Abstract
In 3T3-L1 adipocytes, both insulin and endothelin 1 stimulate glucose transport via translocation of the GLUT4 glucose carrier from an intracellular compartment to the cell surface. Yet it remains uncertain as to whether both hormones utilize identical pathways and to what extent each depends on the heterotrimeric G protein Gαq as an intermediary signaling molecule. In this study, we used a novel inducible system to rapidly and synchronously activate expression of a dominant inhibitory form of ADP-ribosylation factor 6, ARF6(T27N), in 3T3-L1 adipocytes and assessed its effects on insulin- and endothelin-stimulated hexose uptake. Expression of ARF6(T27N) in 3T3-L1 adipocytes was without effect on the ability of insulin to stimulate either 2-deoxyglucose uptake or the translocation of GLUT4 or GLUT1 to the plasma membrane. However, the same ARF6 inhibitory mutant blocked the stimulation of hexose uptake and GLUT4 translocation in response to either endothelin 1 or an activated form of Gαq, Gαq(Q209L). These results suggest that endothelin stimulates glucose transport through a pathway that is distinct from that utilized by insulin but is likely to depend on both a heterotrimeric G protein from the Gq family and the small G protein ARF6. These data are consistent with the interpretation that endothelin and insulin stimulate functionally different pools of glucose transporters to be redistributed to the plasma membrane.
In recent years, it has been appreciated increasingly that a ubiquitous mechanism for controlling cellular transport functions is by altering the subcellular distribution of highly specific carrier proteins. A prototype for such processes has been the insulin-dependent activation of glucose transport, in which a sequestered intracellular pool of GLUT4, a specialized hormone response sugar transporter isoform, is rapidly translocated to the cell surface (4). Despite considerable investigation, the precise intracellular pathways trafficked by GLUT4 as well as the key regulatory mechanisms have proven refractory to a detailed understanding. Nonetheless, it is clear that exocytosis of GLUT4 represents the major insulin-regulated event and that transporters continue to recycle in the presence of insulin (31). Moreover, the signaling pathway initiated by insulin appears to depend on the activity of phosphatidylinositol (PI) 3-kinase to elicit increases in hexose uptake as well as to modulate other metabolic processes (11). Given this information, much attention has focused on trafficking events recognized as dependent on or influenced by the abundance of phosphatidylinositides (9, 11).
ADP-ribosylation factor 6 (ARF6) is a small GTP-binding protein remarkable for its ability to influence both vesicular trafficking and actin cytoskeletal remodeling in mammalian cells. Initially implicated in the regulation of the endocytic pathway, more recent data have demonstrated a role for ARF6 in regulated secretion; moreover, ARF6 appears to play a role in a recycling pathway in which membrane from an endosomal-like compartment is translocated to the cell surface (7, 8, 14, 15, 18, 32, 34). However, it remains unclear whether the intracellular structure to which ARF6 localizes and which cycles to the plasma membrane represents a subpopulation of endosomes or a novel compartment (34). As with other members of the Ras superfamily, ARF6 is activated by a guanine nucleotide exchange factor that releases the GDP from the inactive protein, allowing GTP to bind and convert ARF6 to its activated form (29). While the relevant in vivo exchange factors for ARF6 remain uncertain, ARF6 activity appears to be regulated by PI 3-kinase. A class of PI-3,4,5-trisphosphate-dependent exchange proteins demonstrate activity for ARF6 in vitro and, in some cases, colocalize with ARF6 in vivo (reviewed in references 10 and 12). Thus, given that both PI 3-kinase and ARF6 have been shown to regulate vesicular trafficking at the plasma membrane, it has been proposed that PI 3-kinase regulates vesicular trafficking by controlling the activation state of ARF6 (10). Such a model has also proven attractive in regard to insulin-regulated glucose uptake. Results of peptide inhibition studies using permeabilized cells have suggested that in 3T3-L1 adipocytes, ARF6 may be involved in the insulin-stimulated translocation of GLUT4 vesicles to the plasma membrane (27). However, subsequent experiments using 3T3-L1 adipocytes expressing a GTP-binding-deficient mutant of ARF6 were unable to establish such a role for the G protein (42). In contrast, ARF6 was found to regulate the insulin-stimulated secretion of adipsin from 3T3-L1 adipocytes (42).
It has been reported recently that growth factors acting through the heterotrimeric G protein Gq promote the translocation of an ARF6-containing vesicular compartment to the cell surface (6). In 3T3-L1 adipocytes, stimulation of an analogous pathway results in translocation of GLUT4-containing vesicles to the plasma membrane, thus increasing glucose uptake (22–24, 41). Specifically, endothelin 1 stimulates glucose transport through the type A endothelin, G-protein-coupled receptor via a mechanism that depends on activation of a member of the Gq family of heterotrimeric G proteins. In the accompanying article, Bose et al. present evidence that the Gα11 isoform may be the physiologically relevant isoform in adipocytes (5). In some studies, the ability of Gαq/11 to stimulate GLUT4 translocation has been dependent on p110α, the catalytic subunit of PI 3-kinase (22). Since insulin-stimulated GLUT4 translocation is also dependent on the activation of p110α, it has been suggested that the endothelin and insulin signaling pathways converge at PI 3-kinase and use a common mechanism to accelerate glucose transport (20, 22). However, since others have not been able to demonstrate a role for PI 3-kinase in mediating GLUT4 translocation following endothelin stimulation, the endothelin pathway downstream of a Gq family member remains unclear (5, 24, 41). Moreover, Gαq/11 has been proposed to be of general importance in signaling to glucose transport, participating as a necessary factor in the regulation of this process by insulin as well as receptors which couple to heterotrimeric G proteins in a classical manner (23, 24). However, it still remains possible that insulin and endothelin activate glucose transport by distinct mechanisms, with only the latter mediated by Gαq. Thus, in an effort to better understand the downstream events leading to alterations in membrane protein trafficking in 3T3-L1 adipocytes, we have assessed the role of the small G protein ARF6 in the regulation of glucose uptake by endothelin and insulin. To accomplish this, we developed a novel inducible system that allows for the efficient and synchronous expression of genes in terminally differentiated 3T3-L1 adipocytes. We have used this system to confer expression of a dominant inhibitory construct of ARF6, ARF6(T27N), in these cells. We show that endothelin- but not insulin-stimulated increases in hexose uptake and GLUT4 translocation are dependent on ARF6, thus functionally distinguishing the pathways through which each of the stimuli increase glucose uptake.
MATERIALS AND METHODS
Virus and general reagents.
The recombinant adenovirus expressing Cre recombinase (AdCre) was a generous gift from Frank L. Graham, McMaster University (Hamilton, Ontario, Canada) (3). The GTPase-deficient, activated Gαq(Q209L) mutant recombinant adenovirus was a generous gift from Jerrold M. Olefsky, University of California, San Diego (La Jolla) (1). The generation and characterization of the polyclonal sheep anti-GLUT4 and the polyclonal sheep anti-GLUT1 antisera have been previously described (21). Affinity-purified rhodamine-conjugated donkey anti-sheep antibody was purchased from Jackson ImmunoResearch (West Grove, Pa.). Affinity-purified rabbit anti-hemagglutinin (HA), mouse anti-ARF6, and all horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.). The rabbit anti-green fluorescent protein (GFP) antiserum was purchased from Clontech (Palo Alto, Calif.). Bovine serum albumin (BSA) was purchased from Calbiochem. [1,2-3H]-2-deoxy-d-glucose was purchased from NEN Life Sciences Products (Boston, Mass.). Endothelin 1 and all other chemicals were purchased from Sigma.
