Summary
Background
Early diagnosis is crucial for patients with pancreatic ductal adenocarcinoma (PDAC). The AXL receptor tyrosine kinase is proteolytically processed releasing a soluble form (sAXL) into the blood stream. Here we explore the use of sAXL as a biomarker for PDAC.
Methods
AXL was analysed by immunohistochemistry in human pancreatic tissue samples. RNA expression analysis was performed using TCGA/GTEx databases. The plasma concentrations of sAXL, its ligand GAS6, and CA19-9 were studied in two independent cohorts, the HMar cohort (n = 59) and the HClinic cohort (n = 142), including healthy controls, chronic pancreatitis (CP) or PDAC patients, and in a familial PDAC cohort (n = 68). AXL expression and sAXL release were studied in PDAC cell lines and murine models.
Findings
AXL is increased in PDAC and precursor lesions as compared to CP or controls. sAXL determined in plasma from two independent cohorts was significantly increased in the PDAC group as compared to healthy controls or CP patients. Patients with high levels of AXL have a lower overall survival. ROC analysis of the plasma levels of sAXL, GAS6, or CA19-9 in our cohorts revealed that sAXL outperformed CA19-9 for discriminating between CP and PDAC. Using both sAXL and CA19-9 increased the diagnostic value. These results were validated in murine models, showing increased sAXL specifically in animals developing PDAC but not those with precursor lesions or acinar tumours.
Interpretation
sAXL appears as a biomarker for early detection of PDAC and PDAC–CP discrimination that could accelerate treatment and improve its dismal prognosis.
Funding
This work was supported by grants PI20/00625 (PN), RTI2018-095672-B-I00 (AM and PGF), PI20/01696 (MG) and PI18/01034 (AC) from MICINN-FEDER and grant 2017/SGR/225 (PN) from Generalitat de Catalunya.
Abbreviations: PDAC, Pancreatic ductal adenocarcinoma; sAXL, Soluble AXL; CP, Chronic pancreatitis; CA19-9, Carbohydrate antigen 19-9; RTKs, Receptor tyrosine kinases; PanINs, Pancreatic intraepithelial neoplasias; IPMNs, Intraductal papillary mucinous neoplasms; ROC, Receiver operating curve; AUC, Area under the curve; IQR, Interquartile range; IHC, Immunohistochemistry; FDA, Food and drug administration; GAS6, Growth arrest-specific factor 6; TCGA, The Cancer Genome Atlas; GTEx, Genotype-tissue expression; HCC, Hepatocellular carcinoma
Keywords: PDAC, AXL, Pancreas, Biomarker, Differential diagnosis
Research in context.
Evidence before this study
Pancreatic cancer, and in particular pancreatic ductal adenocarcinoma (PDAC), is one of the tumours with the worst prognosis. It is currently the third leading cause of cancer-related deaths in developed countries and is predicted to become the second cause by 2030. One of the reasons for this low survival rate is a late diagnosis, which is the consequence of the lack of bona fide biomarkers for early PDAC detection. Surgical resection is the only curative option for PDAC patients but applies to less than 20% of them, due to late diagnosis. Tissue overexpression of the AXL receptor tyrosine kinase in PDAC correlates with an unfavourable prognosis but at present has no use in the clinic. AXL can be proteolytically processed releasing a soluble form (sAXL) into the bloodstream. Here we explore the use of sAXL in plasma as a biomarker for PDAC early detection.
Added value of this study
Soluble AXL levels in plasma were significantly higher in the PDAC group than in chronic pancreatitis or healthy controls. Consequently, sAXL was found to be a potential biomarker to differentiate PDAC from chronic pancreatitis. An increase in sAXL occurred early in the development of the disease, and it was confirmed in established mouse models of the disease.
Implications of all the available evidence
Early and differential diagnosis of severe pancreatic cancers is an unmet clinical problem. To our knowledge, this is the first study of sAXL as a biomarker in this context. Importantly, sAXL was able to discriminate pancreatic cancer from chronic pancreatitis, adding value to the established biomarkers. The inclusion of sAXL in screening panels would provide additional diagnostic value and help clinicians to expedite surgical resection. The results indicate that AXL targeting could be a potential PDAC treatment for specific patients.
Alt-text: Unlabelled box
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most frequent (>90%) type of pancreatic cancer and presents a major health concern due to its high mortality, ranking among the deadliest tumours.1 This cancer is rarely detected in its initial stages2 and can quickly become a metastatic, therapy-resistant disease, with an average five-year survival of 3% after diagnosis.3 The dismal prognosis of these patients emphasizes the urgency to identify novel biomarkers for early diagnosis, which would allow surgical resection (currently the only option for curative therapy). In addition, these biomarkers would be important for clinical monitoring of high-risk patients, such as those harbouring germline genetic mutations linked to higher PDAC incidence or familial PDAC, as well as patients with chronic pancreatitis (CP).
Currently, PDAC diagnosis relies upon imaging procedures. Although recent studies have identified the genetic landscape of PDAC, and several genetic signatures for specific subtypes have been proposed,4, 5, 6, 7 no gene expression or mutation panel is currently widely used for patient stratification. Secreted biomarkers have been widely studied for PDAC diagnosis in blood samples,8 but to date the carbohydrate antigen 19-9 (CA19-9) is the only FDA-approved biomarker.9,10 However, the inadequate sensitivity and specificity (mean values of 79% and 82%, respectively) of CA19-9 makes it unsuitable as a diagnostic tool for PDAC; thus, its clinical use is restricted to monitoring treatment response and recurrence in patients already diagnosed with PDAC.8, 9, 10 In particular, detection of the CA19-9 biomarker in blood results in false negatives for people with mutations in the FUT3 gene (who make up 5–10% of the world population), as they do not express the Lewis antigen. Moreover, CA19-9 is expressed at high levels in non-malignant pancreatic inflammatory conditions, such as CP, leading to false positives.
A known risk factor associated with the PDAC aetiology is CP, a long-standing inflammation of the pancreas with episodes of acute inflammation. CP is a severe disorder with an annual prevalence close to 0.05%, and it leads to a significant reduction of quality of life. Genetically engineered mouse models of PDAC have demonstrated that pancreatic inflammation accelerates PDAC initiation and progression.11 CP represents a continuum that ranges from recurrent acute pancreatitis, presenting either no or subtle architectural changes of the gland that are difficult to identify by imaging diagnostic tools,12 to advanced CP, which shows architectural gland distortions such as calcifications, side branches, parenchymal atrophy, and main ductal abnormalities.13 At these advanced stages, lesions detected in follow-ups (e.g., by computed tomography scan, magnetic resonance imaging or endoscopic ultrasound) can be indistinguishable from PDAC. Thus, differential diagnosis between both diseases is challenging, even after performing fine needle aspiration, as targeting the sampling site may be difficult since inflammation and cancer display similar characteristics in imaging studies. In addition, symptoms for both PDAC and CP can be similar and vague, such as upper abdominal pain, nausea and vomiting, digestive symptoms, weight loss, and diabetes. Given that early diagnosis is critical for clinical decisions about PDAC, biomarkers that differentiate pancreatic inflammation from PDAC are being actively sought.14
The TAM receptor family of receptor tyrosine kinases (RTKs) comprises three genes in humans: TYRO3 (GeneID:7301), AXL (GeneID:558), and MerTK (GeneID:10461). The TAM receptors share a common modular architecture, composed of two immunoglobulin-like and two fibronectin III-like modules extracellularly, a transmembrane region, and a conserved tyrosine kinase domain. Further, they share two vitamin K-dependent protein ligands, growth arrest-specific factor 6 (GAS6) and protein S (PROS1), which are present in plasma and have the capacity to bind phosphatidylserine exposed in the cellular membrane of activated or apoptotic cells. While TAM receptors are not essential for embryological development, they play important roles in tissue homeostasis especially in tissue repair, controlling cell proliferation and survival, and immune regulation.15 Not surprisingly, these receptors are known to be involved in cancer progression and metastasis.16 In several cancer types, including PDAC, high levels of expression of the AXL protein in tissue samples correlates with adverse prognosis.17,18 Further functional in vitro and in vivo studies have identified AXL as a putative target for pharmacological or immunological therapy in PDAC.19,20
The TAM receptors undergo an enzymatic processing in the cell membrane through an ADAM10/ADAM17-specific shedding mechanism that cleaves the extracellular part of the receptors, releasing a soluble form. In particular, soluble AXL (sAXL) is present in human blood, where it forms a complex with its ligand GAS6,21 and has been detected at high levels in different pathologies, such as heart failure, certain inflammatory conditions, and several tumours.21, 22, 23, 24, 25, 26, 27 In this study, we explored whether sAXL detection in plasma could be used as a novel tool for PDAC diagnosis. We analysed the expression of total AXL and sAXL in tissue and blood samples from human normal and pathological pancreas from different cohorts of patients, as well as in pancreatic cancer cell lines. Using a third independent patient cohort of familial hereditary pancreatic cancer and two well-characterized mouse models of pancreatic cancer, we validated the potential of sAXL for early detection of PDAC. Our results support the use of circulating sAXL determination as an accurate non-invasive diagnostic tool for PDAC, in particular for PDAC–CP discrimination. These results have promising implications for oncologists and PDAC patient management.
Methods
Ethics
This study was approved by the Ethics Committees of Parc de Salut Mar (CEIm-Parc de Salut Mar), Hospital Clinic of Barcelona and Ramon y Cajal University Hospital, Madrid, and was implemented in accordance with the Helsinki Declaration of Principles. The Ethics Committees of PRBB (Barcelona) and CNIO (Madrid) approved animal experiments. Project numbers are indicated below.
