The high-resolution structure of a putative short-chain reductase from the commercially important bacterium Paraburkholderia xenovorans is reported. P. xenovorans degrades organic wastes such as polychlorinated biphenyls.
Keywords: SSGCID, structural genomics, Paraburkholderia xenovorans, oxidoreductases, education and training, detoxification, Seattle Structural Genomics Center for Infectious Disease
Abstract
Paraburkholderia xenovorans degrades organic wastes, including polychlorinated biphenyls. The atomic structure of a putative dehydrogenase/reductase (SDR) from P. xenovorans (PxSDR) was determined in space group P21 at a resolution of 1.45 Å. PxSDR shares less than 37% sequence identity with any known structure and assembles as a prototypical SDR tetramer. As expected, there is some conformational flexibility and difference in the substrate-binding cavity, which explains the substrate specificity. Uniquely, the cofactor-binding cavity of PxSDR is not well conserved and differs from those of other SDRs. PxSDR has an additional seven amino acids that form an additional unique loop within the cofactor-binding cavity. Further studies are required to determine how these differences affect the enzymatic functions of the SDR.
1. Introduction
Paraburkholderia xenovorans is a model bacterium used to study unique pathways, notably the ability of this genus of bacteria to degrade polychlorinated biphenyls and other organic waste products (Francova et al., 2004 ▸; Tehrani et al., 2014 ▸). The genome of P. xenovorans has been sequenced and is one of the largest bacterial genomes studied to date. P. xenovorans has diverse functions, including nitrogen fixation, the catabolysis of aromatic compounds and the degradation of various organic wastes (Francova et al., 2004 ▸; Tehrani et al., 2014 ▸). However, the underlying mechanisms of many of these processes are poorly understood. Additionally, unlike some other Paraburkholderia species, P. xenovorans is not pathogenic to humans. The Seattle Structural Genomics Center for Infectious Disease (SSGCID) selected targets from P. xenovorans for high-throughput structural studies to increase the breadth of drug-target structures to aid drug development against human pathogenic Burkholderia species such as B. mallei and B. pseudomallei. Here, we present the structure of one of these target proteins, a short-chain dehydrogenase/reductase (SDR) which shares less than 37% sequence identity with any published structure. P. xenovorans SDR (PxSDR) is predicted to be a type II fatty-acid synthetase and NAD(P)(H)-dependent oxidoreductase involved in the metabolism of diverse molecules, including lipids, amino acids, carbohydrates, cofactors, hormones and xenobiotics, or other compounds. The structure reported here may offer insights into the metabolism of small molecules by P. xenovorans.
2. Materials and methods
2.1. Macromolecule production
Cloning, expression and purification were conducted as part of the Seattle Structural Genomics Center for Infectious Disease (SSGCID; Myler et al., 2009 ▸; Stacy et al., 2011 ▸) following standard protocols described previously (Bryan et al., 2011 ▸; Choi et al., 2011 ▸; Serbzhinskiy et al., 2015 ▸). The full-length putative short-chain reductase (PxSDR, UniProt Q13GE3) was PCR-amplified from genomic DNA using the primers shown in Table 1 ▸. The resultant amplicon was cloned into the ligation-independent cloning (LIC; Aslanidis & de Jong, 1990 ▸) expression vector pBG1861 encoding a noncleavable 6×His fusion tag (MAHHHHHHM-ORF). Plasmid DNA was transformed into chemically competent Escherichia coli BL21(DE3)R3 Rosetta cells. The plasmid containing His-PxSDR was expression-tested and 2 l of culture was grown using auto-induction medium (Studier, 2005 ▸) in a LEX Bioreactor (Epiphyte Three Inc.). The expression clone for PxSDR, BuxeA.00010.c.B1.GE39410, is available at https://www.ssgcid.org/available-materials/expression-clones/.
