Abstract
Malaria is a fatal disease among children in malaria-prone locations such as Addis Zemen and Woreta because of their weak immune systems. Despite the severity of the disease in children, the majority of research conducted in Ethiopia has focused on adult populations rather than children. Furthermore, there is no data on malaria prevalence, risk factors, or parasite density among children in the Addis Zemen and Woreta catchment areas. Therefore, this study was aimed at filling the above gap in the study area. About 422 children were enrolled in the study by systematic sampling technique. A capillary blood sample was collected from each child to do blood film. The overall prevalence of malaria among children attending South Gonder health institutions was 14.7%. The majority of parasite density was moderate parasitemia followed by low parasitemia, giving 71.0% and 16.0%, respectively. Malaria parasite infection was linked to a history of malaria and the presence of stagnant water near a home, but utilization of insecticide-treated bed nets was found to be protective against the infection. Therefore, health education should be strengthened on proper utilization of bed nets, indoor residual spraying, removing stagnant water by discarding old tires that may collect rainwater, and removing debris from streams so streams flow more freely.
Keywords: malaria parasitemia, malaria risk-factors Plasmodium species
Background
Malaria is a life-threatening vector-borne disease caused by Plasmodium species (Plasmodium spp.), which is spread by female anopheline mosquito bites. 1 It is primarily distributed in tropical climates with plenty of stagnant water, where the parasite-carrying mosquito vector breeds. 2 Human malaria is caused by 5 medically important Plasmodium spp. The parasites causing malaria are Plasmodium falciparum (P. falciparum), Plasmodium vivax (P. vivax), Plasmodium malaria (P. malaria), Plasmodium ovale (P. ovale), and Plasmodium Knowlesi (P. kwowlesi). 3 P. falciparum is the deadliest and most virulent Plasmodium spp., accounting for 50% of all malaria infections globally and 75% in Sub-Saharan Africa. 4
Malaria cases are estimated to reach over 229 million worldwide, with over 409 000 deaths. The African region continues to carry a disproportionately large share of the global malaria burden with 94% of all malaria cases worldwide. 5 Malaria accounts for the majority of morbidity and mortality in Sub-Saharan Africa, where the illness burden, disability, and death rates are particularly high in children under the age of 5. According to WHO estimates from 2008, 243 million cases of malaria resulted in 863 000 fatalities in Sub-Saharan Africa, with more than 80% of deaths occurring in children under the age of 5. 6
It is one of Ethiopia’s most important public health issues by causing both mortality and morbidity. Around 75% of the country’s land mass is favorable to malaria transmission, with an estimated 68 million individuals at risk for getting the disease. 7 According to an Ethiopian federal ministry of health report, a total of 1 620 885 malaria suspected cases from 2015 to 2019 showed that 25% (410 409) of them were positive for malaria, with P. falciparum accounting for 65% of the positive cases and P. vivax accounting for the rest. 8
The majority of malaria illness in high-transmission locations affects young children rather than adults and causes mortality due to severe malaria, hypoglycemia, and cerebral malaria. 9 Between 2007 and 2015, the prevalence of malaria in Ethiopia seemed to have decreased from 0.9% to 0.5%, while the number of suspected cases, malaria admissions, and malaria deaths decreased from 2016 to 2019. 8
In Ethiopia, malaria is one of the 10 top leading causes of morbidity and mortality among children. It is common in children, which ranges from 16% to 54% in the country. 10 It remains a public health problem in the country due to low coverage of insecticide-treated bed nets (ITN), low coverage of indoor residual spraying, drug resistance to malaria, insecticide resistance of malaria vectors, poor access to health care, migration of people from malaria endemic areas to non-endemic areas, and false microscopic results. Malaria transmission is also seasonal and erratic in Ethiopia. 8 As a result, protective immunity is often poor, putting people of all ages at danger, especially children.
