Abstract
Diabetes and gestational diabetes (GD) are areas of concern worldwide. GD can eventually lead to serious development of diabetic retinopathy (DR) during pregnancy or worsening of an already existing DR. GD confers future risk of diabetes, both in the mother and fetus, further complicating their lives. DR in pregnant women has been intriguing in terms of understanding the prevalence, assessing risk factors causing pathogenesis, and problems associated with treating them. Pregnancy itself is a risk factor for progression of DR. Physiological changes such as metabolic, vascular, immunologic, and hormonal changes that occur during pregnancy can cause development as well as worsening of DR. This can eventually lead to permanent visual loss if not addressed on time. Timing of laser, choice of treatment for diabetic macular edema with laser, intravitreal anti-vascular endothelial growth factor agents (VEGF), and intravitreal steroids pose a serious challenge in managing these patients without causing damage to the mother and fetus. This review article showcases the prevalence, risk factors, and pathogenesis, outlines the management of DR in pregnancy, and recommends guidelines based on the available evidence. PubMed and MEDLINE searches were performed pertaining to the prevalence of GD in India, DR in pregnancy, risk factors for progression of DR, role of vasoactive mediators in DR, role of angiopoietic factors in DR, hormonal influence of DR, role of growth factors in DR, use of fluorescein and indocyanine green angiography, retinal lasers, anti-VEGF agents, intravitreal steroids, anesthesia, and retinal surgery, all pertaining to pregnancy and guidelines and recommendations for managing DR in pregnancy.
Keywords: Diabetic retinopathy in pregnancy, gestational diabetes, investigations, management in pregnancy, pathogenesis and risk factors
India is now the diabetes capital of the world. Globally, India occupies second place with 77 million diabetic cases between 20 and 79 years of age for the year 2019. This is expected to increase to 101 million and 134.2 million by 2030 and 2045, respectively. Prevalence of diabetes in the population older than 65 years in India was 12.1 million, which is expected to reach 18 million in 2030 and 23.2 million in 2045. There were approximately 43.9 million people with undiagnosed diabetes in India in 2019.[1]
Gestational diabetes (GD) can affect both the mother and fetus. In the 1970s, proliferative diabetic retinopathy (PDR) was an indication for termination of pregnancy. Pregnancy is known to worsen diabetic retinopathy.[2] However, with the advent of screening procedures and advancements in the management of gestational diabetes, women are able to continue with their pregnancy with good control and good outcome for both the mother and the baby. Diabetes should be kept under control throughout pregnancy whether it started before pregnancy or detected during pregnancy.
Diabetic retinopathy (DR) that occurs during pregnancy has been shown to regress during the postpartum period.[3,4] Prevalence of DR at the first examination in women with type 1 diabetes was 57%–62%, and 17%–28% in women with type 2 diabetics. DR was shown to worsen in women with type 1 diabetes compared to type 2 diabetes.[5,6,7]
This review article addresses the prevalence, metabolic, hormonal, vascular, and immunologic factors that lead to the pathogenesis of diabetic retinopathy in pregnancy and the recommendations for the management of DR in pregnancy in addition to the follow-up protocol during the postpartum period.
Method of Literature Search
PubMed and MEDLINE searches were performed pertaining to the prevalence of GD in India, DR in pregnancy, risk factors for progression of DR, role of vasoactive mediators in DR, role of angiopoietic factors in DR, hormonal influence of DR, role of growth factors in DR, use of fluorescein and indocyanine green angiography, retinal lasers, anti-VEGF agents, intravitreal steroids, anesthesia, and retinal surgery, all pertaining to pregnancy and guidelines and recommendations for managing DR in pregnancy.
Hyperglycemia in Pregnancy
The World Health Organization (WHO) and the International Federation of Gynecology and Obstetrics (FIGO) classifies hyperglycemia in pregnancy (HIP) as either gestational diabetes (GD) or diabetes in pregnancy (DIP). Gestational diabetes (GD) is diabetes detected for the first time during pregnancy and may occur any time (predominantly) after 24 weeks. DIP is pregnant women who have a history of diabetes or hyperglycemia that was diagnosed for the first time during pregnancy and meets the WHO criteria of diabetes in the nonpregnant population. DIP can occur even during the first trimester of pregnancy. Most HIP cases are estimated to be GD and are between 75% and 90%.[1]
Maternal and fetal complications associated with GD
There are few maternal and fetal complications that can be associated with GD [Table 1].[1]
Table 1.
Maternal and fetal complications associated with GD
| Maternal Complications | Foetal complications |
|---|---|
| Eclampsia | Neonatal jaundice |
| Preeclampsia | Prematurity |
| Caesarian delivery | Macrosomia |
| GD in future pregnancy | Hypoglycemia |
| Type 2 DM in the future | Respiratory distress |
| CVD in the future | Stillbirths |
| Shoulder dystocia |
DM=Diabetes mellitus; CVD=Cardiovascular disease
Maternal risk factors that can predispose to GD
There are various risk factors that predispose to GD.[1]
Obesity or increase in body mass index
Family H/O diabetes
Polycystic ovarian syndrome
Increasing age
Physical inactivity
GD in previous pregnancy
Previous stillbirth
Previous delivery of baby with macrosomia
Habitual smoking
Criteria for diagnosing GD
Criteria for gestational diabetes have been laid down by the WHO in 1999, International Association of Diabetes and Pregnancy Groups (IADPSG), Diabetes in Pregnancy Study Group in India (DIPSI), FIGO, National Institute for Health and Care Excellence (NICE), American Diabetes Association (ADA), American College of Obstetricians and Gynecologists (ACOG), Australasian Diabetes in Pregnancy Society (ADIPS), and WHO in 2013. There are different criteria that are used in India [Table 2].[1]
Table 2.
Overall view of the criteria that are used in India
| Criteria | Method | Fasting blood glucose (mg/dl) | 1-h blood glucose (mg/dl) | 2-h blood glucose (mg/dl) |
|---|---|---|---|---|
| WHO (1999) | Fasting OGTT with 75 gm of glucose | ≥126 mg/dl | - | ≥140 |
| IADPSG/WHO (2013) | Fasting OGTT with 75 gm of glucose | ≥92 | ≥180 | ≥153 |
| DIPSI | Non-fasting OGTT with 75 gm of glucose | - | - | ≥140 |
OGTT=Oral glucose tolerance test; This screening test is usually done between 24 and 28 weeks of pregnancy but should be conducted much earlier in pregnancy in high-risk women
Comparison of various criteria for the diagnosis or screening of GD
The sensitivity and specificity of various criteria are shown in Table 3.[8]
Table 3.
Sensitivity and specificity of DIPSI criteria in comparison to WHO criteria and IADPSG criteria
| Non-fasting 2 hour VBG | Sensitivity (%) when compared to WHO criteria | Specificity (%) when compared to WHO criteria | Sensitivity (%) when compared to IADPSG criteria | Specificity (%) when compared to IADPSG criteria |
|---|---|---|---|---|
| ≥ 100 mg/dl | 85.5 | 47.7 | 78.3 | 47.5 |
| ≥ 110 mg/dl | 72.8 | 68.6 | 65.1 | 69 |
| ≥ 140 mg/dl (DIPSI criteria) | 27.7 | 97.7 | 22.6 | 97.8 |
VBG=Venous blood glucose; The IADPSG later came to be called WHO criteria 2013
The above study showed that not taking fasting blood glucose into consideration might lead to missing 72.3% of GD as would otherwise be diagnosed with WHO (1999) criteria and 77.4% with IADPSG criteria.
