (11), we postulated that 177Lu-PSMA would be considered of value for further study if 50% or more patients met the primary endpoint and not worthy if fewer than 40% achieved the primary endpoint. A sample size of 200 patients was required to distinguish between a 40% and a 50% PSA RR with a 78% power (2-sided binomial test with α 0.05 and β 0.20).
We used descriptive statistics including median and interquartile range (IQR) for continuous variables and number and percentage for categoric variables. We present percentage changes in serum PSA levels as a waterfall plot. Kaplan–Meier analysis was used to calculate PSA PFS, pain PFS, and OS by PSA RRs. We used the log-rank test to evaluate the association between treatment arm and patient outcome. The Fisher exact test determined the association between treatment arm and PSA RRs. We tested each endpoint at a 2-sided significance level of 0.05.
In a post hoc analysis, the effect of treatment activity (6.0 vs. 7.4 GBq) on outcome data was adjusted for baseline factors (i.e., ECOG performance score, number of previous chemotherapy lines [0–1 vs. 2], and visceral disease) in multivariate cox/logistic regression models. Hazard ratios/odds ratios and their 95% CIs were derived.
Because of the early study termination, we tested whether the comparison of the 2 activity groups (6.0 vs. 7.4 GBq) would likely have held up in the originally proposed study population of 200 patients with a post hoc conditional power calculation simulation (12). This assumes that the additional patients required to complete the originally planned study cohort exhibit characteristics similar to those of the patients enrolled. The method applies random samples and 1,000 iterations to account for sampling variability. If this calculation yields around a conditional power calculation of 80% (i.e., P < 0.05 in 80% of the 1,000 simulations), then the difference in treatment regimen–associated outcomes would be statistically different.
Statistical analyses were performed using SPSS, version 22 (IBM) and STATA, version 15 (StataCorp LLC).
RESULTS
Enrollment and Baseline Characteristics
We enrolled 51 patients with progressive mCRPC between November 2017 and July 2018 (Supplemental Fig. 1). Eight of 51 (16%) patients were excluded after enrollment because of disease progression (n = 4/8, 50%), negative PSMA PET (n = 2/8, 25%), death (n = 1/8, 13%), or screen failure (n = 1/8, 13%). Forty-three of 51 (84%) patients received at least 1 cycle of 177Lu-PSMA: 14 of 43 (33%) and 29 of 43 (67%) in the 6.0- and 7.4-GBq groups, respectively.
Baseline characteristics are provided in Table 1. In the overall study population, median baseline PSA levels and doubling times were 27.4 ng/mL (IQR 9.5–115.6) and 1.5 mo (IQR 1.0–2.3), respectively. Twenty-two of 43 patients (51%) had received ≥ 2 chemotherapy regimens, and 35 of 43 (82%) underwent treatment with both abiraterone and enzalutamide before 177Lu-PSMA. Twenty-nine of 43 (67%) patients had > 20 metastasis on PSMA PET.
TABLE 1.
Characteristics of Study Population at Baseline
