Visual Abstract
Keywords: melanoma, head and neck, sentinel lymph node biopsy, false-negative
Abstract
Sentinel lymph node (SN) biopsy (SNB) has proven to be a valuable tool for staging melanoma patients. Since its introduction in the early 1990s, this procedure has undergone several technologic refinements, including the introduction of SPECT/CT, as well as radioguidance and fluorescence guidance. The purpose of the current study was to evaluate the effect of this technologic evolution on SNB in the head and neck region. The primary endpoint was the false-negative (FN) rate. Secondary endpoints were number of harvested SNs, overall operation time, operation time per harvested SN, and postoperative complications. Methods: A retrospective database was queried for cutaneous head and neck melanoma patients who underwent SNB at The Netherlands Cancer Institute between 1993 and 2016. The implementation of new detection techniques was divided into 4 groups: 1993–2005, with preoperative lymphoscintigraphy and intraoperative use of both a γ-ray detection probe and patent blue (n = 30); 2006–2007, with addition of preoperative road maps based on SPECT/CT (n = 15); 2008–2009, with intraoperative use of a portable γ-camera (n = 40); and 2010–2016, with addition of near-infrared fluorescence guidance (n = 192). Results: In total, 277 patients were included. At least 1 SN was identified in all patients. A tumor-positive SN was found in 59 patients (21.3%): 10 in group 1 (33.3%), 3 in group 2 (20.0%), 6 in group 3 (15.0%), and 40 in group 4 (20.8%). Regional recurrences in patients with tumor-negative SNs resulted in an overall FN rate of 11.9% (group 1, 16.7%; group 2, 0%; group 3, 14.3%; group 4, 11.1%). The number of harvested nodes increased with advancing technologies (P = 0.003), whereas Breslow thickness and operation time per harvested SN decreased (P = 0.003 and P = 0.017, respectively). There was no significant difference in percentage of tumor-positive SNs, overall operation time, and complication rate between the different groups. Conclusion: The use of advanced detection technologies led to a higher number of identified SNs without an increase in overall operation time, possibly indicating an improved surgical efficiency. Operation time per harvested SN decreased; the average FN rate remained 11.9% and was unchanged over 23 y. There was no significant change in postoperative complication rate.
One fifth of all cutaneous melanomas occur in the head and neck region (1). Sentinel lymph node (SN) biopsy (SNB) for head and neck melanoma was introduced at our institute in the early 1990s for patients with clinically localized disease (2,3). SNB improves survival in node-positive patients, and the tumor status of the SN is the strongest prognostic factor (4). The more accurate staging facilitates selection of patients for adjuvant therapy and for trials (5).
Detection of SNs in the head and neck is often challenging because of the complex anatomy, and interlacing lymph vessels can yield unexpected drainage patterns to multiple and bilateral sites (6). Moreover, nodes in the head and neck region, especially in the parotid gland, are easily overlooked on lymphoscintigraphy because they are near the injection site, where most of the radioactive tracer remains. These factors are responsible for a median false-negative (FN) rate of 20.4% in the reviewed reports, with a range of 3.3%–44% (6–9).
