Abstract
Background
Acute limb ischaemia (ALI), the sudden and significant reduction of blood flow to the limb, is considered a vascular emergency. In the general population, the incidence is estimated as 14 per 100,000. Prognosis depends on the time it takes to diagnose the condition and begin appropriate treatment. Standard initial interventional treatments include conventional open surgery and endovascular interventions such as catheter‐directed thrombolysis (CDT). Percutaneous interventions, such as percutaneous thrombectomy (PT, including mechanical thrombectomy or pharmomechanical thrombectomy) and ultrasound‐accelerated thrombolysis (USAT), are also performed as alternative endovascular techniques. The proposed advantages of PT and USAT include reduced time to revascularisation and when combined with catheter‐directed thrombolysis, a reduction in dose of thrombolytic agents and infusion time. The benefits of PT or USAT versus open surgery or thrombolysis alone are still uncertain. In this review, we compared PT or USAT against standard treatment for ALI, in an attempt to determine if any technique is comparatively safer and more effective.
Objectives
To assess the safety and effectiveness of percutaneous thrombectomy or ultrasound‐accelerated thrombolysis for the initial management of acute limb ischaemia in adults.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL, the World Health Organization (WHO) International Clinical Trials Registry Platform, and ClinicalTrials.gov to 3 March 2021. We searched reference lists of relevant studies and papers.
Selection criteria
We included randomised controlled trials (RCTs) that compared PT (any modality, including mechanical thrombectomy (aspiration, rheolysis, rotation) or pharmomechanical thrombectomy) or USAT with open surgery, thrombolysis alone, no treatment, or another PT modality for the treatment of ALI.
Data collection and analysis
Two review authors independently selected the studies, assessed risk of bias, extracted data, performed data analysis, and assessed the certainty of evidence according to GRADE. Outcomes of interest were primary patency, amputation rate, major bleeding, clinical success, secondary patency, and adverse effects.
Main results
We included one RCT in this review. This study had a total of 60 participants and compared USAT with standard treatment (CDT). The study included 32 participants in the CDT group and 28 participants in the USAT group.
We found no evidence of a difference between USAT and CDT alone for the following evaluated outcomes: amputation rate (risk ratio (RR) 1.14, 95% confidence interval (CI) 0.17 to 7.59); major bleeding (RR 1.71, 95% CI 0.31 to 9.53); clinical success (RR 1.00, 95% CI 0.94 to 1.07); and adverse effects (RR 5.69, 95% CI 0.28 to 113.72). We rated the certainty of the evidence as very low for these outcomes. We downgraded the certainty of the evidence for amputation rate, major bleeding, clinical success, and adverse effects by two levels due to serious limitations in the design (there was a high risk of bias in critical domains) and by two further levels due to imprecision (a small number of participants and only one study included). The study authors reported 30‐day patency, but did not report primary and secondary patency separately. The patency rate in the successfully lysed participants was 71% (15/21) in the USAT group and 82% (22/27) in the CDT group. The study authors did not directly report secondary patency, which is patency after secondary procedures, but they did report on secondary procedures. Secondary procedures were subdivided into embolectomy and bypass grafting. Embolectomy was performed on 14% (4/28) of participants in the USAT group versus 3% (1/32) of participants in the CDT group. Bypass grafting was performed on 4% (1/28) of participants in the USAT group versus 0% in the CDT group. As we did not have access to the specific participant data, it was not possible to assess these outcomes further.
We did not identify studies comparing the other planned interventions.
Authors' conclusions
There is insufficient evidence to assess the safety and effectiveness of USAT versus CDT alone for ALI for our evaluated outcomes: amputation rate, major bleeding, clinical success, and adverse effects. Primary and secondary patency were not reported separately. There was no RCT evidence for PT. Limitations of this systematic review derive from the single included study, small sample size, short clinical follow‐up period, and high risk of bias in critical domains. For this reason, the applicability of the results is limited. There is a need for high‐quality studies to compare PT or USAT against open surgery, thrombolysis alone, no treatment, or other PT modalities for ALI. Future trials should assess outcomes, such as primary patency, amputation rate, major bleeding, clinical success, secondary patency, and adverse effects.
Keywords: Adult, Humans, Endovascular Procedures, Fibrinolytic Agents, Fibrinolytic Agents/therapeutic use, Ischemia, Ischemia/drug therapy, Ischemia/therapy, Thrombectomy, Thrombolytic Therapy, Thrombolytic Therapy/adverse effects
Plain language summary
What are the benefits and risks of percutaneous blood clot removal (thrombectomy) and dissolution of blood clot accelerated by ultrasound waves (ultrasound‐accelerated thrombolysis) for treating acute limb ischaemia?
Key messages
Because of a lack of robust evidence, the benefits and risks of percutaneous blood clot removal (the removal of a blood clot via a needle puncture in the skin, known as percutaneous thrombectomy) or ultrasound‐accelerated thrombolysis (where ultrasound waves are sent by a specific device into the blood vessel to accelerate the dissolution of a blood clot) for the initial treatment of acute limb ischaemia are unclear.
Future research in this area should focus on the effectiveness of treatment options such as blood clot removal, catheter‐directed thrombolysis (drug delivery into the blood clot to dissolve the clot), and open surgery (to remove the clot and improve blood flow), as well as investigating any unwanted effects of these treatments.
What is acute limb ischaemia and how is it treated?
Acute ischaemia is a common condition that affects the limbs. Caused by a sudden and significant reduction of blood flow to the limb, acute limb ischaemia can result in pain, paralysis, pallor, and coldness, and in severe cases, it can lead to amputation.
Standard treatments for the initial management of acute limb ischaemia include open surgery and catheter‐directed thrombolysis. Other treatments for the initial management for acute limb ischaemia are percutaneous thrombectomy and ultrasound‐accelerated thrombolysis.
What did we want to find out?
We wanted to find out if percutaneous thrombectomy or ultrasound‐accelerated thrombolysis are better than catheter‐directed thrombolysis or open surgery at improving blood flow and reducing amputation, bleeding, and other risks.
What did we do?
We searched for randomised controlled studies that examined percutaneous thrombectomy or ultrasound‐accelerated thrombolysis and compared these with either catheter‐directed thrombolysis or open surgery in adults with acute limb ischaemia. In randomised controlled studies, the treatments or tests people receive are decided at random, and these usually give the most reliable evidence about treatment effects. We compared and summarised the results of the identified study and rated our confidence in the evidence, based on factors such as methods and size of the study.
What did we find?
We found one study that involved 60 people with acute limb ischaemia. The study lasted 30 days and compared ultrasound‐accelerated thrombolysis with catheter‐directed thrombolysis. The study did not present the results in a way that could tell us whether ultrasound‐accelerated thrombolysis, compared with catheter‐directed thrombolysis, had an effect on blood flow, and it is unclear if these treatments have an effect on amputation, bleeding, and other risks. Thus, we are very uncertain about the results.
What are the limitations of the evidence?
We have very little confidence in the evidence because it is possible that people in the study were aware of what treatment they received. The study did not provide information about important effects of the treatments (blood flow), and the study involved only a small number of people.
How up to date is this evidence?
The evidence is up to date to March 2021.
