Abstract
Widespread vaccination is a principal strategy to mitigate the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and lessen the global burden of coronavirus disease 2019 (COVID-19). Information is rapidly evolving about the impact of SARS-CoV-2 vaccines on the immune and endocrine systems. This case series heightens clinical awareness of possible thyroid effects and conveys knowledge of what to monitor, which are fundamental components of public health and pharmacovigilance. We present a case series of Graves disease following mRNA SARS-CoV-2 vaccination, with symptoms and altered thyroid function tests developing within 7 days of the first dose in 2 women aged 38 and 63 years, and 28 days after the second dose in a 30-year-old man. New-onset Graves disease occurred following administration of mRNA vaccines against SARS-CoV-2. Based on the timing of signs and symptoms relative to administration of the vaccine and the absence of other probable causes, we consider the vaccine as a potential contributor to the diagnosis. The viral spike protein, delivered indirectly through an encoded mRNA vaccine, may be capable of triggering an inflammatory cascade and immune response triggering thyroid dysfunction.
Keywords: case report, COVID-19 vaccines, endocrinology, Graves disease, immunology, pharmacovigilance, SARS-CoV-2
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–producing coronavirus disease 2019 (COVID-19) pandemic is markedly affecting mortality and the global economy. As a principal countermeasure, rapid vaccine development using the mRNA platform and deployment in adults and adolescents is mitigating COVID-19 hospitalizations and deaths.
As knowledge accrues about the effect of newly developed vaccines on organ systems, we are learning that SARS-CoV-2 vaccines, like COVID-19 disease, may affect endocrine physiology, particularly thyroid physiology. In addition to the occurrence of Graves disease following COVID-19 infection, 1 this condition has recently been documented following SARS-CoV-2 vaccination.2-4
Here, we present a series of 3 adults presenting to a Cornell-affiliated hospital or endocrinology clinic in New York City between January and April of 2021 with new-onset thyroid dysfunction following SARS-CoV-2 vaccine administration (Table 1).
Table 1.
Demographic, Clinical, and Laboratory Parameters of Patients.
| 1 | 2 | 3 | |
|---|---|---|---|
| Age (years) | 38 | 63 | 30 |
| Sex | Female | Female | Male |
| US vaccine a | Pfizer-BioNTech | Moderna | Pfizer-BioNTech |
| Days following vaccination (N) b | 5 days after first dose | 7 days after first dose | 28 days after second dose |
| Clinical presentation | Tachycardia, fever, abdominal pain | Pruritic rash | Palpitations, tremor, weight loss, irritability |
| Initial TSH | <0.008 µIU/mL | 0.011 µIU/mL | <0.005 µIU/mL |
| Initial fT4 | 108 pmol/L | 30.9 pmol/L | 22.9 pmol/L |
| Initial T3/fT3 | T3 10.3 nmol/L | T3 4.6 nmol/L | T3 2.5 nmol/L |
| TSI c | (+) >40 IU/L | (+) 0.95 IU/L | |
| TSHrAb d | (+) 32 IU/L | (+) 22 IU/L | |
| TPO e | (+) 1730 IU/mL | (+) 1149 IU/mL | (–) 15 IU/mL |
| Reference intervals | TSH 0.45-4.5 µIU/mL fT4 10.6-22.8 pmol/L T3 0.9-2.8 nmol/L TSI range < 0.5 IU/L TSHrAb range < 1.75 IU/L TPO range 0-9 IU/mL |
TSH 0.55-4.78 µIU/mL fT4 11.6-23.2 pmol/L T3 0.9-2.8 nmol/L TSHrAb range <1.75 IU/L TPO range 0-9 IU/mL |
TSH 0.45-4.5 µIU/mL fT4 10.6-22.8 pmol/L T3 1.1-2.76 nmol/L TSI range <0.55 IU/L TPO range 0-34 IU/mL |
| Imaging | U/S: heterogeneous, hypervascular, enlarged gland | U/S: heterogeneous, hypervascular gland Scintigraphy: diffuse increased activity |
None |
| Treatment | Methimazole, beta-blocker | None | Methimazole, beta-blocker |
Abbreviations: TSH, thyrotropin; fT4, free thyroxine; TSI, thyroid stimulating immunoglobulin; TSHrAb, thyrotropin receptor antibody; TPO, thyroperoxidase.
