Abstract
Purpose:
To determine the relationship between diabetic retinopathy (DR) and diabetic peripheral neuropathy (DPN), and their associated risk factors.
Methods:
We conducted a cross-sectional analysis on 500 patients who attended the Endocrinology department at a quaternary health care center in Kerala between November 2017 and April 2018. Patients above the age of 30 years with type 2 diabetes mellitus (DM) were included. They underwent a detailed medical history, dilated fundus examination for DR, assessment and grading of DPN, and blood investigations. Among these, 49 randomly selected patients without DR had peripapillary retinal nerve fiber layer (RNFL) and ganglion cell inner plexiform layer (GCIPL) assessed by optical coherence tomogram. RNFL and GCIPL changes in different grades of neuropathy were evaluated.
Results:
Out of 500 patients, 303 (60.6%) were males and 197 (39.4%) were females. Prevalence of DR was 48% and DPN 71.8%. Risk factors for the development of DR included duration of DM >15 years, HbA1c (glycated hemoglobin) greater than 6.5%, serum creatinine more than 1.5 mg/dl, and the presence of DPN. There was a statistically significant association between DR and DPN. There was significant thinning of GCIPL in patients with moderate to severe neuropathy without DR.
Conclusion:
There is a significant association between DR and DPN and their severities. There are early changes in inner retinal layers of diabetic patients without microvascular changes of DR. These neurodegenerative changes parallel DPN in the course of DM. Our study stresses the importance of multidisciplinary approach in the management of diabetes and its complications.
Keywords: Diabetic neuropathy, diabetic retinopathy, microvasculopathy, neurodegeneration
Diabetes mellitus (DM) is one of the biggest healthcare problems in the world. The disease is strongly associated with microvascular and macrovascular complications, causing coexisting diabetic retinopathy (DR), diabetic peripheral neuropathy (DPN), and diabetic nephropathy. Diabetic retinopathy, which was previously considered to be only a microvascular complication of diabetes, has recently been found to have a neurodegenerative component as well.[1] DPN is defined as peripheral nerve dysfunction in a patient with DM in whom other causes of peripheral nerve dysfunction have been excluded.[2] DPN accounts for hospitalization more frequently than any other complication of DM and is the most frequent cause of nontraumatic amputation.[2] The clinical classification systems in DR are solely based on the retinal microvascular changes, and all the treatment modalities currently available in DR target vascular pathology. Neuropathy is now considered a part of DR but is still not included in classifications. However, the recent evidence for neurodegeneration is prompting the introduction of neuroprotective treatments to reduce the damage to the retina in DR.[3]
The International Clinical DR Severity scale is based solely on microvascular changes. DR is graded into mild nonproliferative diabetic retinopathy (NPDR), moderate NPDR, severe NPDR, and proliferative diabetic retinopathy (PDR).[4] However, in patients who have no retinopathy or even mild DR, retinal neurodegenerative changes have been described. These neurodegenerative changes include apoptosis of several populations of retinal cells with consequent reduction in thickness.[5]
DPN may be mild with only slight subjective sensory complaints, or severe with autonomic disturbances and painful dysesthesias. Several tools have been developed to quantify sensory thresholds. The vibration perception threshold (VPT) expressing large fiber function can be examined with a biothesiometer and is known to be affected early in DPN.[6,7] The wide measurement range and the excellent reliability makes biothesiometer a valuable research tool to quantify the loss of sensation. Biothesiometer measurements are not affected by the acute metabolic control as indicated by high HBA1c values.[8] VPT results are not significantly affected by the presence of foot callus or by limb temperature.[9]
We aimed to study the relationship between DR and DPN. We also aimed to elucidate the risk factors and find a time course of DPN and DR in the course of diabetes. We tried to evaluate the relationship between diabetic retinal neurodegeneration and diabetic peripheral neuropathy.