Summary of findings
Summary of findings 1. Ultrasound‐accelerated thrombolysis compared to thrombolysis alone for initial management of acute limb ischaemia.
| Ultrasound‐accelerated thrombolysis compared to thrombolysis alone for initial management of acute limb ischaemia | ||||||
| Patient or population: adults with ALI Setting: hospital Intervention: USAT Comparison: thrombolysis alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with thrombolysis alone | Risk with USAT | |||||
|
Primary patency follow‐up: 30 days |
See comment | See comment | 60 (1 RCT) |
‐ | Schrijver 2015 evaluated the outcome 30‐day patency. No distinction was made between primary and secondary patency. Schrijver 2015 reported that the patency rate in the successfully lysed standard thrombolysis participants was 82% (22/27) versus 71% (15/21) in the USAT participants (P = 0.35). | |
| Amputation rate follow‐up: 30 days | Study population | RR 1.14 (0.17 to 7.59) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | ‐ | |
| 63 per 1000 | 71 per 1000 (11 to 474) | |||||
| Major bleeding follow‐up: 30 days | Study population | RR 1.71 (0.31 to 9.53) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | ‐ | |
| 63 per 1000 | 107 per 1000 (19 to 596) | |||||
| Clinical success ‐ increase in ABI by at least 0.2 follow‐up: 30 days | Study population | RR 1.00 (0.94 to 1.07) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | Increase in ABI by at least 0.2 was achieved by all participants. | |
| 1000 per 1000 | 1000 per 1000 (940 to 1000) | |||||
|
Secondary patency follow‐up: 30 days |
See comment | See comment | 60 (1 RCT) |
‐ | Schrijver 2015 evaluated the outcome 30‐day patency. No distinction was made between primary and secondary patency. Secondary procedures were subdivided into embolectomy and bypass grafting. Embolectomy was performed on 3% (1/32) of the standard thrombolysis participants versus 14% (4/28) of the USAT participants (P = 0.18). Bypass grafting was performed on 0% of the standard thrombolysis participants versus 4% (1/28) of the USAT participants (P = 0.47). | |
|
Adverse events follow‐up: 30 days |
Study population | RR 5.69 (0.28 to 113.72) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | Schrijver 2015 reported combined distal embolisation and iatrogenic dissection in 7% (2/28) of USAT participants and 0% (0/32) of the standard thrombolysis participants. | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ALI: acute limb ischaemia; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio: USAT: ultrasound‐accelerated thrombolysis | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by two levels due to a high risk of bias in more than two critical domains. bWe downgraded by two levels for imprecision as only one study with a small number of participants was included.
Background
Description of the condition
Acute limb ischaemia (ALI) is defined as sudden and significant reduction of blood flow to the limb, with the onset of symptoms usually occurring fewer than two weeks from presentation. This loss of blood flow leads to limb ischaemia of differing severity, which may promote various complications, including the risk of major amputation and death (Norgren 2007). ALI manifests with various symptoms and signs, such as pain, paralysis, paraesthesia, pulselessness, pallor, and poikilothermy. Due to the severity of complications and the rapid progression to possible irreversible damage, ALI is considered a vascular emergency. The estimated incidence in the general population is 14 per 100,000 (Dormandy 1999). There is no difference in the incidence between men and women (Purushottam 2014).
The most frequent cause of ALI is an embolism (a blocked vessel caused by a thrombus (blood clot) that has travelled and reduced the blood flow in a blood vessel); native artery thrombosis (reduced blood flow due to a thrombus formed in one's own artery, including thrombosed popliteal artery aneurysm); thrombosis of a stented artery (reduced blood flow because of a thrombus formed in an artery that has a stent); trauma; and graft or reconstruction occlusions (Katsanos 2017; Norgren 2007).
The most common sources of emboli are the heart: either thrombi formed as a result of atrial fibrillation or mural thrombi (thrombi that attach to the wall of a cardiac chamber) formed after a myocardial infarction (Rutherford 2014). Other risk factors associated with an embolism are aortic thrombus, myxoma (non‐cancerous tumour) of the left ventricle, prosthetic valves, or valves damaged by rheumatic fever. Thrombosis in people with peripheral arterial disease is usually caused by atherosclerosis or occlusion of a prosthetic graft in people who have undergone previous surgical procedures (Falluji 2014).
The clinical presentation of ALI varies according to the presence or absence of collateral circulation in the affected limb (Falluji 2014). People with atherosclerotic disease may have a more developed collateral circulation because of a pre‐existing ischaemic condition, which can lead to a more variable presentation of the symptoms and subsequent neuromuscular impairment (Rutherford 2014).
Limb ischaemia is defined as subacute when presentation to the clinical team is more than 14 days after the onset of symptoms (Schernthaner 2014). The most common aetiologies in people with later presentation of symptoms are thromboses of native arteries and graft occlusions (Norgren 2007).
The severity of ALI is graded according to Rutherford's classification (Rutherford 1997): the limb can be classified as viable (I), marginally threatened (IIa), immediately threatened (IIb), and irreversible (III). Rutherford's classification uses the evaluation of sensory loss, muscle weakness, and Doppler signals (arterial and venous) to guide treatment and suggest prognosis.
Complementary investigations may suggest aetiology and help with planning therapy; however, they should not delay treatment in cases of severe ischaemia. The prognosis depends on the time it takes to diagnose and begin appropriate treatment (six to eight hours from the onset of the symptoms is considered the maximum time to achieve revascularisation in cases with severe ischaemia). Delays should be avoided as they may result in irreversible damage, more severe ischaemia, and reperfusion injury (Fukuda 2015). Duplex ultrasonography and computed tomography angiography may help to define the location of the occlusion and the patency of other vascular territories. Computed tomography angiography has better results in the aortoiliac territory but requires the use of contrast material. Angiography or arteriography are important components in the assessment of people with ALI due to the potential for inclusion in an eventual therapeutic procedure, such as endovascular treatment (thrombolysis, percutaneous thrombectomy, angioplasty, or stenting (Rutherford 2014)).
At initial presentation of ALI, typically 45% of the limbs are classified as viable, 45% of the limbs are classified as threatened, and 10% of the limbs are classified as having irreversible ischaemia (Norgren 2007). ALI classified as class I (viable) may require elective revascularisation or may be treated with medical therapy; ALI classified as class II (threatened) generally requires revascularisation (Rutherford 2014).
Description of the intervention
The standard interventional treatment modalities for ALI are endovascular interventions and open surgery (Rutherford 2014). According to the American College of Cardiology/American Heart Association (AHA/ACC) guideline, the choice of intervention depends on local resources and patient factors, in particular, the aetiology and severity of the ischaemia (class I recommendation; level of evidence C – limited data (Gerhard‐Herman 2017)). The European Society of Vascular Surgery (ESVS) guideline recommends urgent revascularisation in people with ALI classified as Rutherford II (class I recommendation; level of evidence C – limited data); revascularisation is also indicated for people with ALI classified as Rutherford I after image evaluation and discussion of the case (class I recommendation; level of evidence C – limited data (Aboyans 2018)).
In the USA, conventional open surgery is used more often than endovascular interventions (Eliason 2003). Open surgery is preferred in cases of proximal embolism of the lower limbs (e.g. above the inguinal ligament) and when there is no associated atherosclerosis. Surgery consists of thrombus removal and may be combined with control arteriography and catheter‐directed thrombolysis (CDT) (Norgren 2007). According to the AHA/ACC guideline, surgical embolectomy may be useful for people with embolic ALI and a viable limb (class IIa recommendation; level of evidence C – limited data (Gerhard‐Herman 2017)).
Endovascular interventions are less invasive and have a lower morbidity associated with the procedure compared with open surgery (Norgren 2007). In CDT, thrombolytic enzymes are injected inside the thrombus to dissolve the thrombus, which may potentially reveal the aetiological factor (e.g. stenosis or occlusions) and guide further treatment. There is an increased risk of bleeding and stroke associated with CDT (Darwood 2018). There is no evidence of a difference in limb salvage, amputation, or death at 30 days, six months, or one year between initial thrombolysis or initial open surgery (Darwood 2018). According to the AHA/ACC guideline, CDT may be used for people with ALI and a viable limb (class I recommendation; level of evidence A (Gerhard‐Herman 2017)).
