Abstract
Aortic carboxypeptidase-like protein (ACLP) is a member of a diverse group of proteins that contain a domain with similarity to that of the Dictyostelium discoideum protein discoidin I. The discoidin domain has been identified in mammalian milk fat globule membrane proteins, blood coagulation factors, and receptor tyrosine kinases, where it may facilitate cell aggregation, adhesion, or cell-cell recognition. Here we show that ACLP is a secreted protein that associates with the extracellular matrix (ECM). During mouse embryogenesis, ACLP is abundantly expressed in the ECM of collagen-rich tissues, including the vasculature, dermis, and the developing skeleton. We deleted the ACLP gene in mice by homologous recombination. The majority of ACLP−/− mice die perinatally due to gastroschisis, a severe disruption of the anterior abdominal wall and herniation of the abdominal organs. ACLP−/− mice that survived to adulthood developed nonhealing skin wounds. Following injury by a dermal punch biopsy, ACLP−/− mice exhibited deficient wound healing compared with controls. In addition, dermal fibroblasts isolated from ACLP−/− 18.5-day-postconception embryos exhibited a reduced proliferative capacity compared with wild-type cells. These results indicate that ACLP is an ECM protein that is essential for embryonic development and dermal wound healing processes.
Interactions between cells and the extracellular matrix (ECM) are important in the regulation of basic cellular functions, such as proliferation, differentiation, adhesion, and migration (1, 10). These interactions also govern most physiological and pathological processes, including fetal development, angiogenesis, and wound healing.
We have identified a novel protein, aortic carboxypeptidase-like protein (ACLP), that is expressed highly in vascular smooth muscle cells of blood vessels, the expression of which increases with smooth muscle cell differentiation (16). The carboxyl terminus of mouse ACLP is identical to that of a cDNA-encoded protein designated adipocyte enhancer binding protein 1 (AEBP1) and reported to be a DNA-binding transcriptional repressor (6). We demonstrated previously that the AEBP1 cDNA is most likely a partial clone of mouse ACLP lacking 410 N-terminal amino acids (16).
ACLP contains a domain with similarity to the slime mold protein discoidin I (2). In addition, ACLP contains a signal peptide at its amino terminus and a region with structural similarity to the pro-hormone-processing metallocarboxypeptidases at its carboxyl terminus (5). This structure of tandem discoidin and carboxypeptidase domains also occurs in two proteins related to ACLP, CPX-1 and CPX-2 (17, 28). Like ACLP, CPX-1 and CPX-2 are missing several amino acid residues required for catalytic activity toward standard carboxypeptidase substrates, leading to the hypothesis that these proteins potentially function as binding proteins rather than active carboxypeptidases (28). An additional protein in this subfamily of metallocarboxypeptidases is CPZ. CPZ does not contain a discoidin domain, but instead has a frizzled-like domain at its N terminus (23, 27). Frizzled domains are present in many secreted proteins that modify the Wnt signaling pathway (21, 26). Recently, CPZ was shown to be secreted from cells and to associate with the ECM (19).
We hypothesized that ACLP, like other proteins with signal peptides and discoidin domains, is a secreted protein that functions in the extracellular environment. We investigated the subcellular localization of ACLP and analyzed its expression during mouse embryonic development. To elucidate the biological function of ACLP, we targeted the ACLP gene in mice. Here we show that ACLP has an essential role during embryonic development and in adult wound healing processes.
MATERIALS AND METHODS
Cell isolation and culture.
Mouse aortic smooth muscle cells (MASMC) were isolated from the aorta of 18.5-day-postconception (dpc) mouse embryos essentially as described previously (20) and characterized by smooth muscle α-actin and calponin immunostaining (data not shown). Dermal fibroblasts were isolated from the skin explants of 18.5-dpc mouse embryos as described previously (4) and used between passages 2 and 4. Mouse embryo fibroblasts were obtained from 14.5-dpc embryos and cultured as described previously (7).
Subcellular fractionation.
