Abstract
β-Aminoisobutyric acid (BAIBA) is a myokine that is secreted from skeletal muscles by the exercise. Recently, increasing evidence has suggested the multifocal physiological activities of BAIBA. In this study, we investigated whether L-BAIBA has protective effects on rat pheochromocytoma (PC12) cells. Cultured PC12 cells were stimulated with L-BAIBA. Western blot analyses revealed that L-BAIBA stimulation significantly increased the phosphorylation of AMPK and Akt. In contrast, no effect was observed on neurite outgrowth by L-BAIBA. To investigate the effects of L-BAIBA on oxidative stress, PC 12 cells were exposed to hydrogen peroxide (H2O2) with and without L-BAIBA. Hydrogen peroxide significantly increased reactive oxygen species (ROS) production and apoptosis in PC12 cells. Pretreatment with L-BAIBA suppressed H2O2-induced ROS production and apoptosis, which was abolished by the inhibition of AMPK by compound C. On the other hand, the inhibitory effects of L-BAIBA on oxidative stress-induced apoptosis were abolished by the inhibition of both AMPK and PI3K/Akt. In conclusion, we demonstrated that L-BAIBA confers protection against oxidative stress in PC12 cells by activating the AMPK and PI3K/Akt pathways. These results suggest that L-BAIBA may play a crucial role on protection of neuron-like cells and become a pharmacological agent to treat neuronal diseases.
Abbreviations: BAIBA, β-Aminoisobutyric acid; DMEM, Dulbecco’s modified Eagle’s medium; FITC, fluorescein isothiocyanate; FBS, fetal bovine serum; GPCR, G protein-coupled receptor; MRGPRD, Mas-related G protein-coupled receptor type D; PPARγ, peroxisome proliferator-activated receptor gamma; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1-alpha; ROS, reactive oxygen species; TUNEL, TdT-mediated dUTP nick-end labeling
Keywords: β-Aminoisobutyric acid (BAIBA), Neuron, Oxidative stress, Hydrogen peroxide, AMPK, PI3K/Akt
1. Introduction
Exercise is known to have beneficial effects on cognitive disease and neurodegenerative disease, such as Alzheimer disease and Parkinson’s disease (Ahlskog, 2011, Ellis and Rochester, 2018, Mattson, 2012, Valenzuela et al., 2020). Various mechanisms of the beneficial effects by exercise in neuronal diseases are considered such as direct exercise effects, indirect exercise effects, improvement of mitochondrial function and release of circulating factors from skeletal muscles (Burtscher et al., 2021).
Skeletal muscles produce and secrete cytokines and other peptides that are classified as myokines (Pedersen, 2011). β-Aminoisobutyric acid (BAIBA) is a myokine and its production increases during exercise (Pedersen, 2011). BAIBA is produced by the catabolism of thymine and is associated with exercise-induced activation of peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1-alpha (PGC-1α) (Roberts et al., 2014). Recently, increasing evidences have suggested that BAIBA has multifocal physiological activities, such as browning of white fat and the upregulation in hepatic β-oxidation via PPARα (Roberts et al., 2014). In another study of high-fat diet-induced obesity, BAIBA suppressed the gain of body fat, steatosis and inflammation, glucose intolerance, and hypertriglyceridemia (Begriche et al., 2008). Hepatic endoplasmic reticulum stress and apoptosis were suppressed by the effect of BAIBA in mice with type 2 diabetes, which was induced by a combination of streptozotocin and high-fat diet (Shi et al., 2016). BAIBA decreased insulin resistance and ameliorated the free fatty acid oxidation enzyme expression in the muscle cells through AMPK and PPARδ activation (Jung et al., 2015). Furthermore, BAIBA markedly abolished Angiotensin II-stimulated ROS accumulation and repaired renal fibrosis in a mouse model of obstructed kidneys (Wang et al., 2017). While these studies have demonstrated the multifocal effects of BAIBA in various cell types, its role in neurons remains unclear.
There are two enantiomers of BAIBA because it has a chiral center: D-BAIBA and L-BAIBA. (Vemula et al., 2017) In human, exercise induced a 13% and 20% increase in D-BAIBA and L-BAIBA from baseline respectively (Stautemas et al., 2019). The L-isoform was the greater potency compared to the D-isoform on osteocyte viability (Kitase et al., 2018). In this study, we use L-BAIBA for our studies.
The key challenge addressed in this study was to understand whether L-BAIBA has protective effects on neuronal cells. Oxidative stress resulting from excessive reactive oxygen species (ROS) production is a critical mediator of neuronal diseases, such as Alzheimer’s disease, Parkinson’s disease, and cognitive impairment (Butterfield and Halliwell, 2019, Fernandez et al., 2019, Hemmati-Dinarvand et al., 2019). Herein, we demonstrate that L-BAIBA confers neuroprotection against oxidative stress via activation of the AMPK and PI3K /Akt pathways.
2. Experimental procedures
2.1. PC12 culture
Rat pheochromocytoma (PC12) cells were purchased from American Type Culture Collection (ATCC Manassas, VA, USA). The PC12 cells were cultured in RPMI1640 (Gibco, Gaithersburg, MD) supplemented with 5% fetal bovine serum (FBS; Gibco), 10% horse serum (Gibco), and 25 U / ml penicillin/streptomycin. The cells were incubated in a humidified incubator in 5% CO2 at 37 °C.
