Abstract
Background:
Despite the fact that cervical cancer is preventable disease, it is the fourth most frequently diagnosed cancer and leading cause of cancer death in women. An estimated 604 000 women were diagnosed with cervical cancer worldwide and 342 000 women died from the disease. Therefore, the purpose of this study was to determine the prevalence and factors associated with cervical cancer among women attended cervical cancer screening center in Gahandi memorial Hospital.
Methods:
An institutional-based cross-sectional study was conducted at Gahandi Memorial Hospital in which simple random sampling technique was used to select 422 registration books of women who visited the hospital between May 2015 and May 2019. Texts, tables, and graph were used to present results. Binary logistic regression with a P-value of <.25 and multivariate logistic regression with a P-value of <.05 were used to determine the association between independent variables and outcome variable.
Results:
In this study, from the total of 422 women screened with visual inspection with acetic acid (VIA) screening test, 23.5% of them were found to be positive for VIA test. From those who were diagnosed positive with VIA screening test, about 10.1 % were identified with high grade lesions. Having multiple sexual partners (AOR = 1.83, 95% CI: 1.21-3.29), being HIV-positive (AOR = 2.22, 95% CI:1.10-4.69), having a history of Sexual Transmitted Infection (STI) (AOR = 6.76, 95% CI: 1.14-3.90), and beginning sexual intercourse at early age (AOR = 1.38, 95% CI: 1.20-5.13) were factors associated with cervical cancer.
Conclusion:
The study concluded that the high prevalence of cervical cancer. Having multiple sexual partners, being Human Immune Deficiency Virus (HIV) positive, having STI history and early initiation of sexual intercourse were factors associated with cervical cancer. Therefore, avoiding multiple sexual partners, delaying of early sexual contact, and self-protection from STI infections might help to prevent cervical cancer.
Keywords: Cervical cancer, Ethiopia, hospital, retrospective study
Background
Cervical cancer is a type of cancer in which the cells of the cervix develop abnormally and form a tumor. Adenocarcinoma and squamous cell carcinoma are the 2 most common histologic forms of cervical cancer. 1 Cervical cancer is the most common malignancy in women worldwide, with data indicating that 24.6 million people are suffering from cancer.2,3 Cervical cancer is the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death in women, with an estimated 604 000 new cases and 342 000 deaths worldwide in 2020. 2 According to a WHO survey from 2015, the global incidence, death, and prevalence of cervical cancer were 7.9%, 7.5%, and 9%, respectively. In Africa, there were 715 000 new cancer cases and 542 000 cancer deaths recorded. 4 In Sub-Saharan Africa, the incidence rate was 25.2%, the death rate was 23.2%, and the prevalence was 27.6%. Cervical cancer incidence, death, and prevalence were 17.3%, 16.5%, and 18.2% in Ethiopia, respectively. 2 Cervical cancer prevalence rates in Ethiopian women per 100 000 people per year are projected to be 23%. 5 Cervical cancer was confirmed to be the second most common cancer diagnosed in Ethiopian adult women, after breast cancer.4,6,7
Cervical cancer is very common among women living in low-resource environments, according to the literature. About 85% of the cases and 88% of the deaths due to cervical cancer occurred in developing countries. Women in low- and middle-income countries have 35% higher average life risk of cervical cancer than women in high-income countries. 8 Cervical cancer is more prevalent in women over 50 years old in developing countries, but it is becoming more common in women between the ages of 15 and 49 in developing countries. 9 Ethiopia has a population of 20.9 million women aged 15 and above who are at risk of cervical cancer. Before the Addis Tesfa Initiative was launched in 2009, Ethiopia did not have routine access to cervical cancer screening or care for pre-cancerous cervical lesions. 10 The cost of cervical cancer is exceedingly high after it has spread to invasive cervical cancer. According to various surveys, cervical cancer screening is practiced at just 23% of the time in most developing countries.3,8,11 The study in the North, Ethiopia showed that among the participants who know about cervical cancer screening, only 14.7% of them practiced cervical cancer screening. 12 Lack of confidence in the quality of care has arisen as a major barrier to VIA screening and cryotherapy treatment implementation. 13 Cervical cancer prevalence varies by region in Ethiopia, with concentrations of 6.7% in Alameda textile factory in North Ethiopia, 14 16.5% in Yirgalem General Hospital in Southern Ethiopia, 15 and 15.7% in Arba Minch town in Southern Ethiopia. 10 Clients with HIV ADS, a history of sexually transmitted infections, and an early age of sexual contact, smoking cigarette and being infected by Human Papilloma Virus (HPV), long-term use of oral contraceptives, and being commercial sex workers, are all risk factors for cervical cancer.2,4,5,7,15-17
Despite the growing number of cervical cancer cases in Ethiopia, the prevalence and risk factors of cervical cancer data from the primary health care facilities are scarce. 5 In addition, recent evidence in Ethiopia indicates that only 0.6% of women aged 18to 69 years were screened for cervical cancer every 3 years. 3 Therefore, this study aimed to assess the prevalence and factors associated with cervical cancer among women who attended cervical cancer screening cancer in Gahandi memorial Hospital from May 2015 to May 2019.