Generation of plasmid constructs.
The inducible plasmid generated in this study is a derivative of the murine retroviral vector pLNCX1, which contains a neomycin resistance (Neor) gene to allow for the selection of stably infected cells (Clontech Laboratories Inc., Palo Alto, Calif.). The loxP sequence 5′ to the Neor cassette was generated by annealing the synthetic oligonucleotides 5′-GAT CCG CTA GCA GTT AAC CGG TAT AAC TTC GTA TAG CAT ACA TTA TAC GAA GTT ATT TAA ATG-3′ and 5′-GAT CCA TTT AAA TAA CTT CGT ATA ATG TAT GCT ATA CGA AGT TAT ACC GGT TAA CTG CTA GCG-3′ and cloning them into the BclI site in pLNCX1. The second loxP sequence, 3′ to the Neor cassette, was generated by annealing the synthetic oligonucleotides 5′-GAT CAA TAA CTT CGT ATA GCA TAC ATT ATA CGA AGT TAT CAA TTG GCC TAG GTC TCG AGT A-3′ and 5′-AGC TTA CTC GAG ACC TAG GCC AAT TGA TAA CTT CGT ATA ATG TAT GCT ATA CGA AGT TAT T-3′ and cloning them between the unique BamHI and HindIII sites. Insertion of this loxP sequence also removed the cytomegalovirus immediate-early promoter region. Proper orientation and fidelity of the loxP sites was determined through sequence analysis of the indicated regions. The resulting plasmid was designated pLPNPX1, following the standard nomenclature for retroviral constructs. The HA-tagged human ARF6(T27N) construct, a generous gift from Julie G. Donaldson, National Institutes of Health (Bethesda, Md.), and enhanced GFP (EGFP) from plasmid pEGFP-N1 (Clontech Inc.) were then cloned into pLPNPX1, using the unique XhoI/BglII sites and the XhoI/NotI sites, respectively. All constructs were sequenced, and no errors were found.
Cell culture, retroviral infection, and adenoviral infection.
3T3-L1 fibroblasts were cultured and differentiated as described (19). Prior to analysis 9 days following the initiation of differentiation, adipocytes were serum starved for 2 h in Leibovitz L-15 medium containing 0.2% BSA at 37°C in room air.
Stably transfected sublines of 3T3-L1 fibroblasts containing the indicated inducible retroviral vector were generated through the use of retrovirus-mediated gene transfer. To achieve this, a 60-mm-diameter culture of 293T cells was transiently transfected using calcium phosphate with 2 μg each of the two pantropic retroviral packaging constructs, pVSV G and pCgp, a generous gift from Michael H. Malim, University of Pennsylvania (Philadelphia), and a pLPNPX1 derivative containing either EGFP or ARF6(T27N). Cell-free viral supernatants were harvested at 24 h and used to infect 3T3-L1 fibroblasts. Stably infected populations were selected 48 h later in medium containing G418 (600 μg/ml [active concentration]), pooled, and maintained in G418-containing medium until initiation of differentiation or infection with AdCre, whichever came first. To induce expression in the transduced populations, fibroblasts were infected with AdCre at a multiplicity of infection of 4,000 for 24 h at 37°C in a minimal volume of Dulbecco modified Eagle medium containing 0.5% BSA. Differentiated adipocytes were infected similarly for 48 h, with the initiation of infection beginning on day 3 of differentiation for AdCre infection and on day 6 for infection with Gαq(Q209L). All experiments on adipocytes were performed 9 days postdifferentiation.
Glucose uptake and GLUT1 and GLUT4 translocation assays.
The methods for measuring 2-deoxy-d-glucose uptake rates and plasma membrane GLUT1 and GLUT4 levels by the plasma membrane sheets assay have been described elsewhere (16). Digital image acquisition, processing, and automated quantitation of plasma membrane fluorescence intensity were performed using MetaMorph imaging system software (Universal Imaging Corporation, West Chester, Pa.). This system of automated quantitation uses the signal obtained by staining the lipid components of the remaining plasma membranes to create a mask that is then used to identify areas to measure the fluorescence intensity from corresponding images displaying GLUT1 or GLUT4 staining. Using this method, six randomly selected fields of view each containing 60 to 100 plasma membrane sheets were analyzed per experiment. All experiments were performed a minimum of three times, and the data presented represent the average ± standard error of the mean (SEM) of these replicates.
Protein immunoblotting and Northern analysis.
For Western blot analysis of expressed proteins, 25 μg of total cellular lysate, obtained by lysis in 2% sodium dodecyl sulfate (SDS)–66 mM Tris (pH 7.5), was submitted to SDS-polyacrylamide gel (PAGE) electrophoresis on a 12.5% polyacrylamide gel under reduced conditions. Following transfer to polyvinylidene difluoride membranes, the membranes were subjected to immunoblotting using the antibodies indicated in the figures and HRP-conjugated secondary antibodies. Western blots were developed using enhanced chemiluminescence (Amersham Pharmacia Biotech). For Northern analysis of the transduced retroviral message, 5 μg of total cellular RNA was fractionated by electrophoresis in a 0.8% agarose gel in the presence of 50% formamide. Following transfer to a nylon membrane, the retroviral mRNA was detected with a random-primed DNA probe generated to a 0.6-kb portion of the retroviral transcript 3′ to the second loxP site.
RESULTS
Generation of a Cre-inducible retroviral vector.
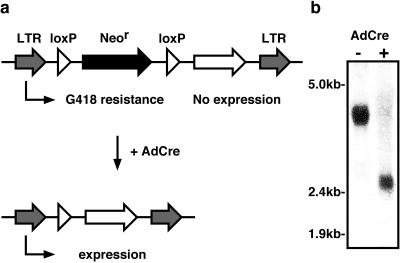
Technical limitations have made it difficult to conditionally express genes in differentiated 3T3-L1 adipocytes. Recombinant vectors based on retroviruses and adenovirus as well as electroporation are now frequently used to introduce genes into 3T3-L1 cells, but all suffer from serious deficiencies. Given these limitations, we sought to develop a new system for inducible expression that utilizes many of the strengths of both retroviruses and adenovirus but eliminates their drawbacks. Using the retroviral vector pLNCX as a backbone, annealed synthetic oligonucleotides were used to create loxP sites on either side of the Neor gene, generating pLPNPX1. In doing so, the original cytomegalovirus immediate-early promoter was removed. This created a retroviral vector that, when inserted into the host cell's genome, generates a single polycistronic mRNA transcribed from the viral promoter contained within the 5′ long terminal repeat (LTR). This single mRNA contains coding regions for both the Neor gene followed by the gene of interest. Since the Neor gene is first, it is translated and provides an efficient means to select stably infected cells. Translation of the inducible gene is extremely rare due to the lack of an internal ribosomal entry site in the polycistronic mRNA (Fig. 1a). However, upon infection of the cells with AdCre, homologous recombination between the two loxP sites flanking the Neor gene removes this region, thereby moving the inducible cDNA into position as the first open reading frame and allowing it to be expressed (Fig. 1a).
FIG. 1.