Malaria is a killer disease of children in malaria-prone areas like Addis Zemen and Woreta because of their weak immune systems. Despite the severity of the disease in children, the majority of research conducted in Ethiopia has focused on adult populations rather than children. Furthermore, there is no data on malaria prevalence, risk factors, or parasite density among children in the Addis Zemen and Woreta catchment areas. As a result, knowing the malaria status of the children in the study area is crucial to designing successful intervention methods. Therefore, the primary objective of this study was to determine the prevalence, parasite density, and associated risk factors and of malaria among children in the study area.
Methods
Study Setting
The South Gonder zone is located in the Amhara Regional State of Ethiopia. It is 660 km from Ethiopia’s capital, Addis Ababa. East Gojjam and Bahir Dar border South Gondar on the south, West Gojjam and Bahir Dar on the southwest, Lake Tana on the west, North Gondar on the north, Wag Hemra on the northeast, North Wollo on the east, and South Wollo on the southeast. The capital of the Zone is Debre Tabor. Woreta and Addis Zemen are 2 other small towns in the zone. The zone is located at 11°39′59.99″ north latitude and 38°00′0.00″ east longitude. The Zone’s elevation spans from 1500 to 3600 m above sea level. The Zone has a total population of 2 711 987, according to South Gonder Zone population projection data, with 1 360 427 (50.16%) men and 1 351 560 (49.84%) women. Out of the total population, 2 483 382 (91.57%) were rural dwellers and the rest were urban dwellers.
Because of climatic variables and the lowland nature of the locations, Addis Zemen and the Woreta catchment areas are recognized for having a high incidence of malaria cases. As a result, we conducted our research at 1 hospital and 1 health center in the Zone: Addis Zemen hospital and Woreta health center.
Study Design and Period
A prospective cross-sectional study design was conducted in the selected health institutions of the South Gonder zone from April to September, 2020.
Sample Size and Sampling Technique
The sample size was calculated using the single proportion formula below, with a maximum proportion of 50%. Then, the initial sample size was calculated to be 384 after considering a 95% confidence interval (z = 1.96) and a 5% marginal error (d = .05); after computing a 10% nonresponse rate, the final sample size was 422.
The study participants were proportionally chosen from Addis Zemen Hospital and Woreta Health Center. The total number of children expected to attend the 2 health facilities during the study period was 2340 based on average daily children flow (8.5 and 4.5 average children flow in Addis Zemen Hospital and Woreta Health Center, respectively).
During the 6-month study period, 1530 (8.5 × 30 × 6) children were admitted to Addis Zemen hospital, while 810 (4.5 × 30 × 6) children were admitted to Woreta health center. As a result, the number of children in each health facility was divided by the total population to obtain the proportion (1530/2340 in Addis Zemen and 810/2340 in Woreta) to determine the total number of children included in the study from both health facilities. The proportion of children in Addis Zemen hospital and Woreta health center was 0.655 and 0.34, respectively, after calculations.
To estimate the actual number of children recruited in the study from each study site, each proportion was multiplied by the total sample size, which was 422. Therefore, 276 (0.655 × 422) children from Addis Zemen hospital and 146 (0.345 × 422) children from Woreta health center were included in the study. Finally, the sampling interval was computed by dividing the total number of the children by the sample size. Then, a systematic random sampling technique was used and the study participants were chosen after every 5 children who visited both health facilities.
Study Population
The study populations were malaria suspected children who visited Addis Zemen hospital and Woreta health center.
Eligibility Criteria
Children whose parents or guardians provided written informed consent were included in the study. Children who had received antimalarial chemotherapy 42 days prior to data collection were excluded since it is considered that the parasite is eliminated from the blood within 42 days of treatment, and re-infection can occur after that time. 11
Data Collection
Socio-demographic and associated risk factors assessment
A standardized questionnaire was designed in an English version to collect data on socio-demographic and associated risk factors for malaria. Then it was translated into Amharic, the native language. Then, 10% of the questionnaires that had been prepared were pre-tested. Finally, trained health professionals conducted the interview.
