Abstract
Stem cell transplantation has been applied to treat spinal cord injury (SCI) in clinical trials for many years. However, the clinical efficacies of stem cell transplantation in SCI have been quite diverse. The purpose of our study was to systematically investigate the efficacy of stem cell transplantation in patients with SCI. The PubMed, Web of Science, Ovid-Medline, Cochrane Library, China National Knowledge Infrastructure, VIP, Wanfang, and SinoMed databases were searched until October 27, 2020. Quantitative and qualitative data were analyzed by Review Manager 5.3 and R. Nine studies (n = 328) were included, and the overall risk of bias was moderate. The ASIA Impairment Scale (AIS) grading improvement rate was analyzed in favor of stem cell transplantation group [odds ratio (OR) = 6.06, 95% confidence interval (CI): 3.16–11.62, P < 0.00001]. Urodynamic indices also showed improvement in bladder function. In subgroup analyses, the results indicated that in patients with complete (AIS A) SCI, with the application of cell numbers between n*(107–108), two cell types (i.e., bone marrow–derived mesenchymal stem cells and bone marrow mononuclears), and treatment time of more than 6 months, stem cell transplantation was more beneficial for sensorimotor function (P < 0.05 for all groups). The risk of fever incidence in the stem cell transplantation group was 4.22 (95% CI: 1.7–10.22, P = 0.001), and principal component analysis (PCA) suggested it was more related to transplanted cell numbers. Thus, stem cell transplantation can promote functional recovery in SCI patients. Moreover, the type and quantity of transplanted stem cells and treatment time are important factors affecting the therapeutic effect of stem cell transplantation in SCI. Further studies are needed to evaluate the effects and elucidate the mechanisms of these factors on stem cell therapy in SCI.
Keywords: spinal cord injury, stem cell transplantation, meta-analysis, AIS grading, urodynamic index
Introduction
Spinal cord injury (SCI) is a grievous neurological disease caused by traumatic and nontraumatic injuries, which leads to different degrees of sensorimotor injury and sphincter dysfunction. The incidence of SCI reaches 0.015‰ to 0.04% and the cases exceed 1 million in North America1–3. In addition, in Japan, the proportion of SCI in trauma patients is increasing annually 4 . Meanwhile, the healthcare cost is extremely high and can reach as much as $7 billion a year 1 . Studies have also shown that the incidence of SCI increases with age, peaking at 46 and 60 5 . SCI is still incurable because of high disability, and there is no suitable therapy to improve functional recovery 6 .
Currently, many therapies, such as surgery7–9, medication10–12, physical treatment13,14, and traditional Chinese therapy, have been applied to SCI, but the clinical efficacy of these therapies is not satisfactory. Stem cells have a great application prospect due to the ability to renew themselves and differentiate into functional cells. In recent years, stem cells, including mesenchymal stem cells (MSCs)15,16, neural stem cells (NSCs) 17 , embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs) 18 , were frequently used in basic experimental research and clinical studies for SCI. These stem cells have the ability to deliver growth factors, provide trophic support, improve the microenvironment, regulate the inflammatory response, and remyelinate15,19–21. All of them can live within the host spinal cord for a period of time, differentiating into neurons and glial cells, and then they can promote the recovery of spinal cord functions to different degrees 15 . However, there are inconsistencies in the efficacy of clinical trials. A case report has shown that a patient has significant improvement in sensory function and lower limb muscle strength recovery after MSCs and CD34 cells in combination with intrathecal injection22,23. But studies have shown that transplanting MSCs with lumbar puncture (LP) or injecting MSCs into the lesion site for treating SCI has no functional recovery, or the functional improvement between the treatment and control groups is not significant23–25.
Thus, it is necessary to critically review these trials with respect to methodology, trial design, transplantation strategies (i.e., cell numbers, transplantation methods, and cell types), and outcome indicators [i.e., ASIA Impairment Scale (AIS) grading and urodynamic index]. This meta-analysis aims to provide a comprehensive reference for treating SCI with stem cell transplantation using different cell numbers, cell types, and transplantation methods. We also analyzed the urodynamic index and adverse reactions to provide the impetus for further studies.
Method
Protocol and Registration
The protocol of the meta-analysis was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (No. INPLASY202140034).
Search Strategy
The PubMed, Web of Science, Ovid-Medline, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP, Wanfang, and SinoMed databases were searched up to October 27, 2020. The search terms included “spinal cord injuries,” “stem cell transplantation,” “progenitor cell transplantation,” “cell transplantation,” and “clinical trials” in combination with the Boolean operators “OR” and “AND.” The detailed search strategies are listed in Supplementary Table 1.
Inclusion and Exclusion Criteria
All studies were screened according to inclusion and exclusion criteria 26 . The inclusion criteria included (1) study subjects: patients with SCI; (2) intervention: stem cell transplantation; (3) outcome indicators: (a) sensorimotor function indicator: AIS grading and (b) urodynamic indices; and (4) study types: clinical control trials (CCTs) or randomized controlled trials (RCTs).
The exclusion criteria included (1) study subjects: animals; (2) the outcome indicators in the study did not include those listed in the inclusion criteria; and (3) the study was not a CCT or RCT, such as case reports, reviews, and economics or satisfaction studies.
Data Extraction
Two researchers independently reviewed the included studies and extracted information based on uniform standards, including the first author’s name, publication year, country, methodological characteristics (study types, allocation, blinding), sample size and basic information (average age, gender), the degree of SCI before treatment, treatment measures (cell types, cell numbers, transplantation methods), the outcome indicator (AIS grading and urodynamic index), and adverse reactions. An AIS grading that increased by a level or more before and after treatment was considered efficient. In all included studies, information was cross-examined. Inconsistencies were discussed, and then, a third researcher determined the final result.
Assessment of Quality
The quality of the included studies was assessed by the Cochrane manual. The evaluation items included (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. According to the extracted information, each item of the included studies had three levels: “low risk of bias,” “unclear risk of bias,” or “high risk of bias.” The bias of publication was assessed by the funnel plot and Egger’s test.
Statistical Analysis
Data were collected by Microsoft Excel 2016, and meta-analysis was performed by Review Manager 5.3. For sensorimotor function indicator and adverse events, dichotomous data were assessed using odds ratio (OR) or risk ratio (RR) with 95% confidence intervals (95% CIs) and P values. The heterogeneity evaluation adopted chi-square test or I2 test. I2 < 50% or P > 0.1 was interpreted as low heterogeneity, and a fixed-effects model was used; otherwise, a random-effects model was used. For the urodynamic index, a systematic review was conducted due to a small number of studies and inconsistent data. Principal component analysis (PCA) was conducted by prcomp function of factoextra package in R software to evaluate the correlation between the incidence of adverse events and treatment measures. To verify the robustness of the conclusions, a sensitivity analysis was performed by computing the impact of excluding individual studies from the analysis.