Methods
This is a cross-sectional study on 500 patients who attended the Endocrinology department at a quaternary health care center in Kerala between November 2017 and April 2018. Patients with type 2 diabetes mellitus above the age of 30 years were included in the study. Individuals with peripheral neuropathy due to causes other than diabetes were excluded. Enrolled patients underwent a detailed medical history including duration of DM, history of hypertension or any other comorbidities, neuropathic symptoms, history of smoking and alcoholism, dilated fundus examination to determine and grade DR, and assessment of diabetic peripheral neuropathy. Blood investigations including HbA1c (glycated hemoglobin), creatinine, and serum cholesterol levels were recorded.
Diabetic retinopathy grading was done by a single ophthalmologist using the International Clinical DR Severity scale.[4] When two eyes of one patient had different diabetic retinopathy stages, the more severe stage was allocated to the subject.
Neuropathy assessment
Neuropathy assessment was done by biothesiometry (vibration perception threshold). This technique is a simple, sensitive, noninvasive, and comfortable method for daily screening of neuropathies with impaired vibration perception threshold.[6] The biothesiometer is an instrument that measures the threshold of appreciation of vibration sense. It is held perpendicular to the skin and rested with its own weight at the site of examination. The foot is placed flat upon a foam cushion. Examination is done on the marked points as shown in Fig. 1 on the plantar aspect, dorsum of the foot, and ankle. Subjects are first familiarized with the sensation. The amplitude of the stimulus (measured in V) is gradually increased until the threshold of vibratory sensation is reached and the stimulus is perceived by the patient. The VPT is determined at each site in succession. The average value of VPT at all sites is taken as the perception threshold. It has 80% sensitivity and 98% specificity for detection of neuropathy.[9] By biothesiometer, a score of 0–15 is no neuropathy, 16–20 is mild, 21–25 is moderate neuropathy, and >25 is severe neuropathy.[10]
Figure 1.
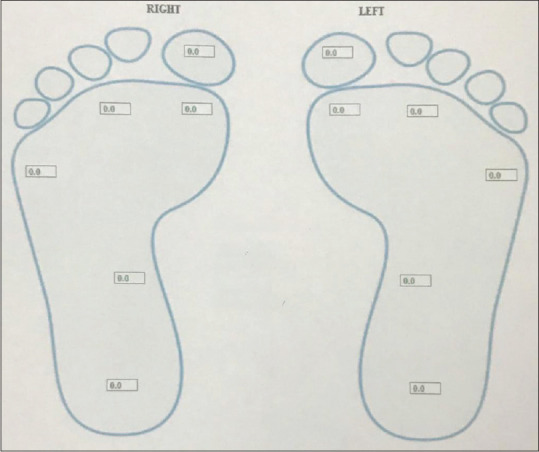
Sites of VPT testing on the plantar aspect of foot by biothesiometer
Relationship between DR and DPN and their risk factors were assessed in these patients. Data collected included age, gender, history of smoking, alcoholism, duration of diabetes, blood pressure, Hb A1c, serum creatinine, and serum cholesterol.
Apart from this, we studied the peri-papillary RNFL (retinal nerve fiber layer) and macular GCIPL (ganglion cell inner plexiform layer) in 49 randomly selected patients without DR with visual acuity of 20/30 or better and clear media. Tomographic images were obtained using the Cirrus HD-OCT (Carl Zeiss, Meditec Dublin, CA, USA) after pupillary dilation by a single, well-trained technician. Patients who had a healthy optic nerve, good view of the retina, and had given consent for further evaluation in the retina clinic for visual acuity and OCT were selected. Those patients with any maculopathy or macular edema, glaucoma or optic disc/optic nerve diseases, or any other retinal conditions and high refractive errors were excluded. RNFL and GCIPL changes in different grades of diabetic neuropathy were analyzed.
The GCIPL maps were based on macular protocol centered on fovea with automated measurement of GCIPL. Given that OCT can detect diabetes-related compromise in multiple layers of the retina, we sought to assess the changes in peripapillary RNFL and macular GCIPL in various grades of DPN even before DR changes set in. For the purpose of analysis of these 49 patients, patients without DPN and mild DPN were grouped together, and moderate and severe DPN were grouped together, and the right eye (dominant side) of our patients were chosen for analysis.
