Graphical abstract
Keywords: Nephrotoxicity, Geophila obvallata, Cadmium, Fractions, Nephro-toxicant
Highlights
-
•
Cadmium-induced toxicity results in oxidative stress in kidney tissues.
-
•
Cadmium intoxication upregulates metallothionine and kidney injury molecule’s mRNA.
-
•
Geophila obvallata extract confers protection against cadmium-induced toxicity.
-
•
Geophila obvallata extract pre-treatment upregulates OH-1 and NQ-O1 gene elements.
Abstract
Cadmium (Cd) is a known nephro-toxicant. This research determined the ameliorative effects of Geophila obvallata crude methanol extract (Me70) and its fractions on Cd-induced nephrotoxicity in Wistar rats. Male rats were randomly allocated to nine (9) groups of six (6) rats each. Group I was given normal water and feed only. Group II received 0.3 mg Cd/L only for two weeks. Groups III, IV, V, VI and VII were pre-exposed to aqueous, ethyl acetate, hexane, butanol and chloroform fractions of Me70, each at a dose of 50 mg/kg bwt for fourteen (14) days prior to treatment with 0.3 mg Cd/L for a further two weeks. Group VIII was pre-exposed to Me70 at a dose of 50 mg/kg bwt for fourteen (14) days, prior to administration of 0.3 mg Cd/L for fourteen (14) days, while group IX received 50 mg/kg.bwt of Me70 only for fourteen (14) days. The treatments were administered daily for twenty-eight (28) days via oral gavage. Cadmium levels in the kidney tissues of Cd-treated rats increased significantly while their body weights decreased relative to control. The levels of kidney function markers in the plasma, lipid peroxidation levels, catalase activity, metallothionine (MT-1) and kidney injury molecule-1 (KIM-1) mRNA were increased significantly, while glutathione (GSH) levels, activities of superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione-s-transferase (GST), and levels of nuclear factor erythroid-2-related factor 2 (Nrf-2), peroxisome proliferator-activated receptor –gamma coactivator- 1-alpha (PGC-1α), heme oxygenase-1 (HO-1) and NADH quinone oxidoreductase-1 (NQ-O1) were decreased significantly in rats treated with Cd-only. Overall, pre-treatment with Me70 produced better protection from the toxic effects of cadmium induction in experimental rats.
1. Introduction
Cadmium is a non-biological metal that can accumulate in living systems, resulting in oxidative stress in cells [1]. Drinking water polluted with cadmium is one of the major ways by which humans are exposed to Cd toxicity [2]. According to the WHO [3], the acceptable threshold limit for a toxicant like Cd in potable-water is 0.005 mg/dL. However, recent studies carried out across Nigerian states have confirmed that the concentration of Cd/L in surface water (0.3 mg Cd/L) was 220 folds above the designated threshold limit [4]. Cadmium (Cd) is often used for inducing oxidative damage in the vital organs of experimental models [5] through the following mechanisms: production of unstable reactive oxygen species (ROS) in target organs [6] and disruptions in cellular antioxidant systems [7].
In recent times, beneficial health and well-being has being linked to the consumption of herbs and vegetables [8]. Medicinal plants are endowned with bio-compounds with protective and therapeutic principles that can help in the management of ailments including nephrotoxicity associated with metal ion poisoning [9]. Geophila obvallata (Rubiaceae) is one of such medicinal herbs used in ethno-medicine for treating kidney disease in the coastal areas of West Africa. This edible creeping plant grows best in shady places and can be found extensively distributed across West tropical Africa [10]. Preliminary studies by this author has shown that the crude methanol extract (Me70) had a significantly higher concentration of bioactive principles with antioxidant properties such as phenols, flavonoids and alkaloids as well as strong reducing powers and scavenging potential against unstable atoms in vitro than the aqueous extract, non-toxic at 100 mg/kg bwt [11,12]. Asagba and Obi [13] had previously reported that drinking-water contaminated with 0.3 mg Cd/L can cause alterations in major biochemical indices of experimental rats. In the absence of documented scientific data on the potential of Geophila obvallata in literature, this research explored the protective effect of Me70 and its fractions in reversing cadmium-induced nephrotoxicity in male Wistar rats.
2. Material and methods
2.1. Plant identification, extraction and fractionation
Geophila obvallata leaves were collected and identified by Dr. H.A. Akinnibosun, a taxonomist working at the University of Benin, Nigeria. A representative sample was preserved at the Plant Biology and Biotechnology Department with number UBHa 0312, for future references. Pulverized leaves of Geophila obvallata (900 g portions) were extracted in 70 % methanol to obtain the hydro-methanolic crude extract (Me70) using soxhlet extraction. The evaporated extract was then freeze-dried to yield a brownish extract (117.52 g) and stored at 4 °C. Portions of the lyophilized extract were reconstituted in H2O (340 mL) and partitioned separately using solvents with ascending polarity (hexane, ethyl acetate, chloroform and butanol; 250 ml each). A rotary evaporator was used to dry the extracts to obtain the corresponding fractions [14]. The weights of the various residues obtained after solvent-solvent fractionation were: 2.15 g (hexane), 3.11 g (ethyl acetate), 0.96 g (chloroform), 5.6 g (butanol), and 4.9 g (aqueous). The fractions were stored at about 4 °C prior to analysis.
