Abstract
Purpose:
To evaluate (i) the distribution of postoperative endophthalmitis (POE) in patients who underwent cataract surgery, (ii) risk factors in diabetic versus nondiabetic patients, and (iii) distribution of POE in those who had undergone rapid reduction of preoperative blood sugar levels versus those with normal blood sugar levels.
Methods:
Medical records were reviewed from January 1995 to July 2021. In total, 391 eyes of 391 patients who developed POE after cataract surgery were studied. Patients with POE were divided into Group A, patients with diabetes (n = 128), and Group B, patients without diabetes (n = 263), and the associations of various clinical factors in the two groups were studied. Patients with diabetes with raised random blood sugars (RBS) preoperatively were subjected to a rapid reduction of blood sugar (RBS <200 mg%) to be considered eligible for surgery. Microbiological profile of patients was examined.
Results:
The cumulative incidence of POE over 26 years was 0.09%. Those who underwent a rapid reduction in preoperative blood sugar levels had higher rates of POE (53.1%) compared with (46.9%) those with blood sugar levels under control (P = 0.486). Men with diabetes had 1.634 times higher odds of POE (P = 0.048), and those with diabetes and hypertension had 3.961 times greater odds of having POE (P < 0.001) when adjusted for age, alcohol, smoking, and socioeconomic strata and presence of posterior capsule rupture. Positive culture results were observed in 45/128 (35%) patients with diabetes and 71/263 (27%) patients without diabetes. Staphylococcus epidermidis was the most commonly identified organism and was detected in 10/45 (22%) in those with diabetes and 21/71 (29%) in those without diabetes of all the culture-positive cases.
Conclusion:
In patients with POE, the odds are greater for men with diabetes, those with a history of hypertension, as well as those who undergo a rapid reduction of preoperative blood sugar.
Keywords: Cataract, diabetes, endophthalmitis, postoperative, vitrectomy
Postoperative endophthalmitis (POE) is a sight-threatening complication of cataract surgery.[1,2,3] The best means to prevent POE remains controversial as doing large studies required to investigate an uncommon problem is often difficult. The best way for prophylaxis based on current clinical evidence is the preoperative preparation with 5% povidone–iodine solution; however, the benefits of altering other perioperative factors remain uncertain.[4,5,6,7]
Diabetes mellitus (DM) has been associated with increased rates of infections, which may be partially explained by a decreased T-cell-mediated immune response or an impaired neutrophil function.[8,9,10] There exists consensus statements of various professional associations regarding the cutoffs for blood sugar levels before cataract surgery. However, clinical evidences related to distribution of endophthalmitis in those with and without diabetes and the risk factors for endophthalmitis in diabetes, such as influence of rapid reduction in preoperative blood sugar versus normal blood sugar is poorly studied.[11]
Preoperative rapid reduction of blood sugar has been shown to be associated with worsening of diabetic retinopathy.[12] However, there are no evidence regarding the rate of endophthalmitis occurring in patients in whom blood sugar was rapidly controlled compared with those in whom blood sugar was already in the normal range before surgery.
The aim of this study was to (i) evaluate the distribution of POE in patients who underwent cataract surgery, (ii) assess the risk factors in diabetic versus nondiabetic group, and (iii) examine the distribution of POE in diabetic patients who had undergone a rapid reduction of blood sugar levels versus those whose already had normal preoperative blood sugar levels.
Methods
This was a single center, institutional, retrospective case record study. Medical and electronic records of all patients who underwent cataract surgery between January 1995 and July 2021 at our institute were reviewed.
Patients with POE either acute or delayed were identified. Diagnosis of POE was based on clinical and/or microbiological findings. Medical records of these patients were studied in detail with regards to demographic profile including socio-economic strata, personal habits, associated systemic diseases, preoperative blood sugar levels, time interval between surgery and blood sugar control, if initial blood sugars were raised, type of cataract surgery, additional procedure performed, time interval between surgery and onset of endophthalmitis, and microbiological profile by culture. Socioeconomic status was defined as “upper” if their monthly salary was above Indian Rupee (INR) 7000 and the criterion was followed till the year 2018. From 2019 to July 2021, the criterion was revised as Indian Rupee (INR) 15,000. Other patients who were not included in the above criterion were considered eligible for a free surgery, postoperative care, and glasses.