Patient samples
For histological studies, samples from normal pancreas (n = 11), CP (n = 14), preneoplastic lesions including Pancreatic intraepithelial neoplasias (PanINs, n = 13), Intraductal papillary mucinous neoplasms (IPMNs, n = 12), and PDAC (n = 17), were obtained from Parc de Salut MAR Biobank (MARBiobanc), Barcelona (Table S1 and S2).
Plasma samples from two independent cohorts were used. A first cohort was from Barcelona-Hospital del Mar (HMar cohort), and comprised samples from 7 healthy controls, 21 patients with CP, and 31 patients with PDAC.28 The second cohort was from Hospital Clínic of Barcelona (HClinic cohort), and comprised samples from 46 healthy controls , 16 patients with CP, and 80 patients with PDAC.29 Tables S1, S3, S4 and S5 summarize the patient characteristics and clinical data corresponding to the plasma samples included in these two independent cohorts. The study was evaluated and approved by the Drug Research Ethics Committee of Parc de Salut Mar (CEIm-Parc de Salut Mar) (2017/7449/I and 2020/9067/I) and Institutional Ethics Committee of Hospital Clinic of Barcelona (HCB/2019/0247). The study incorporates a third cohort of individuals classified as having hereditary or familial PDAC according to the inclusion criteria of the Spanish Familial Pancreatic cancer registry (PANGENFAM) (Ramon y Cajal Health Research Institute, IRYCIS, Madrid), as well as healthy, high-risk family members in a secondary screening program (Ethics Committee number 029-16) (Table S6). This cohort also included cases with no reported hereditary or familial pancreatic cancer syndrome that were classified as sporadic cases.30 All individual participants in the three studies voluntarily signed an informed consent allowing the use of their plasma samples for research purposes.
Animal models
Ela-KRASG12Vp53lox/lox mice carrying specific pancreatic oncogenic mutations in KRAS, and Ela-1-myc transgenic mice, with c-MYC overexpression, were bred and grown as previously reported.31, 32, 33 Animals were sacrificed at 3 to 6 months of age, at which point most mice had developed a pancreatic tumour, as assessed histologically. All animal procedures and protocols were performed with approval from our institutional Animal Care Committee (PRBB, Barcelona, PNM 16-009/9067, and CNIO, Madrid PROEX 257/19).
Immunohistochemistry (IHC)
Biological samples were obtained from Parc de Salut MAR Biobank (MARBiobanc), Barcelona. Paraffin sections (3 μm) were used for IHC analysis, as described.34 Goat anti-AXL polyclonal antibody (R and D Systems Cat# AF154, RRID:AB_354852) or irrelevant IgG (as negative control, Sigma-Aldrich Cat# I5256, RRID: AB_1163599) were used as primary antibodies, and Immpress HRP anti-goat (Vector Laboratories, Cat# MP-7405, RRID: AB_2336526) as secondary antibody. Immunostained samples were analysed by two experts in pancreatic pathology (MI and NM-B), who recorded the intensity and percentage of stained cells to calculate the H-scores.35
Database RNA expression analysis of AXL in PDAC
AXL mRNA expression were downloaded from The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/tcga). Values were normalized using the TMM (Trimmed Mean of M Values) method and expressed as log2 CPM (counts per million).36 RNA expression data of 171 control pancreatic tissue were consulted in GTEx (Genotype-Tissue Expression) database (https://www.gtexportal.org/). For normal versus tumour comparison, data were analysed using the GEPIA2 web server (http://gepia2.cancer-pku.cn/#about).37 Samples from TCGA and GTEx were processed using a uniform bioinformatics pipeline to eliminate batch effect due to distinct computation processing. Survival TCGA Kaplan-Meier analyses and cut-off values were derived from Human Protein Atlas (https://www.v20.proteinatlas.org). Comparison of AXL RNA expression data from normal pancreas versus PanINs was derived from Ayars et al.38 and RNA data from control versus CP and PDAC was obtained from Abdollahi et al (E-EMBL-6).39
Human pancreatic cancer cell lines
Five human pancreatic cancer cell lines were studied: SK-PC-1, adenocarcinoma cells of the exocrine pancreas with epithelial-like morphology (RRID:CVCL_4054); PANC-1, a pancreatic epithelioid carcinoma cell (RRID:CVCL_0480); Hs 766T adenocarcinoma metastatic cells found in lymph nodes (RRID:CVCL_0334); RWP-1, a pancreatic adenocarcinoma cell line derived from a liver metastasis (RRID:CVCL_4373); and Capan-1, a pancreatic ductal adenocarcinoma derived from metastatic sites in the liver (RRID:CVCL_0237). Cells were obtained from the Cell Culture Maintenance Unit (IMIM Core Facilities, Hospital del Mar Medical Research Institute, Barcelona), and validated by short tandem repeat (STR) profile or direct purchase from official repositories (ATCC or ECACC). Cells were cultured in DMEM 10% foetal bovine serum, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin in a humidified incubator at 37 °C with 5% CO2.
Immunoblotting
AXL expression levels in cellular extracts and cultured cells conditioned media were analysed by standard immunoblotting. Briefly, 10% SDS-PAGE was electroblotted onto a nitrocellulose membrane (GE Healthcare). After transfer, membranes were blocked, washed, and incubated overnight at 4 °C with anti-AXL antibodies (Santa Cruz Biotechnology Cat# sc-1096, RRID:AB_630894; and R and D Systems Cat# AF154, RRID:AB_354852) and goat anti-pyruvate kinase (Chemicon Cat# AB1235). Secondary antibodies conjugated to HRP (Thermo Fisher Scientific Cat# 31402, RRID:AB_228395) were used to develop the specific signal by chemoluminiscence using Pierce substrate (Thermo Fisher Scientific) in a ChemiDoc Imaging System (Biorad).
RNA quantification
Total RNA was purified using GenElute mammalian total RNA kit (Sigma Aldrich) according to the manufacturer's instructions for attached cells. The RNA concentration and purity were analysed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). RevertAid First Strand cDNA Synthesis Kit (Thermofisher) was used to retrotranscribe the RNA. Real-time qPCR was performed with SybrTM Green PCR Master Mix (Life Technologies), 25 ng of cDNA, and forward and reverse primers for either AXL (Invitrogen) or (as a control) the housekeeping gene GAPDH. Alternatively, AXL expression was determined using a Taqman specific probe (Hs01064444) using TATA-binding protein (TBP) as housekeeping control. To analyse the samples, the fold-change in relative gene expression was calculated using the ΔΔCT method.
Measurement of plasma levels of sAXL, GAS6, and CA19-9 by ELISA
The levels of human sAXL and GAS6 in plasma or culture media were quantified with the human ELISA kits (R and D Systems) according to manufacturers’ protocols. Plasma samples were diluted 1:50 for the analysis, while culture media were diluted 1:10. The proteins were detected by absorbance determination at 450–570 nm. Plasma levels of CA19-9 were measured by an electrochemiluminescent technique in a Cobas 4000 analyser (Roche Diagnostics) at the Laboratori de Referència de Catalunya (Barcelona, Spain) and at the CORE laboratory (Hospital Clínic, Barcelona). Murine plasma sAXL and GAS6 levels were quantified with the respective mouse ELISA kits (R and D Systems) according to manufacturers’ protocols. Plasma samples were diluted 1:50 for the analysis. The proteins were detected by absorbance determination at 450–570 nm. Biomarker determinations were performed blinded to which group each sample belonged.
Statistical analysis
Data analyses were carried out using the SPSS software (IBM SPSS statistics version 23). Statistical significance was set at p < 0.05 (*p < 0.05; **p < 0.01; ***p < 0.001). As ELISA measurements of sAXL, GAS6, and CA19-9 showed skewed distributions, data were described as median and interquartile range (IQR), specifying quartiles 25 and 75 in the supplementary tables (Q25, Q75), and the nonparametric analyses of Mann-Whitney or Kruskal-Wallis were applied. Multivariate analysis (age, gender, diabetes and obstructive jaundice) was performed in HClinic cohort through median regression models to rule out the effect of these factors in sAXL, GAS6 and CA19-9 determinations. Logistic regression models and receiver operating characteristic (ROC) curve analyses were used to study the potential of sAXL, GAS6, or CA19-9, to discriminate PDAC from CP or healthy controls. Results were given as area under curve (AUC) and standard error with 95% confidence limits (Table S7). The cut-off values maximizing Youden index were derived from this analysis, except indicated otherwise in which we primed a better balance between sensitivity and specificity (Table S8). For the combination of two markers, multivariable logistic regression models were performed, considering sAXL and GAS6 as continuous variables and CA19-9 dichotomized. For Kaplan Meier survival analysis, all patients were followed up until death or last hospital admission, and the cut-off point was given by maximally selected rank statistic using the web based tool Cutoff Finder.40 Patients with missing data were excluded in the analyses of such variables.
Role of funders
Funding sources had no role in writing, data collection, analysis, or interpretation, or any aspect pertinent to the study. The corresponding authors had full access to all data and had final responsibility for the decision to submit the manuscript for publication.
Results
Expression of AXL in tissue samples from normal and pathological human pancreas
Overexpression of the AXL protein in pancreatic cancer tumours has been previously described.17,18,41 However, AXL expression has not been investigated in pathological conditions associated with high risk for developing PDAC, such as CP or preneoplastic lesions. Here, we analysed AXL expression by immunohistochemistry (IHC) in normal and pathological tissue pancreatic samples, including CP, pancreatic intraepithelial neoplasias (PanINs), intraductal papillary mucinous neoplasms (IPMNs), and PDAC (Fig. 1, Table S2). In general, no AXL expression was observed in the normal pancreas and no expression or only a diffuse expression was observed in CP lesions. Overall, the strongest AXL expression was observed in IPMN and PDAC samples (Fig. 1a and 1b). However, AXL expression was heterogeneous, with no expression in some tumours (see IHC quantification using the H-score; Fig. 1b). Thus, AXL is frequently overexpressed in pancreatic cancer and is already induced in precursor lesions (PanIN and IPMN) but not during inflammatory conditions like CP. These data were confirmed using RNA expression data from 179 cases of PDAC (TCGA database) and 171 pancreatic control tissues (GTEx database; Fig. 1c), as well as RNA expression from other previous works including PanIN lesions, CP and PDAC (Figure S1).38,39
Fig. 1.
