Abstract
Of all the eye conditions in the contemporary Indian context, diabetic retinopathy (DR) attracts the maximum attention not just of the eye care fraternity but the entire medical fraternity. Countries are at different stages of evolution in structured DR screening services. In most low and middle income countries, screening is opportunistic, while in most of the high income countries structured population-based DR screening is the established norm. To reduce inequities in access, it is important that all persons with diabetes are provided equal access to DR screening and management services. Such programs have been proven to reverse the magnitude of vision-threatening diabetic retinopathy in countries like England and Scotland. DR screening should not be considered an endpoint in itself but the starting point in a continuum of services for effective management of DR services so that the risk of vision loss can be mitigated. Till recently all DR screening programs in India were opportunistic models where persons with diabetes visiting an eye care facility were screened. Since 2016, with support from International funders, demonstration models integrating DR screening services in the public health system were initiated. These pilots showed that a systematic integrated structured DR screening program is possible in India and need to be scaled up across the country. Many DR screening and referral initiatives have been adversely impacted by the COVID-19 pandemic and advocacy with the government is critical to facilitate continuous sustainable services.
Keywords: Diabetes mellitus, diabetic retinopathy, India, review, screening
Diabetes mellitus (DM) and its complications are a leading challenge to health systems globally and for attaining universal health coverage. The pillars of universal health coverage are accessibility and financial protection and diabetes and its complications test both these pillars. This is because nearly half the population with diabetes is unaware of its diabetic status and even among those who know their diabetic status, access to comprehensive diabetic care services is a major barrier.[1] Additionally, evidence shows that people with diabetes incur significant out-of-pocket expenditure, both direct and indirect.[2]
Magnitude and Trends in Diabetes in India
Diabetes mellitus is now recognized as a significant public health concern in India. The International Diabetes Federation 2019 Atlas projected that 77 million people in India are living with diabetes, with an age-adjusted prevalence of 10.4% (95% Confidence Interval [CI]: 8.4–13.0) among the population aged 20–79 years of age.[1] This is projected to increase to 101 million by 2030 and 134.2 million by 2045.[1] The projections show that India will continue to maintain the 2nd rank in diabetes magnitude, just next to China over the next two decades. These estimates are also corroborated by a major nationwide survey covering 15 States in India, conducted by the Indian Council for Medical Research (ICMR) which shows an age-standardized prevalence of 7.3% (95% CI: 7.0–7.5).[3] The ICMR study also observed that the prevalence varies across the country and that in addition to the urban areas, people hailing from the lower socio-economic strata also showed high prevalence rates.[3]
A recent systematic review of prevalence studies conducted in India found that the reported prevalence estimates varied from a low 1.9% to a high of 25.2% and that 70% of the studies were undertaken in the southern part of India.[4] Therefore, the ICMR study provides a representative national estimate of diabetes. A meta-analysis of 1.7 million Indians documented that the prevalence of diabetes increased in both rural and urban India from 2.4% and 3.3% in 1972 to 15.0% and 19.0%, respectively, in year 2015–2019. Therefore, it can be safely concluded that the evidence points to a major epidemic of diabetes in India, which does not show signs of abating.[5] Another worrisome feature in India is the shift to diabetes initiation at much younger ages and the significant out-of-pocket expenditure incurred by people and families with diabetes.[2]
Magnitude of Diabetic Retinopathy in India
Globally, the number of people with diabetic retinopathy (DR) is expected to grow from 126.6 million in 2010 to 191 million by 2030 and some studies estimate that the number of vision threatening diabetic retinopathy (VTDR) will increase from 37.3 million to 56.3 million.[6] Recent global estimates for the period 2015–2019 were generated from studies using retinal photography.[7] This review of 32 studies from 21 countries (2 from South Asia-India; Nepal) covering 543,448 people with diabetes showed that the overall prevalence for any DR in the South East Asia region was 12.5% (range: 9.9%–15.4%), nonproliferative diabetic retinopathy (NPDR) was 11.8% (range: 9.1%–14.7%), proliferative diabetic retinopathy (PDR) was 3.5% (range: 0.5%– 6.7%), and diabetic macular edema (DME) was 6.0%, compared to global estimates of 27.0% for the presence of any DR, comprising predominantly of NPDR (25.2%), PDR (1.4%), and DME 4.6%.[7] Another systematic review, which included 59 population-based studies and extrapolating data from the 2019 IDF Diabetes Atlas, observed that the global prevalence of any DR was 22.27% (95% CI: 19.73–25.03), and VTDR was 6.17% (95% CI: 5.43–6.98).[8] The study projected that by 2020 there were 103.12 million people with any DR and 28.54 million with VTDR, and that by 2045, these numbers would increase to 160.5 million and 44.82 million, respectively. Therefore, the authors concluded that the burden of DR would continue to remain high and the numbers should guide the public health interventions for DR.[8]
It has been observed that 51% of all those with blindness due to DR globally (n = 424,400) and 56% of those with visual impairment due to DR (2.1 million) come from the Asia-Pacific Region.[9] India has contributed significantly to the global evidence on the prevalence of DR. Recent studies (2015–2020) show that the prevalence of DR among people with diabetes in India varies between 10% and 16.9% [Table 1].[10,11,12,13,14,15] Most of these studies covered rural habitations and many used the Rapid Assessment of Avoidable Blindness survey methods. The advantage of this is that data can be analyzed quickly and can expeditiously be used for planning interventions.