| Characteristic | Overall (n = 43) | 6.0 GBq (n = 14) | 7.4 GBq (n = 29) |
|---|---|---|---|
| Age (y) | 74 (68–78) | 76 (70–79) | 72 (65–78) |
| Time since diagnosis of PCa (y) | 7 (4–17) | 8 (5–17) | 7 (4–15) |
| Gleason grade group at diagnosis* | |||
| ≥4 | 25 (64%) | 9 (69%) | 16 (62%) |
| PSA (ng/mL) | 27.4 (9.5–115.6) | 31.3 (12.6–160.2) | 26.1 (9.5–124.4) |
| PSA doubling time (mo) | 1.5 (1.0–2.3) | 1.3 (1.0–1.7) | 1.8 (1.0–3.2) |
| Total alkaline phosphatase (U/I) | 87 (67–125) | 82 (60–175) | 94 (69–117) |
| Hemoglobin (g/dL) | 12.0 (10.9–13.2) | 12.1 (11.2–12.9) | 11.6 (10.8–13.3) |
| Platelets (103/mL) | 208 (160–245) | 207 (163–356) | 208 (158–238) |
| ECOG performance status | |||
| 0 | 13 (30%) | 8 (57%) | 5 (17%) |
| 1 | 21 (49%) | 4 (29%) | 17 (59%) |
| 2 | 9 (21%) | 2 (14%) | 7 (24%) |
| Pain at baseline (BPI score) | |||
| No pain | 21 (49%) | 4 (28%) | 17 (58%) |
| Mild (1–4) | 11 (26%) | 5 (36%) | 6 (21%) |
| Moderate to severe (5–10) | 11 (26%) | 5 (36%) | 6 (21%) |
| Previous mCRPC systemic treatments | |||
| Chemotherapy regimen lines | |||
| 0 | 11 (26%) | 4 (29%) | 7 (24%) |
| 1 | 10 (23%) | 4 (29%) | 6 (21%) |
| 2 | 12 (28%) | 3 (21%) | 9 (31%) |
| ≥3 | 10 (23%) | 3 (7%) | 7 (24%) |
| Abiraterone | 41 (95%) | 13 (93%) | 28 (97%) |
| Enzalutamide | 37 (86%) | 13 (93%) | 24 (83%) |
| Abiraterone + enzalutamide | 35 (82%) | 12 (86%) | 23 79%) |
| 223Ra | 14 (33%) | 4 (29%) | 10 (35%) |
| Prior lines of mCRPC systemic treatment | |||
| 1 | 4 (9%) | 1 (7%) | 3 (10%) |
| ≥2 | 39 (91%) | 13 (93%) | 26 (90%) |
| ≥3 | 31 (72%) | 10 (71%) | 21 (72%) |
| ≥4 | 25 (58%) | 8 (57%) | 17 (59%) |
| Extent of disease on PSMA-PET | |||
| ≤20 metastases | 14 (33%) | 4 (29%) | 10 (34%) |
| 2 metastases | 29 (67%) | 10 (71%) | 19 (66%) |
| Sites of disease on PSMA PET | |||
| Node only (N1 or M1a) | 3 (7%) | 1 (7%) | 2 (7%) |
| Bone only (M1b) | 9 (21%) | 3 (21%) | 6 (21%) |
| Node + bone (M1b and [N1 or M1a]) | 15 (35%) | 7 (50%) | 8 (28%) |
| Visceral (M1c with/without any other site)† | 15 (35%) | 3 (21%) | 12 (41%) |
Data missing for 4 patients.
Visceral includes lung, liver, rectum, pancreas, peritoneal, brain, and adrenal.
BPI = bone pain index.
Data are median, with IQR in parentheses, or n (%).
The cutoff date for follow-up was June 25, 2020. Median follow-up for patients who survived was 24.8 mo (IQR 22.9–28.8).
Efficacy Endpoints
PSA RRs
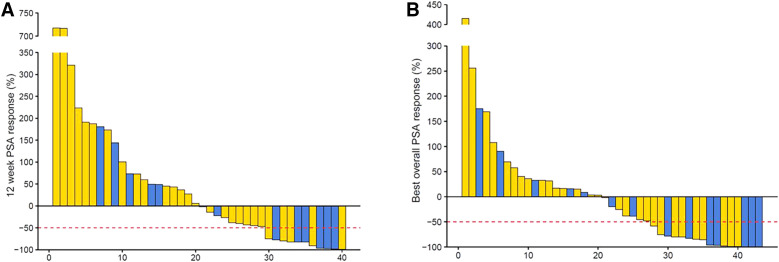
PSA RR after 2 cycles was available in 40 of 43 patients (93%). Overall PSA RR was 11 of 40 (28%; 95% CI 14.6–43.9) and 16 of 43 (37%; 95% CI 23.0–53.3) after 2 cycles (primary endpoint) and at any time, respectively (Fig. 1; Table 2). There was no difference of PSA RRs between the 2 treatment arms after 2 cycles (P = 0.12) or at any time (P = 0.31). The median time to best PSA response was 8.9 wk (IQR, 6.9–25.1) in all 43 patients and 28.8 wk (IQR, 15.2–36.2) in the 16 PSA responders.
FIGURE 1.
Waterfall plots showing PSA changes relative to baseline after 2 cycles of 177Lu-PSMA (A) and any time during treatment (B).
TABLE 2.
