Abstract
The action of insulin to recruit the intracellular GLUT4 glucose transporter to the plasma membrane of 3T3-L1 adipocytes is mimicked by endothelin 1, which signals through trimeric Gαq or Gα11 proteins. Here we report that murine Gα11 is most abundant in fat and that expression of the constitutively active form of Gα11 [Gα11(Q209L)] in 3T3-L1 adipocytes causes recruitment of GLUT4 to the plasma membrane and stimulation of 2-deoxyglucose uptake. In contrast to the action of insulin on GLUT4, the effects of endothelin 1 and Gα11 were not inhibited by the phosphatidylinositol 3-kinase inhibitor wortmannin at 100 nM. Signaling by insulin, endothelin 1, or Gα11(Q209L) also mobilized cortical F-actin in cultured adipocytes. Importantly, GLUT4 translocation caused by all three agents was blocked upon disassembly of F-actin by latrunculin B, suggesting that the F-actin polymerization caused by these agents may be required for their effects on GLUT4. Remarkably, expression of a dominant inhibitory form of the actin-regulatory GTPase ARF6 [ARF6(T27N)] in cultured adipocytes selectively inhibited both F-actin formation and GLUT4 translocation in response to endothelin 1 but not insulin. These data indicate that ARF6 is a required downstream element in endothelin 1 signaling through Gα11 to regulate cortical actin and GLUT4 translocation in cultured adipocytes, while insulin action involves different signaling pathways.
Insulin, the major hormone regulating glucose transport in mammals, stimulates sugar uptake from the circulation into muscle and adipose tissues. Insulin binding to its unique receptor on the cell surface initiates a chain of events that leads to an increase in cell surface localization of GLUT4, the major insulin-sensitive glucose transporter isoform in mammals (6, 10, 13, 20, 64). In the basal state, this transporter is directed to and retained within specific intracellular compartments by a mechanism that requires the unique COOH terminus of the GLUT4 protein (16, 42, 66). Insulin regulates the movement of GLUT4 out of its sequestered compartment mostly through a stimulatory effect on its exocytosis but also by inhibiting endocytosis (15). The translocation of GLUT4 in response to insulin requires the activity of phosphatidylinositol (PI) 3-kinase (PI3-kinase) (11, 51). Insulin stimulation of glucose transport is accompanied by accumulation of PI-3,4-phosphate and PI-3,4,5-phosphate, products of PI3-kinase-catalyzed reactions (35). The fungal product wortmannin (51, 33) and the synthetic drug LY294002 (11) inhibit PI3-kinase activity at concentrations that also inhibit stimulation of glucose transport by insulin. Evidence of the involvement of PI3-kinase in insulin-stimulated glucose transport also comes from the observation that microinjection or expression of dominant inhibitory constructs of the p85 subunit of PI3-kinase completely blocks glucose transport (59). Conversely, expression of the activated form of the catalytic p110 subunit of PI3-kinase partially mimics the effect of insulin on glucose transport (44).
In addition to insulin, various other stimuli can induce the translocation of GLUT4 vesicles to the plasma membrane. Introduction of guanosine 5′-O-(3-thiotriphosphate) (GTPγS), a nonhydrolyzable GTP analogue, into adipocytes rapidly stimulates GLUT4 translocation to the plasma membrane by a tyrosine kinase-dependent mechanism (3, 18, 56). Contraction (26) and hypoxia (73) in skeletal muscle have also been shown to enhance glucose uptake in a wortmannin-insensitive mode, indicating they do not operate through PI3-kinase. The 5′-AMP protein kinase, which is responsive to cell stress, may be involved in these effects (8). Adenosine has also been reported to activate glucose transport or potentiate insulin action in white and brown adipose tissues (32, 39, 62). In rat epitrochlearis and soleus muscles, removing adenosine with adenosine deaminase or blocking its action with an adenosine receptor antagonist markedly reduces the responsiveness of glucose transport to stimulation by insulin (23). The A1 adenosine receptor is tightly coupled to the heterotrimeric G protein Gi (21). Agents which downregulate Gi [prostaglandin E1 and N6-(2-phenylisopropyl)adenosine] have been shown to decrease the maximal response of the cells to insulin by ∼30% (22, 23). Several other hormones like bradykinin, endothelin 1, and platelet-activating factor stimulate GLUT4 translocation and increase glucose uptake in 3T3-L1 adipocytes and CHO-T cells by a mechanism involving the Gq family of trimeric G proteins (37, 38). Consistent with these studies, recent work has also demonstrated that expression of a constitutively active form of Gαq in cultured adipocytes causes GLUT4 translocation (31, 34). The Gαq family consists of five isoforms, Gα15, Gα16, Gα14, Gα11, and Gαq (61). Gα14 is found primarily in stromal and epithelial cells, while Gα15 and Gα16 are found in cells derived from the hematopoietic lineage (2).
Despite recent advances, the precise mechanism by which insulin and these other factors regulate GLUT4 translocation is unknown. However, it is established that the ability of insulin to markedly stimulate GLUT4 translocation and glucose uptake is limited to muscle and adipose tissues. GLUT4 heterologously expressed in other cell types is targeted to the correct intracellular membranes but fails to translocate to the plasma membrane or to mediate enhanced glucose uptake in response to insulin (24). These data indicate that unique components of the GLUT4 translocation machinery are expressed in 3T3-L1 adipocytes, an excellent model system for insulin action, and not in undifferentiated 3T3-L1 fibroblasts, which do not contain GLUT4 and do not respond to insulin.
In an effort to identify new components in this pathway, we have constructed subtractive cDNA libraries consisting of genes highly expressed in insulin-responsive tissues. One of the genes identified as being highly expressed in adipocytes compared to other tissues is Gα11, a protein most related to Gαq, previously reported to mediate GLUT4 translocation (31, 34). However, it was not clear from these previous studies whether Gα11/q, like insulin, acts through a pathway that requires PI3-kinase (31) or not (34). We report here that in 3T3-L1 adipocytes endothelin 1, which acts through Gα11, or expression of the constitutively active Gα11 [Gα11(Q209L)] stimulates GLUT4 translocation and 2-deoxyglucose uptake by a PI3-kinase-independent mechanism. We also find that both insulin and endothelin 1 cause cortical F-actin assembly, and GLUT4 translocation in response to either of these agents is blocked by latrunculin B, which causes disassembly of F-actin (63). Most importantly, we present strong evidence that the actions of endothelin 1 but not insulin on F-actin and GLUT4 are blocked by dominant inhibitory ADP-ribosylation factor 6 (ARF6), a small GTP-binding protein known to modulate actin polymerization (55). These data are consistent with the hypothesis that the initial steps of GLUT4 regulation by insulin and Gα11 are different, but that they converge at or before some step that mobilizes cortical F-actin filaments. The results also raise the hypothesis that mobilization of actin assembly by insulin or agents that activate Gα11/q may facilitate or mediate GLUT4 translocation to the plasma membrane.
MATERIALS AND METHODS
Materials.
Wild-type Gα11 and the constitutively active form Gα11(Q209L) cDNA constructs were kindly provided by Hiroshi Itoh (Tokyo Institute of Technology, Tokyo, Japan). The pCDNA3-GLUT4-GFP was a gift from Jeffrey E. Pessin (University of Iowa, Iowa City). Rabbit polyclonal antibodies specific to Gα11 and Gαq were purchased from Santa Cruz Biotechnology, Inc., and Calbiochem, respectively. Endothelin 1 was purchased from American Peptide Company. Recombinant human insulin used to stimulate 3T3-L1 adipocytes was kindly donated by Eli Lilly and Company. [1,2-3H]2-deoxy-d-glucose was purchased from ICN. Wild-type 293 (293WT) and 293A cells were purchased from the American Type Culture Collection and Quantum Biotechnology, respectively. Rhodamino-phalloidin was purchased from Molecular Probes. Unless otherwise specified, all other chemicals were purchased from Sigma.
Cell culture.
3T3-L1 fibroblasts were grown and differentiated as described before (25), with the following changes. Differentiation of fibroblasts to adipocytes was induced after 8 days by incubating the cells for 3 days with high-glucose Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 5 μg of insulin per ml, 0.25 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 50 U of penicillin per ml, and 50 μg of streptomycin sulfate per ml. The cells were incubated for an additional 3 days in the same medium without dexamethasone and 3-isobutyl-1-methylxanthine and then maintained for an additional 3 to 6 days in high-glucose DMEM containing 10% fetal bovine serum. 3T3-L1 adipocytes were used between 9 and 12 days after the start of differentiation.
For 2-deoxyglucose uptake assays, differentiated 3T3-L1 adipocytes were treated with 0.25% trypsin and 0.5 mg of collagenase per ml in phosphate-buffered saline (PBS) and were reseeded at 150,000 cells/well of a 24-well plate.
293WT and 293A cells were grown and maintained in high-glucose DMEM supplemented with 10% calf serum, 50 U of penicillin per ml, and 50 μg of streptomycin sulfate per ml.
Construction of HA-tagged Gα11(Q209L).
The hemagglutinin (HA) epitope was inserted into pCMV5-Gα11(Q209L) as described by Wilson and Bourne (70). Briefly, site-directed mutagenesis was performed using high-fidelity Vent polymerase enzyme, which replicates both plasmid strands in the presence of mutant oligonucleotides containing the HA epitope (CGGGAGGTCGATGTGGAGAAGGTCTATCCTTATGATGTTCCTGATTATGCAGCCATCAAGA CGCTGTGGAGTGAC). This replaced the amino acids EHQYVN (125 to 130) in Gα11(Q209L) with the HA epitope DVPDYA. Mutagenesis was verified by sequencing. pCMV5-HA-Arf6(T27N) was prepared as described previously (40).