Methods
Study design, period, and setting
A retrospective cross-sectional study was performed among 422 registration books of women who visited Gahandi memorial Hospital cervical cancer screening center between May 2015 and May 2019 from 9876 women screened for cervical cancer. Gahandi memorial Hospital is located in Addis Ababa, Ethiopia, in Kirkos sub-city. It is the country’s largest public hospital, with about 230 inpatient beds and cervical cancer treatment facility that opened 15 years ago.
Source and study population
All women who attended cervical cancer screening cancer in Gahandi memorial Hospital from May 2015 to May 2019 were source population whereas all women who attended cervical cancer screening center in Gahandi memorial Hospital from May 2015 to May 2019 and randomly included in the study were study population.
Eligibility criteria
All women attended cervical cancer screening and whose had completed documents were included in the study and those whose medical records were incomplete were excluded from the study.
Sample size determination
A single population proportion sample size determination formula was used with the assumption of 50% proportion of cervical cancer, 5% margin of error, and 95% desired level of confidence interval and considering a 10% non- response rate, 422 sample size was determined for this study.
Data collection tool and procedures
The standardized questionnaire was generated based on previous studies.12,14,15 It contains the study subjects’ socio- demographic, reproductive, and behavioral characteristics. First list of all women who have attended cervical cancer screening and diagnosis center at Gahandi memorial hospital from May 2015 to May, 2019 were retrieved from cervical cancer screening registration books of the hospital. The information obtained from the hospital records showed that about 9876 women were screened for cervical cancer in the 5 years period (May 2015-May, 2019). Then, 422 registration books of women who had screened in the 5 years period were selected by simple random sampling (Table Random number). Two diploma nurses collected the data and one public health professional supervised the data collection process. The screener clinic used visual inspection with acetic acid (VIA) methods. In this procedure cervix is visualized with the naked eye under a good light source at least 1 minute after applying 3% to 5% acetic acid. If the test results are negative, no acetowhite lesions will be found. If the VIA test result is positive, it shows Sharp, distinct, well-defined, dense (opaque/dull or oyster white) acetowhite area with or without raised margins touching the squamocolumnar junction (SCJ); leukoplakia and warts.
Data quality assurance
Every day after data collection, the collected data was reviewed for completeness and accuracy to ensure data integrity. Data collectors and supervisor undertook 2 days of training. Before the actual data collection, the questionnaire was pre-tested at Yekatit 12 Hospital. The data collectors were closely monitored, and the collected data were inspected and double-checked for accuracy.
Data processing and analysis
The data was entered into a computer using EPI-data version 3.1 and then exported to SPSS version 23 for analysis. Results were presented by texts, tables, and graph. Both bivariate and multivariate logistic regression analysis were used “to determine factors associated with cervical cancer”. All variables with a P-value of less than .25 in the binary logistic regression analysis were chosen for the “multivariable logistic regression analysis after checking for Multicollinearity using VIF”. Those variables which showed significant association in bivariate logistic regression analysis and those that had no Multicollinearity were entered to multivariate logistic regression analysis to identify independent predictor of cervical cancer. Finally, P-value <.05, and AOR with 95% CI were used to identify predictor variables.
Ethical approval and consent to participate
This study was conducted in accordance with the declaration of Helsinki. Ethical clearance was obtained from the Ethical review committee of Rift Valley University with reference number CMHS-R 3012/2020. Formal letter of cooperation was written to Gahandi memorial Hospital administrations to get permission to conduct the study. Direct consent from the study participant was waved due to retrospective nature of the study. Oral informed consent was obtained from Gahandi memorial Hospital and administration office after explaining the objective and aim of the study. Confidentiality was maintained by omitting their name, and personal identification of participants.