Laboratory Data Collection
Blood sample collection, processing, and examination
Capillary blood was taken aseptically from the fingers of the children using a blood lancet to do thin and thick blood films at Addis Zemen hospital and Woreta health center laboratory. The blood film was left to dry in the air. The thin blood film was then fixed with absolute methanol, and both thin and thick blood films were stained for 10 minutes with 10% Giemsa stain. The slides were then washed with distilled water and air dried. Finally, the stained slides were systematically examined by laboratory technologists at the health institutions. Oil emersion objective (100×) was used to detect Plasmodium parasites in thick smears and identify the species of Plasmodium parasites in thin smears.
The asexual forms of the parasites, such as trophozoite and schizont, were counted manually until 200 WBC were counted using a tally counter to assess parasitemia. The following formula was used to compute the number of parasites per microliter of blood, with the total leukocyte count of the patient considered to be 8000/μl of blood. 12
The parasitemia was then divided into 4 categories: low (1000 parasites/μl of blood), moderate (1000-4999 parasites/μl of blood), high (5000-99 999 parasites/μl of blood), and hyperparasitemia (100 000 parasites/μl of blood).13,23
Quality Control
Known malaria positive and negative slides were used to test the quality of the Giemsa stain. Ten percent of all slides were chosen at random and cross-checked by qualified laboratory technologists who were blind for the first result. Before beginning the analysis, the quality of the generated data was checked for completeness and clarity.
Data Analysis
Data was entered and analyzed by SPSS version 23. Sociodemographic parameters and the prevalence rate were calculated using descriptive statistics. Binary logistic regression was used to analyze the associated risk factors of malaria. After performing bivariate logistic regression analysis, variables with a P value of .2 were adjusted using a multivariate logistic regression model by stepwise variable selection to control for possible confounding factors. Finally, factors with P values ≤.05 were considered statistically significant.
Operational Definition
Children: Individual pediatric patients from birth to 15 years old who were taken to health facilities because of having malaria symptoms like fever, shivering, sweating, headache, and fatigue.
House Hole: Is any crack or crevice in the house through which mosquitoes could enter.
House Structure: The material from which a house is constructed, such as cement or mud.
Stagnant Water: Is a water body that may include waste, rainwater, ponds, natural water bodies, and standing water that remains after flooding.
Results
Socio-Demographic Characteristics of the Study Participants
There were 230 (54.5%) males and 192 (45.5%) females among the 422 children who participated in the study. The participants in the study ranged in age from 2 months to 15 years, with an average age of 5.79 years and a 4.46 year standard deviation. In terms of age, 207 (49.1%), 112 (26.5%), and 103 (24.4%) of the children were less than or equal to 5, 6 to 10, and 11 to 15 years, respectively. The majority of the children came from rural areas (53.8%), while the rest came from urban areas (Table 1).
Table 1.
Sociodemographic Characteristics of Children Visiting Health Institutions in the South Gonder Zone From April to September, 2020.
