Abstract
Background:
Though people who inject drugs (PWID) make up the majority of the hepatitis C virus (HCV) epidemic, concerns about adherence often exclude PWID from receiving direct-acting antiviral (DAA) medication. The most effective models of HCV care to promote sustained virologic response (SVR) and high adherence need to be evaluated.
Methods:
We conducted a prospective cohort study in three opioid treatment programs (OTPs) in the Bronx, NY. Participants, in collaboration with providers, chose one of three models of onsite care: directly observed therapy (mDOT), group treatment (GT), or self-administered individual treatment (SIT). SVR12, daily adherence, and participant characteristics were compared between groups.
Results:
Of 61 participants, the majority were male (62%) and Latino (67%), with a mean age of 53 (SD 9). Participants received DAAs via one of three models of care: mDOT (21%), GT (25%), or SIT (54%). The majority (59%) used illicit drugs during treatment. Overall, SVR12 was 98% with no differences between models of care: mDOT (100%), GT (100%), and SIT (97%) (p = 1.0). Overall, daily adherence was 73% (SD 16); 86% among those who chose mDOT compared to 71% among those who chose GT (p < 0.01) and 73% among those who chose SIT (p < 0.01).
Conclusion:
Despite ongoing illicit drug use and suboptimal adherence, SVR12 was high among PWID treated onsite at an OTP using any one of three models of care. Shared decision making in real world settings may be key to choosing the appropriate model of care for PWID.
Keywords: HCV, PWID, OAT, Patient centered interventions
Introduction
People who inject drugs (PWID) make up a considerable burden of the global hepatitis C virus (HCV) infections, with an estimated 6.1 million PWID living with HCV worldwide, and accounting for nearly a quarter of all new HCV infections (Grebely, Larney & Peacock, 2019). In the United States, the overwhelming majority of the five million Americans chronically infected with HCV are PWID, (Alter, Kruszon-Moran & Nainan, 1999) with at least 70% of incident HCV infections due to injection drug use (Suryaprasad, White & Xu, 2014). HCV is the leading cause of cirrhosis and hepatocellular carcinoma in the US, and results in over 15,000 deaths annually (Ly, Hughes, Jiles & Holmberg, 2016). Now, direct-acting antiviral (DAA) oral therapies offer cure rates over 95%, have few side effects, (AASLD/IDSA, 2017) and are associated with decreased mortality (Backus et al., 2011; Dieperink et al., 2014; van der Meer, Veldt & Feld, 2012) and viral transmission (Grebely, Matthews, Lloyd & Dore, 2013; Hellard, McBryde & Sacks Davis, 2015; Martin, Thornton & Hickman, 2016; Martin, Vickerman & Grebely, 2013). Despite the fact that PWID represent the majority of prevalent and incident cases of HCV in the US and other developed countries (Grebely & Dore, 2014; Hajarizadeh, Grebely & Dore, 2013), treatment of HCV in PWID has been minimal both in the US and globally (Alavi, Raffa & Deans, 2014; Grebely, Genoway & Khara, 2007; Hellard, Sacks-Davis & Gold, 2009; Mehta, Genberg & Astemborski, 2008; Midgard, Bramness, Skurtveit, Haukeland & Dalgard, 2016; Treloar, Hull, Dore & Grebely, 2012, 2010).
Given the ease of DAA treatment, we have an opportunity to treat HCV in PWID onsite in settings where they are already accessing care, such as opioid treatment programs (OTPs).In the US, over 375,000 patients receive methadone or buprenorphine from approximately 1500 opioid treatment programs (OTPs) (Alderks, 2017), and conservative estimates suggest that over 60% of PWID in OTPs are infected with HCV (Novick & Kreek, 2008). Though most PWID are willing to undergo HCV treatment (Stein, Maksad & Clarke, 2001; Strathdee, Latka & Campbell, 2005; Walley, White, Kushel, Song & Tulsky, 2005; Zeremski, Dimova & Zavala, 2014), continued reservations among providers that PWIDs may not achieve cure with DAA therapy due to suboptimal adherence has contributed to the dearth of HCV treatment uptake among PWID (Asher et al., 2016; Grebely, Raffa & Lai, 2009; Mehta et al., 2008). PWID do indeed face many adherence challenges, including substance use disorder, mental illness, homelessness, lack of positive social support, and low HCV-related knowledge (Harris & Rhodes, 2013; Mravcik, Strada & Stolfa, 2013; Rich, Chu & Mao, 2016).
To mitigate these barriers to adherence, we developed intensive models of onsite treatment for HCV in OTPs in the Bronx, New York (NY) that could potentially improve treatment outcomes for PWID. The goal of this prospective cohort study was to determine sustained virologic response (SVR) and adherence rates among PWID receiving onsite HCV treatment at OTPs through one of three intensive models of care – directly observed therapy (mDOT), group treatment (GT), and self-administered individual treatment (SIT) - selected by the participant and provider together. Additionally, we determined if there were different participant characteristics of those choosing each treatment model. Finally, we assessed the relationship between illicit drug use and adherence.
Methods
Study design
We conducted a prospective cohort study in three OTPs in the Bronx, NY, in which we offered oral DAA HCV treatment using three models of care. Participants, in collaboration with primary care providers, chose to receive treatment through one of the three models: mDOT, GT, or SIT. These participants declined enrollment in a randomized trial (the PREVAIL trial; outcomes published elsewhere) (Akiyama et al., 2019), or were ineligible because providers/patients were not willing for the participant to be randomized to one of the three models of care. Instead participants received HCV treatment based on patient-provider preference. As such, these participants are unique in that they, with providers, chose which intervention to receive. This study was approved by Albert Einstein College of Medicine Institutional Review Board.
Participant recruitment and eligibility
A convenience sample of HCV-infected participants were prospectively enrolled between May 2014 and November 2015, with a goal of recruiting at least 50 participants. Potential participants were referred by clinicians if eligible for HCV treatment by the American Association for the Study of Liver Diseases/Infectious Diseases Society of America (AASLD/IDSA) guidelines, (AASLD/IDSA, 2017) and were assessed for study inclusion using an oral screener and confirmatory chart review. Eligible participants for this study were: 18 years of age or greater, English or Spanish speaking, HCV genotype 1 or 4, psychiatrically stable, willing to receive HCV therapy onsite at the OTP, and able to provide informed consent. Exclusion criteria included: decompensated cirrhosis, pregnancy/breast-feeding, and less than one-year life expectancy determined by clinician.
