Abstract
Purpose:
To study the profile of sight-threatening diabetic retinopathy (STDR), its association with various factors affecting it, and awareness of diabetic retinopathy (DR) among patients with diabetes mellitus (DM) attending a tertiary care center in Kashmir.
Methods:
In this prospective cross-sectional study, 625 consecutive patients with DM were assessed for STDR. Demographic/clinical data were obtained. Early treatment diabetic retinopathy study (ETDRS) criteria were used to grade fundus photographs. Severe nonproliferative DR, proliferative DR, and/or macular edema were classified as STDR. Optical coherence tomography was used to confirm the diagnosis of macular edema.
Results:
The mean age of patients was 56.36 ± 9.29 years. The male-to-female ratio was 0.92:1. The majority (99.36%) of patients had type 2 DM. STDR was seen in 208 (33.28%) patients. Non-sight-threatening diabetic retinopathy (NSTDR) was seen in 173 (27.68%) patients. Eye care was sought by 313 (50.08%) patients for the first time. STDR had a significant association with difficulty in accessing the health care facilities, duration of diabetes, uncontrolled diabetes, presence of other diabetes complications, use of insulin, and hypertension (P < 0.05 for all). Awareness that diabetes can affect eyes showed a significant association with age, gender, educational status, duration of diabetes, glycemic status, DR, and STDR (P < 0.001 for all).
Conclusion:
STDR is a common complication in diabetes and is duration- and glycemic control-dependent. Understanding the factors associated with STDR can help in making strategies for its prevention. Spreading awareness regarding STDR at the community level in the Kashmir valley is crucial in this regard.
Keywords: Awareness, diabetic retinopathy, Kashmir, profile, sight-threatening diabetic retinopathy
Diabetes mellitus (DM) imposes a tremendous burden on healthcare and economies worldwide, with projections of 552 million patients by 2030.[1] India will have approximately 80 million diabetics by 2030.[2] Uncontrolled diabetes leads to significant macrovascular and microvascular complications.[3,4]
Diabetic retinopathy (DR) is a well-known microvascular complication of diabetes.[5] Globally, DR is the commonest cause of blindness in working-age populations.[6,7] Approximately 2.6% of global blindness is attributed to DR.[8] In 2020, DR accounted for approximately 3.2 million visually impaired and blind people globally.[9] In India, the prevalence of DR ranges from 7.3% to 26.2%.[10,11,12,13,14,15]
Patients with diabetes continue to suffer from impaired visual performance before the appearance of overt damage to the retinal microvasculature and subsequent sight-threatening complications.[16] DR is associated with a longer diabetic duration, higher glycosylated hemoglobin (HbA1c), higher systolic blood pressure (SBP), lower body mass index, and raised blood urea concentration.[17]
The Early Treatment of Diabetic Retinopathy Study (ETDRS) scale is commonly used to classify DR into two stages based on the extent of microvascular damage and ischemia.[18] These are nonproliferative diabetic retinopathy (NPDR) and the more advanced, proliferative diabetic retinopathy (PDR). NPDR can be subclassified into mild, moderate, and severe NPDR. Fundus findings in the nonproliferative stage include microaneurysms, intraretinal hemorrhages, cotton wool spots, venous abnormalities, and intraretinal microvascular abnormalities. PDR is a serious condition that encompasses neovascularization of the retina, optic disc, and/or angle, and advanced eye diseases such as vitreous hemorrhage, rubeosis, and tractional retinal detachment. The ETDRS also defined clinically significant macular edema (CSME), which is caused due to vascular leakage and is a major cause of decreased vision in these patients.[19]
Sight-threatening diabetic retinopathy (STDR) comprises severe NPDR, PDR (including advanced diabetic disease), or CSME.[5] Despite therapeutic advances, the management of DR remains challenging. Newer interventional modalities include intravitreal vascular endothelial growth factor inhibitors and intravitreal steroids such as triamcinolone, dexamethasone, and fluocinolone. Steroids can be given intravitreally as injections or inserted as long-term implants.[20,21,22,23] The introduction of optical coherence tomography (OCT) and fundus fluorescein angiography (FFA) has also revolutionized the management of DR. For PDR, pan-retinal photocoagulation remains a mainstay therapy, although it is an inherently destructive procedure.[24]
A study done in India to assess awareness about diabetes reported that approximately 50% of the participants had not even heard about diabetes.[25] Increasing the awareness about diabetes complications is the first step toward the prevention of visual impairment due to diabetes and possibly also in preventing other diabetes complications.[26]
Despite DR being a common cause of visual loss in India, hardly any data is available regarding DR from Kashmir. Therefore, this study was aimed at studying the profile of STDR, its association with various factors affecting it, and awareness about the effects of diabetes on the eye in patients with DM attending a tertiary care hospital in Kashmir, India.