Vaccine administration under Food and Drug Administration Emergency Use Authorization.
Time interval from vaccine dose to onset of thyroid-related symptoms.
TSI: Case 1: Semi-quantitative chemiluminescent immunoassay, ARUP Laboratories; Case 3: Semi-quantitative immunoassay, Labcorp.
TSHrAb: Case 1, 2: Quantitative electrochemiluminescent immunoassay, ARUP Laboratories.
TPO: Case 1, 2: Quantitative chemiluminescent immunoassay, ARUP Laboratories, Case 3: Electrochemiluminescence immunoassay, Labcorp.
Case 1
A 38-year-old woman received the SARS-CoV-2 vaccine (Pfizer-BioNTech, New York, New York) and 5 days later presented to the emergency department with sudden onset left lower quadrant abdominal pain. Her presentation was consistent with thyroid storm given her fever, tachycardia to 140 beats per minute, and gastrointestinal symptoms (Burch-Wartofsky score 55).
Thyroid function tests revealed suppressed thyrotropin (TSH) of <0.008 µIU/mL, elevated free thyroxine (fT4) of 108 pmol/L, and elevated total triiodothyronine (T3) of 10.3 nmol/L. Thyrotropin receptor antibody (TSHrAb), thyroid-stimulating immunoglobulin (TSI), and anti-thyroid peroxidase (TPO) levels were all elevated. Ultrasonography revealed a diffusely enlarged thyroid gland with heterogeneous echogenicity and increased vascularity consistent with Graves. She has no personal or family history of thyroid or autoimmune disease. A computed tomography (CT) angiogram performed 1 year prior incidentally revealed thyroid gland enlargement measuring 7.3 cm in transverse diameter. She was treated with methimazole at a total daily dose of 60 mg titrated up to 80 mg along with propranolol 40 mg 3 times daily. After 2 months, fT4 decreased to 66 pmol/L, and after 3 months, it normalized to 23 pmol/L, while on methimazole, 80 mg daily in divided doses. After a discussion of the risks and benefit of administering the second vaccine, she decided to postpone the dose. She reported improved energy level and denied recurrence of palpitations or abdominal pain.
Case 2
A 63-year-old woman received the first dose of the SARS-CoV-2 vaccine (Moderna, Cambridge, Massachusetts) and 1 week later developed a pruritic rash on her neck and upper chest. She had no palpitations, heat intolerance, distal tremor, or diarrhea. She received her second vaccine dose 31 days after the first dose. Four days after receiving the second vaccine dose, thyroid function tests were drawn, which revealed a suppressed TSH of 0.011 µIU/mL and elevated fT4 of 30.9 pmol/L and T3 of 4.6 nmol/L. She had elevated TSHrAb, TSI, and TPO antibody levels. Thyroid ultrasound demonstrated a heterogeneous hypervascular gland along with 2 solid isoechoic nodules measuring 1.4 and 2.3 cm. She has no personal or family history of thyroid disease. Except for a sister and aunt with lupus, there is no other family history of autoimmune disease. She received no recent intravenous contrast. No antithyroid medication or bet-blocker was initiated as the patient was asymptomatic. After 6 months, the TSH remained suppressed at 0.01 µIU/mL with a high-normal fT4 of 21 pmol/L. On thyroid scintigraphy, radiotracer activity was high throughout both thyroid lobes with an elevated 24-hour uptake of 41%, consistent with Graves. The patient remained asymptomatic and no antithyroid medication was initiated.