Treatment setting
All HCV care and treatment occurred onsite at one of the three OTPs. All participants, regardless of model of care, obtained an initial HCV evaluation from an OTP provider (primary care physician or physician assistant). Participants in each model of care obtained an HCV viral load, basic metabolic panel, and liver function tests at treatment weeks 4, 8, and 12, and in some cases 24 (for those who received 24 weeks of DAA therapy). Participants received either combination therapy of sofosbuvir/ledipasvir or sofosbuvir/simeprevir for 8–24 weeks. All medication was packaged in weekly electronic blister packs, and participants received one week of medication at a time. Almost all participants (greater than 95%) treated for HCV at all three OTPs have Medicaid. In New York, Medicaid does not restrict HCV medication access based on either fibrosis level or drug use (Barua et al., 2015).
Models of care (Study groups)
Participants, in collaboration with OTP clinicians, determined the model of care in which they wanted to receive HCV therapy. These participants did not want to participate in a concurrent randomized controlled trial, the PREVAIL study, or were deemed ineligible because providers/patients were not willing for the participant to be randomized to one of the three models of care. As such, these participants were unique in that they, with providers, chose which intervention to receive HCV care. Generally, some providers advocated for mDOT for participants whom they believed may have barriers to adherence. Other participants preferred group due to the weekly peer support, or chose mDOT for the greater organization; the majority of participants chose SIT for both privacy and simplicity.
Modified directly observed therapy (mDOT)
Participants receiving mDOT obtained all clinical care from an OTP physician; mDOT was performed by nurses at the OTP pick-up windows. Number of directly observed oral doses of DAAs varied based on number of days the participant attended the clinic to receive methadone or buprenorphine. We considered this intervention to be “modified” DOT since not all oral medication doses were observed by nurses. Depending on methadone pick-up schedule and dosing frequency, certain doses cannot be observed (i.e., weekend doses, and doses taken on non-clinic days). Forty percent of patients picked up 1–2 days per week, 38% picked up 5–6 times per week, and 22% 3–4 times per week; even participants with infrequent methadone schedules still opted for mDOT. In mDOT, non-observed doses were packaged in electronic blister packs as take-home doses for self-administration, and participants were asked to return the blister packs to the nurses at the next clinic visit whether or not they had taken the pills. For observed doses, OTP clinic nurses notified clinicians when doses were declined, assessed for side effects, and referred participants to onsite clinicians as necessary.
Group treatment (GT)
This model, described in detail elsewhere (Stein et al., 2012), was developed by adapting models of HCV peer-based support (Sylvestre & Zweben, 2007). GT occurred weekly and participants received medication blister packs at each group. New participants were first oriented to the group and met other patients and the treatment team (physician and physician assistant). The treatment team presented an overview of the HCV epidemic and its impact on drug users, HCV natural history, and risks, benefits, and efficacy of HCV treatment. GT protocol was discussed, with time allowed for discussion. Weekly GT meetings had five components: 1) brief physical exams; 2) psychosocial support from peers and providers; 3) HCV education; 4) side effect management; and 5) closing meditation on positive health. Between six and 12 participants attended each GT group, and group entry was rolling.
Self-administered individual treatment (SIT)
Participants receiving SIT obtained all clinical care from an OTP provider and received all HCV medications, packaged in weekly blister packs. All medications were self-administered at home.
Study assessments
All demographic and clinical data were obtained through chart review at the end of the study period. Adherence was measured in all three groups using weekly electronic Med-ic® blister packs, which have a 99.6% event accuracy (time of dose removalcorrectly recorded within ± 2 min). Missed doses were not returned to the participants, and treatment regimens were not extended past treatment end date. Participants were compensated $10 dollars for each blister pack that was returned.
Other variables measured were drug use, fibrosis level, and baseline depression. Drug use in the last six months was defined as any urine toxicology positive for cocaine, benzodiazepine, or opiates. All participants had urine toxicology tests performed as per OTP protocol at least once monthly. Frequent drug use was defined as having positive urine toxicology tests greater than 50% of the time. Cirrhosis was defined as having a FibroSure ® test greater or equal to 0.75. Depression was assessed per chart review of the patients’ medical record, and participants were considered to have depression if it was part of the medical problem list.
Study outcomes
The primary outcome was SVR12, defined as an HCV RNA below the limit of quantitation using the COBAS TaqMan real-time reverse transcriptase–polymerase chain reaction assay version 1.0 (< 43 IU/mL) and version 2.0 (< 15 IU/mL) after October 2014 (Roche Diagnostics) at least 12 weeks after treatment completion. Secondary outcomes were end of treatment response (ETR), defined as a negative HCV PCR at the completion of all medication, sustained virologic response 4 weeks post treatment (SVR4), defined as a negative HCV PCR 4 weeks after completion of all medication, and adherence. Adherence was measured rigorously in all three arms using electronic Med-ic® blister packs and visual analog scale. Adherence was computed using daily, weekly, and window timeframes (see Box). Sofosbuvir/simeprevir was packaged in the same blister, such that one “dose” of the blister pack contained both pills.
Adherence outcomes
- Daily time-frame adherence: participants received credit only if medication is popped out of blister pack within the correct day
- 0 – 1 doses credited each day
- Adherence is reported at 100% if all of the daily dose was popped out within 24-hour period
- Weekly time-frame adherence: participants received credit if medication is popped out of the weekly blister pack on any day and time within the week
- 0 –7 doses credited each week
- Participant adherence is reported at 100% even if they pop out all 7 days of doses at the same time
Window Time-frame adherence: Participants received credit if dose(s) were taken within a window based on 25% of the dosing interval. For example, a participant scheduled to take once daily medication at 10 AM received credit if the dose was taken between 4 AM and 4 PM.
Statistical analysis
SVR12, daily adherence, and participant characteristics were reported in percentages/frequencies and compared between study groups, using Chi-square or Fisher’s exact tests. We compared SVR12 rates for those receiving sofosbuvir/ledipasvir and sofosbuvir/simeprevir to large registration trials (ION-1 and OPTIMIST-1) (Afdhal, Reddy & Nelson, 2014; Kwo, Gitlin & Nahass, 2016) by analyzing whether the confidence intervals for our study SVR12 rates crossed the confidence intervals of the registration trial SVR12 rates.
