Abstract
We evaluated the outcomes of resection of small acoustic neuromas using the transcanal transvestibular endoscopic approach. Two patients with a small acoustic neuroma were treated using this approach. The sizes of the tumors were 11 × 6 mm and 12 × 10 mm. Both tumors were removed completely without residual tumor tissue, and damage to the facial nerve and cochlear nerve was avoided. No patients developed postoperative vertigo, aggravation of postoperative facial paralysis, severe pain, or permanent postoperative complications. The patients were followed up for 6 months, and none developed recurrence. Resection of small acoustic neuromas by the transcanal transvestibular endoscopic approach is a simple and safe technique that achieves excellent functional results.
Keywords: Acoustic neuroma, internal auditory meatus, transcanal transvestibular approach, endoscopic approach, case report, tumor resection
Introduction
An acoustic neuroma, also known as a vestibular schwannoma, generally originates from the vestibular nerve. It is an uncommon cause of hearing loss. A vestibular schwannoma is a slowly growing benign tumor and the most common lesion of the cerebellopontine angle. Treatments include expectant care with repeat scanning to assess tumor growth in elderly people as well as surgery with or without stereotactic radiotherapy. 1 Otologists and neurosurgeons choose different types of surgery depending on the size and location of the tumor. Endoscopic surgery and microsurgery are used to remove tumors. With the technological progress of diagnosis and treatment, the treatment goal for acoustic neuroma has developed from early tumor control to a larger emphasis on the preservation of brain nerve function. Preservation of auditory nerve function is more difficult than preservation of facial nerve function. Cochlear function is difficult to retain after surgery by the translabyrinthine approach, which was historically the most commonly used approach to remove acoustic neuromas. Surgical techniques and instruments were developed to build on the strengths and minimize the limitations of endoscopic surgery. We often perform endoscopic resection of small acoustic neuromas using the transcanal transvestibular approach. Compared with the translabyrinthine approach and other approaches, this surgical approach has the advantages of full exposure and less trauma. Our surgical method preserves the function of the cochlea and provides a reference for future cochlear implantation. It is a surgical method worth popularizing.