Cells were fractionated into cytosolic and microsomal fractions as described previously (29). Confluent MASMC were washed with phosphate-buffered saline (PBS) and scraped into homogenization buffer (30 mM Tris [pH 7.5], 150 mM NaCl, 0.25 M sucrose) containing protease inhibitors (Complete; Roche, Indianapolis, Ind.). Cells were homogenized with a Polytron (Brinkmann Instruments, Westbury, N.Y.), and insoluble material was removed by centrifugation at 10,000 × g. The cytosolic fraction was separated from the microsomal membrane fraction by centrifugation at 100,000 × g for 1 h at 4°C. ECM was extracted by the method of Knudsen et al. (12). The soluble components from confluent MASMC were removed by washing the cells in PBS containing 0.5% Triton X-100. Monolayers were then extracted with 25 mM NH4OH and washed extensively with 20 mM Tris (pH 7.4), 150 mM NaCl, and 0.05% Tween 20. The remaining ECM was dissolved in 20 mM glycine-HCl (pH 2.7) for 1 h at room temperature, boiled, and analyzed by Western analysis as described previously (16).
Proliferation assays.
Cell proliferation was measured by plating 104 cells/well in 12-well culture dishes. At specific intervals, cells were collected by trypsinization and counted in a Coulter cell counter (Beckman Coulter, Fullerton, Calif.). Cell proliferation was normalized to the cell number at 24 h. Where indicated, comparisons between groups were made by factorial analysis of variance followed by Scheffe's test. Significance was accepted at P < 0.05.
Genomic cloning and targeted disruption of ACLP.
To generate an ACLP gene targeting construct, we isolated genomic clones from a 129 SvJ mouse λDASH II genomic library (Stratagene, La Jolla, Calif.) by hybridization with a 32P-labeled fragment of the ACLP cDNA. Exon and intron junctions were determined by sequence comparison with the mouse ACLP cDNA (GenBank accession no. AF053943). A targeting vector was constructed by replacing exon 7 through most of exon 16 with a PGK-neo cassette. A thymidine kinase cassette was ligated to the 5′ end of the construct for negative selection. D3 embryonic stem (ES) cells were electroporated with the NotI-linearized construct, and ES cells were selected on neomycin-resistant mouse embryo fibroblast feeder layers with G418 and ganciclovir as described previously (30). The surviving colonies were expanded and analyzed by Southern blot analysis with EcoRI-digested genomic DNA. Blots were hybridized with probes derived both 5′ and 3′ to the targeting construct. Two correctly targeted clones (241 and 255) were identified by the smaller EcoRI fragment with both the 5′ and 3′ external probes and injected into C57BL/6 blastocysts (Core Transgenic Mouse Facility, Brigham and Women's Hospital, Boston, Mass.). Chimeric mice were mated to wild-type C57BL/6 mice, and heterozygous offspring were identified by genomic Southern blot analysis of DNA isolated from tail biopsies extracted as described in reference 15. All experiments were performed with mice of a mixed 129 SvJ and C57BL/6 genetic background, and littermates were used as controls.
Dermal wound healing.
To evaluate the wound healing response, wild-type and ACLP−/− mice were subjected to a dermal punch biopsy. Mice were anesthetized, the fur was removed, and a 3-mm full-thickness hole was punched with a sterile disposable biopsy punch (Miltex Instruments, Bethpage, N.Y.). At a given time point, mice were killed, and the injured skin and surrounding tissue were excised and processed as described below. Animal use conformed to Federal guidelines and institutional policies.
Histological analysis.
Immunofluorescence staining of MASMC was performed with cells grown on glass coverslips as described previously (8). MASMC were also stained by an immunocytochemical technique with a biotinylated secondary antibody and a peroxidase 3′, 3-diaminobenzidine (DAB) kit (Vector Laboratories, Burlingame, Calif.). Embryos and adult tissue were fixed in methyl Carnoy's fixative (60% methanol, 30% chloroform, 10% acetic acid), dehydrated, and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin (H&E) or incubated with anti-ACLP serum (16) or anti-filaggrin antibodies (Covance Research Products, Cumberland, Va.), and signal was detected with peroxidase-conjugated secondary antibodies by using reagents from Vector Laboratories. Controls for the specificity of the ACLP immunostaining included the use of preimmune serum and blocking with ACLP recombinant protein (data not shown).