2.1.1. Experimental animals
Rats weighing 170−200 g were used during this research. They were obtained from a private breeder resident at the University of Benin. The rats were given accommodation inside a well sterilized environment conforming to US revised guidelines. The rats were given access to pelleted feeds from Livestock feeds and mills, Nigeria as well as potable water. Ethical principles regulating research with live animals (rats) were complied with [15] while the ethics committee of the University of Benin gave the approval for protocols employed in the animal study. Fifty-four (54) acclimatized experimental animals were assigned to nine (9) sets of six (6) rats each for this experiment. Group I was given normal water and feed only. Group II received only 0.3 mg Cd/L for two weeks. Groups III, IV, V, VI and VII were pre-exposed to chloroform, butanol, ethyl acetate, hexane and aqueous fractions, each received 50 mg/kg bwt Me70 for fourteen (14) days prior to treatment with 0.3 mg Cd/L for a further two weeks. Group VIII was pre-treated with 50 mg/kg bwt Me70 for fourteen (14) days, prior to administration of 0.3 mg Cd/L for a further two weeks, while group IX received 50 mg/kg bwt Me70 only for fourteen (14) days. The entire experiment lasted for four weeks while treatments were administered via oral gavage [13].
2.2. Sample collection and handling
Prior to the sacrifice day, the rats were fasted overnight and final body weights were taken. Sacrifice was done via lower abdominal incision under isoflurane overdose. Minimally-traumatic cardiac puncture was employed to collect blood samples into sterile plain sample and EDTA bottles for biochemical analyses. Sera obtained from centrifugation of the clotted blood samples were stored at low temperatures (5 °C) for biochemical analysis. Kidney tissues were removed from the rats and cleaned with cool KCl and weighed. Parts of the excised organs were embedded in buffered methanal for histological analysis. The remaining kidney portions were placed in sterile universal containers and frozen at 4 °C prior to homogenization for PCR analysis.
2.2.1. Final body and organ weights study
Records of the kidney and final body weights were taken [16].
2.2.2. Renal tissue cadmium concentration
The renal tissue cadmium level was examined using the protocols of Massanyi et al. [17]. Properly weighed and homogenised kidney tissue sample was dissolved in 1 mL trioxonitrate (V) acid. One day later, the samples were dried at 120 ° C for 5 h, the residue was later, mixed with deionised water and measured at 228.8 nm using (Perkin-Elmer 5100) furnace Atomic Absorption Spectrophotometry (AAS) (μg/g of wet renal tissue).
2.2.3. Kidney function assays
The urease Berthelot method of Fawcet and Scott [18] was used to measure blood urea nitrogen concentration using Bio-diagnostic assay kits (Giza, Egypt). The method of Henry [19] was used to estimate the serum creatinine levels with Teco diagnostic kits (Giza, Egypt).
2.2.4. Biochemical analyses
The methods described by Aebi [20] were used to evaluate catalase activity. The SOD activity was measured using megazyme assay kits [21]. The methods described by Paglia and Valentine [22] were used to evaluate GPx, GST and GSH activity. The level of lipid peroxidation by MDA determination and electrolyte levels were carried out following the methods described by Spanidis [23].
2.2.5. Histological assessment and processing of tissues excised from rats
Sections of the right kidney were taken to water, tainted with hematoxylin dye and dried out using graded doses of absolute alcohol with the tissues spending one hour in each of the alcohol and cleared using xylene for 60 min. Sections were later viewed (400x) using a light microscope while the photomicrographs were processed and interpreted by a histopathologist from the University of Benin Teaching Hospital, Benin City [24].
2.2.6. Gene quantification
The relative gene expressions of the pro-inflammatory markers and anti-apoptotic genes as well as kidney injury molecule-1 (KIM-1) and metallothionine-1 (MT-1) mRNA were evaluated relative to the house keeping gene (GAPDH) using RT-PCR following the method specified by the manufacturer [25].
2.2.7. Data analyses
Results of this research were expressed as mean ± standard deviation (SD) with one-way analysis of variance (ANOVA) method using Graph Pad Prism 5 statistical software. Experimental results with p < 0.001 were significant statistically. a p < 0.001 vs. the control rats; b p < 0.001 vs. the CdCl2-treated rats analyzed using Tukey’s HSD test.