Subjects with POE were stratified into two groups, Group A, patients with diabetes, and Group B, patients without diabetes. At our tertiary referral hospital, after cataract surgery was advised by ophthalmologists, all patients went physical examination by an in-house physician. Patients underwent routine laboratory investigations which included random blood sugar (RBS). Patients who were known to have diabetes but with RBS level <200 mg/dL during the routine lab test were labeled as having “‘diabetes with blood sugar under control.” Patients who were not known to have diabetes but with RBS above 200 mg/dL underwent a postprandial blood sugar (PPBS) testing.
Patients with PPBS within normal limits were labeled as not having diabetes; those with PPBS above the normal limits were labeled as having diabetes and were referred to an in-house physician for effective control of blood sugar to ≤200 mg% and were taken for surgery within a week of initial higher blood sugar value. All these patients and known diabetic patients with RBS level >200 mg/dL were labeled as “having diabetes with raised blood sugar.” The above criteria have been recommended by the All India Ophthalmological Society in the AIOS Guidelines for Endophthalmitis Prevention in Cataract Surgery and also by the A Vision 2020: Right to Sight Guidelines for the Management of Cataract in India.[13,14]
The variables were assessed for normality of distribution. Univariate and multivariate analyses were performed using logistic regression to identify risk factors for POE, and the odds ratios with 95% confidence intervals were calculated. A P value of <0.05 was considered statistically significant. A subsequent analysis was then performed to assess the association of rapid reduction and normal preoperative blood sugar in subjects with diabetes with rate of POE.
Results
Overall, 4,01,033 cataract surgeries were performed between January 1995 and July 2021.
Of these, 391 eyes of 391 patients were diagnosed with POE. The cumulative incidence of POE in our study over this 26-year period was 0.09%.
There were 231 males and 160 females. The average age was 54.7 years. One hundred and twenty-eight patients had DM, and constituted Group A, while two hundred and sixty-three patients had no diabetes and constituted Group B.
Fig. 1 depicts the proportion of patients with and without diabetes who had undergone various types of cataract surgeries. The proportion of patients with no diabetes was higher than those with diabetes when stratified according to the type of cataract surgery.
Figure 1.
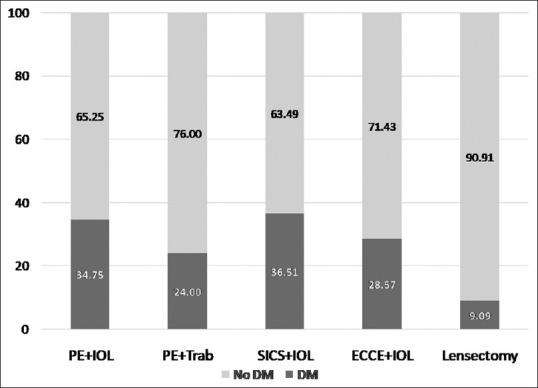
Proportion of subjects with and without diabetes in various types of surgeries in subjects with postoperative endophthalmitis
Table 1 shows the distribution of clinical factors associated with POE in those with and without diabetes. In those with POE, those aged ≥50 years with diabetes were significantly higher in proportion (78.9%) than those without diabetes (64.9%) (P = 0.005). The proportion of men with diabetes (70.8%) was significantly higher compared to men without diabetes (55.1%) (P = 0.003).
Table 1.
