Abstract
The role of inflammation in diabetic retinopathy (DR) is well-established and dysregulation of a large number of inflammatory mediators is known. These include cytokines, chemokines, growth factors, mediators of proteogenesis, and pro-apoptotic molecules. This para-inflammation as a response is not directed to a particular pathogen or antigen but is rather directed toward the by-products of the diabetic milieu. The inflammatory mediators take part in cascades that result in cellular level responses like neurodegeneration, pericyte loss, leakage, capillary drop out, neovascularization, etc. There are multiple overlaps between the inflammatory pathways occurring within the diabetic retina due to a large number of mediators, their varied sources, and cross-interactions. This makes understanding the role of inflammation in clinical manifestations of DR difficult. Currently, mediator-based therapy for DR is being evaluated for interventions that target a specific step of the inflammatory cascade. We reviewed the role of inflammation in DR and derived a simplified clinicopathological correlation between the sources and stimuli of inflammation, the inflammatory mediators and pathways, and the clinical manifestations of DR. By doing so, we deliberate mediator-specific therapy for DR. The cross-interactions between inflammatory mediators and the molecular cycles influencing the inflammatory cascades are crucial challenges to such an approach. Future research should be directed to assess the feasibility of the pathology-based therapy for DR.
Keywords: Complications of diabetes mellitus, diabetic retinopathy, inflammation, treatment of diabetic retinopathy
Diabetic retinopathy (DR) is often considered a sequela to chronic inflammatory stress that results from persistent and clinically unobtrusive activation of multiple harmful cascades in response to an aberrant metabolic memory.[1] The first indications of the involvement of inflammation in DR were provided more than half a century ago by retrospective studies analyzing the effects of rheumatoid arthritis and its therapy on DR.[2] Following multiple studies with experimental, in vitro, and in vivo designs, and scores of well-directed clinical trials which date back to the last millennium, inflammation is now seen as one of the essential elements driving various pathological pathways responsible for the clinical and pathological manifestations of DR.[3] Yet, there is a lapse in our understanding of the linkage between the inflammatory cascades and clinical DR, mainly due to a large number of the mediators and the pathways they result in. The understanding becomes further challenging for the clinician because of the multiple cross-interactions among these mediators.[4]
DR has multiple clinical manifestations, ranging from a staged non-proliferative DR (NPDR) to stages of proliferative DR (PDR) and diabetic macular edema (DME), and inflammation is known to be involved at all these stages.[5] Intravitreal steroid as an anti-inflammatory agent has been successfully employed for the management of chronic DME while other agents have been evaluated.[6,7] However, these agents, seen as “broad-spectrum,” have not been developed for a pathology-based tailored use in the DR that matches specific clinical indicators, and are rather employed as second-line agents only. This is an important reason for the varied results obtained with such therapies.[8] Further, anti-inflammatory agents find minimal use in NPDR and PDR. This is despite the well-documented role of inflammation in these stages, and to date, we do not have any effective anti-inflammatory agent targeting the retardation of DR.
The literature has sufficient pre-clinical evidence favoring the utility of anti-inflammatory agents that target specific inflammatory cascades.[9] However, currently, most of these are not supported by a piece of sufficient clinical-level evidence, though their future use seems likely. As these therapeutic strategies will focus on specific and individual inflammatory cascades,[9] the clinician needs to understand if individual signs of DR can be associated with a particular inflammatory mediator or cascade. The current review evaluates the evidence linking various manifestations of DR with the corresponding inflammatory cascades. We explore whether a clinicopathological correlation can be developed, with a strength sufficient enough to guide pathology-driven therapy.
Methods
PubMed and Google Scholar databases were searched using the following keywords: “inflammation in diabetic retinopathy,” “inflammatory mediators in diabetic retinopathy,” and “pathogenesis of diabetic retinopathy.” Cross-references were also screened from these articles wherever appropriate. Following the initial screening for duplicates and context, the articles published in the English language with available full text were evaluated for suitability, and 224 papers were included in this review. The articles were reviewed for the individual role of inflammatory mediators in DR, inflammatory mediators in association with pathological changes related to DR, correlation of inflammatory mediators with clinical lesions in DR, and individual treatment approaches if present. The data were organized into four sections that discuss the stimulus/source of inflammation in DR, the mediators of inflammation themselves, their role in pathology, and the linkage between inflammation and clinical DR. The data have been summarized in Table 1 and the pathogenesis has been depicted in Figs. 1-3.
Table 1.
Inflammatory mediators in diabetic retinopathy: Evidence, role, and avenues for intervention
| Pathogen | References | Major model | Targeted intervention | Role in DR | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Upregulated/downregulated | Capillary dropout | Pericyte loss | Leakage | Neovascularization | Neurodegeneration | ||||
| TNF-alpha | [35,64,65,66,67,68,83,84,93,99] | Human serum, vitreous, mice | TNF-alpha Inhibitor (Etanercept, infliximab) | ↑ | + | + | + | + | - |
| IL-1ẞ | [22,23,24,25,26,27,28,29,30,31,32,100,101,102,103,104,105,106,107] | mice | IL-1ẞ Inhibitor - Anakinra, minocycline- caspase 1 inhibitor, canakinumab - anti-IL-1β monoclonal antibody | ↑ | + | + | + | + | + |
| IL-2 | [33,34,35,43] | Human aqueous, ERM, mice | - | ↑ | - | - | - | + | - |
| IL-3 | [44,45,108,109] | human aqueous, plasma | - | Lower levels noted in diabetics | - | - | - | - | - |
| IL-4 | [35,46] | Human vitreous | - | ↑ | - | - | - | + | - |
| IL-5 | [35] | Human aqueous | - | ↑ | - | - | - | + | - |
| IL-6 | [35,47,48,49,50,51,52,53,63,87,110,111,112,113,159,161,162,163,164,165,166,167,168,169]\ | HREC, vitreous | IL-6 Inhibitor (EBI-031 antibody) | ↑ | Endothelial apoptosis | - | + | + | - |
| IL-8 | [54,55,56,57,94,108,167,168,175,176,177,178,180,181] | Human vitreous, HUVEC | - | ↑ | - | - | + | + | - |
| IL-10 | [35,58,59,60,61,62,79,80,167,182] | Human aqueous, vitreous | - | Inconclusive | - | -- | - | - | - |
| IL-13 | [81,82] | Human vitreous | IL-13 act as anti-inflammatory agent | Lower levels noted in diabetics | - | - | - | - | - |
| INF-γ | [35,44,67,82] | Human vitreous | - | ↑ | - | - | - | - | - |
| Complement system | [69,70,71,72,73,183,184] | Human vitreous, serum | - | ↑ | - | - | - | + | - |
| VEGF | [41,67,82,85,86,92,93,94,95,96,158,167,185,186,187,188,189,190] | Mice | Anti-VEGF | ↑ | - | + | + | + | + |
| CD 40 | [128,129] | Human blood | - | ↑ | - | - | - | - | - |
| MCP-1 | [44,84,117,118,119,120,121,122,123,175,191,192,193,194,195,196,197,198] | Human vitreous | - | ↑ | - | - | + | + | - |
| MCP-2 | [44] | Human aqueous humor | - | ↑ | - | - | - | - | - |
| NF-κB | [124,125,126,127] | Mice | - | ↑ | + | + | + | + | + |
| Integrin | [74,75,76,77,129,170,199,200,201] | Mice | - | ↑ | + | - | + | - | - |
| VCAM-1, ICAM-1, selectins | [39,40,41,42,92,141,142,159,202,202,203,204,206,207,208,209,210,211] | Human vitreous, serum mice | SAR 1118 | ↑ | + | + | + | + | - |
| Arachidonic acid metabolites | [27,36,38,64,78,212,213] | Mice | COX inhibitors (rofecoxib, LOX inhibitor | ↑ | + | + | + | - | - |
| NO | [114,130,131,132,133,134,135,136,137] | Human, mice | Aminoguanidine | ↑ | + | - | + | + | + |
| PEDF | [214,215,216,217,218] | Human, mice | ACE inhibitor | ↓ | + | - | + | + | + |
ERM - epiretinal membrane, HREC - human retinal endothelial cells, HUVEC - human umbilical vein endothelial cells, PEDF - pigment epithelial-derived factor
Figure 1.
The figure depicts the stimulus of inflammation in diabetes mellitus, the cells (source) responding in the retina to produce the “retinal inflammation,” and the consequent release of inflammatory mediators. AGE: Advanced glycation end products, PKC: Protein kinase C, PARP: Poly-ADP ribose polymerase, RPE: Retinal pigment epithelium, IL: Interleukin, VEGF: Vascular endothelial growth factor, VEGFR: VEGF receptor, MCP: Monocyte chemotactic protein, MIP: Macrophage inflammatory protein, MAC: Membrane attack complex, NF: Nuclear factor, ICAM: Intercellular adhesion molecule, VCAM: Vascular cell adhesion molecule, TNF: Tumor necrosis factor, PDGF: Platelet-derived growth factor, ROS: Reactive oxygen species, NOS: Nitric oxide synthase
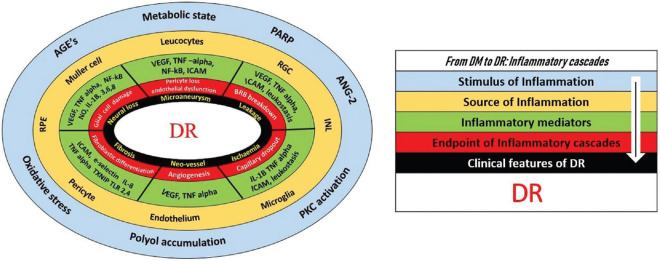
Figure 3.