Case 3
A 30-year-old man received the second dose of the SARS-CoV-2 vaccine (Pfizer-BioNTech) and 4 weeks later developed irritability, palpitations, tremors, and restless sleep. These symptoms were accompanied by a 15-pound weight loss over 6 weeks. Thyroid function tests revealed a suppressed TSH of <0.005 µIU/mL, fT4 minimally elevated at 22.9 pmol/L, and T3 normal at 161 pmol/L. He had an elevated TSI and negative TPO and anti-thyroglobulin antibody (ATA). He has no history of thyroid disease and had a normal TSH level 3 years prior. His mother developed Graves disease postpartum. He has no personal or family history of autoimmune disease. Medication was initiated with methimazole 5 mg and atenolol 25 mg daily. Six weeks later, the TSH remained fully suppressed and fT4 was in the normal range at 14.9 pmol/L. His irritability and restless sleep improved; however, he still endorsed occasional palpitations.
Discussion
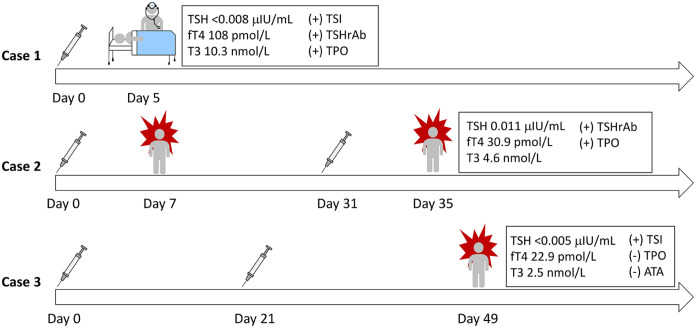
In this case series, we present 3 patients who were vaccinated against SARS-CoV-2 and subsequently developed new-onset Graves disease. Patients ranged in age from 30 to 63 years and 2 were women. Two developed symptoms 5 and 7 days after the first dose of the vaccine and the third developed symptoms 28 days after the second dose (Figure 1).
Figure 1.
Timeline depicts onset of symptoms in relation to severe acute respiratory syndrome coronavirus 2 mRNA vaccine administration in 3 patient cases. The initial thyroid function tests on presentation to their endocrinologist are shown, indicating thyroid abnormalities.
Abbreviations: TSH, thyrotropin; fT4, free thyroxine; TSI, thyroid stimulating immunoglobulin; TSHrAb, thyrotropin receptor antibody; TPO, thyroperoxidase; ATA, anti-thyroglobulin antibody.
Exploring thyroid pathophysiology associated with the SARS-CoV-2 vaccine involves considering how inflammatory processes are triggered by the virus, as a similar mechanism might be driving thyroid disease from the infection and the immunization. Thyroid abnormalities, including autoimmune thyroid disease, have been described following COVID-19.1,5 After initial infection of the respiratory tract, the subsequent pro-inflammatory cascade of cytokine activation, that is, “cytokine storm,” may cause immune-mediated thyroid inflammation.6,7,8 Could the pro-inflammatory state and ensuing thyroid abnormalities observed in COVID-19 patients also occur in those receiving the SARS-CoV-2 vaccine? Three reports have been published of Graves disease after SARS-CoV-2 vaccination. Three days after receiving the Pfizer-BioNTech SARS-CoV-2 vaccine, 2 female health care workers exhibited symptoms of hyperthyroidism and were found to have thyrotoxicosis with imaging findings diagnostic of Graves disease. 2 Two further Graves disease cases were published, 1 patient diagnosed with new-onset Graves following his first dose of SARS-CoV-2 vaccine, and a second patient with recurrent Graves after vaccination following a 17-year period of remission. 3 Finally, a case of exacerbation of existing Graves disease is reported in a 30-year-old woman well controlled on low-dose methimazole who developed worsening hyperthyroid symptoms and thyrotoxicosis following 2 doses of the CoronaVac vaccine, further exacerbated by an additional booster. 4
One mechanism by which a vaccine could trigger a deleterious inflammatory response is through an adjuvant in the formulation. While advantageous in developing an antigen-specific immune response, adjuvants can also trigger autoimmune disease such as Graves in genetically susceptible individuals.9,10 While the novel SARS-CoV-2 mRNA vaccines do not include adjuvants, the mRNA is not only an immunogen but also could act as an adjuvant due to the RNA’s intrinsic immunostimulatory properties. 11 However, there is no definitive mechanistic explanation for autoimmune disease developing after vaccination and epidemiological studies to date do not support the hypothesis of adjuvant-induced autoimmune disease. 12