Schematic of inducible retroviral vector. (a) The retroviral vector, designated pLPNPX1, contains the retroviral LTRs and a selectable Neor from the retroviral vector pLNCX for selection of infected cells. The Neor gene is flanked by loxP sequences and immediately followed by a multiple cloning site into which the cDNA of interest can be cloned. Since the only promoter is in the 5′ LTR, a single polycistronic message is generated from the integrated retrovirus which allows for the translation of the Neor gene but does not permit translation of the open reading frame cloned downstream of it. This provides for the efficient selection of infected cells without expression of the introduced gene product. When expression of the gene of interest is desired, the cells are infected with AdCre. The Cre recombinase catalyzes recombination between the two loxP sites, thus removing the Neor cassette and allowing translation of the foreign gene. (b) 3T3-L1 fibroblasts stably transduced with the inducible retrovirus were mock infected (−) or infected with AdCre (+). Total RNA was collected 3 days following the infection and subjected to Northern blot analysis with the retroviral probe that falls outside the loxP sites. A representative hybridization pattern is presented, with the approximate locations of the molecular markers indicated. The two predominant species represent genomic transcripts before (3.8 kb) and after (2.6 kb) excision of the Neor cassette.
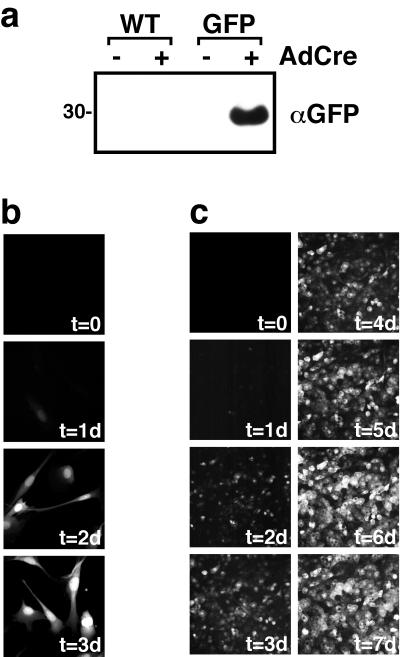
To demonstrate that only one transcript is generated from the inserted retrovirus and that the desired recombination event occurs as predicted, Northern blot analysis of the retroviral transcript was performed. 3T3-L1 preadipocytes were infected with a recombinant retrovirus generated from a plasmid in which the ARF6 cDNA was inserted into the multiple cloning site downstream of the second loxP sequence in pLPNPX1. Since the insert is 0.6 kb, the integrated retrovirus would be predicted to generate a genomic transcript of about 3.8 kb before Cre-catalyzed homologous recombination and 2.6 kb after excision of the Neor cassette (Fig. 1a). As shown in Fig. 1b, Northern blot analysis of RNA extracted from stably transduced 3T3-L1 fibroblasts prior to infection with AdCre revealed a single RNA species of the expected ∼3.8-kb size. Three days following infection with AdCre, the transcript size was reduced by ∼1.2 kb to ∼2.6 kb (Fig. 1b), consistent with the loss of the ∼1.2-kb Neor gene contained between the loxP sites. Under these conditions, there was little detectable full-length transcript remaining, indicating excision in virtually all of the cells expressing the retroviral mRNA. To demonstrate the utility of this system for the expression of genes of interest, we cloned a cDNA encoding GFP into the inducible retrovirus vector and used this recombinant retrovirus to generate pools of G418-resistant 3T3-L1 preadipocytes. Western blot analysis of lysates obtained from these cells prior to infection by AdCre showed no detectable GFP. However, 3 days following infection of the same polyclonal population of 3T3-L1 fibroblasts with AdCre, GFP was easily detected by Western blotting (Fig. 2a). As expected, infection of parental, nontransgenic 3T3-L1 fibroblasts did not lead to the expression of GFP. The time course for induction of GFP expression in 3T3-L1 fibroblasts was relatively rapid, such that expression could be easily observed at 24 h following infection with AdCre, and by 48 h uniform, robust expression was achieved (Fig. 2b). In differentiated 3T3-L1 adipocytes, the time required for induction of GFP expression was slightly longer, but a uniformly high level of expression was still obtained in 85 to 90% of cells by 5 to 7 days following infection with AdCre (Fig. 2c). The increased time required for expression in differentiated 3T3-L1 adipocytes is likely to reflect the relative resistance of these cells compared to fibroblasts to infection with AdCre.
FIG. 2.
Expression of a reporter GFP can be tightly controlled with the AdCre-inducible retrovirus. (a) Wild-type (WT) 3T3-L1 fibroblasts or 3T3-L1 fibroblasts stably infected with a version of the inducible retrovirus designed to encode GFP were mock infected (−) or infected with AdCre (+). Three days later, total cellular lysates were collected and subjected to SDS-PAGE and Western immunoblotting with anti-GFP antisera. A representative blot is presented. Size is indicated in kilodaltons (b) 3T3-L1 fibroblasts that express GFP in response to AdCre infection were fixed at various times following infection with AdCre at time zero (t = 0). A representative composite photomicrograph depicting the relative levels of GFP fluorescence at the indicated number of days (xd) following infection with AdCre is presented. (c) 3T3-L1 adipocytes that express GFP in response to AdCre were infected with AdCre at time zero, and the fluorescence due to GFP from the same field of view was monitored daily. A representative composite photomicrograph depicting the relative levels of GFP fluorescence at the indicated number of days following infection with AdCre is presented. In panels b and c, time zero represents the signal from cells immediately prior to infection.
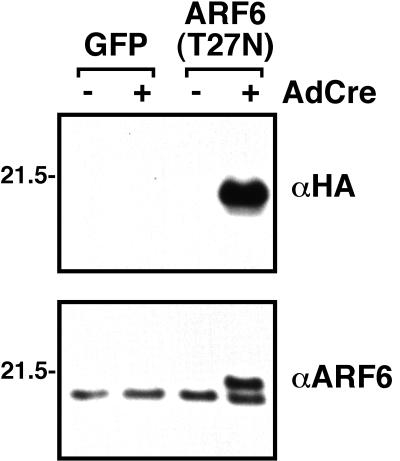
We have taken advantage of this retroviral system that drives highly regulated expression of foreign gene products to study the effects of ARF6(T27N) in 3T3-L1 adipocytes. Consistent with prior experience in other tissue culture systems, it was not possible to obtain significant numbers of 3T3-L1 fibroblasts stably expressing ARF6(T27N); constitutive expression of the mutant protein yielded few G418-resistant cells, and those that survived grew slowly and displayed abnormal morphology (data not shown). For this reason, a cDNA encoding ARF6(T27N) was cloned into pLPNPX1, retrovirus was prepared by transient transfection, and 3T3-L1 preadipocytes were infected with the recombinant retrovirus. Pools of G418-resistant cells were expanded and noted to be indistinguishable from parental 3T3-L1 cells with respect to growth kinetics and morphology. As shown in Fig. 3, Western immunoblot analysis of lysates obtained from these cells indicated that they also displayed highly regulated expression of ARF6(T27N). In lysates probed with an antibody which recognizes the HA epitope tag fused to the carboxyl terminus of the ARF6(T27N) protein, expression of the epitope-tagged transgene was detected only after Cre-catalyzed excision of the Neor cassette following infection with AdCre; no immunoreactive protein was detected in the GFP-expressing cell line (Fig. 3, top). Upon Western blotting with an antibody against ARF6, expression of the epitope-tagged ARF6(T27N), which displays slightly slower electrophoretic mobility than the endogenous ARF6, is visualized only following induction by AdCre (Fig. 3, bottom). Use of the ARF6 antibody also allows us to estimate the level of overexpression of the mutant protein at one to two times that of endogenous ARF6 (Fig. 3). Though this modest level of overexpression is optimal for the experiments described below because it minimizes the possibility of nonspecific inhibition of other ARF-dependent pathways, we have observed as much as 50- to 100-fold overexpression of other proteins with this system (data not shown).