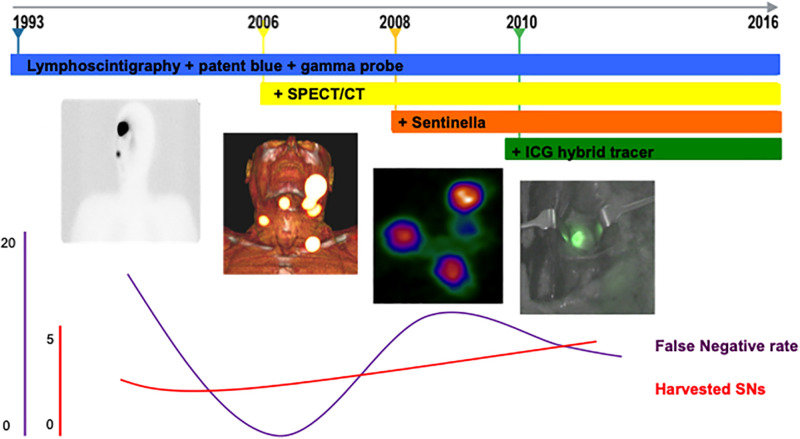
In recent years, various complementing SNB technologies have facilitated the procedure for lymphatic mapping. First, 99mTc-nanocolloid was used for dynamic and static lymphoscintigraphy to map the lymphatic drainage and for intraoperative γ-ray detection probe tracing in combination with patent blue to visualize the afferent lymph vessels and the SNs (10,11). Because of disadvantages such as allergic reactions, coloring of the skin, and fast shiftingof the dye to lymph nodes and pathways, patent blue is now omitted in the head and neck (12–15). The addition of preoperative SPECT/CT visualized SNs in their anatomic context (16). With the introduction of intraoperative use of a portable γ-camera, a better overview of the SNs in the surgical field was provided (17). Lastly, a complex of indocyanine green (ICG) with 99mTc-nanocolloid was implemented to integrate near-infrared fluorescence imaging into the procedure (18–20). This facilitated detection of superficial (<1 cm deep) lymphatic vessels and SNs, using a dedicated fluorescence camera. So, the currently used technologies enable preoperative visualization of the lymphatic drainage pattern, imaging of the SNs within the surrounding anatomy, intraoperative tracing of radioactive SNs, and visualization of the afferent lymphatic ducts and lymph nodes.
The purpose of this study was to determine the impact of the sequential technical advances, over a period of 23 y, on SNB outcomes in patients with melanoma in the head and neck region. The primary endpoint was the FN rate. Secondary endpoints were number of harvested SNs, duration of the procedure, operation time per harvested SN, and postoperative complication rate.
MATERIALS AND METHODS
This retrospective analysis concerned 277 patients with primary cutaneous head and neck melanoma who underwent reexcision and SNB at The Netherlands Cancer Institute between December 1993 and January 2016. SNB was performed for at least pT1b head and neck melanoma without clinical lymph node involvement as determined by palpation, ultrasound, and ultrasound-guided fine-needle aspiration cytology of suggestive lymph nodes according to the guidelines of the Dutch Head and Neck Society based on the eight edition of the American Joint Committee on Cancer–Union for International Cancer Control TNM classification (21). Patients who had a history of previous melanoma in the head and neck region or had already undergone wide excision or radiotherapy of the melanoma site were not eligible.
The medical charts were reviewed for tumor characteristics, overall operation time (from start of surgery to closure of incision), operation time per harvested SN (overall operation time divided by number of SNs), and SNB characteristics, as well as other clinicopathologic features. The institutional review board approved this retrospective study, and the requirement to obtain informed consent was waived. All procedures involving patients were in accordance with the ethical standards of the Medical Ethical Committee of The Netherlands Cancer Institute, and conformed with the Declaration of Helsinki (1964) and later amendments.
Imaging and SNB
An SN was defined as any lymph node receiving direct lymphatic drainage from the primary tumor (22). Depending on the patient admission date, different lymph node mapping techniques were used for 4 different groups. Group 1 (n = 30) covered the period 1993–2005, when preoperative lymphoscintigraphy as well as an intraoperative γ-ray detection probe (Neoprobe; Johnson and Johnson Medical) and patent blue (Laboratoire Guerbet) were used. Group 2 covered 2006–2007, when preoperative SPECT/CT (Symbia; Siemens) was added to visualize SNs in their anatomic habitat. Patent blue was omitted after 2007. Group 3 (n = 40) reflected 2008 and 2009 and included the addition of intraoperative use of a portable γ-camera (Sentinella; OncoVision). Group 4 (n = 192) covered 2010–2016 and included the addition of near-infrared fluorescence guidance in lieu of patent blue using the hybrid tracer ICG-99mTc-nanocolloid and a fluorescence camera (Photo Dynamic Eye, Photo Dynamic Eye-Modality, or Fluorescence Imaging System-00; Hamamatsu Photonics K.K.).
