Abstract
Introduction
Digital technology creates the opportunity to develop and evaluate new tools, such as smartphone applications, to support integrated atrial fibrillation management. This study aimed to develop, evaluate, and validate a new, integrated care application (AF-EduApp) mainly focusing on targeted atrial fibrillation education to improve patient self-care capabilities and therapy adherence.
Methods
The newly developed AF-EduApp, available for Android and iOS, consists of six different modules. The prototype was validated and optimized for its usability and functionality at Jessa Hospital Hasselt and Antwerp University Hospital in two phases: (1) validity evaluation with interviews of an expert panel with 15 healthcare professionals and 10 atrial fibrillation patients, and (2) a pilot study of 1 month with 20 atrial fibrillation patients.
Results
Both experts and patients found that the application aids atrial fibrillation management. Based on the input of patients and experts, the main optimizations concerned the medication module (patient choice on setting reminder; interactivity of reminders with a “taken” or “snooze” function) and development of a clinical dashboard for the caregivers allowing telemonitoring of measurements and feedback to the patients. After the pilot study (n = 20), 16 patients indicated they wanted to use the app for a longer period. The measurement (27%) and education (17%) modules were the two most used modules with a significant improvement in knowledge (71.9% to 87.5%; P = 0.013).
Discussion
The AF-EduApp received a positive evaluation from health professionals and atrial fibrillation patients. Further development should be focused on the medication module and improvement of the clinical dashboard.
Keywords: Atrial fibrillation, patient education, knowledge, mHealth, integrated care, medication adherence, mobile application
Introduction
The daily use of smartphones and tablets is increasing and has clearly also spread to the elderly. 1 The use of different multimedia devices provides new opportunities in the management of chronic diseases, since mobile health (mHealth) technologies can now be brought towards the elderly patients. 2 Atrial fibrillation (AF) is the most common cardiac arrhythmia with a prevalence of 8.8 million European adults over 55 years old in 2010 which will further increase over the next years. 3 AF is a chronic disease that can benefit from interconnected transmural care (i.e. healthcare based on co-operation and coordination between general and specialized caregivers with the use of mHealth technologies). This arrhythmia is associated with increased morbidity and mortality due to potentially life-threatening complications such as stroke and heart failure (HF). 4
Treatment of AF is based on three pillars: (I) oral anticoagulation (OAC) medication to reduce the risk for stroke, (II) symptom control with rate and rhythm control, and (III) tackling risk factors (e.g. hypertension, overweight) to optimize care and to reduce AF recurrences. 5 However, implementation by caregivers and adherence by patients to these three pillars is still suboptimal.6,7 The most recent AF guidelines indicated that a more structured and efficient care system including the three pillars and with the patient in a central role (i.e. integrated AF care) is required to optimize patients’ outcomes. 5 Integrated care has been shown to contribute to improved outcome measures for AF patients and increased patient awareness about their treatment. 8 Therefore, patient education and involvement are fundamental. Our research group has shown that reinforced personal, 9 but also online, 10 education about AF and its treatment improved and maintained patients’ AF knowledge. Currently, non-vitamin K antagonist oral anticoagulants (NOACs) are in most situations preferred over vitamin-K antagonists (VKA) given their relative effectiveness, safety, and convenience.5,11 Given their short anticoagulation effect, strict adherence to the correct dosing regimen is crucial. Notably, previous studies have demonstrated that adherence to NOAC (defined as the period of days covered with medication) is less than 80% in more than 20% of the AF patients.12,13 Our research group has shown that telemonitoring of NOAC intake with direct feedback positively impacted therapy adherence to NOACs. 14 Currently, a multicenter randomized trial in more than 1000 AF patients is being conducted, using these elements (i.e. reinforced in-person or online education, patient involvement to improve self-care capabilities and telemonitoring for therapy adherence to OAC), to study the impact on clinical outcomes. 15
In order to actively involve patients in the different aspects of their treatment and connect them to their caregivers, there is an urgent need for new strategies. The rise in smartphone and tablet use creates opportunities to develop new digital strategies. The in-house developed AF-EduApp builds on the approach of telecoaching based on education and monitoring, by actively involving the patients through their smartphones. The main aim of this study was to validate the AF-EduApp by an expert panel and to assess its usability in a first pilot study in which its effect on the knowledge level of AF patients was assessed.