2.2. Western blot analysis
PC12 cells were stimulated with L-BAIBA (100 µM; Sigma, St Louis, MO) at the indicated time points. The cells were then lysed, and total proteins were extracted using a lysis buffer. Equal amounts of proteins were subjected to SDS-PAGE (10–12% gel). We performed western blotting with antibodies against Akt, phospho-Akt (Ser 473), AMPK, and phospho-AMPK (Thr 172). The antibodies were obtained from Cell Signaling Technology (Beverly, MA). Immunoblotting was performed and the target proteins were detected using ECL Plus western blotting substrate kit (Thermo Scientific, MA).
2.3. Neurite outgrowth
PC12 cells were plated (density, 5 ×10⁴ cells/cm2) in culture dishes coated with type IV collagen (Cellmatrix ®, Nitta Gelatin, Osaka, Japan). After culturing the cells for 24 h, the media was carefully replaced with Dulbecco’s modified Eagle’s medium (DMEM; Wako, Osaka, Japan) containing L-BAIBA (100 µM) supplemented with 1% FBS. The positive control cells were treated with 50 ng / ml nerve growth factor (NGF; Sigma). After 6 days, PC12 cells were fixed with 10% formalin and were incubated with primary antibodies, anti-neurofilament (Sigma). Next, PC12 cells were incubated with goat anti-Mouse IgG (H+L), Alexa Fluor Plus 488 (Thermo Scientific). Neurite outgrowth was assessed using a Keyence fluorescence microscope (B2-X710; Osaka, Japan). Neurite outgrowth was measured as the total length of neurites, calculated using ImageJ plug-in NeuronJ.
2.4. Measurement of ROS levels
PC12 cells were plated at a density of 1 × 104cell / cm2 on type IV collagen-coated glass bottom dishes. After 48 h of incubation, the culture medium was replaced with DMEM supplemented with 1% FBS. L-BAIBA (100 μM) was added and the cells were incubated for 1 h. After pretreatment, the PC12 cells were exposed to hydrogen peroxide (H2O2, 300 µM) for another 1 h. The cells were then treated with CellROX Green reagent (Molecular probe, Eugene, OR) at a final concentration of 5 μM for 30 min and images were obtained using a Keyence fluorescence microscope (B2-X710; Osaka, Japan). The signals generated due to ROS production were quantified using ImageJ software. The fluorescent signal of ROS was normalized to the total number of cells (Kitase et al., 2018).
2.5. Cell viability
PC12 cells were incubated in DMEM containing 1% FBS and 100 µM L-BAIBA for 1 h, followed by stimulation with 300 µM H2O2. After 12 h, the trypan blue staining was used to detect dead cells. Cell viability was expressed as the ratio of live cells per total cells.
2.6. Annexin V–fluorescein isothiocyanate (FITC) assay and TdT-mediated dUTP nick-end labeling (TUNEL) assay
The apoptotic ratio was analyzed using the MEBCYTO Apoptosis Kit (MBL, Aichi, Japan), according to the manufacturer's protocol. Briefly, PC12 cells were incubated in culture medium containing inhibitors; LY294002 (Sigma-Aldrich, Darmstadt, Germany)-a PI3K inhibitor, compound C (Sigma-Aldrich)-an AMPK inhibitor, and MU6840 (Key Organics, Camelford, UK)-a receptor agonist for Mas-related G protein-coupled receptor type D (MRGPRD), for 30 min, followed by stimulation with L-BAIBA (100 µM) before exposure to 300 µM H2O2. After 12 h, the cells were washed and stained with annexin V–FITC and Hoechst 33342.
TUNEL assay was performed using the MEBSTAIN Apoptosis TUNEL Kit Direct (MBL, Aichi, Japan). PC12 cells were fixed with 4% formalin neutral-buffered solution for 1 h at 37 ℃ and incubated with TdT reaction solution for labeling of the 3′-terminal of cellular DNA. The cells were then labeled with fluorescein-dUTP. Images were obtained using a fluorescence microscope (Keyence).
2.7. Animal
Male Sprague–Dawley (SD) rat was obtained from Chubu Kagakushizai (Nagoya, Japan) at 6 weeks of age. This study was approved by the Institutional Animal Care and Use Committees of Aichi Gakuin University (AGUD 318), and the experiment was carried out following national guidelines and the relevant national laws on the protection of animals.
2.8. mRNA expression analyses
Rat was killed by CO2 inhalation. Dorsal rood ganglion (DRG) were removed from SD rat (n = 1) and immersed in an RNAlater ribonucleic acid stabilization reagent (QIAGEN, Hilden, Germany). RNA of PC12 cell and DRG was extracted by a RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. The cDNA was synthesized using ReverTra Ace (Toyobo, Osaka, Japan). TaqMan Gene Expression Assay primers and probes for Mrgprd were purchased from Thermo Scientific. Real-time quantitative PCR was performed and monitored by a LightCyclerR 480 Instrument II (Roche, Basel, Switzerland). The relative quantity was calculated by the ΔΔCt method using β2 microglobulin as the endogenous control. PCR products were observed by agarose gel (Wako, Osaka, Japan) electrophoresis with ethidium bromide (Sigma) staining.
2.9. Analyses of intracellular signaling pathway involved in the protective effects of L-BAIBA
To clarify the signaling pathway, PC12 cells were pretreated with one of the following inhibitors for 30 min before L-BAIBA treatment: LY294002, compound C, and MU6840. The control cells were treated with vehicle control/saline.