For lymphoscintigraphy, the radiopharmaceutical (99mTc-nanocolloid or ICG-99mTc-nanocolloid, 80 MBq for 1-d procedure and 120 MBq for 2-d procedure) was injected intradermally around the tumor or biopsy wound in 4 deposits of 0.1 mL (23). Immediately after injection, anterior and lateral dynamic planar imaging was performed for 10 min to identify first-echelon SNs and distinguish these from higher-echelon nodes. This was followed by acquisition of 5-min static images roughly 10 min and 2 h after injection to identify SNs in other (aberrant) regions. SPECT/CT imaging was performed directly after the 2-h static imaging. SN locations were marked on the skin and indicated on SPECT/CT key images (so-called surgical road maps), which were transferred to a picture archiving and communication system. The imaging results were discussed with the head and neck surgeon before the operation.
When used in the operation (before 2007), 1 mL of patent blue was injected intradermally around the melanoma site and the area was massaged for 5 min. Subsequently, the SNs were traced using a combination of the probe and the patent blue. To maintain visibility of the lymphatic ducts, patent blue injection was repeated every 90 min. With the introduction of a portable γ-camera, pre- and intraoperative images were acquired.
With the advent of the hybrid tracer, a dedicated near-infrared fluorescence camera was also incorporated. When a presumed SN was roughly located using the γ-ray detection probe and portable γ-camera, the lights in the operating room were dimmed and its precise location was determined using fluorescence imaging.
To verify completion of SNB, the wound was inspected and palpated and intraoperative imaging (fluorescence or portable γ-camera) was repeated. When a residual signal was observed at the location of the original SN, this node was considered a missed SN or part of a cluster of multiple adjacent SNs and also removed.
Histopathologic Examination
Following the recommendations of the European Organization for Research and Treatment of Cancer, multiple levels of the SN were analyzed using hematoxylin and eosin staining and immunohistochemistry for melanocytic differentiation antigens S-100 and glycoprotein 100/human melanoma black 45 (1993–2003) or melanocyte-antigen/melanoma antigen recognized by T cell 1 (2004 onward). Since February 2014, our protocol changed from examining 3 levels to examining 6 levels 50–150 μm each with hematoxylin and eosin staining and immunohistochemistry (24).
Follow-up
SN-negative patients were followed every 3 mo in year 1, every 6 mo in years 2–5, and annually thereafter. SN-positive patients were followed every 3 mo in years 1–2 and every 6 mo in years 3–10.
Statistical Analysis
The procedure was considered to be FN if a recurrence developed in the nodal region from which a tumor-free SN had been removed without any signs of local, other regional, or distant tumor activity. The FN rate was calculated by dividing the number of patients who presented with nodal recurrence after a tumor-negative SNB by the sum of those with a true-positive SN (TP) and those with nodal recurrence (FN/(TP + FN)), which is 1 − sensitivity (25–27).
Descriptive statistics are presented with means or medians and with 95% CIs or interquartile ranges or, in the case of nominal data, with numbers. In cases of continuous or ordinal variables, 1-way ANOVA or χ2 tests, respectively, were performed to assess the difference between the different imaging techniques. Significance was defined as a P level of less than 0.05. All statistical analyses were performed using SPSS, version 22.0, or STATA, version 13.
RESULTS
Clinicopathologic Features and Follow-up
Clinicopathologic features for the entire cohort are described in Table 1. Age differed among the 4 groups (Table 2). The Breslow thickness decreased over the years (P = 0.003). Follow-up details are presented in Table 2.
TABLE 1.
Baseline Characteristics
| Characteristic | Data |
|---|---|
| Sex | |
| Female | 100 (36.1%) |
| Male | 177 (63.9%) |
| Age at SNB (y) | 59 (46–68) |
| Location of primary tumor | |
| Scalp | 77 (27.8%) |
| Face | 103 (37.2%) |
| Ear | 55 (19.9%) |
| Nose | 14 (5.1%) |
| Neck | 28 (10.1%) |
| Breslow (mm) | 2.2 (1.5–3.6) |
| T* | |
| T1b | 21 (SNB+, 7.7 [4.8%]) |
| T2 | 94 (SNB+, 34.3 [13.8%]) |
| T3 | 93 (SNB+, 34.1 [30.1%]) |
| T4 | 65 (SNB+, 23.8 [26.2%]) |
| Ulceration | |
| Absent | 195 (70.9%) |
| Present | 69 (25.1%) |
| Unknown | 11 (4.0%) |
According to eighth edition of American Joint Committee on Cancer–Union for International Cancer Control TNM classification.