Case presentation
The reporting of this study conforms to the CARE guidelines. 2
In October 2020, two patients with an acoustic neuroma of the internal acoustic meatus were treated using the transcanal transvestibular endoscopic approach at the Department of Otolaryngology, Shenzhen Second People’s Hospital/The First Affiliated Hospital of Shenzhen University, Shenzhen, China. This study was approved by the Institutional Review Board of the University. The diagnoses were based on the patients’ medical history, physical examination findings, and magnetic resonance imaging (MRI) findings.
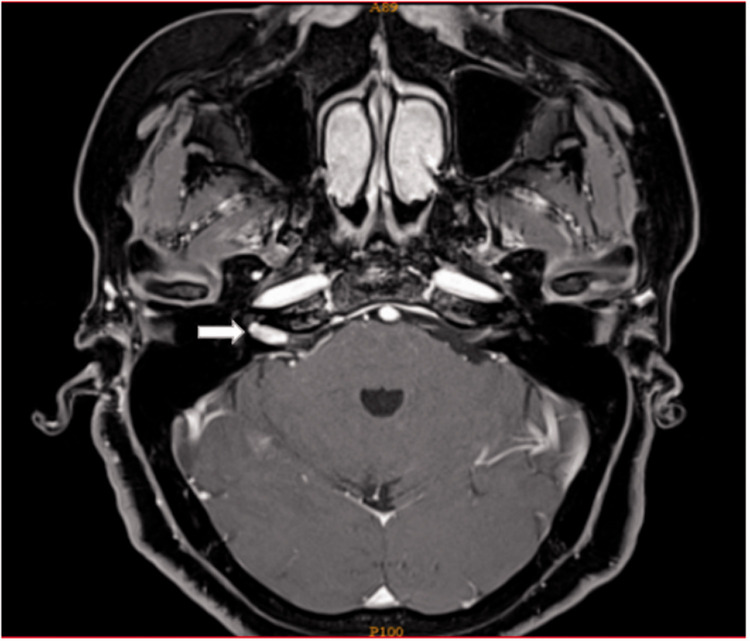
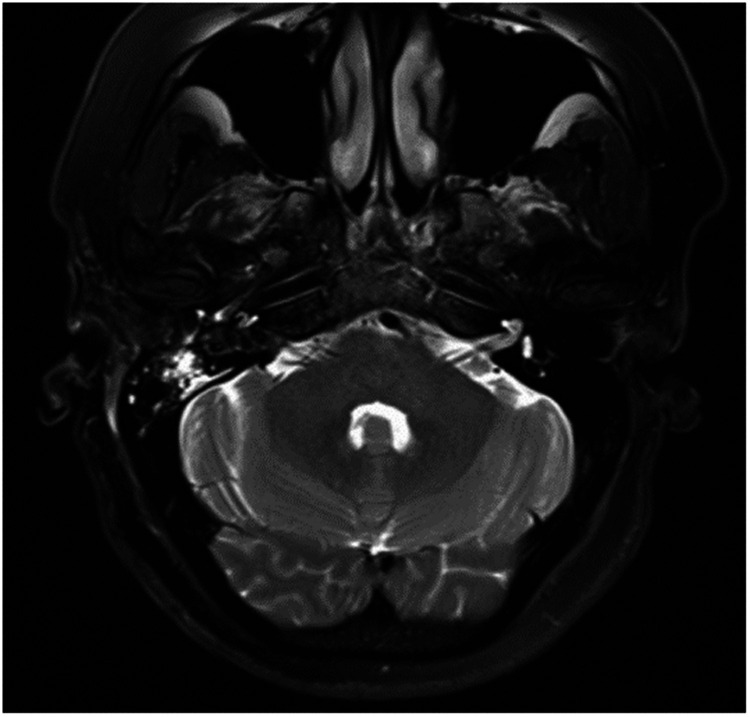
The first patient, a woman in her 50s, presented with a 1-month history of tinnitus and hearing loss. She was unable to hear loud sounds in her right ear. She had no headache, dizziness, or facial paralysis. The patient showed no significant improvement in the hearing loss or tinnitus after taking an oral glucocorticoid for 1 week. Twenty days after drug withdrawal, the patient visited our hospital again. A physical examination revealed complete eardrums and no effusion in the tympanum. Pure-tone audiometry indicated a threshold of 70 dB in the right ear. Preoperative gadolinium-enhanced MRI showed a small (11- × 6-mm) right-sided tumor (Figure 1).
Figure 1.
Small acoustic neuroma (11 × 6 mm).
The second patient, a man in his 30s, presented with a 2-month history of right-sided hearing loss and right-sided facial paralysis. He had persistent tinnitus, occasional vertigo, no ear discharge, and no ear pain. Oral glucocorticoids and neurotrophics were administered for 2 weeks; however, the patient’s symptoms did not improve, and he visited our hospital again 1 month later. A physical examination revealed complete eardrums and no effusion in the tympanum. The right-sided facial paralysis was House–Brackmann stage III. Pure-tone audiometry indicated a threshold of 75 dB in the right ear. Preoperative gadolinium-enhanced MRI showed a small (12- × 10-mm) right-sided tumor.