FIG. 3.
The expression of ARF6(T27N) can be tightly controlled by AdCre. 3T3-L1 preadipocytes were stably infected with a version of the inducible retrovirus designed to encode either GFP or ARF6(T27N) following AdCre infection. Adipocytes from these GFP and ARF6(T27N) lines were then either mock infected (−) or infected with AdCre (+). Total cellular lysates were prepared 6 days later and subjected to SDS-PAGE and Western immunoblotting with the antisera indicated. Even following extended exposure, virtually no HA-tagged ARF6(T27N) could be detected prior to infection with AdCre. Sizes are indicated in kilodaltons.
Expression of a dominant inhibitory ARF6 protein does not block insulin-stimulated glucose uptake but inhibits uptake stimulated by the endothelin/Gαq pathway.
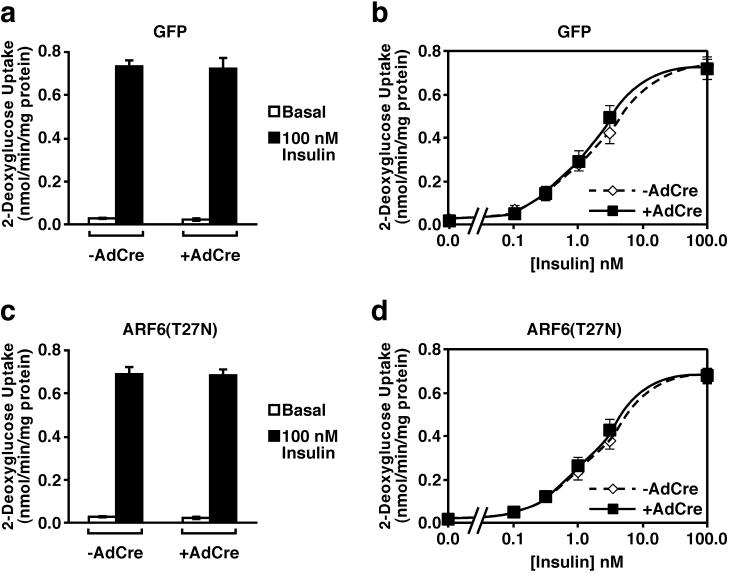
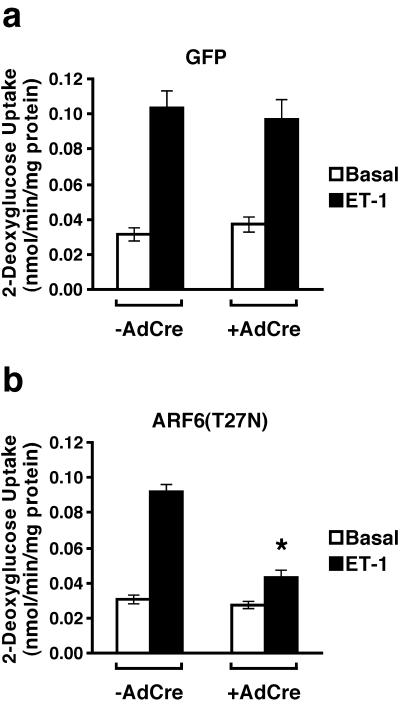
3T3-L1 adipocytes infected with virus containing cDNAs encoding either GFP or ARF6(T27N) were infected with AdCre, allowing expression of the ectopically expressed proteins, and hexose uptake was measured. Induction of expression of GFP by AdCre did not alter the rates of either basal or insulin-stimulated 2-deoxyglucose uptake (Fig. 4a). Similarly, expression of ARF6(T27N) upon infection with AdCre did not affect significantly either basal or insulin-stimulated 2-deoxyglucose uptake (Fig. 4b). The latter data are consistent with what has been reported for another GTP-binding-deficient mutant of ARF6 (42). To exclude an effect of the dominant negative ARF6 on the sensitivity of the cell to insulin, 2-deoxyglucose uptake was measured in the presence of submaximal insulin concentrations. Dominant negative ARF6 did not alter the ability of insulin to stimulate 2-deoxyglucose uptake at any concentration of the hormone tested (Fig. 4c and d).
FIG. 4.
Expression of a dominant negative mutant of ARF6 in 3T3-L1 adipocytes does not affect insulin-stimulated hexose uptake. Six days following induction of GFP (a and b) or ARF6(T27N) (c and d) expression by infection with AdCre, 3T3-L1 adipocytes were treated with the indicated concentration of insulin for 20 min or left in medium without hormone, and the uptake of 2-[3H]deoxyglucose was measured. Nonspecific uptake measured in the presence cytochalasin B in the absence of insulin was subtracted from all values. The results shown are the means ± SEM of four independent experiments, each performed in triplicate.
Since Gq-coupled receptors stimulate both the translocation of an ARF6-containing vesicular compartment and GLUT4-containing vesicles to the plasma membrane, we decided to test whether the GLUT4 translocation caused by treatment of 3T3-L1 adipocytes with endothelin 1 was dependent on ARF6. Endothelin 1 activates 2-deoxyglucose uptake in 3T3-L1 adipocytes three- to fivefold; as expected, induction of GFP expression by infection of control cells with AdCre did not significantly alter this response (Fig. 5a). However, when AdCre was used to initiate the expression of a dominant inhibitory form of ARF6, the ability of endothelin 1 to stimulate 2-deoxyglucose uptake was inhibited by ∼60% (Fig. 5b).
FIG. 5.
Expression of a dominant inhibitory mutant of ARF6 in 3T3-L1 adipocytes inhibits the ability of endothelin-1 (ET-1) to stimulate hexose uptake. Six days following induction of GFP (a) or ARF6(T27N) (b) expression by infection with AdCre, 3T3-L1 adipocytes were treated with 10 nM endothelin 1 for 20 min or left in medium without agonist, and the uptake of 2-[3H]deoxyglucose was measured as in Fig. 4. The results shown are the means ± SEM of four independent experiments, each performed in triplicate. The asterisk denotes P = 0.0004 comparing endothelin 1-stimulated ARF6(T27N) 3T3-L1 adipocytes not infected and infected with AdCre. The endothelin 1-stimulated ARF6(T27N) 3T3-L1 adipocytes infected with AdCre are also significantly different (P = 0.02) compared to the endothelin 1-stimulated GFP cells infected with AdCre.