Qualitative data are number and percentage; continuous data are median and interquartile range. Percentages may not equal 100 because of rounding.
TABLE 2.
Patient and Tumor Characteristics
| Characteristic | Overall, 1993–2016 (n = 277) | Group 1, lymphoscintigraphy, 1993–2005 (n = 30) | Group 2, SPECT/CT, 2006–2007 (n = 15) | Group 3, portable γ-camera, 2008–2009 (n = 40) | Group 4, hybrid tracer, 2010–2016 (n = 192) | P |
|---|---|---|---|---|---|---|
| Median age | 59 (IQR, 46–68) | 49 (IQR, 39–62) | 53 (IQR, 45–66) | 54 (IQR, 45–68) | 60 (IQR, 50–70) | 0.007* |
| Breslow thickness | 2.2 (IQR, 1.5–3.6) | 3.0 (IQR, 1.8–4.9) | 2.9 (IQR, 1.9–6.0) | 2.2 (IQR, 1.5–3.4) | 2.0 (IQR, 1.4–3.5) | 0.003* |
| Mean excised SNs (n) | 3.8 (CI, 3.5–4.1) | 2.8 (CI, 2.0–3.5) | 2.5 (CI, 1.9–3.2) | 3.3 (CI, 2.7–3.8) | 4.2 (CI, 3.8–4.6) | 0.003* |
| Mean tumor-positive SNs (n) | 1.5 (CI, 1.3–1.7) | 1.8 (CI, 0.9–2.7) | 1.3 (CI, 0–2.0) | 1.2 (CI, 0.7–1.6) | 1.5 (CI, 1.2–1.7) | 0.90* |
| Median operation time (min) | 115 (IQR, 87–153) | 118 (IQR, 96–149) | 149 (IQR, 90–164) | 98 (IQR, 77–142) | 115 (89–154) | 0.74* |
| Median time per SN (min) | 38 (IQR, 25–54) | 51 (IQR, 33–71) | 54 (IQR, 39–66) | 40 (IQR, 30–51) | 34 (IQR, 22–51) | 0.017* |
| Complications (n) | 12 (4.3%) | 2 (7.1%) | 1 (7.1%) | 3 (8.1%) | 6 (3.1%) | 0.49† |
| Surgeons (n) | 14 | 6 | 5 | 7 | 10 | NA |
| Median follow-up (mo) | 41 (IQR, 25–65) | 85 (IQR, 31–138) | 106 (IQR, 65–125) | 69 (IQR, 53–88) | 35 (IQR, 22–53) | NA |
One-way ANOVA.
χ2 (exact) test.
NA = not applicable.
Percentages may not equal 100 because of rounding.
Operative and Postoperative Findings
Between 1993 and 2016, 14 head and neck surgeons performed SNB our institute. In all 277 patients, all preoperatively visualized SNs were identified during surgery. A mean of 3.8 (95% CI, 3.5–4.1) SNs per patient was excised in a median operation time of 115 min (interquartile range, 87–153 min) (Table 2). The overall operation time remained stable over the years (P = 0.74). The number of harvested SNs increased over time (P = 0.003), whereas the number of tumor-positive SNs remained equal. The operation time per SN decreased with the advancing technology (P = 0.017).
Twelve patients (4.3%) developed postoperative complications that were related to SNB. The complication rate was similar among the groups. Hemorrhage required treatment in 7 patients: 1 in group 1 (3.3%), 1 in group 2 (6.7%), 1 in group 3 (2.5%), and 4 in group 4 (2.1%). Wound infection developed in 1 patient in group 3 (2.5%) and 2 in group 4 (1.0%). One patient in group 1 (3.3%) developed transient facial nerve palsy, and a case of transient spinal accessory nerve dysfunction was seen in group 3.