Doughnut chart representing the inflammatory pathogenesis of DR. The outermost blue circle represents the stimulus of inflammation developing outside the retina as a consequence of the metabolic state. The second circle shows the beginning of the retinal inflammation and the cells being stimulated as the source to produce the cytokines. The circles further in have been categorized to show how the inflammatory mediators are getting involved in the cascades to lead on to specific pathologic and cellular level changes in the retina that subsequently manifests as a clinical finding, completing the circle of diabetes mellitus (DM) to diabetic retinopathy (DR). There is tremendous overlapping at all levels of this process, horizontally as well as vertically. AGE: Advanced glycation end products, PKC: Protein kinase C, PARP: Poly-ADP ribose polymerase, ANG: Angiotensin, INL: Inner nuclear layer, RPE: Retinal pigment epithelium, RGC: Retinal ganglion cell
Stimulus and Source of Inflammation
Chronic persistent hyperglycemia in type 2 diabetes mellitus (DM) leads to a pro-inflammatory diabetic milieu in the retina. This abnormal metabolic state is reflected by altered platelet physiology and function, hypercoagulable state, altered metabolic pathways, free radical accumulation, oxidative stress, and cellular hypoxia.[10,11] Sustained hyperglycemia acts as a key link in the development of the pro-inflammatory state where various pathways including polyol accumulation, advanced glycation end products (AGEs), oxidative stress, and activated protein kinase C (PKC) acts as a ‘stimulus’ for the expression of various mediators of inflammation.[12] These stimuli subsequently affect the specific cells or pathways within the cellular and vascular domains of the retina to initiate a cascade of inflammation. Following the stimulus from these by-products of DM, the inner retina (mainly the inner nuclear layer), retinal ganglion cells, diversified microglial cells along with the components of the inner blood-retinal barrier (iBRB), Müller cells, retinal pigment epithelium, endothelial cells, and pericytes act as the prime source of inflammation.[12]
The retinal ganglion cells (RGCs) and astrocytes located in the inner retina act as the prominent cellular sites for the expression of monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1a (MIP-1a).[13] the accumulation of free radicals and hypoxia in the inner retina augments the expression of the MCP-1 and MIP-1a genes in these cells.[13] Hyperglycemia and associated hypertensive milieu increase the angiotensin II (Ang-II) production, which along with the increased oxidative stress and AGEs stimulates the expression of nuclear factor (NF)-кB.[14] Cells in the inner and outer nuclear layers, ganglion cell layer, and retinal pericytes are activated by these stimuli, leading to the phosphorylation of an inhibitory protein of NF-кB and its rapid degradation followed by the release of NF-кB.[15] NF-кB is present in nearly all cell types and has wide biological and inflammatory activity, including the promotion of gene transcription of several cytokines and chemokines.[16] the increased -glial cell, -caspase 1 and -caspase 3 activity along with the hypoxia stimulates the Müller glial cells, endothelial cells, macrophages, and neutrophils to increase the expression of Interleukin (IL)-1β [Figs. 1 and 2].[17]
Figure 2.
The figure depicts the actions of the individual inflammatory mediators and the resulting cascades causing the retinal pathology in diabetic retinopathy. NF: Nuclear factor, ICAM: Intercellular adhesion molecule, VCAM: Vascular cell adhesion molecule, IL: Interleukin, TNF: Tumor necrosis factor, MAC: Membrane attack complex, VEGF: Vascular endothelial growth factor, MCP: Monocyte chemotactic protein, MIP: Macrophage inflammatory protein PECAM: Platelet endothelial cell adhesion molecule, NOS: Nitric oxide synthase, MAPK: Mitogen-activated protein kinase, ERK: Extracellular signal-regulated protein, ECM: Extracellular matrix, BRB: Blood-retinal-barrier, ANG: Angiotensin
The microglial cells, which traverse a wide portion of retinal thickness, get activated in response to varied diabetic stimuli. Hyperglycemia, AGEs, oxidative stress, and PKC lead to reactive gliosis with the activation of the microglial cells.[18] Once activated, the microglial cells can assume any of the several phenotypic states, some inflammatory, others anti-inflammatory. In the inflammatory context, they accelerate the production of various cytokines and chemokines including tumor necrosis factor-alpha (TNF-α), IL-1, IL-6, IL-8, and C-reactive protein (CRP). Moreover, elevated levels of IL-1, IL-6, interferon-gamma (IFN-γ), and TNF-α further activate more microglial cells and initiate a vicious circle of inflammation.[9] Hyperglycemia and dyslipidemia also lead to the deposition of C5b-9, a terminal product of complement activation within the retinal microcirculation which ultimately results in altered inflammatory signaling pathways, further exaggerating the inflammatory milieu. The Müller cells are the main source of increased vascular endothelial growth factor (VEGF) expression in the diabetic retina along with a significant contribution from the endothelial cells, astrocytes, and retinal pigment epithelial cells (RPEs).[19] Hyperglycemia, increased oxidative stress, hypoxia, and the synergistic proliferating effect of cytokines like IL-6 and IL-1β act as stimuli for the increased expression of the VEGF-A from these sources [Figs. 1 and 2].[19]
Several stimuli like oxidative stress, dyslipidemia, and poly- Adenosine diphosphate (ADP) ribose polymerase (PARP) activation lead to the upregulation of adhesion molecules (vascular cell adhesion molecule [VCAM-1],: Intercellular adhesion molecule [ICAM-1], integrins) from the endothelial cells and on the surface of the leukocytes,[20] whereas the endothelial cells and platelets act as a prime reservoir for the increased production of P-selectins and E-selectins, another class of adhesion molecules.[21] All these factors eventually culminate in leukostasis with a resultant inflammatory insult as detailed later [Figs. 1 and 2].
Inflammatory Mediators
A large number of inflammatory mediators have been identified. These are responsible for multiple pathologies that later manifest clinically too. Therapeutic avenues have been researched with varying levels of evidence to target these. These individual mediators are summarized in Table 1 and Figs. 1 and 2.
2a Interleukins
Interleukin 1β (IL-1β)
The role of IL-1β in DR is well identified.[22] It is produced as an inactive precursor pro-IL-1β which is cleaved by protease caspase 1 to its active form.[22,26,27,28] It also activates NF-κB which further increases the expression of other pro-inflammatory cytokines.[22] A high caspase 1 activity in the retina of diabetic animals, as well as humans, shows its role in the amplification of IL-1β activity.[23,24] Minocycline, an inhibitor of caspase 1 is known to blunt the diabetes-led increase of IL-1β in the retina.[25] the IL-1R antagonist Anakinra has also been shown to reduce the endothelial dysfunction in diabetic mice. Similarly, systemic IL-1β inhibition with canakinumab in diabetic patients resulted in decreased vessel leakage and resolution of macular edema.[29,30,31,32]
Interleukin 2 (IL-2)
The IL-2 activates T lymphocytes, NK cells, B lymphocytes, and monocytes.[33] Its role in DR has been correlated to its elevated levels. The IL-2 along with other inflammatory mediators leads to endothelial apoptosis and retinal leukostasis resulting in ischemia, thereby, culminating in vascular leakage and neovascularization [Fig. 2]. Johnsen-Soriano et al.[34] showed increased levels of IL-2 in diabetic mice as compared to controls suggesting a possible role in DR. the aqueous analysis of diabetic patients found IL-2 to be higher in the PDR group than in NPDR and controls.[35] Similarly, its levels were noted to be raised in a study of epiretinal membranes obtained from the PDR patients.[43]
Interleukins-3, 4, and 5 (IL-3, IL-4, IL-5)
The role of IL-3 in DR is inconclusive. Its levels were increased in the aqueous humor of diabetics without DR and lower in patients with DR.[44] Similarly, in a study by Hang et al.[45] in the plasma of diabetics, the IL-3 concentration was lower in the diabetics than in controls. The aqueous levels of IL-5 and vitreous levels of IL-4 were seen to be comparatively higher in PDR, as compared to NPDR and non-DR patients, signifying a plausible association with PDR.[35,46]
Interleukin 6
IL-6 is produced acutely by macrophages and microglia on coming in contact with pathogen-associated molecular patterns (PAMP) and danger-associated molecular patterns.[47,48,49] PAMPs are microbial patterns that are recognized directly by the host immune system through cell receptors. The major PAMPs include nucleic acids, surface glycoproteins (GP), lipoproteins (LP), and membrane components. IL-6 is an important mediator in the pathogenesis of DR causing vascular inflammation and endothelial dysfunction.[50] It can directly affect the endothelial cells and disrupt the blood-retinal barrier (BRB) or even induce VEGF production.[50,51,52,53]
Interleukin 8 (IL-8)
IL-8 is a pro-inflammatory cytokine and serves as a chemoattractant for neutrophils and also activates the T cells.[54] It causes endothelial cell death and leukostasis along with other interleukins and TNF-alpha, resulting in ischemia, promoting leakage, and neovascularization [Fig. 2]. The high levels of IL-8 have been found in the vitreous of PDR patients and its levels correlated with PDR activity.[55] the aqueous levels of IL-8 were also significantly elevated in patients with DME refractory to anti-VEGF treatment suggesting its inflammatory role in leakage.[56] The effect of IL-8 on the endothelium was studied experimentally on the human vascular endothelial cell lines which showed that the IL-8 downregulated the tight junctions of the endothelium in a dose and time-dependent manner, thereby, altering its permeability.[57] It, therefore, is a promising target for managing refractory DME.
Interleukin 10 (IL-10)
It is an anti-inflammatory cytokine that decreases the synthesis of IL-1 and TNF-α.[58] It is also postulated to downregulate the VEGF expression, thereby, reducing angiogenesis.[59] Its concentration was low in the aqueous of patients with DR as compared to the controls with a significant negative correlation. Hence, the study concluded that low levels of IL-10 are pathogenetic in DR,[60] which has been noted by other studies too.[55,61,62] However, a significantly higher concentration of IL-10 was noted in the vitreous of PDR patients by Mao et al.[79] and a similar result was noted by Paine et al.[80] too. Thus at the moment, its utility in clinical manifestations and therapeutics of DR is debatable.
Interleukin 13 (IL-13)
IL-13 is produced by the T cells and dendritic cells. In a study by Sai et al., the lower levels of IL-13 were observed in the vitreous of DR patients as compared to other inflammatory mediators, whereas other studies have noted higher levels in the vitreous of patients with PDR suggesting its role in the formation of fibrous membranes.[81,82] Therefore, its role in the management of DR is currently debatable just like IL-10.
2b TNF-α
TNF-α is a pro-inflammatory cytokine produced by microglia, Müller cells, macrophages, neutrophils, and T cells in response to various stimuli.[83] TNF-α is involved in various biological processes including the upregulation of adhesion molecules, proliferation, differentiation, and cell death, and is involved in the pathogenesis of DR and intraocular inflammation.[84] It can alter the distribution of tight junction proteins and increase the BRB permeability and leukocyte adhesion to the retinal endothelial cells.[64,65,66,84] A significantly higher level of TNF-α was found in the serum of the PDR patients suggesting its role in PDR.[67] In another study involving type 1 and type 2 DM mice, increased microvascular apoptotic cell and acellular capillaries were noted. On administration of Pegsunercept (a recombinant soluble TNF-receptor-1 which has been modified by the attachment of polyethylene glycol), significantly reduced the pericyte loss and the acellular capillaries were noted.[66] the administration of infliximab in the laser-refractory-DME patients led to a significant improvement, indicating the pathogenic role of TNF- α.[68] Therefore, therapy targeting the TNF-α cascade is a relevant option in at least the advanced cases of DR.