| Sociodemographic characteristics | Number (%) |
|---|---|
| Sex | |
| Male | 230 (54.5) |
| Female | 192 (45.5) |
| Age (years) | |
| ≤5 | 207 (49.1) |
| 6-10 | 112 (26.5) |
| 11-15 | 103 (24.4) |
| Residence | |
| Rural | 227 (53.8) |
| Urban | 195 (46.2) |
| Father’s educational status | |
| Unable to write and read | 166 (39.3) |
| Elementary school | 106 (25.1) |
| High school | 83 (19.1) |
| Higher education | 67 (15.9) |
| Mother’s educational status | |
| Unable to read and write | 214 (50.7) |
| Elementary school | 90 (21.3) |
| High school | 89 (21.1) |
| Higher education | 29 (6.9) |
Prevalence of Malaria and Its Density
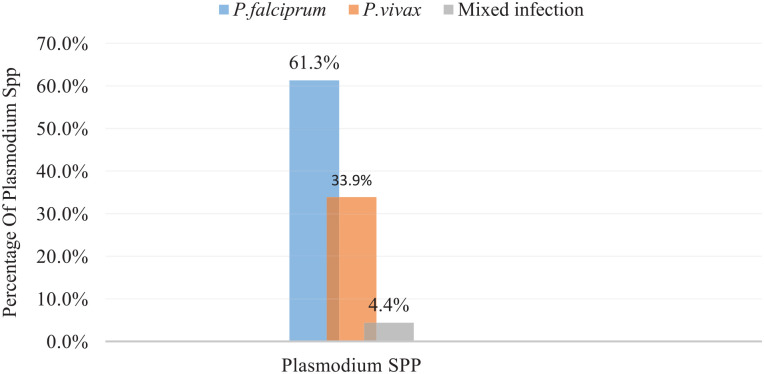
Malaria was found in 14.7% (95% CI 11.4-18.0) of the study participants. P. falciparum and P. vivax were found in 38 (61.3%) and 21 (33.9%) of the infected children, respectively. Mixed infections were found in 3 (4.8%) of the children (Figure 1).
Figure 1.
Plasmodium spp. distribution among children attending health institutions in the South Gonder Zone, North West Ethiopia, from April to September 2020.
Mixed infection = infection by both P. falciparum and P. vivax.
The geometric mean parasite density was 38 572.58 parasites/μl of blood, with minimum and maximum parasite densities of 4800 and 68 000 parasites/μl of blood, respectively. Moderate parasitemia was the most common, followed by low parasitemia, which accounted for 44 (71%) and 10 (16.1%) of parasite density, respectively. High parasitemia was found the least, accounting for 8% of the total (12.9%). Males were found to have a larger prevalence of moderate malaria parasitemia than females, accounting for 73.7% of the total. But there was no statistical difference in parasite density between males and females (P = .956). Although moderate parasitemia had the highest parasite density in 6 to 10 age groups, there was no statistical significance across age groups in terms of parasitemia (P = .291). Even though there was no statistical difference (P = .956), 72.4% of rural residents had moderate parasitemia compared to urban residents (Table 2).
Table 2.
Distribution of Malaria Parasite Density by Age, Sex, and Residence Among Children Visiting Health Institutions in the South Gonder Zone From April to September, 2020.
| Sociodemographic variables | Parasite density | P-value | |||
|---|---|---|---|---|---|
| Low (%) | Moderate (%) | High (%) | Total (%) | ||
| Sex | |||||
| Males | 6 (15.8) | 28 (73.7) | 4 (10.5) | 38 (61.3) | .956 |
| Females | 4 (16.7) | 16 (66.7) | 4 (16.6) | 24 (38.7) | |
| Total | 10 (16.0) | 44 (71.0) | 8 (13.0) | 62 (100) | |
| Age | |||||
| ≤5 | 6 (15.8) | 27 (71.0) | 5 (13.2) | 38 (61.3) | .291 |
| 6-10 | 1 (6.7) | 13 (86.6) | 1 (6.7) | 15 (24.2) | |
| 11-15 | 3 (33.3) | 4 (44.5) | 2 (22.2) | 9 (14.5) | |
| Total | 10 (16.0) | 44 (71. 0) | 8 (13.0) | 62 (100) | |
| Residence | |||||
| Rural | 4 (13.8) | 21 (72.4) | 4 (13.8) | 29 (46.8) | .956 |
| Urban | 6 (18.2) | 23 (69.7) | 4 (12.1) | 33 (53.2 | |
| Total | 10 (16.0) | 44 (71.0) | 8 (13.0) | 62 (100) | |
Risk Factors Analysis
Bivariate logistic regression was used to examine all possible malaria risk factors. The respondents’ sex, residence, and presence of a man-made or natural dam around their dwelling were not statistically associated with malaria infection in bivariate logistic regressions. However, the age of the respondents, their history of malaria parasite infection, the existence of stagnant water near their residence, the usage of ITNs, the presence of a house hole, the number of house windows, and the structure of their homes were all factors associated with malaria (P < .05).All variables with a P-value of <.2 were adjusted to a multivariate logistic regression model to control for possible confounding effects.