SNB Outcomes
A tumor-positive SN was found in 59 patients (21.3%) (Table 3). In group 1, 33.3% of the patients had a tumor-positive SN; in group 2, 20.0%; in group 3, 15.0%; and in group 4, 20.8%. There was no significant difference among the groups. Eight patients with tumor-negative SNs and no disease elsewhere had recurrence in their nodal region, resulting in an overall sensitivity of 88.1% and a FN percentage of 11.9. Two patients had an FN procedure in group 1 (16.7%), none in group 2, 1 in group 3 (14.3%), and 5 in group 4 (11.1%). FN nodes were located infraauricularly and retroauricularly in the parotid gland at levels II and V. With the exception of the level II recurrence, all were located near the primary melanoma, where most of the injected radioactivity remained. Follow-up details and recurrences are visualized in Table 4.
TABLE 3.
SNB Outcomes
| Outcome | Overall, 1993–2016 (n = 277) | Group 1, lymphoscintigraphy, 1993–2005 (n = 30) | Group 2, SPECT/CT, 2006–2007 (n = 15) | Group 3, portable γ-camera, 2008–2009 (n = 40) | Group 4, hybrid tracer, 2010–2016 (n = 192) |
|---|---|---|---|---|---|
| SNB-negative (n) | 210 (75.8%) | 18 (60%) | 12 (80%) | 33 (82.5%) | 147 (76.6%) |
| SNB-positive (n) | 59 (21.3%) | 10 (33.3%) | 3 (20%) | 6 (15%) | 40 (20.8%) |
| FN rate (n) | 8 (11.9%) | 2 (16.7%) | 0 (0%) | 1 (14.3%) | 5 (11.1%) |
| Sensitivity | 88.1% | 83.3% | 100% | 85.7% | 88.9% |
| Specificity | 100% | 100% | 100% | 100% | 100% |
TABLE 4.
Follow-up (in Months) and Recurrences
| Parameter | Overall (n = 277) | SNB-negative (n = 218) | SNB-positive (n = 59) |
|---|---|---|---|
| Follow-up | 41 (25–65) | 44 (24–67) | 37 (23–60) |
| Follow-up recurrence | 33 (18–60) | 35 (19–61) | 26 (10–52) |
| Recurrence | 81 (29%) | 50 (23%) | 31 (53%) |
| Local | 21 | 11 | 10 |
| Regional | 17 | 12* | 5 |
| Distant | 43 | 27 | 16 |
Consisted of 8 FN nodes, 3 locoregional recurrences, and 1 contralateral node recurrence with simultaneous distant metastasis.
Qualitative data are number and percentage; continuous data are median and interquartile range.
DISCUSSION
This study assessed the sequential impact of SPECT/CT, intraoperative use of a portable γ-camera, and intraoperative fluorescence guidance using a hybrid tracer on SNB in patients with melanoma in the head and neck region. With the introduction of these sophisticated techniques, more SNs were harvested per patient whereas the duration of the operations remained the same. So, SNs were identified more quickly, improving the surgical workflow. In this respect, the increasing experience of the individual surgeons in SN identification should also be considered. The greater number of SNs could also imply an increase in postoperative morbidity, but this was not the case. The observed downward trend in FN rate over time from 16.7 to 11.1 could suggest that some—tumor positive—SNs were missed in the early days.
Several large series of patients with head and neck melanomas report the removal of an average of 2.0–2.5 SNs per patient, which is less than the 3.8 in our entire population (8,28,29). Although the current data suggest that this higher yield is due to the portable γ-camera and the fluorescence imaging, depiction of extra sentinel nodes by SPECT/CT in the preoperative work-up cannot be neglected (30). The high tissue penetration of radioactive γ-rays in combination with the high spatial resolution of the fluorescence signal increased the identification of SNs within clusters of lymph nodes (23). If the SN could not be identified separately, the entire cluster was harvested and all nodes were classified as SNs. Since not all of these were necessarily on a direct drainage pathway from the tumor, the lack of intraoperative visualization of afferent lymph vessels may thus inadvertently have increased the number of “SNs.”
Our tumor-positive SNB rate of 21.3% is higher than for melanomas elsewhere in the body (31,32). In a systematic review of 12 studies on head and neck melanoma, de Rosa et al. demonstrated an average tumor-positive rate of 15.1% (9). The high number of thick melanomas in our first cohort may well explain our relatively high rate of tumor-positive SNs.