Results
Socio-demographic characteristics of the study participants and cervical cancer screening results
In this study, from a total of 422 registration books of women reviewed; 23.5% was found to be VIA positive. From those who were screened positive with VIA screening test, about 10.1% were identified with high grade lesions (grade II and III) while 4.5% of them were found with low-grade lesions (Grade I). The average age of the women who came for screening in this sample was 40.14 years, and the majority of the patient’s age was over 35 years. Three-fourths, (74.4%) of the patients were married, 78.9% had a secondary or higher education, and 67.5% were unemployed by profession. In terms of reproductive features, 86.3% of the study subjects were nulliparous, while 76.5% used contraceptives. In addition, 25.1% of the participants had STI history, 12.3% were positive for HIV; 66.6% of them were <18 years when they had the first sexual intercourse and 25.4% of participants had history of multiple sexual partners (Table 1).
Table 1.
Socio demographic characteristics and result of screening for women attended cervical cancer diagnosis center at Gahandi memorial hospital from 2016 to 2019.
| Variables | Frequency | Percentage |
|---|---|---|
| Age in years | ||
| 18-29 | 40 | 9.6 |
| 30-39 | 165 | 39.2 |
| 40-49 | 137 | 32.5 |
| ⩾ 50 | 83 | 19.7 |
| Educational status | ||
| Cannot read and write | 14 | 3.3 |
| Read and write | 24 | 5.7 |
| Primary school | 46 | 10.9 |
| Secondary school | 230 | 54.5 |
| Tertiary school | 108 | 25.6 |
| Marital status | ||
| Single | 24 | 5.7 |
| Married | 331 | 78.4 |
| Divorced | 39 | 9.2 |
| Widowed | 28 | 6.6 |
| Parity | ||
| Nulliparous | 364 | 86.3 |
| Multiparous | 58 | 13.7 |
| HIV status | ||
| Positive | 52 | 12.3 |
| Negative | 370 | 87.7 |
| Number of the sexual partner | ||
| Single | 315 | 74.6 |
| Multiple | 107 | 25.4 |
| History of STI | ||
| Yes | 106 | 25.1 |
| No | 316 | 74.9 |
| History family planning | ||
| Yes | 323 | 76.5 |
| No | 99 | 23.5 |
| Age at first intercourse | ||
| <18 | 281 | 66.6 |
| ⩾18 | 85 | 20.1 |
| Unknown | 56 | 13.3 |
| VIA-screening result | ||
| Positive | 99 | 23.5 |
| Negative | 323 | 76.5 |
| Lesion grade | ||
| Low grade –I | 19 | 4.5 |
| High grade II | 30 | 10.1 |
Abbreviation: VIA, visual inspection of cervix with acetic acid.
Lesion distribution by age category
The result showed that about two-fifths of the screened women (39.3%) were in the age group 30 to 39 years. Correspondingly, about 49.4%, 42.2%, and 60.0% of the result showed that VIA Positive, low grade lesion (grade I) and high-grade lesion (grade II and III) were found in this age-group (30-39 years) respectively (Table 2).
Table 2.
Lesion distribution by age category among women attended cervical cancer screening center at Gahandi memorial hospital from 2015 to 2019.
| Variables |
Women screened |
VIA- positive |
Low-grade lesion Grade 1 |
High-grade lesion II&III |
|---|---|---|---|---|
| Age in years | No (%) | No (%) | No (%) | No (%) |
| 18-29 | 40 (9.5) | 25 (25.2) | 7 (36.6) | 4 (13.3) |
| 30-39 | 165 (39.3) | 49 (49.4) | 8 (42.2) | 18 (60.0) |
| 40-49 | 137 (39.2) | 17 (17.2) | 3 (15.9) | 5 (16.7) |
| ⩾50 | 83 (19.7) | 8 (8.2) | 1 (5.3) | 3 (10.0) |
| Total | 422 (100) | 99 (100) | 19 (100) | 30 (100) |
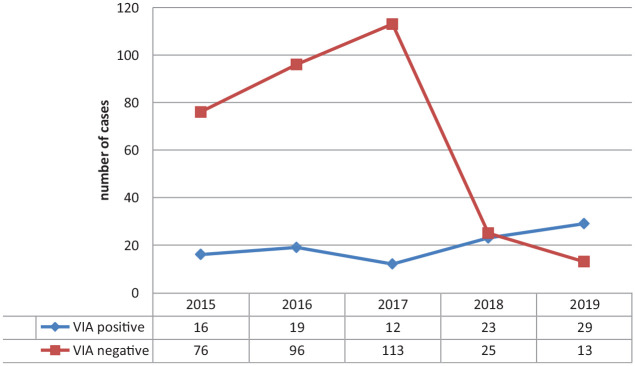
Trends of cervical cancer at the Gahandi memorial hospital
From the selected sample, majority of the VIA positive result were recorded in 2019. Cervical cancer was found to be on the rise in the study area, according to the findings (Figure 1).