Rat tissue extract preparation and Gα11 and Gαq protein immunoblot analyses.
Tissues from Sprague-Dawley male rats were removed and immediately frozen in liquid nitrogen. The tissues were then homogenized in 20 mM HEPES buffer (pH 7.2) with 1% sodium dodecyl sulfate (SDS), aprotinin (5 μg/ml), leupeptin (5 μg/ml), 1 mM benzamidine, 100 mM phenylmethylsulfonyl fluoride, 25 mM sodium fluoride, 1 mM sodium vanadate, and sodium pyrophosphate, using a Polytron homogenizer. The protein concentration of the extracts were determined using the bicinchoninic acid protein assay reagent (Pierce). Samples were diluted to 2 mg/ml with Laemmli sample buffer and kept frozen at −20°C; 25 μg of the each tissue extract was resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel and transferred to nitrocellulose membranes for immunoblotting with a Gα11 or Gαq-specific antibody.
Construction of recombinant adenoviruses.
Recombinant adenoviruses encoding the constitutively active Gα11(Q209L) and dominant negative ARF6[ARF6(T27N)] were constructed as described in He et al. (27). Briefly, the cDNA for Gα11(Q209L) was cloned into the HindIII site and the cDNA for HA-ARF6 (T27N) into the NotI-EcoRV sites of the shuttle vector pAdTrack-CMV. The remaining protocol for producing the recombinant adenovirus was the same for both Gα11(Q209L) and HA-ARF6(T27N). The shuttle vector plasmid was then linearized with PmeI and purified by phenol-chloform extraction and ethanol precipitation. The linearized pAdTrack-CMV-Gα11(Q209L) was electroporated into electrocompetent Escherichia coli BJ5183 having the pAdEasy-1 plasmid to generate the recombinant adenoviral vector DNA pAd-Gα11(Q209L). Kanamycin-resistant clones were analyzed by comparing their supercoiled sizes relative to pAdEasy-1. Clones containing inserts were further tested by BamHI and PacI restriction endonuclease digestions. Once confirmed, the recombinant plasmids were transformed into E. coli XL-1 Blue competent cells for large-scale amplification. This recombinant adenoviral vector DNA was then digested with PacI, purified as before, and transfected into 293 cells. Transfected cells were monitored for green fluorescent protein (GFP) expression and collected 7 to 10 days after transfection. After four cycles of freezing in a methanol-dry ice bath and rapid thawing at 37°C, the viral lysate was used to infect another round of 293 cells. To generate high-titer viral stocks, 293 cells were infected at a multiplicity of infection of 1 to 5 and grown for 2 days, at which time the virus was harvested as described above.
Cell treatments.
Differentiated 3T3-L1 adipocytes were serum starved for 12 to 18 h in DMEM supplemented with 0.5% bovine serum albumin (BSA). The cells were then treated with 100 nM wortmannin for 15 min or with 30 μM nocodazole or 5 μM latrunculin B for 1 h, followed by stimulation with 100 nM insulin or 10 nM endothelin 1 for 30 min. For adenovirus infection, 3T3-L1 adipocytes were infected at a multiplicity of infection of 50 PFU/cell with either a control virus expressing GFP or the recombinant adenovirus encoding GFP and Gα11(Q209L) or ARF6(T27N) in DMEM with 0.5% BSA for 2 h with intermittent shaking. The infected cells were then incubated overnight at 37°C in a 5% CO2 incubator in the same medium. This media was replaced with DMEM containing 10% serum next morning. All the experiments were started after about 30 h of infection. This protocol resulted in an infection efficiency of at least 80% of adipocytes.
pCMV5-HA-Arf6(T27N), pCMV5-HA-Gα11(Q209L), and pcDNA3-GFP-GLUT4 were transfected into differentiated 3T3-L1 adipocytes by electroporation as described by Min et al. (46). Briefly, 4 × 106 3T3-L1 adipocytes were electroporated with 200 μg of pCMV5-HA-Gα11(Q209L) or pCMV5-HA-Arf6(T27N) and 50 μg of GFP-GLUT4 and then distributed equally in a six-well plate. Serum starvation of these transfected cells was started after 30 h.
Plasma membrane lawn assay.
Plasma membrane lawns from 3T3-L1 adipocytes were generated by a technique similar to the one described by Martin et al. (43). Membrane lawns were fixed on the coverslips with 3.7% formaldehyde for 10 min, washed twice with PBS, and incubated for 45 min with 2% BSA in PBS. For quantitation of GLUT4 the coverslips were incubated with a 1:1,000 dilution of rabbit anti-GLUT4 polyclonal antibody in PBS–0.5% Tween 20. Coverslips were washed five times for 3 min each and were incubated with a 1:1,000 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit mixed with 10 μg of rhodamine-conjugated wheat germ agglutinin (WGA) per ml (to quantitate plasma membranes). The coverslips were washed as before and postfixed for 10 min with 3.7% formaldehyde followed by a final wash with PBS and distilled water. They were then mounted on slides with 90% glycerol in PBS (DABCO) and viewed with a 60× objective on a Nikon Diaphot 200 inverted microscope coupled to a Bio-Rad MRC1024 processing unit. Images were analyzed by the Lasersharp processing software. All experiments were done in duplicate, and at least eight images were collected from each set.
GLUT4 rim assay.
Differentiated 3T3-L1 adipocytes were transfected with the plasmids encoding GFP-GLUT4 and HA-ARF6(T27N). After 40 h, the cells were serum starved for 4 h and then stimulated with 100 nM insulin for 45 min or 10 nM endothelin 1 for 30 min. The cells were then washed with ice-cold PBS, fixed with 3.7% formaldehyde as before, permeabilized with PBS plus 1% serum plus 0.5% Triton X-100 (P buffer), and incubated with anti-HA antibody followed rhodamine-conjugated secondary antibody to detect HA-ARF6(T27N)-transfected cells.
2-Deoxyglucose uptake.
Glucose uptake in 3T3-L1 was determined essentially as described by Frost and Lane (19). Cells reseeded on 24-well plates were infected as described above. Prior to glucose uptake, the cells were washed twice with PBS and then serum starved for 2 h at 37°C in Krebs-Ringer phosphate buffer (130 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.3 mM MgSO4, 10 mM Na2HPO4 [pH 7.4]) supplemented with 0.5% BSA and 2 mM sodium pyruvate. Cells were then treated as described above in the same buffer with 100 nM wortmannin for 15 min prior to stimulation with 100 nM insulin or 10 nM endothelin 1 for 30 min. Sugar uptake was initiated by the addition of [1,2-3H]2-deoxy-d-glucose to a final assay concentration of 0.1 mM for 5 min at 37°C. Assays were terminated by three rapid washes with 1 ml of ice-cold Krebs-Ringer phosphate buffer. Cells were solubilized with 0.4 ml of 1% Triton X-100, and 3H was determined in 4 ml of the scintillant. Nonspecific deoxyglucose uptake was measured in the presence of 20 μM cytochalasin B and was subtracted from each determination to obtain specific uptake.
Visualization of F-actin with rhodamine-phalloidin.
The treated cells were washed twice with ice-cold PBS and then fixed with 3.7% formaldehyde for 10 min at room temperature. They were then permeabilized with P buffer for 15 min and stained with anti-HA polyclonal (R4289) (1:1,000 in P buffer) for 2 h at room temperature followed by FITC-conjugated goat anti-rabbit immunoglobulins (1:1,000 in P buffer) and rhodamine-phalloidin (1 U/ml in P buffer) for 30 min at room temperature.
RESULTS
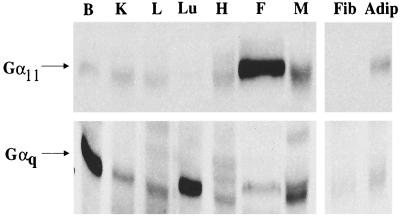
A cDNA clone encoding Gα11 was identified as a potential regulator of insulin action from a subtractive cDNA library that is enriched in genes highly expressed in insulin-responsive adipose tissue (A. D. Cherniack, J. M. Buxton, S. M. C. Nicoloro, S. B. Waters, M. Emoto, A. Bose, and M. P. Czech, unpublished data). To verify that Gα11 is indeed expressed at high levels in insulin-responsive tissues, different rat tissues were tested for the expression of Gα11 or Gαq protein by Western blot analysis using antibodies specific to either isoform. As shown in Fig. 1, Gα11 protein was predominantly expressed in fat whereas Gαq protein was highly expressed in brain, lung, and skeletal muscle. These results suggest that Gα11 is the Gαq isoform predominantly expressed in insulin-sensitive 3T3-L1 adipocytes and in primary fat cells. The high expression level of Gα11 in this major insulin-sensitive tissue is consistent with its potentially important role in regulating GLUT4 translocation in these cells.
FIG. 1.
Expression of Gαq and Gα11 in various rat tissues. Rat tissue extracts were prepared from brain (B), kidney (K), liver (L), lungs (Lu), heart (H), fat (F), skeletal muscle (M), 3T3 L1 fibroblasts (Fib), and adipocytes (Adip); 25-μg aliquots of the extracts were resolved by SDS-PAGE on 10% gels, electrophoretically transferred to nitrocellulose, blocked, and subsequently incubated with Gαq- or Gα11-specific antibodies as described in Materials and Methods.