Percutaneous thrombectomy (PT) and ultrasound‐accelerated thrombolysis (USAT) are performed as alternative endovascular techniques. They encompass different techniques, or modalities, which aim to remove the embolus or the thrombus (or both) from circulation and restore blood flow. These modalities can be mechanical (including aspiration/suction, rheolysis, rotation); mechanical and combined with pharmacological thrombolysis (pharmomechanical thrombectomy (Kasirajan 2001)); or they can use ultrasound to accelerate thrombolysis. According to the AHA/ACC guideline, PT may be used in association with CDT for people with ALI and a viable limb (class IIa recommendation; level of evidence B – non‐randomised (Gerhard‐Herman 2017)) (Björck 2020).
The benefits of PT versus open surgery or thrombolysis are uncertain (Veenstra 2020). Limb salvage after PT in different aetiologies has not yet been fully verified (Liang 2019).
How the intervention might work
Like surgery and thrombolysis, the aim of PT and USAT is to restore limb perfusion.
Percutaneous thrombectomy includes percutaneous mechanical thrombectomy or pharmomechanical thrombectomy (Purushottam 2014). The proposed advantages of PT include reducing:
the time to revascularisation;
the dose and the time of infusion of thrombolytic agents when combined with CDT; and
the risk of associated complications, such as major bleeding (Purushottam 2014).
To perform all forms of PT, the clinical team needs to have the necessary equipment and supplies available in a timely manner (Hynes 2012).
Percutaneous mechanical thrombectomy
Aspiration thrombectomy
In aspiration thrombectomy, the surgeon inserts a large‐lumen catheter that has a thin wall and, with a large volume syringe, then withdraws part or all of the embolus or thrombus from arterial circulation (Norgren 2007). Aspiration thrombectomy can also be performed with endovascular devices that use vacuum aspiration as the mechanism of action (de Donato 2021). The possible complications of aspiration thrombectomy are dissection of the intimal layer of the vessel in areas with atherosclerotic plaque, distal embolisation, and proximal thrombus movement (Hynes 2012).
Rheolytic thrombectomy
In rheolytic thrombectomy, the surgeon uses pressurised and pulsatile saline to fragment and macerate the thrombus. This generates a low‐pressure zone (Venturi – Bernnoulli effect) that facilitates aspiration and withdrawal of the thrombus from circulation (Leung 2015). Potential complications of this technique are embolisation and haemolysis (which may lead to bradyarrhythmia and renal failure (Rutherford 2014)). Intrathrombus thrombolytic agents may be administered during the procedure (Hynes 2012).
Fragmentation or rotational thrombectomy
In fragmentation or rotational thrombectomy, a catheter is inserted and spun at a high frequency, which fragments the thromboembolic material. Some devices will also aspirate the fragments into a collection bag (Lichtenberg 2013). Possible complications of this technique include distal embolisation of thrombus particles (Rutherford 2014).
Pharmomechanical thrombectomy
Some devices have been developed to combine mechanical thrombectomy with the delivery of thrombolytic agents. This is referred to as pharmomechanical thrombectomy. The aim of this technique is to reduce the dose of the medication and the time required to dissolve the thrombus (Rutherford 2014). Potential complications of pharmomechanical thrombectomy are bleeding, distal embolisation, rethrombosis, and arterial injury (Gandhi 2018).
Ultrasound‐enhanced thrombolysis
In the last decade, ultrasound‐enhanced thrombolysis (also known as ultrasound‐accelerated thrombolysis) has been used. Low‐intensity and high‐frequency waves are used to break down fibrin fibres, increasing the permeability of the thrombus and exposing fibrinogen receptors to thrombolytic medication (Schrijver 2015). The aim of this modality is to accelerate the process of thrombolysis. A possible adverse event of this therapy is the potential for the device system to overheat and cause vessel injury (Rutherford 2014).
Why it is important to do this review
Although PT and USAT have been used over the last two decades, no systematic review currently assesses this treatment for people with ALI. This review aimed to present all of the available evidence for PT and USAT in the initial management of ALI, to aid decision‐making for patients and healthcare professionals, including vascular surgeons and interventional radiologists.
Objectives
To assess the safety and effectiveness of percutaneous thrombectomy or ultrasound‐accelerated thrombolysis for the initial management of acute limb ischaemia in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) or quasi‐RCTs that assessed percutaneous thrombectomy (PT, i.e. mechanical thrombectomy (such as aspiration/suction, rheolysis, rotation) or pharmomechanical thrombectomy) or ultrasound‐accelerated thrombolysis (USAT) for the management of acute limb ischaemia (ALI). We excluded studies that compared thrombolysis alone with open surgery as this topic is covered by another Cochrane Review (Darwood 2018).
Types of participants
We included studies with adult participants (at least 18 years old) who were clinically diagnosed with ALI, classified as I or II in Rutherford's classification (Rutherford 1997). We also considered participants with subacute limb ischaemia, i.e. limb ischaemia that has lasted longer than 14 days but shorter than 21 days since the onset of symptoms.
Types of interventions
We evaluated the effects of PT or USAT for the treatment of people with ALI. Percutaneous thrombectomy includes mechanical thrombectomy (aspiration, rheolysis, rotation) or pharmomechanical thrombectomy, resulting in the following possible comparisons:
PT or USAT (any modality) versus open surgery;
PT or USAT (any modality) versus each other, thrombolysis alone, or no treatment.
Types of outcome measures
Primary outcomes
Primary patency: vessel patency at 6 months and 12 months after the intervention, as measured by image evaluation (Doppler ultrasound, tomography, or angiography)
Amputation rate: number of participants undergoing major amputations (defined as amputation either above the ankle in the lower limb or above the wrist in the upper limb) at 12 and 24 months after the intervention
Major bleeding: defined as bleeding that causes a haemoglobin level drop of 3 g/dL or more, requires transfusion, requires surgical intervention for control, or requires vasoactive intravenous drugs; cardiac tamponade; intracranial haemorrhage; intraocular bleed compromising vision, type 3; or fatal bleeding, type 5. We used the Bleeding Academic Research Consortium (BARC) score for this definition (Mehran 2011).
Secondary outcomes
Clinical success: defined as an improvement by at least one category in Rutherford's classification for ALI, or elevation of the ankle‐brachial index by at least 0.2 at 6 and 12 months after the intervention
Secondary patency: patency of the vessel after secondary interventions at 6 and 12 months after the primary intervention, as measured by image evaluation (Doppler ultrasound, tomography, or angiography)
Adverse effects: embolism to new vascular territories, vessel dissection
Search methods for identification of studies
We did not restrict based on language or publication status.
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year, or publication status restrictions:
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 3 March 2021);
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 2) via the Cochrane Register of Studies Online (CRSO);
MEDLINE Ovid (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (searched 1946 to 3 March 2021);
Embase Ovid (searched 1974 to 3 March 2021);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; searched 3 March 2021).
The Information Specialist modelled search strategies for other databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6, Lefebvre 2011). We provide search strategies for major databases in Appendix 1.
The Information Specialist searched the following trials registries on 3 March 2021:
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov).
Searching other resources
We reviewed the bibliographies of the studies identified by the search for further relevant references. We contacted specialists in the field and study authors to request information on any possible unpublished data. We searched the grey literature by consulting the OpenGrey database (opengrey.eu).
