Abstract
Owing to messenger RNA's unique biological advantages, it has received increasing attention to be used as a therapeutic, known as mRNA-based gene therapy. It is critical to have an ideal strategy of mRNA gene therapy for glioma, which grows in a special environment. In the present study, we screened out a safe and efficient transfection reagent for intracranial delivery of synthetic mRNA in mouse brain. First, in order to analyze the effect of different transfection reagents on the intracranial delivery of mRNA, the synthetic luciferase mRNA was wrapped with two different transfection reagents and microinjected into the brain at the fixed point. The expression status of delivered mRNA was monitored by a small animal imaging system. The possible reagent-induced biological toxicity was evaluated by behavioral and blood biochemical measurements. Then, to test the therapeutic effect of our intracranial delivery mRNA model on glioma, synthetic modified tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mRNA was used as an example of therapeutic application. This model demonstrated that synthetic mRNA could be successfully delivered into the brain using commercially available transfection reagents, and TransIT-mRNA showed better results than in vivo-jetPEI kit. This model can be applied in precise targeting and personalized gene therapy of glioma.
Keywords: gene therapy, synthetic mRNA, intracranial delivery, TRAIL, glioma
Graphical abstract
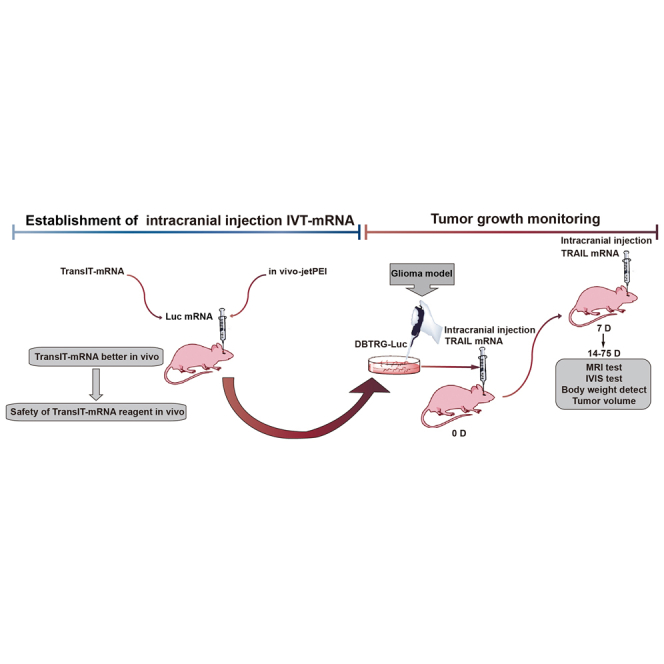
This study explores the TransIT-mRNA for intracranial transfection for the first time and provides an intracranial synthetic mRNA delivery model for preclinical studies of mRNA-based gene therapy.
Introduction
DNA-based gene delivery has been widely used in most preclinical and clinical studies of gene therapy, in which plasmids and viral vectors are commonly utilized as target gene carriers. However, the use of DNA-based gene delivery has been limited by its drawbacks, especially the possibility of insertional mutagenesis and targeted cells restricted on the dividing cells.1 It is critical to explore a safe and efficient gene delivery system for gene therapy. Owing to the unique biological nature of messenger RNA (mRNA), it has received great attention for use as a therapeutic, known as mRNA-based gene therapy. Synthetic mRNA drugs provide an approach in which the robust and tunable production of a therapeutic protein is possible, by avoiding the need for costly manufacturing of proteins in bioreactors. Associated with these unique features is the vision that utilizing synthetic mRNA will help address challenges in newly emerging technologies such as targeted genome engineering, generation and reprogramming of stem cells, as well as production of on-demand personalized vaccines. Clinical trials have investigated naked or protamine-complexed mRNA vaccines that are delivered either intradermally or intramuscularly.2,3 The subsequent coronavirus disease 2019 (COVID-19) pandemic rapidly affected the health and economy of the world, and the production of in vitro synthetic mRNA-based vaccines is a promising recent development in the production of vaccines; relying on its unique advantages, in vitro synthetic mRNA-based vaccines have been developed and entered clinical trials faster than other vaccine products.4, 5, 6 Immunotherapy with DCs(Dendritic cells) electroporated with synthetic mRNA was shown to be safe in patients with cancer.7,8 The mRNA can be translated into therapeutic proteins or peptides in the cytoplasm without entering the nuclei, so that it is able to work on both static and dividing cells without the risk of insertional mutagenesis in the host cells. The main concern about mRNA's therapeutic application is its relative short life span inside the cell. In recent years, along with the discoveries of 5' mRNA anti-reverse cap analogs (ARCAs), poly(A) tails, and the insertion of additional untranslated regions, foreign mRNA's stability and translation efficiency can be significantly enhanced in host cells. In addition, it is easy to transfect into target cells, because the construct size of synthetic mRNA is much smaller than that of corresponding DNA plasmid. The novel modified mRNA constructs have become more attractive alternatives to the most commonly used DNA-based gene carriers.
Glioblastoma multiforme (GBM) is the most common and deadly malignant primary tumor of the brain. Despite the progressive development of surgical techniques and adjuvant therapies, the therapeutic outcomes of GBM treatment have remained unsatisfactory for decades. In addition to GBM's biological features, such as complex cellular composition, diffuse invasiveness, and capacity to escape conventional therapies, the existence of blood-brain barrier (BBB) and brain-tumor cell barrier (BTB) in the brain further increases the intractability of GBM.9 Therefore, under certain circumstances, intracranial injection of synthetic mRNA may be an ideal choice for the treatment of gliomas.
TNF-related apoptosis-inducing ligand (TRAIL) selectively bound to the death receptor 4 (DR4) and death receptor 5(DR5), and induced the apoptosis pathway in tumor cells leading to tumor cell death.10 Because of drug resistance, off-target toxicities, short half-life, and specifically in gene therapy due to the limited uptake of TRAIL genes by cancer cells,11 In the present study, we screened out a safe and efficient transfection reagent for intracranial delivery of synthetic mRNA in mouse brain. Meanwhile, we used this method to deliver TRAIL-mRNA, which significantly inhibited the growth of intracranial gliomas. This mouse model of intracranial delivery of synthetic mRNA could provide additional options for mRNA-based gene therapy.
Results
Expression verification of synthesized Luc-mRNA and TRAIL-mRNA
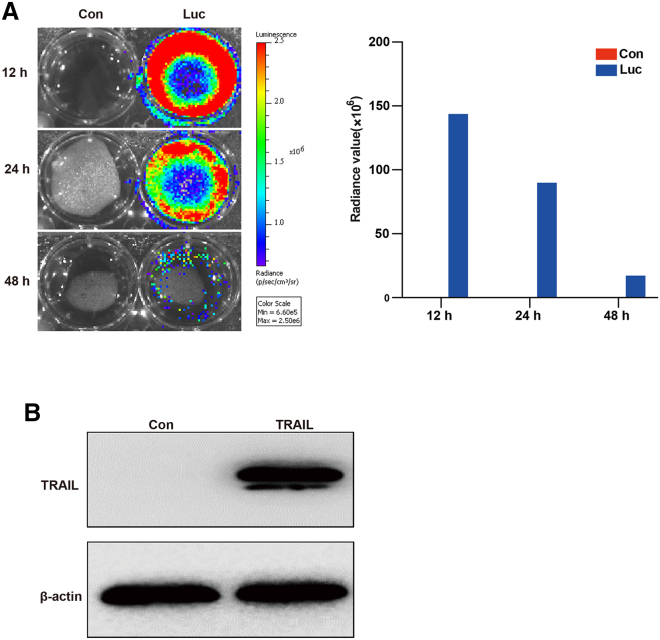
The expression of luciferase mRNA (Luc-mRNA) and TRAIL-mRNA was verified in 293T cells. All transfection procedures were performed using the TransIT-mRNA kit, after transfection of 293T cells with Luc-mRNA, the bioluminescence was detected at 12 h, 24 h, and 48 h using the IVIS Spectrum system. The luciferase expression was maintained for at least 48 h (Figure 1A). To verify the expression of TRAIL-mRNA, TRAIL-mRNA was transfected into 293T cells and detected by western blot. As shown in Figure 1B, the expression of TRAIL was highly upregulated by TRAIL-mRNA transfection.