2.10. Statistical analysis
Statistical analysis was performed using GraphPad Prism Software (version 4.0; GraphPad Software Inc., San Diego, CA, USA). Student’s t-test or two-way analysis of variance was used in the statistical analysis. The results are expressed as mean ± standard error (SE).
3. Results
3.1. L-BAIBA increases the phosphorylation of AMPK and Akt in PC12 cells
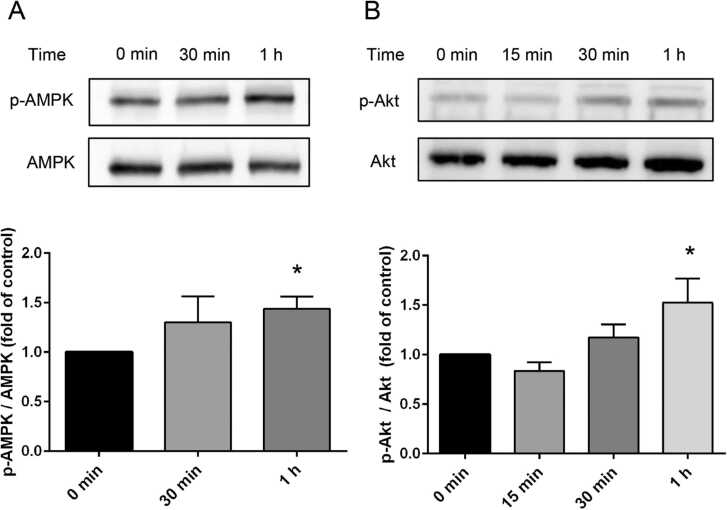
We investigated the effects of L-BAIBA on the activation of AMPK and Akt in PC12 cells. AMPK and Akt phosphorylation was significantly increased by L-BAIBA. One hour after L-BAIBA stimulation, the phosphorylation of AMPK and Akt was significantly increased by 1.4-fold and 1.5-fold, respectively (Fig. 1A, B) (p < 0.05).
Fig. 1.
L-BAIBA augments (A) p-AMPK and (B) p-Akt expression. Western blot analysis of p-AMPK, AMPK, p-Akt, and Akt protein expression levels. Time course of changes in Akt and AMPK phosphorylation after treatment with 100 µM L-BAIBA. The intensities of the protein bands were quantified and normalized to that of the control (baseline). * p < 0.05, versus control. Data are presented as the mean ± SE values. n = 4–5.
3.2. L-BAIBA has no effects on neurite outgrowth
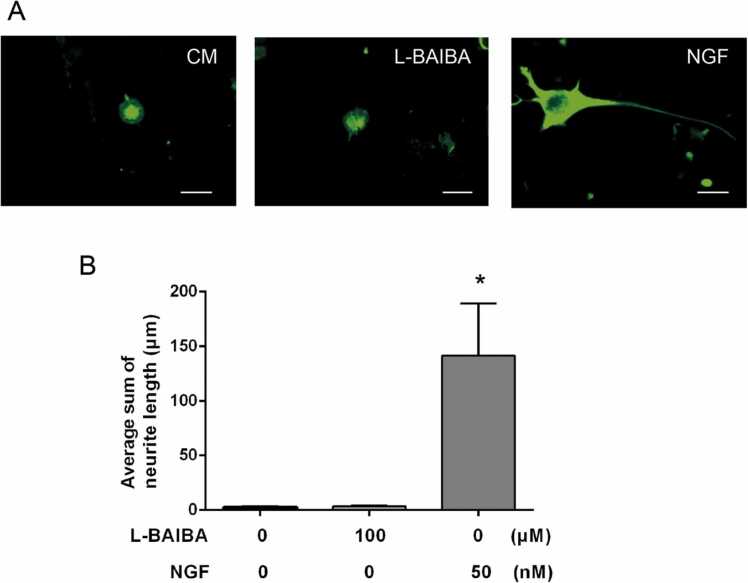
We investigated whether L-BAIBA affects neurite outgrowth in PC12 cells (Fig. 2A). Neurite outgrowth was evaluated based on the total length of neurites (Fig. 2B). NGF, which was used as a positive control, significantly increased neurite length in PC12 cells as compared to the control (p < 0.001). On the other hand, L-BAIBA, at a concentration of 100 μM, did not affect the neurite length of PC12 cells.
Fig. 2.
The effects of L-BAIBAon neurite outgrowth in PC 12 cells. Neurite outgrowth was visualized by immunohistochemical staining of neurofilament. (A) Representative images of PC12 cells after treatment with L-BAIBA (100 µM) or NGF (50 nM; control) were shown. The PC12 cells were incubated for 6 days in a control medium with L-BAIBA or NGF. (B) Quantification of neurite outgrowth in PC12 cells after incubation with L-BAIBA or NGF for 6 days. Ten cells were measured in several fields selected randomly from each culture dish. * p < 0.05, versus control group. Data are presented as the mean ± SE values. Scale bar = 20 µm.