Statistical analysis
The analysis was performed in SPSS v. 22. Baseline characteristics of continuous variables were summarized as mean with standard deviation and categorical variables were reported as frequency with percentage. Diabetic retinopathy and diabetic neuropathy were taken as the main outcome variables. Other outcome variables were severe NPDR/PDR and severe DPN. Association of baseline variables with outcome variables was assessed by Chi-square test. Univariate and multivariate logistic regression were used to find the risk factor association with these outcome variables separately. The risk factors were reported using the odds ratio and 95% confidence interval and P. Multivariate analysis was performed on those variables that were found to be significant at 5% level in the univariate analysis. To know the statistically significant association between GCIPL and vibration perception threshold, Karl Pearson correlation was used.
Results
Out of 500 patients, 303 (60.6%) were males and 197 (39.4%) females. Mean age of the study group was 58.89 ± 10.57 years. Mean duration of DM was 14.19 ± 8.40 years. Most of the patients (89.9%) had uncontrolled blood sugars (HbA1c >6.5%). Diabetic foot ulcer was present in 34 (6.8%) patients. All patients with diabetic foot ulcers had severe neuropathy. Systolic hypertension (>140 mm Hg) was seen in 25.3% and diastolic hypertension (>80 mm Hg) in 26.9%. Abnormal total cholesterol (>200 mg/dl) was seen in 21.8% of patients. The demographics, clinical, and laboratory characteristics of the patients are given in Table 1.
Table 1.
Demographic, clinical, and laboratory characteristics of study patients
| Variables | Number (%) | |
|---|---|---|
| Age | <40 years | 268 (53.6) |
| >40 years | 232 (46.4) | |
| Gender | Male | 303 (60.6) |
| Female | 197 (39.4) | |
| Duration of DM* | <15 years | 254 (50.8) |
| 15-25 years | 199 (39.8) | |
| >25 years | 47 (9.4) | |
| HbA1C† | 6.5 and less | 46 (10.1) |
| >6.5 | 410 (89.9) | |
| Creatinine | <1.5 mg/dl | 408 (90.7) |
| >1.5 mg/dl | 42 (9.3) |
*DM - Diabetes mellitus, †HbA1c - Glycated hemoglobin
Prevalence of DR was 48% and DPN was 71.8%. Prevalence of DPN was found to be 1.5 times that of DR [Table 2]. Univariate regression analysis of patients with DR and without DR showed age more than 40 years, duration of DM >15 years, HbA1c >6.5%, serum creatinine >1.5 mg/dl and DPN to be significantly associated with DR. In patients with DM for more than 25 years, 80.90% had DR. Multivariate regression analysis showed duration of DM, [15–25 years; (OR: 1.91; 95% confidence interval (CI): 1.22–3.00; P - 0.005 or p < 0.05)], [ >25 years; (OR: 3.94; 95% CI: 1.62–9.58; P - 0.003 or p < 0.05)], HbA1c above 6.5% (OR: 3.67; 95% CI: 1.57–8.60; P - 0.003 or p < 0.05), serum creatinine >1.5 mg/dl (OR: 2.34; 95% CI: 0.99–5.52; P - 0.052 or p < 0.05), and DPN (OR: 3.31; CI: 2.97–5.58; P < 0.001) to be risk factors for DR [Table 3].
Table 2.
Prevalence and grading of DR and DPN
| Grading‡ | Number (%) | |
|---|---|---|
| DR* | No | 260 (52) |
| Mild | 81 (16.2) | |
| Moderate | 92 (18.4) | |
| Severe | 53 (10.6) | |
| PDR | 14 (2.8) | |
| DPN† | No | 141 (28.2) |
| Mild | 99 (19.8) | |
| Moderate | 58 (11.6) | |
| Severe | 202 (40.4) |
*DR - diabetic retinopathy. †DPN - diabetic peripheral neuropathy. ‡DR grading based on the International Clinical Diabetic Retinopathy Severity scale
Table 3.