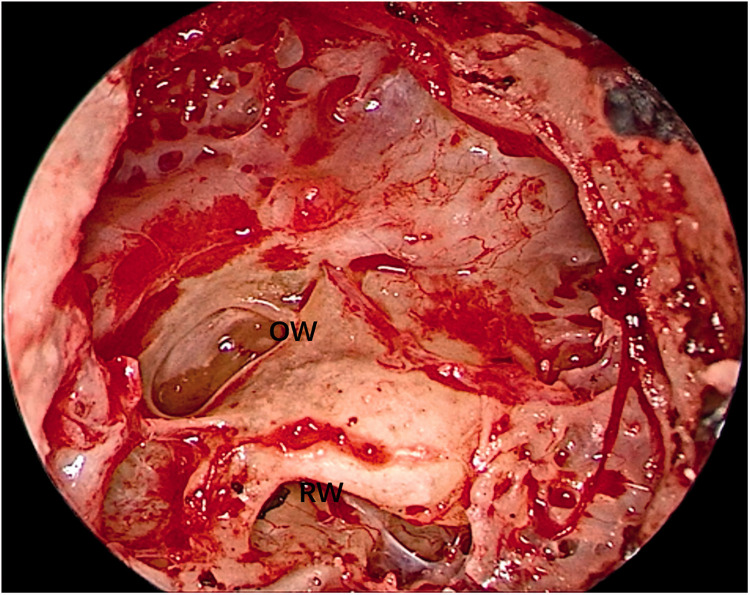
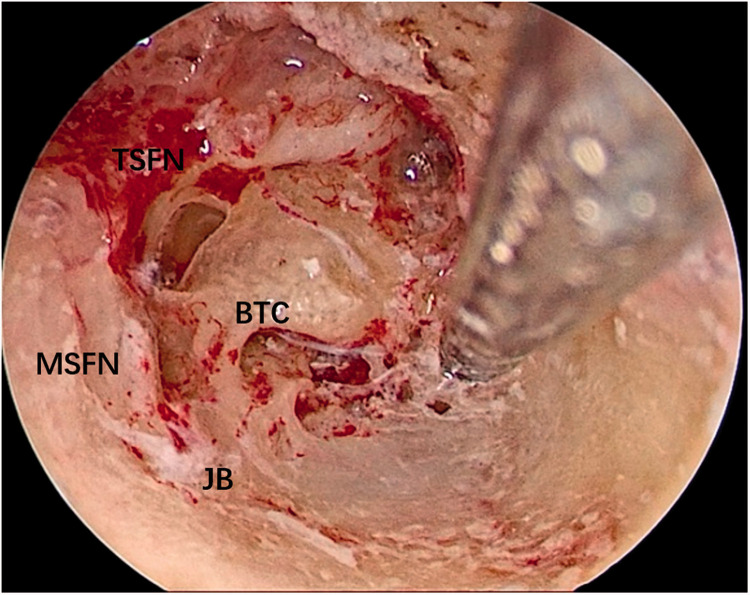
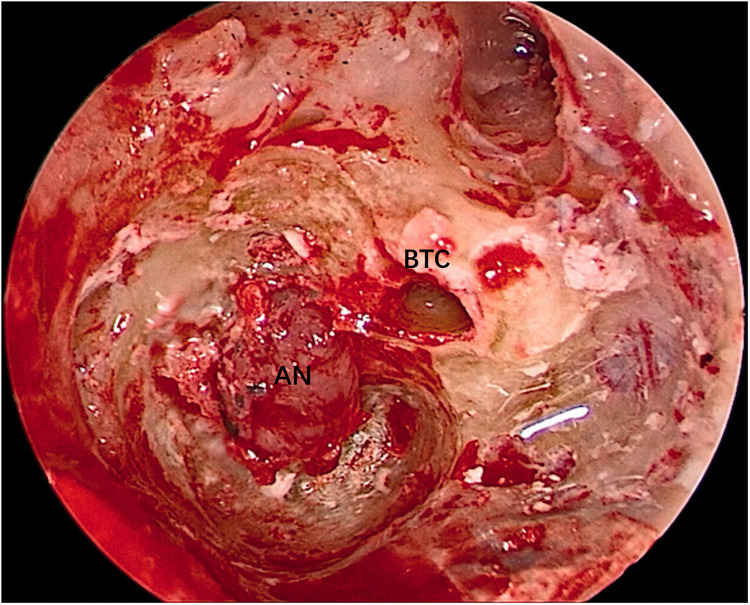
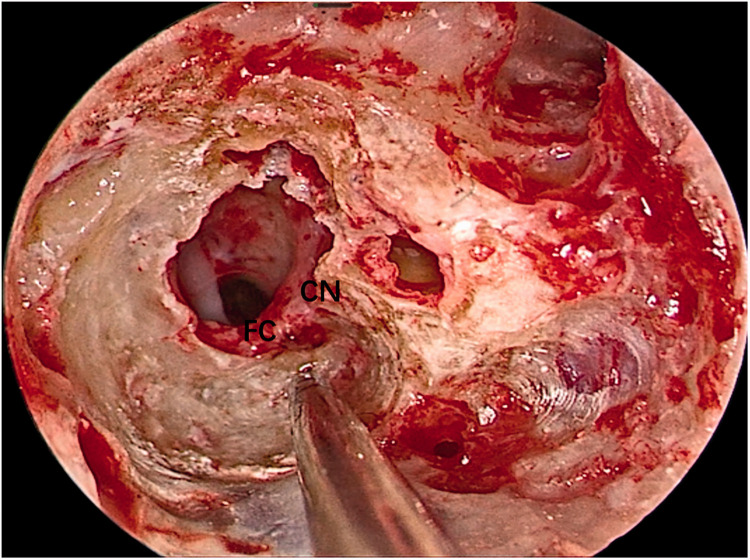
Both operations were performed using oral tracheal intubation under general anesthesia. Both patients were placed in the supine position with the head tilted to the contralateral side. The operative team comprised a chief surgeon, an anesthetist, a circulating nurse, and a scrub nurse. The surgical site was exposed through the external auditory meatus. A 0º, 3-mm-diameter endoscope (Karl Storz, Tuttlingen, Germany) was inserted via the external auditory meatus. Under endoscopic assistance, a circular incision was made in the skin of the external auditory meatus at its bony–cartilaginous junction. The skin was elevated and removed together with the eardrum to gain access to the tympanic cavity. The external auditory meatus bone and scutum were ground with an endoscopic ear drill, and the ossicular chain was removed to expose the whole medial wall of the tympanic cavity (including the vestibule and the round window) (Figure 2). The anatomical boundaries of the surgical field were observed; the anterior boundary was adjacent to the basal turn of the cochlea, the upper boundary was the tympanic segment of the facial nerve, the lower boundary was the jugular bulb, and the posterior boundary was adjacent to the mastoid segment of the facial nerve (Figure 3). The vestibule was exposed to its depth, and the spherical recess was used as a landmark for the fundus of the internal auditory meatus because this recess represents the insertion of the inferior vestibular nerve. The perivestibular bone was removed to widen the vestibular window, and the promontorium tympani was then carefully ground, opening and exposing the basal turn of the cochlea. The cochlea middle turn and top turn did not have to be opened, and the scala tympani was preserved. Once the extensions of the incision were complete and the borders of the tumor could be clearly seen (Figure 4), the facial nerve and cochlear nerve were located deep in the tumor. They were identified and carefully protected while removing the tumor (Figure 5). Finally, the tumor was successfully separated from the internal auditory meatus, and all anatomic areas were thoroughly checked for hemostasis. The cavity was closed using a fat pad harvested from the abdomen to occlude the inner ear and middle ear defects.
