Abstract
Activation of the transcription factor NF-κB is a major effector of the inducible resistance to death receptor-mediated apoptosis. Previous evidence indicates that the combined transcriptional activation of TRAF-1, TRAF-2, IAP-1, and IAP-2 is required to suppress cell death by tumor necrosis factor (TNF). Here we show that NF-κB activation upregulates the caspase 8 inhibitor FLIP, resulting in increased resistance to Fas ligand (FasL) or TNF. Restoration of either the full-length 55-kDa long form of FLIP or an alternatively spliced short form of FLIP in NF-κB null cells inhibits TNF- and FasL-induced cell death efficiently, whereas the expression of IAP or TRAF family members only partially rescues cells from death. Resistance to either FasL- or TNF-induced apoptosis is overcome when cells are incubated in the presence of the protein synthesis inhibitor cycloheximide. This treatment leads to the rapid downregulation of FLIP but not to that of TRAF2. Our findings suggest that FLIP is an important mediator of NF-κB-controlled antiapoptotic signals.
Members of the tumor necrosis factor (TNF) receptor family and their corresponding ligands are critical regulators of apoptosis and various other cellular processes. Some of the receptors (Fas, TNF-R1, TRAIL-R1, TRAIL-R2, TRAMP/DR3, DR6, and EDA-R) contain a cytoplasmic region, called the death domain (DD), which is essential for cell death signaling (18, 22). Signals emanating from Fas and TNF-R1 have been intensively studied (14). Upon receptor activation, the DD of Fas undergoes direct homotypic interaction with a DD in the adapter protein FADD, while FADD recruitment is indirect (via TRADD) in the case of TNF-R1 (4). The death effector domain (DED) at the amino terminus of FADD then recruits pro-caspase 8 via homotypic interaction with its two DEDs. The high local concentration of caspase 8 zymogens facilitates self-processing and assembly of the mature enzyme. Activated caspase 8 initiates apoptosis by subsequent cleavage of downstream caspases (caspase-3, -6, and -7).
Death induced by death receptors is tightly regulated by genes that are activated by the transcription factor NF-κB (25). Modulation of the response in favor of NF-κB protects cells from apoptosis; failure to do so results in increased cell death. At least six NF-κB-responsive genes are involved in this survival amplification loop (26), i.e., those that encode IAP-1 and IAP-2, which block caspase activity (7); that which encodes the Bcl-2 family member A1 (25); and those that encode TRAF-1, TRAF-2 (1), and A20 (21), which are themselves implicated in the NF-κB signaling pathway. However, overexpression of all of these genes affords, at best, partial protection, in particular from death triggered by TNF (25). The only known potent inhibitor of death receptor signals is c-FLIP. Two c-FLIPs have been characterized (23, 24). The full-length 55-kDa-long form of FLIP (FLIPL) exhibits overall structural homology to caspase 8, containing two DEDs that interact with FADD and an inactive caspase-like domain. An alternatively spliced short form of FLIP (FLIPS) contains only the two DEDs and displays reduced antiapoptotic capacity.
We undertook a series of experiments to investigate whether c-FLIP is implicated in the antiapoptotic NF-κB response. Here we provide evidence that FLIP expression is upregulated upon the stimulation of several signaling pathways that are known to trigger activation of the transcription factor NF-κB. Moreover, we found that cells that were rendered highly sensitive to death ligand-induced apoptosis by blocking NF-κB activation could be rescued by expressing FLIP. FLIP may therefore play a key role in the NF-κB-mediated control of death signals.
MATERIALS AND METHODS
Antibodies and materials.
Rabbit anti-TRAF2 polyclonal antibody C20, rabbit anti-cIAP1 polyclonal antibody H-85, and mouse anti-TRAF1 monoclonal antibody H3 were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Rat anti-cFLIP monoclonal antibody Dave II was from Alexis, Lausen Switzerland, and anti-Phospho-IκBa antibody was from Biolab. Anti-tubulin and anti-Flag (M2) antibodies were from Sigma (St Louis, Mo.). Anti-myc tag antibody (9E10) and anti-hemagglutinin (anti-HA) antibody were purchased from Babco (Berkeley, Calif.). Anti-mouse CD40 antibody (hybridoma FGK45) was a gift from Ton Rolink (Basel, Switzerland). Human recombinant ligands (TRAIL, Fas ligand [FasL], and TNF) were obtained from Alexis. Lipopolysaccharide (LPS), phorbol myristate acetate (PMA), ionomycin, and granulocyte-macrophage colony-stimulating factor were from Sigma.