Result
Search Results
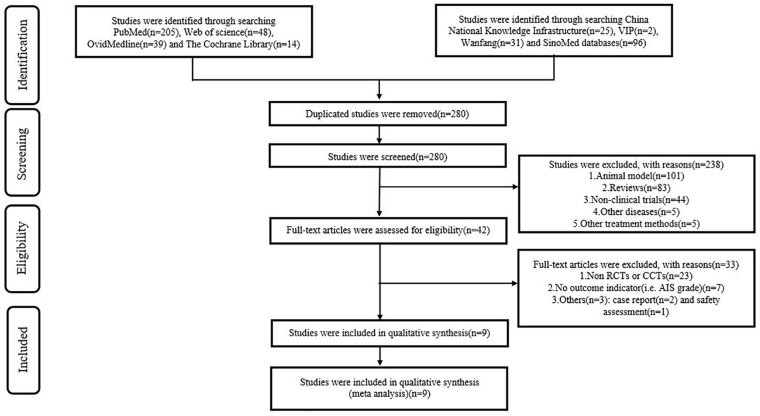
After searching the PubMed, Web of Science, Ovid-Medline, Cochrane Library, CNKI, VIP, Wanfang, and SinoMed databases, we obtained 460 studies in total. We deleted 180 duplicate studies using Endnote X9. A total of 280 studies were excluded after browsing the titles/abstracts, and 238 studies were excluded with reasons of animal models (n = 101), reviews (n = 83), non-clinical trials (n = 44), other diseases (n = 5), and other treatment methods (n = 5). After reading the full text, 33 studies were excluded due to 23 being non-CCTs or non-RCTs, 7 studies not reporting outcome indicators, 2 studies being a case report, and 1 study being a safety assessment. Finally, nine studies27–35 were included in the meta-analysis. The flow chart is shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Fig. 1).
Figure 1.
PRISMA flow diagram.
AIS: ASIA Impairment Scale; CCT: clinical control trial; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT: randomized controlled trial.
Study Characteristics
The study types were mainly phase I/II CCTs. Studies were performed in six countries from Asia, Europe, and Africa. Among the 328 patients in the included studies (188 in the stem cell transplantation group and 144 in the control group), sample sizes ranged from 7 to 50, participants’ average age was 30 to 40 years, and most of them were male (Table 1). A total of 272 patients had severe SCI before treatment, with an AIS grading of A, and 53 patients had an AIS grading of B or C before treatment. All patients received surgery, rehabilitation, physiotherapy, or meditation; the transplantation group adopted stem cell transplantation, whereas the control group did not. Six studies treated SCI by transplanting bone marrow–derived mesenchymal stem cells (BM-MSCs, n = 234), and three studies used one type of umbilical cord–derived mesenchymal stem cells (UC-MSCs, n = 24), human fetal brain–derived nerve stem/progenitor cells (hNSPCs, n = 34), and bone marrow mononuclears (BM-mononuclears, n = 36). The cell transplantation numbers ranged from 106 to 108. A total of 125 patients in three studies received transplant cells via LP, 146 patients in four studies received transplant cells by injection into the lesion site, 36 patients in one study received transplant cells by injection into the cystic cavity and intravenous drip, and 14 patients in one study received transplant cells via two methods (half of them by LP and half by injection into the lesion site). The treatment time, which was the period from transplantation to neurological assessment, ranged from 3 to 12 months (Table 2).
Table 1.
Basic Characteristics of the Study Design of Included Clinical Trials.
| Author | Year | Country | Design | Blinding | Sample size/gender | Average age (years) | |
|---|---|---|---|---|---|---|---|
| SCT | Control | ||||||
| Cheng et al. 29 | 2014 | China | RCT | NR | 10/NR | 14/NR | 35.25 |
| Karamouzian et al. 32 | 2012 | Iran | CCT | NR | 11(7/M, 4/F) | 20(17/M, 3/F) | 33.4 |
| Shin et al. 31 | 2015 | Korea | CCT | NR | 19(16/M, 3/F) | 15(12/M, 3/F) | 37.2 |
| Dai et al. 28 | 2013 | China | RCT | NR | 20(16/M, 4/F) | 20(16/M, 4/F) | 34.9 |
| El-Kheir et al. 34 | 2014 | Egypt | RCT | Single-blind | 50(61M, 9F) | 20 | 16–45 |
| Chernykh et al. 27 | 2007 | Russia | CCT | NR | 18(14/M, 4/F) | 18(12/M, 6/F) | 32.4 |
| Chhabra et al. 30 | 2016 | India | RCT | Single-blind | 14(11/M, 3/F) | 7/NR | 24.9/T |
| Yoon et al. 33 | 2007 | Korea | CCT | Single-blind | 35(26/M, 6/F) | 13(9/M, 4/F) | 41.3 |
| Xie et al. 35 | 2007 | China | RCT | NR | 11(9/M, 2/F) | 13(10/M, 3/F) | 18–49/T, 21–53/C |
C: control group; CCT: clinical controlled trail; F: female; M: male; NR: not reported; RCT: randomized controlled trail; SCT: stem cell transplantation; T: stem cell transplantation group.
Table 2.
Interventions and Outcome Indicators Included in the Study.
| Author | Degree of preinjury | Level of injury | Treatment | Cell types | Cell numbers | Methods | Treatment time | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SCT | Control | SCT | Control | |||||||
| Cheng et al. 29 | AIS A | Thoracolumbar | R +SCT | R | UC-MSCs | 4 × 107 | Injected into lesion site | 6 m | AIS grading, urodynamic examination | |
| Karamouzian et al. 32 | AIS A | 11/Thoracic | 20/Thoracic | Methylprednisolone + P + SCT | Methylprednisolone + P | BM-MSCs | 7 × 105–1.2 × 106 | LP | 6 m | AIS grading |
| Shin et al. 31 | AIS A/B | 19/Cervical | 15/Cervical | S + R + SCT | S + R | hNSPCs | 108 | Injected into lesion site | 12 m | AIS grading |
| Dai et al. 28 | AIS A | 20/Cervical | 20/Cervical | R + SCT | R | BM-MSCs | 2 × 107 | Injected into lesion site | 6 m | AIS grading, residual urine volume |
| El-Kheir et al. 34 | AIS A or B | 10/Cervical, 40/Thoracic |
7/Cervical, 13/Thoracic |
P + SCT | P | BM-MSCs | 2 × 106/kg | LP | 18 m | AIS grading |
| Chernykh et al. 27 | AIS A | 12/Cervical, 2/Thoracic, 4/Lumbar | 8/Cervical, 5/Thoracic, 5/Lumbar | S + SCT | S | BM-mononuclears | NR | Injected into the cystic cavity and intravenous drip | 9.4 ± 4.6 m | AIS grading |
| Chhabra et al. 30 | AIS A | 14/Thoracic | 7/Thoracic | S + R + SCT | S + R | BM-MSCs | 7 × 108–109 | 7/LP, 7/Injected into lesion site | 12 m | AIS grading, ISCIS scores |
| Yoon et al. 33 | AIS A | 23/Cervical,12/Thoracic | 7/Cervical,6/Thoracic | S + SCT | S | BM-MSCs | 1.98 × 108 | Injected into lesion site | 10 m | AIS grading |
| Xie et al. 35 | AIS A/B/C/D | 2/Cervical, 4/Thoracic, 5/Lumbar |
3/Cervical, 4/Thoracic, 6/Lumbar |
R + SCT | R | BM-MSCs | 2–5 × 109 | LP | 3 m | AIS grading, residual urine volume |
AIS: ASIA Impairment Scale; BM-MSCs: bone marrow–derived mesenchymal stem cells; hNSPCs: human fetal brain–derived nerve stem/progenitor cells; ISCIS: International Spinal Cord Injury Scale; LP: lumbar puncture; m: months; NR: not reported; P: physiotherapy; R: rehabilitation; S: surgery; SCT: stem cell transplantation; UC-MSCs: umbilical cord–derived mesenchymal stem cells.