The current overall FN rate of 11.9% concurs with other studies, which included a wide spectrum of melanoma sites (mean, 14.0%; range, 2.8%–32.1%) (25,27,32–34). Although one would intuitively expect innovative detection methods to improve FN rates, we could not establish a significant difference among the groups. Results to improve melanoma staging by ICG vary in the literature. In a large prospective cohort of melanoma patients, ICG and lymphoscintigraphy resulted in higher SN positive rates than the predicted true-positive SNB rate based on the literature and their cohort (35). However, another recent prospective study of 121 melanoma patients demonstrated that the combination of lymphoscintigraphy, probe, and ICG fluorescence improved the SN detection rate only marginally (36).
In addition to the introduction of new detection techniques and advancing surgical skills, the more elaborate histopathologic examination plays an important role in the sensitivity of SNB. After the number of levels of examination of SNs was increased from 3 to 6 in 2014, the number of FN procedures dropped from 8 in 193 patients to none in the subsequent 84.
The variety in definitions of an SN and of an FN SNB hampers meaningful comparison of results of different studies. Some investigators consider the procedure to be FN in cases of a nodal recurrence anywhere after a tumor-negative SNB, whereas in most studies only in-field nodal recurrences count (25,37). Instead of using the formula FN/(TP + FN) (27), some other investigators calculate the rate of FN over the entire group of patients or over the group of tumor-negative SNBs (1,29,38). The differences in follow-up duration are a further limiting factor, as the number of FN cases goes up as more recurrences develop with longer follow-up (9).
There is great geographic variability in the type of radiotracer used. Human serum albumin–based radiocolloids (particle range, 15–100 nm) are generally used in Europe, whereas sulfur colloids (particle range, 20–1000 nm) are commonly used in the United States and antimony sulfide colloids (particle range, 10–15 nm) in Australia (39,40). The more recently approved 99mTc-tilmanocept is now also being studied in the head and neck region (41). No randomized studies have been performed to establish superiority of one radiotracer over another.
The retrospective design, single institution, and relatively small sample size in the time span of the first 2 modalities (Table 3) can also be considered limitations of this study. Although all participating surgeons involved were dedicated head and neck specialists, their substantial number and varying experience might possibly be a limiting factor for the secondary endpoints of the study. Furthermore, the consecutive addition of new technologies without associated randomized studies makes it difficult to discern the independent contribution of each of these.
CONCLUSION
Using advanced image guidance technologies, we found more SNs without increasing the overall operation time or the postoperative complication rate, possibly indicating an improvement of surgical efficiency. Operation time per harvested SN decreased, and the average FN rate remained 11.9% and was unchanged over 23 y.
DISCLOSURE
This work was, in part, supported by the Netherlands Organization for Scientific Research (Rubicon grant [019.171LW.022], VIDI grant [STW BGT11272], and VICI grant [AES BGT 16141]) and by a European Research Council starting grant (ERC-2012-StG-306890). Fijs van Leeuwen acts as a consultant for Hamamatsu Photonics and is chief innovation officer at ORSI Academy. Bart van de Wiel has an advisory role with BMS. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does the technologic evolution of SNB in the head and neck region have an impact on the FN rate?
PERTINENT FINDINGS: This retrospective study found that, over time, there was a higher number of identified SNs with an unchanged FN rate.
IMPLICATIONS FOR PATIENT CARE: The improved outcome of the SNB procedure will make the patient’s prognosis more accurate.