Distribution of diabetes and other demographic variables in postoperative endophthalmitis
| DM, n=128 % | no DM, n=263 % | P | |
|---|---|---|---|
| Age | |||
| <50 years | 21.1% | 35.1% | 0.005 |
| ≥50 years | 78.9% | 64.9% | |
| Gender | |||
| Men | 70.8% | 55.1% | 0.003 |
| Women | 29.2% | 44.9% | |
| Hypertension | |||
| Present | 46.1% | 16.3% | <0.001 |
| Absent | 53.9% | 83.7% | |
| Smoker | 25.8% | 21.3% | 0.321 |
| Nonsmoker | 74.2% | 78.7% | |
| Alcoholic | 6.2% | 2.4% | 0.083 |
| Nonalcoholic | 93.8% | 97.3% | |
| Socioeconomic status | |||
| Lower | 40.6% | 51.3% | 0.047 |
| Upper | 59.4% | 48.7% | |
| Posterior capsule rupture (present) | 5.5% | 3.4% | 0.325 |
| Absent | 94.5% | 96.6% |
DM, Diabetes mellitus; significant P values in bold
Likewise, in those with POE, the proportion of patients with diabetes who had a history of hypertension (46.1%) was significantly higher than those without hypertension (16.3%) (P < 0.001). Patients in upper socioeconomic strata with diabetes (59.4%) were significantly higher in proportion compared to those without diabetes (48.7%) (P = 0.047).
The proportion of patients that had posterior capsule rupture during surgery was 5.5% in those with diabetes and 3.4% in those without diabetes (P = 0.325). However, the distribution of POE among patients with and without diabetes did not differ with respect to history of smoking (P = 0.321) or the use of alcohol (P = 0.083).
Table 2 shows the results of multivariable binary logistic regression to examine for factors associated with POE in patients with and without diabetes. The outcome measure was patients with diabetes versus those without diabetes. Men had greater odds of having diabetes and POE as compared to women with diabetes and POE, OR = 1.634, 95% CI: 1.01, 2.67 (P = 0.045), and in those with diabetes with history of hypertension (OR = 3.978, 95% CI: 2.437, 6.496 (P < 0.001) than those with no history of hypertension.
Table 2.
Association between diabetes and other demographic variables in postoperative endophthalmitis
| Diabetes vs. No Diabetes | OR | 95% CI for OR | P | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Age ≥50 years | 1.479 | 0.869 | 2.515 | 0.149 |
| <50 years (Ref) | 1 | |||
| Gender (Men) | 1.634 | 1.01 | 2.658 | 0.048 |
| Women (Ref) | 1 | |||
| Hypertension (Present) | 3.961 | 2.425 | 6.471 | <0.001 |
| Absent (Ref) | 1 | |||
| Smoking (Yes) | 1.400 | 0.810 | 2.419 | 0.228 |
| Nonsmoker (Ref) | 1 | |||
| Alcohol use (Yes) | 2.193 | 0.710 | 6.777 | 0.173 |
| Not an alcohol user (Ref) | 1 | |||
| Socioeconomicstatus (Lower) | 0.650 | 0.405 | 1.044 | 0.075 |
| Upper (Ref) | 1 | |||
| PC rupture (present) | 1.331 | 0.426 | 4.158 | 0.623 |
| Absent (Ref) | 1 | |||
PC, Posterior capsule; OR, Odds ratio; CI, Confidence interval; significant P values in bold
In patients in Group A, 123 (46.9%) patients had RBS values of ≤200 mg% before the primary cataract surgery, while 68 patients (53.1%) had blood sugar values of >200 mg% before the cataract surgery and therefore were subject to rapid reduction of blood sugar levels in order to be considered for cataract surgery.
The proportion of patients who had rapid control of preoperative blood sugar level was higher (53.1%) than those whose preoperative blood sugar level was already under control (46.9%), although did not reach statistical significance (P = 0.485) and is shown in Fig. 2.
Figure 2.

The difference between rates of POE between subjects who had rapid control of preoperative blood sugar and in those with already controlled blood sugar levels
Table 3 shows the distribution and the profile of microorganisms in patients with POE in the two groups, identified by culture. The microorganisms were stratified based on gram staining. Accordingly, positive culture results were observed in 45/128 (35%) patients with diabetes and 71/263 (27%) patients without diabetes. Staphylococcus epidermidis was identified in 10/45 (22%) in those with diabetes and 21/71 (29%) in those without diabetes of all the culture-positive cases.
Table 3.