Figure 2.
The ossicular chain was removed to expose the whole medial wall of the tympanic cavity.
OW, oval window; RW, round window.
Figure 3.
The anatomical boundaries of the surgical field was observed.
TSFN, tympanic segment of facial nerve; BTC, basal turn of cochlea; MSFN, mastoid segment of facial nerve; JB, jugular bulb.
Figure 4.
The tumor could be clearly seen.
AN, acoustic neuroma; BTC, basal turn of cochlea.
Figure 5.
The facial nerve and cochlear nerve were located deep in the tumor. They were identified and carefully protected when removing the tumor.
FC, facial nerve; CN, cochlear nerve.
Both tumors were removed completely without residual tumor tissue, and damage to the facial nerve was avoided. No patients developed postoperative vertigo, postoperative aggravation of facial paralysis, severe pain, or permanent postoperative complications. All wounds healed without issue. The patients were followed up for 6 months, and none developed recurrence. Postoperative gadolinium-enhanced MRI showed that the tumor was totally removed by the fully endoscopic technique (Figure 6).
Figure 6.
Magnetic resonance imaging was performed to clarify whether the tumor had been completely eliminated 10 days after the surgery.
Discussion
There are different viewpoints on the treatment of small acoustic neuromas. Radiosurgery and observation became increasingly more common after the turn of the century, possibly because of better detection of small and asymptomatic tumors and a greater understanding of the natural history of disease. 3 Although small intracanalicular vestibular schwannomas are commonly observed, progressive hearing loss occurs despite the absence of tumor growth; hence, surgical resection can be performed with the sole aim of hearing preservation in well-informed and eager patients. Both patients described in the present report required surgery, and we obtained informed consent for treatment from both patients.
