Abstract
Purpose:
To assess the perceptions of physicians about diabetic retinopathy (DR) screening, barriers to DR screening, and change in management protocol of Diabetes Mellitus (DM) patients with DR.
Methods:
A cross-sectional descriptive study was conducted using a standard predesigned and pretested structured questionnaire through online mode in the month of April 2021 to assess the criteria used for referral of diabetic patients for DR screening, barriers to DR screening, and the management plan among physicians after the patient has been diagnosed with DR.
Results:
In total, 100 physicians participated in the study. Physicians responded that criteria used for referral for DR screening according to duration was <5 years (n = 0), 5–10 years (n = 60), >10 years (n = 10), and irrespective of the duration (n = 30). According to severity, well-controlled DM without (n = 30) and with other system involvement (n = 50) and uncontrolled DM without (20) and with other system involvement (n = 50) and irrespective of the severity of disease (n = 30) was reported. Physicians (n = 40) responded that patients who were diagnosed with DR belonged to the Type 1 DM category rather than Type 2 DM (P < 0.05). With regard to the barriers and challenges faced in ensuring DR screening, the following themes emerged: no ocular symptoms, lack of compliance, time constraint for the patient, and lack of motivation.
Conclusion:
We found that the preferred practice pattern of physicians regarding referral for DR screening was dependent on the duration of the disease (mostly 5–10 years of the disease) and severity (when other systems were involved). Noncompliance with advice was the major barrier to DR screening.
Keywords: Diabetic retinopathy, perceptions, physicians, referral, consultations
Diabetes mellitus (DM) is a multisystem disease that requires a multidisciplinary approach for management of the disease.[1] Physicians being the primary treating doctors of diabetic patients, it is essential that they ensure timely involvement of other disciplines for management of DM.[2]
Diabetic Retinopathy (DR) is one of the most common complications of DM.[3] According to a recent study, the prevalence of DR in India is 21.7%, more so in insulin-dependent (Type 1) DM.[4] In a study done to assess the perceptions of care and challenges faced by people with diabetes in India, it was reported that 45% of participants already suffered from vision loss due to DR at the time of their first visit to an ophthalmologist.[5] A study done on the uptake of DR screening in a pyramidal eye healthcare model found that 50% at primary level, 40% at secondary, and 2% patients at tertiary level had never undergone a dilated eye examination previously.[6] Most of the DR-related visual impairment can be prevented with early diagnosis and regular follow-up. Further, after diagnosis of DR in patients, it is important that the management plan for diabetic patients is altered.[7]
National Program for Control of Blindness (NPCB) recommends opportunistic screening for identification of DR.[8] Treatment intervention at the early stages of DR can reduce the burden of blindness due to DR. To decrease the burden of preventable blindness due to DR, it is imperative that physicians and ophthalmologists work together.[9] In this study, we intended to evaluate the preferred practice pattern among physicians regarding the referral of DM patients to an ophthalmologist for the screening of DR and to evaluate various measures taken by the physicians in the management protocol after the diagnosis of DR to strengthen the management plan. The information that physicians require from the referral to an ophthalmologist regarding the ocular findings of DM patients was also evaluated in this study.
Methods
After taking approval from the institute's ethics committee, a cross-sectional descriptive study was conducted using a standard predesigned and pretested structured questionnaire through the online mode to evaluate the preferred practice patterns among physicians regarding referral of DM patients for DR evaluation. Inclusion criteria included physicians practicing in India who were accessible online. Exclusion criteria included doctors of other disciplines. The survey was conducted in April 2021.
The questionnaire was designed from existing literature and focus-group interviews of physicians (who were excluded from the study). A pilot study was done (raw alpha value: 0.77). Duplicate entries were not allowed (by limiting the response to one). The time and length of the questionnaire were shared and replying to the questionnaire was considered as their consent to participate in the survey. The data of the responses were stored for a year and have been kept confidential. No personal information was collected and only investigators had access to the information gathered. The questionnaire consisted of 25 questions designed to collect information regarding demographics, area of practice, years of practice, the DR screening schedule advised, barriers in getting screening, management plan after the diagnosis of DR made, known associations between DR and other complications of DM, and to explore the information that physicians require from ophthalmologists in a case of DR.