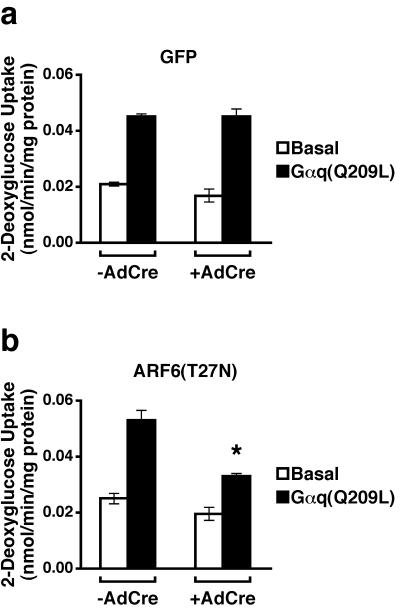
In principle, the effects of ARF6(T27N) on endothelin action might occur at the level of the receptor or at a point in the downstream signaling pathway. Gαq/11, which is thought to mediate the action of endothelin on hexose uptake, has also been implicated as an important modulator of ARF6 (6). To determine whether the effect of ARF6(T27N) on endothelin-induced hexose uptake occurs at a site downstream of the endothelin receptor, we bypassed the latter by increasing 2- deoxyglucose uptake with an adenovirus expressing a constitutively active form of Gαq, Gαq(Q209L). Expression of Gαq(Q209L) in 3T3-L1 adipocytes in which GFP expression had been induced stimulated glucose uptake about twofold (Fig. 6a). Activation of 2-deoxyglucose transport by Gαq(Q209L) was inhibited by ARF6(T27N) to an extent similar to that seen following exposure of cells to endothelin (Fig. 5b and 6b). These data are most consistent with the idea that endothelin produces its effects on hexose uptake via Gαq, or the extremely homologous Gα11 isoform, and that the site of action of ARF6(T27N) is at or after the heterotrimeric G protein.
FIG. 6.
Expression of a dominant negative mutant of ARF6 in 3T3-L1 adipocytes inhibits the ability of activated Gαq to stimulate hexose uptake. Three days following induction of GFP (a) or ARF6(T27N) (b) expression by AdCre, adipocytes were either mock infected (Basal) or infected with a recombinant adenovirus expressing Gαq(Q209L) (multiplicity of infection of ∼20). The uptake of 2-[3H]deoxyglucose was measured 72 h later. The results shown are the means ± SEM of four independent experiments, each performed in duplicate. The asterisk denotes the statistically significant difference (P = 0.003) for Gαq-stimulated ARF6(T27N) cells not infected compared to infected with AdCre and that (P = 0.003) for Gαq-stimulated AdCre-infected ARF6(T27N) cells compared to similarly treated GFP cells.
Expression of a dominant negative ARF6 does not block insulin-stimulated GLUT4 or GLUT1 translocation but does inhibit GLUT4 translocation in response to endothelin.
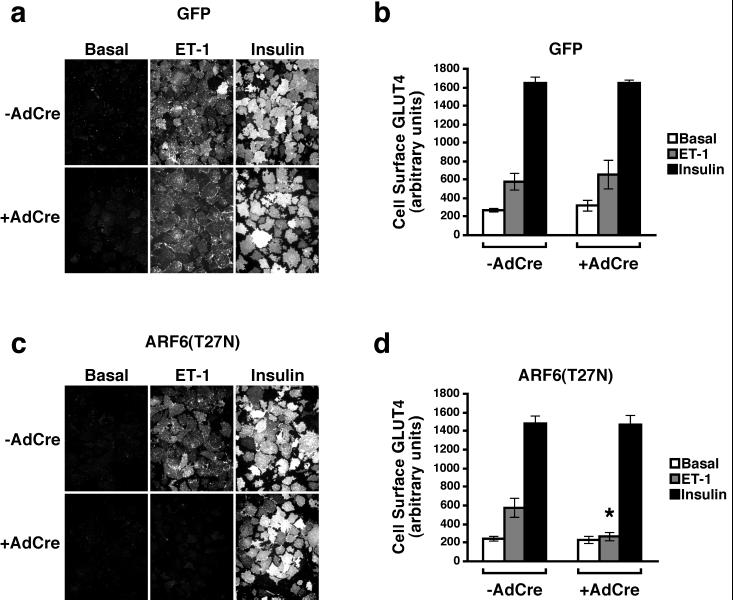
In the basal state, GLUT4 is sequestered in a perinuclear tubulovesicular compartment; upon insulin treatment, the transporter redistributes to the plasma membrane, such that it increases about 10- to 20-fold on the cell surface (4). In 3T3-L1 adipocytes, another transporter isoform, GLUT1, is expressed in greater abundance than GLUT4 and also retained in an intracellular pool. However, intracellular sequestration of GLUT1 is less efficient than that for GLUT4, such that insulin generates only a three- to fivefold increase in plasma membrane GLUT1. Nonetheless, this degree of translocation might well explain relatively modest increases in glucose transport, such as that produced by exposure of cells to endothelin. To assess the extent of translocation of each of the transporters under the conditions described above, we used the plasma membrane sheets assay to monitor the levels of GLUT1 and GLUT4 in the plasma membrane of 3T3-L1 adipocytes. Thus, 3T3-L1 adipocytes in which GFP or ARF6(T27N) expression was induced were treated with insulin or endothelin 1, and plasma membrane sheets were prepared. As shown in Fig. 7a, both hormones readily increased the abundance of GLUT4 on the cell surface of control 3T3-L1 adipocytes. Quantitation of a series of experiments indicated that the pattern of GLUT4 translocation in response to either insulin or endothelin was similar to the ability of each of these stimuli to increase hexose uptake (compare Fig. 7b to Fig. 4 and 5). As expected, induction of GFP by infection of these cells with AdCre was without effect on the GLUT4 translocation evoked by treatment with insulin or endothelin. Similarly, expression of ARF6(T27N) did not reduce the ability of insulin to stimulate GLUT4 translocation (Fig. 7c). In marked contrast, when infection of 3T3-L1 adipocytes by AdCre was used to turn on expression of the dominant inhibitory form of ARF6, endothelin 1 was significantly blocked in its ability to elicit GLUT4 translocation (Fig. 7c and d).
FIG. 7.
Expression of a dominant negative mutant of ARF6 in 3T3-L1 adipocytes inhibits the ability of endothelin 1 to stimulate GLUT4 translocation to the plasma membrane. Six days following induction of GFP (a and b) or ARF6(T27N) (c and d) expression by infection with AdCre, 3T3-L1 adipocytes were exposed to either 10 nM endothelin 1 (ET-1) or 100 nM insulin for 20 min. Plasma membrane sheets were prepared and subjected to immunofluorescence microscopy using polyclonal sheep anti-GLUT4 antibodies and rhodamine-conjugated anti-sheep secondary antibodies. Images were captured with a cooled charge-coupled device camera. (a and c) Composite photomicrographs of a representative experiment depicting the presence of GLUT4 on the plasma membrane under the noted experimental conditions. (b and d) Using a mask created from an identical field of view to identify areas containing plasma membrane, the mean fluorescence intensity from images was quantitated and is presented as the mean ± SEM of three independent experiments. The asterisk denotes the statistically significant difference (P = 0.05) for endothelin 1-stimulated ARF6(T27N) cells not infected compared to infected with AdCre.