Distribution of organisms by culture in the group with and without diabetes
| Microorganism groups | DM | No DM | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| n=128 | % | n=263 | % | ||
| Culture positive cases | 45/128 | 35 | 71/263 | 27 | |
| GP Cocci (Clusters) | |||||
| Coagulase-positive | |||||
| Staphylococcus aureus | 3 | 2.34 | 6 | 2.28 | |
| Coagulase-negative | |||||
| Staphylococcus epidermidis | 10 | 7.81 | 21 | 7.98 | |
| Staphylococcus warneri | 1 | 0.78 | 0 | 0.00 | |
| Staphylococcus lugdunensis | 1 | 0.78 | 0 | 0.00 | |
| Staphylococcus pasteuri | 1 | 0.78 | 0 | 0.00 | |
| Staphylococcus pneumoniae | 0 | 0.00 | 1 | 0.38 | |
| Staphylococcus haemolyticus | 1 | 0.78 | 0 | 0.00 | |
| Staphylococcus hominis | 1 | 0.78 | 1 | 0.38 | |
| Micrococci | 0 | 0.00 | 1 | 0.38 | |
| Dermacoccus nishinomiyaensis | 2 | 1.56 | 0 | 0.00 | |
| GP Cocci (Chains) | |||||
| Viridans streptococci | 1 | 0.78 | 4 | 1.52 | |
| Enterococcus fecalis | 1 | 0.78 | 2 | 0.76 | |
| Streptococcus pneumoniae | 0 | 0.00 | 5 | 1.90 | |
| Streptococcus pyogenes | 0 | 0.00 | 1 | 0.38 | |
| SUBTOTAL | 22 | 17.19 | 42 | 15.97 | 0.862 |
| GP Bacilli | |||||
| Nocardia | 0 | 0.00 | 1 | 0.38 | |
| Cornybacterium | 1 | 0.78 | 2 | 0.76 | |
| Propionibacterium acnes | 1 | 0.78 | 0 | 0.00 | |
| SUBTOTAL | 2 | 1.56 | 3 | 1.14 | 0.844 |
| GN Bacilli (nonfermenting) | |||||
| Alkaligenes fecalis | 4 | 3.13 | 1 | 0.38 | |
| Pseudomonas stutzeri | 0 | 0.00 | 3 | 1.14 | |
| Pseudomonas aeruginosa | 2 | 1.56 | 4 | 1.52 | |
| Pseudomonas sp. | 1 | 0.78 | 0 | 0.00 | |
| Pseudomonas luteola | 0 | 0.00 | 1 | 0.38 | |
| Pseudomonas stutzeri | 0 | 0.00 | 1 | 0.38 | |
| Ralstonia insidiosa | 1 | 0.78 | 0 | 0.00 | |
| Stenotrophomonas maltophilia | 0 | 0.00 | 1 | 0.38 | |
| GN Bacilli (Fermenting Enterobacteriaceae) | |||||
| Proteus vulgaris | 0 | 0.00 | 1 | 0.38 | |
| Citrobacter koseri | 4 | 3.13 | 1 | 0.38 | |
| Enterobacter cloacae | 1 | 0.78 | 1 | 0.38 | |
| Klebsiella pneumoniae | 2 | 1.56 | 0 | 0.00 | |
| Enterobacter | 1 | 0.78 | 0 | 0.00 | |
| Enterobacter aerogenes | 0 | 0.00 | 1 | 0.38 | |
| Providencia alcalifaciens | 2 | 1.56 | 1 | 0.38 | |
| Haemophilus | 0 | 0.00 | 1 | 0.38 | |
| Pantoea sp. | 0 | 0.00 | 1 | 0.38 | |
| SUBTOTAL | 18 | 14.06 | 18 | 6.84 | 0.194 |
| Fungi | |||||
| Aspergillus flavus | 1 | 0.78 | 1 | 0.38 | |
| Ascotricha species | 0 | 0.00 | 1 | 0.38 | |
| Aspergillus terreus | 0 | 0.00 | 1 | 0.38 | |
| Fusarium | 1 | 0.78 | 0 | 0.00 | |
| SUBTOTAL | 2 | 1.56 | 3 | 1.14 | 0.844 |
| Other combinations | |||||
| Pseudomonas Aeurginosa, Kliebsella, Enterococci, Actinobacter | 0 | 0.00 | 1 | 0.38 | |
| Acinetobacter calcoaceticus | 0 | 0.00 | 1 | 0.38 | |
| Capnocytophaga sp. and Aspergillus stellatus | 0 | 0.00 | 1 | 0.38 | |
| S. Epidermidis, Pseudomonas Aeruginosa | 1 | 0.78 | 0 | 0.00 | |
| Sphingomonas paucimobilis, S. Epidermidis, Pseudomonas Aeurginosa | 0 | 0.00 | 1 | 0.38 | |
| Enterobacter aerogenes, Trichosporon beigili | 0 | 0.00 | 1 | 0.38 | |
| SUBTOTAL | 1 | 0.78 | 5 | 1.90 | 0.624 |
| No growth | 83 | 64.80 | 188 | 71.40 | |
| Not done | 0 | 0.00 | 4 | 1.52 | |
| Total n | 128 | 263 | |||
DM, Diabetes mellitus; GP, Gram positive; GN, Gram negative; sp, Species
The proportions of microorganisms in POE were not significantly different in the diabetic group compared to the nondiabetic group [Table 3].
Discussion