RESULTS AND DISCUSSION
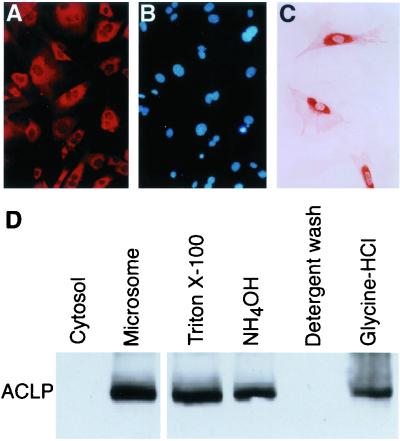
To examine the subcellular localization of ACLP, we performed immunofluorescent staining of cultured MASMC with an ACLP antiserum generated against the carboxyl terminus of ACLP. ACLP was expressed in a predominantly perinuclear pattern (Fig. 1A), indicative of entry into the secretory pathway, and excluded from nuclei as shown by 4′,6′-diamidino-2-phenylindole (DAPI) counterstaining (Fig. 1B). This result was confirmed by immunocytochemistry and light microscopy, which delineated the strong perinuclear expression of ACLP (Fig. 1C). We have previously determined that this antiserum recognizes a single ACLP band on Western blot analysis (16). This indicates that the antiserum is specific for ACLP and does not cross-react with related but substantially smaller CPX-1 and CPX-2 proteins. With this antiserum, we analyzed protein extracts prepared from fractionated cells. By Western analysis, ACLP is present in the 100,000 × g microsomal fraction, but not in the cytosol (Fig. 1D). To examine if ACLP associates with the ECM, MASMC were extracted sequentially by detergent to remove soluble and membrane-associated components and then ammonium hydroxide to remove the remaining soluble proteins, followed by extensive detergent washes to remove any remaining weakly associated proteins. The remaining ECM material was dissolved in glycine-HCl (pH 2.7). ACLP protein was detected in the initial detergent soluble extract, the ammonium hydroxide wash, and the ECM fraction (Fig. 1D). These results demonstrate that ACLP is a secreted protein that associates with the ECM.
FIG. 1.
ACLP associates with the ECM. (A) Immuofluorescent staining of ACLP in MASMC. (B) Nuclei were counterstained with DAPI. (C) Immunocytochemical detection of ACLP delineates its perinuclear localization. (D) ACLP is present in the ECM. Western analysis of protein fractions from MASMC separated into cytosolic and microsomal fractions. Additional cells were sequentially extracted with Triton X-100 and then ammonium hydroxide to remove the remaining soluble proteins and cytoskeleton, followed by extensive detergent washes. The ECM was solubilized in glycine-HCl (pH 2.7).
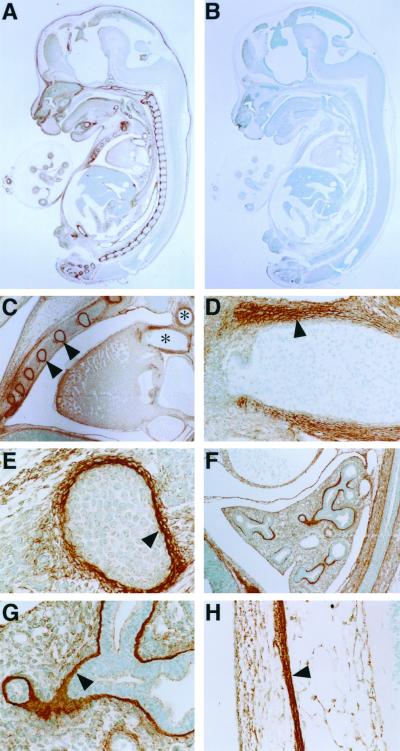
We performed immunostaining of sagittal sections of 15.5-dpc mouse embryos by using ACLP antiserum (Fig. 2A and C to H) or preimmune serum (Fig. 2B). Immunostaining of mouse embryos revealed that ACLP was expressed in several tissues, such as the vascular smooth muscle cells of the larger blood vessels (Fig. 2C and D). In addition, abundant expression was observed in both cartilaginous and bony elements of the developing skeleton, including the skull, vertebrae (Fig. 2A) (data not shown), and perichondrium of the ribs in 15.5-dpc embryos (Fig. 2A, with enlargements in panels C and E). ACLP was expressed in the basement membrane of the airways within the lung (Fig. 2F and G) and in the dermal layer of the skin (Fig. 2H). This expression pattern is similar in some aspects to those of other ECM proteins, including collagens, decorin, and thrombospondin (9, 13, 14).
FIG. 2.