The percentage of detected cases of GD varies with different criteria [Table 4].[9,10,11]
Table 4.
Comparison of various criteria for diagnosing or screening of GD
| Criteria | Percentage detection of cases of GD |
|---|---|
| WHO 1999 vs. WHO 2013 | 9.0% vs. 34.9% |
| 14.6% vs. 18.5% | |
| IADPSG vs. DIPSI | 88.15% vs. 74.34% |
Even worldwide, the prevalence of GD has been significantly higher with IADPSG criteria (6–11 fold) when compared to other criteria.[12]
Prevalence of GD in India
The first study on the prevalence of GD in India was conducted between 1972 and 1975 by the Indian Council of Medical Research (ICMR), which showed the prevalence to be 2.1% and 1.5% in the urban and rural populations, respectively.[13] Prevalence of GD in rural and urban populations across various regions in India is given in Table 5.[14,15,16,17,18]
Table 5.
Prevalence of GD in urban and rural population in various regions of India
| Studies | Regions | Urban prevalence | Rural prevalence |
|---|---|---|---|
| Seshiah et al.[14] (2008) | South India (Tamil Nadu) | 17.8% | 9.9% |
| Khan et al.[15] (2018) | Western India | 15.5% | - |
| Raja et al.[16] (2014) | North India (Kashmir valley) | 7.8% | - |
| Zargar et al.[17] (2004) | North India (Kashmir) | 5.5% | 2.4% |
| Chanda S et al.[18] (2020) | East India (Assam) | - | 16.7% |
Prevalence across various regions in India is given in Table 6.[19,20,21,22,23,24,25] Increased prevalence in urban areas was mostly related to increased maternal age, lack of physical activity, sedentary lifestyle, and increased body mass index associated with urbanization. Lower prevalence in rural areas was attributed to the simple agricultural lifestyle followed by the people.[15,16,17]
Table 6.
Prevalence across various regions in India
| Studies | Region | Prevalence |
|---|---|---|
| Swaminathan et al.[19] | Telangana | 5.4% (0%-11%) |
| Swaminathan et al.[19] | Kerala | 4.5% |
| Seshiah et al. (2004)[20] | Trivandrum | (2.4%-6.7%) |
| Seshiah et al. (2004)[20] | Alwaye | 15.0% 21.0% |
| Swaminathan et al.[19] | West Bengal | 2.3% (0.8%-3.8%) |
| Swaminathan et al.[19] | Assam | 0.23% (0%-0.48%) |
| Swaminathan et al.[19] | Mizoram | 0.16% (0-0.49%) |
| Nielsen et al.[21] | Tamil Nadu | 16.3% |
| Seshiah et al. (2004)[20] | Erode | 18.8% |
| Seshiah et al. (2004)[20] | Ludhiana | 17.5% |
| Siddiqui et al.[22] | Delhi | 14.0% |
| Swami et al.[23] | Maharashtra | 7.7% |
| Nayak et al.[24] | Pondicherry | 27% |
| Rajput et al.[25] | Haryana | 7.1% |
Prevalence of diabetic retinopathy (DR) in pregnancy
Prevalence of DR in GD has been reported to be between 10% and 27%.[26] Prevalence of DR in early pregnancy in type 2 diabetes has been reported as 14% while that in type 1 diabetes ranges between 34% and 72%.[7] Prevalence of DR in India was shown to be 8%.[27]
Risk factors for progression of DR in pregnancy
Diabetes Control and Complications Trial (DCCT)[3] and Diabetes in Early Pregnancy study (DIEP)[28] have discussed the factors leading to and progression of DR in GD.
A. Duration of diabetes
DIEP showed that DR progressed in those who had poor control of diabetes and who had retinopathy at conception. This study showed that the duration of diabetes may not be an important risk factor for a ≥2 step progression of retinopathy as baseline severity of retinopathy but definitely for the development of proliferative DR.[28] The presence of DR was strongly associated with the duration of diabetes as seen in Table 7. The major determinants of progression of retinopathy were duration of diabetes and the severity of preexisting retinopathy [Table 7].
Table 7.
Effect of duration of diabetes on progression of diabetic retinopathy in pregnancy
| Study | Mean duration of Diabetes in years |
|---|---|
| DIEP[28] | >15 years – 50% progression to DR from baseline and 39% progression to PDR<15 years – 55% progression to DR from baseline and 18% progressed to PDR |
| Temple et al.[29] | 10–19 years – 10% <10 years – 0% |
| Axer – Siegel et al.[4] | 15.5±5.3 years – Progression 10.6±6.7 years – No progression |
| Makhwana et al.[27] | 14±6.32 years - 4% overall progression |
B. Metabolic Control
Wang et al.[30] found that worsening of retinopathy was present only in the initial 6–12 months following intensive treatment but significantly reduced thereafter. DIEP study showed that the rate of progression of DR doubled in whom the baseline glycosylated hemoglobin (GHB) was >6 SD above the control mean and had an odds ratio of 2.7 when compared with those whose baseline GHB was within 2 SD of the control mean.[28] Phelps et al.[31] showed that worsening of DR was present in those with poor control of diabetes at presentation and with tight or intensive control of diabetes in the first 6–14 weeks of pregnancy. In Axer–Siegel's study,[4] GHB was higher in the progressive retinopathy group than in the nonprogressive retinopathy group and that in the DR group than in the non-DR group predominantly in the third trimester. DCCT compared the effect of pregnancy in both the “conventional treatment” and the “intensive treatment” group. Patients in the conventional group were switched to the intensive therapy group during the course of pregnancy. In the intensive treatment group, pregnant women had a 1.63-fold greater risk of worsening of DR. In the conventional group, the risk of worsening was 2.48-fold in pregnant women when compared to nonpregnant women.[3]
C. Baseline severity of retinopathy
Presence or absence of retinopathy before pregnancy is a strong predictor of progression of retinopathy during pregnancy [Table 8].
Table 8.
Effect of baseline severity of DR on progression of diabetic retinopathy in pregnancy
| Study | Type of DM | Worsening of DR |
|---|---|---|
| DIEP study[28] | 1 | 10.3% with no DR |
| 18.8 5 with mild NPDR | ||
| 54.8% with moderate to severe NPDR | ||
| 6% with minimal DR and 29% with Moderate NPDR progressed to PDR | ||
| Rahman et al.[6] | 1 | 9.1% with no DR |
| 20% with NPDR | ||
| 58.3% with PDR | ||
| Temple et al.[29] | 1 | 3.7% with no DR 30% with moderate to severe NPDR |
| Vestgaard et al.[32] | 1 | Worsening in 27% |
| Rasmussen et al.[7] | 2 | Worsening in 14% |
| Egan et al.[33] | 1 and 2 | Worsening in 25.9% |
| Axer – Siegel et al.[4] | 1 | Worsening in 77.5% |
| Rosenn et al.[34] | 1 | Worsening in 51% |
| Phelps et al.[31] | 1 | Worsening in 55% |
| DCCT[3] | 1 | 1.63-fold risk of worsening after intensive treatment |
| Makhwana et al.[27] | 1 and 2 | 4% overall progression |
NPDR=Nonproliferative diabetic retinopathy
D. Associated risk factors.
Hypertension (HT)
Rosenn et al.[34] showed progression in 50% with pregnancy-induced HT, 61% with chronic HT (+/− superimposed pregnancy-induced HT), and 55% with any type of HT and progression in 25% with no HT in their study on 154 women. Worsening of DR due to increased systolic blood pressure has been shown by Axer–Siegel et al.[4], Egan et al.,[33] and Vestgaard et al.[32] in their respective studies.