Methods
In this prospective cross-sectional study performed at a tertiary care hospital in Srinagar, Kashmir, from January 2020 to June 2020, 625 consecutive patients were enrolled after obtaining informed consent from the patients or their relatives. Ethical clearance was obtained from the institutional ethical clearance committee.
The sample size was estimated to be 568, assuming the prevalence of DR as 31% among diabetic patients in Kashmir, precision error of 5%, and type 1 (a) error of 1%. It was increased to 625, keeping an additional margin of 10% for the dropouts, if any. Type 1 and type 2 diabetics presenting to the Ophthalmology outpatient department for eye check-ups were included in the study. Patients with advanced glaucoma, severe uveitis, mature cataract, and eye trauma were excluded. Data anonymization was achieved by protecting private or sensitive information by erasing or encrypting identifiers that connected an individual to stored data.
During the study, after the imposition of COVID-19-related restrictions in Kashmir valley toward the end of March 2020, appropriate guidelines such as using a personal protective equipment kit by doctors, use of face mask by the patients, and maintaining a safe distance were followed for examining the patients.
A detailed clinical history was taken and demographic data were obtained. All data were recorded on a proforma. The recruited patients were evaluated by two consultants from the investigating team. A complete ophthalmologic examination was done.
Best-corrected visual acuity (BCVA) was recorded as a Snellen visual acuity (VA). For statistical purposes, Snellen visual acuities were converted to logMAR equivalents. Blindness was defined as VA of <3/60 or a corresponding visual field loss of <10° in the better eye with the best possible correction.[27] Humphrey visual field analyzer was used for assessing visual fields, with a 10-2 testing strategy. Field assessment was performed only at presentation if the vision was equal to or better than 6/60. Patients with field loss due to laser photocoagulation but VA better than 3/60 were not categorized as blind. Anterior segment examination was performed on a slit-lamp microscope. Dilated (tropicamide, 0.5%) fundus examination was done for both the eyes using 90D lenses. Fundus photographs were taken using a Carl Zeiss Visupac FF450 + fundus camera (Germany) for multiple fields using a field of view of 50°. Fundus photographs were graded independently by two experienced ophthalmologists. Refraction was performed wherever possible using a Potec autorefractometer. Intraocular pressure (IOP) was measured using Goldmann applanation tonometry. We used OCT (Carl Zeiss Cirrus 5000 SD-OCT, Germany; Scan protocol: Macular cube, 512 × 128) for the confirmation of macular edema. FFA scan was done, wherever required.
The ETDRS classifications[18] were used for the grading of severity of retinopathy. Patients were classified as having STDR or non-sight-threatening diabetic retinopathy (NSTDR). STDR was defined as severe NPDR, PDR, and/or macular edema in at least one eye.[28,29] Mild and moderate NPDR (without macular edema) were included under the heading of NSTDR.[29]
Glycated hemoglobin (HbA1c) levels were checked on the same day of presentation. Diabetes was deemed as controlled if HbA1C values were <7%. Hypertension was defined as blood pressure (BP) of ≥130/80 mm Hg for conventional office-based measurement.[30] For labeling the patient as hypertensive, history and medical records were taken into consideration. All patients undergoing surgery were subjected to an RTPCR test for COVID-19. Treatment details were recorded.
A questionnaire in the Urdu language, aimed at exploring awareness and knowledge about diabetes and DR, was designed by taking into account the dimensionality of construct, the format of the questionnaire, and items and length of the questionnaire. A preliminary pilot testing was done. The questionnaire items were revised upon reviewing their results. Questions were translated into the Kashmiri language by investigators for the patients non-fluent in the Urdu language. An English version of the questionnaire was also prepared for patients knowing only English (online supplementary file). The answers to the questionnaire by illiterate people/those not knowing the language were reinterpreted by the first author. The questionnaire was standardized to ask all participants precisely the same questions in an identical format and record responses in a uniform manner.
Statistical analysis
The statistical analysis was performed using SPSS version 22. The Student's t-test was used for comparing the normally distributed quantitative data. Chi-square test/Fisher's exact test was used for comparing the categorical data and for testing the association between different variables. P <0.05 was considered statistically significant.
Results
Baseline characteristics of patients with DM are shown in Table 1.
Table 1.