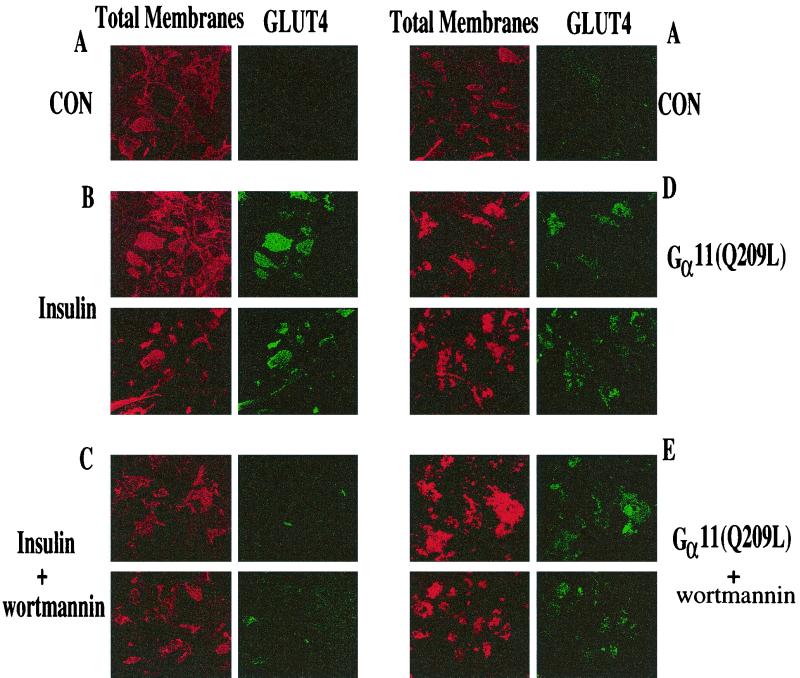
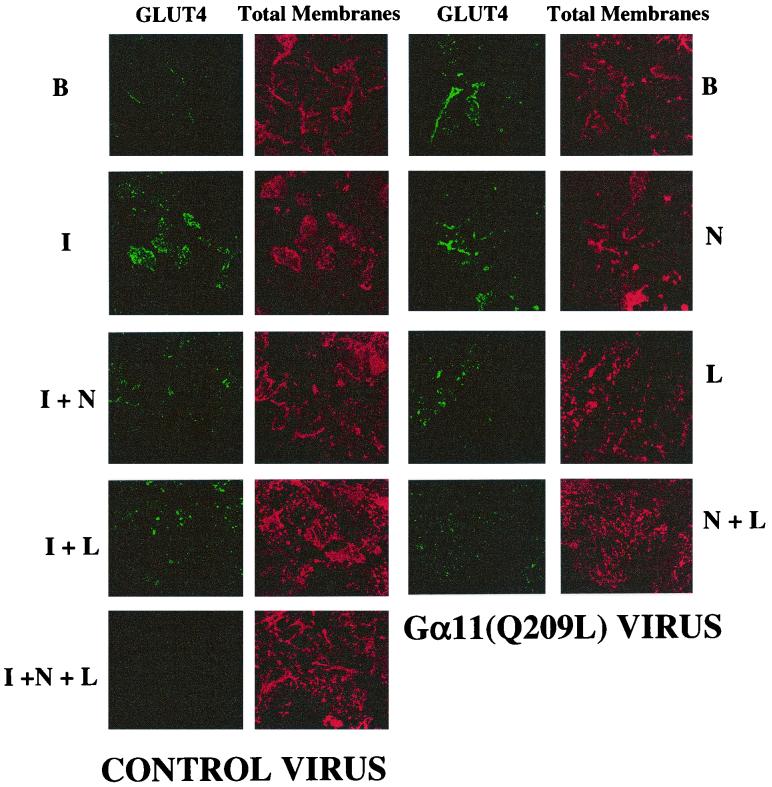
Recent results indicate that introduction of constitutively active Gαq into 3T3-L1 adipocytes causes an increase in plasma membrane GLUT4 and stimulation of glucose transport (31, 34). To test whether activation of Gα11 also mimics insulin action, we constructed adenoviruses that express a constitutively active, GTPase-deficient form of Gα11(Q209L). Western blot analysis of lysate from 3T3-L1 adipocytes infected with these adenoviruses (at 50 PFU/cell) consistently showed a three- to fourfold increase in expression of Gα11 compared to uninfected cells (data not shown). The effect of expressing the activated Gα11 protein on GLUT4 translocation was quantified by detecting GLUT4 on plasma membrane sheets retained on glass coverslips following light sonication of 3T3-L1 adipocytes (43). Insulin causes a significant increase in plasma membrane GLUT4 levels compared to those observed in unstimulated adipocytes (Fig. 2A and B). Expression of the activated Gα11(Q209L) in cultured adipocytes mimics the effect of insulin, resulting in an increase in plasma membrane GLUT4 concentration (Fig. 2D). Insulin-stimulated GLUT4 translocation and glucose uptake are PI3-kinase dependent and can be blocked by treatment of 3T3-L1 adipocytes with the PI3-kinase inhibitor wortmannin at 100 nM (51). This is confirmed by the results shown in Fig. 2C. However, under these same experimental conditions, treatment of cultured adipocytes with 100 nM wortmannin failed to block GLUT4 translocation induced by Gα11(Q209L) expression (Fig. 2E). These data indicate that insulin action on GLUT4 translocation requires wortmannin-sensitive PI3-kinase activity, while the effect of Gα11 is mediated by a mechanism that is independent of this activity.
FIG. 2.
Stimulated GLUT4 levels in plasma membrane lawns of 3T3-L1 adipocytes expressing Gα11(Q209L) is not affected by wortmannin. 3T3-L1 adipocytes were infected with control (CON; A to C) or Gα11(Q209L)-expressing (D and E) adenoviruses as described in Materials and Methods. Overnight serum-starved cells were either untreated (A and D), stimulated with 100 nM insulin for 30 min (B), or treated with 100 nM wortmannin followed by stimulation with 100 nM insulin (C) and 100 nM wortmannin (E) alone for 45 min. The cells were then sonicated, and plasma membrane lawns were fixed and stained for total membranes (red) and GLUT4 (green). The data reflects results from five separate experiments.
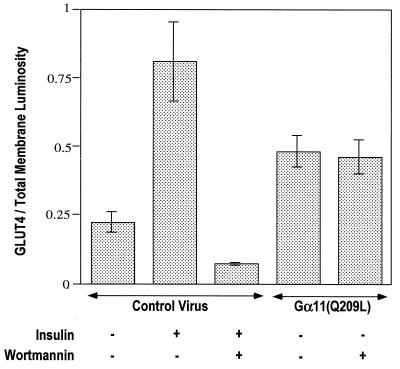
To compare the effects of Gα11(Q209L) and insulin on GLUT4 translocation, the signal intensity of anti-GLUT4 staining of plasma membrane lawns in Fig. 2 was quantified by normalizing to total membranes, estimated by staining with rhodamine-conjugated WGA. The data plotted as ratios of FITC to rhodamine intensities are shown in Fig. 3. Almost twice as much GLUT4 was detected in the adipocyte plasma membrane in response to insulin compared to that detected in response to Gα11(Q209L) expression. These data indicate that Gα11 is not able to mimic the full effect of insulin to cause translocation of GLUT4 to the surface membranes. However, addition of insulin to adipocytes expressing Gα11(Q209L) did not further enhance plasma membrane GLUT4 (not shown).
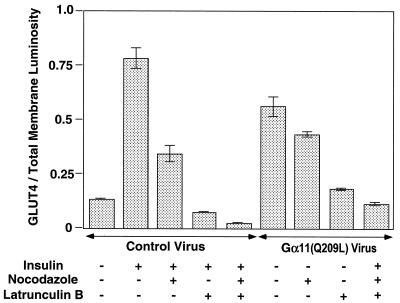
FIG. 3.
Luminosity ratio of GLUT4 to total membrane signals in plasma membrane lawns of recombinant adenovirus-infected 3T3-L1 adipocytes, quantified using Adobe Photoshop 5.0.2. The ratio of the luminosity of the GLUT4 lawns (green) to the luminosity of the corresponding lawn stained with WGA-rhodamine (red) was calculated for each condition and plotted. The average values were calculated from at least 10 data points from five different experiments.
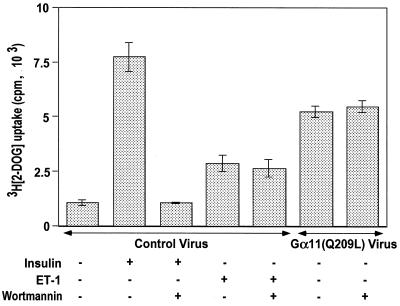
The functional consequence of GLUT4 translocation to the plasma membrane is increased hexose uptake in insulin-stimulated 3T3-L1 adipocytes. Thus, 2-deoxyglucose uptake in Gα11(Q209L)-expressing 3T3-L1 adipocytes was measured to analyze whether the GLUT4 translocated to the plasma membrane can also stimulate hexose uptake (Fig. 4). Insulin stimulation of 3T3-L1 adipocytes infected with control virus caused a sevenfold increase in [3H]2-deoxy-d-glucose uptake compared to unstimulated cells. This stimulated uptake is almost completely inhibited by 100 nM wortmannin and agrees with previously published results that increased hexose uptake in response to insulin stimulation requires PI3-kinase activity (51). Treatment with endothelin 1 of cells infected with control virus results in about a 2.5-fold increase in hexose uptake which is unaffected by 100 nM wortmannin. Gα11(Q209L) expression in the 3T3-L1 adipocytes causes an increase in basal [3H]2-deoxy-d-glucose uptake to 70% of the effect of insulin. However, this increase in hexose uptake due to Gα11(Q209L) was completely unaffected by treatment with 100 nM wortmannin. This result is consistent with the data in Fig. 2 and 3 and indicates that Gα11(Q209L) activates GLUT4 translocation and glucose uptake by a pathway that does not require wortmannin-sensitive PI3-kinase activity.