3. Results
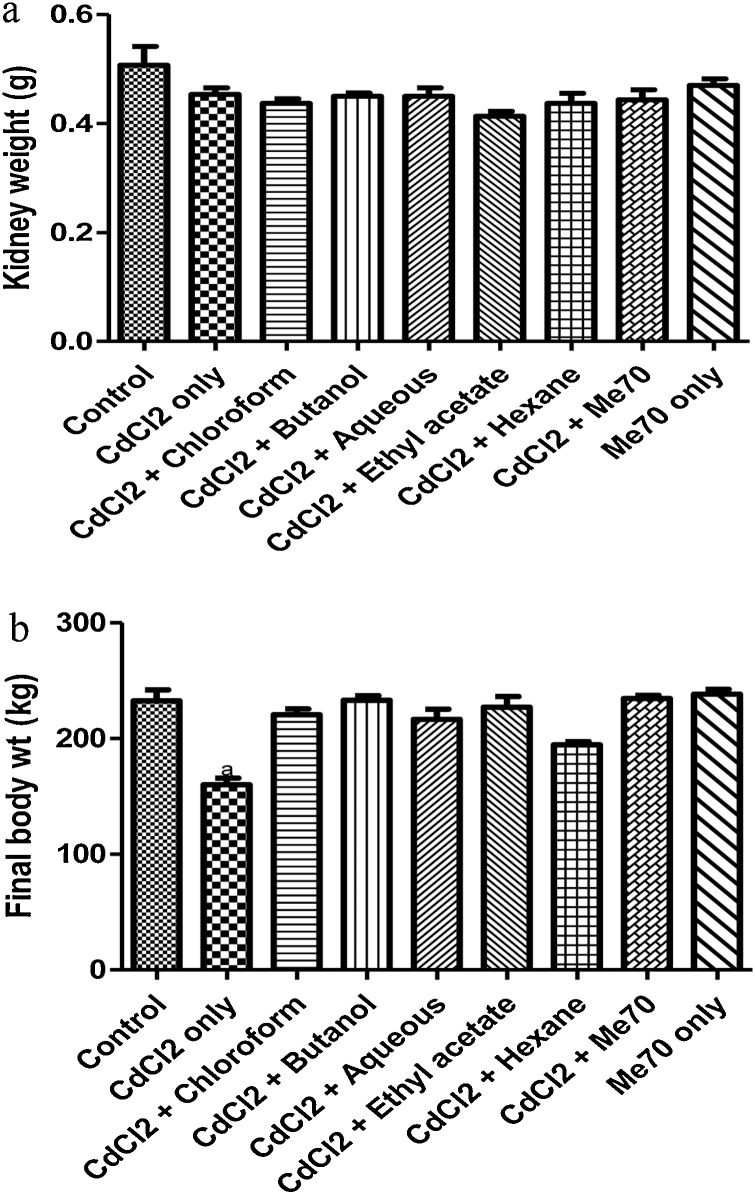
The kidney weight of rats treated with 0.3 mg Cd/L only (Fig. 1a), did not change when compared with the control group. There was a significant decrease (p < 0.001) in the final body weight of rats given 0.3 mg Cd/L only, relative to other experimental groups tested (Fig. 1b).
Fig. 1.
a. Effect of Me70 and its fractions on kidney weight.
b. Effect of Me70 and its fractions on final body weight. ap < 0.001 vs. the control rats analyzed using Tukey’s test.
Cadmium concentration in the kidney of rats given only (0.3 mg Cd/L) cadmium increased significantly (p < 0.001) when compared with the control. A similar trend was seen in the Cd + hexane group when compared with other groups (Table 1).
Table 1.
Cadmium concentration in the kidney of exposed (0.3 mg Cd/L/day) rats.
| Groups | Cadmium ion (μg/g kidney tissue) |
|---|---|
| Control | 1.86 ± 0.00 |
| Cd only | 3.57 ± 0.02a |
| Cd + Chloroform | 2.18 ± 0.00b |
| Cd + Butanol | 2.14 ± 0.03b |
| Cd + Ethyl acetate | 3.08 ± 0.00a, b |
| Cd + Hexane | 3.22 ± 0.04a |
| Cd + Aqueous | 2.16 ± 0.00b |
| Cd + Me70 | 2.13 ± 0.06b |
| Me70 only | 1.98 ± 0.01 |
Values are expressed as mean ± SD.
p < 0.001 vs. the control rats.
p < 0.001 vs. the CdCl2-treated rats analyzed using Tukey’s test.
Lipid peroxidation (MDA) increased in both the Cd-only treated rats and the hexane + Cd group, in renal tissues investigated. In contrast, decreases (p < 0.001) in antioxidant indices (GPx, GSH, GST, CAT and SOD) were mostly seen in the Cd-only group and hexane + Cd group respectively, in the tissues investigated. However, other fractions as well as the Me70 + Cd group showed results similar to the control (Table 2).
Table 2.
Effects of Me70 and fractions on kidney antioxidant indices of Cd exposed rats.
| Groups | MDA (mg/dL of wet tissue) | GPx (unit/mg of wet tissue) | GSH (μmol/g tissue) | GST (unit/mg of wet tissue) | CAT(unit/mg of wet tissue) | SOD (unit/mg of wet tissue) |
|---|---|---|---|---|---|---|
| Control | 2.36 ± 0.27 | 1.34 ± 0.08 | 0.73 ± 0.03 | 3.40 ± 0.08 | 0.68 ± 0.02 | 0.88 ± 0.05 |
| Cd only | 8.29 ± 0.25a | 0.16 ± 0.03a | 0.27 ± 0.03a | 1.64 ± 0.07a | 0.43 ± 0.02a | 0.45 ± 0.02a |
| Cd + Chloroform | 4.67 ± 0.66b | 0.65 ± 0.02b | 0.85 ± 0.02b | 2.16 ± 0.42b | 0.55 ± 0.07b | 0.63 ± 0.03b |
| Cd + Butanol | 3.49 ± 0.09 b | 1.05 ± 0.04b | 0.74 ± 0.03b | 2.22 ± 0.03b | 0.56 ± 0.08b | 0.63 ± 0.05b |
| Cd + Ethyl acetate | 4.80 ± 0.07 b | 0.48 ± 0.04b | 0.86 ± 0.04b | 2.37 ± 0.35b | 0.59 ± 0.05b | 0.70 ± 0.02b |
| Cd + Hexane | 7.27 ± 0.03a | 0.44 ± 0.03a, b | 0.56 ± 0.03a, b | 2.20 ± 0.25b | 0.50 ± 0.02b | 0.54 ± 0.03a |
| Cd + Aqueous | 3.58 ± 0.05b | 0.97 ± 0.03b | 0.76+ 0.02b | 2.33 ± 0.11b | 0.66 ± 0.03b | 0.61 ± 0.14b |
| Cd + Me70 | 3.17 ± 0.50b | 1.16 ± 0.03b | 0.78 ± 0.10b | 2.32 ± 0.06b | 0.59 ± 0.04b | 0.68 ± 0.02b |
| Me70 only | 2.43 ± 0.06 | 1.36 ± 0.03 | 0.71+ 0.10 | 3.30 ± 0.17 | 0.65 ± 0.02 | 0.84 ± 0.05 |
Values are expressed as mean ± SD.
p < 0.001 vs. the control rats.
p < 0.001 vs. the CdCl2-treated rats analyzed using Tukey’s test.