2c Interferon γ (INF-γ)
INF-γ is a cytokine released by helper T-cell class 1 (Th1) activating pro-inflammatory M1 macrophages or microglia and B cells. In independent studies, INF-γ was significantly elevated in the vitreous of DR patients as compared to controls and also in diabetic mice, suggesting the upregulation of Th1 cytokine response in DR.[34,44,82] Its application in the therapy for DR is yet to be proven.
2d Complement system
The initial studies showed complement deposition in the retinal choriocapillaris with decreased levels of complement inhibitors suggesting complement-induced diabetic damage.[69,70] Shahulhameed et al.[71] showed significantly elevated levels of C3ba in the vitreous of PDR patients and the involvement of alternate pathways was also deduced from the upregulation of complement factor H (CFH). Gao et al.[72] reported a similar observation in the vitreous of PDR patients where complement C3 and complement factor I were significantly elevated as compared to the controls. The role of the complement system in neovascularization in DR has been affirmed by other studies as well.[70,73]
2e Integrin
Integrins are cell adhesion receptors mediating cell-cell and cell-extracellular matrix interactions. They have also been associated with various pathological processes, such as vascular leakage, inflammation, neovascularization, and fibrosis.[74] The role of integrin in DME can be well-established from the study by Quiroz-Mercado et al.[75] in which non-inferiority of integrin inhibitor “ALG-1001” over bevacizumab was met with favorable results. In another experimental model, THR-687 a novel integrin receptor antagonist prevented neovascularization.[76] Its role in leukostasis and vascular leakage was confirmed in experimental mice where inhibition of alpha 4 integrin/CD49d significantly attenuated diabetes-induced leukocyte adhesion and vascular leakage.[77]
2f Arachidonic acid metabolites
Arachidonic acid is released from the phospholipids in the cell membranes in response to several stimuli where the cyclooxygenases (COX) and lipoxygenases convert arachidonic acid to leukotrienes and prostaglandins. Cyclooxygenase 2 activity (COX-2) has been noted in retinal endothelial cells of diabetic patients.[78] On administration of COX-2 inhibitor, the diabetes-led upregulation of retinal VEGF, retinal vessel permeability, and leukostasis was found to be inhibited suggesting that COX-2 induces pathological alteration in DR.[36] In a study by Joussen et al.,[64] meloxicam (COX-2 inhibitor) reduced endothelial nitric oxide synthase (eNOS) levels, inhibited NF-κB activation in the diabetic retina, and partially reduced the TNFα levels. Similarly, nepafenac (COX inhibitor) inhibited leukocyte adhesion as well as apoptosis of the retinal capillary cells, and degeneration of retinal pericytes and capillaries.[27]
Arachidonic acid itself may act as a second messenger activator of protein kinase C—an important and established component in the pathogenesis of diabetic retinopathy (DR). Protein kinase C inhibitors have been used in DR. Protein kinase C b Inhibitor-Diabetic Retinopathy Study 2 (PKC-DRS 2), a major clinical trial showed that oral ruboxistaurin led to significant visual improvement in the NPDR eyes more likely due to the reduction in macular edema.[37] the evidence also suggests that leukotrienes and other products of 5-lipoxygenase are involved in DR changes too. Lipoxygenase-derived 5-hydroxyeicosatetraenoic acid (5-HETE) was noted to be elevated in the vitreous of diabetic patients. In 5-lipoxygenase-deficient diabetic mice, degeneration of retinal capillaries, leukostasis, and superoxide generation was found to be inhibited indicating its potential role in these alterations.[38]
2g Cellular adhesion molecules
Adhesion molecules are required for leukocyte migration and adhesion to endothelial cells, leading to leukostasis and endothelial dysfunction. In a study by Joussen et al.,[39] the diabetic mice genetically deficient in ICAM-1 or its ligand (CD18) were protected from the development of lesions of early DR (including capillary degeneration, pericyte loss, and increased permeability) as well as leukostasis. Limb et al.[40] noted significantly elevated levels of soluble vascular cell adhesion molecules (sICAM-1, sVCAM-1, and sE-selectin) in the vitreous of PDR cases. Funatsu et al.[41] reported higher vitreous levels of sICAM-1 in patients with hyperfluorescent DME than in those with minimally fluorescent DME as well as its levels correlated with macular edema. Rao et al.[42] demonstrated that the topical administration of small molecule antagonists of the leukocyte function-associated antigen-1 (LFA-1) significantly reduced retinal leukostasis and blood-retinal-barrier breakdown in rats. Such topical mediators, thus, are very promising as they have a potential for use in both early and late DR, but their utility is yet to be proven.
2h Vascular endothelial growth factor
VEGF is a well-established mediator of DR induced by the ischemic-hypoxic drive of the retina, and its role in leakage and neovascularization is well-known. Various drugs like bevacizumab, ranibizumab, aflibercept, and brolucizumab have been extensively evaluated and found beneficial for advanced DR. However, it has been found that it is a pro-inflammatory molecule too and some patients on anti-VEGF therapy have shown regression of DR too. Its role in leukostasis and BRB breakdown can be judged from the fact that the blockade of endogenous VEGF resulted in significant suppression of these processes in DR.[85] A study by Wang et al.[86] in diabetic mice showed that the inhibition of Müller cell VEGF significantly decreased the expression of TNFα, ICAM-1, and NF-κB. the retinal leukostasis implicated in the DR pathogenesis too is mediated by VEGF. The administration of phosphomannopentaose sulfate (PI-88) (a sulfonated oligosaccharide which inhibits heparanase) led to the inhibition of leukostasis and preservation of the Electroretinogram (ERG) changes in diabetic rats suggesting that it may reverse retinal dysfunction by decreasing the VEGF expression.[115] It has also been reported that pro-inflammatory cytokines such as IL-1β and IL-6 could upregulate the VEGF mRNA expression.[116]
2i Monocyte chemoattractant protein-1 and 2 (MCP-1, MCP-2)
MCP-1 is a chemokine produced by multiple cell lines of the immune system as well as endothelial cells leading to the recruitment and activation of the macrophages and monocytes. It also stimulates VEGF production promoting angiogenesis and fibrosis.[117,118] It is also reported that it significantly alters retinal vascular permeability, angiogenesis, as well as contribution to the DR pathogenesis.[119,120] the higher levels of MCP-1 were noted in the vitreous of the PDR patients and retina of diabetic mice.[121,122,123] MCP-2 was noted to be significantly elevated in the aqueous humor of diabetics without DR as compared to non-diabetics. However, its role in DR and its therapy needs to be further evaluated.[44]
2j NF-κB
It is a transcription factor regulating genes involved in the immune and inflammatory processes as well as cellular proliferation and apoptosis. An overactivated NF-kβ leads to altered gene expression of VEGF, PDGF, and endothelin-1, and also the release of various cytokines including TNF-α, IL-1β, and IL-6. These processes ultimately lead to endothelial apoptosis and angiogenesis.[124,125] The inhibition of NF-κB activation by dehydroxymethylepoxyquinomicin reduced diabetes-induced retinal leukostasis and expression of ICAM-1 and VEGF experimentally.[126] Selective inhibition of NF-κB also resulted in reduced degeneration of the retinal capillaries and expression of inflammatory proteins.[127] Because of its potential cross-interacting broad-spectrum activity, NF-κB is a promising target whose antagonistic therapy will likely curtail DR. Further evidence is, however, required.
2k CD40 Ligand (CD40L)
The CD40L system is known to be present on the platelets. The activated platelets initiate inflammatory reaction of the endothelial cells with CD40L which is shed as soluble CD40L (sCD40L) and can serve as a marker of inflammation.[128] Yngen et al.[129] studied the correlation of sCD40L in type 1 diabetics with and without microangiopathy and found its levels to be significantly elevated in the blood of patients with microangiopathy suggesting it may be a part of the inflammatory cascade in diabetic microangiopathy.
2l Nitric oxide (NO)
It is produced by three nitric oxide synthase (NOS) isoforms that are expressed variably in the retina—endothelial (eNOS), neuronal (nNOS), and inducible (iNOS).[130,131] NO is a regulator of important cellular functions including vascular dilation and inflammation. eNOS and nNOS are expressed constitutively and regulate neuronal and endothelial functions.[114] iNOS is associated with increased severity and accelerated development of DR and is upregulated in DR.[132,133] It has been demonstrated in experimental mice that the deletion of iNOS led to a reduction in the DR changes of capillary degeneration and permeability.[134,135] Aminoguanidine, an inhibitor of NO, has been found to inhibit microvascular complications of diabetes in the retina in experimental models.[132] Several other studies in humans have indicated the role of NO in neurodegeneration and neovascularization.[136,137]
Inflammatory Pathways, Cellular Responses, and Clinicopathological Correlation
Clinically, DR presents with different findings including microaneurysm, edema, ischemia, new vessels, and fibrosis. These findings are used to grade the severity of the disease and assign a stage that determines the entailing management. Neuro-retinopathy has also been realized to be a part of DR now, though not included in the conventional classification systems. The pathologies discussed in Table 1 are responsible for these clinical processes. This section correlates these pathologies to the common clinical findings seen in DR.
Microaneurysm – the loss of structural support to retinal capillaries by pericyte loss and endothelial cell damage leads to microaneurysm formation which on rupture leads to retinal hemorrhage. Eventually, these outpouchings lead on to leakage resulting in edema. Inflammation has been shown to cause pericyte loss as mentioned in Table 1. As explained in Fig. 2, a large number of inflammatory molecules are involved in the pericyte loss and endothelial dysfunction which culminates in aneurysms. A significantly high level of gene expressions and protein concentrations of IL-1β, NF-κB, VEGF, TNFα, TGF-beta, and ICAM-1 were found in retinal pericytes in an experimental model where the cells were exposed to high glucose concentration suggesting changes secondary to inflammation. These changes persisted after the glucose levels became normal, thus, indicating irreversible nature.[138,139] Other inflammatory mediators causing pericytes apoptosis include TNF-alpha and reactive oxygen species.[87]
BRB breakdown and edema – BRB breakdown alters the permeability of retinal capillaries causing leakage eventually culminating in edema and exudation. The role of inflammation in BRB breakdown has been shown to occur by increased leukostasis, cytokines, and growth factors as well as pericyte loss.[88,89,140,170] TNF-alpha and VEGF have also been shown to increase the permeability of BRB. Miyamoto et al. showed that retinal vascular leakage and capillary non-perfusion corresponded to retina leukostasis and the diabetes-led increase in the leukostasis correlated with the elevated levels of ICAM-1. These processes were significantly inhibited by the injections of intravitreal antibody acting against ICAM-1 indicating the role of leukostasis in the BRB breakdown.[69,141,142] Therefore, ICAM-1 antibody can serve as a potential therapeutic line against DR.