Multivariate regression analysis showing risk factors for DR
| Variables | OR* (95 CI†) | P | |
|---|---|---|---|
| Duration of DM‡ | 15-25 years | 1.91 (1.22-3.00) | 0.005 |
| >25 years | 3.94 (1.62-9.58) | 0.003 | |
| HbA1c§ | >6.5% (Abnormal) | 3.67 (1.57-8.60) | 0.003 |
| Creatinine | >1.5 mg/dl (Abnormal) | 2.34 (0.99-5.52) | 0.052 |
| DPN|| | Yes | 3.31 (2.97-5.58) | <0.001 |
| Risk factors for severe NPDR¶ and PDR** | |||
| Duration of DM | 15-25 years | 2.25 (1.13-4.50) | 0.021 |
| >25 years | 2.38 (0.92-6.11) | 0.072 | |
| DPN | Yes | 8.37 (1.970-35.99) | 0.004 |
*OR - Odds ratio, †CI - confidence interval, ‡DM - diabetes mellitus, §HbA1c - glycated hemoglobin, ||DPN - diabetic peripheral neuropathy, DR - diabetic retinopathy, ¶NPDR - nonproliferative diabetic retinopathy, **PDR - proliferative diabetic retinopathy
In patients with severe NPDR and PDR, duration of DM above 15 years, HBA1c greater than 6.5%, creatinine >1.5 mg/dl, and presence of DPN were found to have significant association on univariate analysis. Duration of DM [15–25 years (OR: 2.25; 95% CI: 1.13–4.50; P - 0.021 or p < 0.05)] and DPN (OR: 8.37; 95% CI: 1.97–35.99; P - 0.004 or p < 0.05) were the risk factors for severe NPDR/PDR on multivariate analysis [Table 3].
Multivariate analysis comparing patients with and without DPN showed age more than 60 years (OR: 7.80; CI: 2.99–20.33; P < 0.001), duration of DM >15 years (OR: 1.71; CI: 1.02–2.85; P- 0.041 or p < 0.05), DM >25 years (OR: 5.34; CI: 1.17–24.46; P - 0.031 or p < 0.05), and presence of DR (OR: 3.70; CI: 2.30–6.14; P < 0.001) to be significantly associated with DPN. Risk factors for severe DPN included duration of DM 15–25 years (OR: 2.32; CI: 1.50-3.62; P < 0.001), DM >25 years (OR: 4.13; CI: 1.94–8.82; P < 0.001), creatinine levels more than 1.5 mg/dl (OR: 2.10; CI: 0.99–4.44; P - 0.053 or p < 0.05), and presence of DR (OR: 2.77; CI: 1.80–4.25; P < 0.001).
Seventy-eight percent of patients without DPN had no DR and 22% had DR [Fig. 2], but none had PDR. In patients with severe DPN, 32.7% of patients did not have DR.
Figure 2.
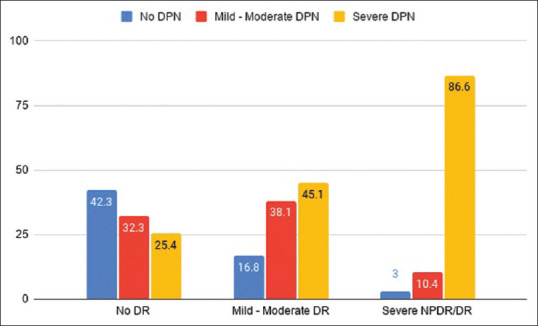
Prevalence and grading of DPN in DR. The association between DR and DPN is statistically significant (P < 0.001)
Only 3% of patients in the severe NPDR and PDR group did not have DPN [Fig. 2] and 86.6% of severe NPDR and PDR group had severe DPN [Fig. 2]. DPN was present in all patients with PDR. Over 25% of patients without DR had severe DPN. There was a significant association of DR with DPN with 86% of patients with DR having DPN (P < 0.001). More than 80% of patients with mild to moderate NPDR had DPN and 58% of patients with no DR had DPN. However, only 21% of patients without DPN had DR and 54% of patients with DPN had DR. Severity of DR increased as the severity of DPN increased and vice versa and there was a significant association between DR and DPN (P < 0.001) [Figs. 2 and 3].
Figure 3.