Age of onset of diabetes
Lauszus et al.[35] showed progression during or after pregnancy in women who had earlier onset of diabetes (14 ± 8 years; range: 1–27 years) in life when compared to those with late-onset diabetes (19 ± 8 years; range: 1–36 years).
Visual Acuity and diabetic macular edema
Vestgaard et al.[32] showed that poor visual acuity and presence of diabetic macular edema at conception were risk factors for further worsening and vision loss in GD.
Pathogenesis
A. Retinal blood flow
Hyperdynamic circulation state is believed to exist in pregnancy with a 40% increase in cardiac output.[36] Autoregulatory mechanisms causing compensatory retinal vessel constriction are deranged in diabetics due to decreased renin–angiotensin system, which in turn results in vascular dilatation, increased blood flow, and worsening of DR.[37] This theory is supported by Hellstedt et al.[38] who demonstrated increased macular blood flow by using blue field entoptic simulation, by Loukovaara et al.[39] who demonstrated increased perimacular blood flow, and by Immonen et al.[40] demonstrating increased retinal capillary blood flow in the inferior macular area during various trimesters and post- partum period. Schocket et al.[41] proposed that decrease in retinal volumetric blood flow may have worsened DR due to hypoxia and retinal ischemia. Klein et al.[42] showed that DR worsened when the duration of diabetes, levels of glycosylated hemoglobin, and baseline retinopathy were added along with an increase in retinal venular diameters.
Optical coherence tomography angiography (OCTA) in pregnancy has shown a decrease in superficial capillary plexus, perfusion density (SCP-PD), and an increase in deep capillary plexus density, perfusion density (DCP-PD), without changes in vessel length density in one study[43] and increase in both SCP (parafoveal and perifoveal area) and DCP in macular area in the pregnant group in another study.[44] There was more capillary dropout in SCP in the diabetic group probably due to hyperglycemia, which got better after delivery, and an increase in DCP in both diabetic and nondiabetic groups in the parafoveal area.[45] Okada et al.[46] showed that the mean foveal avascular zone was less in the pre-pregnancy state than in the antenatal or postpartum state in those with no DR.
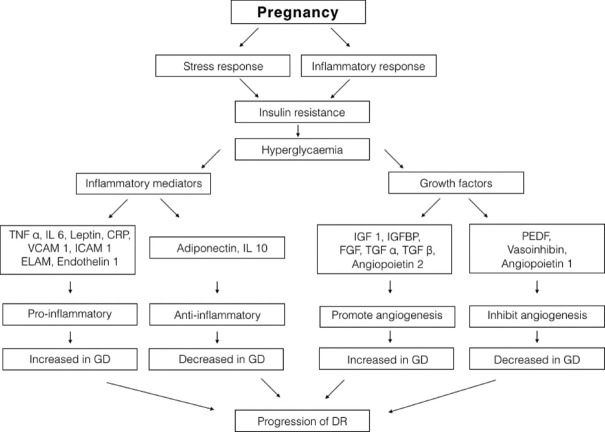
B. Role of growth factors
During pregnancy, growth hormone is replaced by placental growth hormone (PGH), which modulates the secretion of pro-angiogenic factor insulin growth factor (IGF). Both have been shown to be increased in those with PDR and cause progression of DR in pregnancy.[47,48] Loukovaara et al.[49] showed that levels of angiopoietic factors were either similar or low when compared to nondiabetic pregnant women and that they were not associated with progression of DR. However, angiopoetin 2 can accentuate neovascularization in the first trimester if the balance between angipoietic and angiostatic factors is lost in pregnancy. Vasoinhibins obtained from prolactin have not been studied extensively in human pregnant women, but in experimental models has been shown to suppress VEGF-receptor-mediated retinal neovascularization.[50]
C. Role of inflammatory mediators
In pregnancy, placenta that is in a highly inflammatory state causes upregulation of pro-inflammatory factors and down-regulation of anti-inflammatory factors, imbalance of which is implicated in the pathogenesis of PDR.[51,52] Loukovaara et al. and Immonen et al. showed increased levels of CRP in diabetic pregnant women with progression of retinopathy and in women with worse glycemic control but levels of IL-6 or VCAM-1 were similar in both type 1 diabetic and nondiabetic women.[52] Low levels of glycoprotein glycodelin, secreted by the endometrium, which is key in inhibiting E-selectin that promotes leucocyte–endothelium adhesion, has been associated with progression of retinopathy.[53] Similarly, increased levels of endothelin-1 are speculated to cause endothelial damage in pregnancy leading to pregnancy-induced hypertension and progression of DR.[54]
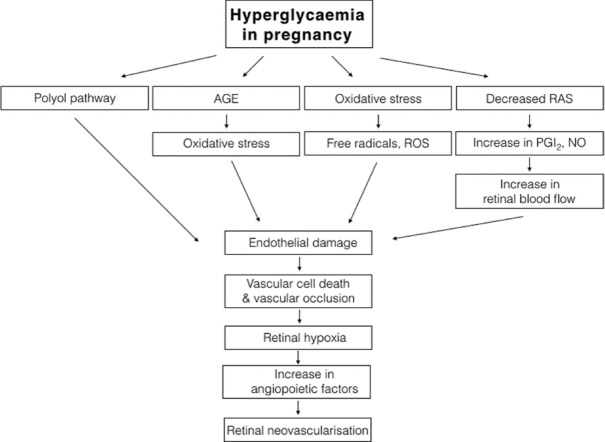
Figs. 1 and 2 show the pathway for pathogenesis of DR and various factors causing progression of DR in pregnancy.
Figure 1.
Pathway for pathogenesis of DR in pregnancy with diabetes (AGE – advanced glycosylated end products, RAS – renin–angiotensin system, ROS – reactive oxygen species, PGI2 – prostacyclin, NO – nitric oxide)
Figure 2.
Role of various growth factors and inflammatory mediators in causing progression of DR in pregnancy with diabetes. (TNF-α – tumor necrosis factor, IL-6 and 10 – interleukin-6 and 10, CRP – C-reactive protein, VCAM-1 – vascular endothelial adhesion molecule-1, ICAM-1 – intercellular adhesion molecule-1, ELAM – endothelial leucocyte adhesion molecule, IGF-1 – insulin growth factor-1, IGFBP – insulin growth factor binding protein, FGF – fibroblast growth factor, TGF α and β – transforming growth factor α and β, PEDF – pigment epithelial derived factor)
Management of DR in Pregnancy
Fluorescein angiography (FA) and Indocyanine green angiography (ICG)
Though fluorescein crosses the placental barrier, studies have shown that FA, when performed before the 15th week, did not induce any adverse effect, and few others reported minimal fetal abnormalities with no major complications.[55,56] The United States Food and Drug Administration (US FDA) and Therapeutics and Goods Administration (TGA) in Australia have placed fluorescein under category 3 and 2 B, respectively. ICG does not cross the placenta or fetus during any stage of pregnancy and is not detected in fetal blood or umbilical cord soon after delivery. It is categorized under category C by the FDA.[57,58]
Retina lasers in pregnancy
Sunness et al.[59] reported that 58% of those diagnosed with PDR in early pregnancy and treated with laser progressed whereas 29% of those diagnosed and treated before pregnancy progressed. Hercules et al.[60] showed that extensive laser showed regression of neovascularization in 63% of women before and after delivery but did not significantly improve the visual acuity. Best et al.[2] and Agardh et al.[61] advocated the use of laser in PDR to promote regression despite concerns regarding macular edema. Chan et al.[62] advised early use of laser in pre-proliferative DR during pregnancy instead of waiting for spontaneous regression as that might give a worse outcome.