Figure 1.
Synthetic Luc-mRNA and TRAIL-mRNA transfection into 293T cells
(A) Bioluminescence was measured at 12 h, 24 h, and 48 h after Luc-mRNA transfection using a small animal image system. Radiance value represents the expression of transfected Luc-mRNA in 293T cells. (B) The expression of transfected TRAIL-mRNA in 293T cells was detected using western blot at 12 h after TRAIL-mRNA transfection.
Establishment of intracranial delivery synthetic mRNA model
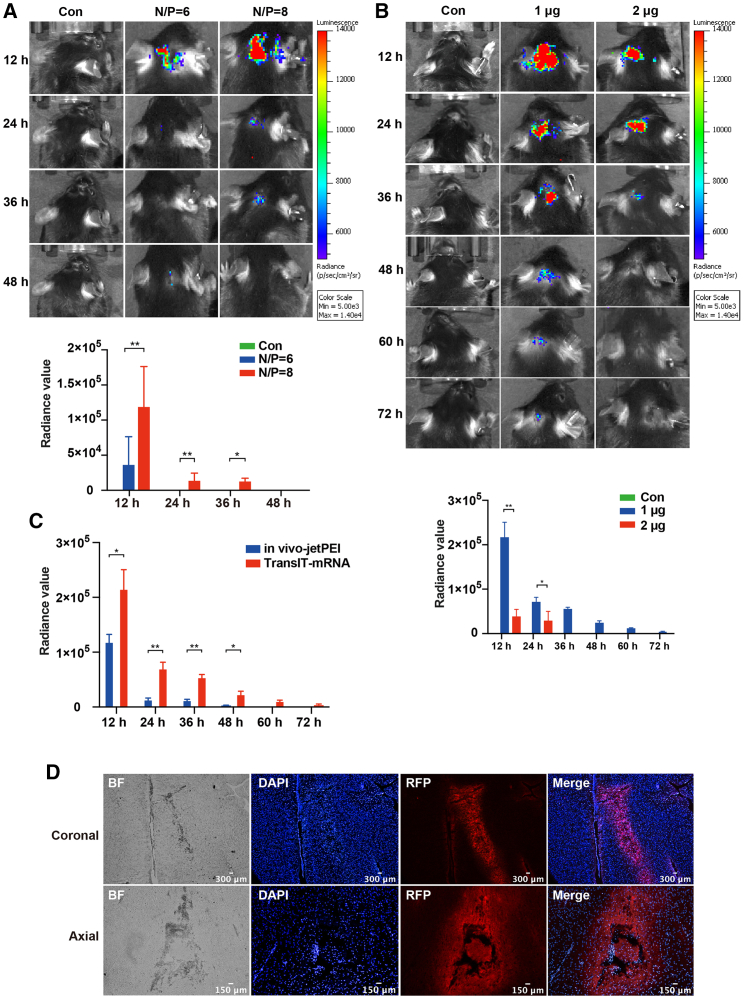
Due to the special structure of brain and the existence of BBB in the brain, there is no good animal model of synthetic mRNA for gene therapy, the main reason being that there is no suitable mRNA transfection reagent for intracranial delivery of mRNA. In order to solve this problem, the two commercially available transfection reagents, in vivo-jetPEI from Polypus Transfection and TransIT-mRNA from Mirus, which have not been used for intracranial mRNA transfection before, were used for the intracranial mRNA delivery. As shown in Figure 2, the intracranial injected synthetic Luc-mRNA was successfully expressed either with jetPEI or with TransIT-mRNA. The peak bioluminescence signals of Luc-mRNA injected with these two reagents appeared in 12 h after intracranial injection (Figures 2A and 2B). However, the bioluminescence intensity produced by Luc-mRNA delivered with TransIT-mRNA was significantly higher than that produced by Luc-mRNA delivered with jetPEI at every time point. As shown in Figure 2C, the duration of luciferase expression was much longer in Luc-mRNA injection with TransIT-mRNA (60 h) than that in Luc-mRNA injection with jetPEI (36 h).
Figure 2.
Intracranial expression of synthetic mRNA delivered with common transfection reagents
(A) The intracranial expression of in vivo-jetPEI-mediated intracranial injection of synthetic Luc-mRNA was detected using an IVIS with N/P = 6 and N/P = 8 at 12 h, 24 h, 36 h, and 48 h. The upper panel shows the representative images at each time point, and lower panel represents the summary. (B) The intracranial expression of TransIT-mRNA-mediated intracranial injection of synthetic Luc-mRNA was detected using an IVIS with 1 μg system and 2 μg system at 12 h, 24 h, 36 h, 48 h, 60 h, and 72 h. The upper panel shows the representative images at each time point, and lower panel represents the summary. (C) The transfection efficiency comparison of the two transfection reagents. One microgram of Luciferase mRNA with TransIT-mRNA or in vivo-jetPEI solution was intracranially injected. ∗p < 0.05; ∗∗p < 0.01. (D) The intracranial expression of TransIT-mRNA-mediated intracranial injection of synthetic RFP-mRNA was detected using a fluorescence microscope.
Because TransIT-mRNA transfection effect and duration were better than jetPEI, we then explored its optimal transfection dose. The results showed that a 1 μg/20 μL (mRNA/TransIT-mRNA) system is the best transfection dose. The peak bioluminescence signals could reach 3.682 × 104 at 12 h after intracranial injection using in vivo-jetPEI kit when the reagent nitrogen to phosphorus (N/P) ratio was 6, and the duration of luciferase expression was 12 h. When reagent N/P increased to 8, the peak bioluminescence signals could reach 1.192 × 105 at 12 h after intracranial injection, and the duration of luciferase expression was increased to 36 h (Figure 2A). The peak bioluminescence signals of TransIT-mRNA kit-mediated 1 μg in vitro synthetic mRNA transfection at 12 h after intracranial injection could reach 2.16 × 105, and duration of luciferase expression was 72 h. However, when the peak bioluminescence of transfected 2 μg of in vitro synthetic mRNA was also at 12 h, the value could reach 3.792 × 104, and the duration of luciferase expression was 24 h (Figure 2B). To compare the transfected efficiency between in vivo-jetPEI kit and TransIT-mRNA kit, we transfected 1 μg of synthetic luciferase mRNA with these two reagents at the same time. The result show that the efficiency and duration of TransIT-mRNA kit were better than those of in vivo-jetPEI kit. At last, we chose TransIT-mRNA kit for downstream experiments.