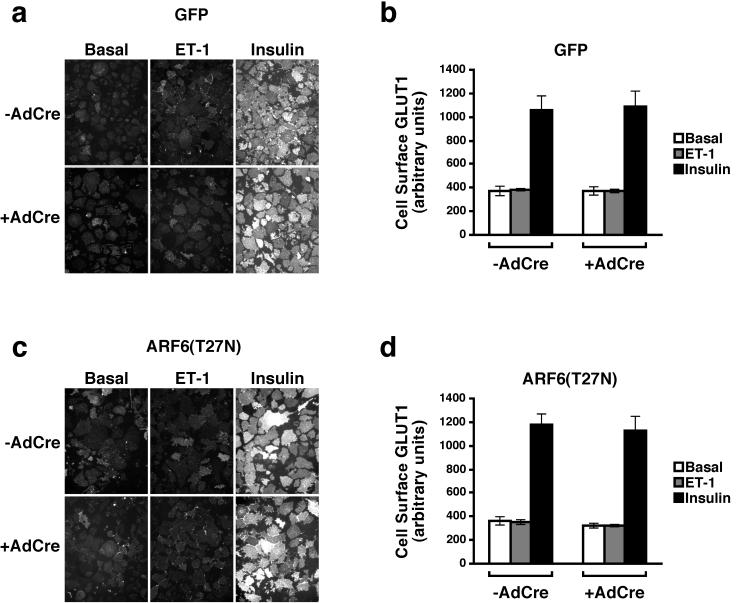
Next, we examined whether endothelin 1 was capable of increasing the abundance of GLUT1 on the surface of 3T3-L1 adipocytes. Insulin stimulated the translocation of GLUT1 to the plasma membrane approximately threefold, and this was unaffected by the expression of either GFP or dominant inhibitory ARF6 (Fig. 8). In contrast, endothelin 1 did not stimulate GLUT1 translocation (Fig. 8). In addition, expression of ARF6(T27N) had no effect on the steady-state levels of GLUT1 on the plasma membrane under basal or endothelin-stimulated conditions (Fig. 8c and d).
FIG. 8.
Effect of endothelin 1 and ARF6(T27N) on the translocation of GLUT1 to the plasma membrane in 3T3-L1 adipocytes. Expression of GFP (a and b) or ARF6(T27N) (c and d) was induced in 3T3-L1 adipocytes, which were then treated with endothelin 1 (ET-1) or insulin, and plasma membrane sheets were prepared as in Fig. 7. Immunofluorescence microscopy was performed with polyclonal sheep anti-GLUT1 antisera. (a and c) Composite photomicrographs of a typical experiment depicting the presence of GLUT1 on the plasma membranes from cells treated as indicated following induction of expression with AdCre; (b and d) fluorescence intensity expressed as the mean ± SEM of three independent experiments.
DISCUSSION
In this study we have described a novel inducible system, useful for the synchronous and quantitative induction of gene products in both dividing and terminally differentiated tissue culture cells, and used it to express a dominant inhibitory form of ARF6 in 3T3-L1 adipocytes. This small GTP-binding protein has been implicated in both vesicular trafficking events and reorganization of the actin cytoskeleton. Two key features have made ARF6 attractive as a potential intermediate in the pathway by which extracellular factors regulate glucose transport. First, ARF6 is required in diverse cell types for translocation of membrane protein from an endosomal-like compartment to the plasma membrane as well as for regulated secretion in chromaffin cells (7, 8, 14, 15, 18, 32, 34, 38). Second, though the precise guanine nucleotide exchange factors responsible for activation of ARF6 continue to be source of some controversy, all likely candidates display dependency on phosphatidylinositides for maximal activity (10, 12). Moreover, there is some direct evidence that both recruitment of these exchange proteins to the plasma membrane and activation of ARF6 in the intact cell are dependent on PI 3-kinase (25, 30, 39, 40). Importantly, stimulation of GLUT4 translocation by insulin and possibly endothelin also depends on the generation of phosphatidylinositides (11, 22). Despite these arguments, our data are in agreement with previous data that do not support strongly a role for ARF6 in the insulin-dependent regulation of glucose transport. In one study, peptides derived from the ARF6 sequence partially inhibited GLUT4 translocation in permeabilized 3T3-L1 adipocytes (27). However, subsequently it was reported that expression of a GTP-binding-deficient mutant of ARF6, ARF6(D125N), did not block insulin-stimulated glucose uptake (42). In this report, we have confirmed the latter result employing a more widely used ARF6 dominant inhibitory mutant, ARF6(T27N). However, we also show that, unlike for insulin, the activation of hexose uptake and GLUT4 translocation in response to endothelin is strongly dependent on ARF6. This result has several implications: (i) it clearly indicates that the pathways by which insulin and endothelin activate glucose transport are distinct; (ii) these data suggest the existence of a novel pool of GLUT4 that is responsive to endothelin; and (iii) they add weight to the proposed link between heterotrimeric G protein signaling and ARF6-dependent events.
ARF6 is remarkable in its capacity to influence both vesicular trafficking and cytoskeleton rearrangements. There is much evidence that expression of a constitutively active ARF6 leads to the redistribution of an endosome-like compartment to the plasma membrane (14, 15, 32, 34, 38). Expression of a dominant inhibitory form of ARF6, such as the ARF6(T27N) used in this study, leads to accumulation of the mutant protein in pericentriolar structures and the prevention of movement of ligands from endosomes to the cell surface (14, 15, 32, 34). Constitutively active ARF6 stimulates the protrusion of peripheral membrane structures rich in actin filaments but morphologically distinct from those elicited by other small G proteins such as Rac or CDC42 (13, 33, 35, 43). The most likely explanation for ARF6's seemingly diverse effects is that it plays a role in the elaboration of new plasma membrane structures, a process that requires cytoskeletal changes as well as the delivery of membrane to the cell surface. Consistent with this idea, ARF6 has been shown to be involved in Fc-mediated phagocytosis in macrophages and cell spreading in fibroblasts (37, 44).
Several recent studies implicating heterotrimeric G proteins in ARF6 action provided the motivation to assess the role of this small G protein in endothelin action in adipocytes. The effects of constitutively active ARF6 are mimicked by addition of aluminum fluoride to cells overexpression the wild-type form of the G protein (34). Aluminum fluoride is known to activate heterotrimeric G proteins, and the likely target of the drug in its regulation of ARF6 is Gαq (2). Boshans et al. have shown that bombesin causes the loading of ARF6 with GTP and the redistribution of ARF6-containing endosomal vesicles to the plasma membrane (6). Moreover, this was accompanied by characteristic actin cytoskeletal rearrangements. Both processes were dependent on Gαq and mimicked by a constitutively active form of Gαq. The key finding in the present report, that ARF6(T27N) blocks endothelin- but not insulin-stimulated GLUT4 translocation, provides strong support for the linkage between Gαq and ARF6 but further suggests that heterotrimeric rather than protein kinase receptor-initiated signaling pathways preferentially couple to ARF6. This is somewhat surprising in view of insulin's potency in activating PI 3-kinase in 3T3-L1 adipocytes and the importance of phosphatidylinositides to the activation of ARF6.
As noted above, the simplest explanation for the differential dependency of insulin and endothelin on ARF6 is that the two hormones elicit translocation of GLUT4 by different mechanisms. While it is possible that endothelin and insulin signaling converge downstream of the former's requirement for ARF6, we favor the idea that the pathways are distinct. Imamura et al. have presented evidence that microinjection of an antibody to either Gαq/11 or p110α, the catalytic subunit of PI 3-kinase, blocks both insulin- and endothelin-induced GLUT4 translocation (22, 23). In partial agreement with Imamura et al., Kanzaki et al. have presented evidence that either microinjection of an antibody against Gαq/11 or expression of the GTPase-activating proteins RGS4 (regulator of G protein signaling 4) and RGS16, which inhibit Gαq, also block insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes (24). It should be noted, however, that other investigators have reported that wortmannin, an inhibitor of PI 3-kinase, does not interfere with endothelin-stimulated hexose uptake (5, 24, 41). In any case, both Imamura et al. and Kanzaki et al. have interpreted their results as indicative of a role for a Gq family member in the signaling cascade leading from the insulin receptor to the stimulation of glucose uptake (23, 24). However, the observation that Gαq- but not insulin-stimulated hexose uptake is blocked by a dominant inhibitory ARF6 argues against this idea (Fig. 4 and 6). One possibility is that a Gαq-dependent but ARF6 independent pathway accounts for this discrepancy. In any case, insulin increases the levels of both GLUT4 and GLUT1 at the plasma membrane, whereas endothelin affects only GLUT4, again emphasizing fundamental differences in the signaling pathways initiated by these hormones (Fig. 7 and 8).