Data collection and analysis
Selection of studies
We evaluated all titles and abstracts of the articles identified by the literature searches. After screening the titles and abstracts, two review authors (STA, DHM) independently assessed the full text of studies that appeared to meet the inclusion criteria. We consulted a third review author (DGC) in case of discrepancies.
Data extraction and management
Two review authors (STA, DHM) independently extracted data from the included study using the Cochrane Vascular data extraction form. We consulted a third review author (DGC) in case of any discrepancies. We entered the data into Review Manager 5 software (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (STA, DHM) independently assessed the risk of bias in the included study using Cochrane's RoB 1, and according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We classified the following domains as low risk of bias, high risk of bias, or unclear risk of bias: selection bias, performance bias, detection bias, attrition bias, outcome reporting bias, and other biases. This information is presented in a risk of bias table and risk of bias summary figures.
Measures of treatment effect
We estimated the effect of treatment in dichotomous variables using risk ratios with their associated 95% confidence intervals, using Review Manager 5 (Review Manager 2014).
Unit of analysis issues
We considered the individual participant to be the unit of analysis.
Dealing with missing data
We contacted the authors of the included study to request missing data or additional information. We used an intention‐to‐treat analysis to analyse the available data.
Assessment of heterogeneity
We intended to use the Chi² test with a significance level set at P < 0.1, included in the forest plot, to assess statistical heterogeneity. We also intended to use the I² statistic to assess inconsistency across included studies. We interpreted the I² statistic according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
0% to 25%: low heterogeneity;
25% to 75%: moderate heterogeneity;
more than 75%: substantial heterogeneity.
Assessment of reporting biases
We intended to explore any publication bias by creating a funnel plot if there were enough included trials in the review (more than 10), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We performed data synthesis with Review Manager 5 (Review Manager 2014). In the absence of substantial heterogeneity among included studies, we intended to use a fixed‐effect model to perform the meta‐analysis. If the statistical heterogeneity among the included studies was substantial (I² > 75%), we intended to use a random‐effects model. If it was not possible to pool data, we intended to provide clear reasons for this and report results narratively.
Subgroup analysis and investigation of heterogeneity
If we identified substantial statistical heterogeneity (I² > 75%) and if sufficient data were available, we intended to perform the following subgroup analyses for all planned outcomes, to investigate the effects of clinical heterogeneity (according to the criteria listed in Assessment of heterogeneity).
Variants of percutaneous thrombectomy (mechanical thrombectomy, such as aspiration, rheolysis, rotation, and pharmomechanical thrombectomy).
Severity of ischaemia: acute versus subacute limb ischaemia.
Severity of ischaemia: immediately threatened limbs (Rutherford IIb) versus Rutherford I or IIa (Rutherford 1997).
Previous interventions (endovascular or open surgery) versus no previous interventions.
Number of patent leg arteries measured by image evaluation (Doppler ultrasound, tomography, or angiography) at baseline (0 patent artery versus 1 patent artery versus > 1 patent arteries).
Aetiology of ischaemia (embolism versus thrombosis).
As we only identified one study for inclusion, it was not appropriate to perform subgroup analyses.
Sensitivity analysis
We intended to perform sensitivity analysis to investigate the impact of study characteristics, including sponsorship, presence of publication bias, and high risk of bias. We would have considered a study as being at high risk of bias if we assessed two or more bias domains at high risk. As we only identified one study for inclusion, it was not possible to perform sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We presented the findings of this review in a summary of findings table, based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to present separate tables for each comparison addressed in this review (see Types of interventions), reporting on the outcomes listed in Types of outcome measures (primary patency, amputation rate, major bleeding, clinical success, secondary patency, and adverse effects) at the clinically most relevant time point. We created the summary of findings table with GRADEpro GDT software (GRADEpro GDT 2018). We followed the GRADE approach to evaluate the certainty of the evidence by considering risk of bias, inconsistency, indirectness, imprecision, and publication bias (Atkins 2004; Higgins 2011). Based on this, we classified the certainty of the body of evidence for the outcomes as high, moderate, low, or very low.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
A flow diagram of the search results is shown in Figure 1.
1.
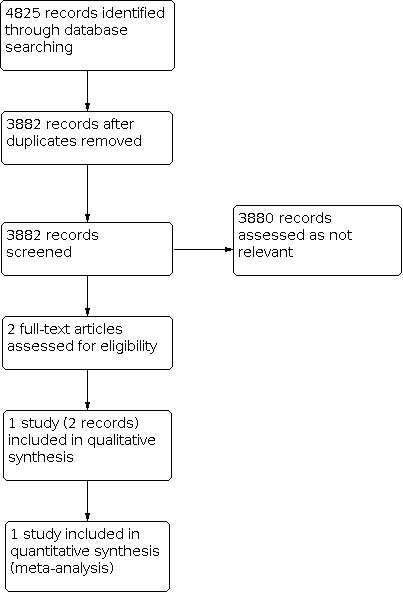
Study flow diagram
Included studies
We identified one completed randomised controlled trial, which compared ultrasound‐accelerated thrombolysis with standard catheter‐directed thrombolysis (CDT) for the initial management of adults with acute limb ischaemia (ALI) (Schrijver 2015). Sixty patients with ALI (Rutherford's classification I or IIa; onset of symptoms between 7 and 49 days) were randomised into two treatment groups, receiving either ultrasound‐accelerated thrombolysis or standard catheter‐directed thrombolysis. Outcomes were assessed on the day of the procedure and 30 days after the intervention. Outcomes were duration of catheter‐directed thrombolysis needed for uninterrupted flow (> 95% lysis), technical success, number of units of urokinase needed for uninterrupted flow, death, major amputation and other adverse events, duration of hospital admission, and 30‐day patency of the treated native artery or bypass graft.
We did not identify randomised controlled trials comparing the other planned interventions.
Excluded studies
We did not identify any excluded studies, i.e. studies we assessed via full text to determine their potential relevance, but which did not eventually meet the inclusion criteria.
Risk of bias in included studies
Our risk of bias assessments for the included study can be seen in Figure 2 and Figure 3. We classified Schrijver 2015 as having a low risk of bias for random sequence generation; unclear risk of bias for allocation concealment and other bias; and high risk for selection, performance, attrition, and selective reporting bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Schrijver 2015 was a randomised controlled trial. Randomisation was undertaken using a computerised randomisation procedure. We classified random sequence generation as at low risk of bias. However, the trial did not detail the method of allocation concealment; therefore, we classified allocation concealment as at unclear risk of bias.
Blinding
Schrijver 2015 did not blind participants or personnel to treatment or during outcome assessment. We classified Schrijver 2015 as at high risk of bias for this domain. Blinding was not possible in the context of this study. Blinding of the outcome assessment (angiography evaluators) was not used due to the visual difference between thrombolysis catheters.
Incomplete outcome data
There were no missing outcome data. We subdivided the attrition bias classification of Schrijver 2015 according to outcome. The included study performed all analyses according to the intention‐to‐treat principle; in some participants in the intervention group, there was technical failure in placing the catheter (therefore, they did not receive the intervention). However, the study did not exclude these participants from the analyses of adverse events. As these participants were not exposed to the risk of the intervention, it could have reduced the detected risk of adverse events. Therefore, we judged Schrijver 2015 as at unclear risk of bias for the outcome adverse effects. We judged attrition bias for the other outcomes as at low risk of bias.
Selective reporting
We classified Schrijver 2015 as at high risk of reporting bias. Two secondary outcomes mentioned in the published study protocol were not reported in the final publication, namely "costs of hospital admission" and "drop of serum fibrinogen concentration to below 1.0 g/L during procedure" (Schrijver 2011).