Table 1.
Prevalence of diabetic retinopathy among known people with diabetes in India (2015-2020)
| Area; Year | Sample; Age | Modality | Prevalence any DR* | Prevalence VTDR** |
|---|---|---|---|---|
| Mumbai urban slums (2017)[10] | 6462; ≥40 years | Fundus Photography | 15.4% | 6.6% |
| Nationwide (2015-19)[11] | 85,135; ≥50 years | Indirect Ophthalmoscopy | 16.9% | NA |
| Pune urban (2017)[12] | 3527; ≥50 years | Indirect Ophthalmoscopy | 14.3% | NA |
| Tamil Nadu rural (2016)[13] | 1190; ≥40 years | Fundus photography | 10% | NA |
| Bihar rural (2016)[14] | 3189; ≥50 years | Indirect Ophthalmoscopy | 15% | 6% |
| Delhi slums (2016)[15] | 11566; ≥40 years | Fundus Photography | 13.5% | NA |
*DR: Diabetic Retinopathy; **VTDR: Vision Threatening Diabetic Retinopathy
Projections show that approximately 3.35–4.55 million persons with diabetes mellitus are at risk of vision-threatening DR (VTDR) in India.[16] This translates to 2,240–3,136 people aged 20 years and above in a population of 1 million. A study estimated that assuming a prevalence of 20% DR among people with diabetes in urban and 10% prevalence of DR in rural India and that 70% of the population normally resides in rural areas, the magnitude of DR would increase to 10.97 million by 2030.[17] These numbers are staggering and can overwhelm the eye care systems in India unless measures for task-sharing and task-shifting are fostered.
Screening for Diabetic Retinopathy
The World Health Organization (WHO) defines screening as ‘the presumptive identification of unrecognized disease or defect by the application of tests, examinations, or other procedures that can be applied rapidly.” Screening tests therefore sort out those who are apparently well but probably have disease from others who are free of the disease being screened. Therefore, screening is not a diagnostic tool but identifies suspects who may have the disease (which needs to be confirmed by applying diagnostic tests). Many a time, people use screening with diagnostic tests synonymously and therein lies the danger of leaning too heavily on screening tools for diagnosis. There are two broad categories of screening: Population-based mass screening and selective screening.[18] Selective screening targets high-risk groups and is therefore more efficient and produces higher yield compared to mass screening.
Selective screening can be further categorized as opportunistic and systematic. Opportunistic screening is sporadic and occurs when a test is offered by a health care professional or when the patient asks the health provider for the test. Opportunistic screening usually is not subjected to strict quality assurance protocols and may not include all those at risk.[19] DR screening camps held intermittently or hospital consultations for walk-in patients fall in this category. In systematic screening active identification of those at risk, maintenance of a register of eligible subjects, and invitation to attend the screening program are essential ingredients.[19] Since the process systematically covers those at risk (i.e. people with diabetes), it ensures better coverage of the population in need.
There is an international acceptance and concurrence that the screening tests for DR should have at least 80% sensitivity, 95 specificity, and <5% technical failure rates, which are also endorsed by the All India Ophthalmological Society (AIOS) guidelines on DR.[20] Screening is only one component of a continuum of DR management and should not be considered as an end-point. Screening is effective only if it is supported by a referral chain and appropriate treatment and follow-up are ensured to close the loop for the patient.