3.3. L-BAIBA prevents oxidative stress-induced ROS production and apoptosis in PC12 cells
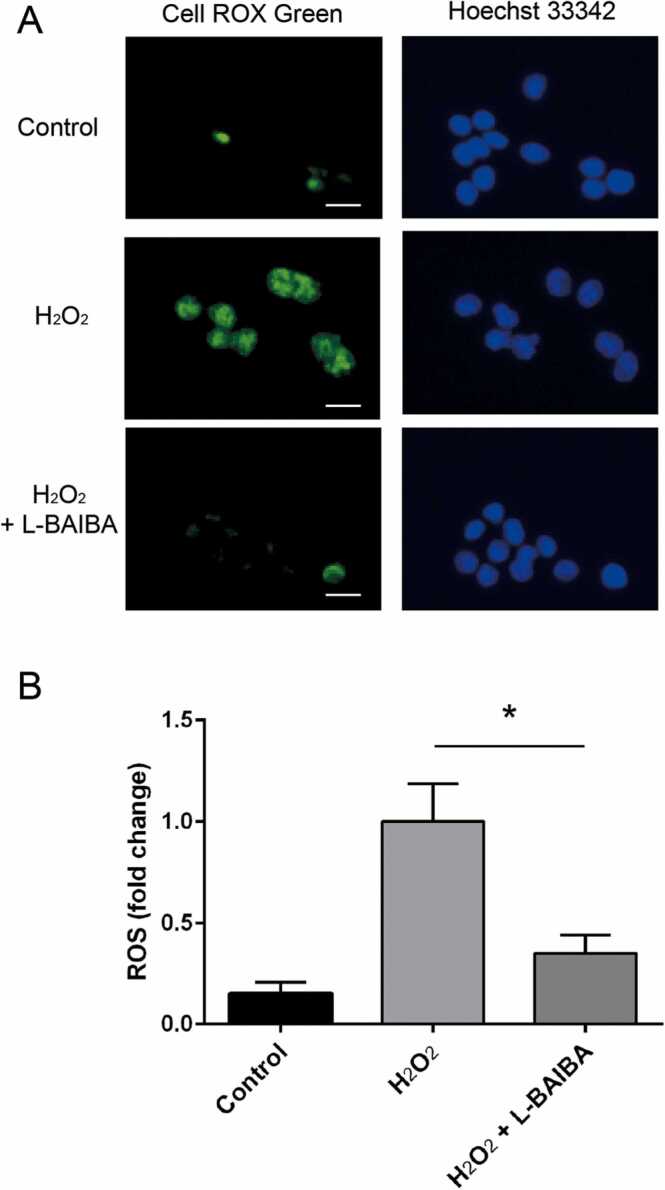
To investigate the protective effects of L-BAIBA against oxidative stress, we induced oxidative stress in PC12 cells with H2O2. ROS production was visualized by green fluorescence using the CellROX Green reagent (Fig. 3A). As shown in Fig. 3B, ROS generation significantly increased by 6.6-fold after H₂O₂ treatment compared with the control (p < 0.05). L-BAIBA significantly prevented H2O2-induced ROS production by 60% (p < 0.05) (Fig. 3B).
Fig. 3.
L-BAIBA decreased H2O2 induced-ROS production. (A) ROS levels were detected using the CellROX reagent and analyzed by fluorescence microscopy. PC12 cells were treated with L-BAIBA (100 μM) for 30 min, followed by the addition of H2O2 (300 µM). Next, the cells were loaded with CellROX and a nuclear dye, Hoechst-33342, and incubated in a control medium for 30 min (B) Quantification of signal intensity normalized by the total number of cells using ImageJ software. * p < 0.05. Data are shown as mean ± SE. n = 6.
3.4. L-BAIBA suppresses oxidative stress-induced apoptosis and cell death in PC12 cells
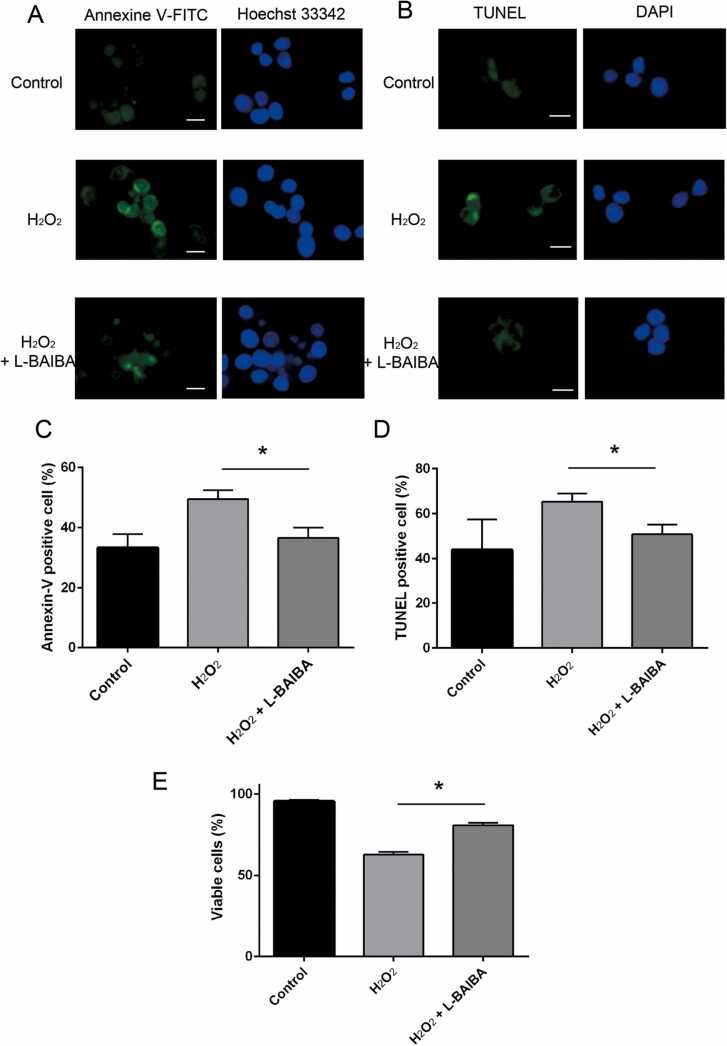
Apoptosis was detected using Annexin V (Fig. 4A) and TUNEL assays (Fig. 4B)·H2O2 treatment increased apoptosis in the Annexin V (Fig. 4C) and TUNEL assays (Fig. 4D) by 16% and 23%, respectively compared with the control; however, L-BAIBA significantly suppressed H2O2-induced apoptosis in PC12 cells by 80% and 58%, respectively, in Annexin V and TUNEL assays, demonstrating the protective effects of L-BAIBA against oxidative stress (p < 0.05).