Methods
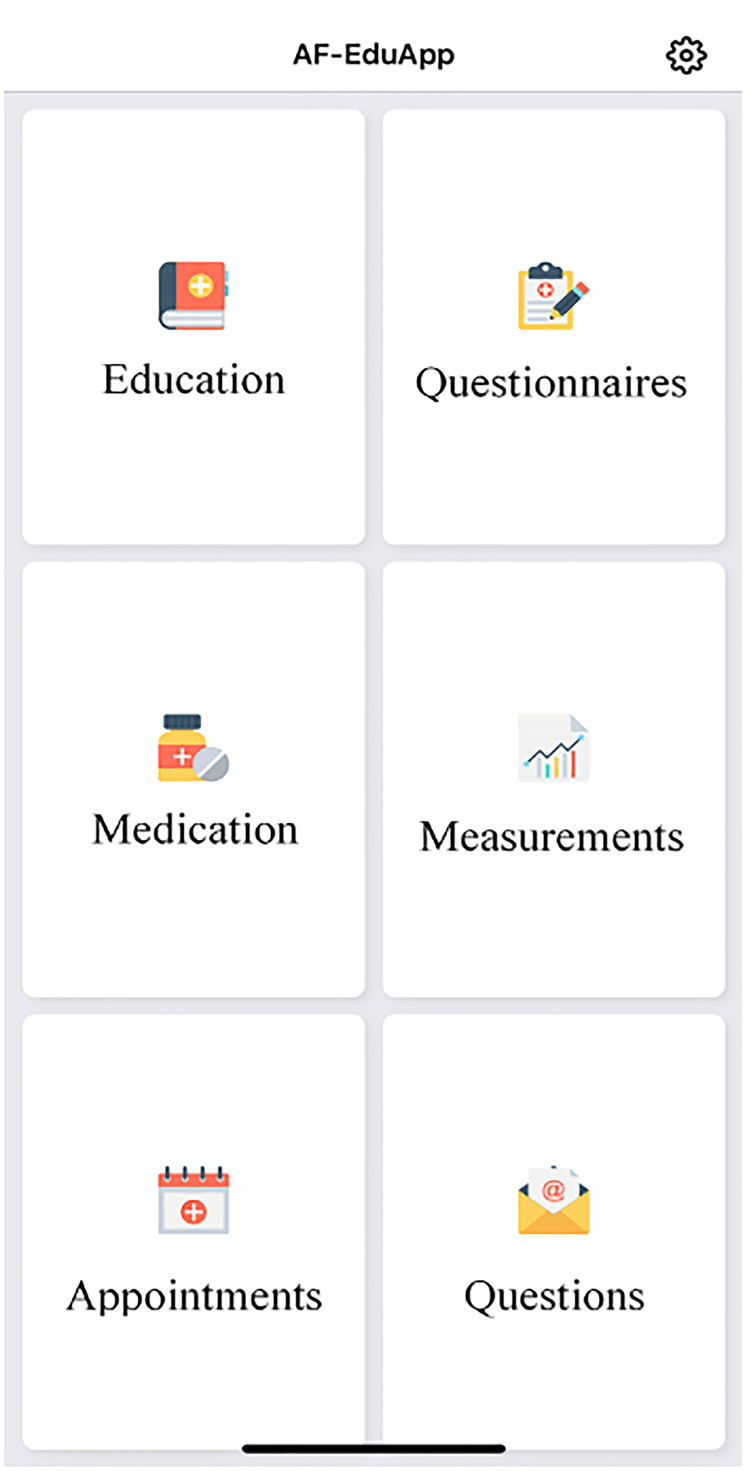
The AF-EduApp (Figure 1) is an in-house developed application built with React Native and connected to a protected FileMaker server as back end. The concept of the AF-EduApp is based on insights gained from previous projects of our research group, focusing on patient education to improve their knowledge, self-care capabilities, therapy adherence to OAC, and hopefully their clinical outcomes.9,10,14,16 The app is only available in Dutch and consists of six different modules. The education module (I) provides information with text, images and videos about AF, its symptoms, detection methods, risk factors, possible complications. Additionally, education about the treatment options is included. Specific education on the different OACs, that is, VKA or NOAC, is also included. An interactive questionnaire module (II) expands on that to improve patients’ education and self-care capabilities. It includes targeted education based on the answers to the Jessa Atrial fibrillation Knowledge Questionnaire (JAKQ). 17 This 16-item questionnaire contains eight questions about AF in general, five about OAC, and three specific questions on VKA or NOAC, depending on the medication of the patient. It also evaluates through other questionnaires patients’ selfcare possibilities, AF related symptoms and quality of life. A third medication module (III) with a list of the Belgian Center for Pharmacotherapeutic Information (BCFI; i.e. a Belgian non-profit organization that provides independent, objective, and evidence-based information about medicines) and with the option to track patients’ medication adherence with interactive reminders. A clinical parameters module (IV) is implemented, in which the patient manually enters specific measurements (i.e. heartbeat, blood pressure, weight, and description of AF episodes). Finally, an appointments module (V) and a questions module (VI) that allows interaction with the study team (currently discontinuous, but expandable to a chat-like functionality) were implemented. Lastly, the app logs the time spent by the user in the different modules. Nevertheless, there is no obligation imposed on how often the patients should use the app. The patient can choose how often the application or modules are used based on their own needs. During the validation phase, the AF-EduApp prototype was available via TestFlight (iOS) and TestFairy (Android), for both smartphone and tablet. Access to the app was possible only with a personal login provided by the study team after written informed consent by the patient.
Figure 1.
Main screen AF-EduApp.
Validation of the AF-EduApp prototype
This paper reports the validation and optimization process of the AF-EduApp, conducted before the start of the randomized prospective AF-EduApp study (NCT03788044) with therapy adherence to OAC as primary outcome parameter. The evaluation and optimization process, which included two phases, was approved by the ethical committee. This was performed in two phases so that there was an interim optimization before patients would use it for a longer period of time during the pilot study. Based on the findings of these two phases, the developer worked on a final optimization before the start of the AF-EduApp randomized controlled trial (RCT, NCT03788044).
Phase 1: content and face validation
First, an expert panel with 15 health professionals and 10 AF patients was asked to give feedback about the prototype’s content and functionality. The health professionals were five electrophysiologists, a cardiologist specialized in cardiovascular prevention and rehabilitation, two AF nurses, a HF nurse, a study nurse specialized in AF, a pharmacist specialized in medication adherence, a general practitioner, a psychologist working at the cardiology department, a member of the mobile health unit of Hasselt University and a researcher specialized in cardiovascular app development. The application was demonstrated to these health professionals in person and afterwards, general questions were asked to gain feedback. Questions were probing whether (I) the app is suitable to increase the knowledge level and medication adherence of AF patients; (II) what is needed so that patients will use the app in the future; (III) the attractivity and ease-of-use of the app; and (IV) whether something should be added or omitted. Ten hospitalized AF patients were recruited at Jessa Hospital Hasselt. The patients were not required to have their own smartphone or tablet. A think aloud protocol (TAP) was performed, in which patients used the app in front of the investigator and said aloud what they thought. Five domains were discussed in person with the patients: (I) is the app easy to use, (II) is the app clear and well structured, (III) would you add or omit certain aspects, (IV) what is needed to use the app in the future, (V) is there something you do not understand. Feedback from this expert panel was analyzed and summarized, allowing the app developer an intermediate optimization.