After adjustment, the presence of a house hole, the structure of the house, and the number of windows in the house were not found to be associated with malaria infection (P > .05). In an adjusted logistic regression analysis, the risk of Plasmodium parasite infection was 88% lower in children under the age of 5 than in children aged 11 to 15 (AOR = 0.279, CI = 0.124-0.624). Malaria risk was 2 times higher in children with a history of malaria than in those who did not (AOR = 2.074, CI = 1.013-4.245). Where there is stagnant water, the prevalence of malaria is 2.2 times more frequent than where there is no stagnant water (AOR = 2.248, CI = 1.143-4.419). Children who did not use ITN were 2.2 times more likely than those who did to be infected with the Plasmodium parasite (AOR = 2.296, CI = 1.166-4.523) (Table 3).
Table 3.
Bivariate and Multivariate Analysis of Associated Risk Factors for Malaria Among Children Visiting Health Institutions at South Gonder Zone From April to September, 2020.
| Associated factors | Malaria status | COR (CI) | AOR (CI) | P Value | |
|---|---|---|---|---|---|
| Positive (%) | Negative (%) | ||||
| Sex* | |||||
| Male | 38 (16.5) | 192 (83.5) | 0.722 (0.416-1.253) | ||
| Female | 24 (11.6) | 168 (88.4) | 1 | ||
| Age in years | |||||
| ≤5 | 38 (18.4) | 169 (81.6) | 0.426 (0.197-0.919) | 0.279 (0.124-0.624) | .002 |
| 6-10 | 15 (13.4) | 97 (86.6) | 0.619 (0.619-1.483) | 0.515 (0.210-1.266) | .148 |
| 11-15 | 9 (8.7) | 35 (91.3) | 1 | ||
| Residence* | |||||
| Rural | 29 (12.8) | 198 (87.2) | 0.719 (0.719-1.234) | ||
| Urban | 33 (17.0) | 162 (83.0) | 1 | ||
| History of malaria | |||||
| Yes | 11 (7.5) | 136 (92.5) | 2.815 (1.418-5.587) | 2.074 (1.103-4.245) | .046 |
| No | 51 (18.5) | 224 (81.5) | 1 | ||
| Presence of stagnant water around residency | |||||
| Yes | 13 (8.2) | 145 (91.8) | 2.542 (1.331-4.853) | 2.248 (1.143-4.419) | .019 |
| No | 49 (18.5) | 215 (81.5) | 1 | ||
| ITN utilization | |||||
| Yes | 48 (19.3) | 201 (82.7) | 1 | ||
| No | 14 (8) | 159 (92) | 2.712 (1.4441-5.095) | 2.296 (1.166-4.523) | .016 |
| Presence of hole in the house | |||||
| Yes | 14 (8.5) | 149 (91.5) | 1.288 (1.288-4.551) | 1.946 (0.989-3.829) | .054 |
| No | 48 (11.4) | 211 (88.6) | 1 | ||
| Presence of man-made or natural dam around residency* | |||||
| Yes | 5 (39.5) | 33 (60.5) | 1.150 (0.431-3.071) | ||
| No | 57 (15) | 327 (85.0) | 1 | ||
| Number of windows in the house | |||||
| 1 | 8 (7.5) | 99 (92.5) | 1 | ||
| 2 | 25 (15.3) | 138 (84.7) | 0.446 (0.193-1.030) | 0.463 (0.193-1.110) | .084 |
| ≥3 | 29 (19.1) | 123 (80.9) | 0.343 (0.150-0.783) | 0.450 (0.019-1.066) | .070 |
| House structure | |||||
| Mud | 55 (13.9) | 341 (86.1) | 2.284 (0.17-5.687) | 2.327 (0.878-6.166) | .089 |
| Cement | 7 (27.0) | 19 (73.0) | 1 | ||
Abbreviations: AOR, adjusted odds ratio; COR, crude odds ratio; CI, confidence interval; ITN, insecticide-treated bed nets.