Detection of AXL in normal and pathological human pancreatic samples. (a) Immunohistochemical analysis of AXL protein from the following human pancreatic samples: normal tissue (n = 11), chronic pancreatitis (n = 14), PanIN lesions (n = 13); IPMN lesions (n = 12); and PDAC (n = 17). Scale bars, 100 μm. (b) Graph shows the AXL staining quantification by H-score. Note that asterisks refer to the comparison of groups with the control, except for the comparisons labelled otherwise. (c) GEPIA2 analysis of AXL RNA levels comparison between normal pancreatic tissue (control, GTEx database, n = 171) and PDAC (TCGA database, n = 179). (d) AXL RNA levels in each subgroup of the different PDAC molecular classifications. From left to right, Collision: C (classical), E (exocrine), QM (quasi-mesenchymal); Moffitt: C (classical), B (basal-like); and Bailey: A (ADEX), P (progenitor), I (immunogenic) and S (squamous). *p < 0.05; **p < 0.01; ***p < 0.001 (Mann-Whitney test).
Recent progresses in cancer genomics have identified two main PDAC subtypes according to their genetic signatures: i) the classical/progenitor subtype, which are normally well-differentiated tumours, and ii) the squamous/basal-like subtype, which have a worse prognosis and low differentiation.4,5,7,9 Thus, we interrogated the TCGA database to gain further insights about AXL expression in pancreatic cancer. Notably, AXL expression was higher in the squamous/basal-like versus classical tumour subtype, using three different genomic classifications (Fig. 1d).4, 5, 6, 7,9 These results indicate that AXL overexpression is observed in most PDAC samples, and that the poorly differentiated, squamous/basal-like pancreatic tumours express the highest levels of AXL.
Expression of AXL in cell lines derived from pancreatic cancer
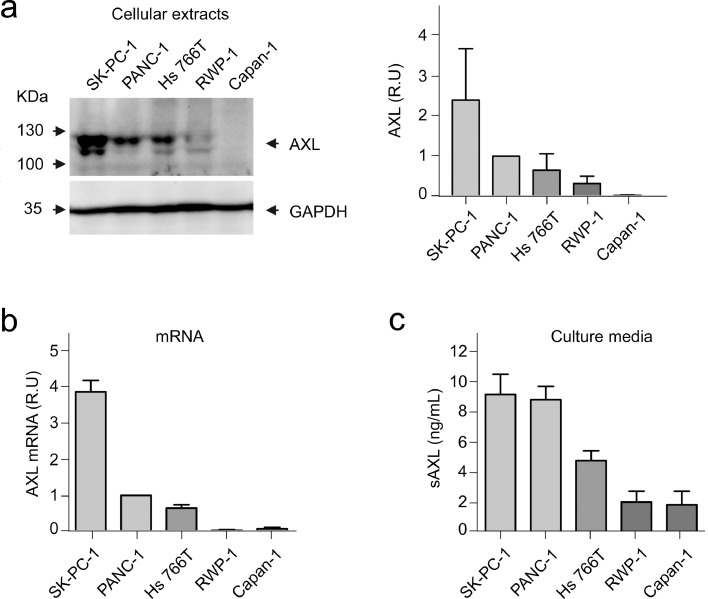
To perform a more exhaustive analysis of AXL expression in PDAC, we explored established human pancreatic cancer cell lines (Fig. 2). Detection of AXL in total cell extracts by immunoblot showed a double band of around 130 KDa, as expected.18 In the cellular extracts of the five cell lines tested, SK-PC-1 showed the highest AXL expression, PANC-1 and Hs 766T cells showed intermediate levels, and RWP-1 and Capan-1 showed the lowest AXL expression (Fig. 2a). This pattern of expression was mirrored at the mRNA level (Fig. 2b). Next, we determined the concentration of processed sAXL in culture media by ELISA. The pattern of expression was consistent with the previous results of total AXL, with sAXL levels highest in the SK-PC-1, PANC-1, and Hs 766T cell lines, and lower in the RWP-1 and Capan-1 cell lines (Fig. 2c). Secreted sAXL levels could be affected by the level of ADAM10/17, the proteases releasing sAXL to the media. These results confirmed the heterogeneity of AXL expression in PDAC and demonstrated that pancreatic tumour cell lines can release the proteolytically processed sAXL fragment to the extracellular milieu.
Fig. 2.
Expression of AXL in pancreatic cancer cell lines. (a) Immunoblot of cellular extracts of SK-PC-1, PANC-1, Hs 766T, RWP-1, and Capan-1 cells (left) using antibodies anti-AXL or anti-GAPDH (as loading control). Bands were quantified by densitometry with respect to GAPDH (right). (b) Total RNA was analysed by RT-qPCR for the relative expression of AXL. Bars represent mean ± SE. (c) Detection of sAXL by ELISA in conditioned media of each cell line after a serum starvation period of 48 h. Bars represent mean ± and SE. R.U., relative units.
Detection of sAXL and GAS6 in plasma from patients with CP or PDAC
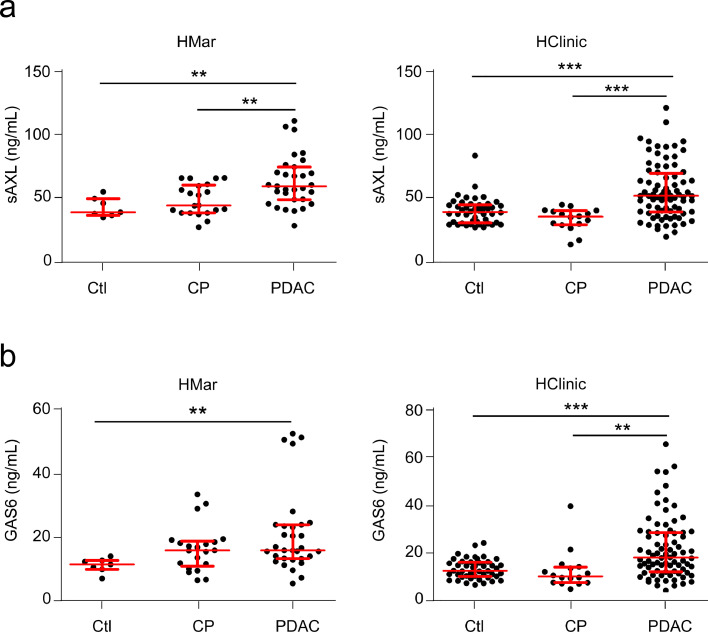
To determine if circulating sAXL can be a diagnostic tool for PDAC patients, we next used ELISA to analyse the levels of both sAXL and GAS6 (the specific ligand of AXL, which binds to and sequesters circulating sAXL in blood) in plasma of healthy controls or patients with CP or PDAC. We used two independent cohorts of plasma samples: HMar cohort (n = 59) and HClinic cohort (n = 142; Tables S1, S3-S5 for clinico-pathological data of patients). To avoid deviations due to cohort definition, sample handling, or processing, we analysed each cohort separately (Fig. 3). In HMar cohort (Fig. 3a, left), the plasma levels of sAXL significantly increased in PDAC patients (median, 59.78 ng/mL; IQR, 25.38) as compared to healthy controls (median, 39.45 ng/mL; IQR, 13.02; p = 0.002) or patients with CP (median, 44.82 ng/ml; IQR, 21.75 p = 0.003). These results were verified in a second larger cohort (HClinic; Fig. 3a, right), where plasma sAXL levels were also significantly increased in PDAC patients (median, 52.66 ng/mL; IQR, 30.08) as compared to healthy controls (median, 40.03 ng/mL; IQR, 14.13; p < 0.0001) or patients with CP (median, 36.34 ng/mL; IQR, 11.05; p < 0.0001). Importantly, statistical significance was maintained in multivariate analysis, discarding age, sex, diabetes and obstructive jaundice as possible confounding factors (Figure S2, Table S5).
Fig. 3.
sAXL and GAS6 plasma levels in human samples from healthy controls or patients with CP or PDAC in two different cohorts. (a) Concentration of the sAXL in the HMar cohort (left panel) or HClinic cohort (right panel), including plasma samples from healthy controls (HMar, n = 7; HClinic, n = 46) or patients with CP (HMar, n = 21; HClinic, n = 16), or PDAC (HMar, n = 31; HClinic, n = 80). (b) GAS6 concentrations in HMar cohort (left panel) or HClinic cohort (right panel) including plasma samples from healthy controls or patients with CP and PDAC, as before. *p < 0.05; **p < 0.01; ***p < 0.001 (Mann-Whitney test).
Determination of GAS6 concentration by ELISA in the same samples (Fig. 3b) showed similar patterns to sAXL, with a significant increase in PDAC patients (median, 15.88 ng/mL; IQR, 10.62, for the HMar cohort and median, 18.14 ng/mL; IQR, 16.34, for the HClinic cohort) as compared to healthy control samples (median, 11.47 ng/mL; IQR 2.79; p = 0.002, for HMar cohort and median, 12.66 ng/mL; IQR, 5.96; p < 0.0001, for HClinic cohort). No difference was found between the CP and PDAC in the HMar cohort, while significant differences were found in the CP group from the HClinic cohort (median, 15.88 ng/mL; IQR, 7.90; p = 0.328 for HMar cohort and median, 10.31 ng/mL; IQR, 6.44; p = 0.001, for the HClinic cohort). Differences were also significant in the multivariate analysis (Figure S2, Table S5). These results suggest that the GAS6 ligand is bound to circulating sAXL in blood, almost mirroring its plasma levels.