Primary and Secondary Endpoints Results
| Outcome measure | Overall (n = 43) | 6.0 GBq (n = 14) | 7.4 GBq (n = 29) | Hazard ratio (95% CI) | P |
|---|---|---|---|---|---|
| Primary endpoint | |||||
| PSA response after 2 cycles | |||||
| No. of evaluable patients | 40 | 13 | 27 | ||
| PSA decline ≥ 50% after 2 cycles | 11 (28%, 95% CI 15–44) | 6 (46%, 95% CI 19–75) | 5 (19%, 95% CI 6–38) | — | 0.12* |
| Secondary endpoint | |||||
| Best PSA response | |||||
| No. of evaluable patients | 43 | 14 | 29 | ||
| Best PSA response ≥ 50% | 16 (37%, 95% CI 23–53) | 7 (50%, 95% CI 23–77) | 9 (31%, 95% CI 15–51) | — | 0.31* |
| Pain response | |||||
| No. of evaluable patients | 18 | 7 | 11 | ||
| Patients with pain improvement (n) | 12 (67%) | 6 (86%) | 6 (55%) | — | 0.31* |
| Pain PFS | |||||
| Median (mo) | 8.2 (95% CI 3.9–12.5) | 5.4 (not reached) | 8.2 (95% CI 2.3–14.1) | 0.96 (0.35–2.66) | 0.94 |
| Post hoc analysis | |||||
| OS | |||||
| Median (mo) | 14.0 (95% CI 10.1–17.9) | 15.8 (95% CI 11.8–19.4) | 13.5 (95% CI 10.0–17.0) | 0.94 (0.46–1.92) | 0.87 |
P values compare the 6.0- and 7.4-GBq treatment arms using exact Fisher test.
Biochemical PFS
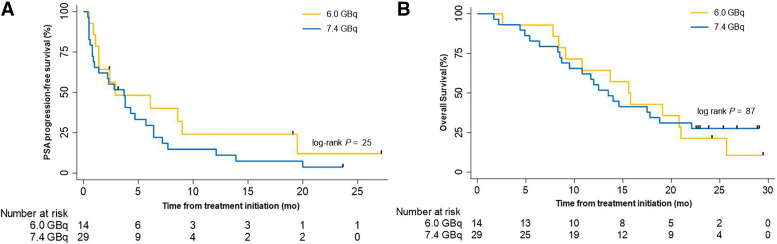
At the end of follow-up, 2 of 43 patients (5%) were alive without PSA progression. The median PSA PFS was 3.7 mo in the overall study population (95% CI 2.0–5.4). It was 2.9 mo (95% CI 0.0–9.0) and 3.7 mo (95% CI 1.9–5.6) in the 6.0- and the 7.4-GBq groups (P = 0.25), respectively (Fig. 2; Table 2; Supplemental Fig. 2).
FIGURE 2.
Survival Kaplan–Meier curves. Kaplan–Meier curves for PSA PFS (A) and OS (B) by treatment arm. Tick marks indicate censored data. The log-rank test is given with P < 0.05 considered significant.
Bone Pain PFS
The pain RR in evaluable patients was 12 of 18 (67%), 6 of 7 (86%), and 6 of 11 (55%) in the overall study population, the 6.0-GBq group, and the 7.4-GBq group, respectively (P = 0.31) (Table 2). Pain PFS was 8.2 mo (95% CI 3.9–12.5), 5.4 mo (95% CI not reached), and 8.2 mo (95% CI 2.3–14.1) in the overall study population, the 6.0-GBq group, and the 7.4-GBq group, respectively (P = 0.94) (Supplemental Fig. 3; Table 2).