Fig. 4.
L-BAIBA decreased H2O2-induced apoptosis. PC12 cells were treated with 100 μM L-BAIBA for 30 min, followed by stimulation with 100 µM H2O2 for 12 h. (A) PC12 cells were treated with Annexin V–FITC (green) and Hoechst 33342 (blue) for 15 min (B) Apoptosis of PC12 cells was detected by the TUNEL assay. (C) Quantification of Annexin V–FITC-positive cells normalized by the total number of cells. n = 10–12. (D) Quantification of TUNEL-positive cells normalized to the total number of cells. n = 10–11. (E) PC12 cells were incubated in DMEM containing 1% FBS and 100 µM BAIBA for 1 h, followed by stimulation with 300 µM H2O2. After 12 h, the trypan blue staining was used to detect dead cells. Cell viability was expressed as the ratio of live cells per total cells. *p < 0.05. Data are shown as mean ± SE.
The percentage of cell viability was presented in Fig. 4E. Cell viability significantly decreased after H₂O₂ treatment by 32%. BAIBA significantly prevented H2O2-induced cell death by 55%.
3.5. Gene expression of MRGPRD in PC12 cells
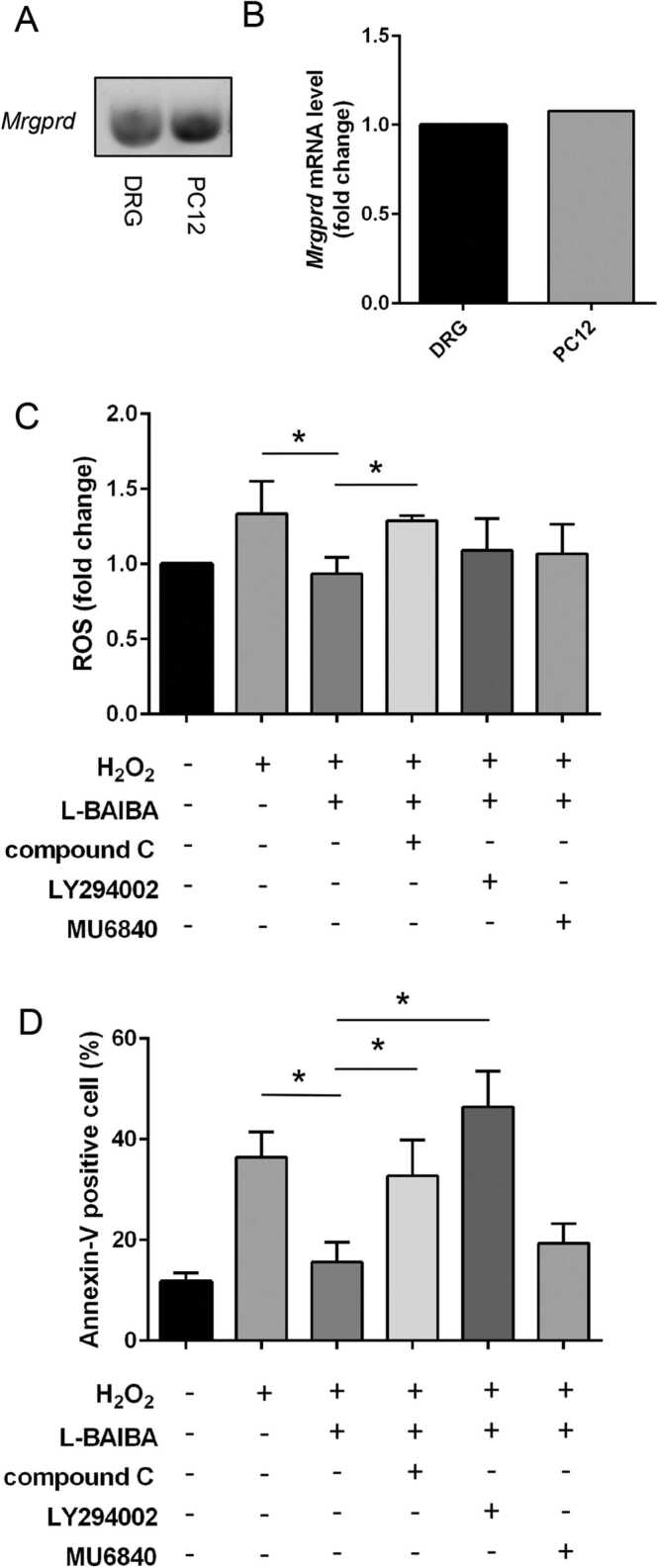
To investigate whether PC12 cells express MRGPRD, a receptor of L-BAIBA, we analyzed gene expressions of MRGPRD in PC12 cells and DRG. As shown in Fig. 5A and B, both PC12 cells and DRG expressed MRGPRD gene.
Fig. 5.