In order to analyze whether this model can deliver mRNA to the target area of the brain, we used a brain localizer to locate the caudate nucleus, and then injected synthetic red fluorescent protein (RFP) mRNA. Twelve hours after injection, the brain tissue samples were taken. Bright-field image merged with DAPI and RFP is shown to reveal the synthetic RFP-mRNA could be correctly expressed in the caudate nucleus (Figure 2D), and the expression of synthetic RFP-mRNA is shown on the coronal and axial planes separately. These results indicate that we have successfully constructed an animal model of targeted delivery of mRNA in the brain.
The evaluation of biochemical toxicity of intracranially injected Luc-mRNA with TransIT-mRNA
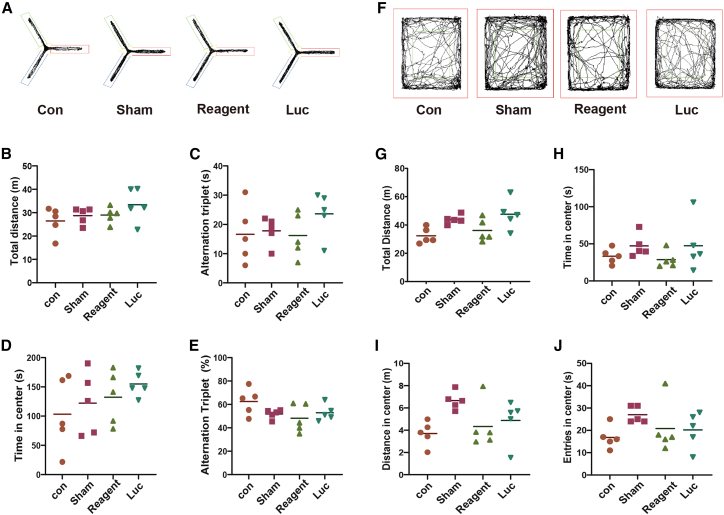
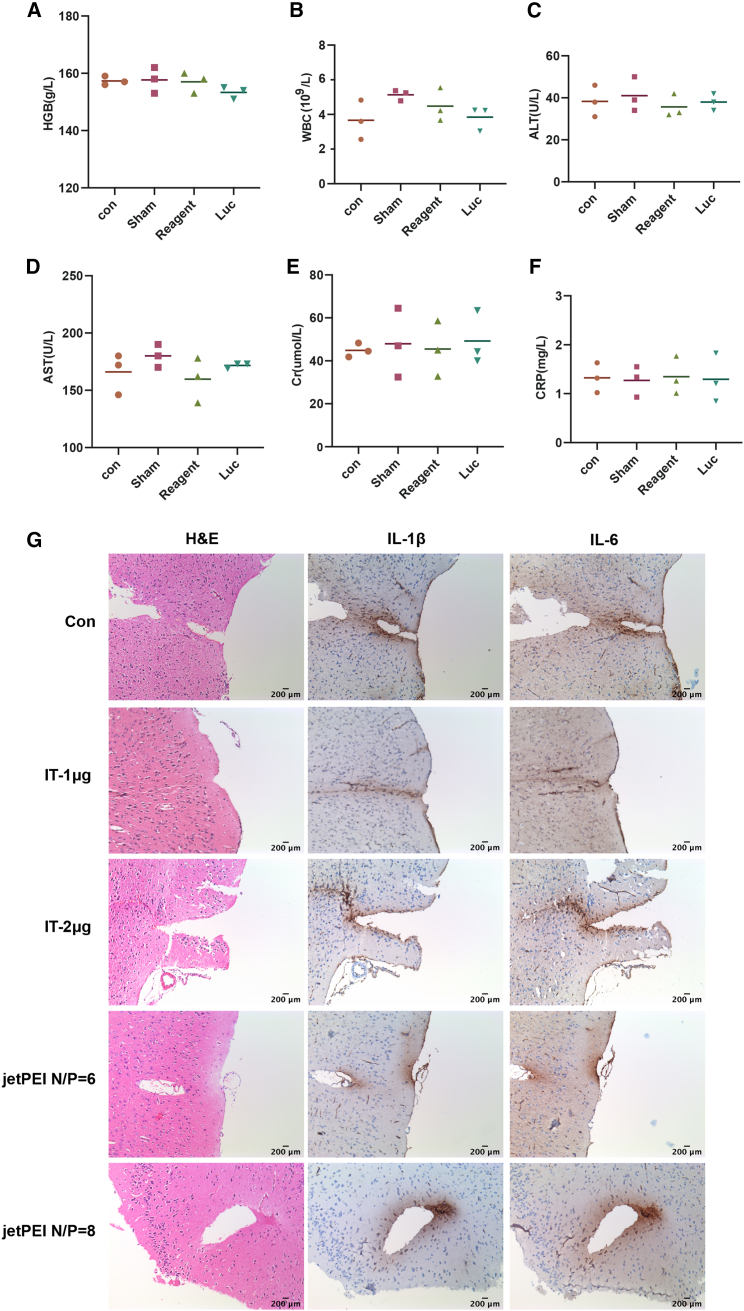
To detect the safety of TransIT-mRNA in intracranial delivery of synthetic mRNA, the optimal dose of 1 μg of mRNA was selected for analysis. After injecting the 1-μg mRNA mixture, all mice remained healthy without any toxic symptoms or behavioral abnormalities. No significant transfection reagent-related neurovirulence was detected by either Y maze (Figures 3A–3E) or open field (Figures 3F–3J) tests. There were also no significant differences between TransIT-mRNA injection and controls on all determined blood biochemical parameters, including hemoglobin (HGB), white blood cells (WBCs), alanine aminotransferase (ALT), aspartate transaminase (AST), Cr, and C-reactive protein (CRP) (Figures 4A–4F). To investigate the effect of the transfection on local inflammation, interleukin (IL)-1β and IL-6 levels were measured by immunohistochemistry (Figure 4G). All these results suggest that TransIT-mRNA reagent-assisted synthetic mRNA delivery is safe for in vivo intracranial administration.
Figure 3.
Behavior evaluation of mice receiving intracranial injection of synthetic Luc-mRNA
Four groups of 8-week-old C57 mice were intracranially injected with synthetic Luc-mRNA in TransIT-mRNA solution or TransIT-mRNA reagent alone. The behavior tests were performed 14 days after injection. (A–E) Y maze test. (A) Infrared video recorder monitored the activity trajectory of mice in Y maze within 8 min. The overall traveled distances in the Y maze were recorded. (B) Total distances of the mice traveled in the Y maze. (C) Alternation triplet times of the mice traveling in the Y maze. (D) Total times of the mice traveling in the center of the Y maze. (E) The percentage of alternation triplet of the mice traveled in Y maze. (F–J) Open field test. (F) Infrared video recorder recorded the activity trajectory of mice in open field within 10 min. (G) Total distance the mice traveled in the open field. (H) Total times of the mice traveled in the center of open field. (I) Total distance of the mice traveled in the center of open field. (J) Total times of the mice entering the center of the open field.
Figure 4.
Safety evaluation of TransIT-mRNA in vivo by routine blood examination and immunohistochemistry
(A) HGB values in mouse serum. (B) WBCs in mouse blood. (C) ALT values in mouse serum. (D) Aspartate aminotransferase values in mouse serum. (E) Cr values in mouse serum. (F) CRP values in mouse serum. (G) The expressions of inflammatory factors IL-1β and IL-6 in brain tissues were measured by immunohistochemistry.