OS
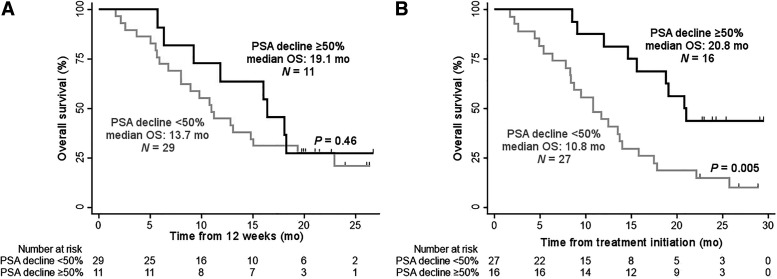
At the end of follow-up, 12 of 14 (86%) and 25 of 29 (87%) of patients had died in the 6.0- and 7.4-GBq arms, respectively. The median OS of the overall study population was 14.0 mo (95% CI 11.8–19.4). The injected activity was not associated with OS: 15.6 (95% CI 11.8–19.4) versus 13.5 mo (95% CI 10.0–17.0) in the 6.0- and the 7.4-GBq arms (P = 0.87), respectively (Fig. 2; Table 2; Supplemental Fig. 2). Patients who experienced a PSA decline ≥ 50% at any time (best response; n = 16/43, 37%) had a significantly longer OS than those who did not (27/43, 63%): median: 20.8 versus 10.8 mo; P = 0.005 (Fig. 3). However, no significant difference was observed when comparing the OS of patients who had a PSA decline ≥ 50% after 2 cycles only (n = 11/40, 28%) with those who did not (n = 29/40, 72%): median: 19.1 versus 13.7 mo; P = 0.46 (Fig. 3).
FIGURE 3.
Kaplan–Meier curves for OS by PSA response after 2 cycles (A) and at any time (B). Tick marks indicate censored data. The log-rank test is given with P < 0.05 considered significant.
After adjusting for baseline factors (ECOG, number of previous chemotherapy regimen [0–1 vs. 2], visceral disease), the treatment activity (6.0 vs. 7.4 GBq) remained not associated with treatment outcomes (P values > 0.05, multivariate cox/logistic regression models, Supplemental Table 1).
The post hoc conditional power calculation simulation assumed a comparable demographic and disease distribution for 157 simulated patients (to obtain the initially planned population of 200 patients). Randomly sampling (with replacement) 86 patients from the 6.0-GBq cohort and 71 patients from the 7.4-GBq cohort and repeating this process 1,000 times yielded a significant difference (P < 0.05) between activity effects on outcome in only 47 of 1,000 simulations (4.7%).
DISCUSSION
This prospective randomized phase 2 study compared two 177Lu-PSMA treatment activity levels in patients with mCRPC who progressed after conventional treatments. PSA RR, PSA PFS, pain RR, and OS did not differ between the 2 activity arms (6.0 vs. 7.4 GBq). This study is, to our knowledge, the first attempt to compare prospectively 2 activity regimens of 177Lu-PSMA therapy. The results are in line with a retrospective study comparing 2 similar treatment activity levels of 177Lu-PSMA (6.0 vs. 7.5 GBq) (13).
The primary efficacy endpoint (i.e., PSA RR after 2 cycles of ≥ 40% in the whole cohort) was not met, possibly because of premature study closure at 36% of the planned enrollment (71/200). This study closure was prompted by the IND sponsorship transfer to Endocyte Inc. and the opening of the phase 3 registration VISION trial (NCT03511664). The current PSA RR is lower than those reported in the Australian prospective phase 2 clinical trials, after 2 cycles (28% vs. 50% in the LuPSMA trial), and at any time point (38% vs. 64% in the LuPSMA trial and 66% in TheraP Trial) (4,6). More rigorous patient selection that included 18F-FDG PET to exclude patients with hyperglycolytic but low PSMA-expressing lesions resulted in improved PSA RR. Dual-tracer PSMA/18F-FDG PET phenotyping can improve patient selection to 177Lu-PSMA therapy and this approach should be further implemented in future prospective trials. However, despite different PSA RRs, OS was similar (median: 14.0 vs. 13.7 mo in the LuPSMA trial) (5). Of note, the quality of life improvement previously reported was also observed in our cohort: pain levels improved in 67% of the evaluable patients (4–6). Further studies on patients reported outcomes are warranted.
A comparative metaanalysis suggested that 177Lu-PSMA was less toxic, induced higher PSA RR (mean frequency 44% vs. 22%) and possibly improved OS (median of 14 vs 12 mo; P = 0.33) compared with other third-line treatments for mCRPC, such as enzalutamide and cabazitaxel (14). The multicenter prospective randomized TheraP trial comparing 177Lu-PSMA with cabazitaxel confirmed these findings with higher PSA RR (66% vs. 44%) and less grade 3–4 adverse events (33% vs. 53%) in the 177Lu-PSMA arm (6). Improvement of OS with 177Lu-PSMA will be critical for regulatory approval, and the results of the VISION trial NCT03511664 (best supportive/standard care vs. 177Lu-PSMA + best supportive/standard care) are awaited.