Intravitreal anti-VEGF injections during pregnancy
Anti-VEGF use in pregnancy has not been substantiated through large prospective studies.
Table 9 gives the details of different anti-VEGF use and their safety profile during various stages of pregnancy.
Table 9.
Use of intravitreal anti-VEGF agents for diabetic retinopathy in pregnancy
| Study | Timing of anti-VEGF | Condition given | Number of injections | Adverse effect (Mother or fetus) |
|---|---|---|---|---|
| Sarmad et al.[63] (1 patient - Bevacizumab) | 4-5 weeks of pregnancy | PDR with DME | 2 (1 in each eye) | None |
| Rosen et al.[64] (1 patient - Bevacizumab) | Second Trimester | CNVM following PIC | 1 | None |
| Tarantola et al.[65] (4 patients - Bevacizumab) | 17, 21, 26, and 31 weeks of gestation Lactation and early pregnancy Preconception and 1, 9, 14, 20, 26, and 32 weeks of pregnancy 23 and 36 weeks in successive pregnancies |
CNVM in sarcoid CNVM in PIC CNVM in POHS CNVM IN POHS |
RE – 4 RE – 14 BE – 9 RE - 2 |
None None None None |
| Petrou et al.[66] (2 patients - Bevacizumab) | 5 weeks of pregnancy 4 weeks of pregnancy |
PDR Myopic CNVM |
LE -1 LE - 1 |
Miscarriage at 7 days Miscarriage at 10 days |
| Sullivan et al.[67] (4 patients - Bevacizumab) | 19 days of gestation 21 days of gestation 24 days of gestation 2 preconception and 1 at 20 days of gestation |
Idiopathic Juxtafoveal CNVM CNVM in PIC PDR PDR |
1 1 BE – 1 each BE – 1 each before conception and 1 more in one eye after conception |
None None None Preeclampsia and urgent caesarian at 29 weeks. Infant intubation for bradycardia and respiratory distress |
| Jouve et al.[68] and Sarhianaki et al.[69] (1 patient each – Ranibizumab) | Third trimester | ICNM | None |
CNVM=Choroidal neovascular membrane; PIC=Punctate inner choroidopathy; POHS=Presumed ocular histoplasmosis syndrome; ICNM=Idiopathic choroidal neovascular membrane
Intravitreal steroids in diabetic macular edema (DME) in pregnancy
There is not much literature evidence to support the use of intravitreal steroids in pregnancy. Smaller studies have brought out their use and safety profile during various stages of pregnancy [Table 10].
Table 10.
Use of intravitreal steroids for diabetic retinopathy in pregnancy
| Study | Type of IV steroid | Improvement in BCVA | Improvement in CFT | Adverse effect |
|---|---|---|---|---|
| Fazelat et al.[70] (2 eyes of 1 patient) | 0.05 ml of 40 mg/ml of Triamcinolone acetonide |
Before OU – 20/40 After RE – 20/20 LE – 20/25 |
Before RE – 578±5µ LE – 667±8µ After RE – 159±5µ LE – 202±6µ |
Nil |
| Concillado et al.[71] z(10 eyes of 5 patients) | 700 µgm of slow-release dexamethasone implant (Ozurdex) | Before Mean ETDRS letters - 63 (Range 50-77) After Increase of ≥5 ETDRS letters |
Before Mean CFT: 535 µm (range: 239-727 µm) After >145 µm reduction in CFT |
Transient increase in IOP in 3/8 eyes |
IV= Intravitreal; ETDRS=Early Treatment Diabetic Retinopathy Treatment Study; BCVA=Best-corrected visual acuity; CFT=Central foveal thickness; IOP=Intraocular pressure
Vitreo-retinal surgery and anesthesia
General anesthesia can be avoided in pregnancy as the procedure of intubation remains tedious and has a tendency to cause rebleeding of a retinal hemorrhage from vasodilatation due to hypoxia and hypercarbia or due to an increase in retinal venous pressure and intracranial pressure. There is also fear of medication passing into the fetus. The difficulty of pregnant women lying on their backs throughout the surgical procedure should not be overlooked.[72]
Management of delivery and postpartum period
Vaginal delivery has been associated with Valsalva-induced vitreous hemorrhage in those with untreated PDR. Though there is no concrete evidence, ADA recommends the use of epidural anesthesia with assisted second stage to avoid pushing or caesarian delivery. There are reports of women with PDR showing regression after delivery.[72]
Discussion
Prevalence of GD in India varies because of the diverse population based on geography and ethnicity, rural and urban population, and different diagnostic criteria. As far as screening or diagnostic tests are concerned, IADPSG has shown increased prevalence probably because of inclusion of fasting blood glucose and lower threshold of fasting blood glucose. However, many authors feel that diagnosis based on fasting blood sugar may not be appropriate for mothers who will have to travel long distances to come to hospitals for a checkup. However, few authors have insisted on fasting blood glucose levels for fear of underestimating GD. Tests used should be feasible and affordable for patients.[9,10,11,12] Adverse fetal and maternal outcomes are associated with GD and obesity either in isolation or in combination as shown by Hyperglycemia and Adverse Pregnancy Outcome Study (HAPOS).[73] Urbanization along with its other disadvantages has shown an increased prevalence of diabetes in pregnancy too.[14,15,16,17,18]
Studies have shown that besides the duration of diabetes, poor control of diabetes and presence of DR at conception are major determinants. Rapid control of diabetes has shown DR progression particularly in the first 6–14 weeks of pregnancy. DCCT showed that progression of DR was more in the intensive treatment group. Studies have shown that baseline severity of DR is a strong predictor of progression of DR during pregnancy. Association with HT has been shown to worsen DR.[3,4,28] Pregnancy hormones such as human placental lactogen (HPL) are believed to worsen DR in pregnancy due to its growth hormone-like action.[47] Pregnancy also induces a hyperdynamic state, reducing peripheral vascular resistance, which in turn adds shear stress to the endothelium at the capillary level. This along with decreased renin–angiotensin system adds more stress to an already compromised retinal vasculature, leading to neovascularization.[2,36,37]
Hyperglycemia in pregnancy can lead to vascular damage through the polyol pathway, advanced glycation end products, and by oxidative stress, which in turn causes hypoxia and stimulates angiogenesis.[74] Retinal ischemia caused by damage to the retinal capillaries induces the formation of angiopoietic factors, causing progression of DR and permanent visual loss. Among many angiopoietic factors, VEGF (VEGF-A) deserves special mention and has been implicated in DR and its levels in vitreous are high in those with PDR.[75] DR is also an inflammatory state wherein inflammatory mediators cause interaction between blood cells (leucocytes) and retinal capillary endothelial cells. Increased levels of ICAM-1, VCAM-1, ELAM-1, IL-6, IL-8, and CRP have been found in those with DR. Decrease in contrast sensitivity and loss of nerve fiber layer defects have been found in early DR or even before the occurrence of DR.[76,77]
Various studies have shown decreased progression of severe NPDR, preproliferative DR, and PDR after treating with laser photocoagulation. Though DR regresses after delivery, it is prudent not to wait for spontaneous regression; instead, give laser to prevent further progression during the postpartum period.[59,60,61,62]
Early Diabetic Treatment Retinopathy Study (ETDRS) recommends the use of grid or focal laser for clinically significant macular edema (CSME).[78] Though ETDRS guidelines are not specific for pregnancy, Rasmussen et al.[7] and Vestgaard et al.[32] supported the use of macular laser at the earliest to prevent irreversible damage. Nicolo et al.[79] recommended the use of micropulse laser for foveal involvement where the safety of conventional laser might be of concern. Agardh et al.[61] in a woman with bilateral edema managed to observe as it was not amenable to conventional laser treatment and managed it postpartum with macular grid laser.
