Abstract
Purpose:
To investigate the urine protein (UP) and urine creatinine (UC) ratio in diabetes mellitus and report its influence as a risk factor for the presence and severity of diabetic retinopathy (DR).
Methods:
In total, 150 diabetic patients presenting to the outpatient department were included. Detailed history with informed consent and ophthalmic examination, including visual assessment, external ocular examination, anterior segment evaluation, dilated fundus examination by slit-lamp biomicroscopy, and indirect ophthalmoscopy, was done. The early morning spot urine sample was used to determine spot urine protein creatinine ratio. Association with hypertension, fasting blood sugar (FBS), and HBA1C (glycosylated Hb) were also noted.
Results:
Urinary PCR increased with the severity of the diabetic retinopathy (P < 0.001). HbA1c, FBS, and duration of diabetes had a direct correlation with urine PCR. ROC curve analysis showed that the optimal PCR cut-off value for predicting the risk of onset DR was 0.65. Retinopathy progressed with increasing urine PCR. Spot urine PCR strongly correlates with stages of diabetic retinopathy and proteinuria measured in 24-h urine samples.
Conclusion:
The study showed that urine PCR can be a marker for risk and progression of diabetic retinopathy.
Keywords: Diabetes, diabetic retinopathy, retinopathy, spot urine protein creatinine ratio, Urine PCR
Diabetes mellitus (DM) is a health issue of importance, with a risk of turning into a global epidemic in the coming years. It leads to a range of systemic complications that have a considerable impact on both the patient and the society because the affected people are in their prime years of productivity. Approximately 80% of the diabetics are found in developing and underdeveloped countries and are increasing at alarming rates.[1,2] India has the largest number of affected people, which was estimated to be 66.8 million.[2,3]
Potential complications (microvascular and macrovascular) can be avoided by early diagnosis and intervention.[4] Diabetic eye disease is an end-organ microvascular complication, the main indicators being diabetic retinopathy, cataract, and glaucoma.[5]
Diabetic retinopathy (DR) is a leading cause of blindness in the world. Furthermore, patients with DR are at a much greater risk of diabetic nephropathy, limb amputations, stroke, coronary heart disease, and death.[6] Duration of diabetes mellitus, hypertension, and poor glycemic control[7,8] are considered the main risk factors for the development or progression of diabetic retinopathy. The risk of vision loss increases with the progression of the stage of diabetic retinopathy. Thus, early detection and identification of the risks for diabetic retinopathy are crucial.[9,10]
Glomerular hyperfiltration and proteinuria begin before overt nephropathy or early clinically evident diabetic retinopathy.[11] Diabetic nephropathy contributes to approximately 20% of cases of chronic renal failure. Collecting accurate 24-h urine samples is inconvenient, especially in an outpatient setting, and the best considered alternative method for quantitative evaluation of proteinuria is the measurement of urine protein to creatinine ratio in a spot urine sample.[12]
Spot urine protein to creatinine ratio is an alternative yet reliable approach that has been proposed for many years. As long as the glomerular filtration rate remains constant, both protein and creatinine excretion rates are assumed to be fairly constant during the day. It was found that the mean intra-individual variation in the protein:creatinine ratio was 38.6%, whereas that of the protein excretion was 96.5%.[13,14] Thus, considering that urine protein:creatinine ratio is a better and more stable indicator compared to only urinary protein, in this study, we measured the urine protein (UP):urine creatinine (UC) ratio of people with diabetes and assess their stage of retinopathy and the correlation with each other.
Methods
Data collection
A random sample of 150 previously or newly diagnosed diabetes mellitus (male and female) were included in the study. After informed consent, participants were interviewed for their demographic and diabetic history. The clinical protocol included elicitation of detailed medical history, including the age of onset of diabetes, duration, treatment history (systemic medications), family history of diabetes, history of hypertension, detailed ocular examination, and biochemical parameters such as serum creatinine, HbA1c, FBS, and urine PCR.
Patients with urinary tract infection, glomerulonephritis due to systemic conditions, malignancies, collagen vascular disorders, or any other systemic condition causing proteinuria and pregnant women were excluded.