Status of systematic DR screening in India
Till recently, screening for DR in India was purely opportunistic and functioned as a vertical activity with no identification of the denominator population of persons with diabetes in a geographic delimited region and a lack of coordination between the physician treating diabetes and the ophthalmologists treating DR. There were some isolated examples of synergy but it was not approached in a systematic planned program mode. With opportunistic screening as the only modality, there is a risk that early disease is missed as people report to an eye facility only when they have a vision loss. This was amply demonstrated in a situational analysis conducted in major metropolitan areas which showed that people with DR, reported to an eye facility only when they had some degree of visual loss.[21] This defeats the principle of screening.
With the huge workload for managing DR, it is time that DR screening and management programs evolve into an ophthalmologist-supported rather than continue to be an ophthalmologist led screening program. Any approach which frees up time from an already busy schedule of an ophthalmologist in a spirit of effective partnerships and task sharing, will help to improve reach of screening programs for DR. Such an approach has also been endorsed strongly by the AIOS which recommends that any health care professional who has been trained suitably can screen for DR. It can be ophthalmologists, optometrists, ophthalmic assistants, trained eye technicians, NCD nurses, or physicians.[20]
Whatever approach is used, the overall goal of a DR screening program should be the prevention of VTDR. Recent efforts to cast the screening net wider by training nonophthalmologist eye care personnel have proved successful. A pilot project observed that the competencies of optometrists to screen for DR after a structured training were very good.[22] The study observed that the sensitivity and specificity for detection of any DR were 95% and 79%, respectively, and the sensitivity and specificity for VTDR were 88% and 90%, respectively.[21] These results are very encouraging and can open the door to adapting the English National Health Services protocols on screening and grading.[23] The England DR program has succeeded in reducing the magnitude of VTDR in England by using a mix of personnel to photograph, read, grade, and manage DR.[23] However, the overall responsibility of the program will rest on the ophthalmologist's shoulders.[20]
A working group of ophthalmic and diabetes experts was constituted recently by the Vision Academy, a Bayer educational initiative to propose evidence-based recommendations for screening for DR.[24] The group recommended that an effective DR screening program should include an accreditation system and require staff to demonstrate evidence of ongoing training. It was stated that trained primary care physicians have been found to grade retinal photographs with acceptable accuracy when compared with ophthalmologists. The group also recommended that in many countries/regions, screening can and should take place outside the ophthalmology clinic. The group opined that induction of mydriasis may improve the specificity of DR detection but involved longer examination time and greater patient discomfort.[24]
It was only in the last five years that a proactive positive trend has emerged in the country. Generous grants first from the Queen Elizabeth Diamond Jubilee Trust, UK and then the UK Research and Innovation (UKRI) through the Global Challenges Research Fund have facilitated the thinking on systematic screening programs embedded in a comprehensive diabetic care model.[16,25] The Queen's Trust initiative developed potential models were mapped against the Wilson–Jungner criteria for screening, to see if there was a good fit with the criteria[18] [Table 2].
Table 2.
Wilson-Jungner criteria applied to Queen's Trust Project
| Criteria | Diabetic Retinopathy | Queen’s Trust Pilot Projects |
|---|---|---|
| Condition should be an important public health problem | Evidence shows that 10%-20% people with diabetes have DR and if not detected in time, it can lead to irreversible vision loss | Areas with higher prevalence of diabetes and therefore with risk of higher prevalence of DR identified compared to other areas |
| There should be an accepted treatment for patients with recognized disease | Effective treatment is available for DR though affordability can be an issue | An effective system for screening, referral, and management of DR was supported |
| Facilities for diagnosis and treatment should be available | Skills, infrastructure, and access are patchy and discriminate against people with diabetes in rural areas | Physicians from the identified districts and NCD Clinic Nurses/Female Health Workers along with ASHA sensitized; Paramedical Ophthalmic Assistants/Officers/NCD Clinic Nurses/Ophthalmologists were skilled and equipment including Fundus Cameras were provided at the district hospital and CHC |
| There should be a recognizable latent or early symptomatic stage | DR has a long latent window and takes 15-20 years to lead to vision loss in most cases | All persons with diabetes registered with NCD Clinics were offered fundus imaging to prevent VTDR |
| There should be a suitable test or examination process | Noninvasive screening tests are available | Nonmydriatic fundus cameras and skills to use the same were provided to all identified districts |
| The test should be acceptable to the people | Undilated fundus examination is acceptable to most people but there is more hesitancy for dilated fundus examination | High compliance rates for screening using nonmydriatic fundus imaging was seen. |