Phase 2: patient pilot study
In a second phase, 20 AF patients were recruited between June 2019 and July 2019 at two Belgian tertiary centers, namely the Jessa Hospital Hasselt and Antwerp University Hospital (UZA), to use the app for 1 month. The Ethics Committees of the participating centers approved the pilot study. Patients hospitalized or presenting at the out-patient visit of the cardiology department of the two centers were asked to participate. Eligible patients (i.e. documented AF diagnosis, having a tablet or smartphone with an internet connection and able to read and speak Dutch) signed an informed consent if they were willing to participate. At the start of the pilot study and after 1 month, the patients needed to fill in the JAKQ. The app-user data were collected from the app to determine the time spent on the app and the different functions used by the patient. After 1 month of usage, the patients returned to the hospital and filled in a questionnaire about their satisfaction with the app. This questionnaire consisted of both open and multiple-choice questions (i.e. general questions about the patient, the different modules and the app in general) (see Supplemental methods). Lastly, clinical and demographic data were collected from the patients’ medical record.
Statistical analysis
Statistical analyses were performed using SPSS 26 (IBM Corporation, Armonk, USA). Continuous variables were described as median and interquartile range (IQR) due to the low sample size. To compare the scores of the JAKQ between baseline and month one, the Wilcoxon Signed-Rank test was used. Because patients were followed up over time and user-data was analyzed, participation statuses (yes/no) obtained from the same patient were expected to be correlated. Ignoring correlation would typically result in the underestimation of standard errors and hence wrong conclusions. 18 Therefore, a generalized estimating equation (GEE) model was constructed using participation as binary outcome, a logit link function and an autoregressive working correlation. 19
Results
Development and validation of the AF-EduApp prototype
Phase 1: content and face validation
In general, both healthcare professionals (n = 15; median age 37 years (IQR: 25–58); five males) and AF patients (n = 10; median age 66 years (IQR: 45–80); six males) were positive about the initiative to inform AF patients and improve their therapy adherence by an app. The app and its functions were clear and well organized according to the patients. Main comments (i.e. positive feedback, problems, and opportunities) given by the expert panel are bundled in Table 1, together with the solutions implemented during the first optimization phase. Screenshots of the application before and after optimization are available in Supplemental results Table 1. During this first phase, the main focus was on improving the medication tab before the pilot study, as therapy adherence is one of the primary focuses of the AF-EduApp project. Some suggestions were not addressed during this optimization phase, but have been updated in a later phase as indicated in italics in Table 1.
Table 1.
Main comments given by the expert panel during interviews with the possible solutions, that is, positive feedback (✓), problems (✗), and opportunities (!?). Implemented changes are in roman font; suggestions that will be addressed in the future are in italics.
| Feedback content & face validation | Optimization | |
|---|---|---|
| AF-EduApp overall | ✗ Improve font size of the text | Start-up of technical development |
| ✗ Simplify functions and buttons | Improved with more blank space and enlarged buttons | |
| Education | ✓ Clear AF information with text, video’s and photos | |
| !?Easy functions: Search function, extend FAQ tab, cross-references | Search function and cross-references were not yet implemented due to technical complexity | |
| !?Personalization: information on specific AF procedures, omitting components that do not apply to the patient (e.g. smoking, alcohol abuse, overweight), add push notification on certain information | Not yet implemented because a focus on medication module during this phase | |
| ✗ Improve structure of the education | Restructured information with extended FAQ as the first module | |
| Questionnaire | ✓ Positive on targeted education | |
| !? Pop-ups indicating the availability of new questionnaires | Several reminders were added when questionnaires are available | |
| !? Improve progress bar with text | Motivational text is implemented e.g. “eight out of the sixteen questions to go” | |
| ✗ Confusing abbreviations of questionnaire names | Changed the abbreviations of the questionnaires with a clear description (i.e. “Knowledge questionnaire” instead of JAKQ) | |
| ✗ Improve the window with the JAKQ score at the end of the questionnaire | Motivational text implemented with reference to the education module | |
| Medication | !? Make an interactive reminder | Patients can indicate in the app whether the medication has been taken or if they want to snooze the reminder for 15 min. For Android, the reminders are added in the patient’s calendar app |
| !? Only a physician could add or change the medication list and add extra information about the medication | As the main goal is to motivate patients themselves to adhere to the medication, they can still add and adapt their list | |
| ✗ Problems and flexibility with setting a reminder | They can indicate the specific hour instead of the time of the day (i.e. morning, afternoon, evening, night) and patients can choose to set a reminder or not | |
| ✗ Visibility to add medication to the list | The “ + ” button is replaced with a larger, green “add medication” button | |
| Measurement | !? Add extra parameters (e.g. INR value, glucose value) and personalization based on medical background | Not yet implemented because the focus on medication module during this phase |
| !? Self-monitoring possibilities | Not yet implemented because the focus on medication adherence | |
| !? Possibility to send parameters to a physician and receive feedback | Not yet implemented in the first optimization phase, but started with making it technically possible | |
| ✗ No possibility to add notes | Implemented and presented with a flag in the measurement overview | |
| ✗ Simplicity of the graph: add “today” and “last week” button | A “last week” and “last month” button is added and they can choose a specific time window | |
| Appointment | ✗ Add information about the appointment | Patients can add information and this is shown in the overview with a flag |
| ✗ Add alerts for an appointment | Patients can install a reminder and choose when they want a reminder (minutes/days before the appointment) | |
| Question | ✓ Ask questions directly to a healthcare provider | |
| ✗ Who will receive the mails | Information is added about who will receive and answer the question |
JAKQ: Jessa atrial fibrillation knowledge questionnaire; FAQ: frequently asked question; INR: international normalized ratio.