Diabetes was diagnosed when the fasting plasma glucose (FPG) level was ≥126 mg/dL (7.0 mmol/L) and/or the 2-h postprandial glucose level was ≥200 mg/dL (11.1 mmol/L) and/or when the patient was treated for diabetes by a physician.[15] Blood pressure was measured using standardized techniques.[16]
A random spot urine sample was collected on the test day in a sterile container and sent for evaluation. Urine protein was estimated using the pyrogallol red molybdate method and urine creatinine by modified Jaffe's method. Urine PCR was calculated by dividing the value of spot urine protein (mg/dl) by the spot urine creatinine (mg/dl) and expressed as a ratio. The ratio was further converted to mg/g in order to classify it into mild, moderate, and severe.
Diabetic retinopathy
All patients underwent a complete ophthalmic examination that included visual acuity measurement, intraocular pressure measurement, slit-lamp examination of the anterior segment, and fundus examination after dilatation using direct and indirect ophthalmoscopy performed by retina specialists. DR was identified and classified based on the presence of any of these characteristic lesions: microaneurysm, dot and blot hemorrhages, hard exudates, cotton wool spots, venous beading, intra-retinal microvascular abnormalities, retinal new vessels, vitreous hemorrhage, fibrous proliferation, tractional retinal detachments, or previous laser therapy.[17,18,19] Any DR was defined by the presence of at least one definite microaneurysm in one or both eyes.[18] The Early Treatment Diabetic Retinopathy Study (ETDRS) grading[20,21,22,23,24,25,26] system was used for grading of DR by retina specialists.
Diabetic kidney disease
Diabetic kidney disease (DKD) was defined as eGFR less than 60 mL/min/1.73 m2 and/ or albuminuria 30 mg/mg or more.
According to the urine protein creatinine ratio, it was divided into three groups: mild (<150), moderate (150–500), and severe (>500) in mg/g.[27,28]
Statistical analysis
Statistical analysis was performed using STATISTICAL PACKAGE SPSS VERSION 17.0., correlation by AN0VA ANALYSIS OF VARIANCE, and P < 0.05 was considered statistically significant. ROC curves were used to determine sensitivity, specificity, and cut-off values of PCR at different stages of DR.
Results
Gender and age
There were 95 (63.3%) male and 55 (36.7%) female participants. The mean age of the patients was 58.7 years (range: 32–82 years) with a standard deviation of 10.59 years.
It was observed that the majority of the patients belonged to the age group of 60–70 years, which accounts for 46 (30.7%) out of 150 patients.
Clinical characteristics
Duration of diabetes ranged from newly detected to more than 15 years. Eighty-one (54%) patients were diagnosed as diabetics for less than 5 years, 44 (29.3%) for 5 to 15 years, 17 (11.3%) for 10–15 years, and 8 (5.3%) patients for more than 25 years. Further, 88 (58.7%) patients were on treatment with oral hypoglycemic agents (OHAs) whereas 62 (41.3%) were on treatment with insulin with or without OHAs. The majority of subjects (129/150) had HbA1c values between 6.5 and 8.
Association between urine PCR and diabetic retinopathy
A total of 300 eyes of 150 patients were examined for diabetic retinopathy. In total, 160 eyes (53.3%) were diagnosed to have mild NPDR, 92 eyes (30.7%) had moderate NPDR, 7 (2.3%) had severe NPDR, and 41 eyes (13.7%) had PDR. Proteinuria values from spot urine protein–creatinine were obtained: 60 (20%) had mild proteinuria, 68 eyes (22.7%) had moderate proteinuria, and 172 (57.3%) had severe proteinuria.
A variable correlation was seen with proteinuria and diabetic retinopathy. Among the 160 eyes with mild NPDR, 46 (28.8%) had mild proteinuria, 50 (31.2%) had moderate proteinuria, and 64 (40%) had severe proteinuria. Among the moderate NPDR group of eyes, 10 (10.9%) had mild proteinuria, 11 (12%) had moderate proteinuria, and 71 (77.2%) had severe proteinuria. Among the PDR group, the majority, that is, 34/41 eyes (82.9%) had severe proteinuria.