Analysis of sAXL and GAS6 expression during PDAC progression and prognostic value
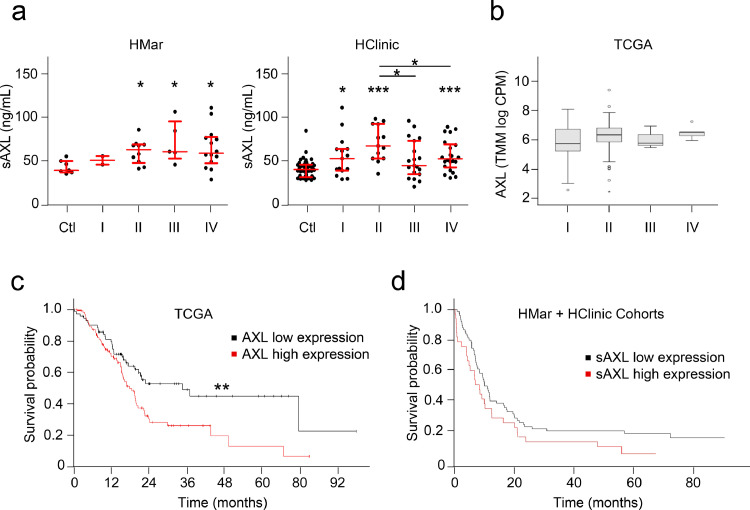
To determine whether sAXL levels reflects PDAC progression, we next classified plasma samples from PDAC patients according to disease clinical staging and compared to controls, for both independent cohorts (Fig. 4a). Samples grouped according to the TNM staging revealed that stage II, III and IV showed higher sAXL concentrations compared to controls but with no significant differences among them observed in a Kruskal-Wallis test (Fig. 4a). These data were confirmed by TCGA RNA database (Fig. 4b). As expected, the concentration of GAS6 in our cohorts and TCGA database showed no differences in the groups classified according to TNM staging (Kruskal-Wallis test) (Figure S3a, b). These data suggest that sAXL could be a good biomarker for early diagnosis of PDAC, as its overexpression is detected at the initial stages of the disease independently of its clinical stage.
Fig. 4.
Analysis of sAXL plasma levels during PDAC progression and prognostic value. (a) Left panel, determination of sAXL plasma levels in HMar cohort samples from controls (n = 7) or PDAC patients segregated by clinical stage: I (n = 2), II (n = 9), III (n = 4), IV (n = 14). Right panel, sAXL in HClinic cohort according to stage: Ctl (n = 46); I (n = 15); II (n = 13); III (n = 19); IV (n = 22). Data were analysed by Mann-Whitney test, all asterisks refer to control unless otherwise specified *p < 0.05; ** p < 0.01; *** p < 0.001. Data were also analysed with Kruskal-Wallis test (HMar, p = 0.628; HClinic, p = 0.056). (b) TCGA AXL RNA expression in different tumour stages: I (n = 21), II (n = 150), III (n = 3), IV (n = 5). Kruskal-Wallis p = 0.148. (c) Prognosis of patients according to their AXL RNA levels was analysed in TCGA data. A cut off at 14.09 FPKM (fragments per kilobase of transcript per million mapped reads) was set to discriminate low (n = 73) or high expression groups (n = 103), p = 0.0096 (log rank test). (d) Prognosis of patients in both cohorts according to their sAXL plasma levels was analysed using a cut off at 67.28 ng/mL (p = 0.068), which best discriminated patients with low (n = 74) and high (n = 33) sAXL plasma levels in terms of survival.
The prognostic value of AXL RNA expression was explored by interrogating data from TCGA dataset, which confirmed that increased RNA expression of AXL in tumour samples correlated with significant shorter survival (Fig. 4c, p = 0.0096). A similar analysis for GAS6 showed a trend towards shorter survival in patients with high GAS6 expression (Figure S3c, p = 0.12). We also studied the prognostic value of sAXL and GAS6 in our cohorts by analysing whether their plasma levels correlate with patient survival data. Interestingly, a trend towards decreased survival in patients expressing high levels of sAXL and GAS6 was observed (p = 0.068; p = 0.14, respectively; Fig. 4d and Figure S3d).
Altogether, these results suggest that sAXL could be a non-invasive biomarker for PDAC diagnosis, and that AXL RNA expression could also predict patient prognosis. Moreover, although GAS6 levels show quite similar results, sAXL performed better as a biomarker for PDAC, as high levels of GAS6 can also be occasionally found in CP.
Validation of sAXL as candidate biomarker for PDAC diagnosis by ROC curve analysis
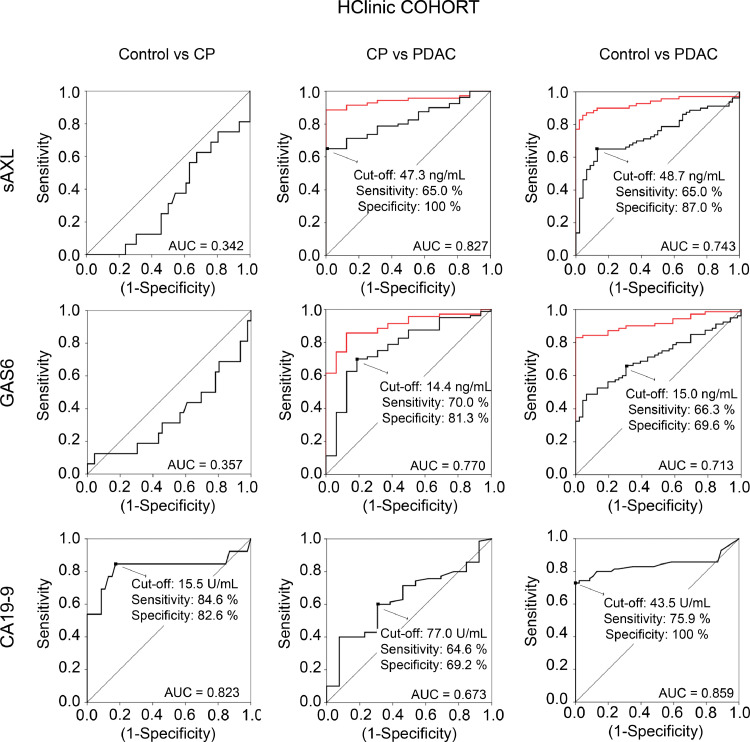
We next performed a univariable or multivariable logistic regression models to evaluate the diagnostic value of the sAXL and GAS6 candidate markers alone or combined with the well-recognized biomarker CA19-9 antigen (Figs. 5, S4, and S5, and Tables S7 and S8). In both cohorts, the mean values of CA19-9 were significantly higher in the PDAC group (as expected from published data) but also in the pancreatitis group, as compared to controls (Figure S4). When we performed receiver operating characteristic (ROC) curves in HClinic cohort for sAXL, GAS6, and CA19-9, all three significantly distinguished PDAC groups from control groups, with AUC values of 0.743, 0.713 and 0.859 respectively (Fig. 5 and Table S7). Importantly, sAXL performed best for discriminating those patients with PDAC from those with CP, with an AUC of 0.827 as compared to 0.770 for GAS6, and of 0.673 for CA19-9. Similar results were obtained in the HMar cohort, although with less statistical power due to the smaller size of the groups (Figure S5; Table S7). Therefore, our data suggest that sAXL is a better parameter than CA19-9 to differentiate CP from PDAC.
Fig. 5.
Sensitivity and specificity of sAXL, GAS6, and CA19-9 plasma levels for discriminating PDAC, CP and healthy controls. ROC analysis in the HClinic cohort to compare the performance of sAXL, GAS6 and CA19-9 to discriminate between PDAC, CP and controls. Area under the curve (AUC), cut-off values maximizing Youden index (except for CA19-9 in CP vs PDAC, see Methods) and sensitivity/specificity values are depicted in the graph. Red lines show ROC curves for sAXL or GAS6 combined with CA19-9.
To assess whether a combination of these markers could increase diagnostic accuracy for clinical use, we calculated the values of sAXL, GAS6, and CA19-9 that maximized the Youden index for the comparison of PDAC samples to CP or control groups (Figs. 5 and S5, and Table S8). Using these cut-off points as diagnostic tools (Table S8), we found that a combination of the established thresholds for sAXL and GAS6 in the HClinic cohort improved their sensitivity and their individual discriminatory powers, by reducing the number of false negatives when comparing either controls or CP versus PDAC patients (Table S8). In addition, combined high sAXL or GAS6 levels and high CA19-9 levels showed a very good sensitivity (91.3%) and specificity (100%) for distinguishing PDAC patients from the control group. Interestingly, when these criteria were applied to the CP–PDAC differential diagnosis, the combination of either with CA19-9 also significantly improved the sensitivity (89.9% for sAXL/CA19-9 and 87.3% for GAS6/CA19-9) and specificity (100% and 92.3%, respectively ) as compared to either biomarker alone. Thus, while sAXL outperformed CA19-9 in differential diagnosis of PDAC versus CP, the combined panel of CA19-9 and sAXL (or GAS6) improved the diagnostic potential for PDAC, especially for the differential diagnosis with CP.