Other potential sources of bias
We classified Schrijver 2015 as at unclear risk of other bias because it is not clear why the study excluded participants with symptom onset less than seven days and participants classified as Rutherford's classification IIb. The study author declared that some research materials were provided free of charge by the supplier.
Effects of interventions
See: Table 1
Ultrasound‐accelerated thrombolysis versus thrombolysis alone
We identified one study, which compared ultrasound‐accelerated thrombolysis with standard catheter‐directed thrombolysis (CDT) for the initial management of adults with ALI (Schrijver 2015).
Primary outcomes
Primary patency
Schrijver 2015 did not directly evaluate this outcome as no distinction was made between primary or secondary patency. Schrijver 2015 assessed patency rate 30 days after the procedure and reported that the patency rate in the successfully lysed participants in the ultrasound‐accelerated thrombolysis group was 71% (15/21) versus 82% (22/27) in the standard CDT group (P = 0.35).
Amputation rate
Schrijver 2015 assessed the outcome of major amputation 30 days after the procedure and reported that the major amputation rate in the ultrasound‐accelerated thrombolysis group was 7% (2/28) versus 6% (2/32) in the standard CDT group (risk ratio (RR) 1.14, 95% confidence interval (CI) 0.17 to 7.59; 1 study, 60 participants; very low‐certainty evidence) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Ultrasound‐accelerated thrombolysis versus thrombolysis alone, Outcome 1: Amputation rate
Major bleeding
Schrijver 2015 subdivided the outcome of major bleeding into severe bleeding and moderate bleeding. For this review, we considered severe bleeding as major bleeding. The rate of severe bleeding in the ultrasound‐accelerated thrombolysis group was 11% (3/28) compared to 6% (2/32) in the standard CDT group (RR 1.71, 95% CI 0.31 to 9.53; 1 study, 60 participants; very low‐certainty evidence). The rate of moderate bleeding in the ultrasound‐accelerated thrombolysis group was 4% (1/28) versus 0% in the standard CDT group (P = 0.47) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Ultrasound‐accelerated thrombolysis versus thrombolysis alone, Outcome 2: Major bleeding
Secondary outcomes
Clinical success
Schrijver 2015 reported an increase in the ankle‐brachial index (ABI) of 0.57 ± 0.31 in the ultrasound‐accelerated thrombolysis group versus 0.56 ± 0.33 in the standard CDT group (RR 1.00, 95% CI 0.94 to 1.07; 1 study, 60 participants; very low‐certainty evidence) (Analysis 1.3). Although the ABI is a continuous variable, we analysed this outcome as a dichotomous outcome (increase in the ABI by at least 0.2 or not); we concluded from the available information that the increase in the ABI by at least 0.2 was achieved by all participants. Schrijver 2015 did not report an improvement in categories in Rutherford's classification for ALI after the intervention.
1.3. Analysis.

Comparison 1: Ultrasound‐accelerated thrombolysis versus thrombolysis alone, Outcome 3: Clinical success
Secondary patency
Schrijver 2015 did not directly evaluate the outcome of secondary patency, but did report on secondary procedures, defined as those performed after failed thrombolysis to achieve revascularisation, i.e. rescue procedures. Schrijver 2015 subdivided secondary procedures into embolectomy and bypass grafting. Fourteen per cent of participants (4/28) in the ultrasound‐accelerated thrombolysis group had embolectomy versus 3% (1/32) of participants in the standard CDT group (P = 0.18). Four per cent of participants (1/28) in the ultrasound‐accelerated thrombolysis group had bypass grafting versus 0% of participants in the standard CDT group (P = 0.47).
Adverse effects
Schrijver 2015 reported combined distal embolisation and iatrogenic dissection in 7% (2/28) of participants in the ultrasound‐accelerated thrombolysis group and 0% (0/32) in the standard CDT group (RR 5.69, 95% CI 0.28 to 113.72; 1 study, 60 participants; very low‐certainty evidence) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Ultrasound‐accelerated thrombolysis versus thrombolysis alone, Outcome 4: Adverse events
Discussion
Summary of main results
This review aimed to assess the safety and effectiveness of percutaneous thrombectomy or ultrasound‐accelerated thrombolysis (USAT) for the initial management of acute limb ischaemia (ALI) in adults. The only study that met the inclusion criteria was Schrijver 2015, which analysed USAT compared to catheter‐directed thrombolysis (CDT) alone.
Schrijver 2015 did not make a distinction between primary and secondary patency and only evaluated the outcome 30‐day patency. Six participants had secondary procedures (embolectomy and bypass) (one in the standard thrombolysis group and five in the ultrasound‐accelerated thrombolysis group). As we did not have access to the specific data from these participants, it was not possible to perform further analyses for these outcomes.
With a single small study, there is insufficient evidence to assess the safety and effectiveness of ultrasound‐enhanced thrombolysis and catheter‐directed thrombolysis for ALI for the outcomes of amputation rate, major bleeding, clinical success, and adverse effects.
Overall completeness and applicability of evidence
The objective of this review was to assess the effectiveness and safety of percutaneous thrombectomy or USAT for the initial management of ALI in adults. However, our search did not identify any randomised controlled trials investigating modalities of percutaneous thrombectomy (defined in the published Cochrane Review protocol as mechanical thrombectomy (aspiration, rheolysis, rotation), and pharmomechanical thrombectomy, Araujo 2019).
We identified only one randomised controlled trial, which assessed USAT compared to standard treatment (thrombolysis alone) in adults. The trial included a real‐life population and used a short clinical follow‐up period (30 days). As we were only able to include one study, we could not perform pooled analysis.
The included study did not directly evaluate our primary outcome primary patency and our secondary outcome secondary patency; it made no distinction between primary and secondary patency. We made an attempt to contact the study author requesting additional data about these outcomes, but to date, we have not received a response. Schrijver 2015 evaluated our other primary (amputation rate and major bleeding) and secondary outcomes (clinical success and adverse effects), which we included in the review.
We were not able to fully address the objective of this review for the following reasons: the inclusion of only one study (Schrijver 2015), we could not investigate all relevant modalities of percutaneous thrombectomy, and the study did not report two relevant outcomes (primary and secondary patency) separately.
In this Cochrane Review, we found very low‐certainty evidence that there is no clear difference between USAT and thrombolysis alone for the outcomes amputation rate, major bleeding, clinical success, and adverse effects.
This result has great relevance for clinical practice since percutaneous thrombectomy or USAT for initial management of ALI are procedures performed routinely in large centres around the world. Although there are many types of percutaneous thrombectomy, which are used to treat an increasing number of people, there is no solid evidence for its use.
A further limitation of this systematic review is the included study's small sample size. We expected to find more clinical trials with more participants in our search. In addition, we expected to find longer follow‐up periods for participants assigned to the evaluated interventions. These limitations alongside issues related to lack of blinding and selective reporting contribute to the risk of bias and decrease the certainty of the evidence. For this reason, the applicability of the results is limited.
Quality of the evidence
The certainty of the evidence provided by the study in our only comparison is summarised in Table 1. We assessed the certainty of the evidence as very low for amputation rate, major bleeding, clinical success, and adverse effects at 30 days following the procedure. We downgraded the certainty of the evidence for amputation rate, major bleeding, clinical success, and adverse effects by two levels due to serious limitations in the design (there was a high risk of bias in critical domains) and by two further levels because of imprecision (small number of participants and only one study included).
Potential biases in the review process
There was only one included study for the outcomes evaluated, so we were not able to pool analyses.
Agreements and disagreements with other studies or reviews
We identified one small randomised controlled trial for inclusion (Schrijver 2015), so we were unable to perform a combined analysis. Our search did, however, retrieve some non‐randomised studies on the proposed subjects of percutaneous thrombectomy and ultrasound‐accelerated thrombolysis for ALI.