The result (Table 3) showed an increase (p < 0.001) in the concentration of blood urea nitrogen and creatinine in the serum of the Cd-only group, chloroform + Cd and hexane + Cd groups respectively. However, other groups showed similar results as the control.
Table 3.
Effects of Me70 and its fractions on kidney function indices in Cd treated rats.
| Groups | BUN (mg/dL) | Serum creatinine (mg/dL) |
|---|---|---|
| Control | 29.39 ± 1.86 | 0.25 ± 1.01 |
| Cd only | 68.45 ± 0.89a | 0.78 ± 0.02a |
| Cd + Chloroform | 57.66 ± 1.28a | 0.66 ± 0.02a |
| Cd + Butanol | 46.65 ± 0.07b | 0.46 ± 0.02b |
| Cd + Ethyl acetate | 41.56 ± 0.38b | 0.52 ± 0.00b |
| Cd + Hexane | 61.99 ± 0.58a | 0.68 ± 0.02a |
| Cd + Aqueous | 49.55 ± 2.24b | 0.48 ± 0.01b |
| Cd + Me70 | 33.28 ± 0.01b | 0.42 ± 0.02b |
| Me70 only | 32.67 ± 0.30 | 0.38 ± 0.02 |
Values are expressed as mean ± SD.
p < 0.001 vs. the control rats.
p < 0.001 vs. the CdCl2-treated rats analyzed using Tukey’s test.
The result (Table 4) showed an increase in serum K+ in the Cd-only group, chloroform and hexane fractions respectively when compared with the control group. In contrast, serum HCO3−, Na+and Cl− concentration reduced (P < 0.001) significantly in the Cd-only group, chloroform, ethyl acetate and hexane fractions respectively when compared to other groups. However, the Me70, butanol and aqueous groups showed similar results as the control.
Table 4.
Effects of Me70 and its fractions on serum electrolytes in cadmium exposed rats.
| Groups | HCO3− (mg/dl) | Na+ (mg/dl) | K+(mg/dl) | Cl−(mg/dl) |
|---|---|---|---|---|
| Control | 10.43 ± 0.04 | 101.77 ± 0.56 | 28.24 ± 0.25 | 78.59 ± 0.04 |
| Cd only | 9.18 ± 0.04a | 58.87 ± 0.02a | 41.27 ± 0.25a | 46.36 ± 0.42a |
| Cd + Chloroform | 10.17 ± 0.02 | 70.52 ± 0.01a, b | 39.69 ± 0.36a | 66.23 ± 0.48b |
| Cd + Butanol | 10.19 ± 0.02 | 96.43 ± 0.76b | 29.08 ± 0.50b | 66.09 ± 0.35b |
| Cd + Ethyl acetate | 10.29 ± 0.04 | 66.76 ± 0.54a | 29.24 ± 1.14b | 55.11 ± 0.38a, b |
| Cd + Hexane | 10.27 ± 0.02 | 61.80 ± 0.45a | 38.88 ± 0.48a | 65.71 ± 0.50b |
| Cd + Aqueous | 10.17 ± 0.03 | 87.80 ± 0.48b | 28.09 ± 0.49b | 56.09 ± 0.22a, b |
| Cd + Me70 | 10.20 ± 0.01 | 95.80 ± 0.85b | 29.16 ± 0.06b | 65.80 ± 0.33b |
| Me70 only | 10.18 ± 0.04 | 98.45 ± 0.23 | 26.89 ± 0.79 | 66.17 ± 0.04 |
Values are expressed as mean ± SD.
p < 0.001 vs. the control rats.
p < 0.001 vs. the CdCl2-treated rats analyzed using Tukey’s test.
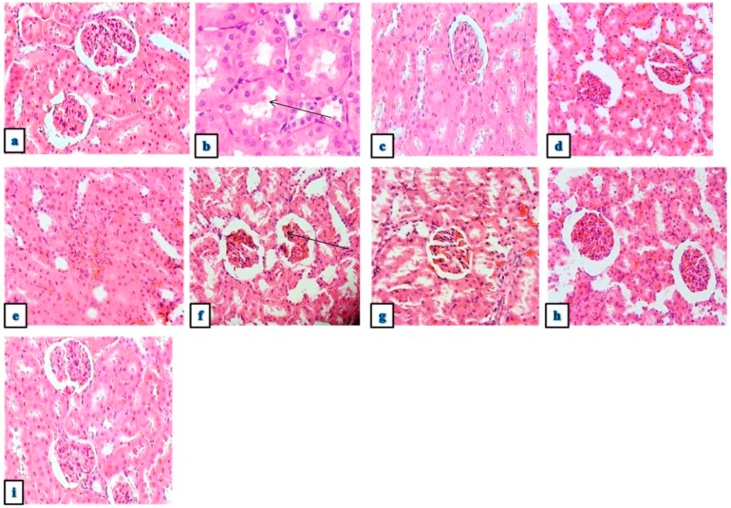
Photomicrographs of the kidney histology (Fig. 2a-i) showed that the rats pre-treated with ethyl acetate and hexane fraction showed signs of necrosis and inflammations similar to the Cd-only treated group while rats pre-treated with aqueous, butanol fractions and Me70 showed no signs of pathology.