Therapeutic application of synthetic TRAIL-mRNA through intracranial injection in orthotopic glioma mouse model
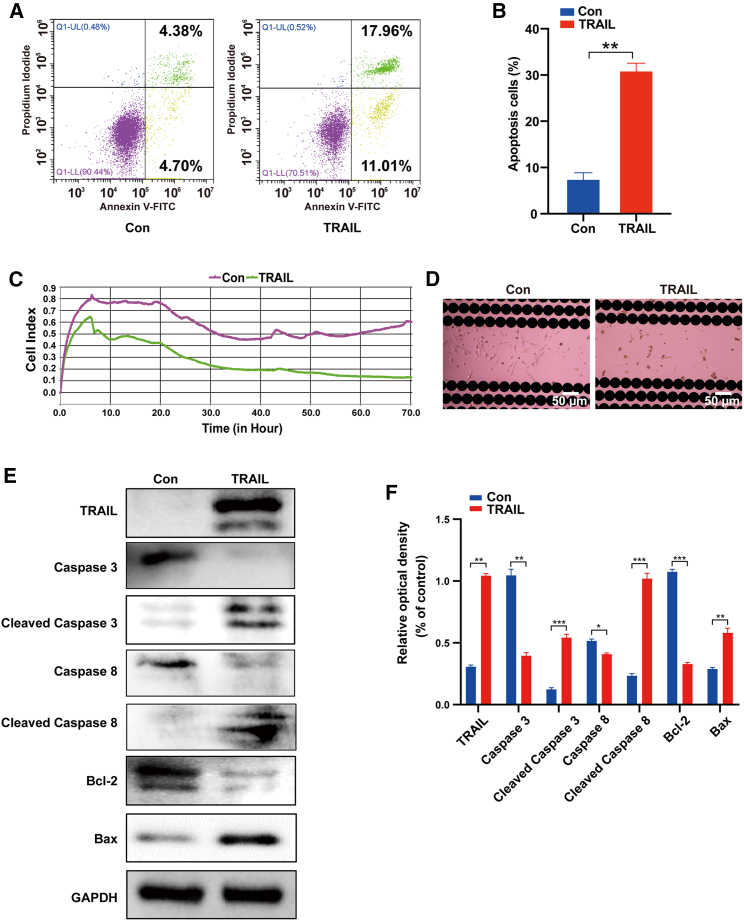
In order to test the effect of intracranial delivery mRNA system on glioma, we selected TRAIL-mRNA, which has a killing effect on glioma. The effect of synthetic TRAIL-mRNA on the viability of DBTRG(Denver Brain Tumor Research Group)-Luc glioma cells was determined using the Real-Time Cell Analyzer (RTCA), and TRAIL-induced apoptosis was detected by flow cytometric analysis. As shown in Figures 5A and 5B, the mean apoptotic population of normal DBTRG-Luc cells was 4.38% + 4.7%; however, the apoptotic population of DBTRG-Luc cells transfected with 1 μg of TRAIL-mRNA was 11.01% + 17.96%. RTCA results indicated that synthetic TRAIL-mRNA significantly inhibited the viability of DBTRG-Luc cells (Figures 5C and 5D). Figures 5E and 5F show the results of immunoblotting analysis of apoptosis-related proteins in DBTRG-Luc cells after TRAIL-mRNA transfection for 24 h. DBTRG-Luc cells expressed similar amounts of total caspase-3 and caspase-8. However, the cleaved form of caspase-3, caspase-8, and Bax were obviously upregulated by the treatment of synthetic TRAIL-mRNA transfection. Bcl-2 was downregulated by synthetic TRAIL-mRNA transfection. These results indicate that TRAIL-mRNA synthesized in vitro has good activity to inhibit glioma.
Figure 5.
The effects of synthetic TRAIL-mRNA on DBTRG-Luc glioma cells
(A and B) Flow cytometric analysis (A) and statistic (B) of synthetic TRAIL-mRNA induced apoptosis in DBTRG-Luc cells using annexin V-FITC/PI. (C) Cell viability determined by RTCA at an interval of 5 min until the end of 72 h. (D) After the experiment, the cells remaining in the E-plate culture plate were observed under the microscope at 400×. (E and F) The expression levels (A) and statistic (B) of apoptosis-related proteins in DBTRG-Luc cells determined by western blot at 24 h after TRAIL-mRNA transfection. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
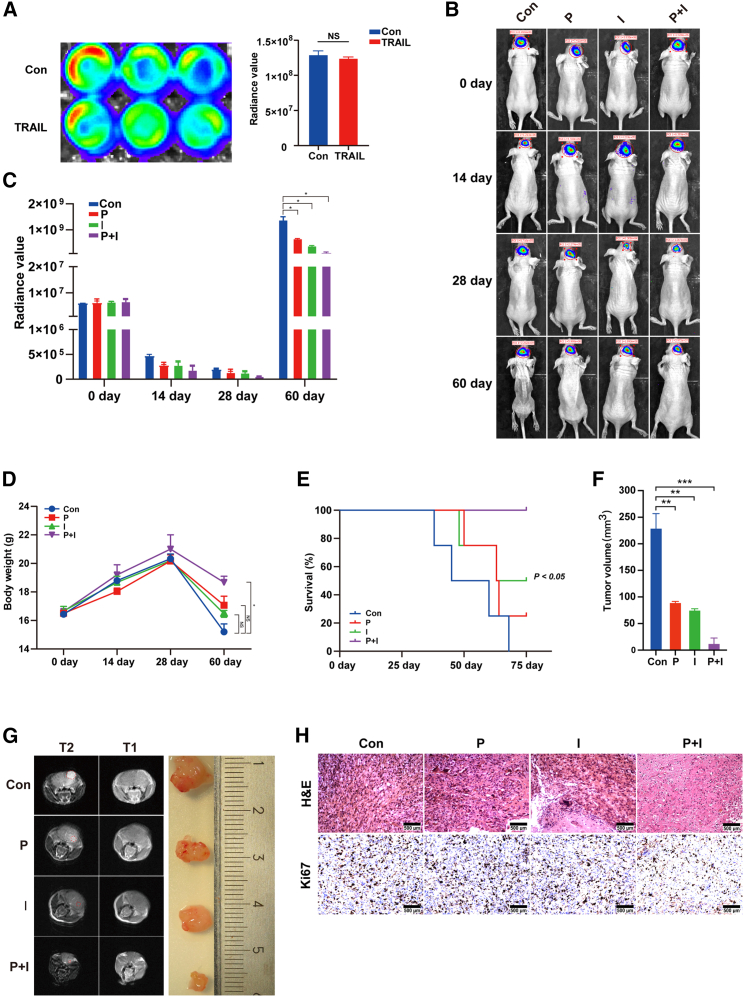
A DBTRG-Luc cell-derived xenografted glioma mouse model was used to test the therapeutic application of synthetic TRAIL-mRNA through intracranial injection. For intracranial xenografts, mice were anesthetized and placed in a stereotaxic platform; 3 × 105 cells resuspended in 4 μL of complete medium were injected 2 mm lateral and 1 mm anterior to the bregma, 2 mm below the skull (n = 5). Bioluminescence from the tumor was monitored at 0 days, 14 days, 28 days, and 60 days using the IVIS Spectrum imaging system (Caliper Life Sciences). As shown in Figure 6A, the intensity of luminescence in DBTRG-Luc cells pretreated with synthetic TRAIL-mRNA for 6 h was not different from DBTRG-Luc cells without synthetic TRAIL-mRNA pretreatment. DBTRG-Luc cell-derived and TRAIL-mRNA-pretreated DBTRG-Luc cell-derived xenografted glioma mouse models were used to further evaluate the effect of intracranial injection of synthetic TRAIL-mRNA. The tumor growth was significantly inhibited by both TRAIL-mRNA pretreatment and TRAIL-mRNA intracranial injection (Figures 6B and 6C). Figures 6D and 6E show the changes of body weight and animal survival rate under different conditions. The tumor size was measured, and the tumor volume was calculated (Figure 6F). At the endpoint, tumor size was detected with brain MRI scan and the measurement of isolated tumors (Figure 6G). The inhibitory effect of injected TRAIL-mRNA on tumor cell proliferation was also confirmed by immunohistochemical staining of tumor tissues (Figure 6H). This result shows that the method of intracranial delivery of TRAIL-mRNA could successfully inhibit the growth of glioma.