In cases where there is refractory DME, Diabetic Retinopathy Clinical Research (DRCR) Network showed greater effectivity with steroids than laser after 4 months in terms of visual acuity and retinal thickness, while laser was proved superior to intravitreal triamcinolone at 3 years.[80,81] Though, first-trimester use of systemic steroids has shown formation of oral clefts,[82] low birth weight with topical steroids[83] and craniofacial abnormalities in animal models with triamcinolone, systemic absorption of triamcinolone has been shown to be minimal.[84] Intravitreal steroids have been shown to cause cataract and an increase in intraocular pressure.[85]
Evidence for use of anti-VEGF in pregnancy is based on case reports or small case series only. Hence, it may not be possible to arrive at a correlation between anti-VEGF agents and feto-maternal complications. From the available evidence, bevacizumab given within 5 weeks of pregnancy has caused more problems than when given after 5 gestational weeks.[64,65] In the case of miscarriage reported by Sullivan et al.,[67] the woman had risk factors of diabetes and hypertension. Ranibizumab given in pregnant women during the 3rd trimester for CNVM has been reported to be without any adverse effect for the mother or fetus.[68,69] Thus, calculation of the time of injection from last menstrual date to the time of conception is important. Careful counseling and advice for injection should be done only when the benefit to the mother outweighs the risk to the fetus. RESTORE extension study[86] demonstrated that visual acuity at 3 years in those who had delayed injections by 12 months was similar to those who had prompt treatment. Hence, withholding anti-VEGF treatment during pregnancy for 6–9 months may not severely affect the vision.
Chan et al.[62] recommended parsplana vitrectomy (PPV) for nonclearing VH, PPV with scleral buckle (SB) ± C3Fb or silicone oil for combined TRD and rhegmatogenous RD in the postpartum period. Surgery can be performed under local anesthesia. Among local anesthetics, lidocaine, etidocaine, and prilocaine hydrochloride are classified under categories A and B in FDA and hence are redeemed safe. Bupivacaine and mepivacaine are class C category drugs that can cause fetal bradycardia and hence should be avoided in pregnancy. Subtenons injection of anesthesia has been advocated for vitrectomy and SB during pregnancy by Errera et al.[72]
Recommendations
Gestational diabetes: Diabetic retinopathy screening is not required and does not pose an increased risk for DR during pregnancy.[87]
-
Pregnant patients with preexisting diabetes
-
Preconception
- Dilated and comprehensive eye examination at the first appointment (unless in the last 6 months) for those who seek preconception care.
- Rapid optimization of blood sugar control should be deferred until retinal examination and assessment.
- Statins and drugs that block the renin–angiotensin system should be stopped before conception or at the first antenatal visit if still being taken.
- Blood glucose in those with severe NPDR and PDR before conception should be reduced slowly to near normal over a 6-month period.
- The importance of controlling risk factors before and during pregnancy and maintaining a target HbA1C as close to normal as possible should be explained.
- Postponement of pregnancy can be discussed until the ocular disease is treated and stabilized.
- In case of DME, indications and treatment should be followed as usual. If need for anti-VEF or triamcinolone is indicated, then contraception 1 month before, during, and 3 months after the last treatment should be advised.
-
During pregnancy
- Retinal examination at first antenatal visit with tropicamide drops and with digital imaging (unless in the last 3 months) – NICE[89]
- Those with no or minimal NPDR should be evaluated at first and third trimester, those with mild retinopathy, every trimester and those with moderate to severe and PDR every month (ADA)[88]
- No to mild or moderate NPDR – every 3-12 months and severe NPDR or worse – 1-3 months (AAO PPP)[87]
- Laser treatment should be considered to prevent vision loss during preconception and in those with high-risk PDR (within four weeks), center-involving DME (focal or macular grid laser or can be observed throughout pregnancy and postdelivery) and severe NPDR (if only one eye) during pregnancy (ADA)[88]
- Anti-VEGF and triamcinolone can be avoided during pregnancy for fear of teratogenicity.
- DR should not be considered as a contraindication to vaginal delivery. However, in women with untreated PDR, vaginal delivery can be associated with retinal and vitreous hemorrhage. Hence, assisted second stage delivery or caesarian delivery should be considered by liaising with the obstetrician and ophthalmologist (ADA, NICE, RCO, and ICO)[88,89,91,92]
-
After delivery
- Dilated retinal examination should be considered 1–2 months after delivery in women with treated or untreated DME and those with mild, moderate, or severe NPDR during pregnancy and followed up until 12 months. Management and further follow-up should be based on the findings.[90]
-
(Recommendations are based on IDF, NICE, ADA, ICO, RCO, AAO-PPP, WHO – South-East Asia, Management of DR and diabetic eye disease in India by London School of Hygiene and Topical Medicine, The Queen Elizabeth Diamond Jubilee Trust, and Indian Institute of Public Health, Hyderabad).
IDF – International Diabetes Federation.
ICO - International Council of Ophthalmologists.
RCO - Royal College of Ophthalmologists.
AAO PPP - American Academy of Ophthalmology – Preferred Practice Patterns.