Figure 1.
Prevalence of cervical cancer at the Gandhi memorial hospital, from 2015 to 2019.
Factors associated with cervical cancer at Gahandi memorial Hospital
Early age at first sexual intercourse, having multiple sexual partners, being HIV positive, and having STI history were identified as the predictor variables of cervical cancer at P value of less than .05 in multiple logistic regression analysis.
The study indicated that the odds of getting cervical cancer were 1.38 times higher among women who started sexual intercourse at age less than 18 years old compared to their counterparts (AOR = 1.38, 95% CI: 1.20-5.13). As opposed to women who had a single intimate relationship, women who had more than one sexual partner in the past had a two-fold elevated chance of cervical cancer (AOR = 1.83, 95% CI: 1.21-3.29). HIV-positive women were 2.22 times more likely to develop cervical cancer compared to HIV-negative women (AOR = 2.22, 95% CI: 1.10-4.69). As compared to their colleagues, women with a history of sexually transmitted disease were 6.76 times more likely to develop cervical cancer (AOR: 6.76, 95% CI: 1.14-3.9) (Table 3).
Table 3.
Factors associated with cervical cancer at Gahandi memorial hospital, 2020 (multivariate logistic regression analysis).
| Variable | Positive for cervical cancer n (%) | Negative for cervical cancer n (%) | COR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| Parity | ||||
| Nulliparous | 50 (11.8) | 313 (74.2) | 0.91 (0.36-0.45) | 0.10 (0.04-1.40) |
| Multipara | 8 (1.9) | 51 (12.1) | 1 | 1 |
| HIV status | ||||
| Positive | 10 (2.4) | 42 (10.0) | 2.54 (1.38-4.66) | 2.22 (1.10-4.69)* |
| Negative | 48 (11.4) | 322 (76.3) | 1 | 1 |
| Age at first intercourse | ||||
| ⩾18 | 13 (3.1) | 79 (18.7) | 1 | 1 |
| <18 | 33 (7.8) | 234 (55.5) | 2.78 (1.20-6.37) | 1.38 (1.20-5.13)* |
| Unknown | 63 (14.9) | 63 (14.9) | 1.17 (0.43- 3.17) | 1.11 (0.58-10) |
| STI history | ||||
| Yes | 10 (2.4) | 74 (17.5) | 7.11 (4.32-11.71) | 6.76 (1.14-3.9)* |
| No | 48 (11.8) | 290 (68.2) | 1 | 1 |
| Number of sexual partners | ||||
| One | 43 (10.2) | 280 (66.4) | 1 | 1 |
| Multiple | 15 (3.5) | 84 (19.9) | 2.16 (1.33- 3.51) | 1.83 (1.21-3.29)* |
Abbreviations: AOR=, adjusted odd ratio; CI, confidence interval; COR, crude odds ratio.
Statistically significant at P-value <.05.
Discussion
According to the results of this report, 23.5% of women who visited the Gahandi Memorial Hospital’s cervical cancer screening and diagnosis center were screen positive with VIA test. This finding is higher than other Ethiopian studies such as a North Ethiopian institution-based cross-sectional study that found a prevalence of 6.7%, 18 study conducted at Yirgalem General Hospital, that reported a prevalence rate of 16.5%, 14 study done at pathology centers in Ethiopia which indicated a prevalence of 18.2%, 4 and another study conducted at South west Ethiopia which indicated a prevalence rate of 12.9%. 19 The higher prevalence in this study finding may be due to variations in the socio-demographic characteristics of the respondents and study settings. This difference might also be explained by the varying risky habits. This result is also higher than those studies conducted outside of Ethiopia, such as Madagascar (11.3%), Malawi (12.4%), and Nigeria (16%). 20 However, the current study’s prevalence is lower than conducted in Lusaka, Zambia, among HIV-positive women (76%). 21 Differences in test providers’ expertise, as well as the underlying prevalence of other sexually transmitted diseases and reproductive features, may explain the higher prevalence of these trials. Furthermore, it’s likely that variations in women’s sexual behaviors are leading to the spike in prevalence.