Baseline characteristics of patients with diabetes mellitus (n=625)
| Parameter | Number of patients | Percentage |
|---|---|---|
| Age | ||
| <30 years | 4 | 0.64 |
| 31-50 years | 184 | 29.44 |
| 51-70 years | 412 | 65.92 |
| >70 years | 25 | 4.00 |
| Gender | ||
| Male | 300 | 48.00 |
| Female | 325 | 52.00 |
| Residencea | ||
| Rural | 306 | 48.96 |
| Urban | 319 | 51.04 |
| Socioeconomic statusb | ||
| High | 40 | 6.40 |
| Middle | 502 | 80.32 |
| Low | 83 | 13.28 |
| Literacy statusc | ||
| Literate | 301 | 48.16 |
| Illiterate | 324 | 51.84 |
| Smoking status | ||
| Smokers | 163 | 26.08 |
| Nonsmokers | 462 | 73.92 |
| Systemic diseases | ||
| Hypertension | ||
| Present | 168 | 26.88 |
| Absent | 457 | 73.12 |
| Type of diabetes | ||
| Type 1 | 4 | 0.64 |
| Type 2 | 621 | 99.36 |
| Treatment being received | ||
| Insulin only | 84 | 13.44 |
| Combination of insulin and OHAs | 72 | 11.52 |
| Combination of diet, exercise, and drugs | 469 | 75.04 |
a Residence was defined as urban for all places with a municipality, corporation, cantonment board or notified town area committee and all other places meeting the criteria of a minimum population of 5000, at least 75 percent of the male main workers engaged in non-agricultural pursuits and a density of population of at least 400 per sq. km. All areas not categorized as the urban area were considered as rural areas; b Socioeconomic status: High (annual income >Rs. 8,50,000), middle (annual income Rs. 50,000-8,50,000) and low (annual income <Rs. 50,000); c Literate: A person was deemed as literate if he/she could read and write with understanding in any language. A person who could read but could not write was not considered literate; OHA- Oral hypoglycemic agent
The majority (99.36%) of patients had type 2 DM. The male-to-female ratio was 0.92:1. The mean (± SD) age was 56.36 (±9.29) years (age range: 20–80 years). Patients aged more than 50 years accounted for 69.92% of all diabetics. Eighty-four (13.44%) patients had diabetes for ≥10 years. Diabetes was controlled in 59.52% (n = 372) patients.
Self-referral accounted for 55.68% (n = 348) patients, while 34.08% (n = 213) were physician referrals for fundus examination for diabetes and 10.24% (n = 64) were referred by ophthalmologists in rural areas.
There was a significant association of age, gender, residence, duration of diabetes, use of insulin, uncontrolled diabetes, presence of other diabetes complications (diabetic nephropathy, neuropathy, and coronary artery disease [CAD]), and hypertension with DR (P < 0.05 for all). STDR had a significant association with difficulty in accessing the health care facilities, duration of diabetes, uncontrolled diabetes, presence of other diabetes complications (diabetic nephropathy and CAD), use of insulin, and hypertension (P < 0.05 for all) [Table 2].
Table 2.
Profile of diabetic retinopathy and factors associated with its threat to sight
| Parameter | DR (n=381) | P | STHR (n=208) | NSTHR (n=173) | P | |
|---|---|---|---|---|---|---|
|
| ||||||
| Present n=381 | Absent n=244 | |||||
| Age | ||||||
| <30 years | 4 | 0 | <0.0001**** | 4 | 0 | 0.162 |
| 31-50 years | 75 | 109 | 36 | 39 | ||
| 51-70 years | 281 | 131 | 155 | 126 | ||
| >70 years | 21 | 4 | 13 | 8 | ||
| Gender | ||||||
| Male | 204 | 96 | 0.0007*** | 110 | 94 | 0.862 |
| Female | 177 | 148 | 98 | 79 | ||
| Literacy status | ||||||
| Literate | 194 | 107 | 0.100 | 92 | 102 | 0.603 |
| Illiterate | 187 | 137 | 116 | 71 | ||
| Residence | ||||||
| Rural | 225 | 81 | <0.0001**** | 122 | 103 | 0.916 |
| Urban | 156 | 163 | 86 | 70 | ||
| Socioeconomic status | ||||||
| High | 28 | 12 | 0.117 | 15 | 13 | 0.477 |
| Middle | 296 | 206 | 166 | 130 | ||
| Low | 57 | 26 | 27 | 30 | ||
| Smoking status | ||||||
| Smokers | 89 | 74 | 0.061 | 51 | 38 | 0.626 |
| Non-smokers | 292 | 170 | 157 | 135 | ||
| Difficulty in accessing the health care facilities | ||||||
| Yes | 194 | 103 | 0.055 | 116 | 78 | 0.048* |
| No | 187 | 141 | 92 | 95 | ||
| Duration of diabetes | ||||||
| ≤10 years | 321 | 220 | 0.046* | 164 | 157 | 0.002** |
| >10 years | 60 | 24 | 44 | 16 | ||
| Use of Insulin | ||||||
| Yes | 132 | 24 | <0.0001**** | 88 | 44 | 0.0008*** |
| No | 249 | 220 | 120 | 129 | ||
| Control of diabetes | ||||||
| Controlled | 130 | 242 | <0.0001**** | 20 | 110 | <0.0001**** |
| Uncontrolled | 251 | 2 | 188 | 63 | ||
| Other diabetic complications | ||||||
| Nephropathy | ||||||
| Present | 24 | 0 | <0.0001**** | 22 | 2 | 0.0003*** |
| Absent | 357 | 244 | 186 | 171 | ||
| Neuropathy | ||||||
| Present | 23 | 0 | 0.0001*** | 17 | 6 | 0.088 |
| Absent | 358 | 244 | 191 | 167 | ||
| CAD | ||||||
| Present | 58 | 11 | <0.0001**** | 40 | 18 | 0.024* |
| Absent | 323 | 233 | 168 | 155 | ||
| Systemic diseases | ||||||
| Hypertension | ||||||
| Present | 137 | 31 | <0.0001**** | 60 | 77 | 0.002** |
| Absent | 244 | 213 | 148 | 96 | ||
DR - Diabetic retinopathy; STDR - Sight-threatening diabetic retinopathy; NSTDR - Non-sight-threatening diabetic retinopathy; CAD - Coronary artery disease
An inter-observer agreement of 92% was seen regarding grading of the fundus abnormalities. Seventy-one photographs were excluded because of poor quality.