Figure 6.
Therapeutic application of synthetic TRAIL-mRNA through intracranial injection in orthotopic glioma mouse model
(A) The bioluminescent influence of pretreatment of DBTRG-Luc cells with synthetic TRAIL-mRNA transfection for 6 h. (B) The IVIS detected image representatives of different groups (n = 4) at day 0, 14, 28, and 60 and (C) corresponding summary. (D) Mouse body weights measured at day 0, 14, 28, and 60. (E) Mouse survival rates. (F) Tumor growth was monitored by measuring tumor volumes at 60 days and the tumor volumes were calculated by formula: tumor volume (V) = 1/2 × L × W2. (G) Brain MRI scan images and isolated tumor samples. (H) H&E and Hi67 staining of tumor tissues. Con, control; P, pretreatment with synthetic TRAIL-mRNA; I, intracranial injection of synthetic TRAIL-mRNA; P + I, the combination of pretreatment and intracranial injection of synthetic TRAIL-mRNA. ∗p < 0.05.
Discussion
Because mRNA possesses high positive charge and high hydrophilicity, it is usually delivered into cells as a complex with transfection reagent. TransIT-mRNA and jetPEI are commonly used for mRNA transfection in most in vitro studies. TransIT reagent was used for in vivo delivery in some studies, although TransIT-mRNA reagent was exclusively used in vitro.12, 13, 14, 15 For the first time, we established TransIT-mRNA reagent-mediated intracranial synthetic mRNA transfection. It is essential to verify the safety of these reagents in in vivo applications and their effects on the expression of associated genes after intracranial injection.
In the present study, luciferase mRNA was synthesized in vitro and its expression was verified in 293T cells. In order to study the safety of TransIT-mRNA and jetPEI in vivo application and their effects on the expression of Luc-mRNA after intracranial injection, we injected Luc-mRNA wrapped with TransIT-mRNA or jetPEI into the brains of C57BL/6J mice. Considering the volume safety of intracranial injection, we compared the transfection efficiency of a 20-μL system containing 1 μg of Luc-mRNA and a 30-μL system containing 2 μg of Luc-mRNA. After intracranial injection of Luc-mRNA, the longest time at which Luc-mRNA-produced bioluminescence can be detected by IVIS was 72 h for the 20-μL system and 48 h for the 30-μL system respectively. The poor performance of the 30-μL system might be due to the large volume leading to an incomplete injection into the brain. So, the 20-μL system containing 1 μg of Luc-mRNA was used for right caudate nucleus injection in the related in vivo experiments. In addition, according to in vivo-jetPEI manufacturer's instruction, a 5-μL system containing 1 μg of Luc-mRNA at N/P ratio of 6 was injected into the right caudate nucleus. The longest detectable time of Luc-mRNA-produced bioluminescence was 36 h. When the N/P ratio was increased to 8, the bioluminescence intensity was significantly higher than that at N/P ratio 6, but the longest detectable time remained 36 h.
The results of comparative experiments clearly indicate that TransIT-mRNA is much better than jetPEI in terms of assisting intracranial Luc-mRNA delivery. Both of these are the complex of liposomes and polymers, but we are not very clear about the proportions of the specific components. The difference in transfection efficiency may be different from the loading efficiency, release, and degradation speed of the polymer. The composition of in vivo-jetPEI contains cationic polymer PEI. The strong cationic charge of PEI may cause cell death.16 However, our result showed that TransIT-mRNA reagent is safer and non-toxic. The transfection efficiency of the 1 μg is better than the 2 μg, which is related to the size of the formed nanoparticles. The higher the RNA concentration, the larger the vesicles formed, which may also lead to incomplete RNA entrapment, resulting in ineffective delivery. Of course, the transfection volume of 2 μg is too large and requires too much reagent volume, which exceeds the maximum intracranial bearing volume of mice and will reduce the transfection efficiency. TransIT-mRNA is preferred to be used for mRNA delivery through intracranial injection. However, long-term safety is an important prerequisite for it to be routinely used in mRNA-based gene therapy studies. The test results of blood biochemical parameters showed a relatively stable internal environment after intracranial injection of TransIT-mRNA, indicating that the TransIT-mRNA solution was harmless to the metabolism of tested subjects. Two behavioral methods were used to test the potential neurotoxicity of TransIT-mRNA. Fourteen days after the intracranial injection, the results of open field test and Y maze test demonstrated that the behaviors of tested mice were not significantly affected by intracranial injection of TransIT-mRNA reagent. Taken together, TransIT-mRNA reagent-assisted synthetic mRNA delivery is safe for in vivo intracranial administration.
The major reasons that limit the effectiveness of conventional therapies for GBM include tumors' exceptional anatomical location and the existence of BBB. Intracranial injection of synthetic mRNA is able to directly deliver specific anticancer genes to the tumor. TRAIL is an anticancer gene. Its protein product can specifically kill cancer cells without harming normal cells.10,11,17, 18, 19 Owing to its tumor-cell-specific killing effect, TRAIL has been widely used in preclinical and clinical studies.18,20, 21, 22, 23, 24, 25, 26, 27, 28, 29 In the current study, we used synthetic TRAIL-mRNA as an example to verify whether this method of intracranial injection can be applied for the treatment of GBM. First, the tumor-cell-killing effect of synthetic TRAIL-mRNA was verified in vitro with DBTRG-Luc cells. TRAIL binds to its receptor DR5 and triggers apoptosis by driving caspase-8-mediated caspase-3 activation.30 Bax is dispensable for TRAIL-induced caspase-8 activation and subsequent cleavage of Bid but it is crucial for the release of cytochrome c and Smac/Diablo from mitochondria and downstream activation of caspases. Mitochondrial amplification of the death receptor signal in type II cells is achieved by caspase-8-mediated cleavage of the BH3-only protein Bid. The resulting active, truncated Bid (tBid) activates Bax, thereby inducing apoptosome and Smac/Diablo-mediated caspase activation. Thus, Bcl-2 family members play a critical role in modulating TRAIL-mediated cell death in tumor cells.31 In the present study, TRAIL-mRNA-mediated cytotoxicity of DBTRG cells and significant suppression of DBTRG cell-derived tumor are consistent with the results of our previous study,12 in which a mesenchymal stem cell-mediated TRAIL-mRNA transfer approach was applied on DBTRG cell-based GBM study. However, the in vivo antitumor effect of intracranially injected TRAIL-mRNA was investigated with DBTRG-Luc cell-derived xenografted glioma mouse model in this study. TRAIL-mRNA was pretransfected into DBTRG-Luc cells for 6 h. At this time, the mRNA had entered the cells, but the transfection time was too short to cause cell apoptosis. The pretreated DBTRG-Luc cells were used to establish a glioma model that could simulate clinical surgery to resect the tumor; it may also be applied directly to surgical sites after resection of a tumor as a postoperative treatment to kill residual tumor cells and prevent tumor metastasis, and this pretreatment method is defined as group P. In clinical surgical treatment of glioma, after the tumor was resected, a tumor cavity drainage tube was generally indwelled to allow injection of drugs, and group I was to simulate the surgical removal of the tumor, and continue the local drug treatment by intracranial injection. Group P + T was a combination of the two treatments. The antitumor effect of intracranially injected TRAIL-mRNA was comparable with that of pretreatment of DBTRG-Luc cells with TRAIL-mRNA, and the combination of pretreatment and intracranial injection of TRAIL-mRNA showed the best antitumor effect. This result shows that the method of synthetic mRNA intracranial delivery using common transfection reagent is suitable for the experimental studies of intracranial tumors.