Cell culture.
HeLa and HT1080 (human fibrosarcoma) cells, wild type (wt) and I-κB mutant (I-κBmut), were cultured in Dulbecco's modified Eagle's medium (Gibco BRL Life Technologies, Gaithersburg, Md.) supplemented with 10% fetal calf serum and penicillin and streptomycin (each at 50 μg/ml) and grown in 5% CO2 at 37°C. Mouse lymphoma EL4 and A20 cells and Jurkat cells were cultured as described above in RPMI 1640 medium. Dendritic cells were obtained from purified human CD34+ cells as described previously (2). NEMO-deficient cells were a kind gift of S. C. Sun (8).
Retrovirus production and cell transduction.
The retroviral vector pBabe-Puro and generation of viruses have been described previously (17). Human Flag-tagged FLIPL, HA-tagged FLIPS, Flag-tagged TRAF1, Flag-tagged TRAF2, and myc-tagged c-IAP1 were subcloned, respectively, from pcDNA3 plasmids (10, 11) into pBabe-Puro. HT1080 wt or I-κBmut cells (1.5 × 106) (10) were transduced for 16 h with viral supernatants containing Polybrene (8 μg/ml). Cells were washed once in phosphate-buffered saline, harvested, plated in complete medium containing puromycin (2.5 μg/ml), and incubated for 3 days before amplification and subsequent analysis of the multiclonal population.
Cell death and viability assays.
Jurkat cells were harvested, washed with RPMI medium, and plated at a density of 10 × 106/ml prior to stimulation with PMA at 20 ng/ml and 1 mM ionomycin. HT1080 fibrosarcoma cells or transfectants derived therefrom (1.5 × 104 per well) were seeded in 96-well microtiter plates in the presence of the indicated reagents for 16 h, and viability was determined by the methylene blue colorimetric assay (16). For HeLa cells, viability was determined by the PMS-MTS method in accordance with the manufacturer's (Promega, Madison, Wis.) instructions. In some experiments, the number of apoptotic cells was determined by Hoechst staining. To quantitate viability and apoptosis in experiments involving EL4 and A20 cells, phosphatidylserine exposure on apoptotic cells was measured as described previously (13). Briefly, cells (2 × 105) were washed in ice-cold HEPES buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, pH 7.4) supplemented with glucose at 1 mg/ml and 0.5% bovine serum albumin. Fluorescein isothiocyanate-labeled annexin V (Nexins Research, Kattendijke, The Netherlands) was added to a final concentration of 2.5 μg/ml. Cells were incubated for 20 min at 4°C and washed twice with HEPES buffer. Before analysis on a FACScan, propidium iodide (PI) was added (final concentration, 5 μg/ml) to the samples to discriminate necrotic cells (annexin V− PI+) from apoptotic cells (annexin V+ PI− and annexin V+ PI+).
Western blotting.
Cell lysates were prepared in NP-40 buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 10% glycerol, 0.2% NP-40) supplemented with a protease inhibitor cocktail stock solution (Roche Biochemicals, Basel, Switzerland). Cell debris was removed by centrifugation at 10,000 × g for 10 min, and the protein concentration was determined by the Bradford assay (Pierce, Rockford, Ill.). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes by electroblotting, and nonspecific binding sites were blocked by incubation in PBS containing 0.5% Tween 20 and 5% (wt/vol) dry milk. Immunoblot analyses were performed with the indicated antibodies. Bound primary antibodies were visualized with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG), goat anti-rat IgG, or goat anti-mouse IgG (Jackson Immunoresearch Laboratories, West Grove, Pa.) and ECL (Amersham, Freiburg, Germany).
RNase protection assay.
HT1080 wt or I-κBmut cells were treated with TNF for the times indicated in Results. Total RNA was isolated with the RNA INSTAPURE kit (Eurogentech, Seraing, Belgium) in accordance with the manufacturer's recommendations. The presence of transcripts of the indicated apoptosis-related genes, as well as the internal controls L32 and glyceraldehyde-3-phosphate dehydrogenase was analyzed by using the hApo3b, hApo5 Multi-Probe template sets (PharMingen, San Diego, Calif.). Probe synthesis, hybridization, and RNase treatment were performed with the RiboQuant Multi-Probe RNase Protection Assay System (PharMingen) in accordance with the manufacturer's recommendations. Finally, protected transcripts were resolved by electrophoresis on denaturing polyacrylamide gels (5%) and quantified on a PhosphorImager with the ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). To correct signals of protected transcripts of special interest for background intensity, the latter was determined for each individual lane in close proximity to the respective mRNA signal and subtracted from the value of the protected transcript.