In our study, a strong correlation was present for severe proteinuria and proliferative diabetic retinopathy. Among the NPDR group, the correlation between proteinuria and stages of NPDR was variable [Table 1]. Fig. 1 is a graphical representation of Table 1.
Table 1.
PCR (mg/g) with grade of diabetic retinopathy
| GRADE OF DR-RE | Total | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mild NPDRa | Moderate NPDR | Severe NPDR | PDR | ||||
| PCR (mg/g) | Mild | Count | 46 | 10 | 0 | 4 | 60 |
| % | 28.8% | 10.9% | 0.0% | 9.8% | 20.0% | ||
| Moderate | Count | 50 | 11 | 4 | 3 | 68 | |
| % | 31.2% | 12.0% | 57.1% | 7.3% | 22.7% | ||
| Severe | Count | 64 | 71 | 3 | 34 | 172 | |
| % | 40.0% | 77.2% | 42.9% | 82.9% | 57.3% | ||
| Total | Count | 160 | 92 | 7 | 41 | 300 | |
| % | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | ||
Figure 1.
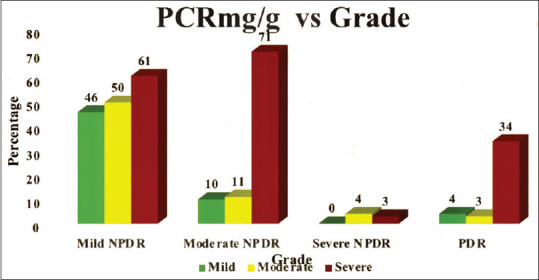
Graphical representation of severity of urine protein creatinine ratio with grading of diabetic retinopathy
Urine PCR (urine protein to urine creatinine ratio/UPUC ratio) and its correlation with diabetic retinopathy were studied (Table 2, with a graphical representation of the same in Fig. 2). Urine PCR values were categorized as <0.2 (normal), 0.2–3.5, and >3.5 (nephrotic range). Among the 160 eyes with mild NPDR, 68 (42.5%) had normal urine PCR of <0.2 whereas the majority (82; 51.2) had urine PCR in the range of 0.2–3.5. Among the moderate NPDR group, the majority (57; 62%) had a urine PCR of 0.2–3.5 and 25 (27.2%) had a urine PCR of >3.5. Three (42.9%) of severe NPDR had a urine PCR of >3.5. In the PDR category, the majority (24; 58.5%) had urine PCR in the nephrotic range. Thus, a strong correlation was seen between spot urine PCR and diabetic retinopathy.
Table 2.
UP: UC ratio with grade of diabetic retinopathy
| GRADE OF DR | Total | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mild NPDRa | Moderate NPDR | Severe NPDR | PDR | ||||
| UP: UC ratio | <0.2 | Count | 68 | 10 | 2 | 4 | 84 |
| % | 42.5% | 10.9% | 28.6% | 9.8% | 28.0% | ||
| 0.2-3.5 | Count | 82 | 57 | 2 | 13 | 154 | |
| % | 51.2% | 62.0% | 28.6% | 31.7% | 51.3% | ||
| >3.5 | Count | 10 | 25 | 3 | 24 | 62 | |
| % | 6.2% | 27.2% | 42.9% | 58.5% | 20.7% | ||
| Total % | Count | 160 | 92 | 7 | 41 | 300 | |
| % | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | ||
Figure 2.
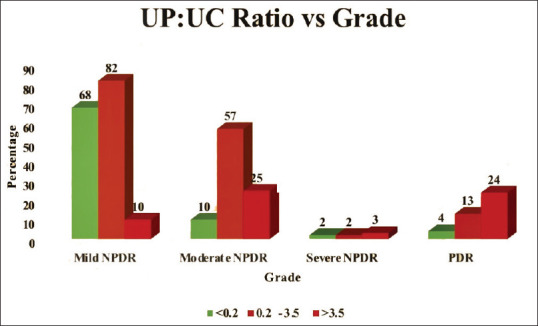
Graphical representation of urine protein creatinine ratio in nephrotic ranges with diabetic retinopathy grading
We found that urine PCR significantly correlated with DR and its severity. Urine PCR levels increased with the severity of diabetic retinopathy. In this study, urine PCR levels were significantly higher at more advanced stages of DR with P < 0.001.