Involvement of AMPK and PI3K/Akt pathway on the anti-oxidative effects of L-BAIBA. (A) Gene expression of MRGPRD, a known receptor of BAIBA, in PC12 cells and DRG. PCR products were observed by agarose gel electrophoresis with ethidium bromide staining. (B) mRNA expression of MRGPRD was analyzed by Real-time quantitative PCR. The relative quantity was calculated by the ΔΔCt method using β2 microglobulin as the endogenous control. (C and D) AMPK and Akt inhibitors reversed the anti-oxidative effects of L-BAIBA.PC12 cells were treated with the inhibitors; LY294002-a PI3K inhibitor, compound C-an AMPK inhibitor, and MU6840-a receptor agonist for MRGPRD, for 30 min before treatment with L-BAIBA (100 μM). Comparative analysis of ROS production (C) and Annexin V–FITC-positive cells (D) in various experimental groups. * p < 0.05. n = 3–4.
3.6. L-BAIBA suppressed oxidative stress-induced ROS production via AMPK activation
To identify the signaling pathways involved in mediating the inhibitory effects of L-BAIBA on H2O2-stimulated ROS production, we evaluated H2O2-induced ROS production in PC12 cells with or without pretreatment with an AMPK inhibitor (compound C) and PI3K inhibitor (LY294002) (Supplemental Fig. 1). As shown in Fig. 5C, inhibition of AMPK abolished the effect of L-BAIBA on H2O2-induced ROS production. On the other hand, inhibition of the PI3K /Akt pathway only partially affected H2O2-induced ROS production. Inhibition of the PI3K /Akt pathway by LY294002 tended to reduce the beneficial effect of L-BAIBA on H2O2-induced ROS production, although the levels of ROS production were not significantly different between the H2O2 +L-BAIBA and H2O2 +L-BAIBA+LY294002 groups.
Inhibition of MRGPRD did not affect H2O2-induced ROS production, suggesting that the inhibitory effect of L-BAIBA on oxidative stress-induced ROS production was not mediated by MRGPRD.
3.7. L-BAIBA suppressed oxidative stress-induced apoptosis via the AMPK and PI3K /Akt pathways
The inhibitory effects of L-BAIBA on oxidative stress-induced apoptosis were abolished by the inhibition of AMPK and PI3K/Akt (p < 0.05) (Fig. 5D). However, MRGPRD inhibition did not reverse the suppressive effect of L-BAIBA on oxidative stress-induced apoptosis. These results indicate that L-BAIBA ameliorated oxidative stress-induced apoptosis in PC12 cells via the AMPK and PI3K/Akt pathways, without the involvement of MRGPRD.
4. Discussion
The present study investigated whether L-BAIBA, an exercise-induced myokine, exhibits neuroprotective effects against oxidative stress. We demonstrated that L-BAIBA suppressed H2O2-induced ROS production via AMPK activation and apoptosis via activation of the AMPK and PI3K /Akt pathways.
BAIBA was identified as a small molecule generated by myocytes expressing PGC-1α. The molecular weight of BAIBA is 103.6 kDa and BAIBA was increased in the plasma of both chronically exercised and muscle-specific PGC-1α transgenic mice (Roberts et al., 2014). The effects of BAIBA was varied such as induction of a brown adipocyte-like phenotype, increase hepatic fatty acid β-oxidation, and decrease of ER stress. In this study, we demonstrated the protective effects of L-BAIBA against oxidative stress. Elevated level of ROS contributes to neurodegeneration and harmful effects on cellular biomolecules (Li et al., 2013). Oxidative stress resulting from ROS accumulation contributes to many neurodegenerative diseases and neurological impairments (Butterfield and Halliwell, 2019, Fernandez et al., 2019, Hemmati-Dinarvand et al., 2019, Lin and Beal, 2006, Martin and Teismann, 2009, Monzón-Sandoval et al., 2020). Aging has also been correlated with increased oxidative DNA damage (Finkel and Holbrook, 2000). Excess H2O2 induces ROS generation, which in turn induces neuronal oxidative damage via oxidation of membrane lipids, cellular proteins, and DNA, and eventually triggers neuronal cell death by apoptosis (Hamdi et al., 2015). We demonstrated that L-BAIBA suppressed H2O2-induced ROS generation in PC12 cells. The antioxidative effects of L-BAIBA are consistent with previous findings that L-BAIBA decreased ROS production in osteocytes and had a bone-protective effect that prevented oxidative stress-induced osteocyte death (Kitase et al., 2018). We revealed that L-BAIBA significantly activated AMPK and Akt in PC12 cells. We found that pretreatment with the antagonist of AMPK, compound C, significantly blocked the effect of L-BAIBA on H2O2-induced ROS production, which indicates that L-BAIBA suppresses H2O2-induced ROS production via the activation of AMPK. Although the precise signaling mechanism how L-BAIBA decrease ROS production is not clear, it is reported that AMPK reduced oxidative stress by the suppression of 26S proteasome activity and consequent suppression of NAD(P)H oxidase expression (Wang et al., 2010).
Excessive ROS production leads to neuronal apoptosis (Maycotte et al., 2010, Radi et al., 2014). We demonstrated that L-BAIBA suppressed oxidative stress-induced apoptosis in PC12 cells by two distinctive assay, Annexin V assay and TUNEL assay (Gavrieli et al., 1992, Vermes et al., 1995). The effects of L-BAIBA on oxidative stress-induced apoptosis in PC12 cells was varied by the detection methods; Annexin V assay 80% and TUNEL assay 58%. Annexin V assay can detect apoptosis from the early phase to the end phase. On the other hand, TUNEL assay detects DNA fragmentation, which is the end phase of apoptosis. Our data suggest that L-BAIBA inhibits oxidative stress-induced apoptosis from the early phase to the end phase.