A significant association between best PSA RR and OS was observed, in line with prior reports (3,5), supporting further investigation of PSA RR as an intermediate surrogacy endpoint for OS.
Findings are limited by an early study closure before completing target enrollment (36%). This was beyond the control of the investigators and resulted in a small sample size. Consequently, the distribution between the 2 treatment groups was also altered (14 vs. 29) as 1:1 randomization was performed centrally for both sites. The premature study termination limits the comparison between the 2 treatment activity groups. However, due to the narrow difference in the 2 tested activities (∼20%, 6.0 vs. 7.4 GBq) even the limited data suggest that there is likely no or only small differences in efficacy between these 2 activities. This is consistent with prior reports that found similar response and toxicity rates to comparable levels of injected activity (6.0 vs. 7.5 GBq) (13). To further test whether the current results of the comparison of the 2 activity groups (6.0 GBq vs. 7.4 GBq) in this cohort of 43 patients would likely have held up in the originally proposed study population of 200 patients, we conducted a post hoc conditional power calculation simulation (12). After 1,000 simulations, only 47 of 1,000 simulations (4.7%) were significant (P < 0.05). Further calculation revealed that around 3,400 patients per group (6,800 total) would have been needed to show a significant difference in effectiveness of the 2-activity regimen (conditional power of 80%).
As another limitation, the study population was heterogeneous regarding prior treatment. The study was self-funded and patients were charged for the study drug (cost recovery, Title 21 CFR 312.8). For ethical reasons, the study therefore allowed various prior systemic therapies for inclusion. To correct for heterogeneity in treatment history and baseline characteristics, we conducted a standard covariate adjustment analysis (Supplemental Table 1). After adjusting for baseline factors including ECOG, number of previous chemotherapy regimen (0–1 vs. 2), and presence of visceral disease, the treatment activity was still not associated with treatment outcome. Thus, administered activity (6.0 vs. 7.4 GBq) did not appear to affect treatment outcome.
To reduce out-of-pocket costs, imaging follow-up modalities were selected by patients and referring oncologists. Thus, a variety of imaging modalities (CT, bone scan, MRI, PSMA, choline, fluciclovine, FDG) were used to assess radiographic progression, which may have increased variance of event data. For instance, PET imaging results in shorter time to progression when compared with conventional anatomic imaging. Because of the lack of standardization, effective conclusions could not be assured. The follow-up imaging analysis is provided in the supplemental material (Supplemental Tables 2 and 3 and Supplemental Fig. 4).
Finally, there was no central blinded review of the screening PSMA PET, and criteria to establish PSMA-target expression were not predefined and left to the discretion of the local investigators. Studies establishing optimal PSMA PET criteria for patient selection and therapy response assessment are warranted.
CONCLUSION
We report here the UCLA study site efficacy results of the prospective phase 2 study RESIST-PC of 177Lu-PSMA for mCRPC after more than 2 y of follow-up. The study closed enrollment before reaching the cohort size because of IND sponsorship transfer to Endocyte Inc. The study population was heterogeneous. PSA RR after 2 cycles and at any time were 28% and 38%. Pain RR was 67%, and the median OS was 14 mo. There was no difference in PSA RR between administration of 6.0 and 7.4 GBq of 177Lu-PSMA. Results justify confirmation with real-world data analysis and further trials to refine and optimize the 177Lu-PSMA therapy administration scheme to improve tumor radiation dose delivery and efficacy.