Risk of Bias in the Included Studies
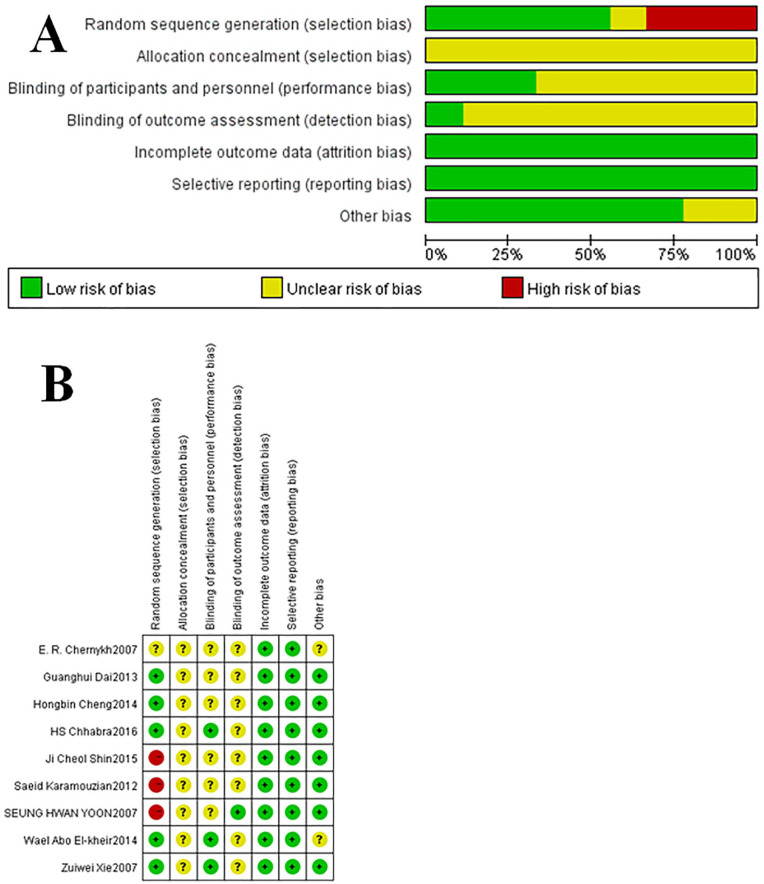
Five studies reported random sequences but did not report specific methods of random sequence generation. No study conducted allocation hiding. Among three studies, a single-blind procedure was reported, in one of which observers did not know the way of group assignment. Only one study had missing data, but the reasons and processing results of missing data were reported. No study had selective reporting or other bias. The overall risk of bias of included studies was assessed as moderate (Fig. 2).
Figure 2.
Risk of bias graph (A) and risk of bias summary (B).
Major Outcomes
Sensory and Motor Function Indicator
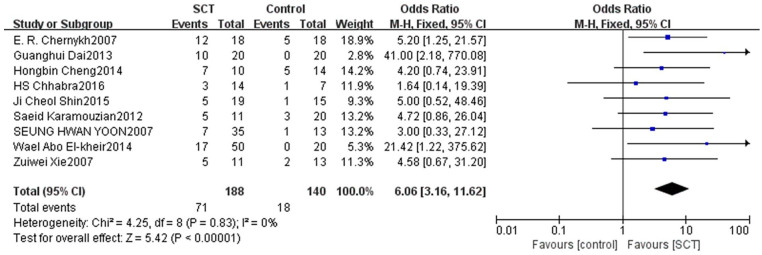
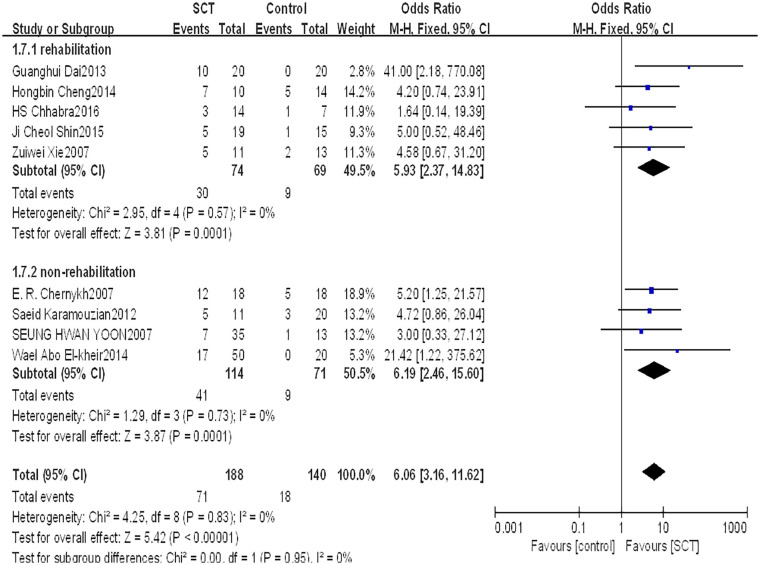
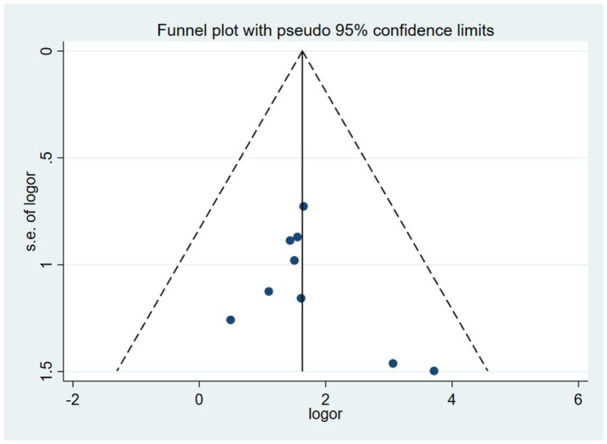
This indicator was assessed among 328 patients in nine studies. The fixed-effects model was used to evaluate the AIS grading improvement rate due to low heterogeneity between studies (P = 0.83, I2 = 0%). The forest plot indicated that compared with the control group, the AIS grading of the stem cell transplantation group was statistically improved (OR = 6.06, 95% CI: 3.16–11.62, P < 0.00001) (Fig. 3). The funnel plot showed that no study was outside the funnel, and Egger’s test indicated that there was no publication bias (P = 0.226) (Fig. 4). The sensitivity analysis confirmed the reliability and stability of the current findings.
Figure 3.
Forest plot and meta-analysis of AIS grading improvement rate.
AIS: ASIA Impairment Scale; CI: confidence interval; SCT: stem cell transplantation.
Figure 4.
Funnel plot for publication bias.
Urodynamic Index
The urodynamic index was reported for 95 patients in four studies (Table 3). The results showed that the bladder function of patients improved after stem cell transplantation compared with that before treatment.
Table 3.
Results of Urodynamic Index.
| Author | Maximum urinary flow rate | Maximum bladder capacity | Residual urine volume | Maximum detrusor pressure | ISCIS scores |
|---|---|---|---|---|---|
| Dai et al. 28 | a | ||||
| Cheng et al. 29 | b | a | b | a | |
| Chhabra et al. 30 | b | ||||
| Xie et al. 35 | a |
ISCIS: International Spinal Cord Injury Scale.
Compared with index before treatment.
Reported the indicator in the study.