In summary, the in vitro synthetic luciferase mRNAs were highly expressed in the mouse brain through intracranial injection, in which the injected mRNAs were wrapped with commonly used TransIT-mRNA transfection reagents. Mouse behavioral and blood biochemical measurements verified the biological safety of related reagents' intracranial application for mRNA transfection. Using synthetic TRAIL-mRNA as an experimental therapeutic example, its intracranial delivery with common transfection reagents significantly inhibited the tumor growth in DBTRG cell-derived xenografted glioma mouse model. TransIT-mRNA is the first in vitro reagent for transfection of mRNA in the world. Surprisingly, we found that TransIT-mRNA is also good for in vivo application. A commercially available in vivo transfection reagent was compared, and it was found that the intracranial transfection efficiency of TransIT was better than that of in vivo-jetPEI. Our study explores the TransIT-mRNA for the intracranial transfection first time and provides an intracranial synthetic mRNA delivery model for preclinical studies of mRNA-based gene therapy.
Materials and methods
In vitro synthesis of Luc-mRNA, RFP-mRNA, and TRAIL-mRNA
Luc-mRNA, RFP-mRNA, and TRAIL-mRNAs were synthesized in vitro as previously described.32 Briefly, the human 5′ UTR with Kozak sequence and 3′ UTR sequence were commercially synthesized by Integrated DNA Technologies (Coralville, IA) and sub-cloned into pcDNA3.3. The DNA templates of human TRAIL and luciferase were obtained from our previously constructed expression vectors through restriction enzyme digestion. MEGAscript T7 kit (Ambion) was used to synthesize mRNAs, whereas m7GpppG was replaced with ARCA cap analog (New England Biolabs) and cytidine and uridine were replaced with 5-methylcytidine triphosphate and pseudouridine triphosphate (TriLink Biotechnologies) respectively. Reactions were sustained for 5 h at 37°C followed by DNase treatment. Then, the reactions were treated with Antarctic Phosphatase (New England Biolabs) for 2 h at 37°C to remove residual 5′-triphosphates. The synthesized mRNAs were purified with Ambion MEGAclear spin columns (Ambion) and quantitated with Nanodrop (Thermo Fisher Scientific).
Cells and animals
293T cells stored by the laboratory were cultured in DMEM with 10% fetal calf serum (FCS), 2 mM L-glutamine, and 1% penicillin-streptomycin solution (all from Invitrogen, Carlsbad, CA) and incubated at 37°C in a humidified atmosphere with 5% CO2. Human glioblastoma cell line (DBTRG) was purchased from American Type Culture Collection (ATCC, Manassas, VA) to be used as target cells in the xenografted tumor model. DBTRG cells were maintained as suggested by ATCC and pre-labeled with luciferase using pGL4.51[luc2/CMV/Neo] vector (Promega Corporation, Madison, WI), according to the manufacturer's protocol.
C57BL/6J and nude mice (female, 6–8 weeks of age) were purchased from the Model Animal Research Center at Nanjing University (Nanjing, China) and housed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocols of the present study were approved by the Animal Care Committee at Hubei University of Medicine (Shiyan, China).
Intracranial injection of synthetic mRNA in mice
The C57BL/6J mice were anesthetized by intraperitoneal injection of 1% sodium pentobarbital. A sagittal incision (1.0–1.5 cm) was made on the scalp, and the calvarium was exposed by blunt dissection. A tiny parietal hole was created on the sagittal suture of the skull. The microinjector was positioned at the right caudate nucleus (1 mm forward and 2 mm right of the anterior fontanelle) and vertically punctured 3 mm. The Luc-mRNA, RFP-mRNA, or TRAIL-mRNA solution (20 μL) was injected at a rate of 2 μL/min through the microinjector. The TransIT-mRNA kit-mediated mRNA solution was composed of 1 μL of synthetic mRNA (1 or 2 μg/μL Luc-mRNA or 1 μg/μL RFP-mRNA, TRAIL-mRNA), 15 μL of Opti-MEM, 2 μL of Boost reagent, and 2 μL of TransIT-mRNA. The in vivo-jetPEI kit-mediated mRNA solution was composed of 1 μL of synthetic mRNA (1 μg/μL Luc-mRNA), 0.12 μL or 0.16 μL of in vivo-jetPEI reagent (N/P ratio 6 or 8), 2 μL of 10% glucose solution, and 0.88 or 0.84 μL DEPC(Diethyl pyrocarbonate) water. After injection, the microinjector was kept in place for 5 min. The mice were injected with RFP-mRNA and, after 12 h, the brain tissues were taken for frozen sections, and the expression of RFP protein in the brain tissues of mice was observed under a fluorescence microscope (Leica, Germany).
Establishment of xenografted tumor model
Since the IVIS Spectrum system is preferentially sensitive to bioluminescence, luciferase gene-transfected DBTRG (DBTRG-Luc) cells were used for the xenograft test. A total of 3 × 105 DBTRG-Luc cells were implanted into the right frontal lobe of nude mice. This xenografted tumor model was used to test the therapeutic effect of synthetic TRAIL-mRNA through intracranial injection. Seven days after in situ implantation of DBTRG-Luc cells, 20 μL of a cocktail solution (containing 1 μg of TRAIL-mRNA) was intracranially injected to each mouse. The bioluminescence was determined at day 0, 14, 28, and 60 using the IVIS Spectrum system. The body weight was recorded at day 0, 14, 28, and 60. Statistical analysis of survival rate of mice was performed at day 75.
Determination of tumor size by MRI
In order to detect the tumor formation of DBTRG-Luc cells, 75 days after in situ implantation of DBTRG-Luc cells, the mice were anesthetized by intraperitoneal injection of 1% sodium pentobarbital, and the tumors size were detected by MRI (General Electric).
Immunohistochemistry
The tumors were isolated from the brain tissues. One section per sample was deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E). Additional sections were immunostained using the automated immunohistochemistry and in situ hybridization staining system Bond RX (Leica Biosystems). Sections were stained for proliferating cells using rabbit anti-Ki67 antibody (1:50, Abcam).
Determination of blood biochemical indexes in exogenous mRNA-injected mice
To assess the toxicity of luciferase mRNA and Mirus reagents, routine blood examinations were performed for both control and Luc-mRNA-injected mice. Blood was collected by cardiac puncture in EDTA-K2 blood collection tubes at the day of sacrifice. The WBC, HGB, AST, alanine transaminase (ALT), creatinine (Cr), and CRP were determined using Mindray BC-6900 Vet animal automatic hematology analyzer. The blood cells of the mice were analyzed by using Mindray BC-5300 Vet animal automatic hematology analyzer (Mindray, China).