We further revealed that L-BAIBA ameliorates H2O2-induced apoptosis in PC12 cells via activation of the AMPK and PI3K /Akt pathways. Although Akt did not significantly affect the inhibitory effects of L-BAIBA on H2O2-induced ROS production, Akt is a key mediator of cell survival(Manning and Cantley, 2007); it is a crucial factor that attenuates neuronal apoptosis (Chen et al., 2020, Okada et al., 2019, Wu et al., 2020). Akt promotes cell survival by blocking the function of pro-apoptotic proteins and processes. Akt negatively regulates Bcl-2 and directly phosphorylates BAD, which protects cells against apoptosis. On the other hand, L-BAIBA had no effect on neurite outgrowth in PC12 cells. These results suggest that L-BAIBA plays a role for neuronal survival, but not for neural network.
MRGPRD is a G protein-coupled receptor (GPCR) which belongs to the Mas-related GPCRs expressed in the dorsal root ganglia. BAIBA is one of the ligands of MRCPRD (Uno et al., 2012). Kitase et al. demonstrated that L-BAIBA protected cells from oxidative stress through MRGPRD (Kitase et al., 2018). We investigated whether the effects of L-BAIBA on PC12 were inhibited by a receptor antagonist for MRGPRD. Although PC12 cells expressed MRGPRD, we found no significant changes in the effect of L-BAIBA on the reduction of ROS following MRGPRD inhibition in PC12 cells, suggesting that L-BAIBA activates AMPK and Akt via alternative receptors in PC12 cells. Although the signaling mechanisms of BAIBA is still obscure, several receptors, such as orphan receptor GPR41 and glycine receptors are proposed to mediate the effects BAIBA.(Begriche et al., 2008;Schmieden and Betz, 1995). Further studies are required to identify the receptors which mediate the protective effects of BAIBA in PC12 cells.
In conclusion, we demonstrated that L-BAIBA has protective effects against oxidative stress in PC12 cells and that these effects mediate via activation of the AMPK and PI3K/Akt pathways. Since BAIBA is secreted by skeletal muscles as a myokine, the protective effects of L-BAIBA against the oxidative stress on PC12 cells suggest the beneficial effects of exercise on neuron. PC12 cells, rat pheochromocytoma cells, are extensively used in neuroscience research and undergo neuronal differentiation in response to NGF. However, PC12 cells are neuron-like cells, but not neuron itself. To investigate the relationship between exercise and neuroprotection, further study is needed to investigate the effects of L-BAIBA on neuron in vivo.
Funding
This work was supported in part by the “Strategic Research AGU-Platform Formation (2008–2012)” Project for Private University from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT).
CRediT authorship contribution statement
Tomomi Minato: Investigation, Formal analysis, Writing – original draft, Visualization. Nobuhisa Nakamura: Methodology, Investigation, Visualization. Tomokazu Saiki: Investigation, Visualization. Megumi Miyabe: Investigation, Validation. Mizuho Ito: Investigation, Validation, Tatsuaki Matsubara: Project administration. Keiko Naruse: Conceptualization, Methodology, Writing – review & editing, Supervision.
Conflicts of Interest
The authors declare they have no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ibneur.2021.12.001.
Appendix A. Supplementary material
Supplementary material.
.
References
- Ahlskog J.E. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77:288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begriche K., Massart J., Abbey-Toby A., Igoudjil A., Lettéron P., Fromenty B. Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity (Silver Spring, Md) 2008;16:2053–2067. doi: 10.1038/oby.2008.337. [DOI] [PubMed] [Google Scholar]
- Burtscher J., Millet G.P., Place N., Kayser B., Zanou N. The muscle-brain axis and neurodegenerative diseases: the key role of mitochondria in exercise-induced neuroprotection. Int. J. Mol. Sci. 2021;22:6479. doi: 10.3390/ijms22126479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Peng J, Sherchan P, Ma Y, Xiang S, Yan F, Zhao H, Jiang Y, et al. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2020;17:168. doi: 10.1186/s12974-020-01853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T., Rochester L. Mobilizing Parkinson’s disease: the future of exercise. J. Parkinsons Dis. 2018;8:S95–S100. doi: 10.3233/JPD-181489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A., Meechan D.W., Karpinski B.A., Paronett E.M., Bryan C.A., Rutz H.L., Radin E.A., Lubin N., Bonner E.R., Popratiloff A., Rothblat L.A., Maynard T.M., LaMantia A.S. Mitochondrial dysfunction leads to cortical under-connectivity and cognitive impairment. Neuron. 2019;102:1127–1142. doi: 10.1016/j.neuron.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi Y., Kaddour H., Vaudry D., Leprince J., Zarrouk A., Hammami M., Vaudry H., Tonon M.C., Amri M., Masmoudi-Kouki O. Octadecaneuropeptide ODN prevents hydrogen peroxide-induced oxidative damage of biomolecules in cultured rat astrocytes. Peptides. 2015;71:56–65. doi: 10.1016/j.peptides.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Hemmati-Dinarvand M., Saedi S., Valilo M., Kalantary-Charvadeh A., Alizadeh Sani M., Kargar R., Safari H., Samadi N. Oxidative stress and Parkinson’s disease: conflict of oxidant-antioxidant systems. Neurosci. Lett. 2019;709 doi: 10.1016/j.neulet.2019.134296. [DOI] [PubMed] [Google Scholar]