Table 1. Macromolecule-production information.
| Source organism | Paraburkholderia xenovorans (strain LB400) |
| DNA source | Genomic DNA, provided by Dr Mary Lidstrom (University of Washington, USA) |
| Forward primer | 5′-CTCACCACCACCACCACCATATGAGTTCAGCAGGAAGATTGCAG-3′ |
| Reverse primer | 5′-ATCCTATCTTACTCACTTAATCGCAGCGGGCGCTCATCC-3′ |
| Expression vector | pBG1861 |
| Expression host | Escherichia coli BL21(DE3)R3 Rosetta cells |
| Complete amino-acid sequence of the construct produced | MAHHHHHHMSSAGRLQGKVALVTGAGCIGPGWGNGRAIAVRFAEEGAHVIAVDRDLASMDATLELVRAAGGSVTPCLCDVTDSASVERLVADSVARCGRVDILVNNVGAPSPGGPVALDEAQWAMQLELNLTTAFLMCKYVLPVMEQQGGGAIVNIASTSGIRWTGAAQVGYAAAKAGMIQMGRVVAVEYAAKNVRVNSVVPGLLHTPMVDTKIAHNQAGGDVELLLRKRQARIPMPFMGDGRDTANAALFLASDEARFVTGTEIVVDGGMSARCD |
His-PxSDR was purified in a two-step protocol consisting of an immobilized metal-affinity chromatography (IMAC) step and size-exclusion chromatography (SEC). All chromatography runs were performed on an ÄKTApurifier 10 (GE) using automated IMAC and SEC programs according to previously described procedures (Bryan et al., 2011 ▸). Thawed bacterial pellets were lysed by sonication in 200 ml buffer consisting of 25 mM HEPES pH 7.0, 500 mM NaCl, 5% glycerol, 0.5% CHAPS, 30 mM imidazole, 10 mM MgCl2, 1 mM TCEP, 250 µg ml−1 AEBSF, 0.025% azide. After sonication, the crude lysate was clarified with 20 µl (25 units µl−1) benzonase and incubated while mixing at room temperature for 45 min. The lysate was then clarified by centrifugation at 10 000 rev min−1 for 1 h using a Sorvall centrifuge (Thermo Scientific). The clarified supernatant was then passed over an Ni–NTA HisTrap FF 5 ml column (GE Healthcare) which was pre-equilibrated with loading buffer composed of 25 mM HEPES pH 7.0, 500 mM NaCl, 5% glycerol, 30 mM imidazole, 1 mM TCEP, 0.025% sodium azide. The column was washed with 20 column volumes (CV) of loading buffer and was eluted with loading buffer plus 250 mM imidazole in a linear gradient over 7 CV. Peak fractions, as determined by UV absorption at 280 nm, were pooled and concentrated to 5 ml. A SEC column (Superdex 75, GE) was equilibrated with running buffer composed of 25 mM HEPES pH 7.0, 500 mM NaCl, 5% glycerol, 2 mM DTT, 0.025% azide. The peak fractions were collected and analyzed for the protein of interest using SDS–PAGE. The SEC peak fractions eluted as a single large peak at a molecular mass of ∼76 kDa, suggesting a dimeric enzyme. The peak fractions were pooled and concentrated to 45 mg ml−1 using an Amicon purification system (Millipore). Aliquots of 200 µl were flash-frozen in liquid nitrogen and stored at −193 K until use for crystallization.
2.2. Crystallization
PxSDR was crystallized by the sitting-drop vapor-diffusion method using the JCSG+ commercial crystallization screen (Rigaku Reagents). Crystals were obtained by mixing 0.4 µl protein solution at 22.5 mg ml−1 with 0.4 µl precipitant (Rigaku Reagents JCSG+ screen condition H11: 0.2 M magnesium chloride, 0.1 M bis-Tris pH 5.5, 25% PEG 3350), equilibrating against a reservoir consisting of 80 µl precipitant and incubating at 287 K (Table 2 ▸). A single crystal was transferred into a cryoprotectant (reservoir solution supplemented with 20% ethylene glycol) and vitrified by plunging into liquid nitrogen before data collection.
Table 2. Crystallization.
| Method | Vapor diffusion, sitting drop |
| Plate type | 96-well, Compact 300, Rigaku |
| Temperature (K) | 287 |
| Protein concentration | PxSDR (BuxeA.00010.c.B1.PS02595) at 22.5 mg ml−1 |
| Buffer composition of protein solution | 25 mM HEPES pH 7.0, 500 mM NaCl, 5% glycerol, 2 mM DTT, 0.025% azide |
| Composition of reservoir solution | Rigaku Reagents JCSG+ screen H11: 0.2 M magnesium chloride, 0.1 M bis-Tris pH 5.5, 25% PEG 3350 |
| Volume and ratio of drop | 0.4 µl protein:0.4 µl reservoir (1:1) |
| Volume of reservoir (µl) | 80 |
2.3. Data collection and processing
X-ray diffraction data were collected on LS-CAT beamline 21-ID-F at the Advanced Photon Source. Data were integrated using XDS and reduced with XSCALE (Kabash, 2010 ▸). Additional data-collection information is provided in Table 3 ▸. The raw images and detailed data-collection information are available for download (https://proteindiffraction.org/project/5jc8/).