In de Athayde Soares 2020, a single‐centre retrospective cohort study, 49 patients with ALI underwent endovascular treatment with pharmomechanical thrombectomy (rheolytic thrombectomy) or CDT. The participants were followed up for 720 days. Eighteen participants underwent pharmomechanical thrombectomy, and 31 underwent CDT. The outcomes evaluated were limb salvage rate, survival rate, and perioperative mortality at 30 days postsurgery. The study was not randomised, and the intervention was chosen according to the decision of the vascular surgeon who admitted the participant. The limb salvage rate was 87.8% in the intervention group and 89.7% in the control group (P = 0.78), and the overall survival rate was 84.7% in the pharmomechanical thrombectomy group and 69.2% in the CDT group (P = 0.82). Perioperative 30‐day mortality was 11.1% (two participants) in the pharmomechanical thrombectomy group and 19.3% (six participants) in the CDT group (P = 0.03).
In Muli Jogi 2018, a retrospective study, 94 patients with ALI underwent 117 percutaneous mechanical thrombectomy (rheolytic and rotational thrombectomy) or CDT procedures. The participants were followed up for 30 days. Twenty‐eight participants underwent percutaneous mechanical thrombectomy, and 89 underwent catheter‐directed thrombolysis. The outcomes evaluated were technical success, clinical success, amputation rate at 30 days, duration of hospitalisation, and 30‐day mortality. The study was not randomised, and the intervention was chosen according to the clinical scenario and the operator’s expertise. Clinical success was achieved in 21 participants (75%) in the percutaneous mechanical thrombectomy group and in 65 participants (73%) in the CDT group (P = 0.837). Technical success was achieved in 19 participants (67.9%) in the percutaneous mechanical thrombectomy group and in 42 participants (47.2%) in the CDT group (P = 0.056). Major amputation at 30 days occurred in two participants (7.1%) in the percutaneous mechanical thrombectomy group and in 15 participants (16.9%) in the CDT group (P = 0.323). The duration of hospitalisation (mean stay) was 6 days in the percutaneous mechanical thrombectomy group versus 12.6 days in the CDT group (P < 0.001). The thirty‐day mortality rate was 3.6% (1) in the percutaneous mechanical thrombectomy group versus 8% (7) in the CDT group (P = 0.425).
In Kronlage 2017, a single‐centre retrospective study, 202 patients with acute and subacute thrombotic occlusions of the lower extremity underwent rotational thrombectomy, local thrombolysis, or a combination of both. The participants were followed up for one year. One hundred and forty‐six participants underwent rotational thrombectomy; 28, thrombolysis; and 28, a combination of rotational thrombectomy and thrombolysis. The outcomes evaluated were overall and amputation‐free survival, as well as patency in a one‐year follow‐up. The study was not randomised, and the intervention was chosen by the interventionalist, dependent on the extension of the occlusion and the availability of the rotational thrombectomy system. Overall survival 12 months after intervention reached 96% in non‐critically ill participants, and amputation‐free survival was 94.3% in all three groups. In the rotational thrombectomy group, primary patency was 63% and for secondary patency it was 85%.
Authors' conclusions
Implications for practice.
There is insufficient evidence to assess the safety and effectiveness of ultrasound‐enhanced thrombolysis (USAT) versus standard treatment (catheter‐directed thrombolysis) for ALI for the evaluated outcomes amputation rate, major bleeding, clinical success, and adverse effects. Our included study did not separately report primary and secondary patency. We did not identify studies that assessed percutaneous thrombectomy. Limitations of this systematic review are the limited availability of clinical trials (one randomised controlled trial identified), the small number of participants, the short clinical follow‐up period, and a high risk of bias in critical domains. For this reason, the applicability of the results is limited. New trials are very likely to have an important impact; they may find different results or strengthen the evidence presented in this systematic review.
Implications for research.
There is a need for high‐quality trials that compare percutaneous thrombectomy (any modality) or USAT against open surgery, thrombolysis alone, no treatment or other percutaneous thrombectomy modalities for ALI. We suggest that these future comparative studies consider the following:
clearly describe the methods of randomisation and allocation concealment;
include a greater number of participants;
perform longer clinical follow‐up periods;
perform blinding of the outcome assessments/assessors;
build a flow diagram with a detailed description of the participant losses throughout the study; and
follow the CONSORT guidelines.
History
Protocol first published: Issue 11, 2019
| Date | Event | Description |
|---|---|---|
| 21 November 2019 | Amended | Affiliation of external reviewer amended in protocol |
Notes
Parts of the Methods section of this protocol are based on a standard template established by Cochrane Vascular.
Acknowledgements
The review authors would like to thank the Cochrane Vascular editorial base staff for their attention and support. Special thanks to the Brazilian Cochrane Centre Study Group (Federal University of São Paulo ‐ UNIFESP/EPM) for their methodological support.
The review authors, and the Cochrane Vascular editorial base, are grateful to the following peer reviewers for their time and comments: Professor Gianmarco de Donato, Department of Medical Science, Surgery and Neuroscience, University of Siena, Italy; Scott Kinlay, MBBS, PhD, Veterans Affairs Boston Healthcare System, Boston, USA / Brigham and Women's Hospital, Boston, USA; Dr Venu Vadlamudi, Associate Professor of Radiology, University of Tennessee Health Science Center, USA; Ratko Peric, Bosnia and Herzegovina.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW | #1 acute limb ischaemia OR Arterial Occlusive Disease* OR Peripheral Vascular Disease* AND INREGISTER #2 Thrombectomy OR Thrombolytic Therapy AND INREGISTER #3 #1 AND #2 |
13.1.20: 28 3.3.21: 21 |
| CENTRAL | #1 MESH DESCRIPTOR Arterial Occlusive Diseases EXPLODE ALL TREES 11791 #2 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 3110 #3 (acute limb ischaemia):TI,AB,KY 9 #4 (acute limb ischaemia):TI,AB,KY 71 #5 ((arter* or vascular or vein* or veno* or peripher*) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 13270 #6 (peripheral adj2 dis*):TI,AB,KY 6401 #7 (leg adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 98 #8 (limb adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 236 #9 (lower extrem* adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 117 #10 (((iliac or femoral or popliteal or femoro* or fempop* or crural) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*))):TI,AB,KY 1934 #11 (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*))):TI,AB,KY 2200 #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 29633 #13 MESH DESCRIPTOR Thrombectomy EXPLODE ALL TREES 266 #14 MESH DESCRIPTOR Thrombolytic Therapy EXPLODE ALL TREES 1649 #15 (((Percutaneous or mechanical or Aspiration or Rheolytic or Fragmentation or rotational or ultrasound enhanced or Pharmomechanical) adj3 thrombectomy)):TI,AB,KY 716 #16 #13 OR #14 OR #15 2489 #17 #12 AND #16 342 |
13.1.20: 342 3.3.21: 23 |
| Clinicaltrials.gov | acute limb ischaemia OR Arterial Occlusive Diseases OR Peripheral Vascular Diseases | Thrombectomy OR Thrombolytic Therapy | 13.1.20: 346 3.3.21: 27 |
| ICTRP Search Portal | acute limb ischaemia OR Arterial Occlusive Diseases OR Peripheral Vascular Diseases | Thrombectomy OR Thrombolytic Therapy | 13.1.20: 2 3.3.21: N/A |