FIG. 4.
Gα11(Q209L) stimulation of 2-deoxyglucose uptake in differentiated 3T3-L1 adipocytes is wortmannin independent. Differentiated 3T3-L1 adipocytes seeded at 150,000/well in 24-well plates were infected with either control or Gα11(Q209L)-expressing adenoviruses. The cells were serum starved for 2 h in Krebs-Ringer phosphate buffer with BSA and pyruvate. The control virus-infected cells were then stimulated with either 100 nM insulin or 10 nM endothelin 1 (ET-1) alone for 30 min or following treatment of the cells with 100 nM wortmannin for 15 min. The cells that were infected with Gα11(Q209L)-expressing adenoviruses were either left untreated or treated with 100 nM wortmannin for 45 min. They were then assayed for 2-deoxyglucose (2-DOG) uptake as described in Materials and Methods. The data represents the average of four separate experiments with each condition tested in triplicate.
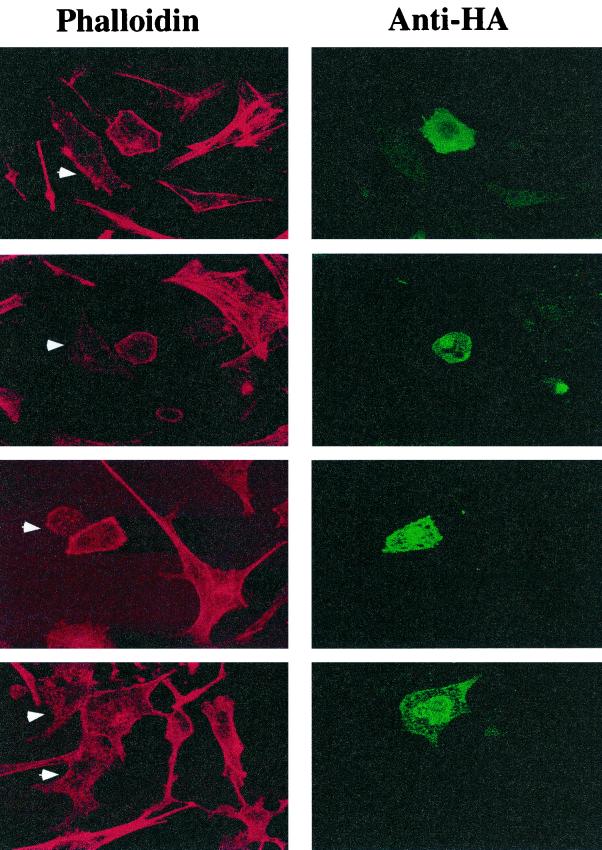
Insulin action modulates F-actin assembly in 3T3-L1 adipocytes (68), primary fat cells (52), and L6 myotubes (65). Furthermore, it was reported that disassembly of polymerized actin in primary adipocytes by incubation with latrunculin B blocks insulin action on GLUT4 translocation (52). These data suggest that actin filament formation may be critically important for movements of GLUT4-containing membranes to the plasma membrane. Recent studies have revealed that Gαq signaling pathways can also regulate cellular actin assembly, causing increased cortical F-actin, which is defined as a continuous cortical actin rim visualized with phalloidin staining (28, 53). In airway smooth muscle cells, endothelin 1 action enhances the appearance of filamentous actin, an effect that is inhibited by treatment of the cells with antisense oligonucleotides against Gαi2 and Gαq (28). We therefore tested whether in 3T3-L1 adipocytes expression of Gα11(Q209L) could mimic the ability of insulin to cause the formation of cortical F-actin. Figure 5 shows the results of a representative experiment where cultured adipocytes transiently transfected with cDNA encoding HA-tagged Gα11(Q209L) were fixed and stained with anti-HA antibody (visualized with FITC-conjugated secondary antibody) and with rhodamine-labeled phalloidin to visualize F-actin. The 3T3-L1 adipocytes in these experiments show a greatly decreased amount of F-actin compared to the fibroblasts in the culture, with little or no evidence of actin stress fibers. Adipocytes expressing the HA-tagged GTPase-deficient Gα11 displayed a markedly enhanced cortical F-actin staining pattern compared to neighboring untransfected adipocytes under basal conditions.
FIG. 5.
F-actin staining in differentiated 3T3-L1 adipocytes transfected with HA-Gα11(Q209L). 3T3-L1 adipocytes were electroporated with pCMV5-HA-Gα11(Q209L) as described in Materials and Methods. The cells were then fixed and stained with anti-HA-FITC and rhodamine-phalloidin to identify transfected cells and visualize F-actin, respectively. The Gα11(Q209L)-transfected cells were identified by staining the cells with anti-HA antibodies. Untransfected adipocytes are indicated by arrowheads. Each panel represents a separate field from a representative experiment.
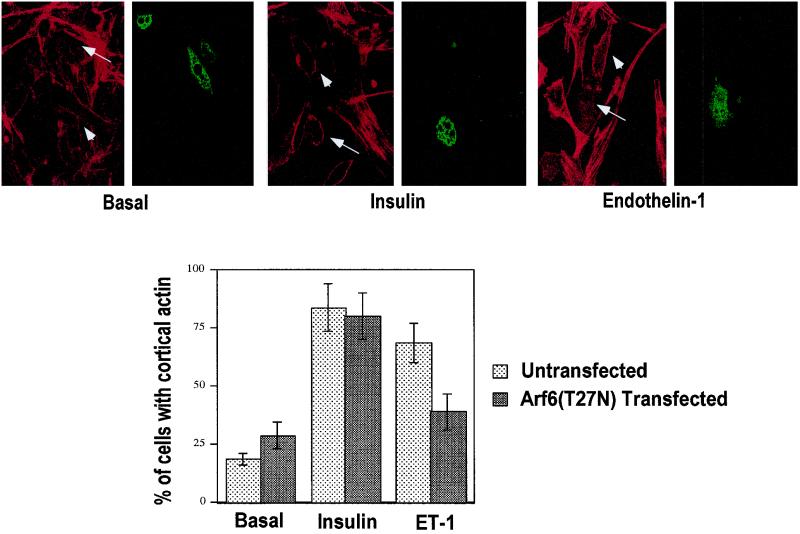
The results shown in Fig. 5 were quantified by manually counting cells in many fields that displayed a continuous cortical actin rim versus those cells that did not. Only 20% of untransfected 3T3-L1 adipocytes displayed a continuous actin rim under basal conditions, while 80 or 75% of the cells showed an actin rim when stimulated by insulin or endothelin 1, respectively. Almost 95% of Gα11(Q209L)-expressing adipocytes displayed bright cortical rims of rhodamine-phalloidin compared to untransfected cells under basal conditions. This striking effect of Gα11(Q209)L to cause cortical F-actin formation in cultured adipocytes is similar to the effects on cortical actin polymerization reported for insulin and endothelin 1 in other cell types and to the effects of these agents that we observe in 3T3-L1 adipocytes (also see Fig. 9 for comparison).
FIG. 9.
Cortical F-actin formation in response to endothelin 1 but not insulin is inhibited in 3T3-L1 adipocytes transfected with HA-ARF6(T27N). Differentiated 3T3-L1 adipocytes were transfected with pCMV5-HA-Arf6(T27N) by electroporation, left untreated or treated with 100 nM insulin or 10 nM endothelin 1 for 30 min, and then fixed and stained with anti-HA-FITC and rhodamine-phalloidin to identify transfected cells and visualize F-actin, respectively (top). The graph shows the percentage of untransfected or transfected cells that displayed cortical actin staining in response to insulin and endothelin 1 (ET-1) (average of five separate experiments).
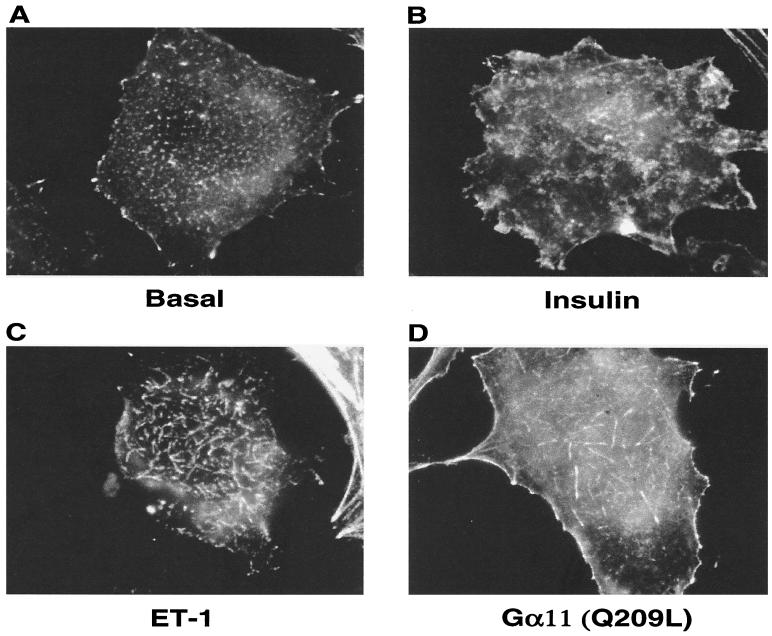
A second approach that was taken to visualize cortical actin assembly involved the use of digital imaging microscopy to analyze optical planes close to the cytoplasmic side of adipocyte surface membranes that are attached to coverslips. Figure 6 shows representative images of such optical planes from adipocytes treated without or with insulin or endothelin 1 or transiently transfected with Gα11(Q209L). Control adipocytes display a random distribution of small punctate structures, while many of the adipocytes exposed to each of the three agents display filamentous actin structures at or near the surface membrane. These structures are strikingly similar to those observed by overexpression of PI 4-phosphate 5-kinase Iα in COS-7 cells and are described as resembling pine needles (60). Almost 90% of the 3T3-L1 adipocytes stimulated with either insulin or endothelin 1 or expressing activated Gα11(Q209L) displayed the actin “pine needle,” whereas less than 20% of control adipocytes dsiplayed similar structures. Taken together, these experiments strongly support the concept that signaling by insulin, endothelin 1, or Gα11(Q209L) triggers molecular events that lead to marked changes in actin assembly near the cell surface membrane of cultured adipocytes.