RESULTS
The short-lived FLIP protein is induced in response to stimuli known to induce NF-κB activation.
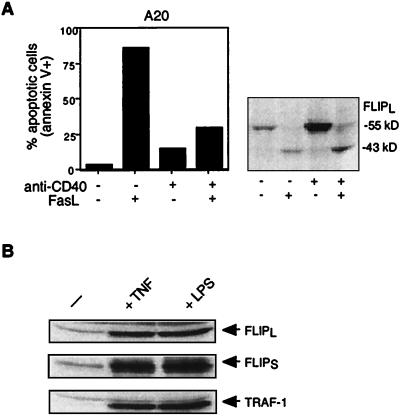
The murine B-cell line A20 is highly susceptible to Fas-mediated apoptosis but can be rescued by signaling through its surface B-cell receptor (6, 27). This effect has been attributed to the upregulation of Bcl-2 family members (6) and/or FLIP (27). The precise signaling pathways which result in the upregulation of the inhibitory proteins is, however, not known. As activation of the transcription factor NF-κB is frequently involved in the protection of cells from apoptosis, we considered the possibility that the gene for FLIP is also a target of the NF-κB transcription factor. Stimulation of the CD40 receptor is known to strongly activate NF-κB via recruitment of several TRAF proteins. Murine A20 B cells were therefore stimulated with agonistic antibodies to CD40 for 6 h before FasL addition. Whereas more than 80% of the cells were killed at a FasL concentration of 100 ng/ml, only 25% underwent apoptosis when cells had been prestimulated with anti-CD40 antibodies (Fig. 1A). Western blot analysis revealed that CD40-stimulated cells had a considerably increased concentration of FLIP, whereas caspase 8 levels remained unchanged (data not shown). Upon recruitment to death receptors, FLIPL is cleaved by caspase 8 between the large and small subunits in the caspase-like region, thereby inhibiting caspase 8 activity (11). In FasL-sensitive cells not prestimulated with TNF or CD40 and thus without increased FLIP levels, only processed FLIP (p43) was detectable after FasL addition, indicating that the entire cellular FLIP pool had been used up to form caspase 8–FLIP heterodimers, thus leaving some caspase 8 unprotected. As a consequence, caspase 8–caspase 8 homodimer formation was possible, resulting in caspase 8 autoactivation and cell death. By contrast, in CD40-prestimulated cells, a substantial amount of the increased concentration of precursor FLIP was still discernible after FasL treatment, indicating that the FLIP concentration was still sufficiently high to block the full processing of all of the caspase 8 molecules that were recruited to the Fas signaling complex (DISC).
FIG. 1.
Anti-CD40, TNF, or LPS stimulation upregulates FLIP and partly protects from FasL-mediated apoptosis. (A) A20 lymphoma cells were pretreated for 24 h with anti-CD40 at 10 μg/ml and treated with recombinant Flag-tagged FasL (33 ng/ml) in the presence of cross-linking anti-Flag antibodies at 1 μg/ml for 8 h. Apoptosis was quantified by annexin V staining, and FLIP protein content was determined by Western blotting with an anti-FLIP antibody. (B) Human dendritic cells were either nontreated (left lane) or incubated with TNF (40 ng/ml) or LPS (10 μg/ml) for 24 h and analyzed by immunoblot assay for the expression of FLIP and TRAF1.
We next investigated the capacity of two other stimuli which induce NF-κB activation to upregulate FLIP expression. Immature human monocyte-derived dendritic cells are resistant to FasL (3, 19), possibly due to the high FLIP levels already present even in nonmature cells (Fig. 1B). Dendritic cell maturation can be achieved by treating cells with agents that trigger NF-κB activation, such as LPS or TNF (5), and this is reflected by the strong increase in the NF-κB-responsive protein TRAF1 (Fig. 1B). We observed that not only the expression of FLIPL but also that of FLIPS was strongly increased in response to a 2-day treatment with LPS and TNF (Fig. 1B).