To compare adherence rates between study groups, we applied mixed-effects linear models to account for within-participant longitudinal correlations by taking first order autoregressive covariance structure; the fixed effects were study group, time, and study group-by-time interactions. To identify participant characteristics that might be associated with adherence, we applied a series of mixed-effects models using the characteristics of interest as main effects, including study group as a covariate. We used SAS v9.4 for all statistical analyses, and statistical significance was declared when p < 0.05.
Results
Between May 2014 and November 2015, 109 participants were screened and 48 were excluded (36 due to enrollment in another HCV study and 12 who were not interested in participation). Of 61 participants enrolled, the majority were male (62%), of Latino ethnicity (67%), with a mean age of 53 (SD 9.3) (Table 1). Most were genotype 1 (97%), HCV treatment naïve (73%), and without cirrhosis (67%). Participants received either sofosbuvir/simeprevir (25%) or sofosbuvir/ledipasvir (75%) via one of three models of care onsite at the OTP clinic: mDOT (21%), GT (25%), or SIT (54%). The majority of participants used illicit drugs during HCV treatment (59%), most commonly opiates or cocaine. More participants in the intensive models of care (mDOT or GT) vs. SIT had depression, HIV, cocaine or opiate use in the previous six months, and any drug use in the previous six months.
Table 1.
Participant characteristics by study group.
| Treatment Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | mDOT | GT | SIT | ||||||
| Characteristic | N | % | N | % | N | % | N | % | p-value |
| Total | 61 | 100 | 13 | 21 | 15 | 25 | 33 | 54 | |
| Age | |||||||||
| (Mean, SD) | 53 | 9 (SD) | 55 | 8 (SD) | 53 | 11 (SD) | 52 | 9 (SD) | 0.74 |
| Gender | |||||||||
| Male | 38 | 62% | 6 | 46% | 9 | 60% | 23 | 70% | 0.34 |
| Hispanic Ethnicity | |||||||||
| Yes | 41 | 67% | 9 | 69% | 8 | 53% | 24 | 73% | 0.41 |
| Cirrhosis | |||||||||
| Yes | 20 | 33% | 3 | 23% | 7 | 47% | 10 | 30% | 0.39 |
| HIV | |||||||||
| Yes | 14 | 23% | 7 | 54% | 1 | 7% | 6 | 18% | 0.01 |
| Depression | |||||||||
| Yes | 38 | 62% | 10 | 77% | 12 | 80% | 16 | 49% | 0.05 |
| Genotype | |||||||||
| 1 | 59 | 97% | 13 | 100% | 14 | 93% | 32 | 97% | 0.61 |
| HCV Treatment Experience | |||||||||
| Yes | 12 | 20% | 1 | 8% | 5 | 33% | 6 | 18% | 0.22 |
| Treatment Medication | |||||||||
| Sofosbuvir/Ledipasvir&&&90 mg/400mg | 46 | 75% | 12 | 92% | 6 | 40% | 28 | 85% | 0.001 |
| Simeprevir150mg/ Sofosbuvir400mg | 15 | 27% | 1 | 8% | 9 | 60% | 5 | 15% | 0.001 |
| Benzodiazepine Use 6 M Prior to HCV Treatment | |||||||||
| Yes | 18/58 | 31% | 5/13 | 39% | 5/14 | 36% | 8/31 | 26% | 0.60 |
| Benzodiazepine Use During HCV Treatment | |||||||||
| Yes | 14/68 | 24% | 5/13 | 39% | 3/14 | 21% | 6/31 | 19% | 0.42 |
| Cocaine Use 6 M Prior to HCV Treatment | |||||||||
| Yes | 20/58 | 34% | 7/13 | 54% | 7/14 | 50% | 6/31 | 19% | 0.03 |
| Cocaine Use During HCV Treatment | |||||||||
| Yes | 13/58 | 22% | 5/13 | 39% | 4/10 | 29% | 4/31 | 13% | 0.13 |
| Opioid Use 6 M Prior to HCV Treatment | |||||||||
| Yes | 24/58 | 41% | 9/13 | 69% | 6/14 | 43% | 9/31 | 29% | 0.05 |
| Opioid Use During HCV Treatment | |||||||||
| Yes | 23/58 | 40% | 7/13 | 54% | 5/14 | 36% | 11/31 | 36% | 0.50 |
| Any Drug Use 6 M Prior to HCV Treatment | |||||||||
| Yes | 35/58 | 60% | 10/13 | 77% | 11/14 | 79% | 14/31 | 45% | 0.040 |
| Any Drug Use During HCV Treatment | |||||||||
| Yes | 34/58 | 59% | 9/13 | 69% | 10/14 | 71.4% | 15/31 | 48% | 0.24 |
| Frequent Drug Use 6 M Prior to HCV Treatment | |||||||||
| Yes | 13/58 | 22% | 4/13 | 21% | 3/14 | 21% | 6/31 | 19% | 0.65 |
| Frequent Drug Use During HCV Treatment | |||||||||
| Yes | 13/58 | 22% | 5/13 | 38% | 4/10 | 29% | 4/31 | 13% | 0.13 |
Abbreviations : mDOT (modified directly observed therapy); GT (group treatment); SIT (self-administered individual treatment); M (month).
HCV treatment outcomes
Overall, 98% (60/61) of participants achieved SVR12 (HCV cure); the one participant that did not achieve SVR12 was lost to follow-up and did not obtain bloodwork for this assessment. This participant was a 46-year-old HIV-negative Hispanic male who had HCV genotype 1, advanced fibrosis, and completed treatment with sofosbuvir/ledipasvir for 12 weeks with an adherence rate of 78.6%. One-hundred percent of participants achieved end of treatment response (ETR) and an undetectable viral load four weeks after treatment completion (SVR4). SVR12 was no different based on HCV model of care received: mDOT (100%), GT (100%), or SIT (97%), p = 1.0; (Fig. 1). SVR12 for the participants receiving sofosbuvir/ledipasvir was 98% (CI 88.5–99.9) and SVR12 for participants receiving sofosbuvir/simeprevir was 100% (CI 78.2–100), no different than seen in registration trials (Fig. 2).