DISCLOSURE
Jeremie Calais is the recipient of grants from the Prostate Cancer Foundation (2020 Young Investigator Award, 20YOUN05), the Society of Nuclear Medicine and Molecular imaging (2019 Molecular Imaging Research Grant for Junior Academic Faculty), the Philippe Foundation Inc. (NY, USA), and the ARC Foundation (France) (International Mobility Award SAE20160604150) and reports prior consulting activities outside of the submitted work for Advanced Accelerator Applications, Blue Earth Diagnostics, Curium Pharma, GE Healthcare, IBA Radiopharma, Janssen, Progenics, POINT Biopharma, Radiomedix, and Telix Pharmaceuticals. Andrei Gafita was the recipient of the Jonsson Comprehensive Cancer Center Fellowship award and the Dr. Christiaan Schiepers postdoctoral fellowship award. Matthias Eiber was a consultant for ABX, Blue Earth Diagnostics, and Progenics and has patent rights on rhPSMA outside of the submitted work. David Ranganathan is an employee and equity holder of RadioMedix. Ken Herrmann received funding from the German Research Foundation (Deutsche Forschungsgemeinschaft grant HE 5247/4-1) and was a consultant for Advanced Accelerator Applications, Amgen, Bayer, Curium Pharma, GE Healthcare, IPSEN, Janssen Pharmaceuticals, BTG, Sirtex, Novartis, ROTOP, and Bain Capital outside of the submitted work. In addition, Ken Herrmann is a board member of and holds equity in Sofie Biosciences; intellectual property is patented by the University of California and licensed to Sofie Biosciences. Ebrahim Delpassand reports equity ownership at Excel Nuclear Oncology Center and RadioMedix. Wolfgang P. Fendler received financial support from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, grants FE1573/1-1/807122 and FE1573/3-1/659216 and DFG Research Training Group 1739); was a consultant for Endocyte and BTG; and received fees from RadioMedix, Bayer, and Parexel outside of the submitted work. Johannes Czernin was supported by the Prostate Cancer Foundation (2019 and 2017 Challenge Award, 19CHAL09, 17CHAL02) and by the Johnson Comprehensive Cancer Center NIH-NCI Cancer Center Support Grant (P30 CA016042) and was a consultant for Endocyte Inc. (VISION trial steering committee), Actinium Pharmaceuticals, and Point Biopharma outside of the submitted work. In addition, Johannes Czernin is a founder and board member of and holds equity in Sofie Biosciences and Trethera Therapeutics; intellectual property is patented by the University of California and licensed to Sofie Biosciences and Trethera Therapeutics. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the efficacy profile of 2 activity regimens of 177Lu-PSMA therapy (6.0 GBq vs. 7.4 GBq) in patients with mCRPC?
PERTINENT FINDINGS: In this prospective randomized phase 2 study that included 43 patients with progressive mCRPC, 177Lu-PSMA therapy resulted in biochemical response in 38%, and the median OS was 14 mo. There was no difference in efficacy between administration of 6.0 and 7.4 GBq of 177Lu-PSMA.
IMPLICATIONS FOR PATIENT CARE: 177Lu-PSMA therapy using and 6.0 and 7.4 GBq is a therapeutic option for patient with mCRPC with a good efficacy.
ACKNOWLEDGMENTS
We thank all the patients and their referring physicians whose willingness to participate made this study possible. We thank the whole staff team of the UCLA Nuclear Medicine and Theranostics Division whose hard work made this study possible.
REFERENCES
- 1. Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. [DOI] [PubMed] [Google Scholar]
- 2. Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. AJR. 2019;213:275–285. [DOI] [PubMed] [Google Scholar]
- 3. Heck MM, Tauber R, Schwaiger S, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:920–926. [DOI] [PubMed] [Google Scholar]
- 4. Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. [DOI] [PubMed] [Google Scholar]
- 5. Violet J, Sandhu S, Iravani A, et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. [DOI] [PubMed] [Google Scholar]
- 7. Vickers AJ. How to randomize. J Soc Integr Oncol. 2006;4:194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 9. Mathias SD, Crosby RD, Qian Y, Jiang Q, Dansey R, Chung K. Estimating minimally important differences for the worst pain rating of the Brief Pain Inventory-Short Form. J Support Oncol. 2011;9:72–78. [DOI] [PubMed] [Google Scholar]
- 10. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walter SD, Han H, Guyatt GH, et al. A systematic survey of randomised trials that stopped early for reasons of futility. BMC Med Res Methodol. 2020;20:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seifert R, Kessel K, Schlack K, Weckesser M, Bögemann M, Rahbar K. Radioligand therapy using [177Lu]Lu-PSMA-617 in mCRPC: a pre-VISION single-center analysis. Eur J Nucl Med Mol Imaging. 2020;47:2106–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Eyben FE, Roviello G, Kiljunen T, et al. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur J Nucl Med Mol Imaging. 2018;45:496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]