ACLP immunostaining on 15.5-dpc mouse embryo. Wild-type 15.5-dpc embryos were fixed in methyl Carnoy's fixative, dehydrated, and embedded in paraffin. Sagittal sections (5 μm) were incubated with a polyclonal ACLP antiserum (A and C to H) or preimmune serum (B) and detected as described in Materials and Methods. (A) ACLP expression in 15.5-dpc embryo. Original magnification, ×10. (B) Preimmune control staining. Original magnification, ×10. (C) Expression in large blood vessels (lumen indicated by ∗) and ribs (arrowheads). Original magnification, ×100. (D) Higher magnification of panel C showing expression surrounding the smooth muscle cells (arrowhead) of a large blood vessel. Original magnification, ×200. (E) Expression in periosteum of rib (arrowhead). Original magnification, ×400. (F and G) Expression in basement membrane of lung (arrowhead). Original magnification, ×400. (H) Dermal layer of skin (arrowhead). Original magnification, ×400.
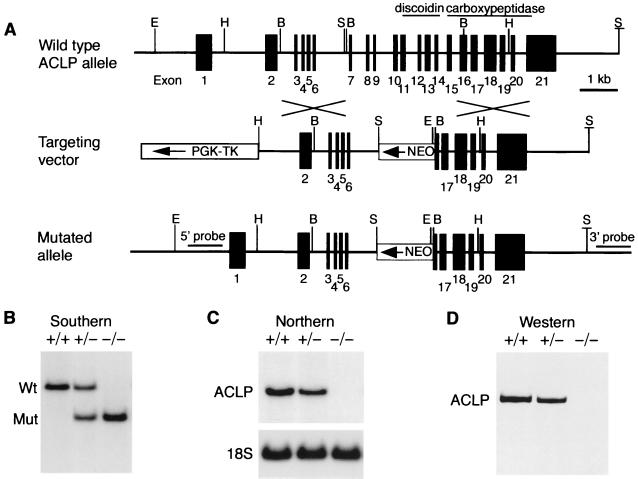
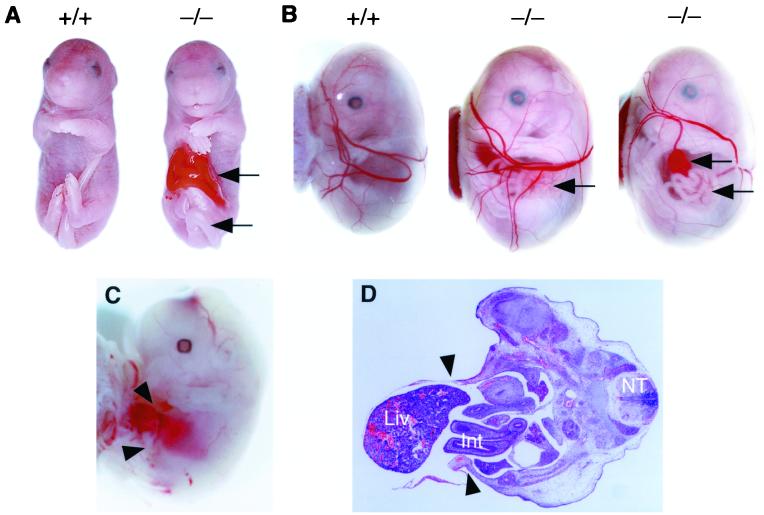
To examine the role of ACLP during embryonic development, we isolated the complete mouse ACLP gene from several overlapping genomic phage clones. Exon and intron junctions were determined by comparison of the genomic sequences to the mouse ACLP cDNA sequence (Fig. 3A) (16). The mouse ACLP gene has many closely spaced small exons (Fig. 3A). The discoidin domain is encoded by exons 11 through 14, while exons 15 through part of 21 encode the carboxypeptidase-like domain (Fig. 3A, top). A targeting construct was generated, electroporated into D3 ES cells, and selected with G418 and ganciclovir as described previously (30). Chimeric mice produced from two correctly targeted ES cell clones were crossed to C57BL/6 mice. ACLP+/− mice were identified by Southern blot analysis (Fig. 3B) and were phenotypically normal. These ACLP+/− mice were crossed to generate homozygous mutant offspring (Fig. 3B). ACLP mRNA and protein were not detected in cells isolated from ACLP−/− 14.5-dpc embryonic fibroblasts (Fig. 3C and D, respectively). The ACLP antiserum was generated against the C terminus; thus, we cannot exclude the possibility that a truncated protein could be generated from exons 1 to 6 of the targeted allele. Because the heterozygote animals are phenotypically normal and we did not detect significant levels of transcript with N-terminal cDNA probes by Northern analysis (data not shown), we believe that the observed phenotype in the ACLP−/− mice is the result of the absence of ACLP and not the gain of function from a truncated protein. From ACLP heterozygote breeding, we observed dead ACLP−/− neonates that were pale and often missing abdominal organs, such as the liver and intestine (data not shown), in both ES cell-derived lines. Of 168 embryos genotyped at 18.5 dpc, 26.2% were ACLP−/−, indicating that most of the lethality occurred around the time of birth. These ACLP−/− 18.5-dpc embryos were equivalent in weight to wild-type and heterozygote littermates (ACLP+/+, 1.25 ± 0.06 g; ACLP+/− , 1.27 ± 0.10 g; ACLP−/−, 1.25 ± 0.13 g). These ACLP−/− embryos exhibited an anterior abdominal wall defect (Fig. 4A) and frequently had a bent or looped tail (Fig. 4A, lower arrow). In humans, gastroschisis is characterized by the extrusion of the abdominal viscera through the ventral body wall without a covering or sac with normal umbilical cord position and morphology (18, 25). To determine