Detection of sAXL in plasma samples from healthy, high-risk individuals from hereditary/familial PDAC patients
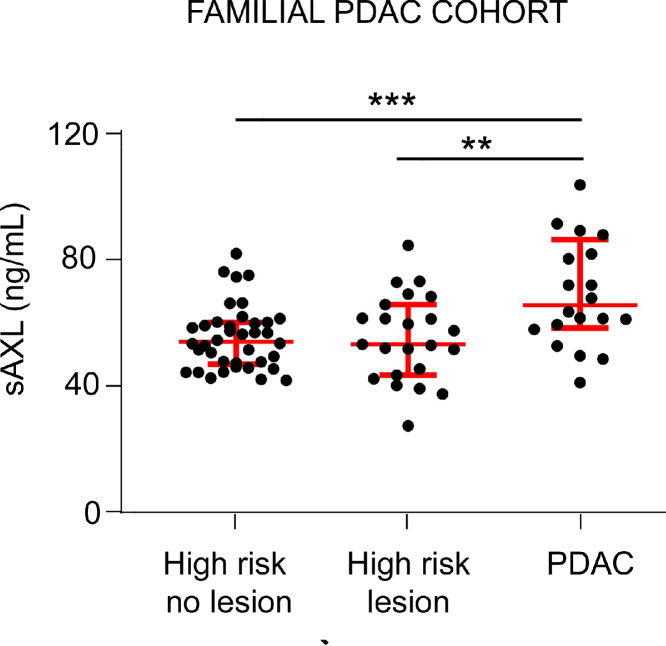
Our results of sAXL plasma levels during PDAC progression after sample stratification suggested that sAXL is elevated already in the initial stages of the disease. Therefore, sAXL emerged as an interesting candidate for screening at-risk PDAC patients, such as individuals with germline pathogenic genetic mutations. We therefore measured the concentration of sAXL in a population (n = 22) of individuals classified as high-risk members of hereditary or familial pancreatic cancer (H/FPC) families according to established inclusion criteria (see Methods) (Fig. 6). These high-risk individuals were participating in a secondary screening program for early PDAC detection, with or without a known genetic cause, and had been diagnosed with pancreatic cysts or IPMN, but with no cases of PDAC at the time of sample extraction (high-risk (HR) lesion cohort). We compared the values with those from a group of patients with PDAC (n = 20; PDAC) and with a second group of high-risk individuals who were participating in the secondary screening program and were negative for pancreatic lesions on imaging (n = 26; HR no lesion cohort). PDAC patients, either H/FPC or sporadic, had a significantly increased sAXL concentration (median, 65.89 ng/mL; IQR, 25.96) as compared to high-risk healthy controls (HR no lesion, median, 56.95 ng/mL; IQR, 23.33; p = 0.0031) (Fig. 6). In the cohort of high-risk with an initial pancreatic lesion, sAXL plasma levels showed also significantly lower levels (HR lesion, median, 53.42 ng/mL; IQR, 23.39) than those detected in PDAC (p = 0.0114), with similar values to those from the high-risk control group with no lesions (p = 0.748) (Fig. 6). Thus, our results showed that sAXL is not increased in people from the high-risk cohort who had not developed PDAC, in accordance to the clinical data from their close follow-up and screening tests.
Fig. 6.
sAXL concentration in plasma samples from a hereditary/familial PDAC cohort. sAXL levels were determined in plasma samples from individuals at high-risk of hereditary/familial pancreatic cancer with no lesions (high risk no lesion, n = 26) or with initial preneoplastic lesions (cysts or IPMNs, high risk lesion, n = 22) and compared to patients with PDAC (n = 20). *p < 0.05; **p < 0.01; ***p < 0.001 (Mann-Whitney test).
Detection of plasma levels of sAXL in mouse models of pancreatic cancer
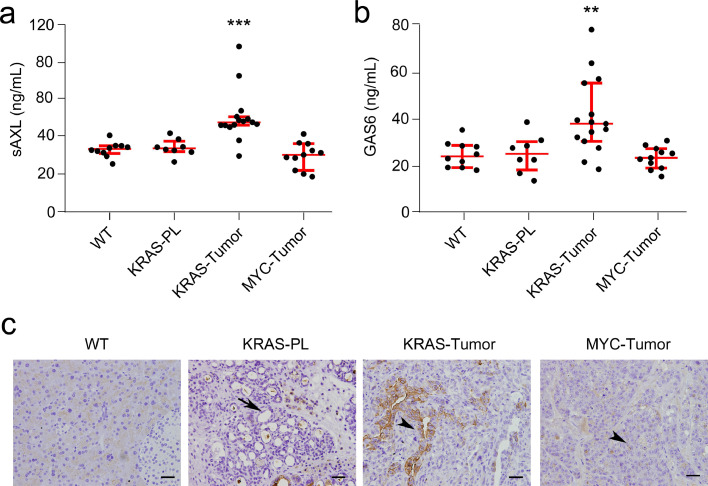
To better understand the correlation of plasma sAXL levels with pancreatic cancer development, we determined the value of these parameters in plasma of mice from two well-described models of pancreatic cancer.11,32 First, we used a KRAS-driven transgenic mouse model, Ela-KrasG12Vp53lox/lox (here, KRAS mice), which expresses the G12V mutated KRAS oncogene under the pancreatic specific elastase promoter and has a homozygous deletion of the p53 gene. These mice initially develop precursor preneoplastic lesions—PanINs—which progress to PDAC at around 3–4 months of age. This model is recognized as one of the best to mirror human disease, as it recapitulates the different stages of PDAC progression as well as its histological hallmarks.11 Plasma samples were collected from the KRAS mice (n = 23), animals were euthanized, and pancreas were analysed histologically to detect the presence of precursor lesions and/or adenocarcinomas. Next, we determined the plasma concentration of sAXL and GAS6 by ELISA (Fig. 7). KRAS mice that developed PDAC (n = 15) had an increased concentration of sAXL (median 47.79 ng/mL; IQR 4.52) in their plasma as compared to WT animals (n = 10) of the same age (median, 33.53 ng/mL; IQR 4.65; p = 0.0003). We also analysed plasma samples from KRAS mice whose pancreas histology showed preneoplastic lesions but not PDAC (n = 8); these mice had sAXL concentrations in plasma similar to control animals (median 33.89 ng/mL; IQR, 4.36; p = 0.590). Next, to validate whether sAXL is a specific biomarker for PDAC, we used the Ela-1-myc transgenic mouse model (here, MYC mice).32 These animals overexpress the c-MYC oncogene under the elastase promoter and develop pancreatic tumours at 3–6 months of age. Plasma samples from MYC mice (n = 11) were analysed by ELISA to determine sAXL levels. Strikingly, and in contrast to the results from KRAS mice, the plasma levels of sAXL were not increased in MYC mice compared to control wild type mice from the same strain (median 30.41 ng/mL; IQR 14.36; p = 0.207). Histological characterization of tumours from the MYC mice showed mostly acinar carcinomas and occasionally mixed ductal/acinar tumours, as expected.32 The value of GAS6 in KRAS-driven preneoplastic or PDACs and c-MYC-driven pancreatic tumours followed a similar pattern to sAXL, although in this case the differences observed were less significant (Fig. 7b). KRAS mutant tumour–positive animals had increased concentration of GAS6 (median, 37.92 ng/mL; IQR, 25.0) in their plasma as compared to control animals (median, 23.90 ng/mL; IQR, 9.72; p = 0.004). Both KRAS mice (with or without preneoplastic lesions) and MYC mice had values similar to WT mice (KRAS: median, 25.01 ng/mL; IQR, 10.11; p = 0.965; MYC: median, 23.32 ng/mL; IQR, 8.35; p = 0.647). Moreover, we performed AXL immunostaining in both mouse models (Fig. 7c). AXL expression was high in ductal adenocarcinomas, while it was barely detected in precursor lesions from the KRAS model. In contrast, acinar tumours from MYC mice were negative, indicating that sAXL is specifically overexpressed in PDAC.
Fig. 7.
Analysis of sAXL and GAS6 in murine PDAC models. (a) sAXL was measured in plasma samples from KRAS or MYC- driven transgenic PDAC mouse models. (b) GAS6 values in plasma samples from KRAS and MYC- driven transgenic PDAC mouse models. *p < 0.05; **p < 0.01; ***p < 0.001 (Mann-Whitney test). (c) AXL immunohistochemistry in mouse samples from normal pancreas of control mice (WT), KRAS-driven pancreatic precursor lesions (KRAS-PL), KRAS-driven PDAC tumours (KRAS-Tumour) and MYC-driven tumours. Arrows indicate precursor lesions whereas arrowheads highlight tumour cells. Bar, 100 µm.
In sum, results from KRAS or MYC mouse models of pancreatic cancer demonstrated that sAXL and GAS6 were specifically increased in plasma of animals with ductal adenocarcinomas but not in those with precursor lesions or acinar tumours, confirming the accuracy of circulating sAXL for early diagnosis of PDAC.