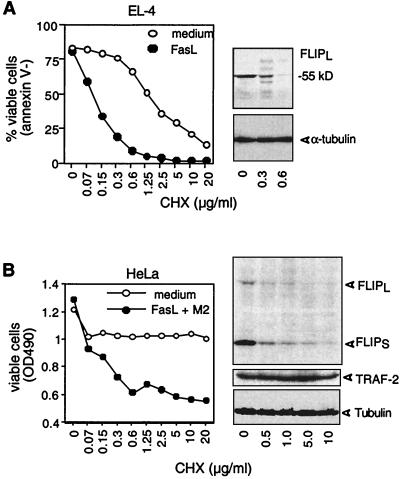
Resistant dendritic cells can be rendered sensitive to FasL by the protein synthesis inhibitor cycloheximide (CHX) (28). This was correlated with the disappearance of FLIPL. We confirmed these observation (data not shown). To explore whether FLIP was also short-lived in other cells, the murine T-cell lymphoma cell line EL-4 and the human adenocarcinoma line HeLa, which are both reasonably resistant to apoptosis when treated with FasL at 100 ng/ml, were incubated with increasing doses of CHX (Fig. 2A and B). In both cell lines, incubation with CHX at 0.5 μg/ml for 16 h sufficed to cause the disappearance of detectable levels of FLIPL protein, whereas levels of α-tubulin or of the antiapoptotic gene TRAF-2 remained unchanged. The same sensitivity to CHX was also observed for FLIPS, which, in contrast to EL-4 cells, is expressed in HeLa cells. As a consequence of the addition of the protein synthesis inhibitor, EL-4 and HeLa cells became sensitive to FasL (Fig. 2A and B) and underwent apoptosis at CHX concentrations that were identical to those required for FLIP downregulation. Thus, FLIP appears to be short-lived in several cell lines, requiring continuous biosynthesis to ensure levels that are required for protection against apoptosis.
FIG. 2.
FLIP downregulation by CHX enhances FasL-induced apoptosis in EL4 and HeLa cells. (A) EL-4 cells were treated with increasing concentrations of CHX in the presence or absence of cross-linked (+M2) FasL at 100 ng/ml for 16 h. Cell viability was subsequently determined by fluorescence-activated cell sorting using annexin V staining. FLIP and tubulin protein contents were determined by immunoblot assay. (B) HeLa cells were treated and analyzed as described above, except that viability was determined by the PMS-MTS method. In addition to FLIP and tubulin contents, TRAF2 protein content was analyzed by immunoblot assay. OD490, optical density at 490 nm.
The gene for FLIP is an NF-κB-responsive gene.
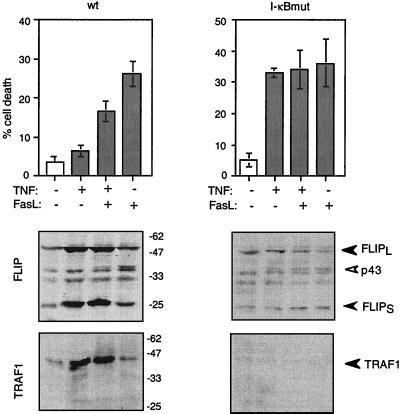
The above-described experiments suggested that FLIP is upregulated in response to signals that lead to NF-κB activation. To corroborate this notion, we took advantage of the HT1080 fibrosarcoma cell line, which expresses a modified form of the NF-κB inhibitor I-κBα that cannot be degraded due to mutated phosphorylation sites (10, 26). In this cell line (I-κBmut), translocation of NF-κB into the nucleus is highly impaired, and thus, synthesis of NF-κB-responsive genes does not occur (10, 26). As a result, the mutant cell line is highly susceptible to death signals such as TNF and FasL (10, 26). Both wt and mutant HT1080 cells were treated with small doses of TNF to stimulate NF-κB activation, which, as expected, led to a high level of expression of NF-κB-responsive TRAF-1 in the wt cells but not in the in the I-κBmut HT1080 cells (Fig. 3A). At the TNF concentration used (50 ng/ml), expression of TRAF-1 was detectable at 4 h and reached a maximum between 10 and 24 h, at a time where no cell death was observed in the I-κBmut cells, and thus the absence of TRAF-1 expression cannot be attributed to cell death (wt HT1080 cells). The low concentration of FLIP which is already present in HT1080 cells before stimulation was increased in wt but not mutant HT1080 cells, indicating that activation of NF-κB is required for FLIP induction (Fig. 3A). Interestingly, the kinetics of induction of the two forms of FLIP differed. While FLIPS was already detectable 4 h after the addition of TNF, induction of FLIPL was slower and peaked between 10 and 24 h, when the levels of FLIPS had already decreased. TNF also induced a considerable induction of FLIP mRNA levels (Fig. 3B), demonstrating that the increase in FLIP protein was, at least in part, due to increased NF-κB-mediated transcription of the gene for FLIP. While the well-described increase in the TRAF-1 and c-IAP-1 messages could be confirmed in our experimental setting, no substantial increase, however, in TRAF-2 and c-IAP-2 was observed. When the resistance to FasL of the HT1080 cells was subsequently analyzed, wt but not I-κBmut cells showed increased resistance to FasL upon TNF pretreatment (Fig. 4), correlating with the increased FLIP levels. Taken together, these results indicate that FLIP, similar to the antiapoptotic TRAF and IAP family members (26), is positively regulated by NF-κB following TNF addition.