FIG. 6.
F-actin rearrangements in 3T3-L1 adipocytes by insulin, endothelin 1, and Gα11(Q209L). 3T3-L1 adipocytes were serum starved and either left untreated (A) or stimulated with 100 nM insulin (B) and 100 nM endothelin 1 (ET-1; C) for 30 min. Samples were fixed, permeabilized, and stained with rhodamine-phalloidin as described in the text. Images were acquired by focusing at the bottom of the cells with an Olympus IX-70 inverted microscope and the Metamorph software (Universal Imaging). 3T3-L1 adipocytes that were transfected with HA-Gα11(Q209L) were fixed and permeabilized following serum starvation. They were then stained with anti-HA-FITC for identifying electroporated cells (D) and rhodamine-phalloidin to visualize F-actin.
Recent results have implicated cortical F-actin as an important element of the mechanism by which GLUT4 is translocated to the cell surface membrane in response to insulin (36, 52, 69). We thus tested whether the effects of Gα11 or insulin on GLUT4 translocation might require F-actin in 3T3-L1 adipocytes by treating the cells with latrunculin B, an agent known to cause disassembly of actin filaments (63). Nocodazole, an agent that causes the dispersion of microtubules, was also used in these experiments. Figure 7 depicts anti-GLUT4 staining of plasma membrane lawns derived from 3T3-L1 adipocytes treated with or without these drugs, individually or in combination, at concentrations that we found could effectively disrupt microtubules and actin filaments. Insulin action on GLUT4 translocation was modestly (about 60%) inhibited by nocodazole treatment of cells, while latrunculin B almost completely inhibited this insulin effect. GLUT4 translocation to 3T3-L1 adipocyte plasma membranes in response to insulin was almost completely inhibited when cells were treated with nocodazole and latrunculin B. Similar results were obtained when these agents were tested in cells infected with recombinant adenovirus expressing Gα11(Q209L) to enhance GLUT4 translocation, although nocodazole had a lesser effect than observed on insulin action. The increased GLUT4 in plasma membranes from adipocytes expressing Gα11(Q209L) was greatly inhibited by latrunculin B or latrunculin B plus nocodazole (Fig. 7). Quantification of these results by analysis of the ratios of anti-GLUT4 signal to WGA signal on the plasma membranes showed that significantly less GLUT4 was present in insulin-treated or Gα11(Q209L)-expressing adipocytes when they were treated with latrunculin B alone or together with nocodazole (Fig. 8). Interestingly, nocodazole alone more strongly inhibited GLUT4 translocation in cells treated with insulin compared with adipocytes infected with recombinant adenovirus expressing Gα11(Q209L) (about 15% inhibition, which is statistically insignificant).
FIG. 7.
Effects of nocodazole and latrunculin B on insulin and Gα11(Q209L)-induced GLUT4 translocation in differentiated 3T3-L1 adipocytes. 3T3-L1 adipocytes differentiated in six-well plates were infected with either control or Gα11(Q209L)-expressing adenovirus as described in Materials and Methods, treated with either nocodazole (N) or latrunculin B (L) alone or in combination (N + L) for 1 h, and stimulated with insulin (I) for 10 min. The plasma membrane lawns were then prepared and stained for GLUT4 (green) and total membranes (red). The data reflect results from four different experiments.
FIG. 8.
Luminosity ratio of GLUT4 to total membrane signals in plasma membrane lawns of nocodazole and latrunculin B-treated 3T3-L1 adipocytes. The total luminescence of the FITC signal (GLUT4) and the rhodamine signal (total membranes) from the plasma membrane lawns was calculated and plotted as in Fig. 4. The data represent the average of four separate experiments.
Next we addressed the important issue of what signaling pathway or pathways may be involved in mediating the effects of endothelin 1 and Gα11 on actin polymerization and GLUT4 translocation. A number of small GTP-binding proteins have been implicated in cortical F-actin formation, including RAC1 (50), CDC42 (49), and ARF6 (55). Notably, recent work has linked Gα11 function to the ARF6 GTPase in connection with its ability to modulate membrane ruffling in HeLa cells, a function dependent on F-actin (7). We therefore transiently transfected the dominant inhibitory construct HA-ARF6(T27N) into cultured adipocytes and assessed by its effect on cortical F-actin fluorescence microscopy (Fig. 9). The presence of this ARF6 inhibitor protein (indicated by arrows in Fig. 9, top). dramatically inhibited the bright rims of rhodamine-conjugated phalloidin that normally delineate F-actin near the plasma membrane of the cultured adipocytes. The majority of cells that were not transfected (indicated by arrowheads) on the same coverslip consistently displayed this intense F-actin signal in response to endothelin 1. In contrast, expression of HA-ARF6(T27N) in 3T3-L1 adipocytes was ineffective in modifying the strong effect of insulin to enhance actin polymerization near the cell surface (Fig. 9). This construct also failed to significantly modify the disposition of actin in untreated adipocytes. These data indicate that endothelin 1, but not insulin, signals to the actin cytoskeleton through a mechanism that requires normal ARF6 function. Quantification of this data is represented in the graph in Fig. 9.
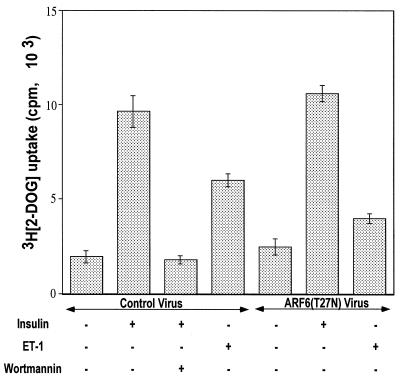
To evaluate a possible connection between ARF6 function and glucose transport regulation the HA-tagged ARF6(T27N) cDNA was engineered into recombinant adenovirus, and 3T3-L1 adipocytes were infected with this virus or control virus prior to addition of insulin or endothelin 1. Figure 10 depicts the results of this protocol on 2-deoxyglucose uptake. In this set of experiments, endothelin 1 stimulated hexose transport about threefold in cultured adipocytes infected with control virus. This effect of endothelin 1 was significantly inhibited by the expression of the dominant inhibitory ARF6 protein. In contrast, similar expression of HA-ARF6(T27N) in the 3T3-L1 adipocytes had no inhibitory effect on basal hexose uptake or on the marked response of 2-deoxyglucose uptake to insulin (Fig. 10). These results strongly support a major role for ARF6 function in the regulation of glucose transport by endothelin 1 but not insulin.
FIG. 10.
Dominant negative ARF6(T27N) inhibits 2-deoxyglucose uptake stimulated by endothelin 1 but not by insulin in 3T3-L1 adipoctes. Differentiated 3T3-L1 adipoctes were infected with ARF6(T27N)-expressing adenoviruses as described in the text. After serum starvation, the cells were treated with insulin or endothelin 1 (ET-1) for 30 min and then assayed for 2-deoxyglucose (2-DOG) uptake as described in the text. The data represents the average of four separate experiments.
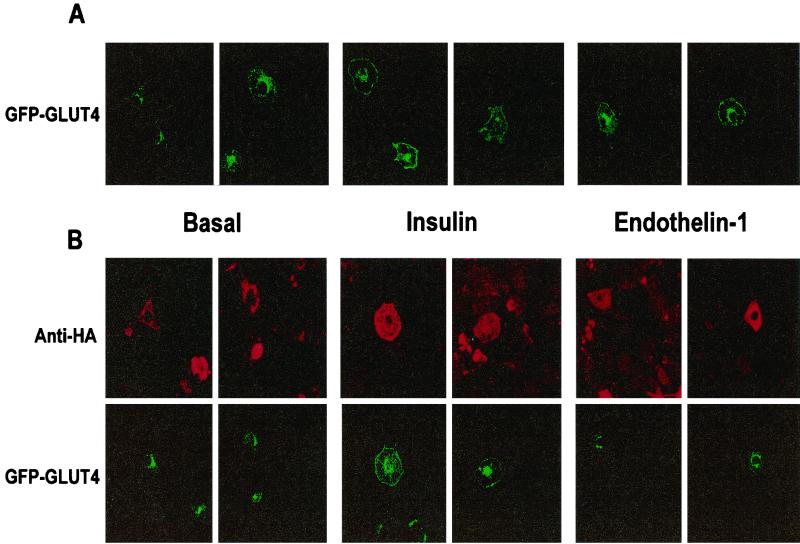
To further test this concept, experiments were also conducted to determine the effect of HA-ARF6(T27N) on GLUT4 translocation. Cultured adipocytes infected with recombinant adenovirus carrying HA-ARF6(T27N) exhibited the normal low amounts of GLUT4 on plasma membrane sheets attached to coverslips as control cells, and the large effect of insulin to increase GLUT4 on these membrane sheets was also unaffected by expression of HA-ARF6(T27N) (data not shown). However, this method was not suitable for assessing endothelin 1 function due to the lower anti-GLUT4 signal obtained in response to this peptide compared to insulin. Instead, we used a method whereby expressed GFP-GLUT4 can be visualized by fluorescence microscopy to move to the cell perimeter in response to insulin (34) or endothelin 1 (71). As shown in Fig. 11A, GFP-GLUT4 protein is concentrated in the perinuclear region of cultured control adipocytes transiently transfected with GFP-GLUT4 cDNA, while insulin or endothelin 1 can be observed to enhance fluorescence from this protein at the cell surface. In cells cotransfected with both HA-ARF6(T27N) and GFP-GLUT4, the GFP signal is also perinuclear in the basal state and can be seen to define the cell perimeter in response to insulin.