In spite of improvement in surgical techniques and infection prevention measures, endophthalmitis continues to be a serious complication of ophthalmic surgery in a small percentage of patients. The rate of endophthalmitis after ocular surgery is approximately 0.12% (range 0.05–0.3%)[2] depending on type of surgery. There are good evidence regarding the ocular and periocular risk factors, contamination of surgical environment, and patient hygienic factors in causing endophthalmitis.[15] However, there is paucity of information regarding the influence of systemic conditions like diabetes in POE, wherein a compromised immune status can lead to infection.[15,16] We found that among the POE, the distribution is similar in both diabetes and no diabetes group. A third (32.7%) of patients with endophthalmitis were diabetic, which is consistent with that observed in the current study. Shimizu et al.[17] also found similar distribution, with 26% of those with endophthalmitis were diabetic subjects. However, among the subjects enrolled in the endophthalmitis vitrectomy study fewer had diabetes (13.8%).[18]
Several studies have reported increased rates of adverse postsurgical events among men including endophthalmitis.[19] We also found a similar trend among people with diabetes. Possible explanations for the higher proportions of in male patients in the diabetes group may include behavioral differences (e.g., adherence to postoperative instructions and antibiotic use) and differences in bacterial flora between the genders.[20,21]
We observed a greater proportion of patients who underwent a rapid reduction of blood sugar levels compared with normal blood sugar group in those with POE. This association has not been reported earlier. Normal levels of blood glucose in the postoperative period results in less rates of infections and decreased morbidity and mortality in both diabetic and nondiabetic patients.[22,23,24]
In people with diabetes, the association between hyperglycemia and the susceptibility to infection is well established.[25] A likely explanation for this could be impairment of neutrophil adherence, chemotaxis, phagocytosis, and intracellular bactericidal activity.[26,27,28] The degree of neutrophil impairment correlates with the degree of hyperglycemia. Though some of the defects can be partially reversed in vivo with intensive insulin treatment, there are changes in vascular permeability and alterations in the normal redox reactions caused by hyperglycemia, which probably needs more sustained control. These changes create a state of pseudohypoxia that further impairs the tissue defenses.[29]
Staphylococcus epidermidis was the most commonly observed organism in both the groups in our study. Staphylococcus epidermidis is a coagulase-negative Gram-positive coccus and is reported to be a common cause of culture-proven endophthalmitis,[30,31] which is again in alignment with our study results. Similar to our study, other studies observed a preponderance of Staphylococcus in subjects with POE with and without diabetes.[11,32]
There are several limitations to the present study. The study was conducted with patients from a single hospital site. Although we adjusted for several factors like age, history of hypertension, smoking, usage of alcohol, and socioeconomic strata, we did not control for other intraoperative factors like prolonged surgical time, etc., which also may have affected the results. As a routine protocol, PPBS testing was performed in all patients with a high RBS levels; however, HbA1c may be an ideal laboratory investigation. In addition, a single RBS can change with various factors such as the quantity of food, inactivity, not enough insulin or oral diabetes medications, side effects from other medications, such as steroids or antipsychotic medications, illness, stress, which can produce hormones that raise blood glucose levels, and menstrual periods, which cause changes in hormone levels.[33] Therefore, many factors could affect the single RBS reading in our study.