FIG. 3.
NFκ-B activation leads to FLIP protein and mRNA upregulation. (A) The HT1080 fibrosarcoma cell line (wt or I-κBmut), stably transfected with a myc-tagged, mutated, nondegradable version of I-κBα, were treated for the indicated periods of time with TNF at 50 ng/ml and analyzed for FLIPL, FLIPS, and TRAF1 expression by immunoblot assay. (B) RNase protection assay analysis of mRNA levels of various apoptosis-related genes in wt or I-κBmut HT1080 cells after treatment with TNF as described above, using different hApo multiprobe template sets. L32 and glyceraldehyde-3-phosphate dehydrogenase (GADPH) were included in each template set as internal controls.
FIG. 4.
NF-κB-mediated upregulation of FLIP protects from Fas-induced apoptosis. HT1080 wt or I-κBmut cells were pretreated for 2 h with TNF at 50 ng/ml, washed with fresh medium, and subsequently incubated in the presence of cross-linked FasL at 100 ng/ml for 8 h. Apoptosis was quantified by Hoechst staining. FLIP and TRAF1 protein contents were analyzed by immunoblot assay. Compared to Fig. 2, a longer exposure time was chosen to reveal the cleavage of FLIPL into its p43 fragment upon treatment with FasL. The values beside the lower left-hand images are molecular sizes in kilodaltons.
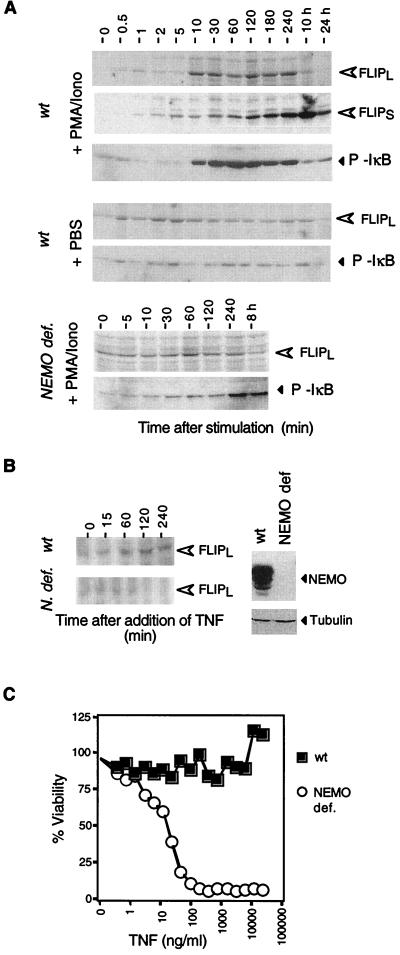
To demonstrate that the NF-κB dependence of FLIP expression is a more general phenomenon and not restricted to a single cell type, we investigated FLIP expression in the Jurkat T-cell line. Assembly of the IKK signalosome and successful signal transmission are dependent on NEMO/IKKγ, which acts as a scaffolding protein (15). We therefore compared FLIP induction by two known stimuli of NF-κB activation in Jurkat cells, i.e., PMA-ionomycin and TNF, in wt Jurkat cells and in Jurkat cells that are genetically deficient in NEMO (8). Upon addition of PMA-ionomycin, levels of FLIPL, which was barely detectable in unstimulated cells, increased 10 min after stimulation and peaked after 2 to 4 h (Fig. 5A). In contrast to HT1080 cells, FLIPS was induced after FLIPL in Jurkat cells. This sequence of events was previously observed in primary T cells (9), indicating that the control of expression of the two FLIP splice variants is cell type specific. When NF-κB was activated with TNF, FLIPL increased with similar kinetics (Fig. 5B). The absolute increase in FLIPL was smaller than in cells stimulated with PMA-ionomycin, which adequately reflects the fact that the latter stimulus is more potent. No or little FLIP protein induction was observed in NEMO-deficient cells not exposed to the stimulus (Fig. 5A and B). The absence of NF-κB activity in NEMO-deficient cells rendered Jurkat cells sensitive to TNF, while the parental cell line was completely resistant to TNF-mediated apoptosis (Fig. 5C).