ROC curve analysis showed that the optimal PCR cut-off value for predicting the risk of onset DR was 0.65. Severe diabetic retinopathy was associated with higher urine PCR. Fig. 3 with Area under the curve (PCR mg/g): 0.644.
Figure 3.
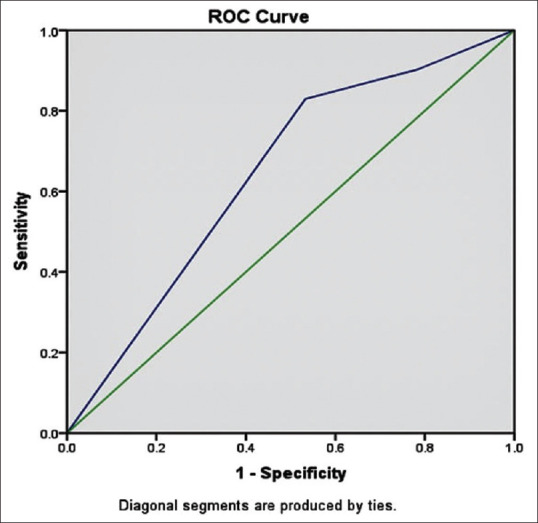
Representing receiver operating characteristic (ROC) curve with area under curve (cut-off value) as 0.644
The study shows that the majority of patients with urine PCR within normal limits had mild diabetic retinopathy. This emphasizes early screening in all diabetics for retinopathy.
Discussion
Proteinuria is considered and proven to be the single best predictor of kidney dysfunction and diabetic nephropathy progression. However, collecting 24-h urine is not always feasible. Spot urine protein urine creatinine ratio (PCR) is another dependable, convenient, and cost-effective measure of renal dysfunction.[13,29] As urine PCR can be easily performed on an outpatient department basis, it can be a convenient alternative in clinical practice. Microalbuminuria is said to be the earliest clinical feature of diabetic nephropathy.[30] Many studies have proved a very strong association between 24-h proteinuria and random spot urine PCR.[29] This study was done to find the correlation between spot urine protein to creatinine ratio and diabetic retinopathy.
Diabetic kidney disease/diabetic nephropathy is a serious complication of both type 1 and 2 DM. Kidney damage can accelerate diabetic retinopathy changes that are associated with raised serum levels of lipoproteins and fibrinogen and increased blood pressure. Diabetic renal disease and retinopathy are a cause of significant mortality and morbidity in diabetics. Thus, early detection and treatment are helpful in improving the quality of life and prevention of blindness in diabetics.
Our study showed a good correlation between proteinuria and proliferative diabetic retinopathy. However, this correlation was not seen in the eyes with nonproliferative disease. In contrast, there was a consistent correlation between urine PCR and retinopathy. The presence of diabetic retinopathy with normal urine PCR suggests the need for retinal examination in all diabetics at the earliest. The urine PCR levels increase with increasing severity of retinopathy. The majority of the proliferative group had urine PCR in the nephrotic range.
This study showed that spot urine PCR was an independent factor associated with DR. The PCR levels increased with the severity of diabetic retinopathy. The majority of patients diagnosed with mild NPDR had urine PCR within normal ranges (<0.2), whereas patients diagnosed with PDR (41) were found to have urine PCR in the nephrotic ranges (>3.5).
Our study also found that the associations of diabetic retinopathy with renal functions are independent of other risk factors such as hypertension, age, and sex. They had a negative correlation with high P values.
Chronic hyperglycemia in diabetics causes capillary endothelial damage, leading to capillary occlusion and nonperfusion of tissues. These microvascular changes occur in both the glomerulus and the retina.[31,32] In DM, the retina is affected due to capillary endothelial cell loss and consequent loss of pericyte, which in turn leads to the development and progression of microvascular features (microaneurysms, hemorrhages, soft exudates, etc.).[33]
The glomerular apparatus shows widespread loss of podocytes and capillary occlusion, causing proteinuria and a decline in renal function. Hence, it is crucial to detect proteinuria and retinopathy at the earliest.[34]
Our study showed that diabetics with higher urine PCR levels had advanced stages of retinopathy. Thus, all diabetics with increasing PCR should have a periodic retinal examination to prevent or retard the progression of diabetic retinopathy.