| The natural history of the condition, including development from latent to declared disease should be adequately understood | Available evidence supports knowledge of the natural history and rate of progression in most individuals. However, there may be rapid progression in proliferative DR or DME | Available evidence on progression of DR and VTDR was used to develop strategies under the project. |
| There should be an agreed policy on whom to treat as patients | National guidelines are available along with ICO guidelines on whom to treat | Guidelines were developed and shared with all mentoring partner institutes |
| The cost of case-finding (including diagnosis and treatment) should be economically balanced in relation to possible expenditure on medical care as a whole | Cost of case-finding is affordable; cost of treatment with some regimens is not affordable, unless covered by insurance schemes; treatment entails a high out-of-pocket expenditure | Screening and DR management services were provided at no cost to the patient |
| Case finding should be a continuous process and not a one-time intervention | Systematic screening is not yet established and many people are screened in temporary camps | Screening activities and referral services were in place from 2016/2017 to the end of the project |
The Queen Elizabeth Diamond Jubilee Trust supported a pilot project to integrate DR screening and management with the existing government public health system at the district and sub-district levels. Support of the National Government and respective State governments was elicited. The pilot envisaged reaching persons with diabetes outside the eye facilities so that persons with diabetes could be identified at an early stage in the natural history of diabetes so as to reduce the risk of VTDR. The National Program for Control of Noncommunicable Diseases (NCD) which was initiated in 2011 embarked on establishing NCD Clinics at all levels of the health system. This afforded a viable sustainable opportunity to embed screening for DR among known diabetics registered at the NCD Clinics for their regular medication and follow-up. Ten districts were identified [Table 3] based on the known prevalence of diabetes and the availability of NCD Clinics and a mentoring hospital in the Nongovernmental Organization (NGO) sector, which had the capacity to support the government public health system. Overall, 66,455 people with diabetes were screened and DR was detected in 16.2% (10,765), while VTDR was detected in 7.5%. There was no existing baseline, but comparing the data from the first year of the project till the end of the implementation phase, a 7-fold increase in the number of people screened was witnessed.[16] The project was able to synchronize the strengths of the available infrastructure and human resources under the National NCD Program and the National Program for Control of Blindness and Visual Impairment. The Trust-supported implementation phase ended in June 2019 but the program continued to be implemented by the government sector with support of mentoring hospitals beyond the project lifespan in most districts, albeit at a lower intensity in many States.
Table 3.
Queen's Trust supported pilot DR screening and management project in India
| State/District | Screening initiated | Screening location | Fundus Imaging | Image Grading | No. screened |
|---|---|---|---|---|---|
| Andhra Pradesh (Viziangaram) | March 2016 | CHC1/DH2 NCD3 Clinics | PMOA/OO4 | DH Ophthalmologist | 5801 |
| Goa Whole State | May 2016 | NCD Clinics CHC, PHC, SDH5 | PMOA/OO | DH Ophthalmologist | 5867 |
| Gujarat (Surat) | Feb 2017 | NCD Clinics in CHC, DH | PMOA/OO | Ophthalmologist | 5972 |
| Karnataka (Tumkur) | January 2016 | NCD Clinics CHC; Mobile Van | PMOAOO; Ophthalmologist | PMOA of Mentoring Hospitals | 6017 |
| Kerala (Thrissur) | Dec 2016 | NCD Clinics, CHC | PMOA/OO | Ophthalmologist of Mentoring Hospital | 18084 |
| Maharashtra (Wardha) | October 2016 | NCD Clinics CHC, PHC, DH | NCD Nurses; PMOA/OO | Ophthalmologist of Mentoring Hospital | 8759 |
| Odisha (Khurda) | Apr 2016 | NCD Clinics CHC | PMOA/OO Ophthalmologist | Ophthalmologist at Mentoring and Capital Hospital | 1672 |
| Rajasthan (Pali) | Aug 2016 | CHC | PMOA/OO | Ophthalmologist at Mentoring Hospital | 3310 |
| Tamilnadu (Tirunelveli) | Nov 2016 | NCD Clinics CHC | NCD Nurses | Ophthalmologist at Medical College and Mentoring Hospital | 6462 |
| West Bengal (Medinapur Paschim) | Jan 2017 | NCD Clinics CHC and DH | PMOA/OO | Ophthalmologist at Mentoring Hospital | 4511 |
| Total | 66,455 |
1 CHC: Community health Centre; 2 District Hospital; 3 NCD: Noncommunicable Disease Clinics; 4 PMOA/OO: Para Medical Ophthalmic Assistant/Ophthalmic Officer; 5 Subdivisional Hospital
During the lifespan of the project, a number of modalities were experimented with to reach persons with diabetes in the different districts. This varied from a static to a mobile approach; from Primary Health Centre (PHC) to Community Health Centre (CHC) as the location for screening; NCD nurse to a paramedical ophthalmic assistant/officer (PMOA/O) being the primary screener; and from standalone DR screening and management to comprehensive management of diabetes and its complications.[16] Approximately 1,000 people with diabetes were screened per 100,000 population, in each defined geographically delimited area. The pilot projects demonstrated for the first time ever in India that integration with the government public health system is possible. In the last three years, there have been very few public health interventions for DR at the population level in India [Table 4]. These studies show that there can be more than one way in which DR screening and management services can be provided in a country so geographically and culturally diverse like India.