Subgroup analysis
Subgroup analysis of different AIS gradings before treatment
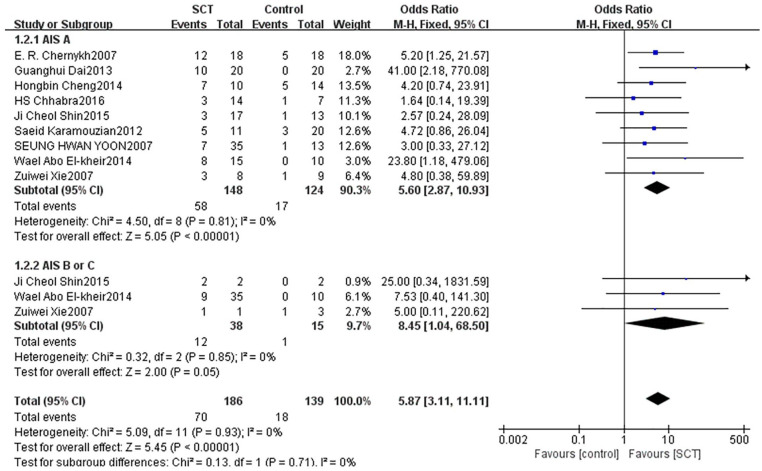
We divided the patients into two subgroups based on their degree of injury before treatment. Eight studies with 272 patients with AIS A reported that the AIS grading significantly improved (OR = 5.60, 95% CI: 2.87–10.93, P < 0.00001). Three studies with 53 patients with AIS B or C reported that the AIS grading improved, but not significantly (OR = 8.45, 95% CI: 1.04–68.50, P = 0.05) (Fig. 5).
Figure 5.
Forest plot and meta-analysis of AIS grading improvement of SCT and control groups in subgroups of different AIS gradings before transplantation.
AIS: ASIA Impairment Scale; CI: confidence interval; SCT: stem cell transplantation.
Subgroup analysis of different cell transplantation numbers
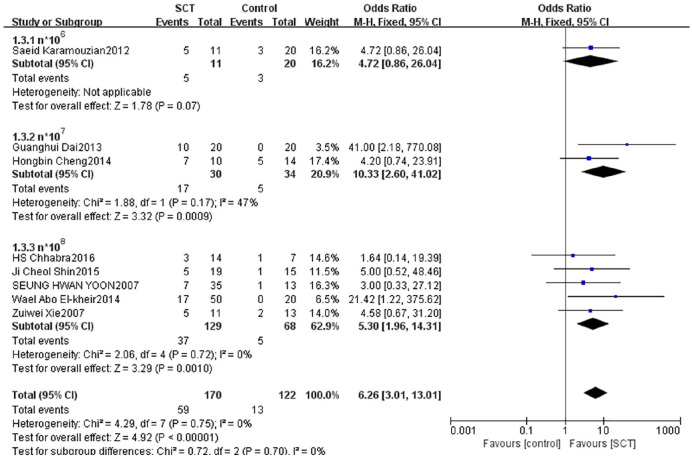
We divided patients into three subgroups based on the cell transplantation numbers. One study with 31 patients reported AIS grading had no statistically significant improvement with n*106 cells between groups (OR = 4.72, 95% CI: 0.86–26.04, P = 0.07). Two studies with 64 patients reported that the AIS grading significantly improved with n*107 cells between groups (OR = 10.33, 95% CI: 2.60–41.02, P = 0.0009). Five studies with 197 patients reported that the AIS grading significantly improved with n*108 cells between groups (OR = 5.30, 95% CI: 1.96–14.31, P = 0.0010) (Fig. 6).
Figure 6.
Forest plot and meta-analysis of AIS grading improvement of SCT and control groups in subgroups of different cell numbers.
AIS: ASIA Impairment Scale; CI: confidence interval; SCT: stem cell transplantation.
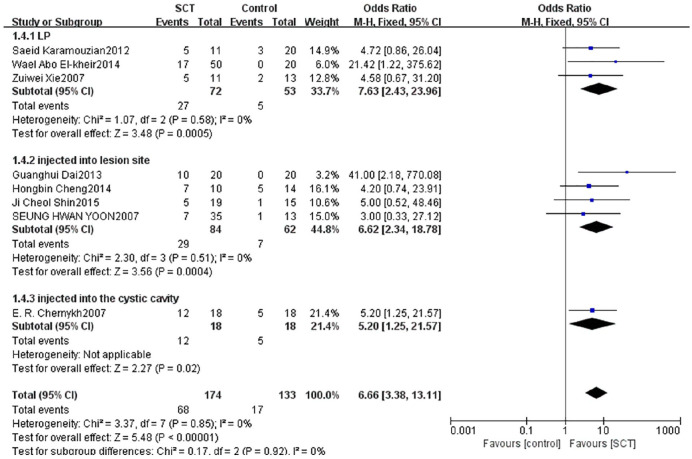
Subgroup analysis of different transplantation methods
We divided patients into three subgroups based on the transplantation methods. Three studies with 125 patients reported that the AIS grading significantly improved by LP between groups (OR = 7.63, 95% CI: 2.43–23.96, P = 0.0005). Four studies with 146 patients reported that the AIS grading significantly improved by injecting into lesion site between groups (OR = 6.62, 95% CI: 2.34–18.78, P = 0.0004). One study with 36 patients reported that the AIS grading significantly improved by injecting into the cystic cavity and intravenous drip between groups (OR = 5.20, 95% CI: 1.25–21.57, P = 0.02) (Fig. 7).
Figure 7.
Forest plot and meta-analysis of AIS grading improvement of SCT and control groups in subgroups of different methods of transplantation.
AIS: ASIA Impairment Scale; CI: confidence interval; LP: lumbar puncture; SCT: stem cell transplantation.
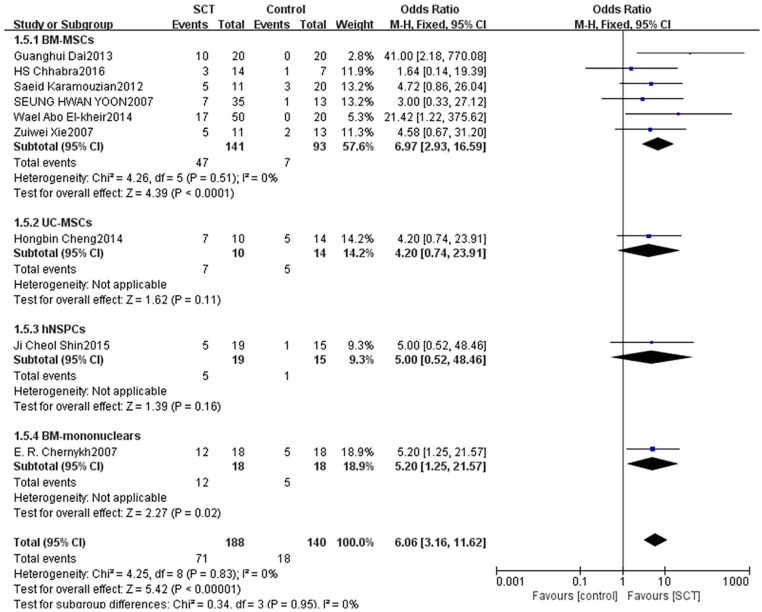
Subgroup analysis of transplanted stem cell types
We divided patients into four subgroups based on cell types. Six studies with 234 patients reported that the AIS grading significantly improved with adopting BM-MSCs between groups (OR = 6.97, 95% CI: 2.93–16.59, P < 0.0001). One study with 24 patients reported that the AIS grading did not significantly improve when adopting UC-MSCs between groups (OR = 4.20, 95% CI: 0.74–23.91, P = 0.11). One study with 34 patients reported that the AIS grading did not significantly improve when adopting hNSPCs between groups (OR = 5.00, 95% CI: 0.52–48.46, P = 0.16). One study with 36 patients reported that the AIS grading significantly improved when adopting BM-mononuclears between groups (OR = 5.20, 95% CI: 1.25–21.57, P = 0.02) (Fig. 8).
Figure 8.
Forest plot and meta-analysis of AIS grading improvement of SCT and control groups in subgroups of different cell types.