Mild to moderate DR (without macular edema) or NSTDR was seen in 173 (27.68%) patients. STDR was seen in 208 (33.28%) patients [Fig. 1]. Table 3 shows the clinical profile of patients with STDR. Of all patients with STDR, 76 (36.54%) patients had VA of <3/60 in at least one eye. Treatment for the eyes in the form of intravitreal injections, laser, or surgery was received by 124 (59.61%) patients. Intravitreal injections of bevacizumab were planned further for 41.82% (n = 87) of these patients; 64 of these were first-time visitors. Of 253 uncontrolled diabetics, 188 (64.30%) had STDR. Of 84 patients with diabetes for ≥10 years, 44 (52.38%) had STDR. Of the remaining 541 patients with a lesser duration of diabetes, 164 (30.31%) had STDR.
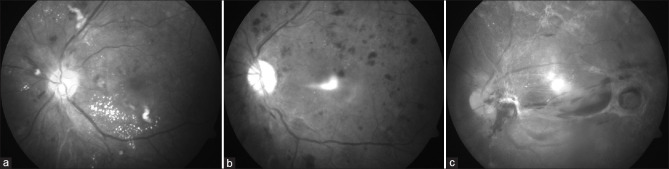
Figure 1.
Fundus photographs of patients with diabetic retinopathy. These patients were unaware of the effects of diabetes on the eye. (a) Moderate NPDR showing scattered flame-shaped hemorrhages and cotton wool spots, with exudates, present inferiorly in the perifoveal area; (b) Severe NPDR showing hemorrhages in all quadrants. An artifact is present centrally; (c) Advanced diabetic eye disease, showing extensive involvement of the posterior pole with fibrotic bands
Table 3.
Clinical profile of patients with sight-threatening diabetic retinopathy (n=208)
| Feature | Number of patients | Percentage |
|---|---|---|
| Treatment History | ||
| IVA | 75 | 36.06 |
| Laser | 25 | 12.02 |
| IVTA | 10 | 4.80 |
| Vitrectomy | 14 | 6.73 |
| Symptoms | ||
| Diminution of vision | 208 | 100 |
| Blindness | ||
| Unilateral | 61 | 29.33 |
| Bilateral | 17 | 8.17 |
| Signs | ||
| Visual Acuity in the worse eye | ||
| Severe visual impairment (6/60-3/60) | 48 | 23.08 |
| Blindness (<3/60) | 76 | 36.54 |
| *Visual Acuity in the better eye | ||
| Severe visual impairment | 34 | 16.35 |
| Blindness | 17 | 8.17 |
| Severe NPDR | 60 | 28.85 |
| PDR | 76 | 36.54 |
| Maculopathy | ||
| Present | 196 | 94.23 |
| Absent | 12 | 5.77 |
| Photocoagulation scars | 25 | 12.02 |
| Hemorrhage (Preretinal/intragel/both) | 32 | 15.38 |
| Retinal detachment | 14 | 6.73 |
| Rubeosis iridis | 6 | 2.88 |
| Other diabetic complications | ||
| Diabetic nephropathy | 22 | 10.58 |
| Diabetic neuropathy | 17 | 8.17 |
| Coronary artery disease | 40 | 19.23 |
IVA - Intravitreal Avastin injection; IVTA - Intravitreal Triamcinolone Acetonide injection; *12 patients had the same vision in both eyes; NPDR - Nonproliferative diabetic retinopathy; PDR - Proliferative diabetic retinopathy
For patients with STDR, the mean logMAR VA was 0.76 ± 0.52 in the better eye and 1.35 ± 0.83 in the worse eye. For patients with NSTDR, the mean logMAR VA in the better eye was 0.31 ± 0.28 and 0.39 ± 0.21 in the worse eye. Mean HbA1c values for STDR and NSTDR were 8.67% and 6.99%, respectively.
Difficulty in accessing health facilities was quoted by 55.76% (n = 116) of patients with STDR. There was a lack of explanation of the disease by health practitioners as per 61.92% (387/625) of respondents. These were the main reasons for late presentation to the ophthalmologist for screening for DR. Thirty-two patients with STDR experienced an improvement in vision after interventions such as intravitreal bevacizumab and vitrectomy.