FIG. 5.
NFκ-B activation leads to FLIP upregulation in Jurkat T cells. (A) Both wt and NEMO (N.)-deficient (def.) Jurkat cells were stimulated for the indicated periods of time with PMA and ionomycin (Iono) (or phosphate-buffered saline [control]) and analyzed for FLIPL, FLIPS, and phosphorylated IκB expression by immunoblot assay. (B) Cells were stimulated with TNF at 100 ng/ml, and FLIPL expression was monitored as in panel A. Expression of NEMO is demonstrated on the right. (C) Cell death induced by TNF in wt and NEMO-deficient cells, respectively.
FLIP protects NF-κB-incompetent cells against death receptor-induced apoptosis.
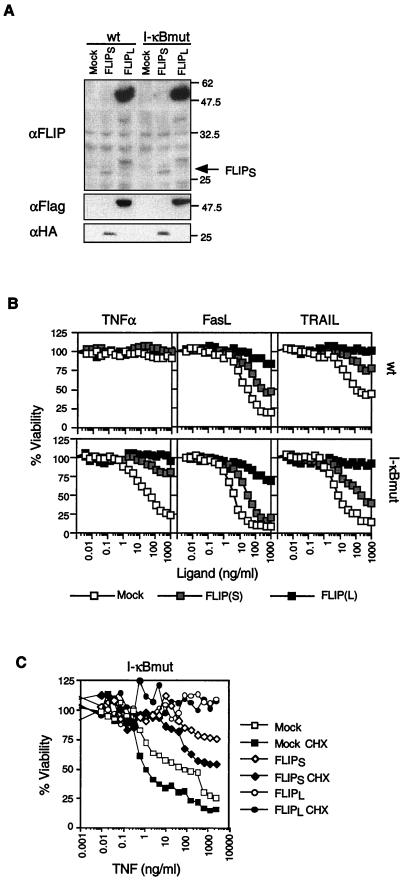
Previous results have suggested that the protective effect of NF-κB stimulation against death receptor (TNF)-induced apoptosis can be mimicked by simultaneously overexpressing the NF-κB-induced proteins TRAF-1, TRAF-2, c-IAP-1, and c-IAP-2 in I-κBmut HT1080 cells, leading to a blockade of caspase 8 activation (26). All of these proteins, however, were, at best, partially protective when overexpressed individually (26). Given that FLIP appears to be the most potent inhibitor of death receptor signaling pathways and that FLIP is induced by NF-κB (see above), we considered the possibility that FLIP plays an even more important role than the TRAF and IAP proteins in the NF-κB antiapoptotic response. If this is so, overexpression of FLIP alone in I-κBmut cells should have a strong protective effect against death receptor signals. In order to avoid the selection of apoptosis-resistant clones that frequently occurs when cells are cultured in drug-containing medium for a longer period of time, we chose to retrovirus infect cells and to analyze whole cell populations without further cloning efforts. FLIPL was highly expressed in several of the cell populations analyzed, and one example is shown in Fig. 6A. In contrast, FLIPS expression was always low, for reasons which are not understood. However, the low expression levels of FLIPS sufficed to protect the I-κBmut cells from the apoptotic effect of TNF (Fig. 6B) while cell death still occurred at high concentrations of FasL and TRAIL. Cells expressing high levels of FLIPL were completely protected not only from TNF-induced cell death but also from FasL- and TRAIL-induced cell death, in agreement with previous results (11). We can exclude the possibility that this protection was due to the reactivation of the NF-κB response in I-κBmut cells, as levels of I-κBmut remained unchanged after viral infection (data not shown). Exogenous FLIP is also short-lived, and treatment of the infected cell population with CHX led to a decrease in FLIP expression after 4 h (data not shown) and to increased sensitivity to TNF in mock-infected and FLIPS-infected cells (Fig. 6C). By contrast, the amount of FLIPL remaining during the CHX treatment (FLIPL is expressed at much higher levels than FLIPS; Fig. 6A) was still enough to afford complete protection from TNF-mediated cell death.
FIG. 6.
FLIP overexpression protects from TNF-, FasL-, and TRAIL-induced cell death. (A) HA-tagged FLIPS, Flag-tagged FLIPL, and empty vectors were introduced into HT1080 wt or I-κBmut cells by retrovirus infection. Cells were selected in puromycin for 3 days before analysis of protein content and function. FLIP expression was determined by immunoblot assay using an anti-FLIP antibody, anti-HA antibody, or anti-Flag antibody. The values on the right are molecular sizes in kilodaltons. (B) Infected pools of cells were treated with increasing concentrations of TNF, FasL, and TRAIL (control) in the presence of cross-linking antibody at 1 μg/ml (with the exception of TNF). Cell viability was determined 16 h after treatment. (C) Cell viability of the indicated cells pretreated or not pretreated with CHX (20 μg/ml for 1 h) after incubation (16 h) with increasing concentrations of TNF.