AIS: ASIA Impairment Scale; BM-MSCs: bone marrow–derived mesenchymal stem cells; CI: confidence interval; hNSPCs: human fetal brain–derived nerve stem/progenitor cells; SCT: stem cell transplantation; UC-MSCs: umbilical cord–derived mesenchymal stem cells.
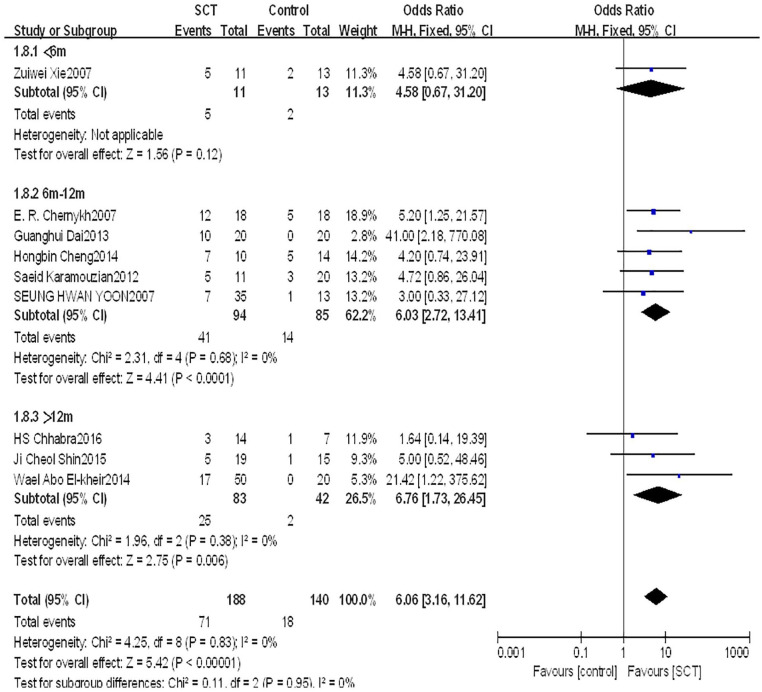
Subgroup analysis of different treatment time after injury
We divided patients into three subgroups based on the treatment time after injury. One study with 24 patients reported that the AIS grading did not significantly improve with treatment time of less than 6 months between groups (OR = 4.58, 95% CI: 0.67–31.20, P = 0.12). Five studies with 169 patients reported that the AIS grading significantly improved with treatment time between 6 and 12 months between groups (OR = 6.03, 95% CI: 2.72–13.41, P < 0.0001). Three studies with 125 patients reported that the AIS grading significantly improved with treatment time of more than 12 months between groups (OR = 6.76, 95% CI: 1.73–26.45, P = 0.006) (Fig. 9).
Figure 9.
Forest plot and meta-analysis of AIS grading improvement of SCT and control groups in subgroups of different treatment time after injury.
AIS: ASIA Impairment Scale; CI: confidence interval; SCT: stem cell transplantation.
Subgroup analysis of whether receiving rehabilitation
We divided patients into two subgroups based on whether they were receiving rehabilitation. Five studies with 143 patients reported that the AIS grading significantly improved with receiving rehabilitation between groups (OR = 5.93, 95% CI: 2.37–14.83, P = 0.0001). Four studies with 185 patients reported that the AIS grading significantly improved with not receiving rehabilitation between groups (OR = 6.19, 95% CI: 2.46–15.60, P = 0.0001) (Fig. 10).
Figure 10.
Forest plot and meta-analysis of AIS grading improvement of SCT and control groups in subgroups of whether receiving rehabilitation.
AIS: ASIA Impairment Scale; CI: confidence interval; SCT: stem cell transplantation.
Adverse Events
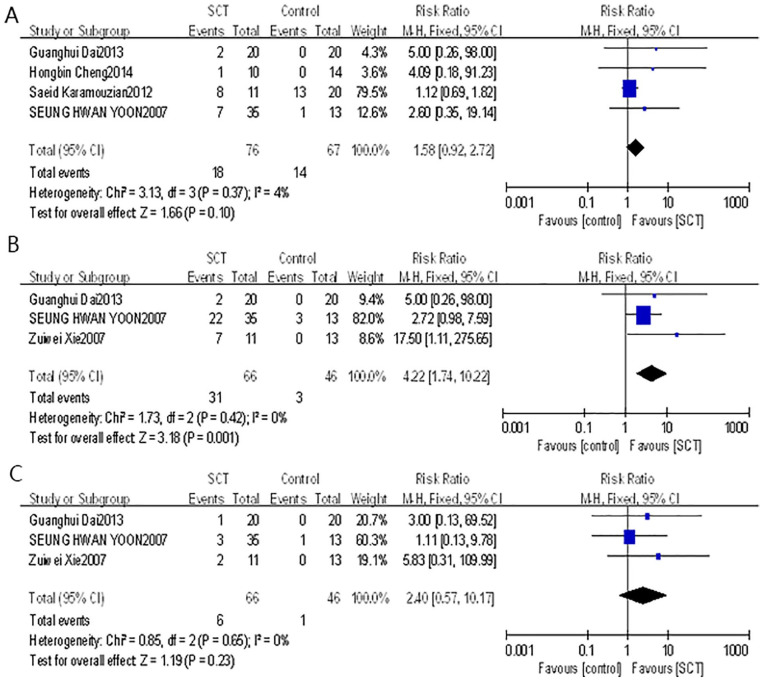
There were four studies that reported neuropathic pain. The forest plot indicated that the average RR of incidence of neuropathic pain in these studies was 1.58 (95% CI: 0.92–2.72, P = 0.10) with low heterogeneity (P = 0.37, I2 = 4%). There were three studies that reported fever. The forest plot indicated that the average RR of incidence of fever in these studies was 4.22 (95% CI: 1.74–10.22, P = 0.001) with low heterogeneity (P = 0.42, I2 = 0%). There were three studies that reported headache. The forest plot indicated that the average RR of incidence of headache in these studies was 2.40 (95% CI: 0.57–10.17, P = 0.23) with low heterogeneity (P = 0.65, I2 = 0%) (Fig. 11). These results suggested that stem cell transplantation increased the risk of fever, and Egger’s test showed that there was no publication bias (P = 0.359). Therefore, PCA was performed to further assess correlation between the studied parameters. The number of variables used to construct the PCA plot is 4, including incidence of fever, cell numbers, cell types, and transplantation methods. The result of Biplot showed that the weights of variation explained by principal component 1 (PC1) and principal component 2 (PC2) are 84.8% and 15.1%, and the incidence of fever was positively correlated with transplanted cell numbers (Fig. 12). However, these adverse events caused by stem cell transplantation were alleviated spontaneously or after symptomatic treatment. In addition, there was no tumor, wound infection, cerebrospinal fluid leakage, intracranial infection, or spinal cord diameter increase reported in these studies. Only one patient was reported to have a suture fracture on the second day after the surgery.
Figure 11.
Forest plot and meta-analysis of incidence of adverse events (A: neuropathic pain; B: fever; C: headache).
CI: confidence interval; SCT: stem cell transplantation.
Figure 12.