Fig. 1.
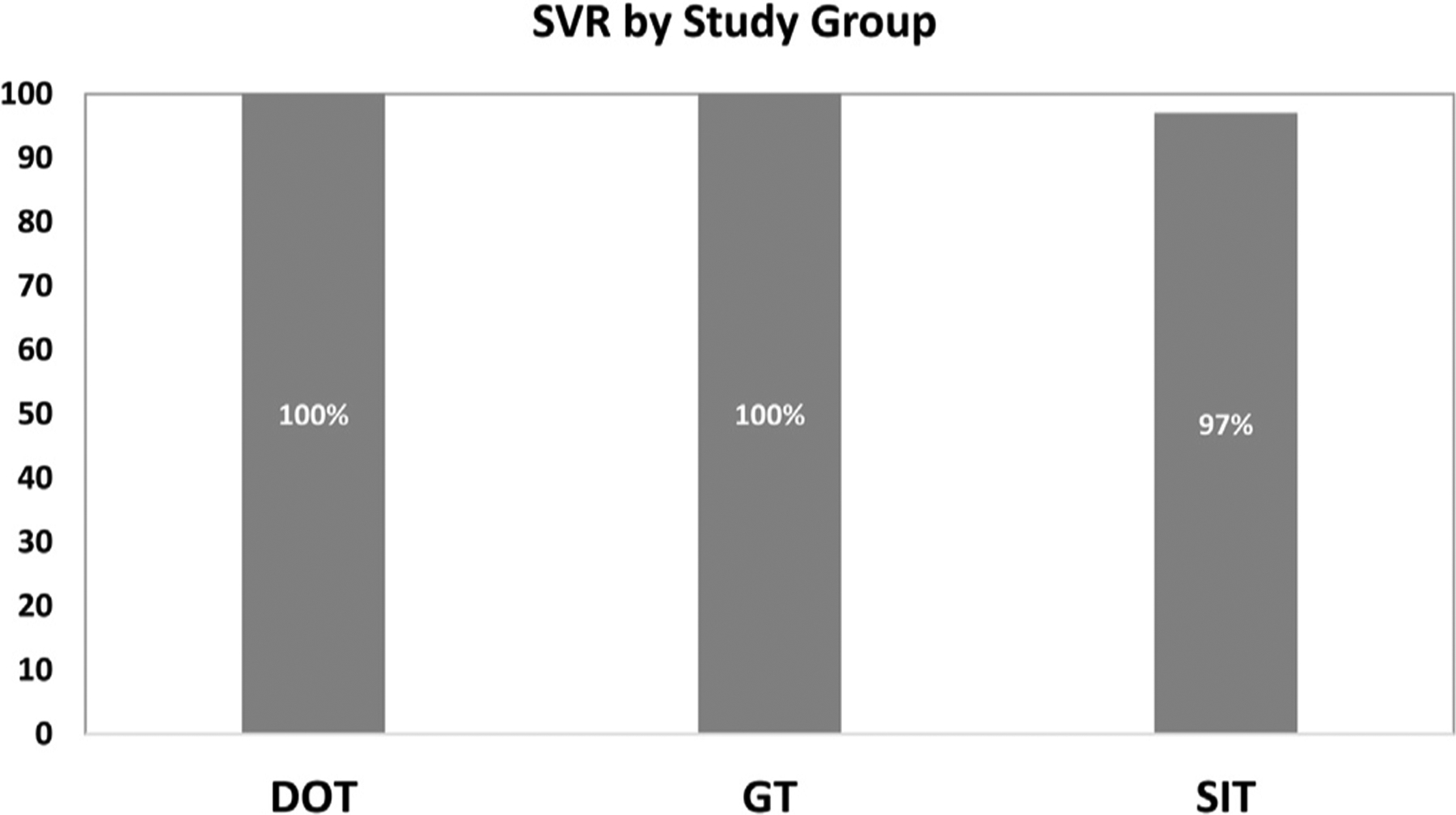
Sustained Virologic Response in each Study Group Abbreviations: mDOT (modified directly observed therapy); GT (group treatment); SIT (self-administered individual treatment).
Fig. 2.
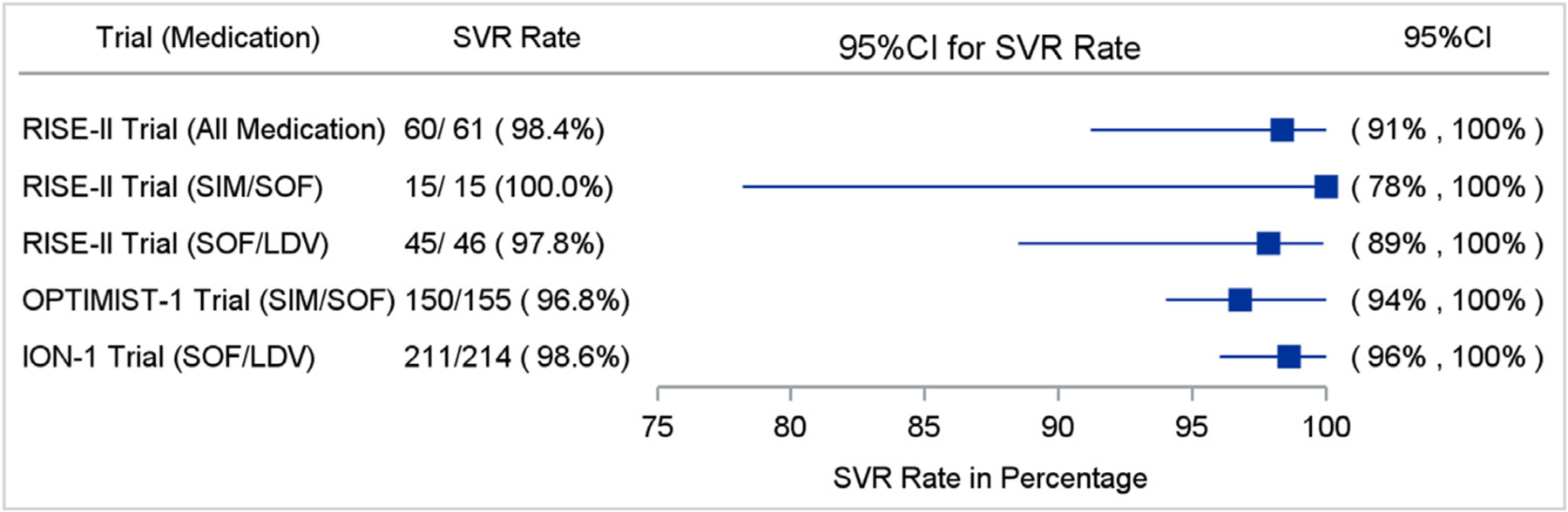
Comparison of SVR12 Rates Between Our Patient-Centered Models of Care and Large Registration Trials Abbreviations: SVR12 (sustained virologic response); SIM (simeprevir); SOF (sofosbuvir); LDV (ledipasvir).