Only completed questionnaires were included in data analysis. The DR screening schedule followed by physicians was noted according to the duration and severity of the disease. Proportions of the patients who got DR screening after being advised were noted as a percentage. Chi-square test was used to find the association between types of DM and DR as perceived by the physicians. Descriptive data gathered regarding barriers to DR screening was thematically analyzed. The association between other complications and DR was noted on a Likert scale. Data were analyzed using SPSS ver 19.0. P < 0.05 was considered as significant.
Results
The questionnaire was sent to 300 physicians online via WhatsApp, Email, and Telegram using Google forms link. A total of 100 physicians responded to our study, with age ranging from <40 to >60 years (<40: n = 38, 40–60: n = 58; >60: n = 4), out of which 80% (n = 80) practiced in urban areas and 20% (n = 20) in rural areas; 90% (n = 90) had been practicing for more than 10 years and 10% (n = 10) had been practicing for 5–10 years. All (n = 100) physicians responded that they have easy access to the services of an ophthalmologist near them. Fig. 1 represents the percentage of DM patients attending OPD according to the duration of the disease (in a week). DR screening practices followed by physicians are shown in Table 1. Practices followed for patients diagnosed with DR are shown in Table 2. Association between DR and other complications in their practice is shown in Table 3. With regard to communication with ophthalmologists or vice versa about the management plan for DM patients, 80% (n = 80) of physicians reported that they communicate sometimes, 10% (n = 10) never communicated, and 10% (n = 10) always communicated. On thematic analysis of the qualitative responses, as regards to information physician sought through a referral from an ophthalmologist about ocular findings, the following themes emerged: Severity and grading of DR, other ocular changes, need for ocular intervention, and frequency of screening and follow-up based on the changes observed. With regard to the barriers and challenges faced to ensure DR screening in DM patients, the following themes emerged: no ocular symptoms, lack of compliance, time constraint for the patients, economic challenges, and lack of motivation among patients.
Figure 1.
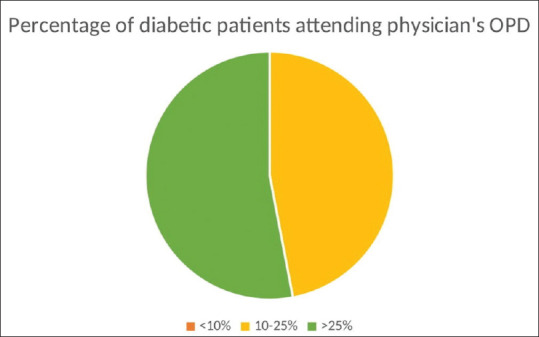
The percentage of DM patients attending OPD according to the duration of the disease (in a week)
Table 1.
DR screening practices followed by physicians
| Criteria for screening for diabetic retinopathy in diabetic patients (according to the duration of diabetes) | <5 years | 5-10 years | >10 years | All patients irrespective of the duration of DM | |
|---|---|---|---|---|---|
| 0 | 60 | 10 | 30 | ||
| Criteria for screening for diabetic retinopathy in diabetic patients (according to the severity of diabetes) | Well-controlled DM with no other system involvement | Well-controlled DM with other system involvement | Uncontrolled DM with no other system involvement | Uncontrolled DM with other system involvement | All types of DM |
| 30 | 50 | 20 | 50 | 10 | |
| Diabetic Retinopathy Screening schedule advised to the patients (thematic analysis) | 6 monthlies | At first visit and then every 6 months | Annual | When patient complains of any ocular symptoms | |
| 50 | 30 | 15 | 5 | ||
| Percentage of patients getting screened for diabetic retinopathy (with report) after being advised | <25% | 25%-50% | >50% | ||
| 50 | 30 | 20 | |||
| Baseline screening of Diabetic Retinopathy done at the time of diagnosis of Diabetes Mellitus in all patients. | Yes | No | Sometimes | ||
| 63 | 0 | 37 | |||
Table 2.