Discussion
Early diagnosis of pancreatic cancer is an unmet challenge in clinical oncology. Here, we show that detection of sAXL in plasma samples represents a novel biomarker for PDAC diagnosis at initial stages (e.g., still resectable) of the disease. Moreover, plasma sAXL levels can discriminate PDAC from CP, a major risk factor for PDAC, with a better accuracy than the CA19-9 biomarker currently used in the clinic. In fact, our data support that blood detection of sAXL and CA19-9 panel by ELISA represents a good strategy for early detection of PDAC. Overexpression of AXL is frequent in cancer, both in hematologic and solid tumours, and plays a crucial role in malignant progression through activation of cell growth, metastasis, angiogenesis, immune evasion, and therapy resistance.42, 43, 44, 45, 46, 47, 48, 49 Therefore, inhibition of AXL has emerged as a potential novel therapeutic target in oncology. In pancreatic cancer, the role of AXL in tumour biology has been less characterized. Histopathological studies have shown an overexpression of AXL in a subset of PDAC patient samples and cell lines that is associated to increased tumour cell invasion and poor prognosis.17,18 These findings led to the proposal of targeting AXL as a novel therapy for this tumour.17,50 Here, we analysed AXL expression by IHC in normal versus pathological pancreas, including CP, precursor lesions (PanIN and IPMN), and PDAC samples. We found that normal pancreas and CP showed almost undetectable levels of AXL, while its expression was high in PDAC and IPMNs, and moderate in PanINs. Intriguingly, we found several cancer samples with low or even no AXL staining, suggesting that activation of AXL expression can be differentially regulated in PDAC subtypes. Interestingly, our analyses from TCGA RNA datasets indicate that AXL expression in PDAC patients is related to specific genetic signatures, being overrepresented in the basal/squamous subgroups, according to the recent genomic classification of PDAC.4, 5, 6, 7 Moreover, the high AXL expression in IPMNs suggests that AXLhigh tumours may arise, at least in part, from these preneoplastic lesions.
The molecular mechanisms leading to AXL overexpression in cancer have not been fully elucidated.51 Genomic amplifications or activating mutations in AXL gene have been rarely found,52,53 suggesting that AXL expression in PDAC is controlled by other transcriptional mechanisms or by translational or post-translational regulation.54,55 In other tumours, several transcription factors have been reported to bind AXL promoter and induce transcription, including the hypoxia-inducible factor-1 (HIF1).56,57 Notably, hypoxia is one of the major hallmarks of PDAC,58 which makes it plausible that the different AXL expression level observed in pancreatic tumours may be related to their hypoxic state. Importantly, PDAC is characterized by a high both intertumoural and intratumoural genetic heterogeneity, which may contribute to different AXL expression levels found in these tumours. Indeed, our TCGA analysis, as well as previous studies,54 have shown that high AXL levels associate with squamous-type tumours. Moreover, invasive cells in the periphery of the tumour show the highest levels of AXL within a given lesion.41 Future studies to better elucidate the contribution of hypoxia, tumour genetic heterogeneity and/or other mechanisms to the variable expression of AXL in PDAC are certainly warranted.
The transmembrane receptor AXL can be proteolytically cleaved to generate a soluble form (sAXL) that can be secreted into the blood stream, suggesting its putative use as a cancer biomarker.42 Here we found that sAXL is significantly increased in plasma samples from patients with PDAC, but not with CP, in two independent cohorts. In accordance with our results in tissue samples and TCGA data, we also found heterogeneity regarding the levels of circulating sAXL among different patients. Importantly, sAXL was found in the conditioned medium from several human PDAC cell lines, indicating that increased levels of sAXL in blood from PDAC patients is likely released by tumour cells. Remarkably, increased sAXL did not correlate with tumour staging in our cohorts, indicating that AXL overexpression is an early event in cancer progression. Notwithstanding, data from our cohorts and the TCGA showed a negative correlation between AXL levels and overall patient survival. Although TCGA RNA data are difficult to interpret and have important limitations, this could be related to the fact that AXLhigh tumours may correspond to the basal/squamous PDAC subtype, which are poorly differentiated tumours with lower prognosis. In agreement with these data, Du et al. have recently reported that pancreatic tumours with high AXL expression have a squamous phenotype displaying markers of epithelial-to-mesenchymal transition, and that inhibition of AXL in mouse models results in more differentiated tumours and increased survival.54 We also analysed a cohort of healthy high-risk people from familial pancreatic cancer families that includes young patients with germline genetic mutations linked to PDAC development. sAXL plasma levels in these individuals were similar to healthy controls, indicating that they were disease-free at that moment. These results agree with clinical history and data from imaging techniques performed during the extensive follow-up of this high-risk cohort, which showed that these individuals had non-PDAC pancreatic lesions. In turn, this independently validates the use of sAXL for detection of the occurrence of PDAC, already elevated at initial PDAC stages, while not in patients with precursor lesions. Moreover, using a KRAS-driven murine PDAC model that fully recapitulates human disease,11 we demonstrated that sAXL is significantly increased in plasma from animals that develop frank pancreatic tumours but not in mice with normal pancreas or with early precursor lesions. Moreover, analysis of plasma samples from mice of a c-MYC-driven PDAC model32 with acinar carcinomas showed no increases in sAXL levels as compared to control mice, indicating that sAXL is a specific biomarker for ductal adenocarcinoma and is not overexpressed in other pancreatic tumour types.
One of the main findings of this study is that sAXL is a blood biomarker specifically overexpressed in PDAC tumours and not in CP, thereby emerging as a promising diagnostic tool for discriminating between these two diseases. CP is an important public health problem that has been related to PDAC initiation.11,59 Diagnostic accuracy for CP is low, in particular for advanced cases, when architectural changes of the gland renders difficult to differentiate inflammatory regions from PDAC by imaging techniques.60 At the level of clinical symptoms, both are silent diseases with non-specific and vague manifestations. Non-invasive diagnostic tools for either CP or PDAC include anatomic imaging techniques –mainly endoscopic ultrasound and radiological studies–, pathology tissue studies, and blood tests. However, despite recent advances in this field, combinations of several of these techniques are usually required.61,62 Here, using two different cohorts, we found that blood levels of sAXL were significantly increased in PDAC patients as compared to patients with CP or healthy controls. Increased levels of sAXL and its ligand GAS6 have been also reported in hepatocellular carcinoma (HCC), although, in contrast to PDAC, they are also increased in inflammatory conditions like cirrhosis and advanced fibrosis.63, 64, 65, 66, 67 Remarkably, although AXL is highly expressed by myofibroblasts in HCC, we found AXL expression specifically in ductal tumour cells but not in the stroma (Fig. 1a), indicating that fibroblasts in liver and pancreas behave differentially with respect to AXL expression and activity and may be responsible for the increased sAXL found in hepatic cirrhosis and fibrosis.
Blood tests for early disease diagnosis offer the advantages of their straightforward performance, fast results, and low cost. CA19-9, the sialylated form of Lewis antigen A, is the best-recognized blood test for PDAC diagnosis but has limitations.8 First, high levels of this carbohydrate are found in several non-malignant pathologies, such as obstructive and inflammatory diseases of the hepato-pancreatobiliary system, leading to false positives.68 Second, CA19-9 levels are also increased in other gastrointestinal tumours, including colorectal cancer and HCC.9,64, 65, 66, 67,69, 70, 71 Moreover, using CA19-9 can give false negative results in the Lewis blood-type negative population (around 10% of the White population). Therefore, clinical utility of CA19-9 for PDAC diagnosis is suboptimal, and identification of more specific and sensitive biomarkers is urgently needed. Importantly, our ROC analysis showed that sAXL detection in plasma (AUC, 0.827) has a higher sensitivity (65%) and specificity (100%) for discriminating PDAC from CP than CA19-9 (AUC, 0.673; sensitivity 46.8%, specificity 92.3%). Indeed, plasma sAXL values in CP patients are similar to healthy controls, indicating that this protein is a more specific biomarker for PDAC than the currently used CA19-9. Importantly, combined determination of CA19-9 and sAXL plasma levels contributed to an increased accuracy of diagnosis (89.9% sensitivity; 100% specificity), reducing the proportion of false negatives.
Our study presents some limitations. First, we found high variability regarding the levels of AXL in plasma and tissue samples from PDAC patients. We found a small proportion of patients who showed sAXL levels similar to healthy individuals, leading to false negatives. This heterogeneity in AXL expression emphasizes the need to combine several biomarkers for a precise PDAC diagnosis. Indeed, our results using the combination of sAXL, and CA19-9 significantly improved the discriminatory power of a single determination of sAXL for CP vs PDAC (in HClinic cohort, 65% sensitivity for sAXL alone versus 89.9% for sAXL combined with CA19-9). Emerging data have also shown improved specificity and sensitivity of combining blood detection of CA19-9 with thrombospondin-272 or with MUC5AC.73 Along the same line, the use of nine metabolites plus CA19-9 was recently reported to be an accurate (>90%) biomarker signature for discriminating CP and PDAC.74 In a previous study, using the same HMar, we showed that plasma levels of galectin-1 (Gal-1) were increased in PDAC as compared to healthy controls; however, high levels of this protein were also found in patients with CP, limiting its effectiveness as a diagnostic biomarker for PDAC.28 Strikingly, a combination of sAXL, Gal-1, and CA19-9 resulted in an increased diagnostic accuracy (100% sensibility and 100% specificity for PDAC vs healthy controls; Table S8), suggesting that ELISA detection using this panel can be optimal for diagnosis of these diseases. Another limitation of our study is the fact that circulating sAXL is also upregulated in HCC64, 65, 66, 67 and in non-cancer related diseases, including heart failure, chronic kidney disease, and rheumatoid arthritis,75 which can lead to false positives, hampering its use as a general screening biomarker. Still, given the low prevalence of PDAC in the general population, the most sustainable option for the healthcare system is to screen the PDAC high-risk populations (i.e. germline mutations, PDAC familial aggregation, and CP patients) to allow early detection and curative treatments. We suggest that detection of sAXL in combination with CA19-9 in this PDAC-suspected population might represent a low-cost/high-accuracy screening tool (91.3% sensitivity, 100% specificity) that can contribute to 30%-40% increase of patient's survival by early detection of the pathology. Finally, the limited size of the two cohorts used in our study makes necessary to implement multicentric studies with larger cohorts in order to establish accurate sAXL cut-off values that can be transferred to the clinical setting, both for PDAC diagnosis and for CP discrimination.