What then is the ARF6-sensitive compartment from which GLUT4 translocates in response to endothelin? While we cannot exclude the possibility that ARF6 functions exclusively as a signaling intermediate, we favor the notion that following exposure of cells to endothelin, ARF6 regulates the trafficking of a pool of glucose transporters in 3T3-L1 adipocytes. Considerable evidence favors the existence of multiple intracellular pools of GLUT4. In brown fat cells in the basal state, about 60% of the GLUT4 is found in tubulovesicular elements distributed throughout the cell, about 10% is in the region of the trans-Golgi network, and the remainder is elsewhere in the cell (36). Following insulin stimulation, the amount of GLUT4 in the tubulovesicular elements and in the region of the trans-Golgi network both decreased by 50%, with a concomitant increase in transporter at all the cell surface and in classical endosomal structures. This has been interpreted as indicative of a distribution of GLUT4 between endosomes and a novel insulin-responsive compartment often referred to as GLUT4 storage vesicles. For example, ablation of endosomes by loading with HRP and treatment with H2O2 and diaminobenzidine clearly distinguishes a resistant pool of GLUT4 that is enriched in the synaptobrevin/vesicle-associated membrane protein 2 (26). In addition, recent reports have provided evidence that either 5′-O-(3-thiotriphosphate) or the serine/threonine protein kinase Akt/protein kinase B stimulates translocation of distinct subsets of intracellular GLUT4 (17, 28). Thus, a plausible explanation of the data presented in this report is that endothelin and insulin provoke translocation of distinct pools of GLUT4. Since the magnitude of the insulin response is greater than that for endothelin, we cannot exclude the possibility that the endothelin-sensitive compartment is also responsive to insulin, but we are unable to discern even a modest inhibition of the insulin effect by ARF6(T27N). The precise nature of the intracellular compartment in which endothelin-responsive GLUT4 resides and which is regulated by ARF6 remains obscure. Our data indicate that endothelin regulates GLUT4 independently of GLUT1 trafficking, even though GLUT1 and GLUT4 have been shown to colocalize on intracellular membranes and insulin stimulates the redistribution of both isoforms to the plasma membrane. Since GLUT1, which is believed to traffic primarily between the plasma membrane and the endosomal system, is not influenced by endothelin, it is unlikely that the hormone simply effects a redistribution of general endosomal proteins. These data are consistent with the endothelin/Gq/ARF6 pathway not affecting the endosomal system in adipocytes but instead affecting a relatively specialized compartment possibly designed for the polarized delivery of membrane protein to membrane protrusions.
The inducible expression system described in this report combines advantageous aspects of retroviral and adenoviral delivery systems while avoiding a number of their limitations. Initiation of expression of the desired construct can be efficiently and synchronously initiated through the use of a single recombinant adenovirus. The system appears to be highly regulated, as retrovirus-infected cells express little or no foreign product prior to recombination. However, following induction with AdCre, 85 to 90% of the cells initiate expression of the inducible product, with a time course in fibroblasts that is similar to that obtained by transient transfections. The 3T3-L1 adipocytes used in this study take slightly longer to initiate expression than other dividing and nondividing differentiated cell types that we have tested with this system (J. T. R. Lawrence and M. J. Birnbaum, unpublished observations). We anticipate that this inducible expression system will have general utility, as recombinant retroviruses are relatively simple to prepare, and only a single recombinant adenovirus is required for expression of any gene product.
ACKNOWLEDGMENTS
We thank Frank L. Graham, McMaster University (Hamilton, Ontario, Canada), Jerrold M. Olefsky, University of California, San Diego (La Jolla), and Julie G. Donaldson, National Institutes of Health (Bethesda, Md.) for the gift of reagents and the Vector Core of the Penn Diabetes Center (P30 19525) for amplification of adenovirus. We also thank Michael P. Czech and Martha S. Jordan for discussions and critical reading of the manuscript.
This work was supported by National Institutes of Health grants DK39615 (M.J.B.) and National Research Service Award for Training in Cell and Molecular Biology GM07229 (J.T.R.L.).
REFERENCES
- 1.Adams J W, Sakata Y, Davis M G, Sah V P, Wang Y, Liggett S B, Chien K R, Brown J H, Dorn G W., II Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Awar O, Radhakrishna H, Powell N N, Donaldson J G. Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant. Mol Cell Biol. 2000;20:5998–6007. doi: 10.1128/mcb.20.16.5998-6007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton M, Graham F L. Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: a molecular switch for control of gene expression. J Virol. 1995;69:4600–4606. doi: 10.1128/jvi.69.8.4600-4606.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnbaum M J. The insulin-sensitive glucose transporter. Int Rev Cytol. 1992;137:239–297. [PubMed] [Google Scholar]
- 5.Bose A, Cherniack A D, Langille S E, Nicoloro S M C, Buxton J M, Park J G, Chawla A, Czech M P. Gα11 signaling through ARF6 regulates F-actin mobilization and GLUT4 glucose transporter translocation to the plasma membrane. Mol Cell Biol. 2001;21:5262–5275. doi: 10.1128/MCB.21.15.5262-5275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boshans R L, Szanto S, van Aelst L, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol Cell Biol. 2000;20:3685–3694. doi: 10.1128/mcb.20.10.3685-3694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caumont A S, Galas M C, Vitale N, Aunis D, Bader M F. Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J Biol Chem. 1998;273:1373–1379. doi: 10.1074/jbc.273.3.1373. [DOI] [PubMed] [Google Scholar]
- 8.Caumont A S, Vitale N, Gensse M, Galas M C, Casanova J E, Bader M F. Identification of a plasma membrane-associated guanine nucleotide exchange factor for ARF6 in chromaffin cells. Possible role in the regulated exocytotic pathway. J Biol Chem. 2000;275:15637–15644. doi: 10.1074/jbc.M908347199. [DOI] [PubMed] [Google Scholar]
- 9.Corvera S, Czech M P. Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 1998;8:442–446. doi: 10.1016/s0962-8924(98)01366-x. [DOI] [PubMed] [Google Scholar]
- 10.Cullen P J, Venkateswarlu K. Potential regulation of ADP-ribosylation factor 6 signalling by phosphatidylinositol 3,4,5-trisphosphate. Biochem Soc Trans. 1999;27:683–689. doi: 10.1042/bst0270683. [DOI] [PubMed] [Google Scholar]