Practices followed for patients diagnosed with DR
| Variable | Responses | |||
|---|---|---|---|---|
| Percentage of diabetic patients with diagnosed diabetic retinopathy in their OPD (P<0.05) | <25% | 25%-50% | >50% | |
| Type I DM | 7 | 7 | 40 | |
| Type II DM | 6 | 15 | 25 | |
| Does the diagnosis of diabetic retinopathy change further line of management | Yes | No | Sometimes | |
| 54 | 13 | 33 | ||
| What changes are made to the line of management after diagnosis of DR (thematic analysis) | Tighter glycemic control and counseling to get regular Ophthalmologic, cardiac and neuro consultation lifestyle changes Control of comorbidities such as hypertension Depends on the severity of the retinopathy |
|||
| Does grading of diabetic retinopathy change the line of management | Yes | No | Sometimes | |
| 54 | 26 | 20 | ||
Table 3.
Association of DR with other diabetic complications
| Name of the complication | Association with DR (Grading on Likert scale) 1 being highly unlikely and 5 being highly likely | ||||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3 | 4 | 5 | |
| Diabetic Nephropathy n=100 | 0 | 15 (15%) | 50 (50%) | 35 (35%) | 0 |
| Peripheral neuropathy n=100 | 0 | 15 (15%) | 46 (46%) | 39 (39%) | 0 |
| CVS diseases n=100 | 1 (1%) | 9 (9%) | 45 (45%) | 45 (45%) | 0 |
| Secondary infections n=100 | 5 (5%) | 35 (35%) | 45 (45%) | 15 (15%) | 0 |
| End-organ disease n=100 | 7 (7%) | 0 | 7 (7%) | 50 (50%) | 36 (36%) |
| Dyslipidemia n=100 | 0 | 7 (7%) | 19 (19%) | 56 (56%) | 18 (18%) |
Value of ≥3 was considered as more likely and <3 was considered less likely
Discussion
The physicians do not directly perform DR screening but an opportunity for getting the screening done exists as they have much greater access to patients with DM than ophthalmologists do. Physicians develop a rapport and can influence more patients with DM to get eye screening by counseling them. Unfortunately, they have a heavy workload, limited time, and many barriers from the patient's perspective, which act as barriers for screening for DR.[10] This descriptive study explores the preferred practice patterns of physicians regarding referral to an ophthalmologist for DR screening, barriers for DR screening, and change in management protocol after diagnosis of DR.
Response rate in our study was 33.3%, which is less compared to other specialist survey studies.[11] This may be due to the current burden of patients due to COVID. The sociodemographic data of the respondents in our study were as follows: the majority (75%) of the physicians were practicing in urban areas, 58% belonged to the age of 40–60 years, and 81% were practicing for more than 10 years. This representation could be due to a convenience sampling of physicians who are accessible online. Many studies have shown that doctors under 60 years (40–60 years) are more likely to respond to the surveys, which was seen in our study too.[10] All (100%) physicians responded that they have easy access to the services of an ophthalmologist, which could be because the participants who responded in this survey were either practicing in urban areas (75%) or semi-urban areas (25%). In contrast, lack of accessibility to ophthalmologists was listed as one of the reasons for the increase in the disease burdens associated with DR by Hazin R et al. in a prospective study.[12] Approximately 60% of the physicians responded that they had more than 25% DM patients in their OPD, with most having diabetes between 5–10 years.