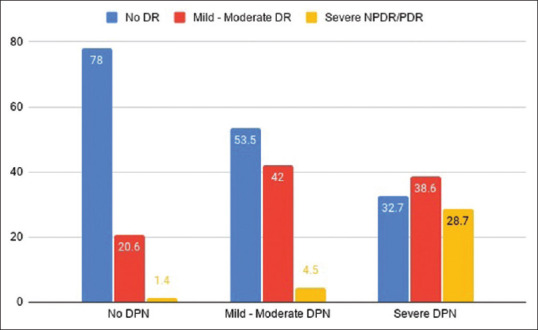
Prevalence and grading of DR in DPN. The association between DR and DPN is statistically significant (P < 0.001)
OCT was done in 49 patients without DR in different grades of DPN. Mean age of 49 patients who underwent OCT was 56.39 ± 8.84 years. Mean duration of DM was 14.7 years. Males constituted 24 (48.97%) and females 25 (51.03%). None of the patients had DR. There was no DPN in 14 (28.58%) patients, mild DPN in 10 (20.40%), moderate DPN in 9 (18.37%), and severe DPN in 16 (32.65%) patients. There was significant thinning of GCIPL and inferior GCIPL in moderate and severe DPN compared to patients with mild and no DPN but no significant difference in RNFL thickness [Table 5]. No significant change in GCIPL or RNFL thickness was found with age, sex, or duration of DM. There was statistically significant negative correlation between neuropathy score by VPT and average GCIPL (r = −0.332, P < 0.020), GCIPL inferior (r = −0.337, P < 0.021), and superior GCIPL (r = −0.373, P < 0.010).
Table 5.
GCIPL and RNFL changes in different stages of DPN without DR
| Variable | Mild and less DPN (Mean±SD)(µm) | Moderate and severe DPN (Mean±SD) (µm) | P |
|---|---|---|---|
| GCIPL Average* | 81.77±9.62 | 75.54±7.68 | 0.016 |
| GCIPL Superior* | 81.96±13.82 | 75.08±11.61 | 0.070 |
| GCIPL Inferior* | 80.44±7.50 | 72.88±11.79 | 0.013 |
| RNFL Average† | 88.64±10.11 | 85.50±10.14 | 0.291 |
*GCIPL - ganglion cell inner plexiform layer, †RNFL- retina nerve fiber layer. Significant P value is shown in bold
Discussion
Diabetes mellitus is a long-standing disease with vascular as well as neural complications that occur with time. DR and DPN are the two most common complications of DM. DR has long been recognized as a microvascular disease and the diagnosis relies on microvascular changes.[11,12] Pericyte loss, apoptosis of endothelial cells, and thickening of the basement membrane collectively contribute to the impairment of the blood–retinal barrier, capillary occlusion, and ischemia.[13] The pathophysiology of DPN is multifactorial and is thought to result from vascular disease occluding the vasa nervorum, endothelial dysfunction, deficiency of myoinositol-altering myelin synthesis and diminishing sodium-potassium adenine triphosphatase (ATPase) activity, chronic hyperosmolarity causing edema of nerve trunks, and effects of increased sorbitol and fructose.[14] Retina is a unique area in the body where we can qualitatively and quantitatively measure vascular and neural pathologies. So far, diabetic retinopathy has been graded in severity based only on the vascular changes, neural changes in diabetic retina are not taken into consideration. The purpose of our study was to evaluate neuropathic as well as vascular changes in DM by looking at the time course of DR and DPN and the neurodegenerative changes in retina that can happen in patients without DR in different stages of DPN.