In this series, none of the patients had intracameral antibiotics. Therefore, this factor has been ruled out. In addition, the presence of posterior capsule rupture during cataract surgery was 5.5% in those with diabetes and 3.4% in those without diabetes and was not significantly associated with POE on multivariable regression analysis, when adjusted for age, gender, socioeconomic strata, history of smoking, and the usage of alcohol.
Conclusion
In conclusion, the current study shows that the rate of POE at a single institution is 0.09% over a 26-year time period. Those with POE had higher proportions of patients who underwent a rapid reduction of preoperative blood sugar levels than those with preoperative blood sugar levels under control. Men with diabetes have greater odds of having POE compared with men without diabetes and the odds are more than double for those with diabetes with a history of hypertension. Given that endophthalmitis can result in poor clinical outcome in the majority of cases, it may be extrapolated that a more cautious approach may be required in case of one-eyed and immunocompromised patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kresloff MS, Castellarin AA, Zarbin MA. Endophthalmitis. Surv Ophthalmol. 1998;43:193–224. doi: 10.1016/s0039-6257(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 2.Kattan HM, Flynn HW, Jr, Pflugfelder SC, Robertson C, Forster RK. Nosocomial endophthalmitis survey. Current incidence of infection after intraocular surgery. Ophthalmology. 1991;98:227–38. [PubMed] [Google Scholar]
- 3.May D, Peyman G. Endophthalmitis after vitrectomy. Am J Ophthalmol. 1976;81:520–1. doi: 10.1016/0002-9394(76)90314-7. [DOI] [PubMed] [Google Scholar]
- 4.Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidoneiodine. Ophthalmology. 1991;98:1769–75. doi: 10.1016/s0161-6420(91)32052-9. [DOI] [PubMed] [Google Scholar]
- 5.Trinavarat A, Atchaneeyasakul L, Nopmaneejumruslers C, Inson K. Reduction of endophthalmitis rate after cataract surgery with preoperative 5% povidone iodine. Dermatology. 2006;212:35–40. doi: 10.1159/000089197. [DOI] [PubMed] [Google Scholar]
- 6.Duthie OM. Discussion: The principles and practice of asepsis in ophthalmic operations. Trans Ophthalmol Soc UK. 1949;69:365–74. [Google Scholar]
- 7.Starr MB. Prophylactic antibiotics for ophthalmic surgery. Surv Ophthalmol. 1983;27:353–73. doi: 10.1016/0039-6257(83)90193-5. [DOI] [PubMed] [Google Scholar]
- 8.Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitue: Analysis of factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabetes Metab. 1992;18:187–201. [PubMed] [Google Scholar]
- 9.Al-Kassab AS, Raziuddin S. Immune activation and T cell subset abnormalities in circulation of patients with recently diagnosed type I diabetes mellitus. Clin Exp Immunol. 2008;81:267–71. doi: 10.1111/j.1365-2249.1990.tb03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrass C. Fc receptor-mediated phagocytosis: Abnormalities associated with diabetes mellitus. Clin Immunol Immunopathol. 1991;58:1–17. doi: 10.1016/0090-1229(91)90144-y. [DOI] [PubMed] [Google Scholar]
- 11.Phillips WB, 2nd, Tasman WS. Postoperative endophthalmitis in association with diabetes mellitus. Ophthalmology. 1994;101:508–18. doi: 10.1016/s0161-6420(13)31268-8. [DOI] [PubMed] [Google Scholar]
- 12.Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen K, Sandvik L, Aagenaes O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: The Oslo study. BMJ. 1985;290:811–5. doi: 10.1136/bmj.290.6471.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AIOS Round Table Meeting 12th January. 2020. Available from: http://www.aios-scientificcommittee.org/wp-content/uploads/2020/02/AIOS-Guidelines-on-DME_10-02-2020.pdf .