Surgeons can remove tumors of the internal auditory meatus in a variety of ways. Generally, two principles must be met: wide intraoperative visibility for safe radical dissection, and minimal functional or cosmetic after-effects. Using the retrosigmoid approach with a small craniotomy is possible even for large schwannomas. 4 The main goal of management of large vestibular schwannomas should focus on maintaining or improving quality of life and making every attempt to preserve facial and cochlear nerve function while ensuring optimal oncological control, thereby meeting patient expectations. 5 Many authors consider the middle fossa approach to be the gold standard approach for resection of small intracanalicular vestibular schwannomas in young patients with serviceable hearing. The implementation of endoscopy with the middle fossa approach, especially for vestibular schwannomas located laterally in the internal auditory meatus, provides a better opportunity for complete resection of the tumor with improved preservation of hearing and facial nerve function. 6 The translabyrinthine approach is the most familiar surgical technique employed by otologists. It is the most direct route to the cerebellopontine angle and internal auditory meatus, and it requires minimum cerebellar retraction. However, it sacrifices any residual hearing in the operated ear. 7
For a small acoustic neuroma limited to the internal auditory meatus, it is difficult to preserve cochlear function after undertaking the translabyrinthine approach. Surgical removal of an acoustic neuroma via the middle cranial fossa approach can be conducted with low morbidity and mortality; however, complete visualization of the tumor is often limited. The most common complication is cerebrospinal fluid leakage. 8 Patients who have undergone surgery for a unilateral acoustic neuroma via the retrosigmoid approach may develop headaches that require some time to recover from. 9 The retrosigmoid and middle cranial fossa approaches more strongly stimulate the brain tissue and are more traumatic, and they are difficult for otolaryngologists to master. Endoscopic surgery has several advantages, including smaller incisions, less tissue damage, and direct vision of a magnified and illuminated operative field. A transcanal transpromontorial approach was developed to reach the inner ear and the cerebellopontine angle through the external auditory meatus. The advantages of this approach are direct visualization of the internal auditory meatus with minimal temporal bone drilling and no need for a craniotomy or manipulation of the dura mater. 10 This procedure usually involves removal of the vestibule and cochlea to expose the internal auditory meatus. The transcanal/transpromontorial endoscopic approach is an effective surgical technique for small intracanalicular acoustic neuroma removal. 11 For small acoustic neuromas confined to the internal auditory meatus, the scala tympani is retained when opening and exposing the cochlea middle turn and top turn. 12 Using our surgical approach, we also found that we did not need to open and expose the cochlea middle turn and top turn, and we avoided damage to the residual hearing of the cochlea by extending the vestibular window and grinding part of the promontorium tympani. We removed the acoustic neuromas directly through the internal auditory meatus. Therefore, we call this the transcanal transvestibular endoscopic approach (the external auditory meatus vestibular approach).
Notably, endoscopic resection of acoustic neuromas via the external auditory meatus vestibular approach should be considered only in selected patients with Koos I to II acoustic neuromas, especially if an artificial cochlear implantation is required, because of the risk of hemorrhage and damage to the cranial nerve. We limited our test subjects to those with internal auditory meatus tumors of <15 mm in diameter, and the patients had no functional hearing. We treated two patients with small, benign acoustic neuromas using the transcanal transvestibular endoscopic approach. We retained the facial nerve, the cochlear nerve, and the auditory nerve. This is a functional surgery. Concurrent or secondary cochlear implants are available if the patient needs them. Although cochlear implants for unilateral deafness are still partially controversial, preserving the cochlear nerve may provide the basis for future cochlear surgery if needed. Patients with unilateral sensorineural hearing loss who accept cochlear implantation obtain good binaural benefits, especially with respect to sound localization. 13
In conclusion, endoscopic resection of acoustic neuromas via the external auditory meatus vestibular approach is efficient, safe, and minimally invasive. In contrast to other approaches, the cochlear function is preserved, which is convenient for future cochlear implants. This is a surgical method worth popularizing.
Footnotes
Author contributions: Tao Chen (primary author) was involved in the study design; data acquisition, analysis, and interpretation; and drafting of the work. Zhenzhang Lu, Yuxiang Zhou, and Duanlong Zhao were involved in the study design, data acquisition, and drafting of the work. Yongtian Lu and Qingguo Meng were involved in the study design, data acquisition, drafting of the work, and critical revision of the work. All authors agree to be accountable for all aspects of the work.