Fig. 2.
a-i. Photomicrographs of the kidney sections obtained from rats (a) Control group showed normal cellular structure without signs of damage (b) Cd-only treated rats showed atrophied glomeruli with degenerated tubules. (c,d) Pre-treatment with chloroform and butanol showed slight improvements in the tubules, however, widened urinary spaces were noticed. (e,f) Pre-treatment with Ethyl acetate and Hexane showed signs of necrosis and cellular damage (g) Pre-treatment with Aqueous fraction showed no pathology (h,i) Pre-treatment with Me70 and Me70 only groups markedly attenuated all renal damages caused by cadmium, using Hematoxylin and eosin (H&E), 400× magnification.
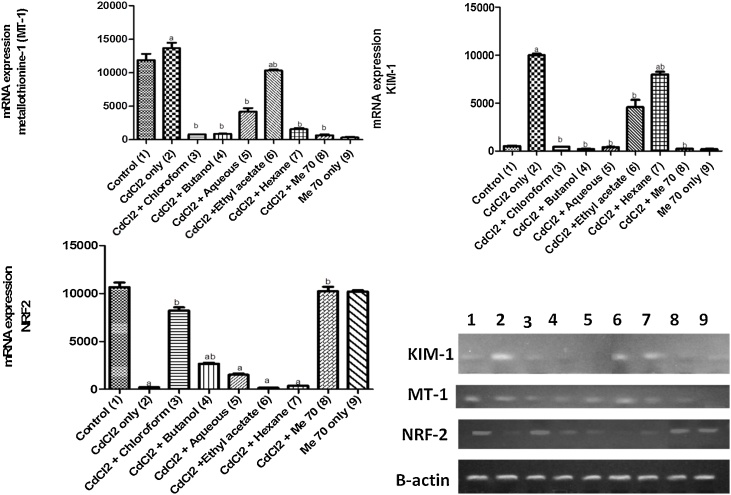
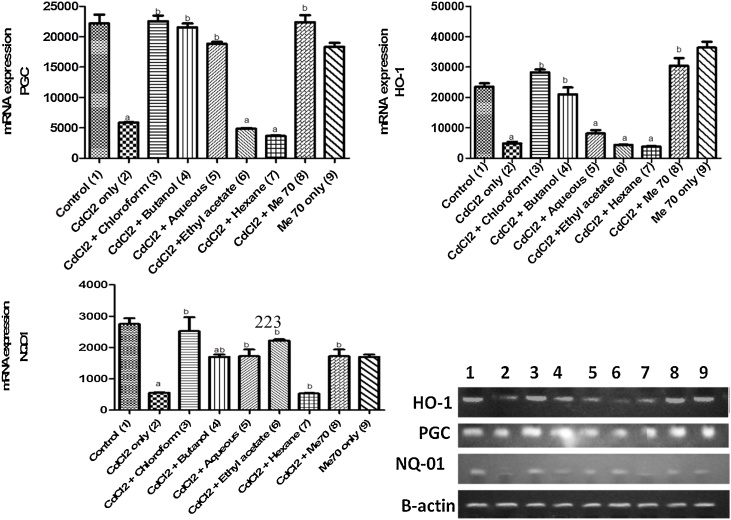
In the RT-PCR study (Fig. 3a-c), metallothionine-1 (MT-1) and KIM-1 mRNA expression was up-regulated while NRF-2, HO-1, NQ-O1 and PGC-1α mRNA (Fig. 4a-c) were down-regulated in the Cd-only exposed group when compared with the control group. The group pre-treated with hexane fraction mostly showed similar results as the Cd-only group. However, there was a significant down-regulation in MT-1 and KIM-1 mRNA as well as an up-regulation in the expression of the antioxidant regulatory element (NRF-2), its downstream genes (HO-1, NQ-O1) and co-factor (PGC-1α) in the rats pre-treated with Me70, butanol and aqueous fractions respectively, when compared with the Cd-only group.
Fig. 3.
a, b and c. Effect of Me70 and other solvent fractions on MT-1, KIM-1 and NRF2 mRNA expressions in Cd-treated rats.
Fig. 4.
a, b and c. Effect of Me70 and fractions on PGC, HO-1, and NQO1 mRNA expressions in Cd- treated rats.
4. Discussion
Nowadays, plant foods and beverages are receiving more scientific attention because of their potential to curb the effect of free radicals in human systems. The data obtained from a pilot study on the phytochemical screening of Geophila obvallata leaves formed the basis for further investigations as it revealed a significantly higher abundance of flavonoids, phenols, saponins, and alkaloids in the methanol crude extract (Me70) [11] as well as in the butanol and aqueous fractions of Me70 when compared to other extracts and fractions. The study also showed that the plant extract possesses powerful radical scavenging and neutralizing bioactive compounds [11]. It has also been reported that persons with mineral deficits (e.g. iron deficiency) as well as menstruating women show a 6% increase in the re-sorption of cadmium than control groups [26,27]. In this study, only male Wistar rats were used to eliminate the physiological bias of one gender on our investigation.