There is limited experimental data concerning SARS-CoV-2 vaccine’s ability to induce autoimmune thyroid disease. Investigators conducting an in vitro study to determine how strongly antibodies to the SARS-CoV-2 spike protein reacted with human tissues found that TPO demonstrated moderate cross-reactivity against spike protein antibodies. They further explained that TPO peptide sequences shared homology with several SARS-CoV-2 proteins, including the spike protein. 13
Our case series corroborates recent case reports of Graves disease occurring after an SARS-CoV-2 mRNA vaccine. Based on the timing of signs and symptoms relative to administration of the vaccine and the absence of other probable causes, we consider the vaccine as a potential contributor to the diagnosis. However, a limitation is the onset of Graves could have occurred independently of the vaccine administration. Clinical awareness of possible effects and knowing what to monitor are necessary components of public health practice and pharmacovigilance. An epidemiological study of incident Graves disease among recently vaccinated and unvaccinated individuals in the general population could clarify the extent of the association between the SARS-CoV-2 vaccine and autoimmune hyperthyroidism. If this case series is further supported by epidemiological studies, it will be important to determine the underlying causal mechanism to optimize vaccine safety in the fight against the COVID-19 pandemic.
Acknowledgments
Dr Andrew J. Martorella, M.W., and N.S.G. contributed patient cases. M.W. performed the literature search and prepared the draft manuscript; B.A. edited and revised the draft and final versions of the manuscript; N.S.G. and B.A. reviewed the final manuscript.
Footnotes
Authors’ Note: Part of this material was previously presented as a poster presentation at the 90th Annual Meeting of the American Thyroid Association on October 2, 2021, and published as a meeting abstract in Thyroid in September, 2021.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
References
- 1. Murugan AK, Alzahrani AS. SARS-CoV-2 plays a pivotal role in inducing hyperthyroidism of Graves’ disease. Endocrine. 2021;73:243-254. doi: 10.1007/s12020-021-02770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of Graves’ disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021; 31:1436-1439. doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 3. Zettinig G, Krebs M. Two further cases of Graves’ disease following SARS-Cov-2 vaccination. J Endocrinol Invest. 2021. doi: 10.1007/s40618-021-01650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with Graves’ disease. Endocrine. 2021;74:226-227. doi: 10.1007/s12020-021-02879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mateu-Salat M, Urgell E, Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J Endocrinol Invest. 2020;43:1527-1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen W, Tian Y, Li Z, Zhu J, Wei T, Lei J. Potential interaction between SARS-CoV-2 and thyroid: a review. Endocrinology. 2021;162:bqab004. doi: 10.1210/endocr/bqab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg MK, Gopalakrishnan M, Yadav P, Misra S. Endocrine involvement in COVID-19: mechanisms, clinical features, and implications for care. Indian J Endocrinol Metab. 2020;24:381-386. doi: 10.4103/ijem.IJEM_440_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruggeri RM, Campennì A, Deandreis D, et al. SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol. 2021;17:737-759. doi: 10.1080/1744666X.2021.1932467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34:101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 10. Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol (Lausanne). 2016;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195-197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olivieri B, Betterle C, Zanoni G. Vaccinations and autoimmune diseases. Vaccines (Basel). 2021;9:815. doi: 10.3390/vaccines9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2020;11:617089. doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]