Some suggestions were given to improve accessibility (Supplemental results Table 1). As elderly often have difficulties using smartphones and tablets, it is crucial that they do not become frustrated by accessibility issues. The font size, size of the buttons, and blank space between them were improved. Completing the questionnaires went smoothly for the patients. Suggestions were mainly on motivational aspects and usability (e.g. reminders, progress bar, and JAKQ score with motivational text or clear questionnaire names). Both patients and health care professionals were enthusiastic about the education module and proposed to further personalize information. The opinions about the medication tab were divided within the patient group. Two patients were more satisfied with a hardware pillbox compared to a digital medication list with reminders. Optimization of the medication module focused mainly on ease of use (e.g. “add medication” button; setting reminders). A lot of attention was paid to the functionality to create interactive medication reminders, that is, pop-ups in which the patient can indicate if they had taken their medication or want to snooze it. In the measurements module, extra functions were implemented to have a better overview and the possibility to add free-text information. Given the complexity (technical and regulatory) of connecting the app to medical files and/or lab results, or other medical devices (e.g. blood pressure monitors, weighing scales), and given that the focus of the first RCT with AF-EduApp is on improving therapy adherence and self-care, these suggestions are kept for future extensions of the application. The integration of measurements into a clinical dashboard for caregivers was implemented in the second optimization.
Phase 2: patient pilot study
In total, 35 patients were invited to participate, of which 15 declined (11 patients had no smartphone or tablet, 3 were not interested, and 1 had no time to participate). On average, these 15 patients were older than the actual study group of 20 patients (median age: 72.0 years (IQR: 60–86) vs 68.5 years (IQR: 45.0–83.0)). The other characteristics of the study group are listed in Table 2. Ten patients indicated that they spent on average ≥1 h/day on their smartphone or tablet, of which 5 indicated they spent >2 h/day on their device.
Table 2.
Characteristics included AF patients.
| AF population (n = 20) | |
|---|---|
| Age (years), IQR | 68.5 (45–83) |
| Male, n (%) | 14 (70) |
| Time since AF diagnosis, n (%) | |
| <1 year | 8 (40) |
| 1–5 years | 8 (40) |
| >5 years | 4 (20) |
| Type of AF, n (%) | |
| First episode | 4 (20) |
| Paroxysmal AF | 14 (70) |
| Persistent AF | 2 (10) |
| OAC treatment, n (%) | |
| No OAC | 3 (15) |
| NOAC | 17 (85) |
| Number of devices, n (%) | |
| Only smartphone | 9 (45) |
| Only tablet | 1 (5) |
| Both smartphone and tablet | 10 (50) |
| Operating system on main device, n (%) | |
| Android | 15 (75) |
| IOS | 5 (25) |
AF: atrial fibrillation; OAC: oral anticoagulant; NOAC: non-vitamin K antagonist oral anticoagulant.
User data
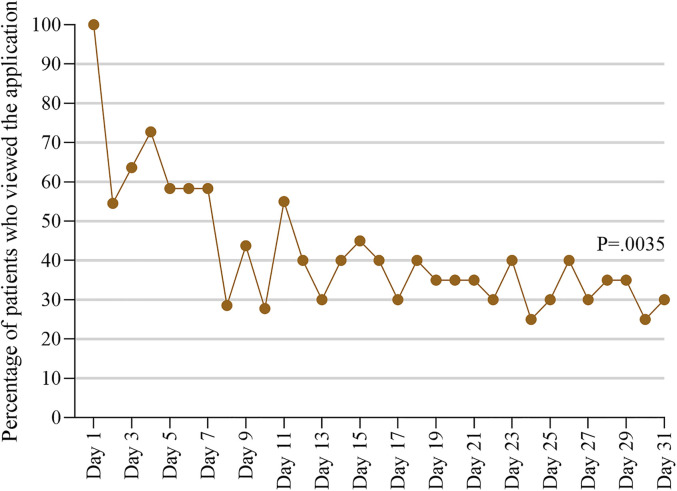
The median time the patients used the app was 12 (IQR: 3–31) days during the pilot study of 1 month. Overall, the odds of app use significantly decreased (P = 0.0035) over time (Figure 2), with 100% of the patients using the application on day 1 and 30% of the patients using it on day 31.
Figure 2.
Percentage of patients (n = 20) who used the app over time.
As shown in Supplemental results Figure 1, the measurement module was the most used module with 8 (IQR: 3–28) days viewed. The education module was the second most used module (5 (IQR: 3–7) days used). On average patients spent 1 min and 59 s (IQR: 39″-6′37″) on the education module per used day. In Supplemental results Figure 2, the median time spent on the different chapters within the education module is shown. Based on the JAKQ scores, patients’ knowledge about their arrhythmia significantly increased (P = 0.013) from 71.9% (IQR: 68.8–85.9) at baseline to 87.5% (IQR: 81.3–93.8) after 1 month (Figure 3). At baseline, 27.8% (5/18) of the patients did not know that they needed to discuss with the physician what to do with their OAC if they had to undergo surgery. After using the application, everyone answered this question correct (P = 0.025). In addition, almost half of the patients on NOAC (n = 17) did not know what to do if they had forgotten their NOAC dose (7/17; 41.2%). After 1 month, this decreased to 5.9% (1/17) (P = 0.014). There was also a positive trend on the knowledge about the negative impact of overweight on AF (60% to 85%; P = 0.059) and about which pain medication is allowed in combination with OAC (66.7% to 94.4%; P = 0.059), although these results did not reach statistical significance.
Figure 3.