American Diabetes Association, the American College of Physicians, and the American Academy of Ophthalmology recommend the following guidelines for DR screening: Type 1 DM patients with onset at 0–30 years should have the first screening examination at 5 years duration, whereas Type 1 DM patients with later-onset and Type 2 DM patients should receive a dilated retinal examination by an ophthalmologist at diagnosis.[13] In India, we follow the opportunistic method of screening; all patients with DM should be screened regularly for sight-threatening DR as it is the most common microvascular ocular complication of diabetes.[14] Despite these recommendations, the practice scenario is quite different. In our study, physicians followed various criteria for referring patients for DR screening. According to the duration of diabetes, 60% of the physicians referred patients who had DM between 5 and 10 years and only 30% referred DM patients irrespective of duration, whereas guidelines followed in India recommend that all patients should be screened irrespective of the duration.[14] In a study done by Khadem et al., the physicians increasingly used duration of diabetes as a criterion, similar to the findings of our study.[15] According to severity of DM, only 10% of physicians responded that they get DR screening done for patients irrespective of severity of DM, and the majority of the physicians get DR screening done for patients when other system involvement occurs (50% in well-controlled and 50% in controlled). Only 20% of physicians responded that they get DR screening for uncontrolled DM without systemic involvement. This emerged as a criterion according to the preferred practice pattern rather than according to various recommended guidelines, which do not use severity of DM as a criterion for referral for DR screening.[13,14] The preferred DR screening schedule advised to the patients by physicians was every 6 months according to 50% of the physicians; at first visit for first time screening for DR and then every 6 months afterward by 30% of the physicians. Further, 15% of the physicians advised annual DR screening checkups to their patients and only 5% advised their patients to get DR screening done if patients developed any ocular symptoms. This is not in accordance with the guidelines but emerged as a preferred practice pattern in the group of physicians under study.
Almost half of the physicians (50%) responded that <25% of the patients get screening done once advised; 30% responded that 25%–50% of patients get screened for DR while 20% responded that more than 50% of patients get screening done for DR. Thus, the percentage of patients getting screened for DR are less, which is in accordance with other studies, which point out that due to certain factors, relatively less number of patients get screening done.[16,17,18] On further exploring the barriers against DR screening from the physician's perspective, the following themes emerged: no ocular symptoms, lack of compliance, time constraints, financial constraints, and lack of motivation among patients. Lack of patient motivation was identified as a barrier in other studies as well.[12,18,19] Hartnett et al.[20] cited inadequate patient education and access to care as a barrier perceived by physicians or primary health care providers, which was not cited in our study as one of the barriers.
In our study, most of the physicians responded that patients who were diagnosed with DR belonged to the Type 1 DM category rather than Type 2 DM (P < 0.05), which is in accordance with other studies.[4] Management protocol was changed by more than half of the physicians (54%) once the patient was diagnosed with DR. Only 13% responded that no change in the management protocol was carried out by them. Grading of severity of DR affected the management protocol of 54% of physicians while 26% responded that it did not make any difference in their management protocol. American Diabetes Association recommends more stringent control of DM associated with complications, and the same was recommended by most of the physicians in our study as well.[21] Changes done in management protocol included strict glycemic control, counseling to get regular ophthalmological consultations, screening for complications occurring in other systems such as neurology and cardiology, lifestyle changes, and control of comorbidities such as hypertension.
With regard to associations between DR and other micro or macrovascular complications, physicians responded that patients having DR were 85% more likely to have nephropathy (>3 on Likert scale). Peripheral neuropathy was observed as more likely to be associated with DR by 85% of physicians (≥3 on Likert scale), CVS diseases by 90% of physicians (≥3 on Likert scale), secondary infections by 60% of physicians (>3 on Likert scale). End-organ diseases and dyslipidemia were suggested to be associated with DR changes by 86% of physicians (>4 on Likert scale) and 74% of physicians (>4 on Likert scale), respectively. Association of DR with other micro and macrovascular complications has been reported by other studies as well.[22,23,24] In a study done by Hazin et al.,[12] the primary contact physicians failed to evaluate these risk factors’ associations with DR in contrast to the physicians in our study.