In conclusion, by analysing sAXL and GAS6 in human plasma from patients with PDAC or CP, this study demonstrated that sAXL is a bona fide biomarker for PDAC early diagnosis and for PDAC–CP discrimination. Our data indicated that ELISA measurement of sAXL in a patient's plasma represents a novel cost-effective test with high specificity and sensitivity, superior to CA19-9 in discriminating PDAC and CP. In addition, combination of CA19-9 and sAXL can achieve great diagnosis accuracy for both PDAC vs healthy controls (91.3% sensitivity; 100% specificity) and PDAC vs CP (89.9% sensitivity; 100% specificity). Moreover, our data on high sAXL levels in the basal/squamous PDAC subtype suggest that this biomarker can be particularly useful for diagnosis in genetically defined subsets of patients, highlighting its putative use in personalized medicine. Therefore, sAXL gives an added diagnostic value and is a suitable biomarker in the clinical management of PDAC patients, with a chance for a drastic improvement in overall survivability of this dismal disease.
Contributors
Conceptualization: Pilar Navarro and Pablo García de Frutos. Investigation: Neus Martínez-Bosch, Helena Cristóbal, Domenico Calafato, Noemí Manero-Rupérez and Mireia Moreno. Formal analysis and resources: Neus Martínez-Bosch, Helena Cristóbal, Mar Iglesias, Meritxell Gironella, Luis Barranco, Laura Visa, Domenico Calafato, Silvia Jiménez-Parrado, Julie Earl, Alfredo Carrato, Albert Morales, Carmen Guerra, Pilar Navarro, and Pablo García de Frutos. Funding acquisition: Pilar Navarro, Pablo García de Frutos, Albert Morales, Meritxell Gironella and Alfredo Carrato. Supervision, data curation and writing the original draft: Pilar Navarro and Pablo García de Frutos. All authors read and approved the final draft.
Declaration of interests
The authors have declared that no competing interest exists.
Acknowledgments
Acknowledgements
We thank Dr. X. Duran for help with the statistical analysis; the Bioinformatics Unit of MARGenomics (Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain) for assistance with bioinformatics data analysis and V.A. Raker for English proofreading and manuscript editing. We also thank patients and their families as well as nurses for sample collection. This work was supported by grants from the Spanish Ministry of Science and Innovation (MICINN)/ Instituto de Salud Carlos III (ISCIII)-European Regional Development Fund (ERDF) (PI20/00625) and the “Generalitat de Catalunya” (2017/SGR/225) to PN; grants from the MICINN (Project# RTI2018-095672-B-I00) to AM and PGdF; grant from the MICINN/ ISCIII-FEDER (PI20/01696) to MG and grant from the MICINN/ ISCIII-FEDER (PI18/01034) to AC. We also acknowledge grants from the Instituto de Salud Carlos III (ISCIII)/ERDF(PT20/00023) and the "Xarxa de Bancs de tumours” sponsored by Pla Director d'Oncologia de Catalunya (XBTC). PN's lab is part of Redes de investigación (Enfermedades Metabólicas y Cáncer RED2018-102799-T), a project run by MINECO. CIBEREHD is funded by Instituto de Salud Carlos III.
Data sharing statement
The data generated during the study to support the findings are available upon request from the corresponding authors (Dr. Pilar Navarro and Dr. Pablo García de Frutos) upon reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103797.
Contributor Information
Pilar Navarro, Email: pilar.navarro@iibb.csic.es.
Pablo García de Frutos, Email: pablo.garcia@iibb.csic.es.
Appendix. Supplementary materials
Caption 1. EBIOM-D-21-03117_Table S1. Clinical characteristics of all patients whose tissue or plasma samples have participated in the study.
Caption 2. EBIOM-D-21-03117_Table S2. AXL expression and clinico-pathological characteristics of PDAC tissue samples.
Caption 3. EBIOM-D-21-03117_Table S3.sAXL, GAS6 and CA19-9 plasma values and clinico-pathological data from HMar cohort.
Caption 4. EBIOM-D-21-03117_Table S4.sAXL, GAS6 and CA19-9 plasma values and clinico-pathological data from HClinic cohort.
Caption 5. EBIOM-D-21-03117_Table S5.Multivariate analysis of HClinic cohort sAXL, GAS6 and CA19-9 between groups, adjusted for gender, age, diabetes and obstructive jaundice.
Caption 6. EBIOM-D-21-03117_Table S6.Description of clinico-pathological characteristics of the familial/hereditary cohort of PDAC.
Caption 7. EBIOM-D-21-03117_Table S7.ROC analysis of sAXL, GAS6 and CA19-9 plasma values in HMar and HClinic cohort.
Caption 8. EBIOM-D-21-03117_Table S8.Cut-offs, sensitivity and specificity values derived from ROC curves for individual or combined determinations of sAXL, GAS6 and CA19-9 plasma values in HMar and HClinic cohorts.
Caption 9. EBIOM-D-21-03117_Figure S1.AXL RNA analysis in previous published literature.
Caption 10. EBIOM-D-21-03117_Figure S2.sAXL, GAS6 and CA19-9 levels in HClinic cohort levels considering obstructive jaundice (a) and diabetes (b).
Caption 11. EBIOM-D-21-03117_Figure S3.Analysis of GAS6 plasma levels during PDAC progression and prognostic value.
Caption 12. EBIOM-D-21-03117_Figure S4.Determination of CA19-9 plasma levels in samples from two independent cohorts.
Caption 13. EBIOM-D-21-03117_Figure S5.ROC analysis in the HMar cohort.
References
- 1.Hidalgo M., Cascinu S., Kleeff J., et al. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology. 2015;15(1):8–18. doi: 10.1016/j.pan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 4.Moffitt R.A., Marayati R., Flate E.L., et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collisson E.A., Sadanandam A., Olson P., et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey P., Chang D.K., Nones K., et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network Electronic address: andrew_aguirre@dfci.harvard.edu, Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32(2):185–203. doi: 10.1016/j.ccell.2017.07.007. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Yang J., Li H., Wu Y., Zhang H., Chen W. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med. 2015;8(7):11683–11691. [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy M.J., Sturgeon C., Lamerz R., et al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol Off J Eur Soc Med Oncol. 2010;21(3):441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shaheri F.N., Alhamdani M.S.S., Bauer A.S., et al. Blood biomarkers for differential diagnosis and early detection of pancreatic cancer. Cancer Treat Rev. 2021;96 doi: 10.1016/j.ctrv.2021.102193. [DOI] [PubMed] [Google Scholar]
- 11.Guerra C., Schuhmacher A.J., Cañamero M., et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11(3):291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Conwell D.L., Lee L.S., Yadav D., et al. American pancreatic association practice guidelines in chronic pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43(8):1143–1162. doi: 10.1097/MPA.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beyer G., Habtezion A., Werner J., Lerch M.M., Mayerle J. Chronic pancreatitis. Lancet (London, England) 2020;396(10249):499–512. doi: 10.1016/S0140-6736(20)31318-0. [DOI] [PubMed] [Google Scholar]
- 14.Brezgyte G., Shah V., Jach D., Crnogorac-Jurcevic T. Non-invasive biomarkers for earlier detection of pancreatic cancer-a comprehensive review. Cancers (Basel) 2021;13(11) doi: 10.3390/cancers13112722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5(11) doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burstyn-Cohen T., Maimon A. TAM receptors, Phosphatidylserine, inflammation, and Cancer. Cell Commun Signal. 2019;17(1):156. doi: 10.1186/s12964-019-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koorstra J.-.B., Karikari C.A., Feldmann G., et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009;8(7):618–626. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song X., Wang H., Logsdon C.D., et al. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer. 2011;117(4):734–743. doi: 10.1002/cncr.25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du W., Phinney N.Z., Huang H., et al. AXL Is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Mol Cancer Res. April 2021 doi: 10.1158/1541-7786.MCR-20-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig K.F., Du W., Cruz V.H., et al. Small-molecule inhibition of axl targets tumor immune suppression and enhances chemotherapy in pancreatic cancer. Cancer Res. 2017;78(1):246–255. doi: 10.1158/0008-5472.can-17-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekman C., Stenhoff J., Dahlbäck B. Gas6 is complexed to the soluble tyrosine kinase receptor Axl in human blood. J Thromb Haemost. 2010;8(4):838–844. doi: 10.1111/j.1538-7836.2010.03752.x. [DOI] [PubMed] [Google Scholar]
- 22.Ekman C., Linder A., Akesson P., Dahlbäck B. Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Crit Care. 2010;14(4):R158. doi: 10.1186/cc9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekman C., Gottsäter A., Lindblad B., Dahlbäck B. Plasma concentrations of Gas6 and soluble Axl correlate with disease and predict mortality in patients with critical limb ischemia. Clin Biochem. 2010;43(10-11):873–876. doi: 10.1016/j.clinbiochem.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Batlle M., Recarte-Pelz P., Roig E., et al. AXL receptor tyrosine kinase is increased in patients with heart failure. Int J Cardiol. 2014;173(3):402–409. doi: 10.1016/j.ijcard.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Ekman C., Site D.F., Gottsäter A., Lindblad B., Dahlbäck B. Plasma concentrations of growth arrest specific protein 6 and the soluble form of its tyrosine kinase receptor Axl as markers of large abdominal aortic aneurysms. Clin Biochem. 2010;43(1-2):110–114. doi: 10.1016/j.clinbiochem.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Liu X., Gong Y., Jia J., et al. Plasma concentrations of sAxl are associated with severe preeclampsia. Clin Biochem. 2014;47(3):173–176. doi: 10.1016/j.clinbiochem.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Lee I.J., Hilliard B.A., Ulas M., et al. Monocyte and plasma expression of TAM ligand and receptor in renal failure: Links to unregulated immunity and chronic inflammation. Clin Immunol. 2015;158(2):231–241. doi: 10.1016/j.clim.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Bosch N., Barranco L.E., Orozco C.A., et al. Increased plasma levels of galectin-1 in pancreatic cancer: Potential use as biomarker. Oncotarget. 2018;9(68):32984–32996. doi: 10.18632/oncotarget.26034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vila-Navarro E., Duran-Sanchon S., Vila-Casadesús M., et al. Novel circulating miRNA signatures for early detection of pancreatic neoplasia. Clin Transl Gastroenterol. 