Some form of DR was seen in 60.96% (n = 381) of patients. Yet, 61.28% (n = 383) patients said they were unsure whether diabetes affected eyes or they had not been advised about regular eye checkups. Of 312 regular patients, 37.82% (n = 118) were unaware that diabetes affects eyes, despite at least one visit to the ophthalmologist. Lack of knowledge regarding diabetes causing blindness was reported by 42.24% (n = 264) patients.
Of the total patients, 313 (50.08%) were seeking eye care the first time. Of these, 177 (56.55%) patients already had some form of DR, 80 (25.55%) had STDR while 282 (90.09%) were unaware if DM affected eyes. Eye care due to visual or other eye-related complaints was sought by 56.23% (n = 176) of first-time visitors; while 32.27% (n = 101) were referred for fundus examination by the treating physicians. Blood sugar levels were perceived as controlled by 465 (74.4%) patients, while 372 (59.52%) patients demonstrated controlled values of HbA1c [Table 4].
Table 4.
Awareness of diabetic retinopathy among all patients with DM (n=625)
| Parameter | Number of patients | Percentage |
|---|---|---|
| Can diabetes affect the eyes? | ||
| Yes | 225 | 36.00 |
| No | 17 | 2.72 |
| Don’t know | 383 | 61.28 |
| Do you think your diabetes is controlled? | ||
| Yes | 465 | 74.40 |
| No | 96 | 15.36 |
| Don’t know | 64 | 10.24 |
| Can diabetes cause blindness? | ||
| Yes | 206 | 32.96 |
| No | 155 | 24.80 |
| Don’t know | 264 | 42.24 |
| Do you think eye check-ups are necessary? | ||
| Yes | 608 | 97.28 |
| No | 17 | 2.72 |
| Don’t know | 0 | 0 |
Awareness that diabetes can affect eyes showed a significant association with age, gender, educational status, duration of diabetes, glycemic status, DR, and STDR (P < 0.001 for all). Awareness that diabetes can cause blindness showed a significant association with age, duration of diabetes, glycemic status, and DR (P < 0.0001 for all) [Table 5].
Table 5.
Association of various epidemiological and clinical factors with awareness regarding affection of eyes by diabetes
| Parameter | Can diabetes affect the eyes? | P | Can diabetes cause blindness? | P | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Yes | No/Don’t know | Yes | No/Don’t know | |||
| Age | ||||||
| ≤50 years | 48 | 140 | 0.0004*** | 35 | 153 | <0.0001**** |
| >50 years | 177 | 260 | 171 | 266 | ||
| Gender | ||||||
| Males | 136 | 164 | <0.0001**** | 108 | 192 | 0.141 |
| Females | 89 | 236 | 98 | 227 | ||
| Educational status | ||||||
| Literate | 148 | 153 | <0.0001**** | 104 | 197 | 0.466 |
| Illiterate | 77 | 247 | 102 | 222 | ||
| Duration of diabetes | ||||||
| ≤10 years | 160 | 381 | <0.0001**** | 158 | 383 | <0.0001**** |
| >10 years | 65 | 19 | 48 | 36 | ||
| Glycemic status | ||||||
| Controlled | 104 | 268 | <0.0001**** | 78 | 294 | <0.0001**** |
| Uncontrolled | 121 | 132 | 128 | 125 | ||
| DR | ||||||
| Present | 177 | 204 | <0.0001**** | 164 | 217 | <0.0001**** |
| Absent | 48 | 196 | 42 | 202 | ||
| Type of DR | ||||||
| STDR | 97 | 111 | <0.0001**** | 120 | 88 | 0.146 |
| NSTDR | 128 | 45 | 86 | 87 | ||
DR - Diabetic retinopathy; STDR - Sight-threatening diabetic retinopathy; NSTDR - Non-sight-threatening diabetic retinopathy
Discussion
We observed that 60.96% of diabetic patients had DR, 27.68% had NSTDR, and 33.28% had STDR. Previous data from India has focused on prevalence patterns in the community setting, mainly in the southern and western states. A major pan-India study estimated the prevalence of DR in India at 21.7% and found positive associations between diabetes and male gender, duration of diabetes more than 5 years, age above 40 years, use of insulin, and history of vascular accidents.[31] A higher frequency of DR among our diabetic patients is due to methodical differences as we determined DR/STDR in patients visiting the hospital for ophthalmic complaints while prevalence has been calculated in the aforementioned study.