Adherence
Overall adherence estimates using the electronic blister packs varied depending on the adherence timeframe that was measured. Overall, adherence estimated by the daily time frame was 73% (SD 16), by the weekly time frame was 90% (SD 11), and by window timeframe was 63% (SD 22). Participants in the mDOT model of care had higher daily adherence rates [86% (95% CI 78, 94] compared to participants in either GT [71% (95% CI 64,78), p < 0.01 v. mDOT) or SIT [73% (95% CI 68, 78), p < 0.01 v. mDOT). (Fig. 3). Similarly, the daily window time frame adherence was significantly higher among mDOT participants [81% (71, 91)] compared to GT [63% (53, 72), p = 0.011 v. mDOT] and SIT [61% (55, 68), p = 0.001 v. mDOT] participants. However, the weekly time-frame adherence was not significantly different across the three groups (p = 0.88): mDOT [93% (89, 97)], GT [94% (90, 97)] and SIT [92% (90, 95)].Differences in daily adherence between study groups were unchanged after adjusting for any drug use in the six months prior to HCV treatment (Fig. 4). After adjusting for study group, participants with benzodiazepine use during treatment had lower daily adherence than those without benzodiazepine use (66% [95% CI 60, 74] vs. 78% [95% CI 74, 83], p < 0.001.
Fig. 3.
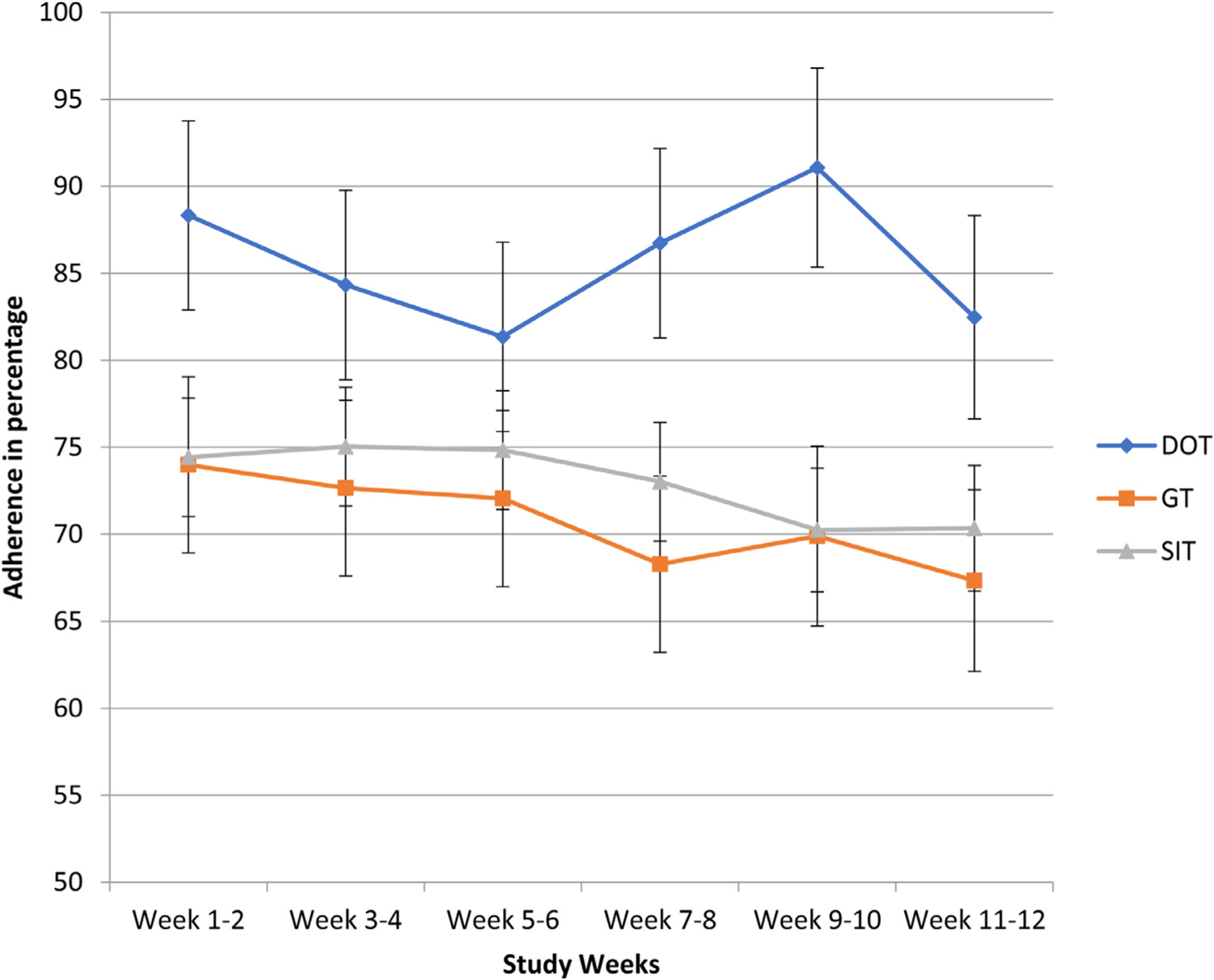
Daily Adherence in each Study Group Over Time.
Fig. 4.