The toxic effect of Cd on the kidney function of Wistar rats was determined by measuring the renal function indices (blood urea nitrogen and creatinine concentration) only. The results showed that the rats that received 50 mg/kg bwt of Me70 and 0.3 mg Cd/L simultaneously had the best reversal in damaging effects of Cd burden evidenced by a decrease in kidney function indices in the serum, similar to the control after 28 days. This study clearly showed that the Me70 was capable of reversing the toxic effect of 0.3 mg Cd/L in drinking water at 50 mg/kg bwt.
The organ and final body weight are important physiological indices used to determine whether the animal is susceptible to the toxic effects of cadmium [16]. In this study, the final body weights of rats that received only cadmium at a dose of 0.3 mg Cd/L significantly decreased even though the kidney weights remained the same. According to Eriyamremu et al. [16], cadmium toxicity disturbs the enzymatic breakdown, absorption and utilization of macromolecules resulting in weight losses in Cd exposed rats. However, rats pre-treated with Me70 and its subsequent fractions showed weights similar to the control probably due to the bioactive principles inherent in the extract as well as increased consumption rate of feeds within the duration of the study. This finding agrees with that of Olayeriju et al. [9] who reported that increased feed conversion rate can be linked with the presence of saponins (aglycone sapogenin) in the extract which may have stimulatory effects on feeding centres in the brain of experimental rats.
Schnellman and Kelly [28] reported that cadmium induction can cause negative alterations in electrolyte homeostasis as well as the activity of Na+/K+- ATPase enzyme. Other reports by Giebisch [29] and Ellison [30] revealed that cadmium exposure, results in disproportionate changes in electrolyte balance of the kidney. This may be due to permeabilizations in the glomerular and proximal tubular cells as seen in the kidney histology photomicrographs (Fig. 2a–i). Our data revealed a significant increase in the serum K+ of rats pre-treated with cadmium alone, chloroform and hexane fractions respectively, while serum HCO3−, Na+and Cl− levels were significantly decreased. This data corresponds with the findings of Hussain [31], who reported that cadmium exposure can cause severe hyperkalemia, hyponatremia and hypochloremia as a result of its ability to compromise membrane integrity via releasing unstable ROS. Also, fluid leakages in the interstitum and tubules can be linked to electrolyte homeostatic imbalance caused by cadmium intoxication [32]. Alternately, rats pre-treated with Me70 showed results similar to the control group, indicating that dominant bioactive principles found in this extract can scavenge ROS, and restore electrolyte equilibrium in experimental rats.
The renal tissues are majorly involved in the elimination of systemic Cd as well as the most sensitive organs to cadmium toxicity [26]. The renal tubular cells and proximal tubules have a higher tendency to accumulate cadmium resulting in a slower excretion rate consequently enhancing its nephrotoxic effects. Its presence in major tissues in small quantities over a period can lead to organ failure and a compromised biological system. In this study, elevations in cadmium concentration were seen in the kidney tissues of rats given only cadmium when compared with the control. The accumulation of cadmium in the kidney was reported to be mainly because renal tissues contain most of the metallothionein which binds cadmium thereby prolonging its half-life resulting in proximal tubular dysfunctions [17,33].
Cadmium once absorbed, has the ability to conjugate with cellular and antioxidant proteins via their SH groups thus inactivating them. The disproportionate balance between antioxidants and ROS proliferation promotes lipid peroxidation and oxidative stress. Previous reports have confirmed that alterations in GSH, CAT, SOD and GPx parameters are an indication of unstable oxygen and nitrogen radicals in major organs due to cadmium overload [34]. A decrease in kidney antioxidants (GSH, GST, CAT, SOD and GPx) alongside a concomitant elevation in lipid peroxidation was observed in the Cd-only and Cd + hexane group, due to cadmium intoxication. The result is the same as reports of other researchers who observed comparable pathological effects on organs of animals treated with cadmium [35]. Cadmium intoxication in target tissues provoked the mass production of unstable radicals (ROS and RNS) which were capable of overwhelming the antioxidant defences of the animal, resulting in functional and structural damages in the cell membrane. Furthermore, rats pre-treated with butanol fraction and Me70 conferred a protective effect on cadmium treated rats. More so, it has been documented that Me70 possesses phytonutrient potentials with ROS quenching properties [11]. Therefore, it’s proposed that the ameliorative effect of Me70 against cadmium induced organ injury is due to its antioxidant qualities and promotion of cellular antioxidant defences.
The transcriptional co-factor (Nrf-2) mediates the anti-oxidant potential of cells and can be triggered by injury or inflammation. It promotes the expression of antioxidant response elements whose downstream gene products are involved in detoxification of ROS via conjugation. It is co-regulated by PGC-1α which works in tandem to co-ordinate cellular protection. Their combined action promotes ROS detoxification by the initiation and transcription of downstream antioxidant gene elements; HO-1 and NQ-O1 in the antioxidant response element signaling pathway [36]. Cadmium is a confirmed nephrotoxicant directly linked with kidney injury in small doses. Consistent with the biochemical findings in this study, RT-PCR analysis showed a down-regulation in the antioxidant response genes studied in the rats that received cadmium alone compared to other groups. This was similar to the reports by Liu et al. [37] that cadmium can significantly inhibit the expression of Nrf-2 and PGC-1α’s genes in the kidney tubules of cadmium treated rats. Its presence in renal tissues led to the down-regulation of antioxidant genes and their downstream protein elements. However, the rats pre-treated with Me70 were able to attenuate the negative effects of Cd-toxicity possibly by inhibiting the repressor protein (Keap-1) that blocks the expression of antioxidants as well as up-regulating HO-1 and NQ-01 genes. The results of this study are similar to the findings of Rafa et al. [36] who reported that pre-treatment with royal jelly attenuated cadmium induced nephrotoxicity in rats.