To confirm the previous findings by Baldwin's group (26) in the context of our experimental setting, we infected cells with viruses containing the genes for TRAF1, TRAF-2, and cIAP1 (Fig. 7). Despite reasonable expression levels of all of these proteins, they were incapable of fully protecting I-κBmut cells against TNF-mediated apoptosis, in agreement with the published results.
FIG. 7.
TRAF1, TRAF2, or cIAP1 overexpression fails to protect HT1080 cells from TNF-, FasL-, and TRAIL-induced cell death. (A) pBape-Flag-tagged TRAF1 and TRAF-2 vectors were introduced by virus infection into HT1080 wt or I-κBmut cells. TRAF1 and TRAF2 expression was determined by Western blot assay using anti-TRAF1, anti-Flag, or anti-TRAF2 antibodies. The sensitivity to TNF-mediated cell death of the cell pools obtained was determined essentially as described in the legend to Fig 5. (B) HT1080 wt or I-κBmut cells were infected with either pBape-myc-tagged cIAP1 or empty vector. Cellular pools were analyzed for resistance to TNF as described above. The values beside the blots are molecular sizes in kilodaltons.
DISCUSSION
In this report, we provide evidence that FLIP is one of several antiapoptotic genes that are under the control of the NF-κB transcription factor. It appears, however, that FLIP is more efficient than TRAF1, TRAF-2, cIAP-1, and c-IAP-2, which have been previously proposed to be responsible for the inhibition of death receptor-induced cell death (26). When expressed at equal levels, FLIPL was found to be by far the most potent inhibitor, and thus, FLIP may have a dominant role in the observed NF-κB-mediated resistance to death ligand-induced apoptosis. c-IAPs may be more important in mitochondrion-induced cell death, as they interact with caspase-9 and caspase-3 but not with caspase 8 (20). Moreover, the overexpression of TRAF1, TRAF-2, cIAP-1, and c-IAP-2 may result in their increased recruitment to signaling complexes, possibly resulting in an augmented NF-κB response. This, in turn, would upregulate FLIP levels and thus indirectly lead to the observed antiapoptotic response of the TRAF1, TRAF-2, cIAP-1, and c-IAP-2 proteins.
We observed that FLIPS and FLIPL are under the control of NF-κB, but interestingly, induction followed different kinetics. In HT1080 cells, FLIPS responded considerably more rapidly to TNF while FLIPS levels dropped at a time when FLIPL protein levels increased. During T-cell activation, the inverse order was observed (9). The reasons for this cell type-specific regulation are unknown.
We have previously shown that FLIPL, but not FLIPS, binds to TRAF2 and RIP, resulting in strong NF-κB activation upon stimulation of death receptor signaling pathways (12). Thus, it is conceivable that FLIP levels that are barely sufficient for protection against death receptors are augmented by an autoamplification loop. In fact, only small variations in FLIP protein levels may decide whether a cell is resistant or sensitive to death mediated by Fas or TNF. It appears that the recruitment of caspase 8–FLIP heterodimers is highly favored over the recruitment of caspase 8–caspase 8 homodimers, and thus, FLIP levels have to exceed caspase 8 levels to be protective. As caspase 8 levels are quite stable, with no or little variation in the presence of various stimuli, only a small increase in FLIP levels may decide whether a cell will respond by dying or living. It is therefore not surprising that FLIP levels are highly controlled. FLIP is an unstable protein and is rapidly degraded when neosynthesis is inhibited (conditions occurring during cell death). Preliminary results indicate that FLIP is ubiquitin modified under certain conditions, which may explain its short half-life. The ring finger domain of IAPs has been found to have ubiquitin E3 ligase activity, resulting in the transfer of ubiquitin to IAP and thus in its own degradation (29). Interestingly, the FLIP-binding protein TRAF2 also contains a ring finger domain, raising the possibility that the limited stability of FLIP is caused by its binding partner. We are currently testing this hypothesis.