Table 4.
Models of systematic DR screening in India
| State/District [Reference] | Modality | Population Covered | Screening lead | Grading Lead |
|---|---|---|---|---|
| Kerala (Thiruvanthapuram)[25] | Nonmydriatic Imaging - NCDa Clinics | 16 PHCsb | PMOA/OOc | Ophthalmologist |
| Goa State[26] | Nonmydriatic Imaging; Comprehensive care of diabetes and all complications - NCD Clinics | 188,640 (all PHCs) | PMOA/OO | Ophthalmologist |
| Tamil Nadu; Tirunelveli[27] |
Nonmydriatic imaging - NCD Clinics |
753,254 18 CHCd/PHC |
NCD Clinic Nurse | Ophthalmologist |
| Maharashtra; Wardha[28] |
Nonmydriatic imaging- Static NCD Clinics and Mobile Van |
1,016,918 6 CHC; DHe; 22 PHC |
PMOA/OO/NCD Clinic Nurses | Ophthalmologist |
| Delhi; Slum dwellers[15] | Nonmydriatic imaging at Vision Centres | 11516 known diabetic; Camps in schools/Vision Centres | Optometrist | Optometrist |
| Karnataka; Tumkur[29] | Nonmydriatic imaging at CHC | 1,249,169; known diabetics registered at NCD clinics supported by mobile van | PMOA/OO | Ophthalmologist |
a NCD: Noncommunicable Disease; b PHC: Primary Health Centre; c PMOA/OO: Para Medical Ophthalmic Assistant/Ophthalmic Officer; d CHC: Community Health Centre; e District Hospital
Impact of COVID-19 on community-based DR screening services
The gains made in the country over the past decade in establishing population-based screening embedded within the community were severely dented by the COVID-19 pandemic. The impact has been seen globally with both the people and providers being apprehensive, but the biggest damage has been in low and middle income countries (LMIC).[30,31] Data from a leading eye care institution in South India showed that in the initial months of “unlocking phase” following the COVID-19 outbreak and the lockdown, there was a 36.7% decline in footfalls compared to the previous years and the impact was seen across all levels of the eye care system.[32] At the height of the pandemic, elective services like community-based screening for DR was halted universally. Though services have been resumed in many countries, the people with diabetes are still hesitant to use services. This can result in a spike of VTDR in the next couple of years.
In India, all the districts where the Queen's Trust projects were initiated were adversely affected in the aftermath of the COVID-19 pandemic and the future resumption and scaling-up of these services by the government is clouded with uncertainty due to the substantial allocations made by the governments for COVID-19. In the larger interest of the persons with diabetes, strong and persistent advocacy efforts are needed with the government, to ward off the dangers of abandoning the threat of increasing rates of VTDR. Across the world, successful DR screening programs are delivered by the government health systems, whether it is the United Kingdom, Canada, Australia or New Zealand. Nongovernmental organizations and researchers can at best try out a proof-of-concept intervention or cover small populations, but the onus of scaling up and sustaining essential health care services rests with the public health systems, funded by public finances and taxes.
The way forward in the post COVID-19 scenario
COVID-19 is neither the first nor the last pandemic or health emergency that will confront the present or future generations. Emergency preparedness which will cause minimal disruption to the delivery of health and eye care needs planned investments in technology, infrastructure, and skilling. It will become increasingly more important to plan effective task-shifting and task-sharing strategies to maintain efficiency of service delivery, without compromising on the quality of care. The past five years have seen rapid progress on the use of technology-supported services. Technology has to be harnessed such that there is a direct benefit to patients by increasing access to health care that patients can receive care closer to their homes.