Ischemia – Inflammation also leads to capillary occlusion and capillary dropout. Inflammatory cytokines including TNFα and IL-1β have been reported to increase caspase 3 activity leading to endothelial cell apoptosis.[22,84] In addition, the capillary occlusion by leukostasis due to the blockage of blood flow can also lead to ischemia.[84] As depicted in Fig. 2, leukostasis itself is a resultant of multiple pathways, especially those involving integrins and cell adhesion molecules. Endothelial cell death is also induced during leukostasis by leucocyte-mediated Fas ligand (FasL) and Fas-mediated apoptosis.[148] The role of leukostasis in DR can be adjudged from the fact that a significant reduction in diabetes-induced capillary degeneration was noted when the proteins required for the adherence of white blood cells to the endothelium (ICAM-1 and CD-18) were deleted in experimental diabetes.[39]
-
Neovascularization – Inflammation has been shown to cause neovascularization. Inhibition of inflammatory cytokines including TNFα attenuated neovascularization.[144,145,146,147] Macrophages and monocytes were also found in the neovascular sprout by Ishida et al.[148] and selective depletion of monocyte lineage with intravitreal clodronate liposomes (dichloromethylene diphosphonate), which act as anti-macrophage agents, led to the suppression of pathological neovascularization. The role of unregulated VEGF in causing the development of new vessels is very well-known.[149]
Neurodegeneration – Neurodegeneration occurs in DR preceding clinical findings in many cases, partly a resultant of neuroinflammation. Microglial activation resulting from a persistent hyperglycemic state has been postulated to be the main factor behind neuroinflammation.[150] An increased microglial count and size have been noted in association with cotton wool spots and microaneurysms.[18] Activated microglia produce pro-inflammatory mediators such as IL-1β, IL-3, IL-6, TNF-α, VEGF, lymphotoxin, MIP-1a, MMPs, NO, ROS, COX-2, and complement factors which promote neuronal cell death.[18,151,152] Further activated Müller cells and astrocytes produce IL-1β and TNF-α, which then induce cytokine IL-8 contributing to neuroinflammation.[153,154] the presence of activated NF-κB had been noted in microglia in a hypoxic neovascular mouse model.[18,127] Topical gents like pigment epithelium-derived growth factor have been used in the mice to prevent microglial activation, and this pathway has also been exploited in phase 3 human trials using neuroprotective agents.[18] However, no therapy targeting specifically neuroinflammation in DR has been evaluated yet.
Fibrosis and traction – Though the role of inflammation in diabetic retinal detachment/tractional changes and fibrosis has not been much explored, Limb et al.[40] evaluated the vitreous of PDR patients. The authors reported significantly elevated levels of sICAM-1 and sE-selectin in the eyes with Tractional retinal detachment (TRD) as compared to the eyes with vitreous haemorrhage (VH) alone. IL-8 and TNF-alpha have also been reported to contribute to fibrosis after initiating ocular angiogenesis.[155] Another study showed that the upregulation of thioredoxin-interacting protein (TXNIP), a pro-inflammatory and proapoptotic protein, played a significant role in promoting retinal fibrosis and blockade of TXNIP-ameliorated retinal fibrosis in diabetic rats.[156] Additionally, toll-like receptors 2 and 4, were also shown to play a significant role in subretinal fibrosis formation.[157] All these are possible future avenues.
Linking Inflammatory Pathways to Clinical Findings and Approach to Therapy
Fig. 3 presents the link between DM, stimulus source of inflammation, the resulting inflammatory mediators, inflammatory cascade-led cellular processes, and the clinical finding manifesting as DR. It is easily seen that there are major overlaps in the proximal cascade, especially at the level of stimulus and source of inflammation. For example, AGEs contribute to the production of NF-κB and also stimulate the glial cells. The former, as discussed before, is a general transcription controlling factor that relates to the production of a host of mediators, is present in almost all the retinal layers, and contributes to the regulation of definitive molecules like VEGF and ICAM. Similarly, as mentioned before, stimulated glial cells can lead to the production of tumor necrosis factor-alpha (TNF alpha), IL-1, IL-6, IL-8, and CRP, apart from inciting neurodegeneration. This would theoretically mean that AGEs alone are sufficient to be considered as responsible for all manifestations of DR. If we consider the source of inflammation, Müller cells are involved in the production of VEGF, IL-1β, and NF-κB, and thus, are involved in almost all the major pathways of DR [Fig. 1]. The overlap is lesser as we come closer to DR centrally in the passage of linkage, but major mediators like VEGF, IL-1B, and TNF-alpha are involved in multiple inflammatory pathways. Further, molecules like VEGF, NO, and ICAM can beget inflammation themselves leading to the production of other mediators. Moving further centrally, the overlaps are too well-known clinically where microaneurysm can lead to leakage, ischemia can lead to new vessels, and new vessels can lead to fibrosis. Thus, there is tremendous overlap and interaction at all steps in the inflammatory cascade, initiating from DM and reaching right up to the clinical manifestations of DR, though the cross-interactions are lesser centrally. Utilizing anti-inflammatory agents in the context of DR should be seen in three contrasting approaches: narrow, tailored, or broad-spectrum.
Narrow-spectrum approach: While attempting to individualize anti-inflammatory therapy with reference to Fig. 3, the inside-out approach (a clinical sign of DR to an inflammatory agent) is easier to adopt than vice versa. This has multiple reasons. First, each individual with DM and other related comorbidities can have different clinical manifestations, which will also vary depending on the stage of DR in terms of predominance. Second, at any given point in time, one pathology of DR may be more responsible for the disease or symptoms while multiple pathologies may have manifested in the retina. Third, clinical examination is an easier done evaluation in comparison to the assessment of ocular inflammatory markers while managing patients. For example, If the clinician is able to identify microaneurysms as the predominant pathology [black circle Fig. 3], it should mean that pericyte loss or endothelial dysfunction is the predominant pathogenesis [red circle Fig. 3]. Such a patient may benefit from anti-inflammatory therapy based on the VEGF, TNF, ICAM, or NF-κB pathways. Beyond this, at the levels of sources [yellow circle Fig. 3] and stimuli of inflammation [blue circle Fig. 3], there are lots of cross-interactions that do not allow an individualized approach. At these levels, any agent is likely to have a “broad-spectrum” action.
Broad-spectrum approach: the anti-inflammatory agents which target “broad-spectrum” targets like IL-1β, VEGF, TNF-α, and NO [Table 1] are likely to act in most stages and manifestations of DR, as compared to a narrow-spectrum target like IL-2 or MCP-1, though the efficiency would be variable.[171] The linkage in Fig. 3 explains why therapies that target a “broad-spectrum” mediator like VEGF work very well and have become so well-adopted into clinical practice, as also the success of the agents that have “broad-spectrum” action like the steroids.[90] This approach has easier application, but its long-term efficiency is questionable.
Tailored approach: At present, the narrow-spectrum approach for individualizing anti-inflammatory therapy to a particular manifestation of DR is challenging, and more research is needed to define the utility of a particular agent for a particular manifestation of DR. The broad-spectrum approach though may work in most cases, it may not be the most efficient in the long term. The tailored approach is another possibility but may require the assessment of ocular inflammatory markers. This approach may be feasible at least in some cases like refractory DME where, for example, the IL-8 pathway may be targeted.[91] Further, deducing the actual rise of a mediator in a particular pathology in restricted cases, as seen in the case of the correlating rise of sICAM-1 and angiographic leakage in the retina, will give vital insights into the “primary” inciting role of these mediators at that stage of the pathology.[92,142] Such approaches are likely to be more efficient in the long term.
Conclusion
Diabetes induces a chronic inflammatory state in the retina that originates due to altered systemic metabolism. Many cells of the retinal neurovascular unit contribute to its occurrence, and it is not limited to any particular retinal layer. As a consequence, a large number of heterogeneous inflammatory mediators are generated which not only participate in multiple inflammatory cascades but also influence each other's production and action. The result is a persistently active intertwined inflammation with entailing cellular responses leading to DR.
The current success of some anti-inflammatory agents in DR can be attributed in part to their “broad-spectrum” nature. Trials conducted in the last century had contrasting results. The Dipyridamole Aspirin Microangiography of Diabetes Study Group (DAMAD) showed a small reduction in the number of microaneurysms (MAs) in an aspirin = treated group whereas the Early Treatment of Diabetic Retinopathy Research Group (ETDRS) found no significant difference in the impact of aspirin on DR.[172,173] the current research has shown promising pre-clinical results with many anti-inflammatory agents in their domains, and in the future, we are likely to see a higher level of evidence as clinical trials unfold. While steroids as broad-spectrum agents have been tested extensively for DR and DME, risuteganib is a prime example of a possible targeted therapy acing at the level of integrins and is entering phase 3 trials. Which patients will benefit from such therapies is, however, yet not known.[174]
We reviewed the role of inflammation in DR in detail and attempted to develop a clinicopathological correlation between the inflammatory mediators and DR. There is a theoretical possibility of a tailored anti-inflammatory approach apart from other approaches discussed in the review. However, we found that the current literature does not present sufficient evidence to build specific linkages between the inflammatory mediators and clinic-pathological findings of DR. This will make the “future choice” of such agents difficult and arbitrary for the clinician, as well as render these therapies inefficient. Many questions need to be answered before the potential of such individualized anti-inflammatory therapies is realized, and that they may be offered in a tailored way to the patients and not just as second-line agents in refractory cases. The search should begin in realizing their actual role as well as the extent of their role in the pathogenesis of DR. Mere extraneous presence of a mediator is not enough to suggest its “therapy-defining role,” rather elucidating the correlation between the mediator activity and clinicopathologic finding should set its “therapy-defining role.” This should be the prime focus of future clinical research evaluating the role of inflammation in DR.
Financial support and sponsorship
Dr BT: Hyderabad eye Research Foundation, DBT Wellcome Trust India Alliance Clinical Research Centre Grant awarded to IHOPE [grant number IA/CRC/19/1/610010]
Dr SB: Hyderabad eye Research Foundation, DBT Wellcome Trust Intermediate Fellowship in Clinical and Public Health Research
Conflicts of interest
There are no conflicts of interest.