The mean duration of diabetes among the 500 patients was 14.19 years. By this time, there were 48% who had DR and 71.8% who had DPN. The prevalence of DPN was 1.5 times that of DR in our study group. There was a strong odds of developing DPN with the duration of diabetes (5.34) as compared to DR (3.94) in our study. These show that DPN is more common than retinopathy as the duration of DM increases. A similar study done in a diabetic clinic by Abdollahi et al.[15] showed a prevalence of 73% and 72% for DR and DPN, respectively, at a mean duration of 12.8 years. The prevalence of DR and DPN was equal in this study. Unlike our study, neuropathy symptoms and change (NSC) questionnaire was used to diagnose DPN, and severity of DPN was not assessed. In their study, 90.9% of patients with PDR had DPN; and 27.8% of patients with DPN had PDR. In our study, all the patients with PDR had DPN and 3.8% of patients with DPN had PDR. Sharma et al.[16] did a study on 100 DM patients with a duration of more than 5 years in ophthalmology clinics and camps, where the prevalence of DR and DPN were 27% and 56%, respectively. Prevalence of DPN was approximately twice that of DR and retinopathy increased 2.75 times in patients with neuropathy (37%) than in patients without DPN (14%).[16]
While 86% of patients with DR and 100% with PDR had DPN, only 57% of patients with DPN and 67.3% of severe DPN had DR in our study. Severity of DR increased as the severity of DPN increased and vice versa [Fig. 3]. Furthermore, 28.7% of patients with severe DPN had severe NPDR/PDR [Fig. 3]. There was no PDR in patients without neuropathy. There were only 1.4% of patients with severe NPDR who did not have DPN [Fig. 3]. In contrast, all PDR except one had severe DPN. The odds of severe NPDR and PDR in DPN was 8.37 but the odds of severe DPN in DR was 2.77. Our results suggest that the presence of DPN may be an indicator of making follow-up of DR more frequent to prevent and detect complications and manage them earliest. A study done by Hwang et al.[17] comparing the occurrence of DR in the presence and absence of diabetic foot ulcer (DFU), which is a severe stage of DPN, showed 4.5% DR in the absence of DFU and 90% in the presence of DFU. Only 0.6% had PDR in the no DFU group and 55% in the DFU group. However, this study did not assess and grade DPN in the no DFU group. Our results suggest that there is a significant number of patients without DR in the presence of DPN, which suggests that DPN occurs earlier in the course of diabetes than diabetic retinopathy.
Duration of DM >15 years, HbA1c above 6.5%, serum creatinine >1.5 mg/dl, and DPN are the risk factors for DR in our study. DPN and duration of DM (15–25 years) were the only risk factors for severe NPDR/PDR [Table 3]. Duration of DM >25 years was not found to be a risk factor for severe NPDR/PDR contrary to other studies.[18] This may be due to the fewer number of severe NPDR/PDR patients (14%) in our study group and less number (10%) of patients with a duration of DM above 25 years. In contrast, duration of DM >15 years was a predictive factor for severe DPN. A study done in South India to assess the risk factors for PDR also did not show duration of DM as a risk factor.[19] Elevated creatinine (>1.5 mg/dl) was found to be a risk factor for DR and severe DPN [Tables 3 and 4].
Table 4.
Multivariate regression analysis showing risk factors for DPN
| Variables | OR* (95% CI†) | P | |
|---|---|---|---|
| Age | Above 60 Years | 7.80 (2.99-20.33) | <0.001 |
| 15-25 Years | 1.71 (1.02-2.85) | 0.041 | |
| Duration of DM‡ | 15 -25 and > 25 | 5.34 (1.17-24.46) | 0.031 |
| Diabetic retinopathy | Yes | 3.70 (2.30-6.14) | <0.001 |
| Risk factors for severe DPN§ | |||
| Duration of DM | 15-25 Years | 2.32 (1.50-3.62) | <0.001 |
| >25 Years | 4.13 (1.94-8.82) | <0.001 | |
| Creatinine | >1.5 mg/dl (Abnormal) | 2.10 (0.99-4.44) | 0.053 |
| Diabetic retinopathy | Yes | 2.77 (1.80-4.25) | <0.001 |
*OR - Odds ratio, †CI - confidence interval, ‡DM - diabetes mellitus, §DPN - diabetic peripheral neuropathy, ||NPDR- nonproliferative diabetic retinopathy, ¶PDR - proliferative diabetic retinopathy
A study done by Srinivasan et al.[20] reported that ganglion cell loss is an independent predictor of DPN. They evaluated GCC loss in 84 patients with type 1 diabetes, 67 with type 2 diabetes, and 42 without diabetes. They also reported OCT as a noninvasive technology that aids in the detection of retinal structural changes in patients with established diabetic neuropathy.[21] Hegazy et al.[22] studied retinal ganglion cell complex changes using spectral-domain OCT in diabetic patients without retinopathy. They found GCIPL focal loss volume was significantly more in diabetic eyes than in normal eyes. Their study concluded the significant GCIPL thinning in diabetes predates retinal vasculopathy, which is mainly focal rather than diffuse. Our study showed significant thinning of both average and focal GCIPL in patients with DPN.