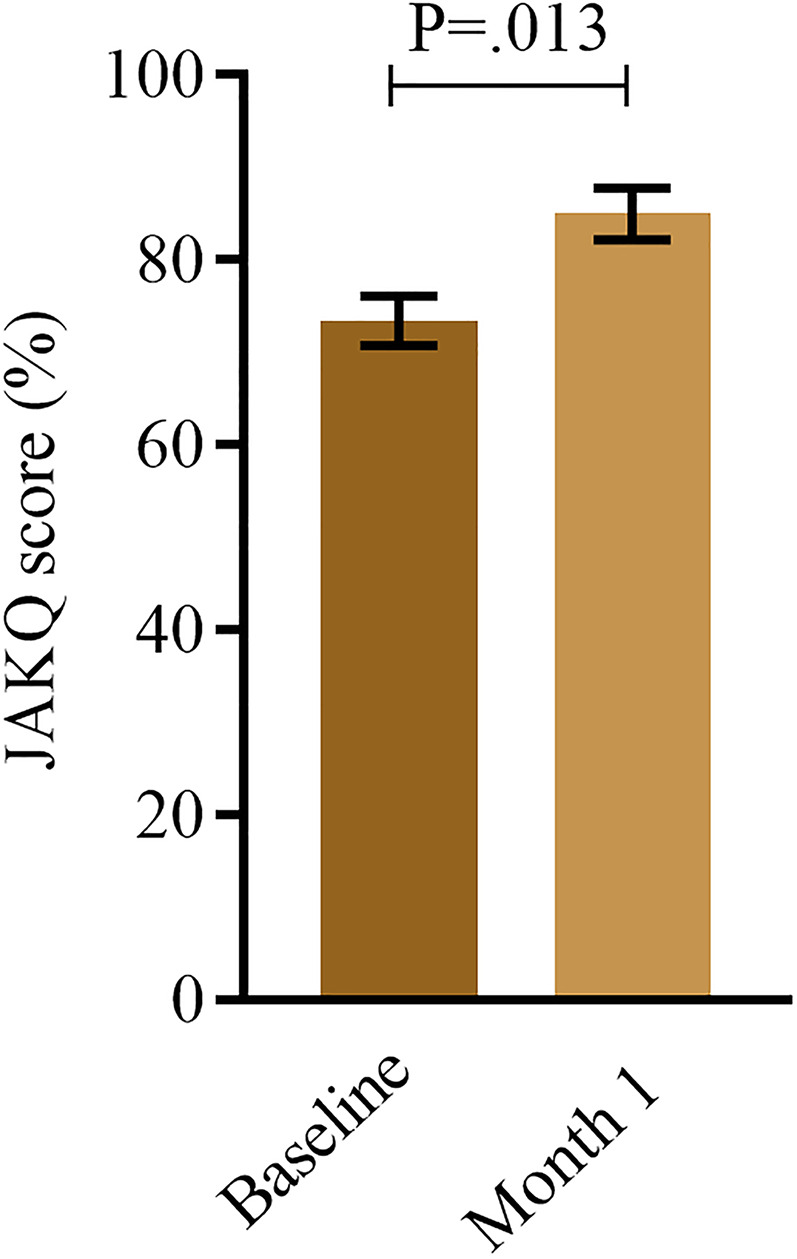
Knowledge level at baseline and after 1 month. Data are shown as bar chart with median and interquartile range (n: 20).
Patients’ satisfaction and final optimization
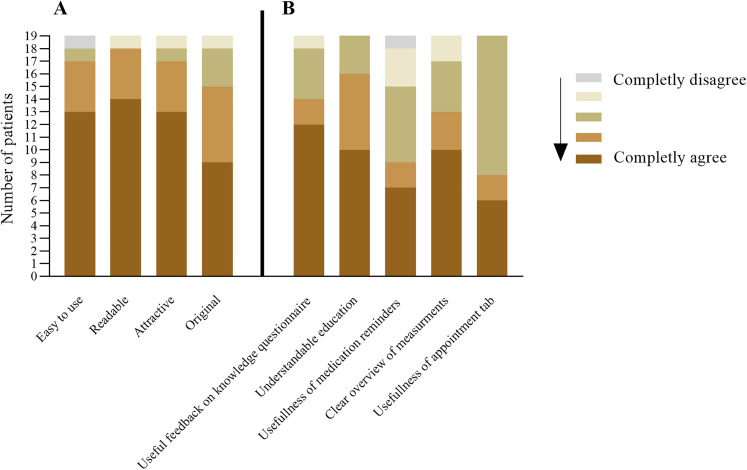
In total, 19 out of 20 patients filled in the satisfaction questionnaire after 1 month usage. Thirteen (68%) indicated that they are interested in information about AF and its treatment and 14 (74%) patients indicated to be more motivated to take care of their health thanks to the application. In addition, 16 patients (84.2%) wanted to use the app for a longer period. Of the patients who did not want to use the app for a longer period, one patient has a too busy lifestyle and another patient admitted that he only used his smartphone to read mails and the newspaper. The third patient indicated that he was not interested in receiving additional information about AF and preferred to have direct contact with the cardiologist. In general, patients were positive on the application itself (Figure 4A) and the different modules (Figure 4B).
Figure 4.
Patient satisfaction about the application in general (A) and the different modules (B) (n: 19).
After completing the pilot study, a final optimization of the prototype of the AF-EduApp took place based on the comments given by the 20 patients (Table 3; screenshots are available in Supplemental results Table 1). During this optimization, the focus was mainly on simplicity of use. In addition, modules were broadened based on the feedback.
Table 3.
Main comments given during the pilot study and the solutions for final optimization, that is, positive feedback (✓), problems (✗), and opportunities (⁉). Implemented changes are in roman font; suggestions that will be addressed in the future are in italics.