Declaration of conflicting interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics statement: All procedures performed in this study were in accordance with the ethical standards of the institutional research committee. This study protocol was reviewed and approved by the institutional review board of the hospital and the clinical research ethics committee of Shenzhen Second People’s Hospital/The First Affiliated Hospital of Shenzhen University (20203357013). Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Data availability statement: Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study was supported by the Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No. SZGSP004).
ORCID iDs
Tao Chen https://orcid.org/0000-0002-3448-3237
Zhenzhang Lu https://orcid.org/0000-0002-6148-8164
References
- 1.Wright A andBradford R.. Management of acoustic neuroma. BMJ 1995; 311: 1141–1144. doi: 10.1136/bmj.311.7013.1141. Erratum in: BMJ 1995 Nov 25; 311(7017): 1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. doi: 10.1111/head.12246. [DOI] [PubMed] [Google Scholar]
- 3.Chan SA, Marinelli JP, Hahs-Vaughn DL, et al. Evolution in management trends of sporadic vestibular schwannoma in the united states over the last half-century. Otol Neurotol 2021; 42: 300–305. doi: 10.1097/MAO.0000000000002891. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Chen LH, Ling F, et al. Removal of vestibular schwannoma and facial nerve preservation using small suboccipital retrosigmoid craniotomy. Chin Med J (Engl) 2010; 123: 274–280. [PubMed] [Google Scholar]
- 5.Starnoni D, Giammattei L, Cossu G, et al. Surgical management for large vestibular schwannomas: a systematic review, meta-analysis, and consensus statement on behalf of the EANS skull base section. Acta Neurochir (Wien) 2020; 162: 2595–2617. doi: 10.1007/s00701-020-04491-7. Epub 2020 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montaser AS, Todeschini AB, Harris MS, et al. Role of endoscopy in resection of intracanalicular vestibular schwannoma via middle fossa approach: technical nuances. World Neurosurg 2018; 120: 395–399. doi: 10.1016/j.wneu.2018.08.215. Epub 2018 Sep 7. [DOI] [PubMed] [Google Scholar]
- 7.Aznmi MN Lokman BS andIshlah L.. The translabyrinthine approach for acoustic neuroma and its common complications. Med J Malaysia 2006; 61: 72–75. [PubMed] [Google Scholar]
- 8.Scheich M, Ginzkey C, Ehrmann Müller D, et al. Complications of the middle cranial fossa approach for acoustic neuroma removal. J Int Adv Otol 2017; 13: 186–190. doi: 10.5152/iao.2017.3585. [DOI] [PubMed] [Google Scholar]
- 9.Aihara N, Yamada H, Takahashi M, et al. Postoperative headache after undergoing acoustic neuroma surgery via the retrosigmoid approach. Neurol Med Chir (Tokyo) 2017; 57: 634–640. doi: 10.2176/nmc.oa.2017-0108. Epub 2017 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchioni D, Gazzini L, Boaria F, et al. Is endoscopic inspection necessary to detect residual disease in acoustic neuroma surgery? Eur Arch Otorhinolaryngol 2019; 276: 2155–2163. doi: 10.1007/s00405-019-05442-4. Epub 2019 Apr 26. [DOI] [PubMed] [Google Scholar]
- 11.Marchioni D, Carner M, Rubini A, et al. The fully endoscopic acoustic neuroma surgery. Otolaryngol Clin North Am 2016; 49: 1227–1236. doi: 10.1016/j.otc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Marchioni D, Veronese S, Carner M, et al. Hearing restoration during vestibular schwannoma surgery with transcanal approach: anatomical and functional preliminary report. Otol Neurotol 2018; 39: 1304–1310. doi: 10.1097/MAO.0000000000001980. [DOI] [PubMed] [Google Scholar]
- 13.Liu JF Dai JS andWang NY.. [Effect of cochlear implantation on sound localization for patients with unilateral sensorineural hearing loss]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2016; 51: 623–630. [In Chinese]. doi: 10.3760/cma.j.issn.1673-0860.2016.08.015. [DOI] [PubMed] [Google Scholar]