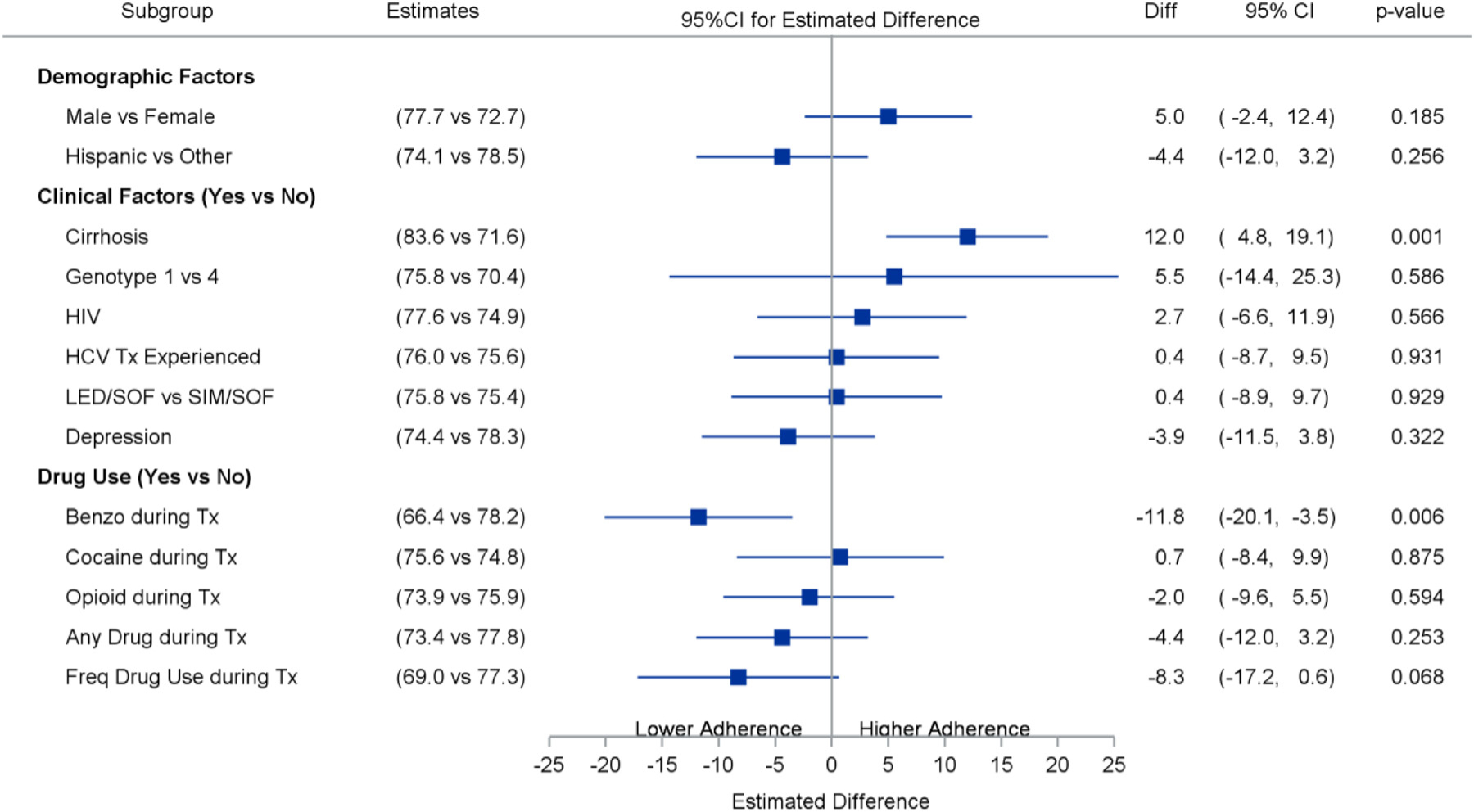
Impact of Participant Characteristics on Adherence.
Discussion
To our knowledge, this is the first study to evaluate patient-provider selected models of HCV care for PWID onsite at an OTP. Despite suboptimal adherence and the majority of participants using illicit drugs during HCV treatment, SVR12 rates were high in all study groups and were similar to large registration trials for these DAA regimens. This is notable since most registration trials of DAA therapies have generally excluded or minimized entry of participants who were either active PWID or on opiate agonist treatment. Additionally, all PWID in this study achieved SVR4, which has a 98% concordance with SVR12 in patients treated with sofosbuvir-based regimens (Yoshida, Sulkowski & Gane, 2015). Though other clinical trials of PWID have demonstrated high SVR12 rates (Dore, Altice & Litwin, 2016; Graf, Mucke & Dultz, 2019; Grebely, Dalgard & Conway, 2018), our results represent one of the highest SVR12 rates for any trial of PWID in the era of DAAs. This study also provides SVR12 data from a real-world setting in which PWID and providers chose the OTP-based model of HCV care through shared decision making.
While most participants still received the traditional SIT model of care, those with characteristics traditionally associated with poor adherence (i.e. those with depression or drug use) were over-represented in the more intensive HCV interventions (mDOT and GT), likely due to provider-patient preference. Despite these characteristics, participants in the mDOT group had higher adherence rates than in GT or SIT, and SVR12 rates were no different between all three groups. In a study conducted among PWID receiving DAAs in Australia, providers and clients chose to deliver enhanced adherence support to a subset of PWID, via increased communication and flexible DOT. Similar to our study, these participants had characteristics traditionally associated with poor adherence such as mental health comorbidities and increased drug use. Despite these characteristics, SVR12 rates among PWID who received the enhanced support were no different than those who did not receive enhanced support. Together, these results suggest that not all PWID require intensive models of care, but rather that patient-centered approaches through which patients at risk for poor adherence can receive treatment through mDOT, GT, or other enhanced support interventions that may improve adherence. Importantly, these intensive models of care should fit the setting in which they are implemented – though mDOT may be easily implemented in OTP programs throughout the world, other health settings with less frequent patient contact might benefit from other intensive models of care.