| Feedback pilot study | Final optimization | |
|---|---|---|
| AF-EduApp overall | ✗ Font size of the text | Patients can adapt the font size in the settings |
| Education | ✓Satisfied about the attractively displayed education | |
| ✗ Personalization: add information based on the medical background of the patient | Extra information on ablation and cardioversion was added. Personalization will be implemented in the future but was not the main focus. | |
| ✗ Size of the images | A zoom function was implemented for images | |
| Questionnaire | ✓Sufficient feedback on knowledge questionnaire | |
| ⁉ Visualization of the JAKQ scores at any time | Will be taken into account after the AF-EduApp study | |
| Medication | ✗ Problems with setting an alarm, mostly with android users as the alarm was added to the own calendar app | A new manner for reminders was implemented in which both IOS and Android users received the reminder from the application itself. In addition, the reminder is muted if the patient already indicated the medication was taken. |
| Measurements | ⁉ Set reminders when patients need to measure a parameter | Not yet implemented, but schedule to add reminders will be discussed with the patient individually |
| ⁉ Possibility to print the parameters to show or send to the physician | Entered parameters are made available in a clinical dashboard | |
| ✗ Problems with adding measurements offline | Offline saving will be implemented in the future but was not the main focus | |
| ✗ Improve layout of the overview of a specific measurement with date and time of measurement | Patients can now remove or change an entered measurement. In addition, they can indicate a certain measurement in the graph and this measurement will light up in the general overview. Date and time of the measurement are added. Besides, a BMI calculator was added and risk zones are colored in the graphs. | |
| ✗ Self-monitoring: connect with devices | Not yet implemented because the focus on medication adherence | |
| Appointment | ✓ Easy to add an appointment and clearly displayed | |
| ✗ Patients use their own calendar app or paper calendar | Despite previous adjustments, patients prefer to use one tool for all their appointments (e.g. paper calendar, smartphone calendar application) | |
| Questions | ✗ Add a phone number in case of an urgent question | Information is added about who they need to call with urgent questions |
In the AF-EduApp, a “start screen” after login was implemented with some general info about the application and the different modules, the possibility to set their height for BMI calculation and font size, and a screen to give consent for the notifications. A full medication list of BCFI was added in the AF-EduApp to make it more user friendly. This way, the patient only has to enter the first letters of the medication, after which a list of options appears. During both phases of validation of the AF-EduApp, it was considered important that measurements were visible for the physician and research team. This was implemented in the final optimization phase of our project. A clinical dashboard was developed where the care team could consult an update of the entered parameters by the patient at any moment of the day (Supplemental results Figure 3).
The app was uploaded to the Google Play Store and Apple App Store for the start of the AF-EduApp study, in which the effect on the adherence for OACs in AF patients will be investigated as primary outcome. The app is not available yet beyond the use in this RCT (NCT03788044).
Discussion
More and more patients have access to smartphones and tablets and these consumer devices are increasingly used for health related purposes.1,20,21 Development of the AF-EduApp aims to take advantage of this opportunity to reach more AF patients to provide integrated and transmural care. Usability (e.g. avoidance of technical difficulties, clarity of information, speed of the system) is of major importance for app compliance, as much as health status, accessibility, perceived utility, and motivation. 22 To improve usability, involvement of patients in the development and optimization of the application is important. Both AF patients and healthcare professionals with various backgrounds were involved in the development and validation of the AF-EduApp.
AF related applications
Several apps related to AF are available in app stores. Most apps are not focusing on education or self-care, but rather on detection and follow-up of AF, following up the heart rhythm and blood pressure, or calculating stroke and bleeding risk scores. A few educational applications for AF patients have been tested: the MyAF, mAF, and the Health Buddies app.16,23,24 The MyAF app provides education to AF patients, and symptoms and quality of life can be assessed and shared with the healthcare provider. 23 The mAF app has an incorporated clinical decision support tool for physicians, patient education programs, and patient self-care components. 24 Lastly, the Health Buddies app was developed to improve knowledge and therapy adherence based on daily challenges between patients and their grandchildren. 16 According to our knowledge, the AF-EduApp and MyAF app are the only two Dutch educational AF applications that are currently in use. 23
Ahmed I. et al. identified three major strategies for medication adherence applications, that is, reminder, educational, and behavioral change strategies. Almost all apps used a reminder function, and behavioral strategy was the second largest category. 25 Education strategy was the least used option. Moreover, the review published by Treskes R. et al. concluded that reminders only are not enough for the bigger population to improve adherence. 26 Educational strategies, ideally including human interaction (e.g. videos, calling), can lead to more patient involvement and are most effective. These three strategies are discussed in more detail and compared between the four applications.
Educational strategies for AF management
The AF-EduApp implements both a reminder and educational strategy. The education module proved to be the second most used module. We demonstrated its impact as there was a significant improvement in knowledge after 1 month. Other studies have shown that app-based education has a real effect on patient’s knowledge. In 2017, Guo Y. et al. performed a randomized controlled pilot study with the mAF app to support AF management. 24 Patients were randomized into two groups, that is, mAF App or standard care. The education provided to the patients was less extensive compared to the AF-EduApp. Nevertheless, the knowledge level assessed with the AF knowledge scale 27 significantly increased after 3 months of using the mAF App. In 2017, our research group validated the Health Buddies app which improved the JAKQ knowledge level from 64.6% to 70.4% after 3 months (P = 0.09), and their insights into medication adherence were improved. 16 In addition, 73% of the patients indicated they found the educational method of the application the most positive aspect of the app.
Reminder strategy for improved adherence
Reminders are the most available function in adherence applications. 25 Also AF-EduApp allows input of the patient’s medication list and related reminders for intake. However, user data taught us that the initial implementation was suboptimal. Even after optimization, the medication module was only the fourth most used module. However, patients were asked to mark their medication intake, but could use this module based on their own needs. The mAF app has no medication module. 24 The MyAF app has a more limited medication list and reminder functionality compared to the AF-EduApp. In the MyAF app, patients can only input the generic name (out of a list) instead of the brand name of their medication, and they cannot install reminders. 23 The Health Buddies application implemented reminders differently: the daily “challenges” for the patient were based on taking their OAC medication, while the adherence was measured with the Medication Event Monitoring System (MEMS). The study showed that patients thought they were taking their medication correctly most of the time (99.0 ± 1.8%), which was not confirmed by objective adherence data of the MEMS (81.8 ± 18.7%). 16 The reminder function of the AF-EduApp will still be used in the RCT. However, patients are free to use it or not. In order to track patients therapy adherence, the MEMS will be used during the whole RCT with intermediary feedback moments and telemonitoring if there is a low therapy adherence.