In this study, early sexual activity was found to be positively associated with cervical cancer. This finding was consistent with the finding from Nigeria, 20 Rwandan, 22 India, 23 and Brazil 24 in which early initiation of sexual practice identified as a positively associated with cervical cancer. This might be because at this age cervical tissue undergoes physiologic changes, transformation zone on the ecto-cervix is enlarged, and exposure to HPV at such times may facilitate infection which may make this area more vulnerable to development of dysplasia, a cervical squamous cancer. In addition an earlier age of sexual practice implies a longer period of sexual activity which increases the chance of developing cervical cancer.9,25
The findings of the present study indicated that history of multiple sexual partners was positively associated with cervical cancer. The finding is consistent with previous studies conducted in Southern and Northern Ethiopia, Nigeria, Rwandan, and Morocco18,20,22 where multiple sexual partners are identified as positively associated with cervical cancer. This could be because women who have had 2 or more lifetime sexual partners are more likely to develop cervical cancer, as the number of sexual partners’ increases; they are more likely to contract the HPV infection, which is the cause of cervical cancer and invasive cervical cancer.
The present study indicated that being HIV positive is positively associated with cervical cancer. This was in line with previous research from Zambia, 21 which found that HIV-positive women are more likely to develop cervical cancer. This could be because women with HIV have a higher rate of HPV co-infection, which leads to higher cancer rates. 26
Limitation of the study
The details of the independent variables were not found in the registration books of the patients since the data was collected from secondary sources. The selected study subjects were women who visited the Gahandi memorial Hospital for cervical cancer screening because of suspecting themselves which may lead to selection bias and make the prevalence of cervical cancer higher than the general population.
Conclusion
The prevalence of cervical cancer was high this study. Age at first sexual intercourse, having multiple sexual partners, being HIV positive, and having history of STI status were the identified as factors associated with cervical cancer. As a result, delaying the age at first sexual intercourse, and the provision of condoms for those who have multiple sexual partners, as well as STIs such as HIV, may play role in the prevention and control of cervical cancer.
Acknowledgments
We would like to express our gratitude to Rift Valley University for providing us fund for the study. We would also like to express our heartfelt gratitude to Gahandi memorial Hospital who provided us relevant information to conduct this research.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Rift Valley University’s research and community service director office provided funding for this study. Aside from providing financial support, the funding body had no involvement in the study’s design, data collection, analysis, and interpretation, or manuscript writing.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed significantly to substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Moreover they participated in drafting the work or revising it critically for important intellectual content. The authors approved the final version to be published. They agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Sharing Statement: On reasonable request, the corresponding author will provide the datasets used in and/or analyzed during the current study.
Ethics Approval and Consent to Participate: This study was conducted in accordance with the declaration of Helsinki. Ethical clearance was obtained from the Ethical review committee of Rift Valley University with reference number CMHS-R 3012/2020. Formal letter of cooperation was written to Gahandi memorial Hospital administrations to get permission to conduct the study. Direct consent from the study participant was waved due to retrospective nature of the study. Oral informed consent was obtained from Gahandi memorial Hospital and administration office after explaining the objective and aim of the study. Confidentiality was maintained by omitting their name, and personal identification of participants.