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | Beamline 21-ID-F, APS |
| Wavelength (Å) | 0.97872 |
| Temperature (K) | 100 |
| Detector | RayoniX MX-225 CCD |
| Crystal-to-detector distance (mm) | 140 |
| Rotation range per image (°) | 1 |
| Total rotation range (°) | 240 |
| Exposure time per image (s) | 1 |
| Space group | P21 |
| a, b, c (Å) | 71.18, 81.08, 86.37 |
| α, β, γ (°) | 90, 94.53, 90 |
| Mosaicity (°) | 0.145 |
| Resolution range (Å) | 50–1.45 (1.49–1.45) |
| Total No. of reflections | 855886 (53861) |
| No. of unique reflections | 165687 (10796) |
| Completeness (%) | 95.8 (84.8) |
| Multiplicity | 5.2 (5.0) |
| 〈I/σ(I)〉 | 25.10 (4.45) |
| R r.i.m. † | 0.042 (0.392) |
| Overall B factor from Wilson plot (Å2) | 12.76 |
Estimated R r.i.m. = R merge[N/(N − 1)]1/2, where N is the data multiplicity.
2.4. Structure solution and refinement
The structure of PxSDR was solved by molecular replacement with MOLREP (Vagin & Teplyakov, 2010 ▸; Lebedev et al., 2008 ▸) using PDB entry 4ni5 (36% sequence identity), an unpublished structure from the SSGCID, as a search model. The structure was refined in Phenix (Liebschner et al., 2010 ▸) with manual model building in Coot (Emsley & Cowtan, 2004 ▸; Emsley et al., 2010 ▸). The quality of the model was assessed using MolProbity (Headd et al., 2009 ▸) and structure-refinement statistics are provided in Table 4 ▸. The structure was deposited in the Protein Data Bank as entry 5jc8. All structure figures were made using PyMOL (DeLano, 2002 ▸).
Table 4. Structure solution and refinement.
Values in parentheses are for the outer shell
| Resolution range (Å) | 50–1.45 (1.48–1.45) |
| Completeness (%) | 95.8 |
| σ Cutoff | F > 1.34σ(F) |
| No. of reflections, working set | 165652 (9476) |
| No. of reflections, test set | 2032 (112) |
| Final R cryst | 0.141 (0.194) |
| Final R free | 0.158 (0.202) |
| No. of non-H atoms | |
| Protein | 7374 |
| Ion | 4 |
| Ligand | 66 |
| Solvent | 1068 |
| Total | 8512 |
| R.m.s. deviations | |
| Bonds (Å) | 0.007 |
| Angles (°) | 0.878 |
| Average B factors (Å2) | |
| Protein | 17.5 |
| Ion | 17.7 |
| Ligand | 33.7 |
| Water | 31.7 |
| Ramachandran plot | |
| Most favored (%) | 96 |
| Allowed (%) | 4 |
3. Results and discussion
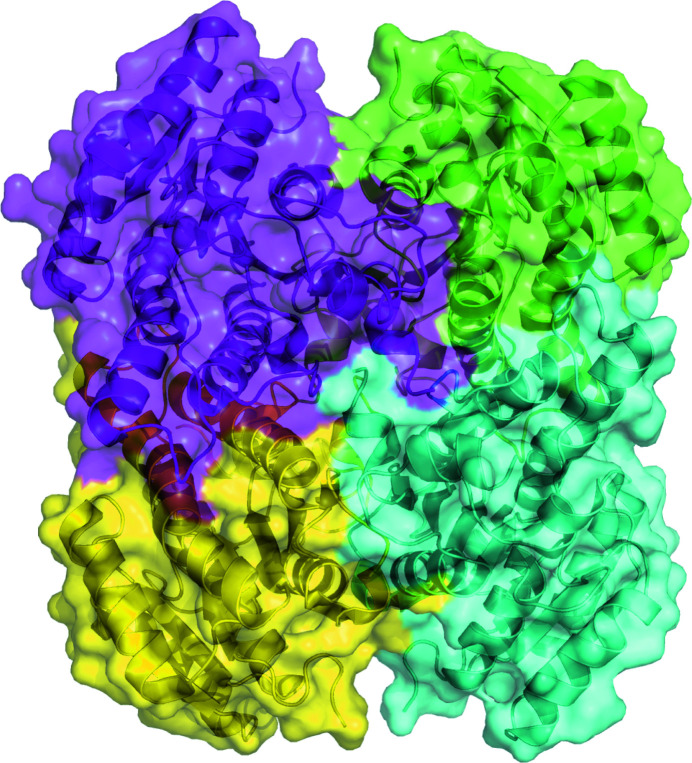
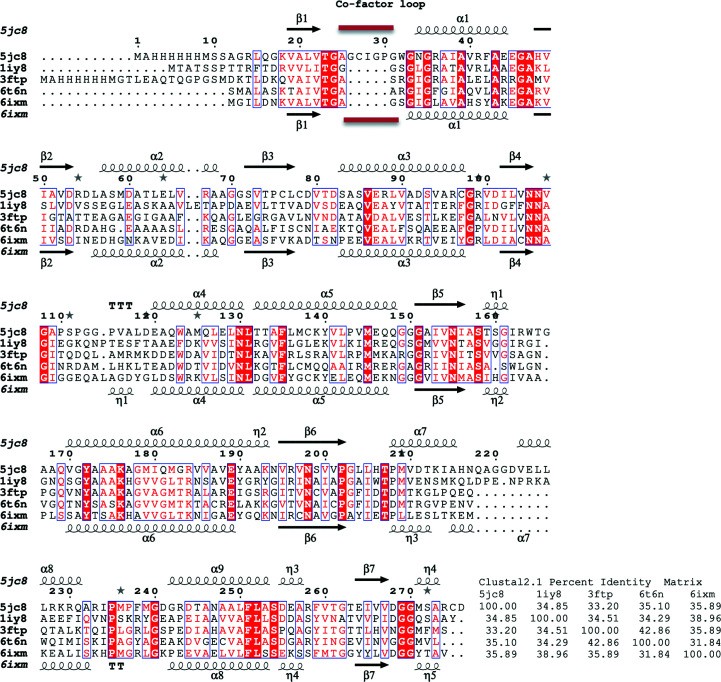
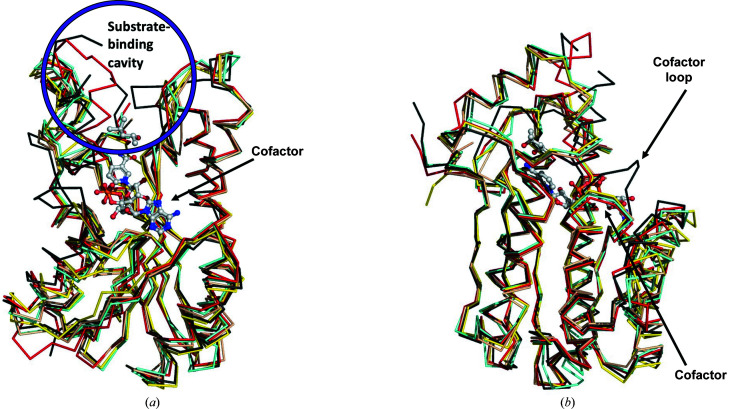