Behavioral change to increase therapy adherence
Behavioral change strategies (e.g. involving family, friends or healthcare providers, possibilities to track adherence, and encouragement based on entered data) is the third important application pillar to improve therapy adherence. 25 The MyAF patient app has a companion app for the healthcare provider, “AF management app.” 23 Patients can invite physicians to access and follow their data via the AF management app. Patients can also keep track of their appointments via the connection with the AF manager app, in which a doctor can add contacts. Within the mAF app, a clinical decision support module is incorporated which calculates the risk for stroke, bleeding and quality of VKA therapy and patients can enter their heart rate and blood pressure (like in AF-EduApp). The application is available for both patient and physician. 24 Our study has shown that physicians and patients appreciate a clinical dashboard as back-end of the patient application. The Health Buddies app made use of gamification between AF patients and their grandchildren to improve therapy adherence. Despite the fact that gamification can cause behavioral change, this limited the Health Buddies app’s usability in daily clinical practice. Out of 410 AF patients, 114 patients were eligible and only 15 patients were willing to participate. 16
The AF-EduApp requires more focus on behavioral change strategies. This was also mentioned during the validation by the healthcare professionals. Possibilities include reminders for patients to measure certain parameters, the possibility to connect the app with medical devices for automatically connected measurements and semi-automated follow-up of the patient through individualized alerts and a clinical dashboard. During the final optimization phase, such a clinical dashboard was implemented (Supplemental results Figure 3). Healthcare providers can see the patient’s entered parameters and use this information for feedback. This will be further improved with patient-tailored pop-ups for the specialist when a patient enters parameters that are in a risk zone (e.g. too high blood pressure). In a study by Park G et al., with focus groups on perception and experiences of mobile technology for medication adherence, the aspect of having personal feedback from a healthcare provider was a common request. 28 Also in our study, one patient preferred to receive information from the physician. More attention is needed on aspects to improve the healthcare provider interface and ways for behavioral change of patients during further development of the application. The possibility to change the application based on the needs of the patients during their AF follow-up and progression is an important factor for app compliance. As most patients use the application less often when they are familiar with the self-management strategies and no new strategies are offered if there is drop-in use. Therefore, patient involvement is an important factor in the development and further improvement of the application. 29
Limitations
Compared with the general AF population (mean age 72 years), the median age of our study patients in both phases (TAP protocol and pilot study) was younger (66 and 68 years, respectively). 30 Older patients may have less knowledge about, or access to smartphone applications which was also seen during the pilot study as the patients who participated were younger compared to the patients that declined (68 years vs 72 years). During both phases, a rather small sample size of patients was included, which is nevertheless representable for a validation and pilot study. This study is followed by an RCT (NCT03788044) in which more patients will be included, and this will be an intention-to-treat trial in which the age difference can be taken into account. However, the main goal of this pilot study was to evaluate the general usability and search for pitfalls in the prototype, which was accessed with a more qualitative research during both TAP protocol and with the satisfaction questionnaire during the pilot study. During the pilot study, a drop-in use was seen over time. However, this also had to do with technical issues that could occur, which may have impacted the decline in use. Therefore, this feasibility study was performed in order to test the app sufficiently and reduce technical problems. Nevertheless, the app has not been developed for daily use, but patients should use the app according to their personal needs. This pilot study did not provide data on impact on therapy adherence and other clinical outcomes for which the follow-up AF-EduApp clinical study is being conducted. Evaluation of the app and how it can be implemented in daily practice may lead to the app also becoming available in languages other than Dutch only.
Conclusions
According to both healthcare professionals and AF patients, the AF-EduApp was considered a worthwhile addition to their AF management. The validation and evaluation showed that the application is clear, attractive, and educational. It provides AF patients with targeted education, which had an impact on knowledge level after 1 month. The application needs further evaluation concerning its usability in an older, less smartphone-savvy population. Based on these results, behavior change strategies can be an added value to the application, in which the clinical dashboard is a first step. The AF-EduApp is now tested in an RCT to assess its impact on therapy adherence and other outcome parameters.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076211067105 for A new smartphone application for integrated transmural care of atrial fibrillation, AF-EduApp: Usability and validation study by Lieselotte Knaepen, Michiel Delesie, Rik Theunis, Johan Vijgen, Paul Dendale, Lien Desteghe and Hein Heidbuchel in Digital Health
Acknowledgments
This study is part of Limburg Clinical Research Center, supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital.
Footnotes
Conflict of interest: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: H.H. did receive personal fees from Biotronik and Pfizer-BMS. He received unconditional research grants through the University of Antwerp and/or the University of Hasselt from Bayer, Boehringer-Ingelheim, Bracco Imaging Europe, Abbott, Medtronic, Biotronik, Daicchi-Sankyo, Pfizer-BMS, and Boston-Scientific, all outside the scope of this work. Rik Theunis is the developer of the application.
Contributorship: L.K. contributed to study design, data collection, data analysis, writing of the article. M.D contributed to conceptualization, study design, data collection, and critically revised the article. R.T contributed to conceptualization and critically revised the article. J.V. contributed to conceptualization and critically revised the article. P.D. contributed to conceptualization and critically revised the article. L.D. contributed to conceptualization, study design, and critically revised the article. H.H. contributed to conceptualization, study design, and critically revised the article.
Ethical approval: This clinical pilot study was approved by the leading Ethics Committee of UZA/UA and taking into account the advice of the local ethics committees (Belgian study number: B300201836720).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an BMS/Pfizer European Thrombosis Investigator Initiated Research Program (ERISTA) grant.
Guarantor: HH.
ORCID iD: Lieselotte Knaepen https://orcid.org/0000-0003-2816-1896
Supplemental material: Supplemental material for this article is available online.