- Jung T.W., Hwang H.J., Hong H.C., Yoo H.J., Baik S.H., Choi K.M. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARdelta-dependent pathway in mice. Diabetologia. 2015;58:2096–2105. doi: 10.1007/s00125-015-3663-z. [DOI] [PubMed] [Google Scholar]
- Kitase Y., Vallejo J.A., Gutheil W., Vemula H., Jähn K., Yi J., Zhou J., Brotto M., Bonewald L.F. Beta-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018;22:1531–1544. doi: 10.1016/j.celrep.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yu S., Wu J., Zou Y., Zhao Y. Sulfiredoxin-1 protects PC12 cells against oxidative stress induced by hydrogen peroxide. J. Neurosci. Res. 2013;91:861–870. doi: 10.1002/jnr.23218. [DOI] [PubMed] [Google Scholar]
- Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H.L., Teismann P. Glutathione--a review on its role and significance in Parkinson’s disease. FASEB J. 2009;23:3263–3272. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- Mattson M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycotte P., Guemez-Gamboa A., Moran J. Apoptosis and autophagy in rat cerebellar granule neuron death: role of reactive oxygen species. J. Neurosci. Res. 2010;88:73–85. doi: 10.1002/jnr.22168. [DOI] [PubMed] [Google Scholar]
- Monzón-Sandoval J., Poggiolini I., Ilmer T., Wade-Martins R., Webber C., Parkkinen L. Human-specific transcriptome of ventral and dorsal midbrain dopamine neurons. Ann. Neurol. 2020;87:853–868. doi: 10.1002/ana.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Enkhjargal B., Travis Z.D., Ocak U., Tang J., Suzuki H., Zhang J.H. FGF-2 attenuates neuronal apoptosis via FGFR3/PI3k/Akt signaling pathway after subarachnoid hemorrhage. Mol. Neurobiol. 2019;56:8203–8219. doi: 10.1007/s12035-019-01668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B.K. Muscles and their myokines. J. Exp. Biol. 2011;214:337–346. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- Radi E., Formichi P., Battisti C., Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014;42 Suppl 3:S125–S152. doi: 10.3233/jad-132738. [DOI] [PubMed] [Google Scholar]
- Roberts L.D., Boström P., O’Sullivan J.F., Schinzel R.T., Lewis G.D., Dejam A., Lee Y.K., Palma M.J., Calhoun S., Georgiadi A., Chen M.H., Ramachandran V.S., Larson M.G., Bouchard C., Rankinen T., Souza A.L., Clish C.B., Wang T.J., Estall J.L., Soukas A.A., Cowan C.A., Spiegelman B.M., Gerszten R.E. Beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Betz H. Pharmacology of the inhibitory glycine receptor: agonist and antagonist actions of amino acids and piperidine carboxylic acid compounds. Mol. Pharmacol. 1995;48:919–927. [PubMed] [Google Scholar]
- Shi C.X., Zhao M.X., Shu X.D., Xiong X.Q., Wang J.J., Gao X.Y., Chen Q., Li Y.H., Kang Y.M., Zhu G.Q. beta-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci. Rep. 2016;6:21924. doi: 10.1038/srep21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stautemas J., Van Kuilenburg A.B.P., Stroomer L., Vaz F., Blancquaert L., Lefevere F.B.D., Everaert I., Derave W. Acute aerobic exercise leads to increased plasma levels of R- and S-β-aminoisobutyric acid in humans. Front. Physiol. 2019;10:1240. doi: 10.3389/fphys.2019.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno M., Nishimura S., Fukuchi K., Kaneta Y., Oda Y., Komori H., Takeda S., Haga T., Agatsuma T., Nara F. Identification of physiologically active substances as novel ligands for MRGPRD. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/816159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P.L., Castillo-García A., Morales J.S., de la Villa P., Hampel H., Emanuele E., Lista S., Lucia A. Exercise benefits on Alzheimer’s disease: state-of-the-science. Ageing Res. Rev. 2020;62 doi: 10.1016/j.arr.2020.101108. [DOI] [PubMed] [Google Scholar]
- Vemula H., Kitase Y., Ayon N.J., Bonewald L., Gutheil W.G. Gaussian and linear deconvolution of LC-MS/MS chromatograms of the eight aminobutyric acid isomers. Anal. Biochem. 2017;516:75–85. doi: 10.1016/j.ab.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Wang H., Qian J., Zhao X., Xing C., Sun B. beta-Aminoisobutyric acid ameliorates the renal fibrosis in mouse obstructed kidneys via inhibition of renal fibroblast activation and fibrosis. J. Pharmacol. Sci. 2017;133:203–213. doi: 10.1016/j.jphs.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang M., Liang B., Xu J., Xie Z., Liu C., Viollet B., Yan D., Zou M.H. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ. Res. 2010;106:1117–1128. doi: 10.1161/circresaha.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Shi X., Luo M., Inam-u-llah U.L., Li K., Zhang M., Ma J., Li Y., Liu Y., Zhang C., Liu X., Li S., Li Q., Chen X., Che X., Piao F. Taurine inhibits neuron apoptosis in hippocampus of diabetic rats and high glucose exposed HT-22 cells via the NGF-Akt/Bad pathway. Amino Acids. 2020;52:87–102. doi: 10.1007/s00726-019-02810-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.