The reported apo structure of PxSDR was determined in the monoclinic space group P21 as a prototypical SDR tetramer (Fig. 1 ▸). Each monomer has the prototypical NADPH Rossmann topology as observed in the architecture of PFAM domain PF00106 or the short-chain dehydrogenases. Specifically, PxSDR has the enoyl-(acyl carrier protein) reductase or 3-oxoacyl-ACP reductase domain architecture otherwise referred to as adh_short_C2. The most similar structures to PxSDR were identified by PDBeFold (http://www.ebi.ac.uk/msd-srv/ssm) analysis using the default threshold cutoffs of 70% for the percentage of secondary structure of the target chain identified in the query protein and of the secondary structure of the query chain (Krissinel & Henrick, 2004 ▸). The closest structure is that of the ketone reductase ChKRED20 from the genome of Chryseobacterium (Li et al., 2019 ▸). This enzyme shares a sequence identity of <36%, with an r.m.s.d. of 1.24 Å for 89% of the matched sequence identity. Wild-type ChKRED20 is an NADH-dependent ketoreductase that reduces over 100 g l−1 ketones for some pharmaceutically relevant substrates and can use 2-propanol as the ultimate reducing agent. All of the closest structures have less than 36% sequence similarity to PxSDR (Fig. 2 ▸). These structures are PDB entry 1iy8, the crystal structure of levodione reductase from Leifsonia aquatica (Sogabe et al., 2003 ▸), PDB entry 3ftp, a 3-ketoacyl-(acyl-carrier-protein) reductase from Burkholderia pseudomallei (Baugh et al., 2013 ▸), PDB entry 6t6n, Klebsiella pneumoniae FabG2(NADH-dependent) in complex with NADH (Vella et al., 2021 ▸), and PDB entry 6ixm, ketone reductase ChKRED20 from the genome of Chryseobacterium (Li et al., 2019 ▸). Interestingly, while all of the other structures have well conserved cofactor-binding domains, an extended loop connecting the first strand in the N-terminus to the first helix creates a larger cofactor-binding cavity in PxSDR (Figs. 2 ▸ and 3 ▸). This additional structural difference between PxSDR and the other structures in the cofactor-binding domain is unique. This is an unexpected difference between PxSDR and the other proteins beyond the expected flexibility in proximity to the substrate-binding cavity. While the flexibility in the substrate-binding cavity explains the specificity of each protein, it is unknown why PxSDR has this unique insertion in the cofactor loop (Figs. 2 ▸ and 3 ▸).