Conclusion
Understanding the prevalence and risk factors causing pathogenesis in DR in pregnancy is essential for proper management, screening, and counseling for future pregnancies. This will in turn pave for a safe maternal and fetal outcome. Managing DR in a pregnant woman poses a big challenge. Control of sugars and associated risk factors is the mainstay of treatment. With the advent of lasers, pre-proliferative DR, PDR, and DME can be managed effectively. Intravitreal steroids can be considered for refractory DME after discussing the pros and cons, preferably in the 2nd or 3rd trimester. Anti-VEGF agents can be withheld because of lack of evidence to support their use or considered as a last resort after proper counseling preferably during the latter half of pregnancy. Mode of delivery, choice of anesthesia, and the anesthetic agent for cesarian as well as retinal surgeries should be explained clearly to patients. Additional emphasis should be given for following up during the postpartum period. Thus, a methodical approach will mitigate the concerns and adverse outcomes regarding managing DR in pregnancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Diabetes Federation (IDF) Online Atlas. 9th ed. 2019. Available from: www.diabetesatlas.org/en/
- 2.Best RM, Chakravarthy U. Diabetic retinopathy in pregnancy. Br J Ophthalmol. 1997;81:249–51. doi: 10.1136/bjo.81.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084–91. doi: 10.2337/diacare.23.8.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axer-Siegel R, Hod M, Fink-Cohen S, Kramer M, Weinberger D, Schindel B, et al. Diabetic retinopathy during pregnancy. Ophthalmology. 1996;103:1815–9. doi: 10.1016/s0161-6420(96)30421-1. [DOI] [PubMed] [Google Scholar]
- 5.Omori Y, Minei S, Testuo T, Nemoto K, Shimizu M, Sanaka M, et al. Current status of pregnancy in diabetic women. A comparison of pregnancy in IDDM and NIDDM mothers. Diabetes Res Clin Pract. 1994;24(suppl):S273–8. doi: 10.1016/0168-8227(94)90261-5. [DOI] [PubMed] [Google Scholar]
- 6.Rahman W, Rahman FZ, Yassin S, Al-Suleiman SA, Rahman J. Progression of retinopathy during pregnancy in type 1 diabetes mellitus. Clin Exp Ophthalmol. 2007;35:231–6. doi: 10.1111/j.1442-9071.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen KL, Laugesen CS, Ringholm L, Vestgaard M, Damm P, Mathiesen ER. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia. 2010;53:1076–83. doi: 10.1007/s00125-010-1697-9. [DOI] [PubMed] [Google Scholar]
- 8.Mohan V, Mahalakshmi MM, Bhavadharini B, Maheswari K, Kalaiyarasi G, Anjana RM, et al. Comparison of screening for gestational diabetes mellitus by oral glucose tolerance tests done in the non-fasting (random) and fasting states. Acta Diabetol. 2014;51:1007–13. doi: 10.1007/s00592-014-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vij P, Jha S, Gupta SK, Aneja A, Mathur R, Waghdhare S, et al. Comparison of DIPSI and IADPSG criteria for diagnosis of GDM: A study in a north Indian tertiary care center. Int J Diabetes Dev Ctries. 2015;35:285–8. [Google Scholar]
- 10.Arora GP, Thaman RG, Prasad RB, Almgren P, Brøns C, Groop LC, et al. Prevalence and risk factors of gestational diabetes in Punjab, North India: Results from a population screening program. Eur J Endocrinol. 2015;173:257–67. doi: 10.1530/EJE-14-0428. [DOI] [PubMed] [Google Scholar]
- 11.Bhavadharini B, Mahalakshmi MM, Anjana RM, Maheswari K, Uma R, Deepa M, et al. Prevalence of gestational diabetes mellitus in urban and rural Tamil Nadu using IADPSG and WHO 1999 criteria (WINGS 6) Clin Diabetes Endocrinol. 2016;2:8. doi: 10.1186/s40842-016-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani Tehrani F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:11. doi: 10.1186/s13098-019-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran A. Epidemiology of diabetes in Indians. Int J Diab Dev Ctries. 1993;13:65–7. [Google Scholar]
- 14.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu)--a community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 15.Khan S, Bal H, Khan ID, Paul D. Prevalence of gestational diabetes mellitus in an urban Indian cohort using diabetes in pregnancy study group in India (DIPSI) criteria–validating one-step approach. Int J Med Med Res. 2018;4:13–9. [Google Scholar]
- 16.Raja MW, Baba TA, Hanga AJ, Bilquees S, Rasheed S, Haq IU, et al. A study to estimate the prevalence of gestational diabetes mellites in an urban block of Kashmir valley (North India) Int J Med Sci Public Health. 2014;3:191–5. [Google Scholar]
- 17.Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res Clin Pract. 2004;66:139–45. doi: 10.1016/j.diabres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Chanda S, Dogra V, Hazarika N, Bambrah H, Sudke AK, Vig A, et al. Prevalence and predictors of gestational diabetes mellitus in rural Assam: A cross-sectional study using mobile medical units. BMJ Open. 2020;10:e037836. doi: 10.1136/bmjopen-2020-037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swaminathan G, Swaminathan A, Corsi DJ. Prevalence of gestational diabetes in India by individual socioeconomic, demographic, and clinical factors. JAMA Netw Open. 2020;3:e2025074. doi: 10.1001/jamanetworkopen.2020.25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seshiah V, Balaji V, Balaji MS, Sanjeevi CB, Green A. Gestational diabetes mellitus in India. J Assoc Physicians India. 2004;52:707–11. [PubMed] [Google Scholar]
- 21.Kragelund Nielsen K, Damm P, Kapur A, Balaji V, Balaji MS, Seshiah V, et al. Risk factors for hyperglycaemia in pregnancy in Tamil Nadu, India. PLoS One. 2016;11:e0151311. doi: 10.1371/journal.pone.0151311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqui S, Waghdhare S, Panda M, Sinha S, Singh P, Dubey S, et al. Regional prevalence of gestational diabetes mellitus in North India. J Diabetol. 2019;10:25. [Google Scholar]
- 23.Swami SR, Mehetre R, Shivane V, Bandgar TR, Menon PS, Shah NS. Prevalence of carbohydrate intolerance of varying degrees in pregnant females in western India (Maharashtra)--a hospital-based study. J Indian Med Assoc. 2008;106:712–4, 735. [PubMed] [Google Scholar]
- 24.Nayak PK, Mitra S, Sahoo JP, Daniel M, Mathew A, Padma A. Feto-maternal outcomes in women with and without gestational diabetes mellitus according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria. Diabetes Metab Syndr. 2013;7:206–9. doi: 10.1016/j.dsx.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Rajput R, Yadav Y, Nanda S, Rajput M. Prevalence of gestational diabetes mellitus and associated risk factors at a tertiary care hospital in Haryana. Indian J Med Res. 2013;137:728–33. [PMC free article] [PubMed] [Google Scholar]
- 26.Hadden DR. Diabetes in pregnancy 1985. Diabetologia. 1986;29:1–9. doi: 10.1007/BF02427272. [DOI] [PubMed] [Google Scholar]
- 27.Makwana T, Takkar B, Venkatesh P, Sharma JB, Gupta Y, Chawla R, et al. Prevalence, progression, and outcomes of diabetic retinopathy during pregnancy in Indian scenario. Indian J Ophthalmol. 2018;66:541–6. doi: 10.4103/ijo.IJO_1062_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew EY, Mills JL, Metzger BE, Remaley NA, Jovanovic-Peterson L, Knopp RH, et al. Metabolic control and progression of retinopathy. The diabetes in early pregnancy study. National Institute of Child Health and Human Development diabetes in early pregnancy study. Diabetes Care. 1995;18:631–7. doi: 10.2337/diacare.18.5.631. [DOI] [PubMed] [Google Scholar]