REFERENCES
- 1. Gomez-Rivera F, Santillan A, McMurphey AB, et al. Sentinel node biopsy in patients with cutaneous melanoma of the head and neck: recurrence and survival study. Head Neck. 2008;30:1284–1294. [DOI] [PubMed] [Google Scholar]
- 2. Morton DL, Wen D-R, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. [DOI] [PubMed] [Google Scholar]
- 3. Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376:2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Brien CJ, Uren RF, Thompson JF, et al. Prediction of potential metastatic sites in cutaneous head and neck melanoma using lymphoscintigraphy. Am J Surg. 1995;170:461–466. [DOI] [PubMed] [Google Scholar]
- 7. Wells KE, Rapaport DP, Cruse CW, et al. Sentinel lymph node biopsy in melanoma of the head and neck. Plast Reconstr Surg. 1997;100:591–594. [DOI] [PubMed] [Google Scholar]
- 8. Shpitzer T, Segal K, Schachter J, et al. Sentinel node guided surgery for melanoma in the head and neck region. Melanoma Res. 2004;14:283–287. [DOI] [PubMed] [Google Scholar]
- 9. de Rosa N, Lyman GH, Silbermins D, et al. Sentinel node biopsy for head and neck melanoma: a systematic review. Otolaryngol Head Neck Surg. 2011;145:375–382. [DOI] [PubMed] [Google Scholar]
- 10. Alazraki N, Glass EC, Castronovo F, et al. Society of Nuclear Medicine. Procedure guideline for lymphoscintigraphy and the use of intraoperative gamma probe for sentinel lymph node localization in melanoma of intermediate thickness 1.0. J Nucl Med. 2002;43:1414–1418. [PubMed] [Google Scholar]
- 11. Brouwer OR, Valdés Olmos RA, Vermeeren L, et al. SPECT/CT and a portable γ-camera for image-guided laparoscopic sentinel node biopsy in testicular cancer. J Nucl Med. 2011;52:551–554. [DOI] [PubMed] [Google Scholar]
- 12. van der Ploeg IMC, Madu MF, van der Hage JA, et al. Blue dye can be safely omitted in most sentinel node procedures for melanoma. Melanoma Res. 2016;26:464–468. [DOI] [PubMed] [Google Scholar]
- 13. Haque RA, Wagner A, Whisken JA, et al. Anaphylaxis to patent blue V: a case series and proposed diagnostic protocol. Allergy. 2010;65:396–400. [DOI] [PubMed] [Google Scholar]
- 14. Howard JD, Moo V, Sivalingam P. Anaphylaxis and other adverse reactions to blue dyes: a case series. Anaesth Intensive Care. 2011;39:287–292. [DOI] [PubMed] [Google Scholar]
- 15. Bézu C, Coutant C, Salengro A, et al. Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol. 2011;20:e55–e59. [DOI] [PubMed] [Google Scholar]
- 16. van der Ploeg IMC, Valdés Olmos RA, Nieweg OE, et al. The additional value of SPECT/CT in lymphatic mapping in breast cancer and melanoma. J Nucl Med. 2007;48:1756–1760. [DOI] [PubMed] [Google Scholar]
- 17. Vermeeren L, Valdés Olmos RA, Klop WMC, et al. A portable γ-camera for intraoperative detection of sentinel nodes in the head and neck region. J Nucl Med. 2010;51:700–703. [DOI] [PubMed] [Google Scholar]
- 18. van den Berg NS, Miwa M, KleinJan GH, et al. (Near-infrared) fluorescence-guided surgery under ambient light conditions: a next step to embedment of the technology in clinical routine. Ann Surg Oncol. 2016;23:2586–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van den Berg NS, Brouwer OR, Schaafsma BE, et al. Multimodal surgical guidance during sentinel node biopsy for melanoma: combined gamma tracing and fluorescence imaging of the sentinel node through use of the hybrid tracer indocyanine green-99mTc-nanocolloid1. Radiology. 2015;275:521–529. [DOI] [PubMed] [Google Scholar]
- 20. Brouwer OR, Klop WMC, Buckle T, et al. Feasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracer. Ann Surg Oncol. 2012;19:1988–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richtlijnen hoofd-hals tumoren. Nederlandse Werkgroep Hoofd-Hals Tumoren website. http://www.nwhht.nl/richtlijnen. Accessed May 27, 2021.