FIG. 11.
Dominant negative ARF6(T27N) inhibits GLUT4 translocation stimulated by endothelin 1 but not GLUT4 translocation stimulated by insulin. Differentiated 3T3-L1 adipocytes were transfected with GFP-GLUT4 alone (A) or with HA-ARF6(T27N) (B) by electroporation. After 48 h, the cells were serum starved for 4 h and then treated with either 100 nM insulin for 45 min or 10 nM endothelin 1 for 30 min. The cells were then fixed and stained with anti-HA antibody followed by rhodamine-conjugated secondary antibody to detect HA-ARF6(T27N)-transfected cells. The data represent four separate experiments.
Consistent with the data in Fig. 10, the expression of HA-ARF6(T27N) protein inhibits the display of GFP-GLUT4 at the cell surface normally seen in response to endothelin 1 (Fig. 11B). Quantification of the GFP-GLUT4 signal in these images reveals that only 5% of cells transfected with GFP-GLUT4 showed GLUT4 rims under basal conditions, which increased to 55 and 40% in cells stimulated with insulin and endothelin 1, respectively. However, when the cells were transfected with both the GFP-GLUT4 and HA-ARF6(T27N) constructs, the number of cells displaying GLUT4 rims decreased dramatically (to ∼20%) in response to endothelin 1, while the dominant inhibitory ARF6 had no effect in basal and insulin-stimulated cells. Thus, our data indicate that dominant inhibitory ARF6 protein inhibits F-actin polymerization and both the stimulation of 2-deoxyglucose uptake and GLUT4 translocation in response to endothelin 1. In contrast, it is without effect on these three responses to insulin (Fig. 9 to 11).
DISCUSSION
The results presented in this paper show that Gα11 signaling mimics the actions of insulin on both actin cytoskeletal rearrangements and the translocation of GLUT4 to the cell surface of cultured adipocytes, though the mechanisms involved clearly differ. Our data also indicate that signaling by endothelin 1 through the Gαq family of trimeric G-protein α subunits not only stimulates glucose transport in 3T3-L1 adipocytes (Fig. 10 and references 30, 31, 34, and 71) but also mobilizes cortical F-actin (Fig. 9). Our results suggest that the Gα11 isoform of the Gαq-related proteins displays unusually high expression in insulin-sensitive murine adipocytes. By both Northern analysis of mRNA (data not shown) and Western blotting using anti-Gα11 antibody, we found a striking and selectively high expression of the Gα11 isoform of Gαq in fat (Fig. 1). Interestingly, significant expression of Gα11 protein was also observed in the other two tissues that exhibit insulin-sensitive GLUT4 regulation, skeletal muscle and heart (Fig. 1). We also find a large increase in Gα11 expression upon differentiation of 3T3-L1 fibroblasts to adipocytes (Fig. 1), consistent with a report that appeared as we were preparing this report (34). In contrast, Gαq expression is lower in fat than in other tissues, although its expression in muscle is higher (Fig. 1). Endothelin 1 stimulates adipocyte glucose transport through a receptor known to signal through the Gαq-type trimeric G proteins (71). Hence, our data show that the stimulation of glucose transport by endothelin 1 in these cells is likely mediated primarily through the Gα11 protein, which is so markedly abundant in fat.
The experiments reported here directly address an important question related to the stimulatory actions of endothelin 1 and Gα11 on glucose transport: what are the underlying signaling mechanisms that mediate their effects on GLUT4? An important clue derives from our findings that endothelin 1 and the constitutively active Gα11(Q209L) share with insulin the ability to mobilize cortical F-actin in cultured adipocytes (Fig. 5 and 9). Very recent work has implicated the GTP-binding protein ARF6 in mediating Gαq signaling to peripheral cytoskeletal rearrangements in response to bombesin in another cell type (7). ARF6 is one of a family of closely related proteins that includes ARF1, which has been implicated in initiating the coating of intracellular membranes necessary for budding (47). ARF6 itself has been linked to both movements of specialized endosomal recycling membranes (1, 9, 54, 55) and F-actin polymerization (17, 29, 74). We therefore tested whether ARF6 may be required for endothelin 1 action through Gα11 in cultured adipocytes and found marked inhibitions of both F-actin assembly and GLUT4 translocation by expressed dominant negative ARF6(T27N) (Fig. 9 to 11). These data strongly suggest the novel concept that ARF6 function is necessary for endothelin 1 action on F-actin polymerization and glucose transport in cultured adipocytes. Recent findings by Lawrence and Birnbaum (41) also indicate that dominant negative ARF6(T27N) inhibits activated Gαq(Q209L)-stimulated glucose transport. This finding along with the data presented here are consistent with the hypothesis that ARF6 is activated as a downstream signaling element of Gα11 in endothelin 1 action on these cell processes. This latter point will require further work to document unequivocally.
The selectivity of the effect of ARF6(T27N) expression in cultured adipocytes to inhibit the actions of endothelin 1 but not insulin is striking (Fig. 9 to 11). The failure of ARF6(T27N) to interfere with insulin action on either actin or GLUT4 agrees with a recent report showing that dominant negative ARF6 expression does not affect insulin action on GLUT4 (72). These data indicate that the insulin signaling pathways to both F-actin mobilization and GLUT4 are distinct from those employed by endothelin 1 and Gα11(Q209L). Yet this conclusion seems to conflict with the interpretation of recent data from two other laboratories (31, 34). In those studies, antibodies against Gαq/11 were reported to block insulin action on GLUT4 translocation when introduced into 3T3-L1 adipocytes, indicating that Gα11 may act downstream of insulin receptor activation (31, 34). Expression in 3T3-L1 adipocytes of the GTPase-activating protein RGS2, which acts to deactivate Gα11, also inhibited insulin action on GLUT4 (31). One possible explanation of these data is that some ARF6-independent function of Gα11 is required for effective insulin signaling or for the membrane trafficking events involved in GLUT4 exocytosis. Perhaps this ARF6-independent function of Gα11 plays a permissive role for insulin action and is not on the direct path of insulin signaling. It is also noted that a constitutively active mutant of ARF6 was reported not to stimulate GLUT4 translocation (72), and we have confirmed this (data not shown). Thus, ARF6 function appears necessary but not sufficient for Gα11-mediated GLUT4 translocation. In any case, the data we present strongly suggest that the signaling events initiated by endothelin 1 and Gα11 to modulate F-actin and GLUT4 require ARF6 function and are distinct from those triggered by insulin.
If the signaling pathways for Gα11 and insulin signaling differ, where do they diverge? Insulin action on GLUT4 is completely dependent on PI3-kinase activity, but there are conflicting reports in the literature on whether Gαq/11 signaling stimulates glucose transport by a mechanism that requires PI3-kinase activity. Imamura et al. suggested that insulin and endothelin 1 stimulate GLUT4 translocation and transport in 3T3-L1 adipocytes via a pathway involving Gαq/11 that is blocked by wortmannin (30, 31). In contrast, Wu-Wong et al. (71) reported that endothelin 1 stimulates glucose transport in 3T3-L1 adipocytes even in the presence of wortmannin. In apparent agreement with this view, Kanzaki et al. (34) found that the effect of activated Gαq to recruit GLUT4 to the cell surface membrane is not sensitive to either wortmannin or expression of a dominant negative construct of the p85 regulatory subunit of PI3-kinase.
The results presented in here also show that Gα11(Q209L)-stimulated GLUT4 translocation and glucose transport in 3T3-L1 adipocytes are independent of the PI3-kinase activity that is required for insulin action (Fig. 2 and 4). Wortmannin at 100 nM had no effect on endothelin 1 or Gα11 action. The reason for this apparent discrepancy is not clear. One possibility is the different concentrations of wortmannin used in the various studies. The study by Kanzaki et al. (34) and the present work show that no effect of 100 nM wortmannin on Gα11 or Gαq is apparent, while Imamura et al. (31) used 300 nM wortmannin to inhibit the actions of Gαq. We could confirm that such high doses of wortmannin do inhibit Gα11(Q209L) action on 2-deoxyglucose uptake in 3T3-L1 adipocytes (data not shown). In these same experiments, we observed that insulin action was blocked by 100 nM wortmannin (Fig. 2 to 4), in agreement with many other reports (10–12). The p85/p110-type PI3-kinase activity is known to be inhibited half-maximally by only 2 nM wortmannin (67), while complete inhibition of PI3-kinase activity occurs at a 100 nM concentration of the drug (35). Thus, perhaps at 300 nM wortmannin other cellular targets of the drug are inhibited (48), accounting for the results of Imamura et al. (31). Further experiments will be required to clarify this issue. Taken together, the data available are consistent with a model whereby insulin signaling diverges from Gα11 signaling at the earliest steps in their respective mechanisms.