Behavioral evaluation of mice injected with synthetic mRNA
According to previous literature reports, behavioral tests have been used for the evaluation of drug-induced potential biological toxicity in animals.33,34 In the present study, open field test and Y maze test were performed to determine the possible transfection-reagent-induced neurotoxicity. These two behavioral tests were conducted in C57BL/6J mice on day 14 post inoculation of Luc-mRNA wrapped with Mirus TransIT-mRNA or Mirus TransIT-mRNA alone. For the open field test, each mouse was placed in the center of a darkened white box (50 × 50 × 38 cm) and monitored using an infrared video tracking system (Ethovision XT 9.0, Noldus Information Technology) for 10 min. A 30-by-30-cm square in the center of the box was defined as the zone, and the peripheral arena was defined as the residual. The distance traveled and time spent in the zone and residual were recorded for further analysis.
For the Y maze test, the Y maze was fabricated from gray plastic and consisted of three arms (21 cm long, 15.5 cm high, 7 cm wide at the bottom, and 10 cm wide at the top) with an angle of 120°. Visual cues were placed outside each arm, and the apparatus was illuminated at 10 lux. Each mouse was placed at the end of one arm and allowed to freely explore the maze for 10 min. An arm entry was defined as all four paws of the mouse being in the arm, and the sequence of arm entries was monitored with a video camera and counted manually. An alternation was defined as successive entries into the three arms on overlapping triplet sets.
Flow cytometry analysis of the apoptosis of synthetic TRAIL-mRNA transfection into DBTRG-Luc cells
Briefly, 1 × 106 DBTRG-Luc cells were seeded in a six-well plate and incubated for 24 h to resume exponential growth. Synthetic TRAIL-mRNA was transfected into DBTRG-Luc cells and incubated for additional 24 h, then the cells were harvested and washed with PBS. The extent of apoptosis was measured through annexin V-FITC apoptosis detection kit (Beyotime, China), and analyzed by flow cytometry software (Beckman, United States). The upper right part represents apoptotic cells undergoing secondary necrosis at the last stage or dead cells (annexin V and phosphatidylinositol [PI] double positive), and the lower right part represents the early-stage apoptotic cell population (annexin V positive and PI negative).
Viability assay of DBTRG-Luc glioma cells
The cell viability was detected by real-time assessment by the xCELLigence cell analyzer (RTCA, Roche, United States) as previously described.32 A volume of 100 μL of DBTRG-Luc cell suspension (5 × 103 cells) was seeded in E-plate 16. All cells were allowed to settle at the bottom of the wells at room temperature (RT) for 15 min, and then incubated at 37°C and 5% CO2. The impedance signals were recorded every 5 min for the first 6 h. After 6 h of baseline measurement, 0.1 μg of synthetic TRAIL-mRNA mixture was added into each well. The impedance signals were recorded using the same time intervals until the end of the experiment (up to 72 h). Cell index (CI) value was defined as relative change in measured impedance compared with background impedance and represented cell status, which is directly proportional to the quantity, size, and attachment forces of the cells.
Immunoblotting analysis of TRAIL-induced apoptosis-related protein expression
Immunoblotting analysis was used to detect the cellular expression of TRAIL-induced apoptosis-related proteins (caspase-3, caspase-8, Bcl-2, and Bax) in DBTRG-Luc cells. Briefly, DBTRG-Luc cells were washed three times with PBS and collected with the cell lysis RIPA buffer (Cowin Bio, China). Cell lysates were incubated on ice for 30 min. Protein concentration was determined using BCA protein assay reagents (Beyotime) according to the manufacturer's protocol. Equal amounts of protein (50 μg in each sample) were loaded in each lane, separated by electrophoresis in 12% SDS polyacrylamide gel, and electrotransferred to nitrocellulose membranes. The membrane was put in blocking buffer for 1 h at RT followed by overnight incubation at 4°C with appropriate primary TRAIL antibody (1:1,000, CST, United States), caspase-3 antibody (1:1,000, CST, United States), caspase-8 antibody (1:500, Proteintech, China), Bcl-2 antibody (1:1,000, CST, United States), and GAPDH antibody (1:4,000, Proteintech, China). The blots were rinsed with TBST(Tris Buffered Saline with Tween®20 ) three times, incubated with horseradish peroxide-conjugated secondary antibody (1:2,000) for 60 min, and detected by chemiluminescence using ECL Hyperfilm (Bio-Rad, United States).
Statistical analysis
Numerical data were expressed as mean ± standard error. Statistical differences between the means for the different groups were evaluated with Prism 8.0 (GraphPad software, La Jolla, CA) using the Student's t test with the level of significance at p < 0.05.
Acknowledgments
Funding: this research was supported by the National Natural Science Foundation of China (nos. 81702482, 82073232, 81700769, 81641028, 81602297), the Biomedical Research Foundation, Hubei University of Medicine (HBMUPI201803), the Hubei Science & Technology Department Foundation (2020CFB558, 2018ACA162), the Key projects of Hubei Education (D20202103), and the Key Projects of Precision Medicine (2016JZ01).
Author contributions
J.L., X.J.T., and L.J.D. contributed to the conception. H.P., X.R.G., and J.J.H. performed all experiments and composed the manuscript. H.P. analyzed and interpreted all the data. X.R.G. and X.J.T. supported the experimental techniques. L.J.D. and B.W. helped write and critically review the manuscript and provided intellectual input. L.Z., R.F., D.K.W., F.L.C., H.C., H.G., J.X.X., and X.H.Z. conceived the study, participated in coordination, and helped in drafting the manuscript. J.L., X.J.T., and L.J.D. supervised all studies. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Longjun Dai, Email: ljdai@mail.ubc.ca.
Xiangjun Tang, Email: tangxiang_jun@163.com.
Jie Luo, Email: luojie_001@126.com.