References
- 1.Forrester JV, Kuffova L, Delibegovic M. The role of inflammation in diabetic retinopathy. Front Immunol. 2020;11:583687. doi: 10.3389/fimmu.2020.583687. doi:10.3389/fimmu.2020.583687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell ED, Field RA. Diabetic retinopathy and rheumatoid arthritis. Lancet. 1964;2:17–8. doi: 10.1016/s0140-6736(64)90008-x. [DOI] [PubMed] [Google Scholar]
- 3.Noda K, Ishida S. Role of chronic inflammation in diabetic retinopathy. Inflamm Regen. 2013;33:230–7. [Google Scholar]
- 4.Antonetti DA, Silva PS, Stitt AW. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 2021;17:195–206. doi: 10.1038/s41574-020-00451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semeraro F, Morescalchi F, Cancarini A, Russo A, Rezzola S, Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. doi: 10.1016/j.diabet.2019.04.002. doi:10.1016/j.diabet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62:231–6. doi: 10.1159/000499540. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead M, Wickremasinghe S, Osborne A, Van Wijngaarden P, Martin KR. Diabetic retinopathy: A complex pathophysiology requiring novel therapeutic strategies. Expert Opin Biol Ther. 2018;18:1257–70. doi: 10.1080/14712598.2018.1545836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Liu H, Rojas M, Caldwell RW, Caldwell RB. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy. 2011;3:609–28. doi: 10.2217/imt.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaštelan S, Tomić M, Gverović Antunica A, Salopek Rabatić J, Ljubić S. Inflammation and pharmacological treatment in diabetic retinopathy. Mediators Inflamm. 2013;2013:1–8. doi: 10.1155/2013/213130. doi:10.1155/2013/213130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 11.Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 2018;108:656–62. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2:96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida S, Yoshida A, Ishibashi T, Elner SG, Elner VM. Role of MCP-1 and MIP-1alpha in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. J Leukoc Biol. 2003;73:137–44. doi: 10.1189/jlb.0302117. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros MC, Frasnelli SC, Bastos Ade S, Orrico SR, Rossa C., Jr Modulation of cell proliferation, survival and gene expression by RAGE and TLR signaling in cells of the innate and adaptive immune response: Role of p38 MAPK and NF-KB. J Appl Oral Sci. 2014;22:185–93. doi: 10.1590/1678-775720130593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang N, Chen XL, Yang HW, Ma YR. Effects of nuclear factor κB expression on retinal neovascularization and apoptosis in a diabetic retinopathy rat model. Int J Ophthalmol. 2015;8:448–52. doi: 10.3980/j.issn.2222-3959.2015.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 17.Hangai M, Yoshimura N, Yoshida M, Yabuuchi K, Honda Y. Interleukin-1 gene expression in transient retinal ischemia in the rat. Invest Ophthalmol Vis Sci. 1995;36:571–8. [PubMed] [Google Scholar]
- 18.Grigsby JG, Cardona SM, Pouw CE, Muniz A, Mendiola AS, Tsin AT, et al. The role of microglia in diabetic retinopathy. J Ophthalmol 2014. 2014 doi: 10.1155/2014/705783. 705783. doi:10.1155/2014/705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19:942. doi: 10.3390/ijms19040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda K, Nakao S, Ishida S, Ishibashi T. Leukocyte adhesion molecules in diabetic retinopathy. J Ophthalmol 2012. 2012 doi: 10.1155/2012/279037. 279037. doi:10.1155/2012/279037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEver RP. Selectins: Lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–6. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 22.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88:1343–7. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Church LD, Cook GP, McDermott MF. Primer: Inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22:189–95. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–30. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 26.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007. 2007 doi: 10.1155/2007/95103. 95103. doi:10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claudio L, Martiney JA, Brosnan CF. Ultrastructural studies of the blood-retina barrier after exposure to interleukin-1 beta or tumor necrosis factor-alpha. Lab Investig. 1994;70:850–61. [PubMed] [Google Scholar]
- 29.Olson JL, Courtney RJ, Rouhani B, Mandava N, Dinarello CA. Intravitreal anakinra inhibits choroidal neovascular membrane growth in a rat model. Ocul Immunol Inflamm. 2009;17:195–200. doi: 10.1080/09273940802710705. [DOI] [PubMed] [Google Scholar]
- 30.Lavalette S, Raoul W, Houssier M, Camelo S, Levy O, Calippe B, et al. Interleukin-1β inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am J Pathol. 2011;178:2416–23. doi: 10.1016/j.ajpath.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallejo S, Palacios E, Romacho T, Villalobos L, Peiro C, SanchezFerrer CF. The interleukin-1 receptor antagonist Anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2014;13:158. doi: 10.1186/s12933-014-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahel M, Becker M, Graf N, Michels S. Systemic interleukin 1 beta inhibition in proliferative diabetic retinopathy: A prospective open-label study using canakinumab. Retina. 2016;36:385–91. doi: 10.1097/IAE.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olejniczak K, Kasprzak A. Biological properties of interleukin 2 and its role in pathogenesis of selected diseases—a review. Med Sci Monit. 2008;14:RA179–89. [PubMed] [Google Scholar]
- 34.Johnsen-Soriano S, Sancho-Tello M, Arnal E, Navea A, Cervera E, Bosch-Morell F, et al. IL-2 and IFN-gamma in the retina of diabetic rats. Graefe's Arch Clin Exp Ophthalmol. 2010;248:985–90. doi: 10.1007/s00417-009-1289-x. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Hwang DK, Song X, Tao Y. Association between aqueous cytokines and diabetic retinopathy stage. J Ophthalmol 2017. 2017 doi: 10.1155/2017/9402198. 9402198. doi:10.1155/2017/9402198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur J Pharmacol. 2003;458:283–9. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]
- 37.Aiello LP, Vignati L, Sheetz MJ, Zhi X, Girach A, Davis MD, et al. Oral protein kinase c β inhibition using ruboxistaurin: Efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the Protein Kinase C b Inhibitor-Diabetic Retinopathy Study and the Protein Kinase C b Inhibitor-Diabetic Retinopathy Study 2. Retina. 2011;31:2084–94. doi: 10.1097/IAE.0b013e3182111669. [DOI] [PubMed] [Google Scholar]
- 38.Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15- lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008;57:1387–93. doi: 10.2337/db07-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb J. 2004;18:1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 40.Limb GA, Hickman-Casey J, Hollifeld RD, Chignell AH. Vascular adhesion molecules in vitreous from eyes with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999;40:2453–7. [PubMed] [Google Scholar]
- 41.Funatsu H, Yamashita H, Sakata K, Noma H, Mimura T, Suzuki M, et al. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005;112:806–16. doi: 10.1016/j.ophtha.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 42.Rao VR, Prescott E, Shelke NB, Trivedi R, Thomas P, Struble C, et al. Delivery of SAR 1118 to retina via ophthalmic drops and its effectiveness in reduction of retinal leukostasis and vascular leakiness in rat streptozotocin (STZ) model of diabetic retinopathy (DR) Invest Ophthalmol Vis Sci. 2010;51:5198–204. doi: 10.1167/iovs.09-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang S, Le-Ruppert KC. Activated T lymphocytes in epiretinal membranes from eyes of patients with proliferative diabetic retinopathy. Graefe's Arch Clin Exp Ophthalmol. 1995;233:21–25. doi: 10.1007/BF00177781. [DOI] [PubMed] [Google Scholar]
- 44.Vujosevic S, Micera A, Bini S, Berton M, Esposito G, Midena E. Proteome analysis of retinal glia cells-related inflammatory cytokines in the aqueous humor of diabetic patients. Acta Ophthalmol. 2016;94:56–64. doi: 10.1111/aos.12812. [DOI] [PubMed] [Google Scholar]
- 45.Hang H, Yuan S, Yang Q, Yuan D, Liu Q. Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol Vis. 2014;20:1137–45. [PMC free article] [PubMed] [Google Scholar]
- 46.Takeuchi M, Sato T, Tanaka A, Muraoka T, Taguchi M, Sakurai Y, et al. Elevated levels of cytokines associated with TH2 and TH17 cells in vitreous fluid of proliferative diabetic retinopathy patients. PLoS One. 2015;10:e0137358. doi: 10.1371/journal.pone.0137358. doi:10.1371/journal.pone. 0137358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida S, Sotozono C, Ikeda T, Kinoshita S. Interleukin-6 (IL-6) production by cytokine stimulated human Müller cells. Curr Eye Res. 2001;22:341–7. doi: 10.1076/ceyr.22.5.341.5498. [DOI] [PubMed] [Google Scholar]
- 49.Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: Inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19010110. doi:10.3390/ijms19010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valle ML, Dworshak J, Sharma A, Ibrahim AS, Al-Shabrawey M, Sharma S. Inhibition of interleukin-6 trans-signaling prevents inflammation and endothelial barrier disruption in retinal endothelial cells. Exp Eye Res. 2019;178:27–36. doi: 10.1016/j.exer.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye EA, Steinle JJ. miR-146a suppresses STAT3/VEGF pathways and reduces apoptosis through IL-6 signaling in primary human retinal microvascular endothelial cells in high glucose conditions. Vis Res. 2017;139:15–22. doi: 10.1016/j.visres.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–41. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 53.Funatsu H, Noma H, Mimura T, Eguchi S. Vitreous inflammatory factors and macular oedema. Br J Ophthalmol. 2012;96:302–4. doi: 10.1136/bjo.2010.181222. [DOI] [PubMed] [Google Scholar]