DM management necessitates a multidisciplinary team with a dynamic flow of information between the treating doctors. Holley and Lee’ s[25] qualitative research study found that primary care providers had poor communication with eye care providers. In our study, 80% of the physicians communicated with ophthalmologists sometimes whereas only 10% ensured communication at all times. The information sought by the treating physicians from the ophthalmologists regarding their referral for ocular findings included severity, grading of DR, other ocular changes, need for intervention, and frequency of screening and follow-up based on changes observed.
Limitations
In our study, it is important to recognize that nonrespondents may differ from participating physicians’ views in ways we were unable to assess and is noted as a possible response bias issue.
Recommendations
Based on our study, we recommend a dynamic flow of information between physicians and ophthalmologists and emphasis on screening of DR in DM patients.
Conclusion
The preferred practice pattern among the majority of physicians regarding referral to an ophthalmologist for the screening of DR in DM patients was duration of the disease between 5 and 10 years and with severity of the disease when it involves other organs. Among the barriers against DR screening, compliance of the patients and time and financial constraints were cited by physicians. Most of the physicians changed their management protocol with tighter glycemic control in patients who were diagnosed with DR. In addition to presence of DR, physicians require ophthalmologists to provide information regarding severity, grading of DR, other ocular changes, need for intervention, and frequency of screening and follow-up based on the changes observed. Monitoring clinical practice patterns through these kinds of surveys enables the educators to address misconceptions and modulate existing programs or develop new ones.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.McGill M, Blonde L, Chan JCN, Khunti K, Lavalle FJ, Bailey CJ. The interdisciplinary team in type 2 diabetes management: Challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol. 2017;7:21–7. doi: 10.1016/j.jcte.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathy JP, Sagili KD, Kathirvel S, Trivedi A, Nagaraja SB, Bera OP, et al. Diabetes care in public health facilities in India: A situational analysis using a mixed methods approach. Diabetes Metab Syndr Obes. 2019;12:1189–99. doi: 10.2147/DMSO.S192336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015;6:489–99. doi: 10.4239/wjd.v6.i3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy in India: The All India ophthalmological society diabetic retinopathy eye screening study 2014. Indian J Ophthalmol. 2016;64:38–44. doi: 10.4103/0301-4738.178144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla R, Gudlavalleti MV, Bandyopadhyay S, Anchala R, Gudlavalleti AS, Jotheeswaran AT, et al. Perception of care and barriers to treatment in individuals with diabetic retinopathy in India:11-city 9-state study. Indian J Endocr Metab. 2016;20(Suppl 1):33–41. doi: 10.4103/2230-8210.179772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingam S, Rani PK, Sheeladevi S, Kotapati V, Das T. Knowledge, attitude and practices on diabetes, hypertension and diabetic retinopathy and the factors that motivate screening for diabetes and diabetic retinopathy in a pyramidal model of eye health care. Rural Remote Health. 2018;18:4304. doi: 10.22605/RRH4304. [DOI] [PubMed] [Google Scholar]