ORCID iD: Berhanu Senbeta Deriba  https://orcid.org/0000-0002-4282-1427
https://orcid.org/0000-0002-4282-1427
References
- 1. Berek JS. ed. Berek and Novak’s Gynecology. 14th ed. Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. [DOI] [PubMed] [Google Scholar]
- 3. WHO/ICO. Information Centre on HPV and Cervical Cancer (HPV Information Centre), Author Summary Report on HPV and Cervical Cancer Statistics in Ethiopia, Ethiopia. WHO/ICO; 2014. www.hpvcentre.net [Google Scholar]
- 4. Loutfi A, Pickering JL. The distribution of cancer specimens from two pathology centers in Ethiopia. Ethiop Med J. 1992;30:13-17. [PubMed] [Google Scholar]
- 5. Bsc EG. Knowledge Attitude and Practice on Cervical Cancer and Screening among Reproductive health Service Clients, Addis Ababa, Ethiopia (Doctoral dissertation, Addis Ababa University). [Google Scholar]
- 6. Singh GK. Rural-urban trends, and patterns in cervical cancer mortality, incidence, stage, and survival in the United States. J Community Health. 2012;37:217-223. [DOI] [PubMed] [Google Scholar]
- 7. The GLOBOCAN database. Cancer Incidence, Mortality, and Prevalence Worldwide. International Agency for Research on Cancer; 2012. [Google Scholar]
- 8. Echelman D, Feldman S. Management of cervical precancers: a global perspective. Hematol Oncol Clin North Am. 2012;26:31-44. [DOI] [PubMed] [Google Scholar]
- 9. Yesuf T. Survival and Associated Factors Among Cervical Cancer Patients in Black Lion Hospital, Addis Ababa, Ethiopia. Addis Ababa Uiversity Libraries; 2012. [Google Scholar]
- 10. Shiferaw N, Salvador-Davila G, Kassahun K, et al. The single-visit approach as a cervical cancer prevention strategy among women with HIV in Ethiopia: successes and lessons learned. Glob Health Sci Pract. 2016;4:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Department of Health. Pretoria. National Guideline for the Cervical Cancer Screening Program. Department of Health; 2000. [Google Scholar]
- 12. Getahun F, Mazengia F, Abuhay M, Birhanu Z. Comprehensive knowledge about cervical cancer is low among women in northwest Ethiopia. BMC Cancer. 2013;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paul P, Winkler JL, Bartolini RM, et al. Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy: experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist. 2013;18:6-12. [DOI] [PubMed] [Google Scholar]
- 14. Hailemariam T, Yohannes B, Aschenaki H, Mamaye E, Orkaido G, Seta M. Prevalence of cervical cancer and associated risk factors among women attending cervical cancer screening and diagnosis center at Yirgalem General Hospital, southern Ethiopia. J Cancer Sci Ther. 2017;9:730-735. [Google Scholar]
- 15. Kassa RT. Risk factors associated with precancerous cervical lesion among women screened at Marie Stops Ethiopia, Adama town, Ethiopia 2017: a case control study. BMC Res Notes. 2018;11:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kinfu S. Prevalence and Predictors of Anxiety and Depression among Cervical Cancer Patients at TikurAnbessa Specialized Hospital, Addis Ababa, Ethiopia. Dissertation. Addis Ababa University; 2019. [Google Scholar]
- 17. Teka T, Kote M, Kejela G, Getachew T. Magnitude and factors associated with precervical cancer among screened women in southern Ethiopia. Adv Public Health. 2019;2019:1-8. [Google Scholar]
- 18. Bayu H, Berhe Y, Mulat A, Alemu A. Cervical cancer screening service uptake and associated factors among age eligible women in Mekelle zone, northern Ethiopia, 2015: a community based study using health belief model. PLoS One. 2016;11:e0149908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gedefaw A, Astatkie A, Tessema GA. The prevalence of precancerous cervical cancer lesion among HIV-infected women in southern Ethiopia: a cross-sectional study. PLoS One. 2013;8:e84519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. WHO. Prevention of Cervical Cancer through Screening Using Visual Inspection with Acetic Acid (VIA) And Treatment with Cryotherapy. A Demonstration Project in Six African Countries: (Malawi, Madagascar, Nigeria,Uganda, The United Republic of Tanzania, And Zambia). WHO; 2012. [Google Scholar]
- 21. Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007;96:1480-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forhan SE, Godfrey CC, Watts DH, Langley CL. A systematic review of the effects of visual inspection with acetic acid, cryotherapy, and loop electrosurgical excision procedures for cervical dysplasia in HIV-Infected women in low- and middle-income countries. J Acquir Immune Defic Syndr. 2015;68:S350-S356. [DOI] [PubMed] [Google Scholar]
- 23. Roopali F, Shashi G, Subash G. Sociodemographic risk factors for cervical cancer in Jammu region of J and k state of India. Asian J Sci Res. 2014;9:105-110. [Google Scholar]
- 24. Ferreira da, Silva I, Koifman RJ, Quinto Santos Souza C, Ferreira de, Almeida Neto O, Koifman S. TP53Genetic polymorphisms and environmental risk factors associated with cervical carcinogenesis in a cohort of Brazilian women with cervical lesions. J Toxicol Environ Health A. 2010;73:888-900. [DOI] [PubMed] [Google Scholar]
- 25. WHO. International Agency for Research on the Cancer Press Release. WHO; 2013:223. [Google Scholar]
- 26. WHO. Guidelines for Screening and Treatment of Precancerous Lesion for Cervical Cancer Prevention. WHO; 2013:7-9. [PubMed] [Google Scholar]