Our study did not exclude patients using angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) drugs, which are found to decrease urine PCR levels.[10] This is a limitation of our study.
Apart from the vision being affected, the presence of DR also notifies a raised incidence of life-threatening systemic vascular complications, such as coronary heart disease, stroke, heart failure, and neuropathy.[35] Therefore, regular ophthalmic examination should be mandated in order to screen and monitor not only DR but also other microvascular and macrovascular complications.
Future population-based cohort studies are needed to further evaluate the prediction of spot urine PCR on the development and progression of DR. Better and improvised screening of DR will lead to early diagnosis and a lower rate of progression to a visually impairing form. Urine PCR is largely used to diagnose diabetic kidney disease, but studies evaluating the use of PCR in the screening process for DR are limited.[36]
Conclusion
The results of our study show that urine protein::creatinine ratio can be a marker for risk and progression of diabetic retinopathy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes. 2015;6:850–67. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi SR. Diabetes care in India. Ann Glob Health. 2015;81:830–8. doi: 10.1016/j.aogh.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Nakagami T, Borch-Johnsen K, Carstensen B, Nhr-Hansen C, Hu G, Tuomilehto J, et al. Age, body mass index and type 2 diabetes - Associations modified by ethnicity. Diabetologia. 2003;46:1063–70. doi: 10.1007/s00125-003-1158-9. [DOI] [PubMed] [Google Scholar]
- 4.Gupta HL, Yadav M, Sundarka MK, Talwar V, Saini M, Garg P. A study of prevalence of health problems in asymptomatic elderly individuals in Delhi. J Assoc Physicians India. 2002;50:792–5. [PubMed] [Google Scholar]
- 5.Abdulghani YS, Ali TO. Original article correlation between central corneal thick- ness and diabetes in Sudanese patients. Natl J Med Res. 2013;3:309–11. [Google Scholar]
- 6.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–56. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 7.Biradar SB, Kallaganad GS, Rangappa M, Kashinakunti SV, Retnakaran R. Correlation of spot urine protein-creatinine ratio with 24-hour urinary protein in type 2 diabetes mellitus patients: A cross sectional study. J Res Med Sci. 2011;16:634–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Sanjari M, Asadikaram GR, Beigzadeh F, Torabian S, Safi Z, Ghaseminejad Tafreshi A. The association between urinary lgM excretion and diabetic retinopathy in diabetic patients. J Diabetes Metab Disord. 2016;15:18. doi: 10.1186/s40200-016-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 10.Lee MK, Han KD, Lee JH, Sohn SY, Hong OK, Jeong JS, et al. Normal-to-mildly increased albuminuria predicts the risk for diabetic retinopathy in patients with type 2 diabetes. Sci Rep. 2017;7:11757. doi: 10.1038/s41598-017-11906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahbazian H, Rezaii I. Diabetic kidney disease;review of the current knowledge. J Renal Inj Prev. 2013;2:73–80. doi: 10.12861/jrip.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Gaspari F, Perna A, Remuzzi G. Cross sectional longitudinal study of spot morning urine protein: Creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BJM. 1998;316:504–9. doi: 10.1136/bmj.316.7130.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore RR, Hirata-Dulas CA, Kasiske BL. Use of urine specific gravity to improve screening for albuminuria. Kidney Int. 1997;52:240–3. doi: 10.1038/ki.1997.326. [DOI] [PubMed] [Google Scholar]
- 14.Koopman MG, Krediet RT, Koomen GCM, Strackee J, Arisz L. Circadian rhythm of proteinuria: Consequences of the use of urinary protein: Creatinine ratios. Nephrol Dial Transplant. 1989;4:9–14. [PubMed] [Google Scholar]