Our study also showed that inner retinal neurodegeneration occurs in diabetic subjects before the onset of any significant diabetic retinopathy and that GCIPL reduction occurs earlier than peripapillary RNFL thinning in diabetic patients without retinopathy. Independent studies have also observed that inferior quadrant (RNFL) thickness is reduced in association with peripheral neuropathy independent of diabetic retinopathy.[23]
Retinal neurodegeneration seems to be paralleling with DPN. There may be common pathways for retinal and peripheral neurodegeneration that are independent of conventional DR risk factors. In recent years, electrophysiological tests such as electroretinograms and visually evoked potentials have shown sensitivity in identifying signs of neurodegeneration of the retina even in the preclinical phase.[24]
Bonnin et al.[25] assessed GCIPL thickness with visual functions in patients with treated diabetic macular edema (DME). They found that despite favorable anatomic response and restoration of a central macular thickness (CMT) after resolution of DME, the GCIPL thickness in DME eyes was lower than that in non-DME eyes and correlated with the VA. Thus, neurodegeneration is clinically significant as it will affect the visual outcome.
Neurodegeneration as an early component of DR introduces the possibility to explore alternative therapies to prevent the onset of vision loss, including neuroprotective therapies targeting individual neurotransmitter systems and general neuroprotective approaches targeting retinal neurodegeneration and DPN. Prospective studies are needed where these patients are followed up to know the development of DR, visual function changes, progression to PDR, or diabetic maculopathy, which might help in the future to plan preventing therapies before the development of microvascular lesions detectable by ophthalmoscopy. It could also act as a potential imaging biomarker to assess the preventive effects of new therapies.
Our study also stresses the importance of multidisciplinary approach in the management of diabetes and its complications. Patients with DPN should be evaluated for DR and ophthalmologists should be referring patients with DR, especially severe NPDR and PDR to a podiatrist for DPN evaluation, which will prevent complications of DR and DPN.
Our study is likely to have recruitment bias as it was done in the endocrinology department at a quaternary center and it is a limitation of our study. We also did not include the cognition tests in our study which may be closely related to DR-related neuronal degeneration.
Conclusion
There is a significant association between DR and DPN and their severities. There are early changes in inner retinal layers of diabetic patients without microvascular changes of DR. These neurodegenerative changes parallel DPN in the course of DM. Our study stresses the importance of multidisciplinary approach in the management of diabetes and its complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stem MS, Gardner TW. Neurodegeneration in pathogenesis of diabetic retinopathy: Molecular mechanisms and therapeutic implications. Curr Med Chem. 2013;20:3241–50. doi: 10.2174/09298673113209990027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82:95–100. doi: 10.1136/pgmj.2005.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunha-Vaz J. Neurodegeneration as an early event in the pathogenesis of Diabetic Retinopathy: A multicentric, prospective, phase II–III, randomised controlled trial to assess the efficacy of neuroprotective drugs administered topically to prevent or arrest Diabetic Retinopathy. EUROCONDOR – EU FP7 Project. Acta Ophthalmol. 2012:90. [Google Scholar]
- 4.Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 5.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–91. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasalová Z. Biothesiometry in the diagnosis of peripheral neuropathies. Cas Lek Cesk. 2002;141:223–5. [PubMed] [Google Scholar]