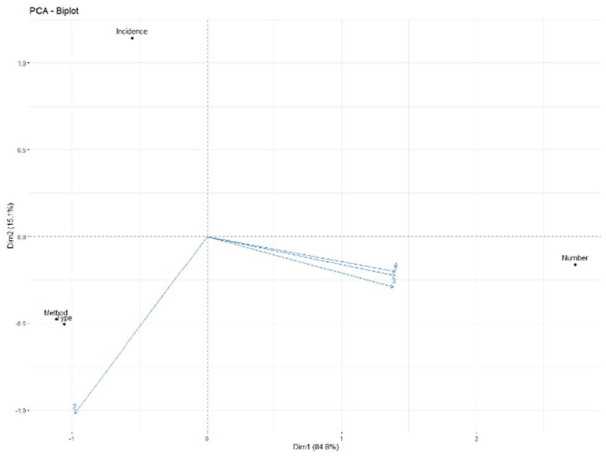
The Biplot between incidence of fever and transplantation measures.
PCA: principal component analysis.
Discussion
In this meta-analysis, nine studies evaluated the clinical efficacy of stem cell transplantation on sensorimotor and urinary function after SCI, and the results indicated that AIS grading significantly improved after stem cell transplantation therapy. In subgroup analyses, our study indicated that stem cell transplantation was more efficient for complete SCI, and the application of BM-MSCs, BM-mononuclears, and cell number between n*(107–108) seemed to be more beneficial. Moreover, urodynamic indices showed that there was improvement in bladder function in SCI patients after treatment.
For patients with AIS A before treatment, there was significant improvement in AIS grading, while for patients with AIS B or C, there was no improvement in AIS grading, suggesting that the therapeutic effect of stem cell transplantation for patients with a severe degree of SCI was better. Studies have indicated that mechanisms of compensation and neural plasticity represent major factors underlying clinical recovery in human SCI 36 . Patients with AIS A have fairly limited and predictable neurological recovery compared with those with AIS B/C/D. Most of the spontaneous neurological recovery in AIS A subjects is likely to occur within the Zone of Partial Preservation30,36. In these clinical trials, the sample size of patients with AIS B or C was small. Therefore, for evaluating the clinical efficacy of stem cell transplantation for patients with AIS B or C, more clinical trials are needed to provide further evidence.
In SCI patients treated with stem cell transplantation in numbers of n*106, the AIS grading was not significantly improved. However, the AIS grading was significantly improved in patients with n*107 and n*108 cell numbers. The results showed that stem cell transplantation could significantly improve AIS grading in high cell number subgroups (107 and 108 of cells), but not in low cell number subgroup (106 cells). This was consistent with the conclusions of the other two meta-analysis of clinical trials, whose results showed that cell numbers of 107 and 108 were more beneficial than 106 for SCI patients37,38. The reason might be that certain stem cell numbers (n*107–108) were necessary for treating SCI patients to ensure the survival, proliferation, and differentiation of transplanted cells 39 . The safety data in preclinical trials, including tolerable level of cell dose, injection location or number, and cell suspension volumes based on neurological examination and neuropathology, provide guidance for the dose and delivery method for clinical trials40,41. The final dose is determined a priori based on stopping and reduction rules for safety and tolerability 41 . Seung Hwan Yoon and his colleagues 33 used a dose of 300 ml, whereas other researchers used a dose of 25 ml 28 . Their results suggested that the larger volume of transplantation may result in more edema, which will increase the risk of secondary injury. 28 Thus, more phase I/II clinical trials are needed to confirm the number and dose of stem cells used in transplantation for SCI with high efficacy and good tolerability.
Among the transplantation methods, the results showed that AIS grade significantly improved in three transplantation methods (LP, injected into the lesion site, and injected into cystic cavity and intravenous drip). To be injected into the lesion site, the cone needs to be opened, and point injection or even multipoint injection is adopted. The surgical wound is large, which increases the chance of wound infection. However, Takahashi et al. 42 concluded that in terms of grafted cell survival and safety, injection into the lesion site is the most effective and feasible method for NS/PC transplantation. Compared with injection into the lesion site, the wound of LP is small. Studies have shown that when BM-MSCs were injected by LP into animal SCI models, BM-MSCs homed toward injured spinal cord tissues 43 . The LP route allows more efficient delivery of cells to the injured cord compared with the intravenous route. However, this route also has limitations. As transplanted stem cells need to migrate to the damaged spinal cord through cerebrospinal fluid (CSF), the number of effective stem cells to reach the therapeutic target is uncertain. A study also raises questions about how long the cells remain in the CSF, what happens, and what effects they have 43 . Compared with the above two methods, injection into the cystic cavity and intravenous drip have a high requirement of the number of stem cells. Moreover, the cystic cavities packaged with stem cells need to have the characteristics of a suitable microenvironment and low immunogenicity to maintain the proliferation and differentiation of stem cells. In addition, other issues such as uncertain number of cells reaching the target and adverse reactions may exist27,44. Furthermore, in choosing the transplantation methods, the patient’s condition and the operating proficiency of the surgeon should also be considered 43 .
About the types of stem cells, the AIS grading of patients was significantly improved after BM-MSC and BM-mononuclear transplantation, but did not significantly improve after hNSPC and UC-MSC transplantation. This difference is due to the differentiation potential of different stem cells. BM-MSCs have different mechanisms to promote the repair of damaged tissues. They not only have an anti-inflammatory effect, but also deliver different growth factors to provide nutritional support and neuroprotection19,45,46. The results of other clinical trials of meta-analysis have also verified the efficiency of BM-MSC transplantation in SCI patients38,47. BM-mononuclears produce neurotrophic factors that stimulate neuronal growth and myelin remyelination. Their cell suspension contains endothelial precursors, which promote angiogenesis and regeneration of nerve tissue 27 . The nerve stem/progenitor cells are heterogeneous and are based on their types and regional origins. Preclinical studies have shown that after transplanting hNSPCs into the epicenter of the injured cord, only 21.3% of hNSPCs differentiate into neurons, and most of them differentiate into gliocyte or even remain in an undifferentiated state31,48. Studies have shown that oligodendrocytes differentiated from hNSPCs are limited48–50. Meanwhile, there are many variables in the differentiated process of hNSPCs after transplantation, including the source of cells, culture technology, cell preparation, and injury models 31 . UC-MSCs, combined with various factors, have neurotrophic, anti-inflammatory, antiapoptotic, and angiogenesis-related effects, which could promote nerve tissue repair 20 . However, our results showed that AIS grading is not significantly improved by the transplantation of these cell types; therefore, for hNSPC and UC-MSC transplantation, more experimental studies and clinical trials are needed to further clarify their therapeutic mechanism and optimize their therapeutic variables.
For the treatment time between stem cell transplantation and neurological assessment, the AIS grading of patients was significantly improved in subgroups of more than 6 months after transplantation, but did not significantly improve in subgroup of less than 6 months. These results indicate that treatment time after injury is also an important variable affecting stem cell transplantation in the treatment of SCI. The reason may be that the nervous functional reorganization after injury is time-dependent51,52.
Rehabilitation therapy is an important method for the treatment of SCI. Therefore, we observed its effect on stem cell transplantation. The AIS grading of patients was significantly improved whether receiving rehabilitation or not. These showed that rehabilitation therapy was not the key factor determining the effectiveness of stem cell therapy. In view of the therapeutic effect of rehabilitation on SCI 53 , more research is needed in the future to determine the effective combination of these two approaches.
Our study found that stem cell transplantation increased the risk of fever, and the incidence of fever was positively related to transplanted cell numbers. Fever is one of the manifestations of engraftment syndrome (ES), and the hospital stay duration is directly related to its occurrence54,55. Meanwhile, fever is a common event after transplantation, regardless of patients’ age or CD34+ cell numbers 56 . Studies showed that leukocyte or T-cell numbers were predictors for fever57,58. More studies, which aim to decrease the risk of fever and improve prognosis, are needed.