A well-established signaling pathway for Gαq/11 is the activation of phospholipase Cβ, which catalyzes hydrolysis of PI 4,5-bisphosphate to form inositol 1,4,5-triphosphate and diacylglycerol. The inositol 1,4,5-triphosphate released into the cytoplasm mobilizes Ca2+ from internal stores, whereas diacylglycerol activates protein kinase C (5). Consistent with this model, GLUT4 translocation in differentiated 3T3-L1 adipocytes in response to endothelin 1 was accompanied by a stimulation in PI hydrolysis (71). However the phospholipase C inhibitor U-73122 (30) and the protein kinase C inhibitor bisindolylmaleimide (71) both failed to block endothelin 1-stimulated glucose transport, strongly suggesting that GLUT4 regulation by Gαq/11 occurs via a novel pathway. Our data strongly implicate ARF6 in this novel Gα11 signaling pathway to GLUT4 (Fig. 9 to 11).
A recent report suggested that in response to agonists, GLUT4 is recruited to the plasma membrane from two different intracellular compartments: the recycling endosomal compartment and a specialized secretory compartment termed GLUT4 storage vesicles (45). Results presented in that report suggested that GTPγS selectively stimulates recycling of GLUT4 via the endosomal system, while insulin regulates this pathway in addition to stimulating exocytosis of GLUT4 from the specialized GLUT4 storage vesicles. Consistent with this concept GTPγS, like Gα11 in the present study, stimulated GLUT4 translocation to a lesser extent than insulin. It is possible that Gαq/11, like GTPγS, also stimulates GLUT4 translocation mostly by modulating endosomal GLUT4 rather than the insulin-sensitive specialized compartment of GLUT4. These considerations are also consistent with the hypothesis that the signaling elements that mediate insulin versus Gα11 actions on GLUT4 are distinct.
The effects of insulin, endothelin 1, and Gα11(Q209L) on 3T3-L1 adipocytes include both the mobilization of cortical F-actin and the regulation of GLUT4 translocation and glucose transport, indicating these two classes of responses may be related. The results presented here showing that disassembly of adipocyte F-actin through the action of latrunculin B blocks the effects of these agents on GLUT4 translocation (Fig. 7 and 8) is consistent with a direct role of cortical F-actin in glucose transport regulation. These data are also consistent with the suggestion that cortical F-actin mobilization is a mechanism for colocalizing PI3-kinase with GLUT4 vesicles, which may be required for GLUT4 translocation (36). Our results are also compatible with the concept, analogous to that developed with other membrane trafficking systems (57, 58), that cytoskeletal elements including microtubules (Fig. 9 and 10), actin filaments, and their motors play integral roles in GLUT4 vesicle movement. It is interesting that ARF6 modulation of endosomal recycling, apparently necessary for membrane ruffling (29), and its induction of actin protrusions at the plasma membrane is reported to be mediated through different regions within the ARF6 sequence (55). Thus, in the case of endothelin 1 action, the effects on actin and GLUT4 may be mediated independently by ARF6 through these two domains. Alternatively, it is possible that the mobilization of cortical F-actin may be necessary for the translocation of GLUT4 in response to both endothelin 1 and insulin. According to this model, the different signaling pathways for insulin and endothelin 1 to regulate GLUT4 would hypothetically converge at the mobilization of the actin cytoskeleton. Within this concept, cortical F-actin formation would be required but not necessarily sufficient for GLUT4 regulation. In this regard, it has been proposed that insulin action on GLUT4 requires two independent signals, one dependent on and one independent of PI3-kinase (4, 14). It is tempting to speculate that the role of one of these two pathways may be to regulate actin cytoskeletal rearrangements. Further attempts to enhance our understanding of the role of actin in regulation of GLUT4 should be of great interest. Current experiments are devoted to unraveling this question.
ACKNOWLEDGMENTS
We sincerely thank Hiroshi Itoh for providing the pCMV5-Gα11 (wild type) and pCMV5-Gα11(Q209L) constructs and Bert Vogelstein for the pAdTrack-CMV and pAdEasy-1 plasmids. We also thank Morris J. Birnbaum and John T. R. Lawrence for discussions and critical reading of the manuscript.
This work was supported in part by National Institutes of Health grant DK30648 (M.P.C.) and by a mentor-based fellowship to A.B. from the American Diabetes Association
REFERENCES
- 1.Al-Anwar O, Radhakrishna H, Donaldson J G. Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant. Mol Cell Biol. 2000;20:5998–6007. doi: 10.1128/mcb.20.16.5998-6007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amatruda T, Steele D, Slepak V, Simon M I. G alpha 16, a G protein alpha subunit specifically expressed in hematopoietic cells. Proc Natl Acad Sci USA. 1991;88:5587–5591. doi: 10.1073/pnas.88.13.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldini G, Hohman R, Charron M J, Lodish H F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT 4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J Biol Chem. 1991;266:4037–4040. [PubMed] [Google Scholar]
- 4.Baumann C A, Ribon V, Kanzaki M, Thurmond D C, Mora S, Shigematsu S, Bickel P E, Pessin J E, Saltiel A R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- 5.Berridge M J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum M J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- 7.Boshans R L, Szanto S, Alest L V, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol Cell Biol. 2000;20:3685–3694. doi: 10.1128/mcb.20.10.3685-3694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brozinick J T, Birnbaum M J. Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J Biol Chem. 1998;273:14679–14682. doi: 10.1074/jbc.273.24.14679. [DOI] [PubMed] [Google Scholar]
- 9.Caumont A S, Vitale N, Gensse M, Galas M C, Casanova J E, Bader M F. Identification of a plasma membrane-associated guanine nucleotide exchange factor for ARF6 in chromaffin cells. Possible role in the regulated exocytotic pathway. J Biol Chem. 2000;275:15637–15644. doi: 10.1074/jbc.M908347199. [DOI] [PubMed] [Google Scholar]
- 10.Charron M J, Brosius F C, Alper S L, Lodish H F. A glucose transport protein expressed predominantly in insulin-responsive tissues. Proc Natl Acad Sci USA. 1989;86:2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheatham B, Vlahos C, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA syntesis and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- 13.Cushman S W, Wardzala L J. Potential mechanism of insulin action on glucose transport in isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- 14.Czech M P. Lipid rafts and insulin action. Nature. 2000;407:147–148. doi: 10.1038/35025183. [DOI] [PubMed] [Google Scholar]
- 15.Czech M P, Buxton J M. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J Biol Chem. 1993;268:9187–9190. [PubMed] [Google Scholar]
- 16.Czech M P, Chawla A, Woon C W, Buxton J M, Armoni M, Tang W, Joly M, Corvera S. Exofacial epitope-tagged glucose transporter chimeras reveal COOH-terminal sequences governing cellular localization. J Cell Biol. 1993;123:127–135. doi: 10.1083/jcb.123.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Souza-Schorey C, Boshans R L, McDonough M, Stahl P D, Alest L V. A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmendorf J S, Chen D, Pessin J E. Guanosine 5′-O-(3-thiotriphosphate) (GTPγS) stimulation of GLUT4 translocation is tyrosine kinase-dependent. J Biol Chem. 1998;273:13289–13296. doi: 10.1074/jbc.273.21.13289. [DOI] [PubMed] [Google Scholar]
- 19.Frost S C, Lane M D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3–L1 adipocytes. J Biol Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- 20.Fukumoto H, Kayano T, Buse J B, Edwards Y, Pilch P F, Bell G I, Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989;264:7776–7779. [PubMed] [Google Scholar]
- 21.Gao Z, Robeva A S, Linden J. Purification of A1 adenosine receptor-G-protein complexes: effects of receptor down-regulation and phosphorylation on coupling. Biochem J. 1999;338:729–736. [PMC free article] [PubMed] [Google Scholar]
- 22.Green A, Walters D J A, Belt S E. Tumor necrosis factor increases the rate of lipolysis in primary cultures of adipocytes without altering levels of hormone-sensitive lipase. Endocrinology. 1994;136:E254–E261. doi: 10.1210/endo.134.6.8194485. [DOI] [PubMed] [Google Scholar]
- 23.Han D H, Hansen P A, Nolte L A, Holloszy J O. Removal of adenosine decreases the responsiveness of muscle glucose transport to insulin and contractions. Diabetes. 1998;47:1671–1675. doi: 10.2337/diabetes.47.11.1671. [DOI] [PubMed] [Google Scholar]
- 24.Haney P M, Slot J W, Piper R C, James D E, Mueckler M. Intracellular targeting of the insulin-regulatable glucose transporter (GLUT4) is isoform specific and independent of cell type. J Cell Biol. 1991;114:689–699. doi: 10.1083/jcb.114.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison S A, Buxton J M, Clancy B M, Czech M P. Insulin regulation of hexose transport in mouse 3T3–L1 cells expressing the human HepG2 glucose transporter. J Biol Chem. 1990;265:20106–20116. [PubMed] [Google Scholar]
- 26.Hayashi T, Hirshman M F, Kurth E J, Winder W W, Goodyear L J. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 27.He T C, Zhou S, DaCosta L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirshman C A, Emala C W. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am J Physiol. 1999;277:L653–L661. doi: 10.1152/ajplung.1999.277.3.L653. [DOI] [PubMed] [Google Scholar]