- 15.Association AD. American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 17.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Diabetic retinopathy preferred practice pattern®. Ophthalmology. 2020;127:P66–145. doi: 10.1016/j.ophtha.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs–An extension of the modified Airlie house classification: ETDRS report number 10. Ophthalmology. 1991;98(5 Suppl):786–806. doi: 10.1016/S0161-6420(13)38012-9. [PubMed] [Google Scholar]
- 19.Mathis A. Diabetic Retinopathy. Elsevier Inc. (4th ed) 1993;43 [Google Scholar]
- 20.Zhang H, Wang J, Ying GS, Shen L, Zhang Z. Diabetic retinopathy and renal function in Chinese type 2 diabetic patients. Int Urol Nephrol. 2014;46:1375–81. doi: 10.1007/s11255-014-0675-4. [DOI] [PubMed] [Google Scholar]
- 21.Waugh J, Hooper R, Lamb E, Robson S, Shennan A, Milne F, et al. Spot protein-creatinine ratio and spot albumin-creatinine ratio in the assessment of pre-eclampsia: A diagnostic accuracy study with decision-analytic model-based economic evaluation and acceptability analysis. Health Technol Assess. 2017;21:1–90. doi: 10.3310/hta21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong TY, Coresh J, Klein R, Muntner P, Couper DJ, Sharrett AR, et al. Retinal microvascular abnormalities and renal dysfunction: The atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15:2469–76. doi: 10.1097/01.ASN.0000136133.28194.E4. [DOI] [PubMed] [Google Scholar]
- 23.Kostraba JN, Klein R, Dorman JS, Becker DJ, Drash AL, Maser RE, et al. The epidemiology of diabetes complications study: IV. Correlates of diabetic background and proliferative retinopathy. Am J Epidemiol. 1991;133:381–91. doi: 10.1093/oxfordjournals.aje.a115892. [DOI] [PubMed] [Google Scholar]
- 24.Lim LS, Cheung CY, Sabanayagam C, Lim SC, Tai ES, Huang L, et al. Structural changes in the retinal microvasculature and renal function. Invest Ophthalmol Vis Sci. 2013;54:2970–6. doi: 10.1167/iovs.13-11941. [DOI] [PubMed] [Google Scholar]
- 25.Parr JC. Retinal vascular disease. Aust N Z J Med. 1978;8:664–5. [Google Scholar]
- 26.Wu L, Fernandez-Loaiza P, Sauma J, Hernandez-Bogantes E, Masis M. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290–4. doi: 10.4239/wjd.v4.i6.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020 Oct;(98)(4S):S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Becker GJ, Wheeler DC, De Zeeuw D, Fujita T, Furth SL, Holdaas H, et al. Kidney disease: Improving global outcomes (KDIGO) blood pressure work group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney International Supplements. 2012;2:337–414. [Google Scholar]
- 29.Lim SK, Malathi T, Yean CW, Peng NK, Tan LP, Wong CM, et al. The utility of urine protein-creatinine ratio and urine albumin-creatinine ratio in estimating proteinuria in chronic kidney disease. Nephrology. 2014;19:72. doi: 10.1111/nep.12236-2. [Google Scholar]
- 30.Hofmann W, Guder WG. A diagnostic programme for quantitative analysis of proteinuria. Clin Chem Lab Med. 1989;27:589–600. doi: 10.1515/cclm.1989.27.9.589. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua J, Lim CXY, Wong TY, Sabanayagam C. Diabetic retinopathy in the Asia-pacific. Asia Pac J Ophthalmol (Phila) 2018;7:3–16. doi: 10.22608/APO.2017511. [DOI] [PubMed] [Google Scholar]
- 33.Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, et al. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:5594–603. doi: 10.1167/iovs.17-21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bek T. Diameter changes of retinal vessels in diabetic retinopathy. Curr Diab Rep. 2017;17:82. doi: 10.1007/s11892-017-0909-9. [DOI] [PubMed] [Google Scholar]
- 35.Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–8. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Song SJ. Current challenges in diabetic retinopathy: Are we really doing better? Endocrinol Metab (Seoul) 2016;31:254–7. doi: 10.3803/EnM.2016.31.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]