| Medline (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present | 1 Arterial Occlusive Diseases/ 2 exp Peripheral Vascular Diseases/ 3 acute limb ischaemia.ti,ab. 4 acute limb ischemia.ti,ab. 5 ((arter* or vascular or vein* or veno* or peripher*) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 6 (peripheral adj2 dis*).ti,ab. 7 (leg adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 8 (limb adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 9 (lower extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 10 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 11 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 12 or/1‐11 13 exp Thrombectomy/ 14 exp Thrombolytic Therapy/ 15 ((Percutaneous or mechanical or Aspiration or Rheolytic or Fragmentation or rotational or ultrasound enhanced or Pharmomechanical) adj3 thrombectomy).ti,ab. 16 or/13‐15 17 12 and 16 18 randomized controlled trial.pt. 19 controlled clinical trial.pt. 20 randomized.ab. 21 placebo.ab. 22 drug therapy.fs. 23 randomly.ab. 24 trial.ab. 25 groups.ab. 26 or/18‐25 27 exp animals/ not humans.sh. 28 26 not 27 29 17 and 28 |
13.1.20: 1977 3.3.21: 110 |
| Embase 1974 to present | 1 Arterial Occlusive Diseases/ 2 Peripheral Vascular Diseases/ 3 acute limb ischaemia.ti,ab. 4 acute limb ischemia.ti,ab. 5 ((arter* or vascular or vein* or veno* or peripher*) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 6 (peripheral adj2 dis*).ti,ab. 7 (leg adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 8 (limb adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 9 (lower extrem* adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 10 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 11 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 12 or/1‐11 13 exp Thrombectomy/ 14 exp fibrinolytic therapy/ 15 ((Percutaneous or mechanical or Aspiration or Rheolytic or Fragmentation or rotational or ultrasound enhanced or Pharmomechanical) adj3 thrombectomy).ti,ab. 16 or/13‐15 17 12 and 16 18 randomized controlled trial/ 19 controlled clinical trial/ 20 random$.ti,ab. 21 randomization/ 22 intermethod comparison/ 23 placebo.ti,ab. 24 (compare or compared or comparison).ti. 25 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 26 (open adj label).ti,ab. 27 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 28 double blind procedure/ 29 parallel group$1.ti,ab. 30 (crossover or cross over).ti,ab. 31 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 32 (assigned or allocated).ti,ab. 33 (controlled adj7 (study or design or trial)).ti,ab. 34 (volunteer or volunteers).ti,ab. 35 trial.ti. 36 or/18‐35 37 17 and 36 |
13.1.20: 1324 3.3.21: 176 |
| CINAHL | S33 S17 AND S32 S32 S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 S31 MH "Random Assignment" S30 MH "Triple‐Blind Studies" S29 MH "Double‐Blind Studies" S28 MH "Single‐Blind Studies" S27 MH "Crossover Design" S26 MH "Factorial Design" S25 MH "Placebos" S24 MH "Clinical Trials" S23 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S22 TX crossover OR "cross‐over" S21 AB placebo* S20 TX random* S19 TX trial* S18 TX "latin square" S17 S12 AND S16 S16 S13 OR S14 OR S15 S15 ((Percutaneous or mechanical or Aspiration or Rheolytic or Fragmentation or rotational or ultrasound enhanced or Pharmomechanical) N3 thrombectomy) S14 (MH "Thrombolytic Therapy") S13 (MH "Thrombectomy") S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 S11 (femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) N2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) S10 ((iliac or femoral or popliteal or femoro* or fempop* or crural) N2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S9 (lower extrem* N2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S8 (limb N2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S7 (leg N2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S6 peripheral N2 dis* S5 (arter* or vascular or vein* or veno* or peripher*) N2 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) S4 TX acute limb ischemia S3 TX acute limb ischaemia S2 (MH "Peripheral Vascular Diseases+") S1 (MH "Arterial Occlusive Diseases+") |
13.1.20: 412 3.3.21: 38 |
Data and analyses
Comparison 1. Ultrasound‐accelerated thrombolysis versus thrombolysis alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Amputation rate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.17, 7.59] |
| 1.2 Major bleeding | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.31, 9.53] |
| 1.3 Clinical success | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| 1.4 Adverse events | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.69 [0.28, 113.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Schrijver 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
USAT: high‐frequency US was delivered using the EKOS EndoWave system (EKOS Corporation, Bothell, Washington, USA) and urokinase was infused through 3 drug lumens containing multiple side holes Standard CDT: 250,000‐IU bolus of urokinase was given, followed by a continuous infusion of 100,000 IU/h using a 5‐F UniFuse Infusion catheter (AngioDynamics, Queensbury, New York, USA) |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Funding | The study authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the supplier provided the EKOS Endowave System free of charge. | |
| Declarations of interest | The study authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer randomised participants. |
| Allocation concealment (selection bias) | Unclear risk | The study paper did not report the method. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not used and was not possible in the context of the study due to the visual difference between thrombolysis catheters. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not used due to the visual difference between the thrombolysis catheters. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not exclude 3 participants in the intervention group who experienced technical failure in placing the catheter from the analysis of the outcomes death and adverse effects. |
| Incomplete outcome data (attrition bias) Techinical success | Low risk | There were no missing outcome data. |
| Incomplete outcome data (attrition bias) 30‐day patency | High risk | At the 30‐day follow‐up, the study considered only the successfully lysed participants when analysing the patency rates. |
| Selective reporting (reporting bias) | High risk | Two secondary outcomes mentioned in the published study protocol were not reported in the final publication: "Costs of hospital admission" and "drop of serum fibrinogen concentration to below 1.0 g/L during procedure". |
| Other bias | Unclear risk | It is not clear why the study excluded participants with symptom onset in less than 7 days and participants classified as IIb in Rutherford's classification. |
ALI: acute limb ischaemia CDT: catheter‐directed thrombolysis CFA: common femoral artery DFA: deep femoral artery F: measure of sheath size IU: international unit SFA: superficial femoral artery US: ultrasound USAT: ultrasound‐accelerated thrombolysis
Differences between protocol and review
We amended the review to separate ultrasound‐accelerated thrombolysis from percutaneous thrombectomy.
Contributions of authors
STA: contact person, guarantor of the review, protocol drafting, addressing clinical comments from the referees, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and future review updates DHM: protocol drafting, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and review drafting DGC: protocol drafting, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and review drafting
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
STA: none known. DHM: none known. DGC: none known.