Variables whose P-values are >.2 and were not adjusted to multivariate logistic regression.
Discussion
Malaria is a public health issue in Sub-Saharan Africa, which includes Ethiopia. In children, it causes cognitive impairment, school absenteeism, and social interaction restrictions in children. 14 Plasmodium infection was found in 14.7% (95% CI = 11.4-18.0) of children attending health institutions in South Gondar, according to the current study. This result is similar to a 14% study conducted in Rwanda. 15 This result was lower than 20.5% in the East Shewa zone of Oromia regional state, 16 43.8% in the Jawi district of Amhara regional state, 17 and 58.2% in Nigeria’s Anambra State. 18 However, the prevalence of malaria in our study was higher than the 2.6% reported in Ghana. 19
The differences in malaria prevalence between our study and others may be due to differences in malaria transmission season or study period, altitude, or study population (eg, there is a sample size difference between our study and others). Furthermore, the ecological niche for mosquito breeding could explain discrepancies in malaria prevalence between our study and others (eg, there was no natural or man-made dam in 91% of the respondents in our case). P. falciparum and P. vivax were the 2 species identified in the blood of the children, accounting for 61.3% and 33.4% of infections, respectively. This result is in line with national Plasmodium spp. prevalence 20 and other previous research. 21 The higher dominance of P. falciparum over P. vivax is related to P. falciparum’s high proliferation in the host cell, the parasite’s ability to infect all ages of RBC, and the parasite’s treatment resistance. 1 Moreover, 4.4% of the children had mixed infections of P. falciparum and P. vivax. The reason for the mixed infection may be due to either simultaneous infection by both species or drug resistance.
The majority of infected children had moderate parasite density, followed by low parasite density, which accounted for 71% and 16% of the children, respectively, while 12.9% had high parasite density. This was consistent with earlier research. 22 However, in East Central Tanzania, a significant proportion of high parasite density was identified, while in Sanja Town, a high proportion of low parasite density was reported.23,24 The variation in parasite density could be attributable to the study participants’ immune condition, age category, and dietary status. Acquired or adaptive immunity does, in fact, provide protection against clinical disease, morbidity associated with parasite density, and new infection by lowering parasite burden. 25
In this study, female children appeared to have a higher infection rate than male children. This is in agreement with research conducted in Nigeria’s Anambra State 18 and Jawi district. 17 According to a study conducted in the Afar region, 26 females were 1.99 times more likely to be infected than males. The comparable prevalence of malaria in males and females in our study could be owing to equivalent exposure of both sexes to female Anopheles mosquitoes at night, when the mosquito bites most effectively.
This study found that children under the age of 5 were less likely to be infected by the Plasmodium parasite than those of a higher age group. This finding is consistent with research conducted in the Oromia region’s East Shewa zone, which found that children aged 10 to 15 were more likely to be infected than children of younger ages. 16 However, the study conducted in Nigeria showed children of 1 to 4 years were the most affected age group. 18 This could be due to older age groups participating in outdoor activities such as agricultural or pastoral activities, which could expose them to exophilic mosquitoes.