if this abdominal wall defect was gastroschisis and not omphalocele, an abdominal wall defect involving the umbilical cord, embryos were examined within the yolk sac at 15.5 dpc (Fig. 4B). At this developmental point in the mouse, the intestines are normally contained within the base of the umbilical cord (11). The ACLP−/− mouse embryos exhibited extrusion of the intestine and often the liver into the amniotic cavity surrounded by the yolk sac (Fig. 4B). This abdominal wall defect was detected as early as 13.5 dpc (Fig. 4C), indicating that the appearance of gastroschisis precedes the normal return of the intestine to the body cavity by 16.5 dpc (11). Histological characterization of these embryos revealed the abnormal protrusion of the liver and intestine through the disruption in the anterior body wall (Fig. 4D). In addition to the ACLP−/− mice, abdominal wall defects have been observed in other induced mouse mutants. Targeted deletion of the homeodomain transcription factor Alx-4 results in abdominal wall defects resembling gastroschisis (22). These mice exhibit additional abnormalities, such as preaxial polydactyly (22), a condition that does not occur in the ACLP−/− animals. Deletion of bone morphogenetic protein 1 (Bmp1), which functions as a procollagen C proteinase, results in a gastroschsisis phenotype. These mice have defects in the ventral body wall that may result from defective collagen fibril formation that leads to an improper folding or adhesion of the amnion (24).
FIG. 3.
ACLP genomic organization and targeted disruption in mice. (A) The mouse ACLP gene contains 21 exons. Exons 11 through 14 encode the discoidin domain, while exons 15 through part of 21 encode the carboxypeptidase-like domain. A targeting construct was designed to replace exons 7 through 16 with a neomycin selection cassette (NEO). E, EcoRI; H, HindIII; B, BamHI; S, SalI; S, SacI. (B) Genotypes were determined by Southern blot analysis of EcoRI-digested genomic DNA with probes flanking the targeting construct (5′ or 3′ probes). The wild-type allele is ∼19 kb, and the targeted allele, detected with the external 5′ or 3′ probes, is smaller due to the internal EcoRI site in the NEO cassette. (C) Northern analysis of RNA from 14.5-dpc mouse embryonic fibroblasts. 18S rRNA oligonucleotide hybridization was used as an RNA loading control. (D Western analysis with an ACLP antiserum directed against the carboxyl terminus confirmed that the absence of ACLP mRNA correlated with the absence of ACLP protein in the knockout embryos.
FIG. 4.
Characterization of gastroschisis in ACLP−/− embryos. (A) Wild-type (18.5 dpc) embryo (left) and ACLP−/− sibling (right) exhibiting a disruption of the anterior abdominal wall with herniation of the intestine (upper arrow) and bent tail (lower arrow). (B) Wild-type 15.5-dpc embryo contained within yolk sac (left) and two examples of ACLP−/− embryos with gastroschisis (center and right). ACLP−/− embryo 15.5 dpc with intestine and colon (center, arrow) projecting through a hole in the body wall into the space created by yolk sac, while another ACLP−/− embryo (right) has extruded liver (upper arrow) and intestine (lower arrow). (C) Gastroschisis in 13.5-dpc ACLP−/− embryo. Arrowheads indicate hole with protruding liver. (D) H&E staining of 13.5-dpc ACLP−/− embryo. Arrowheads indicate anterior portion of body wall. Liver (liv), intestine (Int), and neural tube (NT) are indicated.