- 54.Petering H, Götze O, Kimmig D, Smolarski R, Kapp A, Elsner J. The biologic role of interleukin-8: Functional analysis and expression of CXCR1 and CXCR2 on human eosinophils. Blood. 1999;93:694–702. [PubMed] [Google Scholar]
- 55.Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22:719–22. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 56.Kwon JW, Jee D. Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PLoS One. 2018;13:e0203408. doi: 10.1371/journal.pone.0203408. doi:10.1371/journal.pone.0203408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu H, Huang X, Ma Y, Gao M, Wang O, Gao T, et al. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int J Biol Sci. 2013;9:966–79. doi: 10.7150/ijbs.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubio-Perez JM, Morillas-Ruiz JM. A review: Inflammatory process in Alzheimer's disease, role of cytokines. ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/756357. 756357. doi:10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silvestre JS, Mallat Z, Duriez M, Tamarat R, Bureau MF, Scherman D, et al. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Levy BI Circ Res. 2000;87:448–52. doi: 10.1161/01.res.87.6.448. [DOI] [PubMed] [Google Scholar]
- 60.Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734–46. [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J, Shi B, He S, Yao X, Willcox MD, Zhao Z. Changes to tear cytokines of type 2 diabetic patients with or without retinopathy. Mol Vis. 2010;16:2931–8. [PMC free article] [PubMed] [Google Scholar]
- 62.Cheung CM, Vania M, Ang M, Chee SP, Li J. Comparison of aqueous humor cytokine and chemokine levels in diabetic patients with and without retinopathy. Mol Vis. 2012;18:830–7. [PMC free article] [PubMed] [Google Scholar]
- 63.Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Endothelial permeability and IL-6 production during hypoxia: Role of ROS in signal transduction. Am J Physiol. 1999;277:L1057–65. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- 64.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Döhmen S, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–40. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 65.Sagara M, Satoh J, Zhu XP, Takahashi K, Fukuzawa M, Muto G, et al. Inhibition with N-acetylcysteine of enhanced production of tumor necrosis factor in streptozotocininduced diabetic rats. Clin Immunol Immunopathol. 1994;71:333–7. doi: 10.1006/clin.1994.1094. [DOI] [PubMed] [Google Scholar]
- 66.Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172:1411–8. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gustavsson C, Agardh E, Bengtsson B, Agardh CD. TNF-alpha is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J Diabetes Complications. 2008;22:309–16. doi: 10.1016/j.jdiacomp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Sfikakis PP, Grigoropoulos V, Emfietzoglou I, Theodossiadis G, Tentolouris N, Delicha E, et al. Infliximab for diabetic macular edema refractory to laser photocoagulation: A randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care. 2010;33:1523–8. doi: 10.2337/dc09-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2002;43:1104–8. [PubMed] [Google Scholar]
- 70.Zhang J, Gerhardinger C, Lorenzi M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes. 2002;51:3499–504. doi: 10.2337/diabetes.51.12.3499. [DOI] [PubMed] [Google Scholar]
- 71.Shahulhameed S, Vishwakarma S, Chhablani J, Tyagi M, Pappuru RR, Jakati S, et al. A systematic investigation on complement pathway activation in diabetic retinopathy. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.00154. doi:10.3389/fimmu.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008;7:2516–25. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 73.Muramatsu D, Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Goto H. Correlation of complement fragment C5a with inflammatory cytokines in the vitreous of patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251:15–7. doi: 10.1007/s00417-012-2024-6. [DOI] [PubMed] [Google Scholar]
- 74.Joy SS, Siddiqui K. Molecular and pathophysiological mechanisms of diabetic retinopathy in relation to adhesion molecules. Curr Diabetes Rev. 2019;15:363–71. doi: 10.2174/1573399814666181017103844. [DOI] [PubMed] [Google Scholar]
- 75.Quiroz-Mercado H, Boyer DS, Campochiaro PA, Heier JS, Kaiser PK, Kornfield J, Kuppermann BD, et al. Randomized, Prospective, Double-Masked, Controlled Phase 2b Trial to Evaluate the Safety &Efficacy of ALG-1001 (Luminate®) in Diabetic Macular Edema. Invest Ophthalmol Vis Sci. 2018;59:1960. [Google Scholar]
- 76.Hu TT, Vanhove M, Porcu M, Van Hove I, Van Bergen T, Jonckx B, et al. The potent small molecule integrin antagonist THR-687 is a promising next generation therapy for retinal vascular disorders. Exp Eye Res. 2019;180:43–52. doi: 10.1016/j.exer.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 77.Iliaki E, Poulaki V, Mitsiades N, Mitsiades CS, Miller JW, Gragoudas ES. Role of alpha 4 integrin (CD49d) in the pathogenesis of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:4898–904. doi: 10.1167/iovs.08-2013. [DOI] [PubMed] [Google Scholar]
- 78.El-Asrar AM, Missotten L, Geboes K. Expression of cyclo-oxygenase-2 and downstream enzymes in diabetic fibrovascular epiretinal membranes. Br J Ophthalmol. 2008;92:1534–9. doi: 10.1136/bjo.2008.142182. [DOI] [PubMed] [Google Scholar]
- 79.Mao C, Yan H. Roles of elevated intravitreal IL-1β and IL-10 levels in proliferative diabetic retinopathy. Indian J Ophthalmol. 2014;62:699–701. doi: 10.4103/0301-4738.136220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paine SK, Sen A, Choudhuri S, Mondal LK, Chowdhury IH, Basu A, et al. Association of tumor necrosis factor a, interleukin 6, and interleukin 10 promoter polymorphism with proliferative diabetic retinopathy in type 2 diabetic subjects. Retina. 2012;32:1197–203. doi: 10.1097/IAE.0b013e31822f55f3. [DOI] [PubMed] [Google Scholar]
- 81.de Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol. 1998;102:165–9. doi: 10.1016/s0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- 82.Tsai T, Kuehn S, Tsiampalis N, Vu M-K, Kakkassery V, Stute G, et al. Antiinflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients. PLoS One. 2018;13:e0194603. doi: 10.1371/journal.pone.0194603. doi:10.1371/journal.pone.019460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–82. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, et al. VEGF164 is pro-inflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–62. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spencer BG, Estevez JJ, Liu E, Craig JE, Finnie JW. Pericytes, inflammation, and diabetic retinopathy. Inflammopharmacology. 2020;28:697–709. doi: 10.1007/s10787-019-00647-9. [DOI] [PubMed] [Google Scholar]
- 88.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240–8. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 89.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–15. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 90.Hussain RM, Ciulla TA. Treatment strategies for refractory diabetic macular edema: Switching anti-VEGF treatments, adopting corticosteroid-based treatments, and combination therapy. Expert Opin Biol Ther. 2016;16:365–74. doi: 10.1517/14712598.2016.1131265. [DOI] [PubMed] [Google Scholar]
- 91.Schoenberger SD, Kim SJ, Shah R, Sheng J, Cherney E. Reduction of interleukin 8 and platelet-derived growth factor levels by topical ketorolac, 0.45%, in patients with diabetic retinopathy. JAMA Ophthalmology. 2014;132:32. doi: 10.1001/jamaophthalmol.2013.6203. [DOI] [PubMed] [Google Scholar]
- 92.Jain A, Saxena S, Khanna VK, Shukla RK, Meyer CH. Status of serum VEGF and ICAM-1 and its association with external limiting membrane and inner segment-outer segment junction disruption in type 2 diabetes mellitus. Mol Vis. 2013;19:1760–8. [PMC free article] [PubMed] [Google Scholar]
- 93.Koleva-Georgieva DN, Sivkova NP, Terzieva D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv) 2011;53:44–50. doi: 10.2478/v10153-010-0036-8. [DOI] [PubMed] [Google Scholar]
- 94.Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, et al. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:5594–603. doi: 10.1167/iovs.17-21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuo JZ, Guo X, Klein R, Klein BE, Cui J, Rotter JI, et al. Systemic soluble tumor necrosis factor receptors 1 and 2 are associated with severity of diabetic retinopathy in Hispanics. Ophthalmology. 2012;119:1041–6. doi: 10.1016/j.ophtha.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaul K, Hodgkinson A, Tarr JM, Kohner EM, Chibber R. Is inflammation a common retinal-renal-nerve pathogenic link in diabetes? Curr Diabetes Rev. 2010;6:294–303. doi: 10.2174/157339910793360851. [DOI] [PubMed] [Google Scholar]
- 97.Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–28. [PMC free article] [PubMed] [Google Scholar]
- 98.Tsilimbaris MK, Panagiotoglou TD, Charisis SK, Anastasakis A, Krikonis TS, Christodoulakis E. The use of intravitreal etanercept in diabetic macular oedema. Semin Ophthalmol. 2007;22:75–9. doi: 10.1080/08820530701418243. [DOI] [PubMed] [Google Scholar]
- 99.Rasier R, Artunay O, Gormus U, Yuzbasioglu E, Sengul A, Kukner AS, et al. Interleukin-8 and tumor necrosis factor-琢 levels in vitreous samples from patients with diabetic retinopathy. Guoji Yanke Zazhi (Int Eye Sci) 2013;13:2365–9. [Google Scholar]
- 100.Ridker PM, Everett BM, Thuren T, JG MF, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 101.Dhimolea E. Canakinumab. MAbs. 2010;2:3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.KINERET®(anakinra) U.S. Prescribing Information. [Last accessed on 2019 May 27]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103950s5136lbl.pdf .