New
References
References to studies included in this review
Schrijver 2015 {published data only}
- Schrijver AM, Reijnen MM, Oostayen JA, Tutein Nolthenius RP, Valk PH, Hoksbergen AW, et al. Dutch randomized trial comparing standard catheter-directed thrombolysis versus ultrasound-accelerated thrombolysis for thromboembolic infrainguinal disease (DUET): design and rationale. Trials 2011;12:20. [DOI: 10.1186/1745-6215-12-20] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijver AM, Leersum M, Fioole B, Reijnen MM, Hoksbergen AW, Vahl AC, et al. Dutch randomized trial comparing standard catheter-directed thrombolysis and ultrasound-accelerated thrombolysis for arterial thromboembolic infrainguinal disease (DUET). Journal of Endovascular Therapy 2015;22(1):87-95. [DOI: 10.1177/1526602814566578] [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Aboyans 2018
- Aboyans V, Ricco JB, Bartelink ME, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Revista Espanola de Cardiologia (English ed.) 2018;71(2):111. [DOI: 10.1016/j.rec.2017.12.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI: 10.1136/bmj.328.7454.1490] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Björck 2020
- Björck M, Earnshaw JJ, Acosta S, Bastos Gonçalves F, Cochennec F, Debus ES, et al. Editor's choice - European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of acute limb ischaemia. European Journal of Vascular and Endovascular Surgery 2020;59(2):173-218. [DOI: 10.1016/j.ejvs.2019.09.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Darwood 2018
- Darwood R, Berridge DC, Kessel DO, Robertson I, Forster R. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD002784. [DOI: 10.1002/14651858.CD002784.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Athayde Soares 2020
- Athayde Soares R, Matielo MF, Brochado Neto FC, Pereira de Carvalho BV, Sacilotto R. Analysis of the safety and efficacy of the endovascular treatment for acute limb ischemia with percutaneous pharmacomechanical thrombectomy compared with catheter-directed thrombolysis. Annals of Vascular Surgery 2020;66:470-8. [DOI: 10.1016/j.avsg.2019.11.038] [PMID: ] [DOI] [PubMed] [Google Scholar]
de Donato 2021
- Donato G, Pasqui E, Sponza M, Intrieri F, Spinazzola A, Silingardi R, et al. Safety and efficacy of vacuum assisted thrombo-aspiration in patients with acute lower limb ischaemia: the INDIAN trial. European Journal of Vascular and Endovascular Surgery 2021;61(5):820-8. [DOI: 10.1016/j.ejvs.2021.01.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dormandy 1999
- Dormandy J, Heeck L, Vig S. Acute limb ischemia. Seminars in Vascular Surgery 1999;12(2):148-53. [PMID: ] [PubMed] [Google Scholar]
Eliason 2003
- Eliason JL, Wainess RM, Proctor MC, Dimick JB, Cowan JA Jr, Upchurch GR Jr, et al. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Annals of Surgery 2003;238(3):382-9; discussion 389-90. [DOI: 10.1097/01.sla.0000086663.49670.d1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falluji 2014
- Falluji N, Mukherjee D. Critical and acute limb ischemia: an overview. Angiology 2014;65(2):137-46. [DOI: 10.1177/0003319712470966] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fukuda 2015
- Fukuda I, Chiyoya M, Taniguchi S, Fukuda W. Acute limb ischemia: contemporary approach. General Thoracic and Cardiovascular Surgery 2015;63(10):540-8. [DOI: 10.1007/s11748-015-0574-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gandhi 2018
- Gandhi SS, Ewing JA, Cooper E, Chaves JM, Gray BH. Comparison of low-dose catheter-directed thrombolysis with and without pharmacomechanical thrombectomy for acute lower extremity ischemia. Annals of Vascular Surgery 2018;46:178-86. [DOI: 10.1016/j.avsg.2017.07.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gerhard‐Herman 2017
- Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vascular Medicine (London, England) 2017;22(3):NP1-43. [DOI: 10.1177/1358863X17701592] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2018 [Computer program]
- GRADEpro GDT. Version accessed 7 November 2018. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hynes 2012
- Hynes BG, Margey RJ, Ruggiero N 2nd, Kiernan TJ, Rosenfield K, Jaff MR. Endovascular management of acute limb ischemia. Annals of Vascular Surgery 2012;26(1):110-24. [DOI: 10.1016/j.avsg.2011.05.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kasirajan 2001
- Kasirajan K, Haskal ZJ, Ouriel K. The use of mechanical thrombectomy devices in the management of acute peripheral arterial occlusive disease. Journal of Vascular and Interventional Radiology 2001;12(4):405-11. [DOI: 10.1016/s1051-0443(07)61877-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Katsanos 2017
- Katsanos K, Al-Lamki SA, Parthipun A, Spiliopoulos S, Patel SD, Paraskevopoulos I, et al. Peripheral stent thrombosis leading to acute limb ischemia and major amputation: incidence and risk factors in the aortoiliac and femoropopliteal arteries. Cardiovascular and Interventional Radiology 2017;40(3):351-9. [DOI: 10.1007/s00270-016-1513-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kronlage 2017
- Kronlage M, Printz I, Vogel B, Blessing E, Müller OJ, Katus HA, et al. A comparative study on endovascular treatment of (sub)acute critical limb ischemia: mechanical thrombectomy vs thrombolysis. Drug Design, Development and Therapy 2017;11:1233-41. [DOI: 10.2147/DDDT.S131503] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Leung 2015
- Leung DA, Blitz LR, Nelson T, Amin A, Soukas PA, Nanjundappa A, et al. Rheolytic pharmacomechanical thrombectomy for the management of acute limb ischemia: results from the PEARL Registry. Journal of Endovascular Therapy 2015;22(4):546-57. [DOI: 10.1177/1526602815592849] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liang 2019
- Liang S, Zhou L, Ye K, Lu X. Limb salvage after percutaneous mechanical thrombectomy in patients with acute lower limb ischemia: a retrospective analysis from two institutions. Annals of Vascular Surgery 2019;58:151-9. [DOI: 10.1016/j.avsg.2018.11.025] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lichtenberg 2013
- Lichtenberg M, Stahlhoff FW, Boese D. Endovascular treatment of acute limb ischemia and proximal deep vein thrombosis using rotational thrombectomy: a review of published literature. Cardiovascular Revascularization Medicine 2013;14(6):343-8. [DOI: 10.1016/j.carrev.2013.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mehran 2011
- Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123(23):2736-47. [DOI: 10.1161/CIRCULATIONAHA.110.009449] [PMID: ] [DOI] [PubMed] [Google Scholar]
Muli Jogi 2018
- Muli Jogi RK, Damodharan K, Leong HL, Tan AC, Chandramohan S, Venkatanarasimha NK, et al. Catheter-directed thrombolysis versus percutaneous mechanical thrombectomy in the management of acute limb ischemia: a single center review. CVIR Endovascular 2018;1(1):35. [DOI: 10.1186/s42155-018-0041-1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45(Suppl S):S5-67. [DOI: 10.1016/j.jvs.2006.12.037] [PMID: ] [DOI] [PubMed] [Google Scholar]
Purushottam 2014
- Purushottam B, Gujja K, Zalewski A, Krishnan P. Acute limb ischemia. Interventional Cardiology Clinics 2014;3(4):557-72. [DOI: 10.1016/j.iccl.2014.07.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutherford 1997
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. Journal of Vascular Surgery 1997;26(3):517-38. [DOI: 10.1016/s0741-5214(97)70045-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rutherford 2014
- Rutherford RB. Vascular Surgery. 8th edition. Vol. 2. Philadelphia: Elsevier Inc, 2014. [Google Scholar]
Schernthaner 2014
- Schernthaner MB, Samuels S, Biegler P, Benenati JF, Uthoff H. Ultrasound-accelerated versus standard catheter-directed thrombolysis in 102 patients with acute and subacute limb ischemia. Journal of Vascular and Interventional Radiology 2014;25(8):1149-56. [DOI: 10.1016/j.jvir.2014.03.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schrijver 2011
- Schrijver AM, Reijnen MM, Oostayen JA, Tutein Nolthenius RP, Valk PH, Hoksbergen AW, et al. Dutch randomized trial comparing standard catheter-directed thrombolysis versus ultrasound-accelerated thrombolysis for thromboembolic infrainguinal disease (DUET): design and rationale. Trials 2011;12:20. [DOI: 10.1186/1745-6215-12-20] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veenstra 2020
- Veenstra EB, Laan MJ, Zeebregts CJ, Heide EJ, Kater M, Bokkers RP. A systematic review and meta-analysis of endovascular and surgical revascularization techniques in acute limb ischemia. Journal of Vascular Surgery 2020;71(2):654-68. [DOI: 10.1016/j.jvs.2019.05.031] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Araujo 2019
- Araujo ST, Moreno DH, Cacione DG. Percutaneous thrombectomy for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013486. [DOI: 10.1002/14651858.CD013486] [DOI] [PMC free article] [PubMed] [Google Scholar]