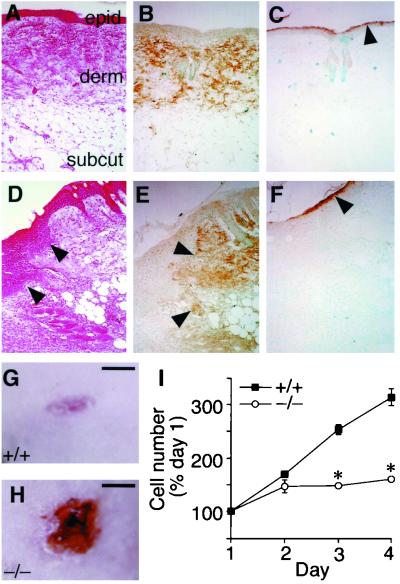
Although most ACLP−/− mice died perinatally from gastroschisis (Fig. 4), some survived to adulthood. From 587 mice of both lines genotyped at 3 weeks of age, 193 (32.9%) were wild type and 355 (60.5%) were heterozygous, while only 39 (6.6%) were ACLP−/−. We observed the appearance of skin lesions in these animals (data not shown). To investigate the expression and function of ACLP in the skin, we performed immunostaining of noninjured wild-type mouse skin. H&E staining on normal mouse skin defines the epidermis composed of differentiated keratinocytes; the dermal layer, which is rich in dermal fibroblasts, blood vessels, and ECM; and the fatty subcutaneous layer (Fig. 5A). Immunostaining with anti-ACLP antibodies detected abundant ACLP expression throughout the dermis of the skin (Fig. 5B). The dermis is rich in ECM, including collagen produced by dermal fibroblasts, and provides a structural support for the epidermis (3). ACLP is not expressed in the keratinocytes of the epidermal layer, identified with the differentiated keratinocyte protein filaggrin (Fig. 5C). Also noted was a low level of ACLP expression throughout the subcutaneous fat (Fig. 5B).
FIG. 5.
ACLP expression in the skin and defective wound healing in ACLP−/− mice. (A to C) Skin from an uninjured wild-type mouse. (A) H&E staining of skin defining epidermis (epid.), dermis (derm.), and subcutaneous fat (subcut.). Original magnification in panels A to F, ×100. (B) ACLP expression in the dermis. (C) Differentiated epidermal keratinocytes identified by filaggrin immunostaining (arrow). ACLP expression during dermal wound healing. (D to F) Dorsal skin 4 days following a 3-mm punch biopsy. (D) H&E staining showing proliferative keratinocytes (arrow). (E) ACLP expression is confined to the uninjured dermis (arrowheads) and is not expressed in the proliferating keratinocytes. (F) Filaggrin immunostaining (arrowhead). Wild-type and ACLP−/− mice were subjected to a 3-mm punch biopsy through the dorsal skin. Representative dermal wounds from ACLP+/+ (G) and ACLP−/− (H) mice 6 days following punch biopsy (bar = 1 mm). (I) Proliferation deficiency in dermal fibroblasts derived from ACLP−/− mice. Dermal fibroblasts were isolated from 18.5-dpc ACLP+/+ and ACLP−/− embryos and plated in 12-well dishes in triplicate. Cells were counted at 24-h intervals and normalized to cell number at 24 h after plating. ∗, P < 0.05 versus ACLP+/+ control.
First, we wanted to examine the expression of ACLP in the normal wound healing process to see if such expression was still limited to the dermal layer. Wild-type mouse skin was subjected to a 3-mm dermal punch biopsy, and after 4 days, skin was collected for analysis. H&E staining revealed a region of proliferating keratinocytes and delineated the border between these cells and the underlying dermis (Fig. 5D). ACLP was expressed throughout the uninjured dermis adjacent to the site of injury (Fig. 5E). ACLP expression was not detected in the proliferative keratinocytes (Fig. 5D) or in the differentiated keratinocytes detected by filaggrin staining (Fig. 5F).