Telemedicine has allowed the transition of health care back into the patient's home. Traditionally, consultations were at the patient's home which moved into the outpatient clinics and hospitals due to increased use of sophisticated diagnostic equipment to make a diagnosis. Telemedicine combines the benefits of diagnostic tools in the patient's home environment using technology to bridge the distance between the patient and the clinic. Video consultations have increased in the post COVID era but the next breakthrough that is required is for patient's to be able to be examined at home or in its vicinity either by self or facilitated by a family member or community volunteer, and then uploading images for being read by a specialist. Till such a breakthrough is available, using mobile vans mounted with fundus cameras which will allow a person with diabetes to be examined from their community setting, close to home may be the need of the future. The imaging can be done by trained nonophthalmic personnel who are located at the nearest PHC or Vision Centers.
With an increasing load of persons with diabetes and with the availability of automated image analysis, it is time to share the task of imaging with NCD clinic nurses and Para Medical Ophthalmic Assistants/Officers (PMOA/OO) and Vision Technicians (VT) rather than Ophthalmologists. A process flow that engages different cadres in the DR care delivery pathway needs to be designed. If NCD Clinic Nurses/PMOA/OO and VT are tasked with imaging and optometrists with grading with the support of automated systems, it can increase the efficiency of the system in delivering DR care. Senior optometrists can be trained to verify a sample of the images read by automated systems and ophthalmologists can verify a sub-sample. The overall responsibility would rest with the Ophthalmologist, without severely compromising their time for direct clinical care.
Artificial intelligence (AI), machine learning (ML), and deep learning (DL) have revolutionized diagnostic acumen across the spectrum of health care. Algorithms developed by many fundus camera manufacturers and other agencies involved in AI and DL have been found to have high sensitivity and specificity >95% even with nonmydriatic images.[33,34]
A recent systematic review which also included a study from India concluded that teleophthalmology was cost-effective based on the results of the economic evaluations.[35] The authors stated that these programs were even more effective in low-income populations and rural diabetic patients who incur high transportation costs. They perceived that with advances in technology including better and faster telecommunication, cloud storage, miniaturization of equipment (smartphones with digital cameras), and automation of retinal image analysis, teleophthalmology screening programs can be optimized even further.
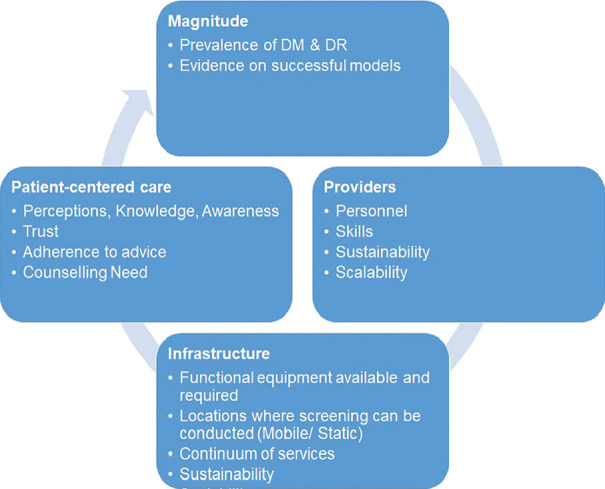
Even with technology-enabled DR screening services, it is to be emphasized that concentrating on targeted populations within a geographically delimited area is critical for success. Since the primary aim of DR screening services is to prevent the progression of VTDR, the priority group that needs to be targeted is known persons with diabetes. One should not combine screening for diabetes with screening for DR. Both are important but parallel activities, and pathways have now been established for the detection of diabetes in the NCD control program. Now it is important that the eye care fraternity develops protocols for sustainable, affordable, scalable DR screening services that are population-based and reach the known persons with diabetes with coverage rates of more than 80% to reduce avoidable visual impairment and blindness from diabetes. A situational analysis framework should be used for planning such services [Fig. 1].
Figure 1.