- 29.Temple RC, Aldridge VA, Sampson MJ, Greenwood RH, Heyburn PJ, Glenn A. Impact of pregnancy on the progression of diabetic retinopathy in type 1 diabetes. Diabet Med. 2001;18:573–7. doi: 10.1046/j.1464-5491.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341:1306–9. doi: 10.1016/0140-6736(93)90816-y. [DOI] [PubMed] [Google Scholar]
- 31.Phelps RL, Sakol P, Metzger BE, Jampol LM, Freinkel N. Changes in diabetic retinopathy during pregnancy. Correlations with regulation of hyperglycemia. Arch Ophthalmol. 1986;104:1806–10. doi: 10.1001/archopht.1986.01050240080044. [DOI] [PubMed] [Google Scholar]
- 32.Vestgaard M, Ringholm L, Laugesen CS, Rasmussen KL, Damm P, Mathiesen ER. Pregnancy-induced sight-threatening diabetic retinopathy in women with type 1 diabetes. Diabet Med. 2010;27:431–5. doi: 10.1111/j.1464-5491.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 33.Egan AM, McVicker L, Heerey A, Carmody L, Harney F, Dunne FP. Diabetic retinopathy in pregnancy: A population-based study of women with pregestational diabetes. J Diabetes Res 2015. 2015 doi: 10.1155/2015/310239. 310239. doi:10.1155/2015/310239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenn B, Miodovnik M, Kranias G, Khoury J, Combs CA, Mimouni F, et al. Progression of diabetic retinopathy in pregnancy: Association with hypertension in pregnancy. Am J Obstet Gynecol. 1992;166:1214–8. doi: 10.1016/s0002-9378(11)90608-5. [DOI] [PubMed] [Google Scholar]
- 35.Lauszus F, Klebe JG, Bek T. Diabetic retinopathy in pregnancy during tight metabolic control. Acta Obstet Gynaecol Scand. 2000;79:367–70. [PubMed] [Google Scholar]
- 36.Thornburg KL, Jacobson S-L, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24:11–4. doi: 10.1016/s0146-0005(00)80047-6. [DOI] [PubMed] [Google Scholar]
- 37.Chen HC, Newsom RS, Patel V, Cassar J, Mather H, Kohner EM. Retinal blood flow changes during pregnancy in women with diabetes. Invest Ophthalmol Vis Sci. 1994;35:3199–208. [PubMed] [Google Scholar]
- 38.Hellstedt T, Kaaja R, Teramo K, Immonen I. Macular blood flow during pregnancy in patients with early diabetic retinopathy measured by blue-field entoptic simulation. Graefes Arch Clin Exp Ophthalmol. 1996;234:659–63. doi: 10.1007/BF00292350. [DOI] [PubMed] [Google Scholar]
- 39.Loukovaara S, Harju M, Kaaja R, Immonen I. Retinal capillary blood flow in diabetic and nondiabetic women during pregnancy and postpartum period. Invest Ophthalmol Vis Sci. 2003;44:1486–91. doi: 10.1167/iovs.02-0293. [DOI] [PubMed] [Google Scholar]
- 40.Immonen IJ, Loukovaara S, Harju M, Kaaja R. Retinal capillary blood flow during pregnancy in patients with diabetes. Invest Ophthalmol Vis Sci. 2002;43:546. doi: 10.1167/iovs.02-0293. [DOI] [PubMed] [Google Scholar]
- 41.Schocket LS, Grunwald JE, Tsang AF, DuPont J. The effect of pregnancy on retinal haemodynamics in diabetic versus nondiabetic mothers. Am J Ophthalmol. 1999;128:477–84. doi: 10.1016/s0002-9394(99)00234-2. [DOI] [PubMed] [Google Scholar]
- 42.Klein BEK, Horak KML, Meuer SM, Mosher AE, Ewen AF, Danforth LG, et al. Retinal vessel diameters confound the relationship of pregnancy to retinopathy and infant outcomes in T1D. J Diabetes Complications. 2019;33:530–4. doi: 10.1016/j.jdiacomp.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Chanwimol K, Balasubramanian S, Nassisi M, Gaw SL, Janzen C, Sarraf D, et al. Retinal vascular changes during pregnancy detected with optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2019;60:2726–32. doi: 10.1167/iovs.19-26956. [DOI] [PubMed] [Google Scholar]
- 44.Kızıltunç PB, Varlı B, Büyüktepe TÇ, Atilla H. Ocular vascular changes during pregnancy: An optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2020;258:395–401. doi: 10.1007/s00417-019-04541-6. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, Wang F. Macular vascular changes in pregnant women with gestational diabetes mellitus by optical coherence tomography angiography. BMC Ophthalmol. 2021;21:170. doi: 10.1186/s12886-021-01927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okada M, Widyaputri F, Rogers S, Nankervis A, Conn J, Shub A, et al. Retinal vascular changes during pregnancy in patients with diabetes mellitus as measured using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2019;60:3024. [Google Scholar]
- 47.Caufriez A, Frankenne F, Englert Y, Golstein J, Cantraine F, Hennen G, et al. Placental growth hormone as a potential regulator of maternal IGF-I during human pregnancy. Am J Physiol. 1990;258:E1014–19. doi: 10.1152/ajpendo.1990.258.6.E1014. [DOI] [PubMed] [Google Scholar]
- 48.Lauszus F, Klebe JG, Bek T, Flyvbjerg A. Increased serum IGF-I during pregnancy is associated with progression of diabetic retinopathy. Diabetes. 2003;52:852–56. doi: 10.2337/diabetes.52.3.852. [DOI] [PubMed] [Google Scholar]
- 49.Loukovaara S, Immonen I, Koistinen R, Rudge J, Teramo KA, Laatikainen L, et al. Angiopoietic factors and retinopathy in pregnancies complicated with type 1 diabetes. Diabet Med. 2004;21:697–704. doi: 10.1111/j.1464-5491.2004.01235.x. [DOI] [PubMed] [Google Scholar]
- 50.Triebel J, Macotela Y, de la Escalera GM, Clapp C. Prolactin and vasoinhibins: Endogenous players in diabetic retinopathy. IUBMB Life. 2011;63:806–10. doi: 10.1002/iub.518. [DOI] [PubMed] [Google Scholar]
- 51.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363–5. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loukovaara S, Immonen I, Koistinen R, Hiilesmaa V, Kaaja R. Inflammatory markers and retinopathy in pregnancies complicated with type I diabetes. Eye. 2005;19:422–30. doi: 10.1038/sj.eye.6701499. [DOI] [PubMed] [Google Scholar]
- 53.Loukovarra S, Immonen IR, Loukavaara MJ, Koistinen R, Kaaja RJ. Glycodelin: A novel serum anti-inflammatory marker in type 1 diabetic retinopathy during pregnancy. Acta Ophthalmol Scand. 2007;85:46–9. doi: 10.1111/j.1600-0420.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- 54.Best RM, Hayes R, Hadden DR, Chakravarthy U, Archer DB. Plasma levels of endothelin-1 in diabetic retinopathy in pregnancy. Eye (Lond) 1999;13:179–82. doi: 10.1038/eye.1999.47. [DOI] [PubMed] [Google Scholar]
- 55.Soubrane G, Canivet J, Coscas G. Influence of pregnancy on the evolution of background retinopathy –Preliminary results of a prospective fluorescein angiography study. Int Ophthalmol. 1985;8:249–55. doi: 10.1007/BF00137653. [DOI] [PubMed] [Google Scholar]
- 56.Halperin LS, Olk RJ, Soubrane G, Coscas G. Safety of fluorescein angiography during pregnancy. Am J Ophthalmol. 1990;109:563–6. doi: 10.1016/s0002-9394(14)70686-5. [DOI] [PubMed] [Google Scholar]
- 57.Fineman MS, Maguire JI, Fineman SW, Benson WE. Safety of indocyanine green angiography during pregnancy: A survey of the retina, macula, and vitreous societies. Arch Ophthalmol. 2001;119:353–5. doi: 10.1001/archopht.119.3.353. [DOI] [PubMed] [Google Scholar]