When ACLP−/− animals were similarly wounded with the 3-mm dermal punch, they had a reduction in the rate or extent of healing. Skin was examined 6 days after injury, and the wild-type mice were substantially healed (Fig. 5G). In comparison, ACLP−/− animals at this time point exhibited delayed or inefficient wound healing (Fig. 5H), indicating that ACLP is essential for the proper wound repair. In a representative experiment with three animals per group, the area of the dermal wounds in ACLP+/+ mice was 2.51 ± 0.29 cm2, compared with 6.31 ± 0.15 cm2 in ACLP−/− mice. Taken together, these data suggest a deficiency of ACLP in the extracellular matrix produced by dermal fibroblasts may contribute to the abnormal wound healing in the ACLP−/− mice.
To address the cellular mechanism for this wound healing deficiency, dermal fibroblasts were isolated from 18.5-dpc embryo skin. The proliferation rate of these cells was significantly lower than in wild-type cells (Fig. 5I). Additionally, these ACLP−/− cells appeared flattened and senescent (data not shown).
Our results demonstrate that ACLP is a secreted ECM protein (Fig. 1) that localizes to the ECM of bone, blood vessels, and skin (Fig. 2). Moreover, in the absence of ACLP, dermal fibroblasts have an impaired proliferative capacity, which in part may contribute to the development of gastroschisis and the inefficient wound healing in the ACLP−/− mice. It remains to be determined whether additional phenotypes in the vasculature and skeleton exist in the ACLP−/− mice or whether structurally related proteins such as CPX-1 and CPX-2, which contain discoidin and carboxypeptidase domains, can compensate for the loss of ACLP in these tissues. Our findings imply that ACLP has important roles in both embryonic developmental processes and adult tissue repair.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL10113 (M.D.L.) and HL57977 (M.-E.L. and S.-F.Y.) and a grant from the March of Dimes Birth Defects Foundation (M.-E.L. and M.A.P.).
We thank Bonna Ith for expert technical assistance.
REFERENCES
- 1.Adams J C, Watt F M. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner S, Hofmann K, Chiquet-Ehrismann R, Bucher P. The discoidin domain family revisited: new members from prokaryotes and a homology-based fold prediction. Protein Sci. 1998;7:1626–1631. doi: 10.1002/pro.5560070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark R A F. Wound repair: overview and general considerations. In: Clark R A F, editor. The molecular and cellular biology of wound repair. 2nd ed. New York, N.Y: Plenum Press; 1996. pp. 3–50. [Google Scholar]
- 4.Dlugosz A A, Glick A B, Tennenbaum T, Weinberg W C, Yuspa S H. Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol. 1995;254:3–20. doi: 10.1016/0076-6879(95)54003-2. [DOI] [PubMed] [Google Scholar]
- 5.Gomis-Rüth F X, Companys V, Qian Y, Fricker L D, Vendrell J, Avilés F X, Coll M. Crystal structure of avian carboxypeptidase D domain II: a prototype for the regulatory metallocarboxypeptidase subfamily. EMBO J. 1999;18:5817–5826. doi: 10.1093/emboj/18.21.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He G P, Muise A, Li A W, Ro H S. A. eukaryotic transcriptional repressor with carboxypeptidase activity. Nature. 1995;378:92–96. doi: 10.1038/378092a0. [DOI] [PubMed] [Google Scholar]
- 7.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 8.Hsieh C-M, Yoshizumi M, Endege W O, Kho C-J, Jain M K, Kashiki S, de los Santos R, Lee W-S, Perrella M A, Lee M-E. APEG-1, a novel gene preferentially expressed in aortic smooth muscle cells, is down-regulated by vascular injury. J Biol Chem. 1996;271:17354–17359. doi: 10.1074/jbc.271.29.17354. [DOI] [PubMed] [Google Scholar]
- 9.Iruela-Arispe M L, Liska D J, Sage E H, Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn. 1993;197:40–56. doi: 10.1002/aja.1001970105. [DOI] [PubMed] [Google Scholar]
- 10.Jones P L, Schmidhauser C, Bissell M J. Regulation of gene expression and cell function by extracellular matrix. Crit Rev Eukaryot Gene Expr. 1993;3:137–154. [PubMed] [Google Scholar]
- 11.Kaufman M H. The atlas of mouse development. San Diego, Calif: Academic Press, Inc.; 1992. [Google Scholar]