Figure 1.
PxSDR is a prototypyical SDR tetramer.
Figure 2.
Structural and primary-sequence alignment of PxSDR with the closest structures identified by PDBeFold. Also shown is the percent identity matrix generated with Clustal2.1. The structures are PDB entry 5jc8 (the apo structure of PxSDR), PDB entry 1iy8 (the crystal structure of levodione reductase from Leifsonia aquatica), PDB entry 3ftp [3-ketoacyl-(acyl-carrier-protein) reductase from Burkholderia pseudomallei], PDB entry 6t6n [Klebsiella pneumoniae FabG2(NADH-dependent) in complex with NADH] and PDB entry 6ixm (ketone reductase ChKRED20 from the genome of Chryseobacterium). The secondary-structure elements shown are α-helices (α), 310-helices (η), β-strands (β) and β-turns (TT). Identical residues are shown in white on a red background and conserved residues are shown in red. This figure was generated using ESPript (Gouet et al., 1999 ▸, 2003 ▸).
Figure 3.
PxSDR superposed on its closest structural orthologues reveals differences in the substrate- and cofactor-binding cavities. (a) The substrate-binding cavities of the proteins, indicated in the blue circle, have differences that are indicative of different substrate specificities. (b) PxSDR in black has a unique loop insertion in the cofactor-binding cavity, while the other proteins have a well conserved cofactor-binding cavity and loops. The superposed structures are PDB entry 5jc8 (apo structure of PxSDR, black), PDB entry 1iy8 (crystal structure of levodione reductase from Leifsonia aquatica, red), PDB entry 3ftp [3-ketoacyl-(acyl-carrier-protein) reductase from Burkholderia pseudomallei, yellow], PDB entry 6t6n [Klebsiella pneumoniae FabG2(NADH-dependent) in complex with NADH, wheat] and PDB entry 6ixm (ketone reductase ChKRED20 from the genome of Chryseobacterium, aquamarine). The cofactor, NADH and substrate, (4R)-2-methylpentane-2,4-diol, are from PDB entry 1iy8. Structures were superposed with PyMOL.
4. Conclusions
While having a prototypical SDR topology, the apo structure of PxSDR reveals conformational flexibility in both the cofactor- and substrate-binding cavities that needs to be further investigated in order to determine the roles of this enzyme in the degradation of organic wastes by P. xenovorans.
Supplementary Material
PDB reference: putative short-chain reductase, 5jc8
Acknowledgments
We thank the SSGCID cloning and protein-production groups at the Center for Infectious Disease Research and the University of Washington. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817).
Funding Statement
This work was funded by National Institute of Allergy and Infectious Diseases grants HHSN272201700059C, HHSN272201200025C, and HHSN272200700057C to Peter J. Myler, Peter J. Myler, and Peter J. Myler; National Institute of General Medical Sciences grant 1U01GM138433-01 to Oluwatoyin A. Asojo.