References
- 1.Vandendriessche K and Marez LD. (2019) Imec digimeter 2019. Digitale mediatrends in Vlaanderen. Accessed on 20.01.2020. via https://www.imec.be/sites/default/files/2020-02/476531-IMEC-Digimeter-Rapport%202020-WEB.PDF
- 2.Wang J, Wang Y, Wei C, et al. Smartphone interventions for long-term health management of chronic diseases: an integrative review. Telemed J e-health : Off J Am Telemed Assoc 2014; 20: 570–583. 2014/05/03. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report From the American heart association. Circulation 2019; 139: e56–e528. 2019/02/01. [DOI] [PubMed] [Google Scholar]
- 4.Chung MK, Refaat M, Shen WK, et al. Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol 2020; 75: 1689–1713. 2020/04/11. [DOI] [PubMed] [Google Scholar]
- 5.Hindricks G, Potpara T, Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of cardio-thoracic surgery (EACTS). Eur Heart J 2020; 2020: 373–498, 2020/08/30. DOI: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 6.Dupree L, DeLosSantos M, Smotherman C. Evaluation of adherence to guideline-directed antithrombotic therapy for atrial fibrillation at hospital discharge. J Cardiovasc Pharmacol Ther 2018; 23: 502–508. 2018/05/29. [DOI] [PubMed] [Google Scholar]
- 7.Koziel M, Simovic S, Pavlovic N, et al. Adherence to the ABC (Atrial fibrillation Better Care) pathway in the Balkan region: the BALKAN-AF survey. Pol Arch Intern Med 2020; 130: 187–195. DOI: 10.20452/pamw.15146. [DOI] [PubMed] [Google Scholar]
- 8.Proietti M, Romiti GF, Olshansky B, et al. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (Atrial Fibrillation Better Care) pathway. Am J Med 2018; 131: 1359–1366.e1356. [DOI] [PubMed] [Google Scholar]
- 9.Desteghe L, Engelhard L, Vijgen J, et al. Effect of reinforced, targeted in-person education using the Jessa atrial fibrillation knowledge questionnaire in patients with atrial fibrillation: a randomized controlled trial. Eur J Cardiovasc Nurs 2018; 13: 1474515118804353. DOI: 10.1177/1474515118804353 [DOI] [PubMed] [Google Scholar]
- 10.Desteghe L, Germeys J, Vijgen J, et al. Effectiveness and usability of an online tailored education platform for atrial fibrillation patients undergoing a direct current cardioversion or pulmonary vein isolation. Int J Cardiol 2018; 272: 123–129. [DOI] [PubMed] [Google Scholar]
- 11.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. 2013/12/10. [DOI] [PubMed] [Google Scholar]
- 12.van den Heuvel JM, Hövels AM, Büller HR, et al. NOACs replace VKA as preferred oral anticoagulant among new patients: a drug utilization study in 560 pharmacies in The Netherlands. Thromb J 2018; 16–23. DOI: 10.1186/s12959-017-0156-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmasi S, Loewen PS, Tandun R, et al. Adherence to oral anticoagulants among patients with atrial fibrillation: a systematic review and meta-analysis of observational studies. BMJ Open 2020; 10: e034778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desteghe L, Vijgen J, Koopman P, et al. Telemonitoring-based feedback improves adherence to non-vitamin K antagonist oral anticoagulants intake in patients with atrial fibrillation. Eur Heart J 2018; 39: 1394–1403. [DOI] [PubMed] [Google Scholar]
- 15.Delesie M, Knaepen L, Dendale P, et al. Effect of targeted education for atrial fibrillation patients: design of the EduCare-AF study. Eur J Clin Investig 2021; 51: e13442. [DOI] [PubMed] [Google Scholar]
- 16.Desteghe L, Kluts K, Vijgen J, et al. The health buddies app as a novel tool to improve adherence and knowledge in atrial fibrillation patients: a pilot study. JMIR Mhealth Uhealth 2017; 5: e98–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desteghe L, Engelhard L, Raymaekers Z, et al. Knowledge gaps in patients with atrial fibrillation revealed by a new validated knowledge questionnaire. Int J Cardiol 2016; 223: 906–914. 2016/09/03. [DOI] [PubMed] [Google Scholar]
- 18.Molenberghs G, Verbeke G. Models for discrete longitudinal data. New York, NY: Springer, 2005. [Google Scholar]
- 19.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73: 13–22. [Google Scholar]
- 20.Guhl E, Althouse AD, Pusateri AM, et al. The atrial fibrillation health literacy information technology trial: pilot trial of a mobile health app for atrial fibrillation. JMIR Cardio 2020; 4: e17162. 2020/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merks P, Religioni U, Arciszewska K, et al. Usability testing and satisfaction of “The Patient Access”: a mobile health application for patients with venous thromboembolic disease. A pilot study. Cardiol J 2020; 27: 891–893. 2020/09/12. DOI: 10.5603/CJ.a2020.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simblett S, Greer B, Matcham F, et al. Barriers to and facilitators of engagement With remote measurement technology for managing health: systematic review and content analysis of findings. J Med Internet Res 2018; 20: e10480. Review 12.07.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotecha D, Chua WWL, Fabritz L, et al. European society of cardiology smartphone and tablet applications for patients with atrial fibrillation and their health care providers. Europace 2018; 20: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Chen Y, Lane DA, et al. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF App trial. Am J Med 2017; 130: 1388–1396.e1386. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed I, Ahmad NS, Ali S, et al. Medication adherence apps: review and content analysis. JMIR Mhealth Uhealth 2018; 6: e62. 2018/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treskes RW, Van der Velde ET, Schoones JW, et al. Implementation of smart technology to improve medication adherence in patients with cardiovascular disease: is it effective? Expert Rev Med Devices 2018; 15: 119–126. 2017/12/23. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks JML, Crijns HJGM, Tieleman RG, et al. The atrial fibrillation knowledge scale: development, validation and results. Int J Cardiol 2013; 168: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 28.Park LG, Ng F, Shim J K, et al. Perceptions and experiences of using mobile technology for medication adherence among older adults with coronary heart disease: a qualitative study. Digit Health 2020; 6: 2055207620926844–2055207620926844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson K, Burford O, Emmerton L. Mobile health apps to facilitate self-care: a qualitative study of user experiences. PLoS One 2016; 11: e0156164. 2016/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in AdultsNational implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors In atrial fibrillation (ATRIA) study. JAMA 2001; 285: 2370–2375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076211067105 for A new smartphone application for integrated transmural care of atrial fibrillation, AF-EduApp: Usability and validation study by Lieselotte Knaepen, Michiel Delesie, Rik Theunis, Johan Vijgen, Paul Dendale, Lien Desteghe and Hein Heidbuchel in Digital Health