Bed net use was found to be a protective factor against malaria, with children who did not use a bed net being 2.2 times more likely to be infected by Plasmodium parasite infection than those who did in our study. This finding is supported by additional research in Jawi district, 17 Oromia region’s East Shewa Zone, 16 and Rwanda. 15 This is because ITN prevents mosquitoes mechanically by stopping mosquitoes from entering the human body or biologically by killing insects that come into touch with it. 27
Children with a history of malaria had 2 times the chance of getting malaria than those who did not in our study. This finding is similar to the study conducted in the Afar Region of Ethiopia. 26 This could be attributable to P. falciparum’s medication resistance and P. vivax’s relapse. 28 Malaria was 2.2 times more likely to be present in respondents’ homes where there was stagnant water than in homes where there was no stagnant water. The results are consistent with research conducted in the Jawi district and the Afar region.17,26
Conclusions
In this study, the overall prevalence of malaria in the selected health institutions of the South Gonder Zone was 14.7%. A higher proportion of moderate parasitemia was observed in the study area. Therefore, health education on proper utilization of ITN, indoor residual spraying, removing stagnant water by discarding old tires that may collect rainwater and by removing debris from streams so streams flow more freely.
Acknowledgments
First and foremost, we want to express our gratitude to the children who visited Addis Zemen Hospital and Woreta Center for their willingness to engage in the research. We also like to express our gratitude to the administrations of Addis Zemen Hospital and Woreta Health Center for allowing us to conduct the research.
Footnotes
Author Contributions: LW: Selected the title, designed the study, wrote the manuscript, analyzed and interpreted the data. ML: Analyzed, analyzed and reviewed the final draft of the manuscript. SD: Analyzed, analyzed and reviewed the final draft of the manuscript. TE: Analyzed, analyzed and reviewed the manuscript. TK: Analyzed, analyzed and reviewed the manuscript. SD: Analyzed, analyzed and reviewed the manuscript. WH: Analyzed, analyzed and reviewed the manuscript. DM: Analyzed, analyzed and reviewed the manuscript. TE: Analyzed, analyzed and reviewed the manuscript. Finally, all authors read and approved the final manuscript.
Availability of Data and Materials: All the data used in this research article are presented within the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Considerations: Ethical clearance was obtained from Debre Tabor University; College of Health Sciences research and ethical review committee (reference number: CHS/250/2012 according to Ethiopian calendar). Moreover, permission letter to perform the study was obtained from Addis Zemen hospital and Woreta health center medical director office. This study was conducted in accordance with the Declaration of Helsinki. After briefly describing the significance of the study, the children’s parents or guardians signed informed written consent. Finally, children who had been infected with the Plasmodium parasite received antimalarial treatment according to the local malaria treatment guidelines.
ORCID iDs: Lemma Workineh  https://orcid.org/0000-0003-0137-3426
https://orcid.org/0000-0003-0137-3426
Tahir Eyayu  https://orcid.org/0000-0002-2041-7183
https://orcid.org/0000-0002-2041-7183
References
- 1. Gillespie S, Pearson RD. Principles and Practice of Clinical Parasitology. John Wiley & Sons; 2003. [Google Scholar]
- 2. Ayele DG, Zewotir TT, Mwambi HG. Prevalence and risk factors of malaria in Ethiopia. Malar J. 2012;11(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Satoskar A, Simon G, Hotez P, Tsuji M. Medical Parasitology. Landes Bioscience; 2009. [Google Scholar]
- 4. Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong’echa JM. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci. 2011;7(9):1427-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges. World Health Organization; 2020. [Google Scholar]
- 6. Crawley J, Chu C, Mtove G, Nosten F. Malaria in children. Lancet. 2010;375(9724):1468-1481. [DOI] [PubMed] [Google Scholar]