- 22. Nieweg OE, Tanis PJ, Kroon BBR. The definition of a sentinel node. Ann Surg Oncol. 2001;8:538–541. [DOI] [PubMed] [Google Scholar]
- 23. KleinJan GH, van Werkhoven E, van den Berg NS, et al. The best of both worlds: a hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur J Nucl Med Mol Imaging. 2018;45:1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chakera AH, Hesse B, Burak Z, et al. EANM-EORTC general recommendations for sentinel node diagnostics in melanoma. Eur J Nucl Med Mol Imaging. 2009;36:1713–1742. [DOI] [PubMed] [Google Scholar]
- 25. Nieweg OE. What is a sentinel node and what is a false-negative sentinel node? Ann Surg Oncol. 2004;11(suppl):169S–173S. [DOI] [PubMed] [Google Scholar]
- 26. Testori A, De Salvo GL, Montesco MC, et al. Clinical considerations on sentinel node biopsy in melanoma from an Italian multicentric study on 1,313 patients (SOLISM-IMI). Ann Surg Oncol. 2009;16:2018–2027. [DOI] [PubMed] [Google Scholar]
- 27. Nieweg OE. False-negative sentinel node biopsy. Ann Surg Oncol. 2009;16:2089–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Wilt JHW, Thompson JF, Uren RF, et al. Correlation between preoperative lymphoscintigraphy and metastatic nodal disease sites in 362 patients with cutaneous melanomas of the head and neck. Ann Surg. 2004;239:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carlson GW, Murray DR, Lyles RH, et al. Sentinel lymph node biopsy in the management of cutaneous head and neck melanoma. Plast Reconstr Surg. 2005;115:721–728. [DOI] [PubMed] [Google Scholar]
- 30. Vermeeren L, van der Ploeg IMC, Valdés Olmos RA, et al. SPECT/CT for preoperative sentinel node localization. J Surg Oncol. 2010;101:184–190. [DOI] [PubMed] [Google Scholar]
- 31. Callender GG, Egger ME, Burton AL, et al. Prognostic implications of anatomic location of primary cutaneous melanoma of 1 mm or thicker. Am J Surg. 2011;202:659–664. [DOI] [PubMed] [Google Scholar]
- 32. Hodges M, Jones E, Jones T, et al. Analysis of melanoma recurrence following a negative sentinel lymph node biopsy. Melanoma Manag. 2015;2:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veenstra HJ, Wouters MWJM, Kroon BBR, et al. Less false-negative sentinel node procedures in melanoma patients with experience and proper collaboration. J Surg Oncol. 2011;104:454–457. [DOI] [PubMed] [Google Scholar]
- 34. Nieweg OE, Veenstra HJ. False-negative sentinel node biopsy in melanoma. J Surg Oncol. 2011;104:709–710. [DOI] [PubMed] [Google Scholar]
- 35. Knackstedt R, Couto RA, Gastman B. Indocyanine green fluorescence imaging with lymphoscintigraphy for sentinel node biopsy in head and neck melanoma. Ann Surg Oncol. 2019;26:3550–3560. [DOI] [PubMed] [Google Scholar]
- 36. de Carvalho CEB, Capuzzo R, Crovador C, et al. Near infrared (NIR) fluorescence is not a substitute for lymphoscintigraphy and gamma probe for melanoma sentinel node detection: results from a prospective trial. Ann Surg Oncol. 2020;27:2906–2912. [DOI] [PubMed] [Google Scholar]
- 37. Saltman BE, Ganly I, Patel SG, et al. Prognostic implication of sentinel lymph node biopsy in cutaneous head and neck melanoma. Head Neck. 2010;32:1686–1692. [DOI] [PubMed] [Google Scholar]
- 38. Chao C, Wong SL, Edwards MJ, et al. Sentinel lymph node biopsy for head and neck melanomas. Ann Surg Oncol. 2003;10:21–26. [DOI] [PubMed] [Google Scholar]
- 39. Vidal-Sicart S, Vera DR, Valdés Olmos RA. Next generation of radiotracers for sentinel lymph node biopsy: what is still necessary to establish new imaging paradigms? Rev Esp Med Nucl Imagen Mol. 2018;37:373–379. [DOI] [PubMed] [Google Scholar]
- 40. Scolyer RA, Thompson JF, Li LX, et al. Failure to remove true sentinel nodes can cause failure of the sentinel node biopsy technique: evidence from antimony concentrations in false-negative sentinel nodes from melanoma patients. Ann Surg Oncol. 2004;11(suppl):174S–178S. [DOI] [PubMed] [Google Scholar]
- 41. den Toom IJ, Mahieu R, van Rooij R, et al. Sentinel lymph node detection in oral cancer: a within-patient comparison between [99mTc]Tc-tilmanocept and [99mTc]Tc-nanocolloid. Eur J Nucl Med Mol Imaging. 2021;48:851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]