References
- 1.Sanders N., Rudolph C., Braeckmans K., De Smedt S.C., Demeester J. Extracellular barriers in respiratory gene therapy. Adv. Drug Deliv. Rev. 2009;61:115–127. doi: 10.1016/j.addr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rittig S.M., Haentschel M., Weimer K.J., Heine A., Muller M.R., Brugger W., Horger M.S., Maksimovic O., Stenzl A., Hoerr I., et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol. Ther.: J. Am. Soc. Gene Ther. 2011;19:990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Su H.H., Yang Y., Hu Y., Zhang L., Blancafort P., Huang L. Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol. Ther.: J. Am. Soc. Gene Ther. 2013;21:358–367. doi: 10.1038/mt.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F., Kream R.M., Stefano G.B. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Med. Sci. monitor: Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vabret N. Preclinical data from SARS-CoV-2 mRNA vaccine. Nat. Rev. Immunol. 2020;20:461. doi: 10.1038/s41577-020-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. New Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diken M., Kreiter S., Selmi A., Türeci O., Sahin U. Antitumor vaccination with synthetic mRNA: strategies for in vitro and in vivo preclinical studies. Methods Mol. Biol. (Clifton, NJ) 2013;969:235–246. doi: 10.1007/978-1-62703-260-5_15. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen L.B., Larsen A.B., Lichota J., Moos T. Nanoparticle-derived non-viral genetic transfection at the blood-brain barrier to enable neuronal growth factor delivery by secretion from brain endothelium. Curr. Med. Chem. 2011;18:3330–3334. doi: 10.2174/092986711796504637. [DOI] [PubMed] [Google Scholar]
- 9.Tang X., Zhang S., Fu R., Zhang L., Huang K., Peng H., Dai L., Chen Q. Therapeutic prospects of mRNA-based gene therapy for glioblastoma. Front. Oncol. 2019;9:1208. doi: 10.3389/fonc.2019.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belkahla H., Herlem G., Picaud F., Gharbi T., Hémadi M., Ammar S., Micheau O. TRAIL-NP hybrids for cancer therapy: a review. Nanoscale. 2017;9:5755–5768. doi: 10.1039/c7nr01469d. [DOI] [PubMed] [Google Scholar]
- 11.Dianat-Moghadam H., Heidarifard M., Mahari A., Shahgolzari M., Keshavarz M., Nouri M., Amoozgar Z. TRAIL in oncology: from recombinant TRAIL to nano- and self-targeted TRAIL-based therapies. Pharmacol. Res. 2020:104716. doi: 10.1016/j.phrs.2020.104716. [DOI] [PubMed] [Google Scholar]
- 12.Karikó K., Muramatsu H., Keller J.M., Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther.: J. Am. Soc. Gene Ther. 2012;20:948–953. doi: 10.1038/mt.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardi N., Secreto A.J., Shan X., Debonera F., Glover J., Yi Y., Muramatsu H., Ni H., Mui B.L., Tam Y.K., et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 2017;8:14630. doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y., Gaudin A., Zhang J., Agarwal T., Song E., Kauffman A.C., Tietjen G.T., Wang Y., Jiang Z., Cheng C.J., et al. A "top-down" approach to actuate poly(amine-co-ester) terpolymers for potent and safe mRNA delivery. Biomaterials. 2018;176:122–130. doi: 10.1016/j.biomaterials.2018.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stadler C.R., Bähr-Mahmud H., Celik L., Hebich B., Roth A.S., Roth R.P., Karikó K., Türeci Ö., Sahin U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med. 2017;23:815–817. doi: 10.1038/nm.4356. [DOI] [PubMed] [Google Scholar]
- 16.Wu L., Zhou W., Lin L., Chen A., Feng J., Qu X., Zhang H., Yue J. Delivery of therapeutic oligonucleotides in nanoscale. Bioact Mater. 2022;7:292–323. doi: 10.1016/j.bioactmat.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han H.R., Park S.A., Ahn S., Jeun S.S., Ryu C.H. Evaluation of combination treatment effect with TRAIL-secreting mesenchymal stem cells and compound C against glioblastoma. Anticancer Res. 2019;39:6635–6643. doi: 10.21873/anticanres.13878. [DOI] [PubMed] [Google Scholar]
- 18.Gamie Z., Kapriniotis K., Papanikolaou D., Haagensen E., Da Conceicao Ribeiro R., Dalgarno K., Krippner-Heidenreich A., Gerrand C., Tsiridis E., Rankin K.S. TNF-related apoptosis-inducing ligand (TRAIL) for bone sarcoma treatment: pre-clinical and clinical data. Cancer Lett. 2017;409:66–80. doi: 10.1016/j.canlet.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 19.Mert U., Sanlioglu A.D. Intracellular localization of DR5 and related regulatory pathways as a mechanism of resistance to TRAIL in cancer. Cell Mol. Life Sci. 2017;74:245–255. doi: 10.1007/s00018-016-2321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duiker E.W., Mom C.H., de Jong S., Willemse P.H., Gietema J.A., van der Zee A.G., de Vries E.G. The clinical trail of TRAIL. Eur. J. Cancer. 2006;42:2233–2240. doi: 10.1016/j.ejca.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Legler K., Hauser C., Egberts J.H., Willms A., Heneweer C., Boretius S., Röcken C., Glüer C.C., Becker T., Kluge M., et al. The novel TRAIL-receptor agonist APG350 exerts superior therapeutic activity in pancreatic cancer cells. Cell Death Dis. 2018;9:445. doi: 10.1038/s41419-018-0478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuckey D.W., Shah K. TRAIL on trial: preclinical advances in cancer therapy. Trends Mol. Med. 2013;19:685–694. doi: 10.1016/j.molmed.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tisato V., Gonelli A., Voltan R., Secchiero P., Zauli G. Clinical perspectives of TRAIL: insights into central nervous system disorders. Cell Mol. Life Sci. 2016;73:2017–2027. doi: 10.1007/s00018-016-2164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu G.S. TRAIL as a target in anti-cancer therapy. Cancer Lett. 2009;285:1–5. doi: 10.1016/j.canlet.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z.C., Liu J.W., Yang C., Li M.J., Wu R.J., Xiong Z.Q. Targeting KPNB1 overcomes TRAIL resistance by regulating DR5, Mcl-1 and FLIP in glioblastoma cells. Cell Death Dis. 2019;10:118. doi: 10.1038/s41419-019-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chyuan I.T., Tsai H.F., Wu C.S., Hsu P.N. TRAIL suppresses gut inflammation and inhibits colitogeic T-cell activation in experimental colitis via an apoptosis-independent pathway. Mucosal Immunol. 2019;12:980–989. doi: 10.1038/s41385-019-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mert U., Adawy A., Scharff E., Teichmann P., Willms A., Haselmann V., Colmorgen C., Lemke J., von Karstedt S., Fritsch J., et al. TRAIL induces nuclear translocation and chromatin localization of TRAIL death receptors. Cancers (Basel). 2019;11:1167. doi: 10.3390/cancers11081167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hope J.M., Lopez-Cavestany M., Wang W., Reinhart-King C.A., King M.R. Activation of Piezo1 sensitizes cells to TRAIL-mediated apoptosis through mitochondrial outer membrane permeability. Cell Death Dis. 2019;10:837. doi: 10.1038/s41419-019-2063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kretz A.L., Trauzold A., Hillenbrand A., Knippschild U., Henne-Bruns D., von Karstedt S., Lemke J. TRAILblazing strategies for cancer treatment. Cancers (Basel). 2019;11:456. doi: 10.3390/cancers11040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Q., Man S.M., Karki R., Malireddi R.K.S., Kanneganti T.D. Detrimental type I Interferon signaling dominates protective AIM2 inflammasome responses during Francisella novicida infection. Cell Rep. 2018;22:3168–3174. doi: 10.1016/j.celrep.2018.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillissen B., Wendt J., Richter A., Richter A., Müer A., Overkamp T., Gebhardt N., Preissner R., Belka C., Dörken B., et al. Endogenous Bak inhibitors Mcl-1 and Bcl-xL: differential impact on TRAIL resistance in Bax-deficient carcinoma. J. Cell Biol. 2010;188:851–862. doi: 10.1083/jcb.200912070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo X.R., Yang Z.S., Tang X.J., Zou D.D., Gui H., Wang X.L., Ma S.N., Yuan Y.H., Fang J., Wang B., et al. The application of mRNA-based gene transfer in mesenchymal stem cell-mediated cytotoxicity of glioma cells. Oncotarget. 2016;7:55529–55542. doi: 10.18632/oncotarget.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q., Wu J., Ye Q., Ma F., Zhu Q., Wu Y., Shan C., Xie X., Li D., Xiaoyan Zhan X., et al. Treatment of human glioblastoma with a live attenuated Zika virus vaccine candidate. mBio. 2018;9 doi: 10.1128/mBio.01683-18. e01683-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong L., Zhou H., Zhao Q., Xue L., Al-Hawwas M., He J., Wu M., Zou Y., Yang M., Dai J., et al. Overexpression of miR-124 protects against neurological dysfunction induced by neonatal hypoxic-ischemic brain injury. Cell Mol. Neurobiol. 2020;40:737–750. doi: 10.1007/s10571-019-00769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]