- 103.Amano K, Okigaki M, Adachi Y, Fujiyama S, Mori Y, Kosaki A, et al. Mechanism for IL-1 beta-mediated neovascularization unmasked by IL-1 beta knock-out mice. J Mol Cell Cardiol. 2004;36:469–80. doi: 10.1016/j.yjmcc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 104.Maruyama K, Mori Y, Murasawa S, Masaki H, Takahashi N, Tsutusmi Y, et al. Interleukin-1 beta upregulates cardiac expression of vascular endothelial growth factor and its receptor KDR/flk-1 via activation of protein tyrosine kinases. J Mol Cell Cardiol. 1999;31:607–17. doi: 10.1006/jmcc.1998.0895. [DOI] [PubMed] [Google Scholar]
- 105.Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R, et al. The role of IL-1β in the early tumor cell-induced angiogenic response. J Immunol. 2013;190:3500–9. doi: 10.4049/jimmunol.1202769. [DOI] [PubMed] [Google Scholar]
- 106.Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37:416–20. doi: 10.3109/02713683.2012.661114. [DOI] [PubMed] [Google Scholar]
- 107.Lim SW, Bandala-Sanchez E, Kolic M, Rogers SL, McAuley AK, et al. The influence of intravitreal ranibizumab on inflammation-associated cytokine concentrations in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59:5382–90. doi: 10.1167/iovs.17-23325. [DOI] [PubMed] [Google Scholar]
- 108.Jonas JB, Jonas RA, Neumaier M, Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina. 2012;32:2150–7. doi: 10.1097/IAE.0b013e3182576d07. [DOI] [PubMed] [Google Scholar]
- 109.Ghodasra DH, Fante R, Gardner TW, Langue M, Niziol LM, Besirli C, et al. Safety and feasibility of quantitative multiplexed cytokine analysis from office-based vitreous aspiration. Investig Ophthalmol Vis Sci. 2016;57:3017–23. doi: 10.1167/iovs.15-18721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zahir-Jouzdani F, Atyabi F, Mojtabavi N. Interleukin-6 participation in pathology of ocular diseases. Pathophysiology. 2017;24:123–31. doi: 10.1016/j.pathophys.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 111.Mesquida M, Leszczynska A, Llorenç V, Adán A. Interleukin-6 blockade in ocular inflammatory diseases. Clin Exp Immunol. 2014;176:301–9. doi: 10.1111/cei.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yun JH, Park SW, Kim KJ, Bae JS, Lee EH, Paek SH, et al. Endothelial STAT3 activation increases vascular leakage through downregulating tight junction proteins: Implications for diabetic retinopathy. J Cell Physiol. 2017;232:1123–34. doi: 10.1002/jcp.25575. [DOI] [PubMed] [Google Scholar]
- 113.Sepah YJ, Nguyen QD, Do DV, Day B, Wakshull E, Stoilov I. Trends for poorer vision outcomes in nAMD and DME patients with higher aqueous humor levels of IL-6. Invest Ophthalmol Vis Sci. 2019;59:1077. [Google Scholar]
- 114.Kubes P, Suzuki M, Granger DN. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma P, Luo Y, Zhu X, Ma H, Hu J, Tang S. Phosphomannopentaose sulfate (PI-88) inhibits retinal leukostasis in diabetic rat. Biochem Biophys Res Commun. 2009;380:402–6. doi: 10.1016/j.bbrc.2009.01.092. [DOI] [PubMed] [Google Scholar]
- 116.Nagineni CN, Kommineni VK, William A, Detrick B, Hooks JJ. Regulation of VEGF expression in human retinal cells by cytokines: Implications for the role of inflammation in age-related macular degeneration. J Cell Physiol. 2012;227:116–26. doi: 10.1002/jcp.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant Protein-1 (MCP-1): An overview. Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–7. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 119.Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: Role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One. 2014;9:e108508. doi: 10.1371/journal.pone.0108508. doi:10.1371/journal.pone.0108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taghavi Y, Hassanshahi G, Kounis NG, Koniari I, Khorramdelazad H. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in diabetic retinopathy: Latest evidence and clinical considerations. J Cell Commun Signal. 2019;13:451–62. doi: 10.1007/s12079-018-00500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brucklacher RM, Patel KM, VanGuilder HD, Bixler GV, Barber AJ, Antonetti DA, et al. Whole genome assessment of the retinal response to diabetes reveals a progressive neurovascular inflammatory response. BMC Med Genomics. 2008;1:26. doi: 10.1186/1755-8794-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hernandez C, Carrasco E, Casamitjana R, Deulofeu R, Garcia-Arumi J, Simo R. Somatostatin molecular variants in the vitreous fluid: A comparative study between diabetic patients with proliferative diabetic retinopathy and nondiabetic control subjects. Diabetes Care. 2005;28:1941–7. doi: 10.2337/diacare.28.8.1941. [DOI] [PubMed] [Google Scholar]
- 123.Zhang W, Rojas M, Lilly B, Tsai NT, Lemtalsi T, Liou GI, et al. NAD (P) H oxidase-dependent regulation of CCL2 production during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:3033–40. doi: 10.1167/iovs.08-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kitada M, Zhang Z, Mima A, King GL. Molecular mechanisms of diabetic vascular complications. J Diabetes Investig. 2010;1:77–89. doi: 10.1111/j.2040-1124.2010.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patel S, Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 2009;61:595–603. doi: 10.1016/s1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- 126.Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–50. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- 127.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007;56:337–45. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]
- 128.Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 129.Yngen M, Östenson CG, Hu H, Li N, Hjemdahl P, Wallén NH. Enhanced p-selectin expression and increased soluble cd40 ligand in patients with type 1 diabetes mellitus and microangiopathy: Evidence for platelet hyperactivity and chronic inflammation. Diabetologia. 2004;47:537–40. doi: 10.1007/s00125-004-1352-4. [DOI] [PubMed] [Google Scholar]
- 130.Toda N, Nakanishi-Toda M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26:205–38. doi: 10.1016/j.preteyeres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 131.Vielma AH, Retamal MA, Schmachtenberg O. Nitric oxide signaling in the retina: What have we learned in two decades? Brain Res. 2012;1430:1112–25. doi: 10.1016/j.brainres.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 132.Zheng L, Kern T. Role of nitric oxide, superoxide, peroxynitrite and poly(ADP-ribose) polymerase in diabetic retinopathy. Front Biosci. 2009;14:3974–87. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- 133.Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Ball S, Berkowitz BA, et al. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin induced diabetes. Diabetologia. 2007;50:1987–96. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]
- 134.Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrosio AF, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:5257–65. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 135.Abu El-Asrar AM, Struyf S, Opdenakker G, Van Damme J, Geboes K. Expression of stem cell factor/c-kit signaling pathway components in diabetic fibrovascular epiretinal membranes. Mol Vis. 2010;16:1098–2107. [PMC free article] [PubMed] [Google Scholar]
- 136.Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, Miller-Arrevillaga G, Villa-Hernández D, Hernández-Godínez PP, et al. Oxidants, antioxidants and mitochondrial function in non-proliferative diabetic retinopathy. J Diabetes. 2014;6:167–75. doi: 10.1111/1753-0407.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abu El-Asrar AM, Desmet S, Meersschaert A, Dralands L, Missotten L, Geboes K. Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. Am J Ophthalmol. 2001;132:551–6. doi: 10.1016/s0002-9394(01)01127-8. [DOI] [PubMed] [Google Scholar]
- 138.Kowluru RA, Zhong Q, Kanwar M. Metabolic memory and diabetic retinopathy: Role of inflammatory mediators in retinal pericytes. Exp Eye Res. 2010;90:617–23. doi: 10.1016/j.exer.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB Induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–48. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- 140.Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharm Ther. 2017;171:30–42. doi: 10.1016/j.pharmthera.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intercellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995;147:642–53. [PMC free article] [PubMed] [Google Scholar]
- 142.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–41. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Joussen AM, Poulaki V, Mitsiades N, Cai WY, Suzuma I, Pak J, et al. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. Faseb J. 2003;17:76–8. doi: 10.1096/fj.02-0157fje. [DOI] [PubMed] [Google Scholar]
- 144.Ishikawa K, Yoshida S, Kadota K, Nakamura T, Niiro H, Arakawa S, et al. Gene expression profile of hyperoxic and hypoxic retinas in a mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2010;51:4307–19. doi: 10.1167/iovs.09-4605. [DOI] [PubMed] [Google Scholar]
- 145.Sato T, Kusaka S, Hashida N, Saishin Y, Fujikado T, Tano Y. Comprehensive gene-expression profile in murine oxygen-induced retinopathy. Br J Ophthalmol. 2009;93:96–103. doi: 10.1136/bjo.2008.142646. [DOI] [PubMed] [Google Scholar]
- 146.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am J Pathol. 2005;166:637–44. doi: 10.1016/s0002-9440(10)62284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–9. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci. 1995;92:905–9. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hernandez Y, Arora S, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J Immunol. 2005;174:1027–36. doi: 10.4049/jimmunol.174.2.1027. [DOI] [PubMed] [Google Scholar]
- 151.Yang LP, Sun HL, Wu LM, Guo XJ, Dou HL, Tso MO, et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investig Ophthalmol Vis Sci. 2009;50:2319–27. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- 152.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–32. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 153.Liu X, Ye F, Xiong H, Hu D, Limb GA, Xie T, et al. Il-1beta upregulates IL-8 production in human Müller cells through activation of the p38 mapk and erk1/2 signaling pathways. Inflammation. 2014;37:1486–95. doi: 10.1007/s10753-014-9874-5. [DOI] [PubMed] [Google Scholar]
- 154.Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–8. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 155.Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117:576–86. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis. 2010;1:e65. doi: 10.1038/cddis.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang Y, Takeda A, Yoshimura T, Oshima Y, Sonoda KH, Ishibashi T. IL-10 is significantly involved in HSP70-regulation of experimental subretinal fibrosis. PLoS One. 2013;8:e80288. doi: 10.1371/journal.pone.0080288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Myśliwiec M, Balcerska A, Zorena K, Myśliwska J, Lipowski P, Raczyńska K. The assessment of the correlation between vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF-alpha), interleukin 6 (IL-6), glycemic control (HbA1c) and the development of the diabetic retinopathy in children with diabetes mellitus type 1. Klin Oczna. 2007;109:150–4. [PubMed] [Google Scholar]
- 159.Adamiec-Mroczek J, Oficjalska-Mlynczak J, Misiuk-Hojlo M. Proliferative diabetic retinopathy-the influence of diabetes control on the activation of the intraocular molecule system. Diabetes Res Clin Pract. 2009;84:46–50. doi: 10.1016/j.diabres.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 160.Behl Y, Krothapalli P, Desta T, Roy S, Graves DT. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58:917–25. doi: 10.2337/db08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maruo N, Morita I, Shirao M, Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131:710–4. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 162.Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, et al. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J. Biol Chem. 1995;270:11463–71. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- 163.Afridi R, Halim MS, Sadiq MA, Hassan M, Agarwal A, Do DV, et al. Can the levels of inflammatory cytokines in the anterior chamber of eyes with diabetic macular edema predict response to therapy? Invest. Ophthalmol. Vis. Sci. 2017;58:950. [Google Scholar]