- 58.Probst P, Paumgartner G, Caucig H, Grabner G. Studies on clearance and placental transfer of indocyanine green during labor. Clin Chim Acta. 1970;29:157–60. doi: 10.1016/0009-8981(70)90237-8. [DOI] [PubMed] [Google Scholar]
- 59.Sunness JS. The pregnant woman's eye. Surv Ophthalmol. 1988;32:219–38. doi: 10.1016/0039-6257(88)90172-5. [DOI] [PubMed] [Google Scholar]
- 60.Hercules BL, Wozencroft M, Gayed II, Jeacock J. Peripheral retinal ablation in the treatment of proliferative diabetic retinopathy during pregnancy. Br J Ophthalmol. 1980;64:87–93. doi: 10.1136/bjo.64.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agardh E. A case of progression of diabetic retinopathy during pregnancy. Acta Ophthalmol Scand. 2002;80:524–30. doi: 10.1034/j.1600-0420.2002.800512.x. [DOI] [PubMed] [Google Scholar]
- 62.Chan WC, Lim LT, Quinn MJ, Knox FA, McCance D, Best RM. Management and outcome of sight-threatening diabetic retinopathy in pregnancy. Eye (Lond) 2004;18:826–32. doi: 10.1038/sj.eye.6701340. [DOI] [PubMed] [Google Scholar]
- 63.Sarmad A, Lip PL. Intravitreal anti-vascular endothelial growth factor in early pregnancy and the complex management of advance diabetic retinopathy and maculopathy during pregnancy. Acta Ophthalmol. 2016;94:e812–3. doi: 10.1111/aos.13083. [DOI] [PubMed] [Google Scholar]
- 64.Rosen E, Rubowitz A, Ferencz JR. Exposure to verteporfin and bevacizumab therapy for choroidal neovascularization secondary to punctate inner choroidopathy during pregnancy. Eye. 2009;23:1479. doi: 10.1038/eye.2008.218. [DOI] [PubMed] [Google Scholar]
- 65.Tarantola RM, Folk JC, Boldt HC, Mahajan VB. Intravitreal bevacizumab during pregnancy. Retina. 2010;30:1405–11. doi: 10.1097/IAE.0b013e3181f57d58. [DOI] [PubMed] [Google Scholar]
- 66.Petrou P, Georgalas I, Giavaras G, Anastasiou E, Ntana Z, Petrou C. Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 2010;88:e136. doi: 10.1111/j.1755-3768.2009.01572.x. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan L, Kelly SP, Glenn A, Williams CP, McKibbin M. Intravitreal bevacizumab injection in unrecognised early pregnancy. Eye (Lond) 2014;28:492–4. doi: 10.1038/eye.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jouve L, Akesbi J, Nordmann JP. Safety and efficacy of ranibizumab for pregnant women in idiopathic choroidal neovascularization. Acta Ophthalmol. 2015;93:e597–8. doi: 10.1111/aos.12611. [DOI] [PubMed] [Google Scholar]
- 69.Sarhianaki A, Katsimpris A, Petropoulos IK, Livieratou A, Theoulakis PE, Katsimpris JM. Intravitreal administration of ranibizumab for idiopathic choroidal neovascularization in a pregnant woman. Klin Monatsbl Augenheilkd. 2012;229:451–3. doi: 10.1055/s-0031-1299207. [DOI] [PubMed] [Google Scholar]
- 70.Fazelat A, Lashkari K. Off-label use of intravitreal triamcinolone acetonide for diabetic macular edema in a pregnant patient. Clin Ophthalmol. 2011;5:439–41. doi: 10.2147/OPTH.S14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Concillado M, Lund-Andersen H, Mathiesen ER, Larsen M. Dexamethasone intravitreal implant for diabetic macular edema during pregnancy. Am J Ophthalmol. 2016;165:7–15. doi: 10.1016/j.ajo.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Errera MH, Kohly RP, da Cruz L. Pregnancy-associated retinal diseases and their management. Surv Ophthalmol. 2013;58:127–42. doi: 10.1016/j.survophthal.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–6. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu C, Yang H, Geng Q, Ma Q, Long Y, Zhou C, et al. Association of oxidative stress biomarkers with gestational diabetes mellitus in pregnant women: A case-control study. PLoS One. 2015;10:e0126490. doi: 10.1371/journal.pone.0126490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitamura Y, Harada C, Harada T. Role of cytokines and trophic factors in the pathogenesis of diabetic retinopathy. Curr Diabetes Rev. 2005;1:73–81. doi: 10.2174/1573399052952596. [DOI] [PubMed] [Google Scholar]
- 76.Kaaja R, Loukovaara S. Progression of retinopathy in type 1 diabetic women during pregnancy. Curr Diabetes Rev. 2007;3:85–93. doi: 10.2174/157339907780598252. [DOI] [PubMed] [Google Scholar]
- 77.Bhatnagar A, Ghauri AJ, Hope-Ross M, Lip PL. Diabetic retinopathy in pregnancy. Curr Diabetes Rev. 2009;5:151–6. doi: 10.2174/157339909788920929. [DOI] [PubMed] [Google Scholar]
- 78.Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 79.Nicolo M, Musetti D, Traverso CE. Yellow micropulse laser in diabetic macular edema: A short-term pilot study. Euro J Ophthalmol. 2014;24:885–9. doi: 10.5301/ejo.5000495. [DOI] [PubMed] [Google Scholar]
- 80.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–9. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diabetic Retinopathy Clinical Research Network. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–51. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oren D, Nulman I, Makhija M, Ito S, Koren G. Using corticosteroids during pregnancy. Are topical, inhaled, or systemic agents associated with risk? Can Fam Phys. 2004;50:1083–5. [PMC free article] [PubMed] [Google Scholar]
- 83.Degenring RF, Jonas JB. Serum levels of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004;137:1142–3. doi: 10.1016/j.ajo.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Tarara RP, Cordy DR, Hendrickx AG. Central nervous system malformations induced by triamcinolone acetonide in nonhuman primates: Pathology. Teratology. 1989;39:75–84. doi: 10.1002/tera.1420390109. [DOI] [PubMed] [Google Scholar]
- 85.Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127:1115–28. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: The RESTORE extension study. Ophthalmology. 2014;121:1045–53. doi: 10.1016/j.ophtha.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 87.American Academy of Ophthalmology- Diabetic Retinopathy- Preferred Practice Patterns. Available from: www.aaojournal.org/article/S0161-6420(19)32092-5/pdf .
- 88.Kitzmiller JL, Block JM, Brown FM, Catalano PM, Conway DL, Coustan DR, et al. Managing preexisting diabetes for pregnancy: Summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31:1060–79. doi: 10.2337/dc08-9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diabetes in pregnancy: Management from preconception to the postnatal period, NICE guidelines Published:25 February 2015. Available from: www.nice.org.uk/guidance/ng3/resources/diabetes-in-pregnancy-management-from-preconception-to-the-postnatal-period-pdf-51038446021 .
- 90.Guidelines for the prevention and management of diabetic retinopathy and diabetic eye disease in India, Version 1, June 2019, London School of hygiene and tropical medicine, The Queen Elizabeth Diamond Jubilee Trust and Indian Institute of Public Health, Hyderabad [Google Scholar]
- 91.The Royal College of Ophthalmologists, Diabetic Retinopathy Guidelines, December 2012. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-301-FINAL-DR-GUIDELINES-DEC-2012-updated-July-2013.pdf. [Google Scholar]
- 92.International Council of Ophthalmology (ICO) Guidelines for Diabetic Eye Care, updated 2017. http://www.icoph.org/downloads/ICOGuidelinesforDiabeticEyeCare.pdf. [Google Scholar]