REFERENCES
- 1.Arch R H, Gedrich R W, Thompson C B. Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- 2.Arrighi J F, Hauser C, Chapuis B, Zubler R H, Kindler V. Long-term culture of human CD34(+) progenitors with FLT3-ligand, thrombopoietin, and stem cell factor induces extensive amplification of a CD34(−) CD14(−) and a CD34(−) CD14(+) dendritic cell precursor. Blood. 1999;93:2244–2252. [PubMed] [Google Scholar]
- 3.Ashany D, Savir A, Bhardwaj N, Elkon K B. Dendritic cells are resistant to apoptosis through the Fas (CD95/APO-1) pathway. J Immunol. 1999;163:5303–5311. [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Bras A, Martinez A C, Baixeras E. B cell receptor cross-linking prevents Fas-induced cell death by inactivating the IL-1 beta-converting enzyme protease and regulating Bcl-2/Bcl-x expression. J Immunol. 1997;159:3168–3177. [PubMed] [Google Scholar]
- 7.Deveraux Q L, Roy N, Stennicke H R, Van Arsdale T, Zhou Q, Srinivasula S M, Alnemri E S, Salvesen G S, Reed J C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harhaj E W, Good L, Xiao G, Uhlik M, Cvijic M E, Rivera-Walsh I, Sun S C. Somatic mutagenesis studies of NF-kappa B signaling in human T cells: evidence for an essential role of IKK gamma in NF-kappa B activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene. 2000;19:1448–1456. doi: 10.1038/sj.onc.1203445. [DOI] [PubMed] [Google Scholar]
- 9.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer J L, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–502. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 10.Irmler M, Steiner V, Ruegg C, Wajant H, Tschopp J. Caspase-induced inactivation of the anti-apoptotic TRAF1 during Fas ligand-mediated apoptosis. FEBS Lett. 2000;468:129–133. doi: 10.1016/s0014-5793(00)01206-0. [DOI] [PubMed] [Google Scholar]
- 11.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schroter M, Burns K, Mattmann C, Rimoldi D, French L E, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka T, Budd R C, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, Tschopp J. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and erk signaling pathways. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 13.Koopman G, Reutelingsperger C P, Kuijten G A, Keehnen R M, Pals S T, van Oers M H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 14.Krammer P H. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Karin M. Signaling pathways leading to nuclear factor-kappa B activation. Methods Enzymol. 2000;319:273–279. doi: 10.1016/s0076-6879(00)19027-5. [DOI] [PubMed] [Google Scholar]
- 16.Micheau O, Solary E, Hammann A, Dimanche-Boitrel M T. Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem. 1999;274:7987–7992. doi: 10.1074/jbc.274.12.7987. [DOI] [PubMed] [Google Scholar]
- 17.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 19.Rescigno M, Piguet V, Valzasina B, Lens S, Zubler R, French L, Kindler V, Tschopp J, Ricciardi-Castagnoli P. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1beta, and the production of interferon gamma in the absence of IL-12 during DC-T cell cognate interaction. A new role for fas ligand in inflammatory responses. J Exp Med. 2000;192:1661–1668. doi: 10.1084/jem.192.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy N, Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarma V, Lin Z, Clark L, Rust B M, Tewari M, Noelle R J, Dixit V M. Activation of the B-cell surface receptor CD40 induces A20, a novel zinc finger protein that inhibits apoptosis. J Biol Chem. 1995;270:12343–12346. doi: 10.1074/jbc.270.21.12343. [DOI] [PubMed] [Google Scholar]
- 22.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter M E. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 23.Tschopp J, Irmler M, Thome M. Inhibition of Fas death signals by FLIPs. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 24.Wallach D. Apoptosis: placing death under control. Nature. 1997;388:123. doi: 10.1038/40516. [DOI] [PubMed] [Google Scholar]
- 25.Wang C-Y, Guttridge D C, Mayo M W, Baldwin A S., Jr NF-κB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C-Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Lobito A A, Shen F, Hornung F, Winoto A, Lenardo M J. Inhibition of Fas-mediated apoptosis by the B cell antigen receptor through c-FLIP. Eur J Immunol. 2000;30:155–163. doi: 10.1002/1521-4141(200001)30:1<155::AID-IMMU155>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Willems F, Amraoui Z, Vanderheyde N, Verhasselt V, Aksoy E, Scaffidi C, Peter M E, Krammer P H, Goldman M. Expression of c-FLIP(L) and resistance to CD95-mediated apoptosis of monocyte-derived dendritic cells: inhibition by bisindolylmaleimide. Blood. 2000;95:3478–3482. [PubMed] [Google Scholar]
- 29.Yang Y, Fang S, Jensen J P, Weissman A M, Ashwell J D. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]