2019;10(4):e00029. doi: 10.14309/ctg.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mocci E., Guillen-Ponce C., Earl J., et al. PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur J Cancer. 2015;51(14):1911–1917. doi: 10.1016/j.ejca.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Guerra C., Barbacid M. Genetically engineered mouse models of pancreatic adenocarcinoma. Mol Oncol. 2013;7(2):232–247. doi: 10.1016/j.molonc.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandgren E.P., Quaife C.J., Paulovich A.G., Palmiter R.D., Brinster R.L. Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc Natl Acad Sci. 1991;88(1):93–97. doi: 10.1073/pnas.88.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerra C., Collado M., Navas C., et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19(6):728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orozco C.A., Martinez-Bosch N., Guerrero P.E., et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor–stroma crosstalk. Proc Natl Acad Sci. 2018;115(16):E3769–E3778. doi: 10.1073/pnas.1722434115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Detre S., Saclani Jotti G., Dowsett M. A quickscore method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson M.D., Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Z., Li C., Kang B., Gao G., Li C., Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayars M., O'Sullivan E., Macgregor-Das A., et al. IL2RG, identified as overexpressed by RNA-seq profiling of pancreatic intraepithelial neoplasia, mediates pancreatic cancer growth. Oncotarget. 2017;8(48):83370–83383. doi: 10.18632/oncotarget.19848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdollahi A., Schwager C., Kleeff J., et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc Natl Acad Sci U S A. 2007;104(31):12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budczies J., Klauschen F., Sinn B.V., et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leconet W., Larbouret C., Chardès T., et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene. 2014;33(47):5405–5414. doi: 10.1038/onc.2013.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gay C.M., Balaji K., Byers LA. Giving AXL the axe: targeting AXL in human malignancy. Br J Cancer. 2017;116(4):415–423. doi: 10.1038/bjc.2016.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gjerdrum C., Tiron C., Hoiby T., et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci. 2010;107(3):1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goruppi S., Ruaro E., Varnum B., Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17(8):4442–4453. doi: 10.1128/MCB.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyette M.A., Duhamel S., Aubert L., et al. The receptor tyrosine kinase AXL is required at multiple steps of the metastatic cascade during HER2-positive breast cancer progression. Cell Rep. 2018;23(5):1476–1490. doi: 10.1016/j.celrep.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Loges S., Schmidt T., Tjwa M., et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115(11):2264–2273. doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z., Lee J.C.J.-.S., Lin L., et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44(8):852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou L., Liu X.-D., Sun M., et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687–2697. doi: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Müller J., Krijgsman O., Tsoi J., et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat Commun. 2014;5 doi: 10.1038/ncomms6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Itzstein M.S., Burke M.C., Brekken R.A., et al. Targeting TAM to tame pancreatic cancer. Target Oncol. 2020;15(5):579–588. doi: 10.1007/s11523-020-00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scaltriti M., Elkabets M., Baselga J. Molecular pathways: AXL, a membrane receptor mediator of resistance to therapy. Clin Cancer Res. January 2016 doi: 10.1158/1078-0432.CCR-15-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cerami E., Gao J., Dogrusoz U., et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao J., Aksoy B.A., Dogrusoz U., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du W., Brekken RA. Does Axl have potential as a therapeutic target in pancreatic cancer? Expert Opin Ther Targets. 2018;22(11):955–966. doi: 10.1080/14728222.2018.1527315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu C., Wei Y., Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18(1):153. doi: 10.1186/s12943-019-1090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rankin E.B., Fuh K.C., Castellini L., et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111(37):13373–13378. doi: 10.1073/pnas.1404848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nalwoga H., Ahmed L., Arnes J.B., Wabinga H., Akslen LA. Strong expression of Hypoxia-inducible factor-1α (HIF-1α) Is associated with axl expression and features of aggressive Tumors in African breast cancer. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erkan M., Hausmann S., Michalski C.W., et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9(8):454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 59.Lowenfels A.B., Maisonneuve P., Cavallini G., et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328(20):1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 60.Duggan S.N., Ní Chonchubhair H.M., Lawal O., O'Connor D.B., Conlon K.C. Chronic pancreatitis: A diagnostic dilemma. World J Gastroenterol. 2016;22(7):2304–2313. doi: 10.3748/wjg.v22.i7.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimastromatteo J., Brentnall T., Kelly K.A. Imaging in pancreatic disease. Nat Rev Gastroenterol Hepatol. 2017;14(2):97–109. doi: 10.1038/nrgastro.2016.144. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Q., Zeng L., Chen Y., et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract. 2016;2016 doi: 10.1155/2016/8962321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bárcena C., Stefanovic M., Tutusaus A., et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J Hepatol. 2015;63(3):670–678. doi: 10.1016/j.jhep.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reichl P., Fang M., Starlinger P., et al. Multicenter analysis of soluble Axl reveals diagnostic value for very early stage hepatocellular carcinoma. Int J Cancer. 2015;137(2):385–394. doi: 10.1002/ijc.29394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staufer K., Dengler M., Huber H., et al. The non-invasive serum biomarker soluble Axl accurately detects advanced liver fibrosis and cirrhosis. Cell Death Dis. 2017;8(10):e3135. doi: 10.1038/cddis.2017.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dengler M., Staufer K., Huber H., et al. Soluble Axl is an accurate biomarker of cirrhosis and hepatocellular carcinoma development: results from a large scale multicenter analysis. Oncotarget. 2017;8(28):46234–46248. doi: 10.18632/oncotarget.17598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tutusaus A., de Gregorio E., Cucarull B., et al. A Functional Role of GAS6/TAM in nonalcoholic steatohepatitis progression implicates AXL as therapeutic target. Cell Mol Gastroenterol Hepatol. 2020;9(3):349–368. doi: 10.1016/j.jcmgh.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ventrucci M., Pozzato P., Cipolla A., Uomo G. Persistent elevation of serum CA 19-9 with no evidence of malignant disease. Dig Liver Dis. 2009;41(5):357–363. doi: 10.1016/j.dld.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Koprowski H., Herlyn M., Steplewski Z., Sears H.F. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212(4490):53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 70.Goonetilleke K.S., Siriwardena A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Thomsen M., Skovlund E., Sorbye H., et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: a BRAF-mutant subset with high CA 19-9 level and poor outcome. Br J Cancer. 2018;118(12):1609–1616. doi: 10.1038/s41416-018-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim J., Bamlet W.R., Oberg A.L., et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci Transl Med. 2017;9(398) doi: 10.1126/scitranslmed.aah5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaur S., Smith L.M., Patel A., et al. A Combination of MUC5AC and CA19-9 improves the diagnosis of pancreatic cancer: a multicenter study. Am J Gastroenterol. 2017;112(1):172–183. doi: 10.1038/ajg.2016.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayerle J., Kalthoff H., Reszka R., et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut. 2018;67(1):128–137. doi: 10.1136/gutjnl-2016-312432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sainaghi P.P., Bellan M., Nerviani A. Role of the Gas6/TAM system as a disease marker and potential drug target. Dis Markers. 2021;2021:1–3. doi: 10.1155/2021/2854925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Caption 1. EBIOM-D-21-03117_Table S1. Clinical characteristics of all patients whose tissue or plasma samples have participated in the study.
Caption 2. EBIOM-D-21-03117_Table S2. AXL expression and clinico-pathological characteristics of PDAC tissue samples.
Caption 3. EBIOM-D-21-03117_Table S3.sAXL, GAS6 and CA19-9 plasma values and clinico-pathological data from HMar cohort.
Caption 4. EBIOM-D-21-03117_Table S4.sAXL, GAS6 and CA19-9 plasma values and clinico-pathological data from HClinic cohort.
Caption 5. EBIOM-D-21-03117_Table S5.Multivariate analysis of HClinic cohort sAXL, GAS6 and CA19-9 between groups, adjusted for gender, age, diabetes and obstructive jaundice.
Caption 6. EBIOM-D-21-03117_Table S6.Description of clinico-pathological characteristics of the familial/hereditary cohort of PDAC.
Caption 7. EBIOM-D-21-03117_Table S7.ROC analysis of sAXL, GAS6 and CA19-9 plasma values in HMar and HClinic cohort.
Caption 8. EBIOM-D-21-03117_Table S8.Cut-offs, sensitivity and specificity values derived from ROC curves for individual or combined determinations of sAXL, GAS6 and CA19-9 plasma values in HMar and HClinic cohorts.
Caption 9. EBIOM-D-21-03117_Figure S1.AXL RNA analysis in previous published literature.
Caption 10. EBIOM-D-21-03117_Figure S2.sAXL, GAS6 and CA19-9 levels in HClinic cohort levels considering obstructive jaundice (a) and diabetes (b).
Caption 11. EBIOM-D-21-03117_Figure S3.Analysis of GAS6 plasma levels during PDAC progression and prognostic value.
Caption 12. EBIOM-D-21-03117_Figure S4.Determination of CA19-9 plasma levels in samples from two independent cohorts.
Caption 13. EBIOM-D-21-03117_Figure S5.ROC analysis in the HMar cohort.