- 12.Knudsen B, Harpel P, Nachman R. Plasminogen activator inhibitor is associated with the extracellular matrix of cultured bovine smooth muscle cells. J Clin Investig. 1987;80:1082–1089. doi: 10.1172/JCI113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyriakides T R, Zhu Y H, Smith L T, Bain S D, Yang Z, Lin M T, Danielson K G, Iozzo R V, LaMarca M, McKinney C E, Ginns E I, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriakides T R, Zhu Y H, Yang Z, Bornstein P. The distribution of the matricellular protein thrombospondin 2 in tissues of embryonic and adult mice. J Histochem Cytochem. 1998;46:1007–1015. doi: 10.1177/002215549804600904. [DOI] [PubMed] [Google Scholar]
- 15.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Layne M D, Endege W O, Jain M J, Yet S-F, Hsieh C-M, Chin M T, Perrella M A, Blanar M A, Haber E, Lee M-E. Aortic carboxypeptidase-like protein, a novel protein with discoidin and carboxypeptidase-like domains, is up-regulated during vascular smooth muscle cell differentiation. J Biol Chem. 1998;273:15654–15660. doi: 10.1074/jbc.273.25.15654. [DOI] [PubMed] [Google Scholar]
- 17.Lei Y, Xin X, Morgan D, Pintar J, Fricker L. Identification of mouse CPX-1, a novel member of the metallocarboxypeptidase gene family with highest similarity to CPX-2. DNA Cell Biol. 1999;18:175–185. doi: 10.1089/104454999315565. [DOI] [PubMed] [Google Scholar]
- 18.Moore K L, Persaud T V N. The developing human: clinically oriented embryology. 6th ed. Philadelphia, Pa: W. B. Saunders Company; 1998. [Google Scholar]
- 19.Novikova E G, Reznik S E, Varlamov O, Fricker L D. Carboxypeptidase Z is present in the regulated secretory pathway and extracellular matrix in cultured cells and in human tissues. J Biol Chem. 2000;275:4865–4870. doi: 10.1074/jbc.275.7.4865. [DOI] [PubMed] [Google Scholar]
- 20.Oakes B, Batty A, Handley C, Sandberg L. The synthesis of elastin, collagen, and glycosaminoglycans by high density primary cultures of neonatal rat aortic smooth muscle. An ultrastructural and biochemical study. Eur J Cell Biol. 1982;27:34–46. [PubMed] [Google Scholar]
- 21.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 22.Qu S, Niswander K D, Ji Q, van der Meer R, Keeney D, Magnusen M A, Wisdom R. Polydactyly and ectopic ZPA formation in Alx-4 mutant mice. Development. 1997;124:3999–4008. doi: 10.1242/dev.124.20.3999. [DOI] [PubMed] [Google Scholar]
- 23.Song L, Fricker L D. Cloning and expression of human carboxypeptidase Z, a novel metallocarboxypeptidase. J Biol Chem. 1997;272:10543–10550. doi: 10.1074/jbc.272.16.10543. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki N, Labosky P A, Furuta Y, Hargett L, Dunn R, Fogo A, Takahara K, Peters D M P, Greenspan D S, Hogan B L M. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122:3587–3595. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- 25.Torfs C, Curry C, Roeper P. Gastroschisis. J Pediatr. 1990;166:1–6. doi: 10.1016/s0022-3476(05)81637-3. [DOI] [PubMed] [Google Scholar]
- 26.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 27.Xin X, Day R, Dong W, Lei Y, Fricker L D. Cloning, sequence analysis, and distribution of rat metallocarboxypeptidase Z. DNA Cell Biol. 1998;17:311–319. doi: 10.1089/dna.1998.17.311. [DOI] [PubMed] [Google Scholar]
- 28.Xin X, Day R, Dong W, Lei Y, Fricker L D. Identification of mouse CPX-2, a novel member of the metallocarboxypeptidase gene family: cDNA cloning, mRNA distribution, and protein expression and characterization. DNA Cell Biol. 1998;17:897–909. doi: 10.1089/dna.1998.17.897. [DOI] [PubMed] [Google Scholar]
- 29.Yet S-F, Pellacani A, Patterson C, Tan L, Folta S, Foster L, Lee W-S, Hsieh C-M, Perrella M. Induction of heme oxygenase-1 expression in vascular smooth muscle cells. A link to endotoxic shock. J Biol Chem. 1997;272:4295–4301. doi: 10.1074/jbc.272.7.4295. [DOI] [PubMed] [Google Scholar]
- 30.Yet S-F, Perrella M A, Layne M D, Hsieh C-M, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee M-E. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Investig. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]