Additionally, though adherence in all three groups was lower than in other studies of PWID (Dore et al., 2016; J. Grebely, Dalgard & Conway, 2018), SVR12 rates were high and all participants achieved an undetectable viral load 4-weeks post treatment completion (SVR4). Based on data from the interferon-era, suboptimal adherence has been defined as less than 80%, which was shown to decrease rates of SVR (McHutchison, Manns & Patel, 2002). Yet other studies evaluating DAA adherence among PWID have also shown imperfect adherence with high SVR rates (Cunningham, Hajarizadeh & Amin, 2020; Norton et al., 2020). The SIMPLIFY and D3FEAT studies were international, multi-site studies of PWID with recent injection drug use, as well as those on OAT, that also used electronic blister packs to measure DAA adherence (Cunningham et al., 2020). Though the median adherence was higher than in our study (92%), nearly half of participants were deemed non-adherent (< 90% adherence), with no difference in SV12 among those non-adherent vs. adherent. Furthermore, there were no differences in SVR12 between those who did and did not miss 7 consecutive DAA doses. Our results, combined with other adherence data, suggest that high adherence may be not necessary for achieving SVR12, and further that the adherence threshold for cure in the era of DAAs may be lower than in the interferon-era.
Importantly, drug use during treatment, including opioid and cocaine use, was not associated with lower adherence rates; however, benzodiazepine use during treatment was associated with a significant reduction in adherence. Given its sedative properties, benzodiazepine use may reduce cognitive function and therefore adherence to medications, particularly given concurrent opioid use in the setting of methadone maintenance therapy. In a study of PWID treated in the interferon era, benzodiazepine use was associated with reduced HCV treatment uptake among PWID (Grebely, Alavi & Micallef, 2016). Though most studies in the era of DAAs have found that illicit drug use does not reduce HCV medication adherence, some have found that specific drugs (such as stimulants and/or alcohol) have led to reduced adherence (Akiyama et al., 2019; Cunningham et al., 2020; Graf et al., 2019). Because all drug use may not have the same impact on HCV medication adherence, more rigorous measures of specific types of drug, patterns of drug use, and polysubstance use may be needed to further elucidate if certain PWID are at an increased risk of non-adherence. That said, current studies in the DAA era have shown that even with non-perfect adherence or reduced adherence among certain PWID, SVR12 rates have not suffered. As such, we are in need of rigorous adherence studies that can more narrowly define lower adherence cut-offs that lead to HCV treatment failure.
This study has several limitations. Because participants were not randomized and due to convenience sampling, our study was underpowered to detect significant differences in SVR12 between groups, we cannot state which model of care would lead to the highest SVR12 rates. However, the shared decision-making process is useful in real world settings and needs further exploration. Participants in this study consisted of an older, urban population and therefore results may not be generalizable to a younger, non-urban/rural population. Finally, we did not examine the decision-making process between the provider and patient that led to the selected model of care.
In conclusion, PWID treated for HCV at an OTP through one of three patient-provider chosen models of care – mDOT, GT, or SIT – had HCV cure rates similar to registration trials. Despite suboptimal adherence and continued illicit drug use, HCV cure rates were high in all three groups. Our results add to existing evidence demonstrating that HCV cure is achievable in PWID. Furthermore, a patient-centered shared decision-making approach to HCV treatment may be the most appropriate model of care for PWID in OTP settings.
Acknowledgments
This study received support from Gilead Sciences and NIDA R01 034086.This study was also supported by the Prisma Health and Health Sciences Center Addiction Research Center.
Footnotes
Declarations of Interest
This study was approved by the Einstein College of Medicine Institutional Review Board. This manuscript is original work and has not been submitted elsewhere for publication. All authors meet criteria for authorship, have contributed significantly to the work, and have seen and approved the manuscript.
Declarations: Dr. Alain Litwin is on advisory boards for Gilead and Merck.
References
- AASLD/IDSA, Recommendations for testing, managing, and treating hepatitis C. Infectious Diseases Society of America/American Association for the Study of the Liver. Available at: http://www.hcvguidelines.org Accessed May 10, (2017).
- Afdhal N, Reddy KR, & Nelson DR (2014). Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. The New England Journal of Medicine, 370 (16), 1483–1493. [DOI] [PubMed] [Google Scholar]
- Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, & Litwin AH (2019). Intensive Models of Hepatitis C Care for People Who Inject Drugs Receiving Opioid Agonist Therapy: A Randomized Controlled Trial. Annals of Internal Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi M, Raffa JD, & Deans GD (2014). Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver International, 34 (8), 1198–1206. [DOI] [PubMed] [Google Scholar]
- Alderks C(2017).Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003–2015 (Update). Available at: https://www.samhsa.gov/data/sites/default/files/report_3192/ShortReport-3192.html. Accessed March 15th [PubMed]
- Alter MJ, Kruszon-Moran D, & Nainan OV (1999). The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. The New England Journal of Medicine, 341 (8), 556–562. [DOI] [PubMed] [Google Scholar]
- Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, & Page KA (2016). Clinicians’ Views of Hepatitis C Virus Treatment Candidacy With Direct-Acting Antiviral Regimens for People Who Inject Drugs. Substance Use & Misuse, 51 (9), 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, & Mole LA (2011). A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clinical gastroenterology and hepatology: The official clinical practice journal of the. American Gastroenterological Association, 9 (6), 509–516 e1. [DOI] [PubMed] [Google Scholar]
- Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, & Taylor LE (2015). Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Annals of Internal Medicine, 163 (3), 215–223. [DOI] [PubMed] [Google Scholar]
- Cunningham EB, Hajarizadeh B, & Amin J (2020). Adherence to once-daily and twice-daily direct-acting antiviral therapy for Hepatitis C infection among people with recent injection drug use or current opioid agonist therapy. Clinical Infectious Diseases, 71 (7), E115–Ee24. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Pocha C, Thuras P, Knott A, Colton S, & Ho SB (2014). All-cause mortality and liver-related outcomes following successful antiviral treatment for chronic hepatitis C. Digestive Diseases and Sciences, 59 (4), 872–880. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Altice F, & Litwin AH (2016). Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Annals of Internal Medicine, 165 (9), 625–634. [DOI] [PubMed] [Google Scholar]