References
- Aslanidis, C. & de Jong, P. J. (1990). Nucleic Acids Res. 18, 6069–6074. [DOI] [PMC free article] [PubMed]
- Baugh, L., Gallagher, L. A., Patrapuvich, R., Clifton, M. C., Gardberg, A. S., Edwards, T. E., Armour, B., Begley, D. W., Dieterich, S. H., Dranow, D. M., Abendroth, J., Fairman, J. W., Fox, D., Staker, B. L., Phan, I., Gillespie, A., Choi, R., Nakazawa-Hewitt, S., Nguyen, M. T., Napuli, A., Barrett, L., Buchko, G. W., Stacy, R., Myler, P. J., Stewart, L. J., Manoil, C. & Van Voorhis, W. C. (2013). PLoS One, 8, e53851. [DOI] [PMC free article] [PubMed]
- Bryan, C. M., Bhandari, J., Napuli, A. J., Leibly, D. J., Choi, R., Kelley, A., Van Voorhis, W. C., Edwards, T. E. & Stewart, L. J. (2011). Acta Cryst. F67, 1010–1014. [DOI] [PMC free article] [PubMed]
- Choi, R., Kelley, A., Leibly, D., Nakazawa Hewitt, S., Napuli, A. & Van Voorhis, W. (2011). Acta Cryst. F67, 998–1005. [DOI] [PMC free article] [PubMed]
- DeLano, W. L. (2002). http://www.pymol.org
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Francova, K., Macková, M., Macek, T. & Sylvestre, M. (2004). Environ. Pollut. 127, 41–48. [DOI] [PubMed]
- Gouet, P., Courcelle, E., Stuart, D. I. & Métoz, F. (1999). Bioinformatics, 15, 305–308. [DOI] [PubMed]
- Gouet, P., Robert, X. & Courcelle, E. (2003). Nucleic Acids Res. 31, 3320–3323. [DOI] [PMC free article] [PubMed]
- Headd, J. J., Immormino, R. M., Keedy, D. A., Emsley, P., Richardson, D. C. & Richardson, J. S. (2009). J. Struct. Funct. Genomics, 10, 83–93. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Krissinel, E. & Henrick, K. (2004). Acta Cryst. D60, 2256–2268. [DOI] [PubMed]
- Lebedev, A. A., Vagin, A. A. & Murshudov, G. N. (2008). Acta Cryst. D64, 33–39. [DOI] [PMC free article] [PubMed]
- Li, T. B., Zhao, F. J., Liu, Z., Jin, Y., Liu, Y., Pei, X. Q., Zhang, Z. G., Wang, G. & Wu, Z. L. (2019). Enzyme Microb. Technol. 125, 29–36. [DOI] [PubMed]
- Liebschner, D., Afonine, P. V., Baker, M. L., Bunkóczi, G., Chen, V. B., Croll, T. I., Hintze, B., Hung, L.-W., Jain, S., McCoy, A. J., Moriarty, N. W., Oeffner, R. D., Poon, B. K., Prisant, M. G., Read, R. J., Richardson, J. S., Richardson, D. C., Sammito, M. D., Sobolev, O. V., Stockwell, D. H., Terwilliger, T. C., Urzhumtsev, A. G., Videau, L. L., Williams, C. J. & Adams, P. D. (2019). Acta Cryst. D75, 861–877.
- Myler, P. J., Stacy, R., Stewart, L., Staker, B. L., Van Voorhis, W. C., Varani, G. & Buchko, G. W. (2009). Infect. Disord. Drug Targets, 9, 493–506. [DOI] [PMC free article] [PubMed]
- Serbzhinskiy, D. A., Clifton, M. C., Sankaran, B., Staker, B. L., Edwards, T. E. & Myler, P. J. (2015). Acta Cryst. F71, 594–599. [DOI] [PMC free article] [PubMed]
- Sogabe, S., Yoshizumi, A., Fukami, T. A., Shiratori, Y., Shimizu, S., Takagi, H., Nakamori, S. & Wada, M. (2003). J. Biol. Chem. 278, 19387–19395. [DOI] [PubMed]
- Stacy, R., Begley, D. W., Phan, I., Staker, B. L., Van Voorhis, W. C., Varani, G., Buchko, G. W., Stewart, L. J. & Myler, P. J. (2011). Acta Cryst. F67, 979–984. [DOI] [PMC free article] [PubMed]
- Studier, F. W. (2005). Protein Expr. Purif. 41, 207–234. [DOI] [PubMed]
- Tehrani, R., Lyv, M. M. & Van Aken, B. (2014). Environ. Sci. Pollut. Res. 21, 6346–6353. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Vella, P., Rudraraju, R. S., Lundbäck, T., Axelsson, H., Almqvist, H., Vallin, M., Schneider, G. & Schnell, R. (2021). Bioorg. Med. Chem. 30, 115898. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: putative short-chain reductase, 5jc8