- 164.Chalam KV, Grover S, Sambhav K, Balaiya S, Murthy RK. Aqueous interleukin-6 levels are superior to vascular endothelial growth factor in predicting therapeutic response to bevacizumab in age-related macular degeneration. J Ophthalmol 2014. 2014 doi: 10.1155/2014/502174. 502174. doi:10.1155/2014/502174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pearlstein DP, Ali MH, Mungai PT, Hynes KL, Gewertz BL, Schumacker PT. Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arter Thromb Vasc Biol. 2002;22:566–73. doi: 10.1161/01.atv.0000012262.76205.6a. [DOI] [PubMed] [Google Scholar]
- 166.Wu J, Zhong Y, Yue S, Yang K, Zhang G, Chen L, et al. Aqueous humor mediator and cytokine aberrations in diabetic retinopathy and diabetic macular edema: A systematic review and meta-analysis. Dis Markers. 2019;23:6928524. doi: 10.1155/2019/6928524. doi:10.1155/2019/6928524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bromberg-White JL, Glazer L, Downer R, Furge K, Boguslawski E, Duesbery NS. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Investig Ophthalmol Vis Sci. 2013;54:6472–80. doi: 10.1167/iovs.13-12518. [DOI] [PubMed] [Google Scholar]
- 168.Kim M, Kim Y, Lee SJ. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines based on optical coherence tomography patterns of diabetic macular edema. Indian J Ophthalmol. 2015;63:312–7. doi: 10.4103/0301-4738.158069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mocan MC, Kadayifcilar S, Eldem B. Elevated intravitreal interleukin-6 levels in patients with proliferative diabetic retinopathy. Can J Ophthalmol. 2006;41:747–52. doi: 10.3129/i06-070. [DOI] [PubMed] [Google Scholar]
- 170.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–52. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang W, Lo A. Diabetic retinopathy: Pathophysiology and treatments. Int J Mol Sci. 2018;19:1816. doi: 10.3390/ijms19061816. doi:10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Effect of aspirin alone and aspirin plus dipyridamole in early diabetic retinopathy. A multicenter randomized controlled clinical trial. The DAMAD Study Group. Diabetes. 1989;38:491–8. [PubMed] [Google Scholar]
- 173.Soni D, Sagar P, Takkar B. Diabetic retinal neurodegeneration as a form of diabetic retinopathy. Int Ophthalmol. 2021;41:3223–48. doi: 10.1007/s10792-021-01864-4. [DOI] [PubMed] [Google Scholar]
- 174.Bhatwadekar AD, Kansara V, Luo Q, Ciulla T. Anti-integrin therapy for retinovascular diseases. Expert Opin Investig Drugs. 2020;29:935–45. doi: 10.1080/13543784.2020.1795639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moyer KE, Saggers GC, Allison GM, Mackay DR, Ehrlich HP. Effects of interleukin-8 on granulation tissue maturation. J. Cell Physiol. 2002;193:173–9. doi: 10.1002/jcp.10160. [DOI] [PubMed] [Google Scholar]
- 176.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 177.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 178.Canataroglu H, Varinli I, Ozcan AA, Canataroglu A, Doran F, Varinli S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul Immunol Inflamm. 2005;13:375–81. doi: 10.1080/09273940490518900. [DOI] [PubMed] [Google Scholar]
- 179.Lee WJ, Kang MH, Seong M, Cho HY. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br J Ophthalmol. 2012;96:1426–30. doi: 10.1136/bjophthalmol-2012-301913. [DOI] [PubMed] [Google Scholar]
- 180.Petrovič MG, Korosec P, Kosnik M, Hawlina M. Association of preoperative vitreous IL-8 and VEGF levels with visual acuity after vitrectomy in proliferative diabetic retinopathy. Acta Ophthalmol. 2010;88:e311–6. doi: 10.1111/j.1755-3768.2010.02030.x. [DOI] [PubMed] [Google Scholar]
- 181.Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158. doi: 10.1371/journal.pone.0008158. doi:10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Figueras-Roca M, Molins B, SalaPuigdollers A, Matas J, Vinagre I, Ríos J, Adán A. Peripheral blood metabolic and inflammatory factors as biomarkers to ocular fndings in diabetic macular edema. PLoS One. 2017;12:e0173865. doi: 10.1371/journal.pone.0173865. doi:10.1371/journal.pone.0173865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Baudouin C, Fredj-Reygrobellet D, Brignole F, Lapalus P, Gastaud P. MHC class II antigen expression by ocular cells in proliferative diabetic retinopathy. Fundam Clin Pharmacol. 1993;7:523–30. doi: 10.1111/j.1472-8206.1993.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 184.Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004;53:2404–11. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- 185.Cao R, Xue Y, Hedlund EM, Zhong Z, Tritsaris K, Tondelli B, et al. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc Natl Acad Sci U S A. 2010;107:856–61. doi: 10.1073/pnas.0911661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Iacono P, Battaglia Parodi M, Bandello F. Anti-vascular endothelial growth factor in diabetic retinopathy. Dev Ophthalmol. 2010;46:39–53. doi: 10.1159/000320008. [DOI] [PubMed] [Google Scholar]
- 187.Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008;27:608–21. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 188.Connolly DT. Vascular permeability factor: A unique regulator of blood vessel function. J Cell Biochem. 1991;47:219–23. doi: 10.1002/jcb.240470306. [DOI] [PubMed] [Google Scholar]
- 189.Rakoczy EP, Ali Rahman IS, Binz N, Li CR, Vagaja NN, de Pinho M, et al. Characterization of a mouse model of hyperglycemia and retinal neovascularization. Am J Pathol. 2010;177:2659–70. doi: 10.2353/ajpath.2010.090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lu M, Perez VL, Ma N, Miyamoto K, Peng HB, Liao JK, et al. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999;40:1808–12. [PubMed] [Google Scholar]
- 191.Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–51. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 192.Chen YL, Chang YJ, Jiang MJ. Monocyte chemotactic protein-1 gene and protein expression in atherogenesis of hypercholesterolemic rabbits. Atherosclerosis. 1999;143:115–23. doi: 10.1016/s0021-9150(98)00285-8. [DOI] [PubMed] [Google Scholar]
- 193.Chen P, Shibata M, Zidovetzki R, Fisher M, Zlokovic BV, Hofman FM. Endothelin-1 and monocyte chemoattractant protein-1 modulation in ischemia and human brain-derived endothelial cell cultures. J Neuroimmunol. 2001;116:62–73. doi: 10.1016/s0165-5728(01)00280-6. [DOI] [PubMed] [Google Scholar]
- 194.Lee PC, Ho IC, Lee TC. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol Sci. 2005;85:541–50. doi: 10.1093/toxsci/kfi101. [DOI] [PubMed] [Google Scholar]
- 195.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ’ opening’ : Signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–28. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 196.Lee YR, Liu MT, Lei HY, Liu CC, Wu JM, Tung YC, et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol. 2006;87:3623–30. doi: 10.1099/vir.0.82093-0. [DOI] [PubMed] [Google Scholar]
- 197.Abraham JR, Wykoff CC, Arepalli S, Lunasco L, Yu HJ, Hu M, et al. Aqueous cytokine expression and higher order OCT biomarkers: Assessment of the anatomic-biologic bridge in the IMAGINE DME study. Am J Ophthalmol. 2021;222:328–39. doi: 10.1016/j.ajo.2020.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tashimo A, Mitamura Y, Nagai S, Nakamura Y, Ohtsuka K, Mizue Y, et al. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med. 2004;21:1292–7. doi: 10.1111/j.1464-5491.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- 199.Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24:640–7. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 200.Allegro Ophthalmics Announces Positive Topline Results from Del Mar Phase 2b Trial Evaluating Luminate®in Patients with Diabetic Macular Edema. Available from: http://www.allegroeye.com/press .
- 201.Barouch FC, Miyamoto K, Allport JR, Fujita K, Bursell SE, Aiello LP, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000;41:1153–8. [PubMed] [Google Scholar]
- 202.Shram MT, Chaturvedi N, Schalkwijk C, Giorgino F, Ebeling P, Fuller JH, et al. Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes;the EURODIAB Prospective Complications Study. Diabetes Care. 2003;26:2165–73. doi: 10.2337/diacare.26.7.2165. [DOI] [PubMed] [Google Scholar]
- 203.Elner SG, Elner VM, Pavilack MA, Todd RF, 3rd, Mayo-Bond L, Franklin WA, et al. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Investig. 1992;66:200–11. [PubMed] [Google Scholar]
- 204.Nishiwaki A, Ueda T, Ugawa S, Shimada S, Ogura Y. Upregulation of P-selectin and intercellular adhesion molecule-1 after retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2003;44:4931–5. doi: 10.1167/iovs.02-1324. [DOI] [PubMed] [Google Scholar]
- 205.Hirose F, Kiryu J, Miyamoto K, Nishijima K, Miyahara S, Katsuta H, et al. In vivo evaluation of retinal injury after transient ischemia in hypertensive rats. Hypertension. 2004;43:1098–102. doi: 10.1161/01.HYP.0000123069.02156.8a. [DOI] [PubMed] [Google Scholar]
- 206.Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, et al. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1) Am J Pathol. 2000;156:1733–9. doi: 10.1016/S0002-9440(10)65044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expressionand initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;160:501–9. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30:65–84. doi: 10.1007/s00281-008-0111-x. [DOI] [PubMed] [Google Scholar]
- 209.Cunha-Vaz JG. Studies on the pathophysiology of diabetic retinopathy. The blood retinal barrier in diabetes. Diabetes. 1983;32:20–7. doi: 10.2337/diab.32.2.s20. [DOI] [PubMed] [Google Scholar]
- 210.Zhu D, Zhu H, Wang C, Yang D. Intraocular soluble intracellular adhesion molecule-1 correlates with subretinal fluid height of diabetic macular edema. Indian J Ophthalmol. 2004;62:295–8. doi: 10.4103/0301-4738.111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Blum A, Pastukh N, Socea D, Jabaly H. Levels of adhesion molecules in peripheral blood correlate with stages of diabetic retinopathy and may serve as biomarkers for microvascular complications. Cytokine. 2018;106:76–9. doi: 10.1016/j.cyto.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 212.Wilkinson-Berka JL, Alousis NS, Kelly DJ, Gilbert RE. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44:974–9. doi: 10.1167/iovs.02-0392. [DOI] [PubMed] [Google Scholar]
- 213.Talaballi R, Zarini S, Sheibani N, Murphy RC, Gubitosi-Klug RA. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;3:1699–708. doi: 10.1167/iovs.09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yamagishi S, Matsui T, Nakamura K, Ueda S, Noda Y, Imaizumi T. Pigment epithelium-derived factor (PEDF): Its potential therapeutic implication in diabetic vascular complications. Curr Drug Targets. 2008;9:1025–9. doi: 10.2174/138945008786786154. [DOI] [PubMed] [Google Scholar]
- 215.Yoshida Y, Yamagishi S, Matsui T, Jinnouchi Y, Fukami K, Imaizumi T, et al. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab Res Rev. 2009;25:678–86. doi: 10.1002/dmrr.1007. [DOI] [PubMed] [Google Scholar]
- 216.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. Faseb J. 2006;20:323–5. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 217.Zheng Z, Chen H, Ke G, Fan Y, Zou H, Sun X, et al. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to pigment epithelium-derived factor ratio: Involvement of a mitochondria-reactive oxygen species pathway. Diabetes. 2009;58:954–64. doi: 10.2337/db07-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Simo R, Hernandez C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res. 2015;48:160–80. doi: 10.1016/j.preteyeres.2015.04.003. [DOI] [PubMed] [Google Scholar]