- 7. Tegegne Y, Asmelash D, Ambachew S, Eshetie S, Addisu A, Jejaw Zeleke A. The prevalence of malaria among pregnant women in Ethiopia: a systematic review and meta-analysis. J Parasitol Res. 2019;2019:8396091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ethiopian Ministry of Health. Ethiopia malaria elimination strategic plan: 2021-2025. December 4, 2020. http://www.moh.gov.et/ejcc/am/ETHIOPIA_MALARIA_ELIMINATION_STRATEGIC_PLAN:_2021-2025
- 9. World Health Organization. Malaria in children under five. 2021. January 5, 2021. https://www.who.int/malaria/areas/high_risk_groups/children/en/
- 10. Aychiluhm SB, Gelaye KA, Angaw DA, et al. Determinants of malaria among under-five children in Ethiopia: Bayesian multilevel analysis. BMC Public Health. 2020;20(1):1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Methods for surveillance of antimalarial drug efficacy. World Health Organization; 2009. [Google Scholar]
- 12. WHO. Basic Malaria Microscopy. Part I. Learner’s Guide, 2nd ed. WHO; 2010. [Google Scholar]
- 13. Kimbi HK, Sumbele IU, Nweboh M, et al. Malaria and haematologic parameters of pupils at different altitudes along the slope of Mount Cameroon: a cross-sectional study. Malar J. 2013;12(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holding PA, Kitsao-Wekulo PK. Describing the burden of malaria on child development: what should we be measuring and how should we be measuring it? Am J Trop Med Hyg. 2004;71(2 Suppl):71-79. [PubMed] [Google Scholar]
- 15. Habyarimana F, Ramroop S. Prevalence and risk factors associated with malaria among children aged six months to 14 years old in Rwanda: evidence from 2017 Rwanda malaria indicator survey. Int J Environ Res Public Health. 2020;17(21):7975. doi: 10.3390/ijerph17217975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haji Y, Fogarty AW, Deressa W. Prevalence and associated factors of malaria among febrile children in Ethiopia: a cross-sectional health facility-based study. Acta Trop. 2016;155:63-70. doi: 10.1016/j.actatropica.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 17. Hailu T, Alemu M, Mulu W, Abera B. Incidence of Plasmodium infections and determinant factors among febrile children in a district of northwest Ethiopia; a cross-sectional study. Trop Dis Travel Med Vaccines. 2018;4:8. doi: 10.1186/s40794-018-0069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nwaorgu O, Orajaka B. Prevalence of malaria among children 1–10 years old in communities in Awka north local government area, Anambra state south East Nigeria. Afr Res Rev. 2011;5(5):264-281. [Google Scholar]
- 19. Kanwugu ON, Helegbe GK, Aryee PA, et al. Prevalence of asymptomatic malaria among children in the tamale metropolis: how does the PfHRP2 CareStart™ RDT perform against microscopy? J Trop Med. Published online December 21, 2019. doi: 10.1155/2019/6457628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. President’s Malaria Initiative. Malaria Operational Plan FY 2014. President’s Malaria Initiative; 2020. [Google Scholar]
- 21. Shiferaw M, Alemu M, Tedla K, Tadesse D, Bayissa S, Bugssa G. The prevalence of malaria in Tselemti Wereda, North Ethiopia: a retrospective study. Ethiop J Health Sci. 2018;28(5):539-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sirak S, Fola AA, Worku L, Biadgo B. Malaria parasitemia and its association with lipid and hematological parameters among malaria-infected patients attending at Metema Hospital, Northwest Ethiopia. Pathol Lab Med Int. 2016;8:43-50. [Google Scholar]
- 23. Chipwaza B, Sumaye RD. High malaria parasitemia among outpatient febrile children in low endemic area, east-central Tanzania in 2013. BMC Res Notes. 2020;13:251. doi: 10.1186/s13104-020-05092-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Worku L, Damtie D, Endris M, Getie S, Aemero M. Asymptomatic malaria and associated risk factors among school children in Sanja town, northwest Ethiopia. Int Sch Res Notices. Published online July 7, 2014. doi: 10.1155/2014/303269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22(1):13-36. doi: 10.1128/CMR.00025-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woday A, Mohammed A, Gebre A, Urmale K. Prevalence and associated factors of malaria among febrile children in Afar region, Ethiopia: a health facility based study. Ethiop J Health Sci. 2019;29(5):613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Birget PL, Koella JC. An epidemiological model of the effects of insecticide-treated bed nets on malaria transmission. PLoS One. 2015;10(12):e0144173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bloland PB; World Health Organization. Drug Resistance in Malaria. World Health Organization; 2001. [Google Scholar]