The presence of Cd in renal tissues has been reported to induce metallothionine production. This is because the renal metal binding protein is cysteine rich (30 %) with the ability to bind divalent metal ions such as cadmium in its thiol groups [27]. This may be responsible for the up-regulation in metallothionine mRNA expression observed in the group treated with cadmium only. However, rats pre-treated with fractions of Me70 showed results similar to the control.
KIM-1 is a trans-membrane protein receptor mostly expressed during acute kidney injury. Patients with acute renal injury usually express elevated KIM-1 mRNA levels compared with normal patients [38]. The early preliminary biomarker for acute and chronic renal injury, KIM-1, was up-regulated in cadmium exposed rats used in this study. This effect was negated by pre-treatment with Me70 which significantly down-regulated the production of KIM-1 mRNA. This is similar to previous findings by Zhang et al. [38]. It should be noted that the similar trends seen in some of the fractions (Cd + hexane; Cd + chloroform groups) in relation to the Cd-only group may be attributed to the presence of metabolites with a weaker protection against Cd toxicity, being more pronounced in these fractions at that dose. However, their negative effects are greatly repressed when the plant is consumed as a whole (Me70) extract, giving credence to their traditional use.
5. Conclusion
The data showed that Geophila obvallata methanol extract (Me70) with its butanol fraction possesses bioactive principles that are potent for renal protection against Cd-induced nephrotoxicity. However further studies are required to determine the mechanisms responsible for its therapeutic effect.
Data availability
Data will be made available on request.
Authors’ contributions
IL and ON participated in the conception, design of the study, data analysis and drafted the manuscript. IL carried out the experiments and data analysis. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors wish to thank the Department of Bioinformatics and Molecular Biology where the research was conducted.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.Kunwar A., Priyadarsini I. Free radicals, oxidative stress and importance of antioxidants in human health. J. Med. All. Sci. 2011;1:53–60. [Google Scholar]
- 2.EPA/600/R-12/618 . US EPA Office of Research and Development; Washington, DC: 2012. Bastian, R. AND Dan Murray. Guidelines for Water Reuse. [Google Scholar]
- 3.World Health Organization (WHO) Cadmium International Programme on Chemical Safety; 2011. Environmental Health Criteria. [Google Scholar]
- 4.Egborge A.B.M. 1994. Handbook on Water Pollution in Nigeria Biodiversity and Chemistry in Warri River. [Google Scholar]
- 5.Adaikpoh M., Obi F. Prevention of cadmium-induced alteration in rat testes and prostate lipid patterns by α-tocopherol. Afr. J. Biol. Res. 2009;3:321–325. doi: 10.5897/AJBR.9000001. [DOI] [Google Scholar]
- 6.Cuypers A., Ambily R.N., Olivier D., Karen S., Emmy Van K. Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int. J. Mol. Sci. 2013;14:6116–6143. doi: 10.3390/ijms14036116. 10.3390%2Fijms14036116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannino G., Ferruggia E., Luparello C., Rinaldi A. Cadmium and mitochondria. Mitochondria. 2009;9:377–384. doi: 10.1016/j.mito.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Mishra S., Pani S.R., Sahoo S. Anti-nephrotoxic activity of some medicinal plants from tribal rich pockets of Odisha. Pharm. Res. 2014;6(3):210–217. doi: 10.4103/0974-8490.132598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olayeriju O., Crown O., Elekofehinti O., Akinmoladun A., Olaleye M., Akindahunsi A. Effect of moonseed vine (Triclisia gilletii Staner) on ethane-1, 2-diol-induced urolithiasis and its reno-toxicity in Wistar albino rats. Afr. J. Urol. 2020;26:4–10. 1186/s12301-020-0018—x. [Google Scholar]
- 10.Burkil H.M. 2nd ed. Royal Botanic Gardens; Kew: 1985. The Useful Plants of West Tropical Africa; pp. 504–505.http://plants.jstor.org/upwta/2_580 [Google Scholar]
- 11.Iserhienrhien L.O., Okolie N.P. Phytochemical screening and in vitro antioxidant properties of methanol and aqueous leaf extracts of Geophila obvallata. Asian J. Res. Biol. 2018;3(2):1–11. doi: 10.9734/AJRB/2018/45052. [DOI] [Google Scholar]
- 12.Iserhienrhien L.O., Okolie N.P. Acute and sub-acute toxicity profile of methanol leaf extract of Geophila obvallata on renal and hepatic indices in Wistar rats. Cog. Food Agric. 2020;6:1–13. doi: 10.1080/23311932.2020.1794240. [DOI] [Google Scholar]
- 13.Asagba S., Obi F. Effects of oral cadmium exposure on renal glomerular and tubular functions in the rat. J. Appl. Sci. Environ. Manag. 2004;8(1):29–32. doi: 10.4314/jasem.v8i1.17222. [DOI] [Google Scholar]