- 29.Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris A J, Frohman M A, Knaho Y. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;5:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 30.Imamura T, Ishibashi K, Dalle S, Ugi S, Olefsky J M. Endothelin-1-induced GLUT4 translocation is mediated via Galpha(q/11) protein and phosphatidylinositol 3-kinase in 3T3–L1 adipocytes. J Biol Chem. 1999;274:33691–33695. doi: 10.1074/jbc.274.47.33691. [DOI] [PubMed] [Google Scholar]
- 31.Imamura T, Vollenweider P, Egawa K, Clodi M, Ishibashi K, Nakashima N, Ugi S, Adams J W, Brown J H, Olefsky J M. G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3–L1 adipocytes. Mol Cell Biol. 1999;19:6765–6774. doi: 10.1128/mcb.19.10.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joost H G, Weber T M, Cushman S W, Simpson I A. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J Biol Chem. 1986;261:10033–10036. [PubMed] [Google Scholar]
- 33.Kanai F, Ito K, Todaka M, Hayashi H, Kamohara S, Ishii K, Okada O, Hazeki O, Ui M, Ebina Y. Insulin-stimulated GLUT4 translocation is relevant to the phospohorylation of IRS-1 and the activity of PI3-kinase. Biochem Biophys Res Commun. 1993;195:762–768. doi: 10.1006/bbrc.1993.2111. [DOI] [PubMed] [Google Scholar]
- 34.Kanzaki M, Watson R T, Artemyev N O, Pessin J E. The trimeric GTP-binding protein (G(q)/G(11)) alpha subunit is required for insulin-stimulated GLUT4 translocation in 3T3L1 adipocytes. J Biol Chem. 2000;275:7167–7175. doi: 10.1074/jbc.275.10.7167. [DOI] [PubMed] [Google Scholar]
- 35.Kelly K L, Ruderman N B. Insulin-stimulated phosphatidylinositol 3-kinase. Association with a 185 kDa tyrosine-phosphorylated protein (IRS-1) and localization in a low density membrane vesicle. J Biol Chem. 1993;268:4391–4398. [PubMed] [Google Scholar]
- 36.Khayat Z A, Tong P, Yaworsky K, Bloch R J, Klip A. Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J Cell Sci. 2000;113:279–290. doi: 10.1242/jcs.113.2.279. [DOI] [PubMed] [Google Scholar]
- 37.Kishi K, Hayashi H, Wang L, Kamohara S, Tamaoka M, Shimizu T, Ushikubi F, Narumiya S, Ebina Y. Gq-coupled receptors transmit the signal for GLUT4 translocation via an insulin-independent pathway. J Biol Chem. 1996;271:26561–26568. doi: 10.1074/jbc.271.43.26561. [DOI] [PubMed] [Google Scholar]
- 38.Kishi K, Muromoto N, Nakaya Y, Miyata I, Hagi A, Hayashi H, Ebina Y. Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes. 1998;47:550–557. doi: 10.2337/diabetes.47.4.550. [DOI] [PubMed] [Google Scholar]
- 39.Kuroda M, Honnor R C, Cushman S W, Londos C, Simpson I A. Regulation of insulin-stimulated glucose transport in the isolated rat adipocyte. cAMP-independent effects of lipolytic and antilipolytic agents. J Biol Chem. 1987;262:245–253. [PubMed] [Google Scholar]
- 40.Langille S E, Patki V, Klarlund J K, Buxton J M, Holik J J, Chawla A, Corvera S, Czech M P. ADP-ribosylation factor 6 as a target of guanine nucleotide exchange factor GRP1. J Biol Chem. 1999;274:27099–27104. doi: 10.1074/jbc.274.38.27099. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence J T R, Birnbaum M J. ADP-ribosylation factor 6 delineates separate pathways used by endothelin 1 and insulin for stimulating glucose uptake in 3T3–L1 adipocytes. Mol Cell Biol. 2001;21:5276–5285. doi: 10.1128/MCB.21.15.5276-5285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall B A, Murata H, Hresko R C, Mueckler M. Domains that confer intracellular sequestration of the Glut4 glucose transporter in Xenopus oocytes. J Biol Chem. 1993;268:26193–16199. [PubMed] [Google Scholar]
- 43.Martin L B, Shewan A, Millar C A, Gould G W, James D E. Vesicle associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3–L1 adipocytes. J Biol Chem. 1998;273:1444–1452. doi: 10.1074/jbc.273.3.1444. [DOI] [PubMed] [Google Scholar]
- 44.Martin S S, Haruta T, Morris A J, Kippel A, Williams L T, Olefsky J M. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3 L-1 adipocytes. J Biol Chem. 1996;271:17605–17608. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- 45.Millar C A, Shewan A, Hickson G R X, James D E, Gould G W. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3–L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min J, Okada S, Coker K, Ceresa B P, Elmendorf J S, Syu L J, Noda Y, Saltiel A R, Pessin J E. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol Cell. 1999;3:751–760. doi: 10.1016/s1097-2765(01)80007-1. [DOI] [PubMed] [Google Scholar]
- 47.Moss J, Vaughan M. Molecules in the ARF orbit. J Biol Chem. 1998;273:21431–21434. doi: 10.1074/jbc.273.34.21431. [DOI] [PubMed] [Google Scholar]
- 48.Naknishi S, Kakita S, Takahashi I, Kawahara K, Tsukuda E, Sano T, Yamada K, Yoshida M, Kase H, Matsuda Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J Biol Chem. 1992;267:2157–2163. [PubMed] [Google Scholar]
- 49.Nobes C D, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 50.Nobes C D, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:226–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- 51.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 52.Omata W, Shibata H, Li L, Takata K, Kojima I. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem J. 2000;346:321–328. [PMC free article] [PubMed] [Google Scholar]
- 53.Ozawa K, Takahashi M, Sobue K. Phase specific association of heterotrimeric GTP-binding proteins with the actin-based cytoskeleton during thrombin receptor-mediated platelet activation. FEBS Lett. 1996;382:159–163. doi: 10.1016/0014-5793(96)00162-7. [DOI] [PubMed] [Google Scholar]
- 54.Radhakrishna H, Al-Anwar O, Khachikian Z, Donaldson J G. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- 55.Radhakrishna H, Klausner R D, Donaldson J G. Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J Cell Biol. 1996;134:935–946. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson L J, Pang S, Harris D S, Heuser J, James D E. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3–L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J Biol Chem. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodionov V I, Hope A J, Svitkina T M, Borisy G G. Functional coordination of microtubule-based and actin-based motility in melanophores. Curr Biol. 1998;8:165–168. doi: 10.1016/s0960-9822(98)70064-8. [DOI] [PubMed] [Google Scholar]
- 58.Rogers S L, Gelfand V. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr Biol. 1998;8:161–164. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- 59.Sharma P M, Egawa K, Huang Y, Martin J L, Huvar I, Boss G R, Olefsky J M. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus mediated gene transfer and its effect on insulin action. J Biol Chem. 1998;273:18528–18537. doi: 10.1074/jbc.273.29.18528. [DOI] [PubMed] [Google Scholar]
- 60.Shibasaki Y, Ishihara H, Kizuki N, Asano T, Oka Y, Yazaki Y. Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J Biol Chem. 1997;272:7578–7581. doi: 10.1074/jbc.272.12.7578. [DOI] [PubMed] [Google Scholar]
- 61.Simon M I, Strathmann M P, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 62.Smith U, Kuroda M, Simpson I A. Counter-regulation of insulin-stimulated glucose transport by catecholamines in the isolated rat adipose cell. J Biol Chem. 1994;259:8758–8763. [PubMed] [Google Scholar]
- 63.Spector I, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsakiridis T, Vranic M, Klip A. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J Biol Chem. 1994;269:29934–29942. [PubMed] [Google Scholar]
- 66.Verhey K J, Birnbaum M J. A Leu-Leu sequence is essential for COOH-terminal targeting signal of GLUT4 glucose transporter in fibroblasts. J Biol Chem. 1994;269:2353–2356. [PubMed] [Google Scholar]
- 67.Virbasius J V, Guilherme A, Czech M P. Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J Biol Chem. 1996;271:13304–13307. doi: 10.1074/jbc.271.23.13304. [DOI] [PubMed] [Google Scholar]
- 68.Vollenweider P, Martin S S, Haruta T, Morris A J, Nelson J G, Cormont N, Le Marchand-Brustel Y, Rose D W, Olefsky J M. The small guanosine triphosphate-binding protein Rab4 is involved in insulin-induced GLUT4 translocation and actin filament rearrangement in 3T3–L1 cells. Endocrinology. 1997;138:4941–4949. doi: 10.1210/endo.138.11.5493. [DOI] [PubMed] [Google Scholar]
- 69.Wang Q H, Bilan P J, Tsakiridis T, Hinek A, Klip A. Actin filaments participate in the relocalization of phosphatidylinositol 3-kinase to glucose transporter-containing compartments and in the stimulation of glucose uptake in 3T3–L1 adipocytes. Biochem J. 1998;331:917–928. doi: 10.1042/bj3310917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson P T, Bourne H R. Fatty acylation of alpha z. Effects of palmitoylation and myristoylation on alpha z signaling. J Biol Chem. 1995;270:9667–9675. doi: 10.1074/jbc.270.16.9667. [DOI] [PubMed] [Google Scholar]
- 71.Wu-Wong J R, Berg C E, Wang J, Chiou W J, Fissel B. Endothelin stimulates glucose uptake and GLUT4 translocation via activation of endothelin ETA receptor in 3T3–L1 adipocytes. J Biol Chem. 1999;274:8103–8110. doi: 10.1074/jbc.274.12.8103. [DOI] [PubMed] [Google Scholar]
- 72.Yang C Z, Mueckler M. ADP-ribosylation factor 6 (ARF6) defines two insulin-regulated secretory pathways in adipocytes. J Biol Chem. 1999;274:25297–25300. doi: 10.1074/jbc.274.36.25297. [DOI] [PubMed] [Google Scholar]
- 73.Yeh J-I, Gulve E A, Rameh L, Birnbaum M J. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem. 1995;270:2107–2111. doi: 10.1074/jbc.270.5.2107. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Q, Calafat J, Janssen H, Greenberg S. ARF6 is required for growth factor- and Rac-mediated membrane ruffling in macrophages at a stage distal to Rac membrane targeting. Mol Cell Biol. 1999;12:8158–8168. doi: 10.1128/mcb.19.12.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]