- 14.Guidelines for the Management of Cataract in India A VISION 2020: The Right to Sight INDIA Publication. Available from: https://www.sightsaversindia.in/wp-content/uploads/2019/03/16480_Cataract_Manual_VISION2020.pdf .
- 15.Vaziri K, Pershing S, Albini T, Moshfeghi D, Moshfeghi A. Risk factors predictive of endogenous endophthalmitis among hospitalized patients with hematogenous infections in the United States. Am J Ophthalmol. 2015;159:498–504. doi: 10.1016/j.ajo.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Mavrakanas TA, de Haller R, Philippe J. Endogenous endophthalmitis in a patient with diabetes and foot osteomyelitis. Can J Diabetes. 2015;39:18–20. doi: 10.1016/j.jcjd.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu N, Shimizu K. Incidence and prevention of endophthalmitis following cataract surgery. Jpn J Clin Ophthalmol (RinshoGanka) 1997;51:211–4. [Google Scholar]
- 18.Doft B, Wisniewski S, Kelsey S, Groer-Fitzgerald S. Diabetes and postcataract extraction endophthalmitis. Curr Opin Ophthalmol. 2002;13:147–51. doi: 10.1097/00055735-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Hatch W, Cernat G, Wong D, Devenyi R, Bell C. Risk factors for acute endophthalmitis after cataract surgery: A population-based study. Ophthalmology. 2009;116:425–30. doi: 10.1016/j.ophtha.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 20.Karimsab D, Razak S. Study of aerobic bacterial conjunctival flora in patients with diabetes mellitus. Nepal J Ophthalmol. 2013;5:28–32. doi: 10.3126/nepjoph.v5i1.7818. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Rubio ME, Rebolledo-Lara L, Martinez-García M, Alarcón-Tomás M, Cortés-Valdés C. The conjunctival bacterial pattern of diabetics undergoing cataract surgery. Eye (Lond) 2010;24:825–34. doi: 10.1038/eye.2009.218. [DOI] [PubMed] [Google Scholar]
- 22.Pomposelli J, Baxter JK, Babineau T, Pomfret EA, Driscoll DF, Forse RA, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 23.Furnary A, Zerr K, Grunkemeier G, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352–60. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 24.Preiser J, Devos P, Van den Berghe G. Tight control of glycaemia in critically ill patients. Curr Opin Clin Nutr Metab Care. 2002;5:533–7. doi: 10.1097/00075197-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Pozzilli P, Leslie RD. Infections and diabetes: Mechanisms and prospects for prevention. Diabet Med. 1994;11:935–41. doi: 10.1111/j.1464-5491.1994.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 26.Geerlings SE, Hoepelman A. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 27.Delamaire M, Maugendre D, Moreno M, Le Goff M, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15:256–60. doi: 10.2337/diacare.15.2.256. [DOI] [PubMed] [Google Scholar]
- 29.Williamson J, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, et al. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–13. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 30.Moloney TP, Park J. Microbiological isolates and antibiotic sensitivities in culture-proven endophthalmitis: A 15-year review. Br J Ophthalmol. 2014;98:1492–7. doi: 10.1136/bjophthalmol-2014-305030. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Ji J, Li S, Wang Z, Tang L, Cao W, et al. Microbiological isolates and antibiotic susceptibilities: A 10-year review of culture-proven endophthalmitis cases. Curr Eye Res. 2017;42:443–7. doi: 10.1080/02713683.2016.1188118. [DOI] [PubMed] [Google Scholar]
- 32.Bilen H, Ates O, Astam N, Uslu H, Akcay G, Baykal O. Conjunctival flora in patients with type 1 or type 2 diabetes mellitus. Adv Ther. 2007;24:1028–33. doi: 10.1007/BF02877708. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Good to know: Factors affecting blood glucose. Clin Diabetes. 2018;36:202. doi: 10.2337/cd18-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]