- Graf C, Mucke MM, & Dultz G (2019). Efficacy of Direct-acting Antivirals for Chronic Hepatitis C Virus Infection in People Who Inject Drugs or Receive Opioid Substitution Therapy: A Systematic Review and Meta-analysis. Clinical Infectious Diseases. [DOI] [PubMed] [Google Scholar]
- Grebely J, Alavi M, & Micallef M (2016). Treatment for hepatitis C virus infection among people who inject drugs attending opioid substitution treatment and community health clinics: The ETHOS Study. Addiction, 111 (2), 311–319. [DOI] [PubMed] [Google Scholar]
- Grebely J, Dalgard O, & Conway B (2018a). Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): An open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol, 3 (3), 153–161. [DOI] [PubMed] [Google Scholar]
- Grebely J, Dalgard O, & Conway B (2018b). Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): An open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. [DOI] [PubMed] [Google Scholar]
- Grebely J, & Dore GJ (2014). Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Research, 104, 62–72. [DOI] [PubMed] [Google Scholar]
- Grebely J, Genoway K, & Khara M (2007). Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. International Journal of Drug Policy, 18 (5), 437–443. [DOI] [PubMed] [Google Scholar]
- Grebely J, Larney S, & Peacock A (2019). Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction, 114 (1), 150–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Matthews GV, Lloyd AR, & Dore GJ (2013). Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: Feasibility and future requirements. Clinical Infectious Diseases, 57 (7), 1014–1020. [DOI] [PubMed] [Google Scholar]
- Grebely J, Raffa JD, & Lai C (2009). Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. Journal of Viral Hepatitis, 16 (5), 352–358. [DOI] [PubMed] [Google Scholar]
- Hajarizadeh B, Grebely J, & Dore GJ (2013). Epidemiology and natural history of HCV infection. Nature Reviews Gastroenterology & Hepatology, 10 (9), 553–562. [DOI] [PubMed] [Google Scholar]
- Harris M, & Rhodes T (2013). Hepatitis C treatment access and uptake for people who inject drugs: A review mapping the role of social factors. Harm Reduction Journal, 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellard M, McBryde E, & Sacks Davis R (2015). Hepatitis C transmission and treatment as prevention - The role of the injecting network. International Journal of Drug Policy, 26 (10), 958–962. [DOI] [PubMed] [Google Scholar]
- Hellard M, Sacks-Davis R, & Gold J (2009). Hepatitis C treatment for injection drug users: A review of the available evidence. Clinical Infectious Diseases, 49 (4), 561–573. [DOI] [PubMed] [Google Scholar]
- Kwo P, Gitlin N, & Nahass R (2016). Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology, 64 (2), 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KN, Hughes EM, Jiles RB, & Holmberg SD (2016). Rising Mortality Associated With Hepatitis C Virus in the United States, 2003–2013. Clinical Infectious Diseases, 62 (10), 1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Thornton A, & Hickman M (2016). Can Hepatitis C Virus (HCV) Direct-Acting Antiviral Treatment as Prevention Reverse the HCV Epidemic Among Men Who Have Sex With Men in the United Kingdom? Epidemiological and Modeling Insights. Clinical Infectious Diseases, 62 (9), 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Vickerman P, & Grebely J (2013). Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology, 58 (5), 1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHutchison JG, Manns M, & Patel K (2002). Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology, 123 (4), 1061–1069. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Genberg BL, & Astemborski J (2008). Limited uptake of hepatitis C treatment among injection drug users. Journal of Community Health, 33 (3), 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgard H, Bramness JG, Skurtveit S, Haukeland JW, & Dalgard O (2016). Hepatitis C Treatment Uptake among Patients Who Have Received Opioid Substitution Treatment: A Population-Based Study. PLoS One, 11 (11), Article E0166451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravcik V, Strada L, & Stolfa J (2013). Factors associated with uptake, adherence, and efficacy of hepatitis C treatment in people who inject drugs: A literature review. Patient Preference and Adherence, 7, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton BL, Akiyama MJ, Agyemang L, Heo M, Pericot-Valverde I, & Litwin AH (2020). Low Adherence Achieves High HCV Cure Rates Among People Who Inject Drugs Treated With Direct-Acting Antiviral Agents. Open Forum Infectious Diseases, 7 (10) Ofaa377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick DM, & Kreek MJ (2008). Critical issues in the treatment of hepatitis C virus infection in methadone maintenance patients. Addiction, 103 (6), 905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich ZC, Chu C, & Mao J (2016). Facilitators of HCV treatment adherence among people who inject drugs: A systematic qualitative review and implications for scale up of direct acting antivirals. BMC Public Health, 16, 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Maksad J, & Clarke J (2001). Hepatitis C disease among injection drug users: Knowledge, perceived risk and willingness to receive treatment. Drug and Alcohol Dependence, 61 (3), 211–215. [DOI] [PubMed] [Google Scholar]
- Stein MR, Soloway IJ, Jefferson KS, Roose RJ, Arnsten JH, & Litwin AH (2012). Concurrent group treatment for hepatitis C: Implementation and outcomes in a methadone maintenance treatment program. Journal of Substance Abuse Treatment, 43 (4), 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Latka M, & Campbell J (2005). Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clinical Infectious Diseases, 40 (Suppl 5), S304–S312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryaprasad AG, White JZ, & Xu F (2014). Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clinical Infectious Diseases, 59 (10), 1411–1419. [DOI] [PubMed] [Google Scholar]
- Sylvestre DL, & Zweben JE (2007). Integrating HCV services for drug users: A model to improve engagement and outcomes. International Journal of Drug Policy, 18 (5), 406–410. [DOI] [PubMed] [Google Scholar]
- Treloar C, Hull P, Dore GJ, & Grebely J (2012). Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug and Alcohol Review, 31 (7), 918–924. [DOI] [PubMed] [Google Scholar]
- Treloar C, Newland J, Rance J, & Hopwood M (2010). Uptake and delivery of hepatitis C treatment in opiate substitution treatment: Perceptions of clients and health professionals. Journal of Viral Hepatitis, 17 (12), 839–844. [DOI] [PubMed] [Google Scholar]
- van der Meer AJ, Veldt BJ, & Feld JJ (2012). Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA, 308 (24), 2584–2593. [DOI] [PubMed] [Google Scholar]
- Walley AY, White MC, Kushel MB, Song YS, & Tulsky JP (2005). Knowledge of and interest in hepatitis C treatment at a methadone clinic. Journal of Substance Abuse Treatment, 28 (2), 181–187. [DOI] [PubMed] [Google Scholar]
- Yoshida EM, Sulkowski MS, & Gane EJ (2015). Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology, 61 (1), 41–45. [DOI] [PubMed] [Google Scholar]
- Zeremski M, Dimova RB, & Zavala R (2014). Hepatitis C virus-related knowledge and willingness to receive treatment among patients on methadone maintenance. Journal of Addiction Medicine, 8 (4), 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]