This meta-analysis also has some limitations. First, the risk of bias in studies was moderate. Most of these studies did not report clearly randomness, blinding method, and allocation concealment, which may make the strength of evidence to weaken. Second, the sample size was small. In the subgroup analyses, we found that there was only one study in some subgroups, and the numbers of patients in the transplantation and control groups were not exactly the same. This may be related to whether the patients were willing or suitable to conduct stem cell transplantation. Moreover, ethical policy between countries, medical healthcare policy, and economic condition are restrictions. Third, the required information was not reported in every study, which led to the incompletion of data. For example, only patients in the transplantation group performed urodynamic tests.
In summary, stem cell transplantation for treating SCI has gradually entered phase I/II clinical trials. The systematic review and meta-analysis indicated that stem cell transplantation for treating SCI can improve AIS grading and bladder function. A reasonable dose of cell transplantation has not been determined. The choice of delivery mode should be based on the actual situation in the treatment process. Large-sample, well-designed clinical trials are needed to update the evidence on the use of stem cell transplantation for SCI.
Supplemental Material
Supplemental material, sj-docx-1-cll-10.1177_09636897211067804 for Evaluation of the Clinical Efficacy of Stem Cell Transplantation in the Treatment of Spinal Cord Injury: A Systematic Review and Meta-analysis by Qiao-Rui Tang, Hui Xue, Qiao Zhang, Ying Guo, Hao Xu, Ying Liu and Jia-Mei Liu in Cell Transplantation
Acknowledgments
Thank you so much for contributions made by all members of Prof. Jia-Mei Liu’s group (Department of Histology and Embryology, College of Basic Medical Sciences, Jilin University).
Footnotes
Author Contributions: Q-rT contributed to designing the study, analyzing and interpreting the data, and drafting the manuscript; HX contributed to supervising the study, interpreting the data, and revising the manuscript; QZ and HX contributed to screening the included studies and extracting data; YG contributed to revising the manuscript; and YL and J-mL contributed to performing the study, interpreting the data, and significantly revising the manuscript. All authors approved the publication of the manuscript.
Ethical Approval: Ethical Approval is not applicable to this study.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (31800895) and Science and Technology Project of Jilin Provincial Department of Education (JJKH20201028KJ).
ORCID iD: Jia-Mei Liu  https://orcid.org/0000-0003-3761-9731
https://orcid.org/0000-0003-3761-9731
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Shah M, Peterson C, Yilmaz E, Halalmeh DR, Moisi M. Current advancements in the management of spinal cord injury: a comprehensive review of literature. Surg Neurol Int. 2020;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–72. [DOI] [PubMed] [Google Scholar]
- 3. Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–16. [DOI] [PubMed] [Google Scholar]
- 4. Tafida MA, Wagatsuma Y, Ma E, Mizutani T, Abe T. Descriptive epidemiology of traumatic spinal injury in Japan. J Orthop Sci. 2018;23:273–76. [DOI] [PubMed] [Google Scholar]
- 5. Chen R, Liu X, Han S, Dong D, Wang Y, Zhang H, Shi J, Zhao C, Yao M. Current epidemiological profile and features of traumatic spinal cord injury in Heilongjiang province, Northeast China: implications for monitoring and control. Spinal Cord. 2017;55:399–404. [DOI] [PubMed] [Google Scholar]
- 6. Ning GZ, Wu Q, Li YL, Feng SQ. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med. 2012;35:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dvorak MF, Noonan VK, Fallah N, Fisher CG, Finkelstein J, Kwon BK, Rivers CS, Ahn H, Paquet J, Tsai EC, Townson A, et al. The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: an observational Canadian cohort study. J Neurotrauma. 2015;32:645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fehlings MG, Vaccaro A, Wilson JR, Singh A, W Cadotte D, Harrop JS, Aarabi B, Shaffrey C, Dvorak M, Fisher C, Arnold P, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS ONE. 2012;7:e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson JR, Singh A, Craven C, Verrier MC, Drew B, Ahn H, Ford M, Fehlings MG. Early versus late surgery for traumatic spinal cord injury: the results of a prospective Canadian cohort study. Spinal Cord. 2012;50:840–43. [DOI] [PubMed] [Google Scholar]
- 10. Wilson JR, Forgione N, Fehlings MG. Emerging therapies for acute traumatic spinal cord injury. CMAJ. 2013;185:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012;1:CD001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C, Teng A, Toups EG, Harrop JS, Aarabi B, Shaffrey CI, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–22. [DOI] [PubMed] [Google Scholar]
- 14. Formento E, Minassian K, Wagner F, Mignardot JB, Le Goff-Mignardot CG, Rowald A, Bloch J, Micera S, Capogrosso M, Courtine G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci. 2018;21:1728–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li LM, Han M, Jiang XC, Yin XZ, Chen F, Zhang TY, Ren H, Zhang JW, Hou TJ, Chen Z, Ou-Yang HW, et al. Peptide-tethered hydrogel scaffold promotes recovery from spinal cord transection via synergism with mesenchymal stem cells. ACS Appl Mater Interfaces. 2017;9:3330–42. [DOI] [PubMed] [Google Scholar]
- 16. Yao L, He C, Zhao Y, Wang J, Tang M, Li J, Wu Y, Ao L, Hu X. Human umbilical cord blood stem cell transplantation for the treatment of chronic spinal cord injury: electrophysiological changes and long-term efficacy. Neural Regen Res. 2013;8:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emgard M, Piao J, Aineskog H, Liu J, Calzarossa C, Odeberg J, Holmberg L, Samuelsson EB, Bezubik B, Vincent PH, Falci SP, et al. Neuroprotective effects of human spinal cord-derived neural precursor cells after transplantation to the injured spinal cord. Exp Neurol. 2014;253:138–45. [DOI] [PubMed] [Google Scholar]
- 18. Khazaei M, Ahuja CS, Fehlings MG. Induced pluripotent stem cells for traumatic spinal cord injury. Front Cell Dev Biol. 2016;4:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunther MI, Weidner N, Muller R, Blesch A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 2015;27:140–50. [DOI] [PubMed] [Google Scholar]
- 20. Chua SJ, Bielecki R, Yamanaka N, Fehlings MG, Rogers IM, Casper RF. The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine. 2010;35:1520–26. [DOI] [PubMed] [Google Scholar]
- 21. Wang LJ, Zhang RP, Li JD. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir (Wien). 2014;156:1409–18. [DOI] [PubMed] [Google Scholar]
- 22. Ichim TE, Solano F, Lara F, Paris E, Ugalde F, Rodriguez JP, Minev B, Bogin V, Ramos F, Woods EJ, Murphy MP, et al. Feasibility of combination allogeneic stem cell therapy for spinal cord injury: a case report. Int Arch Med. 2010;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chhabra HS, Sarda K. Clinical translation of stem cell based interventions for spinal cord injury: are we there yet? Adv Drug Deliv Rev. 2017;120:41–49. [DOI] [PubMed] [Google Scholar]
- 24. Kishk NA, Gabr H, Hamdy S, Afifi L, Abokresha N, Mahmoud H, Wafaie A, Bilal D. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010;24:702–708. [DOI] [PubMed] [Google Scholar]