- 7.Beaser RS, Turell WA, Howson A. Strategies to improve prevention and management in diabetic retinopathy: Qualitative insights from a mixed-methods study. Diabetes Spectr. 2018;31:65–74. doi: 10.2337/ds16-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vashist P, Singh S, Gupta N, Saxena R. Role of early screening for diabetic retinopathy in patients with diabetes mellitus: An overview. Indian J Community Med. 2011;36:247–52. doi: 10.4103/0970-0218.91324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong TY, Sabanayagam C. The war on diabetic retinopathy: Where are we now. Asia Pac J Ophthalmol (Phila) 2019;8:448–56. doi: 10.1097/APO.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Swearingen R. Diabetic eye screening: Knowledge and perspectives from providers and patients. Curr Diab Rep. 2017;17:94. doi: 10.1007/s11892-017-0911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham CT, Quan H, Hemmelgarn B, Noseworthy T, Beck CA, Dixon E, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol. 2015;15:32. doi: 10.1186/s12874-015-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazin R, Colyer M, Lum F, Barazi MK. Revisiting diabetes 2000: Challenges in establishing nationwide diabetic retinopathy prevention programs. Am J Ophthalmol. 2011;152:723–9. doi: 10.1016/j.ajo.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Screening guidelines for diabetic retinopathy. American college of physicians, American diabetes association, and American academy of ophthalmology. Ann Intern Med. 1992;116:683–5. [PubMed] [Google Scholar]
- 14.Raman R, Ramasamy K, Rajalakshmi R, Sivaprasad S, Natarajan S. Diabetic retinopathy screening guidelines in India: All India ophthalmological society diabetic retinopathy task force and Vitreoretinal Society of India consensus statement. Indian J Ophthalmol. 2021;69:678–88. doi: 10.4103/ijo.IJO_667_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khadem JJ, Buzney SM, Alich KS. Practice patterns in diabetic retinopathy: Part 1: Analysis of retinopathy follow-up. Arch Ophthalmol. 1999;117:815–20. doi: 10.1001/archopht.117.6.815. [DOI] [PubMed] [Google Scholar]
- 16.Funatsu H, Hori S, Shimizu E, Nakamura S. Questionnaire survey on periodic ocular examination in Japanese diabetic patients. Am J Ophthalmol. 2003;136:955–7. doi: 10.1016/s0002-9394(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 17.Schmid KL, Schmid LM, Pedersen C. Knowledge of the ocular effects of diabetes among the general population of Australia and the members of diabetes Australia. Clin Exp Optom. 2003;86:91–103. doi: 10.1111/j.1444-0938.2003.tb03067.x. [DOI] [PubMed] [Google Scholar]
- 18.Karishma G, Rambharos F, Gautam L. Factors affecting retinal screening among patients with diabetes in India. Delhi J Ophthalmol. 2021;31:28–31. [Google Scholar]
- 19.Denberg TD, Myers BA, Eckel RH, McDermott MT, Dickinson WP, Lin CT. A patient outreach program between visits improves diabetes care: A pilot study. Int J Qual Health Care. 2009;21:130–6. doi: 10.1093/intqhc/mzn060. [DOI] [PubMed] [Google Scholar]
- 20.Hartnett ME, Key IJ, Loyacano NM, Horswell RL, Desalvo KB. Perceived barriers to diabetic eye care: Qualitative study of patients and physicians. Arch Ophthalmol. 2005;123:387–91. doi: 10.1001/archopht.123.3.387. [DOI] [PubMed] [Google Scholar]
- 21.Summary of revisions: Standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S4–6. doi: 10.2337/dc20-Srev. [DOI] [PubMed] [Google Scholar]
- 22.Lee WJ, Sobrin L, Lee MJ, Kang MH, Seong M, Cho H. The relationship between diabetic retinopathy and diabetic nephropathy in a population-based study in Korea (KNHANES V-2, 3) Invest Ophthalmol Vis Sci. 2014;55:6547–53. doi: 10.1167/iovs.14-15001. [DOI] [PubMed] [Google Scholar]
- 23.Chandy A, Pawar B, John M, Isaac R. Association between diabetic nephropathy and other diabetic microvascular and macrovascular complications. Saudi J Kidney Dis Transpl. 2008;19:924–8. [PubMed] [Google Scholar]
- 24.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocr Metab. 2016;20:546–51. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holley CD, Lee PP. Primary care provider views of the current referral-to-eye-care process: Focus group results. Invest Ophthalmol Vis Sci. 2010;51:1866–72. doi: 10.1167/iovs.09-4512. [DOI] [PubMed] [Google Scholar]