Situational analysis framework for diabetic retinopathy
Conclusion
All efforts should be directed toward preventing, detecting, and managing VTDR to improve the quality of life of persons with diabetes. The screening programs should ensure a high yield by concentrating on reaching the priority population of persons with diabetes. Optimizing the available ophthalmology workforce to provide high-quality clinical services means that task-sharing is critical for the success of any DR screening program. With the tiered architecture of the existing public health system and the availability of committed public funding for eye care, efforts should be made to integrate DR screening and management services with the public health system at all levels, especially so at the district and sub-district levels. Recent pilot programs have demonstrated models of an integrated approach which can be scaled up with continued advocacy with the government.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diab Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Pradeepa R, Mohan V. Prevalence of type-2 diabetes and its complications in India and economic costs to the nation. Eur J Clin Nutr. 2017;71:816–24. doi: 10.1038/ejcn.2017.40. [DOI] [PubMed] [Google Scholar]
- 3.Anjana RM, Mohan D, Pradeepa R, Mahanata J, Narain K, Das HK, et al. Prevalence of diabetes and pre-diabetes in 15 States of India:results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diab Endocrinol. 2017;5:585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 4.Atre S, Deshmukh S, Kulkarni M. Prevalence of type 2 diabetes (T2DM) in India: A systematic review (1994-2018) Diabetes Metab Syndr. 2020;14:897–906. doi: 10.1016/j.dsx.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 5.Ranasinghe P, Jayawerdana R, Gamage N, Sivanandam N, Misra A. Prevalence and trends of the diabetic epidemic in urban and rural India: A pooled systematic review and meta-analysis of 1.7 million adults. Ann Epidemiol. 2021;58:128–48. doi: 10.1016/j.annepidem.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Congdon N, Zheng Y, He M. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60:428–31. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas RL, Halim S, Gurudas S, Sivaprasad S, Owens DR. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diab Res Clin Pract. 2019;157:107840. doi: 10.1016/j.diabres.2019.107840. [DOI] [PubMed] [Google Scholar]
- 8.Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheng N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology. 2021;S0161-6420(21):00321–3. doi: 10.1016/j.ophtha.2021.04.027. doi:10.1016/j.ophtha.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Chua J, Lim CXY, Wong TY, Sabanayagam S. Diabetic retinopathy in the Asia Pacific. Asia Pac J Ophthalmol. 2018;7:3–16. doi: 10.22608/APO.2017511. [DOI] [PubMed] [Google Scholar]
- 10.Mohan S, Singh AK, Rogye A, Sonawane M, Gaonkar R, Srinivasan R, et al. Prevalence of diabetic retinopathy in urban slums: The Aditya Jyot Diabetic Retinopathy in urban Mumbai slums study –Report 2. Ophthalmic Epidemiol. 2017;24:303–10. doi: 10.1080/09286586.2017.1290258. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Vashist P. Indian community eye care in 2020: Achievements and challenges. Indian J Ophthalmol. 2020;68:291–3. doi: 10.4103/ijo.IJO_2381_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni S, Kondalkar S, Mactaggart I, Shamanna BR, Lodhi A, Mendke R, et al. Estimating the magnitude of diabetes mellitus and diabetic retinopathy in an older age urban population in Pune, Western India. BMJ Open Ophthalmol. 2019;4:e000201. doi: 10.1136/bmjophth-2018-000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan R, Singh S, Surya J, Sharma T, Kulothunga V, Raman R. Age of onset of diabetes and its comparison with prevalence and risk factors for diabetic retinopathy in a rural population of India. Ophthalmic Res. 2019;61:236–42. doi: 10.1159/000496732. [DOI] [PubMed] [Google Scholar]
- 14.Poddar AK, Khan TA, Sweta K, Tiwary MK, Borah RR, Ali R, et al. Prevalence and causes of avoidable blindness and visual impairment, including the prevalence of diabetic retinopathy in Siwan district of Bihar, India: A population-based survey. Indian J Ophthalmol. 2020;68:375–80. doi: 10.4103/ijo.IJO_1709_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadhwani M, Vashist P, Singh SS, Gupta N, Malhotra S, Gupta A, et al. Diabetic retinopathy screening programme utilizing non-mydriatic fundus imaging in slum populations of New Delhi, India. Trop Med Int Health. 2018;23:405–14. doi: 10.1111/tmi.13039. [DOI] [PubMed] [Google Scholar]
- 16.Murthy GVS, Gilbert C, Shukla R, Bala V, Anirudh GG, Mukpalkar S, et al. Overview and project highlights of an initiative to integrate diabetic retinopathy screening and management in the public health system in India. Indian J Ophthalmol. 2020;68(Suppl 1):S12–5. doi: 10.4103/ijo.IJO_1964_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das TP, Pappuru RR. Telemedicine in diabetic retinopathy: Access to rural India. Indian J Ophthalmol. 2016;64:84–6. doi: 10.4103/0301-4738.178151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. WHO papers No 34. 1968. [Last accessed on 2021 May 13]. Available from: https://apps.who.int/iris/handle/10665/37650 .