- 14.Weli A.F. Biological and phytochemical studies of different leaves extracts of Pteropyrum scoparium. Beni-Suef Univ. J. Bas. Appl. Sci. 2018;7(4):481–486. doi: 10.1155/2019/2403718. [DOI] [Google Scholar]
- 15.Ward J.W., Elsea J.R. Methods and Techniques. Markel Dekker; New York: 1997. Animal case and use in drug fate and metabolism; pp. 134–156. [Google Scholar]
- 16.Eriyamremu G.E., Asagba S.O., Onyeneke E.C., Adaikpo M.A. Changes in carboxypeptidase A, dipeptidase and Na+/K+ ATPase activities in the intestine of rats orally exposed to different doses of cadmium. Bio Metals. 2005;18:1–6. doi: 10.5897/AJBR2016.0893. [DOI] [PubMed] [Google Scholar]
- 17.Massanyi P., Tataruch F., Slamecka J., Toman R., Jurcik R. Accumulation of lead, cadmium, and mercury in liver and kidney of the brown hare (Lepus europaeus) parameters of albino rats. J. Toxicol. Environ. Health Sci. 2003;2(2):11–16. doi: 10.1081/ESE-120021127. [DOI] [PubMed] [Google Scholar]
- 18.Fawcett J.K., Scott J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. 10.1136%2Fjcp.13.2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry J.B., Todd S., David S. 16th ed. Vol. 260. W.B. Saunders and Co.; Philadelphia PA: 1974. pp. 1–17. (Clinical Diagnosis and Measurement by Laboratory Methods). [Google Scholar]
- 20.Aebi H.E., et al. In: Methods of Enzymatic Analysis. 3rd edition. Bergmeyer H.U., editor. Academic Press; New York: 1984. Catalase; pp. 673–684. [Google Scholar]
- 21.Nishikimi M., Rao N.A., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 22.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70(1):158–169. PMID: 6066618. [PubMed] [Google Scholar]
- 23.Spanidis, et al. Resistance-trained individuals are less susceptible to oxidative damage after eccentric exercise. Oxid. Med. Cell. Longev. 2018;11(1):14–23. doi: 10.1155/2018/6857190. Article ID 6857190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drury R.A.B., Wallington E.A. 5th ed. Oxford University Press; Oxford: 1980. Carleton’s Histological Technique. [Google Scholar]
- 25.Freeman Sf, Martin P., Munoz Js. Hepatology, a Textbook of Liver Disease. 1st ed. Saunders publication; Philedelphia: 1999. Laboratory evaluation of the patient with liver disease; pp. 17–21.10.1007%2Fs12291-013-0310-7 [Google Scholar]
- 26.Freiberg J.J., Tybjaerg-Hansen A., Jensen J.S., Nordestgaard B.G. Nonfasting triglycerides and risk of ischemic stroke in the general population. J. Am. Med. Ass. 1983;300:2142–2152. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 27.Nordberg G.F., Nordberg B.A., Friberg L.M. Handbook on the toxicology of metals. Rep. Dev. Tox. Met. 2007;1:213–249. [Google Scholar]
- 28.Schnellmann R.G., Kelly K.J. Blackwell publishers; Colorado: 2008. Atlas of Kidney Diseases; pp. 241–251. [Google Scholar]
- 29.Giebisch G., Wang T. Effects of Angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am. J. Phys. 1996;39:211–219. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 30.Ellison D.H. Blackwell publishing; Colorado: 2008. Atlas of Kidney Diseases. [Google Scholar]
- 31.Hussain M.A., Piyatida P., da Silva J.A.T., Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2002;2:10–18. doi: 10.1155/2012/872875. [DOI] [Google Scholar]
- 32.Tabassum B., Priya B. Rejuvenation of cadmium induced electrolyte imbalance by Mentha piperita. Int. J. Sci. Res. Publ. 2013;3(5):1–13. [Google Scholar]
- 33.Hollis L., Hogstrand C., Wood C.M. Tissue specific cadmium accumulation, metallothionein induction, and tissue zinc and copper levels during chronic sublethal in relation to the season, age, and sex in the West Slovakian Lowland. J. Environ. Sci. Health. 2001;9:1299–1309. doi: 10.1007/s002440010273. 10.14202%2Fvetworld.2015.537-540 [DOI] [PubMed] [Google Scholar]
- 34.Ognjanovic B.I., Markovic S.D., Etherdevic N.Z., Trbojevic I.S., Stajn A.S., Saicic Z.S. Cadmium induced lipid peroxidation and changes in antioxidant defense system in the rat testes. Reprod. Toxicol. 2010;29:191–197. doi: 10.1016/j.reprotox.2009.11.009. 10.1177%2F0960327112472995 [DOI] [PubMed] [Google Scholar]
- 35.Renugadevi J., Prabu S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010;62:171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Rafa S., Almeer G.I., AlBasher S., SaadAlkahtani D., Ahmed A.E. Royal jelly attenuates cadmium induced nephrotoxicity in male mice. Sci. Rep. 2019;9(5):2800–2819. doi: 10.1038/s41598-019-42368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Liu J., Iszard M.B., Andrews G.K., Palmiter R.D., Klaassen C.D. Transgenic mice that overexpress metallothionein-I are protected from cadmium lethality and hepatotoxicity. Toxicol. Appl. 2019;194:19–33. doi: 10.1006/taap.1995.1227. 10.1016%2Fj.taap.2009.03.026 [DOI] [PubMed] [Google Scholar]
- 38.Zhang H., Cao X., Liu Y. Rapid recovery of high content phytosterols from corn silk. Chem. Cen. J. 2017;11:108–118. doi: 10.1186/s13065-017-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.