- 11.Czech M P, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson J G, Jackson C L. Regulators and effectors of the ARF GTPases. Curr Opin Cell Biol. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 13.D'Souza-Schorey C, Boshans R L, McDonough M, Stahl P D, Van Aelst L. A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Souza-Schorey C, Li G, Colombo M I, Stahl P D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- 15.D'Souza-Schorey C, van Donselaar E, Hsu V W, Yang C, Stahl P D, Peters P J. ARF6 targets recycling vesicles to the plasma membrane: insights from an ultrastructural investigation. J Cell Biol. 1998;140:603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingar D C, Hausdorff S F, Blenis J, Birnbaum M J. Dissociation of pp70 ribosomal protein S6 kinase from insulin-stimulated glucose transport in 3T3–L1 adipocytes. J Biol Chem. 1993;268:3005–3008. [PubMed] [Google Scholar]
- 17.Foran P G, Fletcher L M, Oatey P B, Mohammed N, Dolly J O, Tavare J M. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3 L1 adipocytes by a pathway involving SNAP- 23, synaptobrevin-2, and/or cellubrevin. J Biol Chem. 1999;274:28087–28095. doi: 10.1074/jbc.274.40.28087. [DOI] [PubMed] [Google Scholar]
- 18.Galas M C, Helms J B, Vitale N, Thierse D, Aunis D, Bader M F. Regulated exocytosis in chromaffin cells. A potential role for a secretory granule-associated ARF6 protein. J Biol Chem. 1997;272:2788–2793. doi: 10.1074/jbc.272.5.2788. [DOI] [PubMed] [Google Scholar]
- 19.Garza L A, Birnbaum M J. Insulin-responsive aminopeptidase trafficking in 3T3–L1 adipocytes. J Biol Chem. 2000;275:2560–2567. doi: 10.1074/jbc.275.4.2560. [DOI] [PubMed] [Google Scholar]
- 20.Hausdorff S F, Fingar D C, Morioka K, Garza L A, Whiteman E L, Summers S A, Birnbaum M J. Identification of wortmannin-sensitive targets in 3T3–L1 adipocytes. Dissociation of insulin-stimulaetd glucose uptake and GLUT4 translocation. J Biol Chem. 1999;274:24677–24684. doi: 10.1074/jbc.274.35.24677. [DOI] [PubMed] [Google Scholar]
- 21.Hausdorff S F, Frangioni J V, Birnbaum M J. Role of p21ras in insulin-stimulated glucose transport in 3T3–L1 adipocytes. J Biol Chem. 1994;269:21391–21394. [PubMed] [Google Scholar]
- 22.Imamura T, Ishibashi K, Dalle S, Ugi S, Olefsky J M. Endothelin-1-induced GLUT4 translocation is mediated via Galpha(q/11) protein and phosphatidylinositol 3-kinase in 3T3–L1 adipocytes. J Biol Chem. 1999;274:33691–33695. doi: 10.1074/jbc.274.47.33691. [DOI] [PubMed] [Google Scholar]
- 23.Imamura T, Vollenweider P, Egawa K, Clodi M, Ishibashi K, Nakashima N, Ugi S, Adams J W, Brown J H, Olefsky J M. G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3–L1 adipocytes. Mol Cell Biol. 1999;19:6765–6774. doi: 10.1128/mcb.19.10.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanzaki M, Watson R T, Artemyev N O, Pessin J E. The trimeric GTP-binding protein (G(q)/G(11)) alpha subunit is required for insulin-stimulated GLUT4 translocation in 3T3L1 adipocytes. J Biol Chem. 2000;275:7167–7175. doi: 10.1074/jbc.275.10.7167. [DOI] [PubMed] [Google Scholar]
- 25.Karnam P, Standaert M L, Galloway L, Farese R V. Activation and translocation of Rho (and ADP ribosylation factor) by insulin in rat adipocytes. Apparent involvement of phosphatidylinositol 3-kinase. J Biol Chem. 1997;272:6136–6140. doi: 10.1074/jbc.272.10.6136. [DOI] [PubMed] [Google Scholar]
- 26.Martin S, Tellam J, Livingstone C, Slot J W, Gould G W, James D E. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millar C A, Powell K A, Hickson G R, Bader M F, Gould G W. Evidence for a role for ADP-ribosylation factor 6 in insulin-stimulated glucose transporter-4 (GLUT4) trafficking in 3T3–L1 adipocytes. J Biol Chem. 1999;274:17619–17625. doi: 10.1074/jbc.274.25.17619. [DOI] [PubMed] [Google Scholar]
- 28.Millar C A, Shewan A, Hickson G R, James D E, Gould G W. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3–L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss J, Vaughan M. Molecules in the ARF orbit. J Biol Chem. 1998;273:21431–21434. doi: 10.1074/jbc.273.34.21431. [DOI] [PubMed] [Google Scholar]
- 30.Oatey P B, Venkateswarlu K, Williams A G, Fletcher L M, Foulstone E J, Cullen P J, Tavare J M. Confocal imaging of the subcellular distribution of phosphatidylinositol 3,4,5-trisphosphate in insulin- and PDGF-stimulated 3T3–L1 adipocytes. Biochem J. 1999;344:511–518. [PMC free article] [PubMed] [Google Scholar]
- 31.Pessin J E, Thurmond D C, Elmendorf J S, Coker K J, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 32.Peters P J, Hsu V W, Ooi C E, Finazzi D, Teal S B, Oorschot V, Donaldson J G, Klausner R D. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson J G. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- 34.Radhakrishna H, Donaldson J G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radhakrishna H, Klausner R D, Donaldson J G. Aluminium fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J Cell Biol. 1996;134:935–947. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slot J W, Geuze H J, Gigengack S, Lienhard G E, James D E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song J, Khachikian Z, Radhakrishna H, Donaldson J G. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J Cell Sci. 1998;111:2257–2267. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- 38.Toda K, Nogami M, Murakami K, Kanaho Y, Nakayama K. Colocalization of phospholipase D1 and GTP-binding-defective mutant of ADP-ribosylation factor 6 to endosomes and lysosomes. FEBS Lett. 1999;442:221–225. doi: 10.1016/s0014-5793(98)01646-9. [DOI] [PubMed] [Google Scholar]
- 39.Venkateswarlu K, Cullen P J. Signalling via ADP-ribosylation factor 6 lies downstream of phosphatidylinositide 3-kinase. Biochem J. 2000;345:719–724. [PMC free article] [PubMed] [Google Scholar]
- 40.Venkateswarlu K, Oatey P B, Tavare J M, Cullen P J. Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Curr Biol. 1998;8:463–466. doi: 10.1016/s0960-9822(98)70181-2. [DOI] [PubMed] [Google Scholar]
- 41.Wu-Wong J R, Berg C E, Wang J, Chiou W J, Fissel B. Endothelin stimulates glucose uptake and GLUT4 translocation via activation of endothelin ETA receptor in 3T3–L1 adipocytes. J Biol Chem. 1999;274:8103–8110. doi: 10.1074/jbc.274.12.8103. [DOI] [PubMed] [Google Scholar]
- 42.Yang C Z, Mueckler M. ADP-ribosylation factor 6 (ARF6) defines two insulin-regulated secretory pathways in adipocytes. J Biol Chem. 1999;274:25297–25300. doi: 10.1074/jbc.274.36.25297. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Calafat J, Janssen H, Greenberg S. ARF6 is required for growth factor- and Rac-mediated membrane ruffling in macrophages at a stage distal to Rac membrane targeting. Mol Cell Biol. 1999;19:8158–8168. doi: 10.1128/mcb.19.12.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q, Cox D, Tseng C C, Donaldson J G, Greenberg S. A requirement for ARF6 in Fcγ receptor-mediated phagocytosis in macrophages. J Biol Chem. 1998;273:19977–19981. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]