- 7.Bloom S, Till S, Sönksen P, Smith S. Use of a biothesiometer to measure individual vibration thresholds and their variation in 519 non-diabetic subjects. Br Med J (Clin Res Ed) 1984;288:1793–5. doi: 10.1136/bmj.288.6433.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Deursen RW, Sanchez MM, Derr JA, Becker MB, Ulbrecht JS, Cavanagh PR. Vibration perception threshold testing in patients with diabetic neuropathy: Ceiling effects and reliability. Diabet Med. 2001;18:469–75. doi: 10.1046/j.1464-5491.2001.00503.x. [DOI] [PubMed] [Google Scholar]
- 9.Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17:557–60. doi: 10.2337/diacare.17.6.557. [DOI] [PubMed] [Google Scholar]
- 10.Bharathi C, Roopakala MS, Shivaprasad C, Acharya PT. Diagnostic value of vibration perception threshold in diabetic peripheral neuropathy. Int J Physiol. 2018;6:84–8. [Google Scholar]
- 11.Wang W, Lo ACY. Diabetic retinopathy: Pathophysiology and treatments. Int J Mol Sci. 2018;19:1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes Metab. 2008;10:53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 13.Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: Mechanisms and consequences. Curr Med Chem. 2013;20:3218–25. doi: 10.2174/09298673113209990022. [DOI] [PubMed] [Google Scholar]
- 14.Tomic-Canic M, Brem H. Gene array technology and pathogenesis of chronic wounds. Am J Sure. 2004;188:67–72. doi: 10.1016/S0002-9610(03)00293-9. [DOI] [PubMed] [Google Scholar]
- 15.Abdollahi A, Moghimi S, Tabasi A, Rajabi MT, Sabet B. Neuropathy and retinopathy in diabetes: Is there any association? Int J Ophthalmol. 2009;2:57–60. [Google Scholar]
- 16.Sharma VK, Joshi MV, Vishnoi AA. Interrelation of retinopathy with peripheral neuropathy in diabetes mellitus. J Clin Ophthalmol Res. 2016;4:83–7. [Google Scholar]
- 17.Hwang DJ, Lee KM, Park MS, Choi SH, Park JI, Cho JH, et al. Association between diabetic foot ulcer and diabetic retinopathy. PLoS One. 2017;12:e0175270. doi: 10.1371/journal.pone.0175270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nittala MG, Keane PA, Zhang K, Sadda SR. Risk factors for proliferative diabetic retinopathy in a Latino American population. Retina. 2014;34:1594–9. doi: 10.1097/IAE.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizza AN, Susarla G, Khan R, Wang V, Brustoski K, Sarangapani S, et al. Risk factors for proliferative diabetic retinopathy and diabetic macular edema in the South Indian population. Invest Ophthalmol Vis Sci. 2020;61:4842. [Google Scholar]
- 20.Srinivasan S, Pritchard N, Sampson GP, Edwards K, Vagenas D, Russell AW, et al. Diagnostic capability of retinal thickness measures in diabetic peripheral neuropathy. J Optom. 2017;10:215–25. doi: 10.1016/j.optom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan S, Pritchard N, Sampson GP, Edwards K, Vagenas D, Russell AW, et al. Focal loss volume of ganglion cell com plex in diabetic neuropathy. Clin Exp Optom. 2016;99:526–34. doi: 10.1111/cxo.12379. [DOI] [PubMed] [Google Scholar]
- 22.Hegazy AI, Zedan RH, Macky TA, Esmat SM. Retinal ganglion cell complex changes using spectral domain optical coherence tomography in diabetic patients without retinopathy. Int J Ophthalmol. 2017;10:427–33. doi: 10.18240/ijo.2017.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahidi AM, Sampson GP, Pritchard N, Edwards K, Vagenas D, Russell AW, et al. Retinal nerve fibre layer thinning associated with diabetic peripheral neuropathy. Diabet Med. 2012;29:e106–11. doi: 10.1111/j.1464-5491.2012.03588.x. [DOI] [PubMed] [Google Scholar]
- 24.Pescosolido N, Barbato A, Stefanucci A, Buomprisco G. Role of electrophysiology in the early diagnosis and follow-up of diabetic retinopathy. J Diabetes Res 2015. 2015 doi: 10.1155/2015/319692. 3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnin S, Tadayoni R, Erginay A, Massin P, Dupas B. Correlation between ganglion cell layer thinning and poor visual function after resolution of diabetic macular edema. Invest Ophthalmol Vis Sci. 2015;56:978–82. doi: 10.1167/iovs.14-15503. [DOI] [PubMed] [Google Scholar]