In our study, despite a high prevalence of DR, the risk factors associated with DR were the same. A significant association between DR and other diabetes complications such as neuropathy, nephropathy, and CAD is in agreement with the abovementioned study,[31] which is further supported by a significant association of STDR with other diabetes complications such as nephropathy and CAD. Using DR to predict the onset of other diabetes complications or vice versa was not possible based on our study findings as it was only a cross-sectional study. However, DR has been reported as an independent risk factor for cardiovascular diseases and cardiovascular mortality in previous studies.[32]
Like the Chennai Urban Rural Epidemiology Study (CURES)[11] and the UK Prospective Diabetes Study (UKPDS),[33] we found a significant association between DR and poor glycemic control. Duration of diabetes was significantly associated with the development of DR (P = 0.046), as also seen in another study.[34] It implies that poor glycemic control leads to the development of DR that can worsen with time.
We also observed a significant association between coexisting hypertension and DR as well as STDR, which highlights the importance of strict control of blood pressure in diabetics. Due to coexisting hypertension and diabetes in a significant number of patients, the presence of a significantly higher number of patients with DR than without DR and a higher number of patients with STDR than with NSTDR imply that the presence of hypertension in diabetic patients increases the risk of developing DR as well as loss of vision due to DR. This supports the findings of the UKPDS that aggressive BP control decreased the development of DR and subsequent blindness than less aggressive BP control.[35] As such, hypertension needs to be paid adequate attention in diabetic patients as its inadequate control may accelerate the rate of loss of vision due to DR.
Shah et al.[36] observed DR in 65% of around 6000 diabetics, NPDR in 28.58%, and PDR in 19.51% diabetics. Risk factors for DR observed by them, such as male gender, age >40 years, smoking, hypertension, poor glycemic control, and reluctance in using insulin, were also observed in our study except for smoking. Aggarwal et al.[37] reported NPDR in 79.8% of patients and PDR in 14.6% of patients in a hospital-based study like ours.
Sapkota et al.[29] observed at a specialist eye clinic in China that among the patients who significantly delayed the treatment for DR, 80% of patients had STDR and patients presented with late-stage retinopathy with vision loss. Our figures of 208 (54.59%) patients having STDR out of 381 patients with DR are less in comparison.
Possible reasons for a high proportion of STDR in our study, as compared to community studies,[10,11,12,13,14,15] could be that our cohort was hospital-based, as people with diminution of vision were more likely to report to the hospital. Difficulty in accessing health facilities could be a major reason for the late presentation of patients with DR, besides other factors such as illiteracy, low socioeconomic status, and lack of explanation by the physician regarding the need for proper control of blood sugar. In addition, the inclusion of severe NPDR in our definition of STDR could have increased the number of patients with STDR. Moreover, macular edema was responsible for a large number of patients with STDR.
Given that a majority of patients showed a lack of awareness about diabetes complications, it is vital that patients be informed about the same during the early stages of the disease. A significant association of awareness regarding eyes being affected by diabetes with age, gender, educational status, duration of diabetes, glycemic status, DR, and STDR (P < 0.001 for all) is supported by another study from Jordan, which showed a significant association between awareness of DR and variables such as gender, education, literacy, and blood glucose control.[38] However, a significantly higher number of patients having awareness about the possibility of eyes being affected by diabetes in the DR group implies that these patients probably developed some awareness after having suffered from DR with or without loss of vision and not because they were more health-conscious.
The same logic applies to the presence of a higher number of diabetic patients having the awareness that diabetes can cause blindness among patients with advancing age, increasing duration of diabetes, uncontrolled glycemic status, and DR; this warrants a strong emphasis on proper control of diabetes.[29,39] Therefore, promoting the awareness and knowledge regarding the development of DR due to uncontrolled diabetes among diabetic patients can help in preventing the development of DR by motivating diabetic patients to ensure proper control of diabetes. Our findings also underscore the need for systematic screening of diabetics by ophthalmologists in time, preferably at diagnosis. In our study, 90% of first-time visitors did not know whether diabetes affected eyes. More worryingly, 37.82% of diabetics who had earlier visited the ophthalmologist were also unaware of eye-related complications from diabetes. A large part of our cohort was uneducated; this highlights the importance of awareness campaigns at the public health level. Patients should be encouraged to visit the ophthalmologist regularly according to need and as per recommendations.[40,41] This can be an effective measure toward prevention of DR as early diagnosis and early treatment for retinopathy can reduce the incidence of severe loss of vision in a high percentage of patients with STDR.[6,42] As such, early screening for DR with an efficient and scalable method is highly needed to reduce blindness,[43] which can be achieved only by promoting awareness and knowledge regarding diabetes and its sight-threatening complications at the community level, particularly among patients with diabetes.
Strengths and weaknesses
Our study is the first study assessing the awareness of DR among patients with DM from the Kashmir region. As our study was hospital-based, our data give a representative picture of the profile of DR and its awareness in the region, though it may be slightly biased for the community. Alcohol use was not evaluated for its association with DR for the threat to sight as alcohol is rarely consumed in the region on religious grounds.
Conclusion
STDR is a common complication of diabetes and is duration- and glycemic control-dependent. Understanding the factors associated with STDR can help in making strategies for its primary as well as secondary prevention in the Kashmir region. Spreading awareness regarding STDR at the community level is crucial in this regard.