- 25. Bhanot Y, Rao S, Ghosh D, Balaraju S, Radhika CR, Satish Kumar KV. Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg. 2011;25:516–22. [DOI] [PubMed] [Google Scholar]
- 26. Wan GY, Zheng LY, Li HQ, Yuan H, Xue H, Zhang XY. Effects of enteral nutritional rich in n-3 polyunsaturated fatty acids on the nutritional status of gastrointestinal cancer patients: a systematic review and meta-analysis. Eur J Clin Nutr. 2020;74:220–30. [DOI] [PubMed] [Google Scholar]
- 27. Chernykh ER, Stupak VV, Muradov GM, Sizikov MY, Shevela EY, Leplina OY, Tikhonova MA, Kulagin AD, Lisukov IA, Ostanin AA, Kozlov VA. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull Exp Biol Med. 2007;143:543–47. [DOI] [PubMed] [Google Scholar]
- 28. Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. [DOI] [PubMed] [Google Scholar]
- 29. Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chhabra HS, Sarda K, Arora M, Sharawat R, Singh V, Nanda A, Sangodimath GM, Tandon V. Autologous bone marrow cell transplantation in acute spinal cord injury: an Indian pilot study. Spinal Cord. 2016;54:57–64. [DOI] [PubMed] [Google Scholar]
- 31. Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee H, Jung K, Hwang K, Kim M, Lee IS, Shin JE, et al. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015;2015:630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114:935–39. [DOI] [PubMed] [Google Scholar]
- 33. Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, Choi BH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: phase I/II clinical trial. Stem Cells. 2007;25:2066–73. [DOI] [PubMed] [Google Scholar]
- 34. El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, El Maadawi ZM, Ewes I, Sabaawy HE. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729–45. [DOI] [PubMed] [Google Scholar]
- 35. Xie ZW, Cui GX, Li YZ, Li BW, Zhu SW, Song CZ, Shi Q, Hou H, Shen B. Curative effect of autologous mesenchymal stem cell transplantation on spinal cord injury. J Clin Rehabil Tissue Eng Res. 2007;11(7):1277–79. [Google Scholar]
- 36. Curt A, Van Hedel HJ, Klaus D, Dietz V; EM-SCI Study Group. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma. 2008;25:677–85. [DOI] [PubMed] [Google Scholar]
- 37. Zhao H, Sun QL, Duan LJ, Yang YD, Gao YS, Zhao DY, Xiong Y, Wang HJ, Song JW, Yang KT, Wang XM, et al. Is cell transplantation a reliable therapeutic strategy for spinal cord injury in clinical practice? A systematic review and meta-analysis from 22 clinical controlled trials. Eur Spine J. 2019;28:1092–1112. [DOI] [PubMed] [Google Scholar]
- 38. Li XC, Zhong CF, Deng GB, Liang RW, Huang CM. Efficacy and safety of bone marrow-derived cell transplantation for spinal cord injury: a systematic review and meta-analysis of clinical trials. Clin Transplant. 2015;29:786–95. [DOI] [PubMed] [Google Scholar]
- 39. Li Y, Yu HL, Chen LF, Duan CX, Zhang JY, Li BC. Survival and number of olfactory ensheathing cells transplanted in contused spinal cord of rats. Chin J Traumatol. 2010;13:356–61. [PubMed] [Google Scholar]
- 40. Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levi AD, Okonkwo DO, Park P, Jenkins AL, Kurpad SN, Parr AM, Ganju A, Aarabi B, Kim D, Casha S, Fehlings MG, et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery. 2018;82:562–75. [DOI] [PubMed] [Google Scholar]
- 42. Takahashi Y, Tsuji O, Kumagai G, Hara CM, Okano HJ, Miyawaki A, Toyama Y, Okano H, Nakamura M. Comparative study of methods for administering neural stem/progenitor cells to treat spinal cord injury in mice. Cell Transplant. 2011;20:727–39. [DOI] [PubMed] [Google Scholar]
- 43. Bakshi A, Hunter C, Swanger S, Lepore A, Fischer I. Minimally invasive delivery of stem cells for spinal cord injury: advantages of the lumbar puncture technique. J Neurosurg Spine. 2004;1:330–37. [DOI] [PubMed] [Google Scholar]
- 44. Syková E, Jendelová P, Urdzíková L, Lesný P, Hejcl A. Bone marrow stem cells and polymer hydrogels–two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26:1113–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silvestro S, Bramanti P, Trubiani O, Mazzon E. Stem cells therapy for spinal cord injury: an overview of clinical trials. Int J Mol Sci. 2020;21:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao H, Cheng L, Du X, Hou Y, Liu Y, Cui Z, Nie L. Transplantation of cerebral dopamine neurotrophic factor transducted BMSCs in contusion spinal cord injury of rats: promotion of nerve regeneration by alleviating neuroinflammation. Mol Neurobiol. 2016;53:187–99. [DOI] [PubMed] [Google Scholar]
- 47. Fan X, Wang JZ, Lin XM, Zhang L. Stem cell transplantation for spinal cord injury: a meta-analysis of treatment effectiveness and safety. Neural Regen Res. 2017;12:815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Akesson E, Piao JH, Samuelsson EB, Holmberg L, Kjaeldgaard A, Falci S, Sundström E, Seiger A. Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiol Behav. 2007;92:60–66. [DOI] [PubMed] [Google Scholar]
- 49. Svendsen CN, Caldwell MA, Ostenfeld T. Human neural stem cells: isolation, expansion and transplantation. Brain Pathol. 1999;9:499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–33. [DOI] [PubMed] [Google Scholar]
- 51. Xiao FL, Gao PY, Sui BB, Wan H, Lin Y, Xue J, Zhou J, Qian TY, Wang S, Li D, Liu S. Time-course of changes in activation among facial nerve injury: a functional imaging study. Medicine (Baltimore). 2015;94:e1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Steeves JD, Kramer JK, Fawcett JW, Cragg J, Lammertse DP, Blight AR, Marino RJ, Ditunno JF, Jr, Coleman WP, Geisler FH, Guest J, et al. Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord. 2011;49:257–65. [DOI] [PubMed] [Google Scholar]
- 53. Nam KY, Kim HJ, Kwon BS, Park JW, Lee HJ, Yoo A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cornell RF, Hari P, Drobyski WR. Engraftment syndrome after autologous stem cell transplantation: an update unifying the definition and management approach. Biol Blood Marrow Transplant. 2015;21:2061–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Amini S, Hadjibabaie M, Jahangard-Rafsanjani Z, Ashuri A, Torkamandi H, Ghavamzadeh A. Evaluation of febrile neutropenia in patients undergoing hematopoietic stem cell transplantation. Acta Med Iran. 2014;52:38–42. [PubMed] [Google Scholar]
- 56. Arango M, Combariza JF. Fever after peripheral blood stem cell infusion in haploidentical transplantation with post-transplant cyclophosphamide. Hematol Oncol Stem Cell Ther. 2017;10:79–84. [DOI] [PubMed] [Google Scholar]
- 57. Schnabel B, Schmidmaier R, Franke D, Emmerich B, Straka C. Correlation of residual leukocyte subsets with neutropenic fever during severe leukopenia after high-dose chemotherapy and autologous stem cell transplantation. Cytotherapy. 2006;8: 473–79. [DOI] [PubMed] [Google Scholar]
- 58. Chen Y, Huang XJ, Wang FR, Yan CH, Wang Y, Zhang YY, Han W, Chen H, Liu DH, Liu KY, Xu LP. [Etiological analysis of fever in the first 24 hours following allogeneic peripheral stem cell transfusion]. Zhonghua Nei Ke Za Zhi. 2012;51:179–83. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cll-10.1177_09636897211067804 for Evaluation of the Clinical Efficacy of Stem Cell Transplantation in the Treatment of Spinal Cord Injury: A Systematic Review and Meta-analysis by Qiao-Rui Tang, Hui Xue, Qiao Zhang, Ying Guo, Hao Xu, Ying Liu and Jia-Mei Liu in Cell Transplantation