- 19.Gangwani RA, Lian JX, McGhee SM, Wong D, Li KKw. Diabetic retinopathy screening: Global and local perspective. Hong Kong Med J. 2016;22:486–95. doi: 10.12809/hkmj164844. [DOI] [PubMed] [Google Scholar]
- 20.Raman R, Ramasamy K, Rajalakshmi R, Sivaprasad S, Natarajan S. Diabetic retinopathy screening guidelines in India: All India Ophthalmological Society diabetic retinopathy task force and Vitreoretinal Society of India Consensus Statement. Indian J Ophthalmol. 2021;69:678–88. doi: 10.4103/ijo.IJO_667_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert CE, Babu GR, Gudlavalleti ASV, Anchala R, Shukla R, Pant BH, et al. Eye care infrastructure and human resources for managing diabetic retinopathy in India: The India 11-city 9-state study. Indian J Endocrinol Metab. 2016;20(Suppl 1):S3–10. doi: 10.4103/2230-8210.179768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rani PK, Peguda HK, Chandrasekhar M, Swarna S, Jonnadula GB, James J, et al. Capacity building for diabetic retinopathy screening by optometrists in India: Model description and pilot results. Indian J Ophthalmol. 2021;69:655–9. doi: 10.4103/ijo.IJO_1944_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanlon PH. The English National Screening Program for diabetic retinopathy 2003-2016. Acta Diabetol. 2017;54:515–25. doi: 10.1007/s00592-017-0974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanzetta P, Sarao V, Scanlon PH, Barratt J, Porta M, Bandello F, et al. Fundamental principles of an effective diabetic retinopathy screening program. Acta Diabetol. 2020;57:785–98. doi: 10.1007/s00592-020-01506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivaprasad S, Raman R, Conroy D, Mohan V, Wittenberg R, Rajalakshmi R, et al. The ORNATE India Project: United Kingdom–India Research Collaboration to tackle visual impairment due to diabetic retinopathy. Eye. 2020;34:1279–86. doi: 10.1038/s41433-020-0854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai AK, Usgaonkar UPS, Naik VS, Deshpande M, Shukla R. Comprehensive diabetes care: The Goa model. Indian J Ophthalmol. 2020;68(Suppl 1):S88–91. doi: 10.4103/ijo.IJO_2003_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramakrishnan R, Khadar SMA, Srinivasan K, Kumar H, Vijayakumar V. Diabetes mellitus in Tamil Nadu State –Nonccommunicable diseases nurse model in diabetic retinopathy screening. Indian J Ophthalmol. 2020;68(Suppl 1):S78–82. doi: 10.4103/ijo.IJO_1987_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla AK, Singh S, Sheikh A, Singh S, Gupta G, Daberao R. Diabetic retinopathy screening at primary and community health centers in Maharashtra. Indian J Ophthalmol. 2020;68(Suppl 1):S83–7. doi: 10.4103/ijo.IJO_1915_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy KR, Murthy PR, Murali B, Basavaraju V, Sindhu BS, Churi A, et al. A scalable, self-sustaining model for screening and treatment for diabetic retinopathy in rural Karnataka. Indian J Ophthalmol. 2020;68(Suppl 1):S74–7. doi: 10.4103/ijo.IJO_1943_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malerbi FK, Morales PHA, Regatieri CVS. Diabetic retinopathy screening and the COVID-19 pandemic in Brazil. Arq Bras Oftalmol. 2020;83:5–6. doi: 10.5935/0004-2749.20200070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamali S, Ashrafi E, Mohammadi SF. Personal experience with COVID-19 and community screening of diabetic retinopathy in Iran. J Diabet Sci Tech. 2020;14:737–8. doi: 10.1177/1932296820930045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rathi VM, Reddy RP, Fernandes M, Rath S, Nayak S, Vemuri JPS, et al. The impact of COVID-19 “Unlock-1“on L V Prasad Eye Institute Network in Southern India. Indian J Ophthalmol. 2021;69:695–700. doi: 10.4103/ijo.IJO_3143_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 34.Bawankar P, Shanbhag N, Smitha KS, Dhawan B, Palsule A, Kumar D, et al. Sensitivity and specificity of automated analysis of a single field non-mydriatic fundus photographs by Bosch DR Algorithm –Comparison with mydriatic fundus photography (ETDRS) for screening in undiagnosed diabetic retinopathy. PLoS ONE. 2017;12:e0189854. doi: 10.1371/journal.pone.0189854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avidor D, Loewenstein A, Waisbound M, Nutman A. Cost-effectiveness of diabetic retinopathy screening programs using telemedicine: A systematic review. Cost Eff Resour Alloc. 2020;18:16. doi: 10.1186/s12962-020-00211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]