Research ethics and patient consent
The study was in accordance with the ethical standards of the responsible committee on human experimentation (institutional) and with the Helsinki Declaration of 1964, as revised in 2013 and was approved by the ethical clearance committee of Govt. Medical College, Srinagar, Jammu and Kashmir, India. Written informed consent was taken from all patients for their participation in the study.
Financial support and sponsorship
Treatment of all patients in SMHS Hospital, Srinagar is borne by the Jammu and Kashmir Government, India, and the study did not require any special funding.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are thankful to Prof. R. M. Kaushik for his valuable suggestions during the preparation of the manuscript.
Supplementary file
Questionnaire for testing awareness regarding Diabetic retinopathy
Date:
Patient No.: _________
1. Sex: Male/Female
2. Age: ________
3. How long had you had diabetes for?
Up to 10 years
10–15 years
≥15 years
4. What type of diabetes do you have?
Type 1
Type 2
Don’t know
5. How do you control your diabetes?
Using non-insulin treatment (Diet only/tablets/or both combined)
Using insulin treatment (insulin only or combining insulin with tablet or diet)
6. What is your literacy level?
Able to read and write in Urdu
Unable to read and write in Urdu
Able to converse only in Kashmiri or English
7. Do you smoke?
Yes
No
8. Do you think your diabetes is controlled?
Yes
No
Don’t know
9. Can diabetes affect the eyes?
Yes
No
Don’t know
10. Can diabetes cause blindness?
Yes
No
Don’t know
11. Do you think eye checkups are necessary?
Yes
No
Don’t know
12. Have you attended a diabetic eye examination previously?
Yes
No
Not sure
13. Have you had any treatment in the eye other than glasses (e.g. surgery and LASER) as a result of diabetes?
Yes
No
Not sure
14. Who referred you for ophthalmological treatment?
Self
Physician
Ophthalmologist in periphery
References
- 1.Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational DM: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(Suppl 3):S173–211. doi: 10.1016/S0020-7292(15)30033-3. [DOI] [PubMed] [Google Scholar]
- 2.Pandey SK, Sharma V. World diabetes day 2018: Battling the emerging epidemic of diabetic retinopathy. Indian J Ophthalmol. 2018;66:1652–3. doi: 10.4103/ijo.IJO_1681_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in DM: Distinct or continuum? Indian J Endocrinol Metab. 2016;20:546–51. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53(5 Suppl):S35–42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 6.Valpuesta Martin Y, Pacheco Callirgos GE, Maroto Martín TM, Piriz Veloso M, Hernández Santamaría S, López Gálvez MI. Satisfaction of patients and primary care professionals with a teleophthalmology-based screening programme for diabetic retinopathy in a rural area in Castilla y León, Spain. Rural Remote Health. 2020;20:5180. doi: 10.22605/RRH5180. [DOI] [PubMed] [Google Scholar]
- 7.Bu Y, Shih KC, Kwok SS, Chan YK, Lo AC, Chan TCY, et al. Experimental modeling of cornea wound healing in diabetes: Clinical applications and beyond. BMJ Open Diabetes Res Care. 2019;7:e000779. doi: 10.1136/bmjdrc-2019-000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, et al. Causes of vision loss worldwide, 1990-2010: A systematic analysis. Lancet Glob Health. 2013;1:e339–49. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 9.Oh K, Kang HM, Leem D, Lee H, Seo KY, Yoon S. Early detection of diabetic retinopathy based on deep learning and ultra-wide-field fundus images. Sci Rep. 2021;11:1897. doi: 10.1038/s41598-021-81539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care. 2014;2:e000005. doi: 10.1136/bmjdrc-2013-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: The Chennai urban rural epidemiology study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 12.Namperumalsamy P, Kim R, Vignesh TP, Nithya N, Royes J, Gijo T, et al. Prevalence and risk factors for diabetic retinopathy: A population-based assessment from Theni District, South India. Postgrad Med J. 2009;85:643–8. doi: 10.1136/bjo.2008.147934. [DOI] [PubMed] [Google Scholar]
- 13.Narendran V, John RK, Raghuram A, Ravindran RD, Nirmalan PK, Thulasiraj RD. Diabetic retinopathy among self-reported diabetics in Southern India: A population-based assessment. Br J Ophthalmol. 2002;86:1014–8. doi: 10.1136/bjo.86.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dandona L, Dandona R, Naduvilath TJ, McCarty CA, Rao GN. Population-based assessment of diabetic retinopathy in an urban population in Southern India. Br J Ophthalmol. 1999;83:937–40. doi: 10.1136/bjo.83.8.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradeepa R, Anitha B, Mohan V, Ganesan A, Rema M. Risk factors for diabetic retinopathy in a South Indian type 2 diabetic population –The Chennai urban rural epidemiology study (CURES) eye study 4. Diabet Med. 2008;25:536–42. doi: 10.1111/j.1464-5491.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 16.Berkowitz BA. Preventing diabetic retinopathy by mitigating subretinal space oxidative stress in vivo. Vis Neurosci. 2020;37:E002. doi: 10.1017/S0952523820000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Wei WB, Yuan MX, Yuan SY, Wan G, Zheng YY, et al. Prevalence and risk factors for diabetic retinopathy: The Beijing Communities Diabetes Study 6. Retina. 2012;32:322–9. doi: 10.1097/IAE.0b013e31821c4252. [DOI] [PubMed] [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study Research Group. Early treatment diabetic retinopathy study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98:741–56. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 19.Musat O, Cernat C, Labib M, Gheorghe A, Toma O, Zamfir M, et al. Diabetic macular edema. Rom J Ophthalmol. 2015;59:133–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Cai S, Bressler NM. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Recent clinically relevant findings from DRCR.net Protocol T. Curr Opin Ophthalmol. 2017;28:636–43. doi: 10.1097/ICU.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 21.Diabetic Retinopathy Clinical Research Network. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–51. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–35.e2. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Ophthalmology. Diabetic retinopathy preferred practice pattern guideline updated 2019. [Last accessed on 2021 Jul 30]. Available from: https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp .
- 25.Deepa M, Bhansali A, Anjana R, Pradeepa R, Joshi S, Joshi P, et al. Knowledge and awareness of diabetes in urban and rural India: The Indian Council of Medical Research India Diabetes Study (Phase I): Indian Council of Medical Research India Diabetes 4. Indian J Endocrinol Metabol. 2014;18:379–85. doi: 10.4103/2230-8210.131191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satapathy J, Minj A, Chakrabarti K, Gupta P. Knowledge, awareness and prevalence of diabetic retinopathy among patients of type 2 DM on their first visit to eye department in a tertiary health care center-A hospital-based cross-sectional study. J Evid Based Med Healthc. 2020;7:1770–5. [Google Scholar]
- 27.World Health Organization (WHO) International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD10) WHO Version for Internet. 2016. [Last accessed on 2019 Mar 03]. Available from: http://apps.who.int/classifications/icd10/browse/2016/en#/H54 .
- 28.Ong GL, Ripley LG, Newsom RS, Cooper M, Casswell AG. Screening for sight-threatening diabetic retinopathy: Comparison of fundus photography with automated color contrast threshold test. Am J Ophthalmol. 2004;137:445–52. doi: 10.1016/j.ajo.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Sapkota R, Chen Z, Zheng D, Pardhan S. The profile of sight-threatening diabetic retinopathy in patients attending a specialist eye clinic in Hangzhou, China. BMJ Open Ophthalmol. 2019;4:e000236. doi: 10.1136/bmjophth-2018-000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy in India: The All India Ophthalmological Society diabetic retinopathy eye screening study 2014. Indian J Ophthalmol. 2016;64:38–44. doi: 10.4103/0301-4738.178144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce I, Simó R, Lövestam-Adrian M, Wong DT, Evans M. Association between diabetic eye disease and other complications of diabetes: Implications for care. A systematic review. Diabetes Obes Metab. 2019;21:467–78. doi: 10.1111/dom.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 34.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649–56. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 36.Shah K, Gandhi A, Natarajan S. Diabetic retinopathy awareness and associations with multiple comorbidities: Insights from DIAMOND study. Indian J Endocrinol Metabol. 2018;22:30–5. doi: 10.4103/ijem.IJEM_240_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggarwal RP, Ranka M, Beniwal R, Gothwal SR, Jain GC, Kochar DK, et al. Prevalence of diabetic retinopathy in type 2 diabetes in relation to risk factors: Hospital-based study. Int J Diab Dev Ctries. 2003;23:16–9. [Google Scholar]
- 38.Bakkar MM, Haddad MF, Gammoh YS. Awareness of diabetic retinopathy among patients with type 2 DM in Jordan. Diabetes Metab Syndr Obes. 2017;10:435–41. doi: 10.2147/DMSO.S140841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garoma D, Merga H, Hiko D. Determinants of diabetic retinopathy in Southwest Ethiopia: A facility-based case-control study. BMC Public Health. 2020;20:503. doi: 10.1186/s12889-020-08652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care: The International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125:1608–22. doi: 10.1016/j.ophtha.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, et al. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40:412–8. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kashim RM, Newton P, Ojo O. Diabetic retinopathy screening: A systematic review on patients’ non-attendance. Int J Environ Res Public Health. 2018;15:157. doi: 10.3390/ijerph15010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Shi J, Peng Y, Zhao Z, Zheng Q, Wang Z, et al. Artificial intelligence-enabled screening for diabetic retinopathy: A real-world, multicenter and prospective study. BMJ Open Diabetes Res Care. 2020;8:e001596. doi: 10.1136/bmjdrc-2020-001596. [DOI] [PMC free article] [PubMed] [Google Scholar]