Abstract
Objective
To prospectively assess the efficacy of GelrinC in the treatment of chondral and osteochondral femoral cartilage lesions using morphological (Magnetic Resonance Observation of Cartilage Repair Tissue [MOCART]) and quantitative (T2-mapping) magnetic resonance imaging (MRI).
Design
This study was designed as a prospective single-arm, open label, multicenter study. Morphological magnetic resonance imaging (MRI) for MOCART assessment and T2 mapping was performed 1 week and 6, 12, 18, and 24 months after GelrinC implantation. Evaluation of T2 mapping was based on the assessment of global T2 indices (T2 of the repair tissue [RT] divided by T2 of healthy reference cartilage) and zonal variation.
Results
Fifty-six (20 female) patients were prospectively enrolled. The mean MOCART score significantly increased from baseline to the 24-month follow-up with 88.8 (95% CI, 85.8-91.9; P < 0.001) for all lesions combined as well as 86.8 (95% CI, 83.0-90.6) for chondral lesions and 94.1 (95% CI, 68.55-100) for osteochondral lesions. Furthermore, based on T2 mapping, significant zonal variation of the RT was observed at 24 months (P = 0.039), which did not differ significantly from healthy reference cartilage (P = 0.6).
Conclusion
Increasing MOCART scores were observed throughout the follow-up period, indicative of maturation of the cartilage repair. Significant zonal variation of the RT at 24 months might indicate the transformation into hyaline cartilage–like RT. Slightly differing morphological outcome between chondral and osteochondral lesions, but similar global and zonal T2 indices at 24 months, support the potential of GelrinC as a treatment option for both lesion types.
Keywords: MOCART, T2-mapping, cartilage repair, chondral, osteochondral
Introduction
The treatment of focal femoral cartilage lesions remains clinically challenging. Surgical treatment aims to alleviate symptoms, enhance cartilage regeneration, and repair tissue integrity and preclude or delay the onset of premature osteoarthritis resulting from cartilage lesions. 1 Established cartilage repair techniques include bone marrow stimulating techniques such as microfracture (MFX)2,3 as well as cell-based therapies such as autologous chondrocyte implantation (ACI) 4 and matrix-associated chondrocyte implantation (MACI), 5 which differ in associated morbidity, costs, and outcome.6,7 Driven by the aim to reduce short- and long-term treatment failure rates, research efforts are continuing to develop novel treatment alternatives. However, so far, no technique has been shown to reproducibly and consistently lead to the regeneration of hyaline cartilage. 8
Newly available acellular scaffolds 9 have the advantage of requiring only a single surgical procedure and are made from biodegradable synthetic, natural, or hybrid polymers. These acellular scaffolds provide a matrix onto which mesenchymal stem cells (MSCs), originating from subchondral bone and surrounding cartilage, are thought to attach, differentiate, and develop into new functional tissue.
GelrinC (GelrinC, Regentis Biomaterials, Or Akiva, Israel) is a new biodegradable, acellular hydrogel implant designed for the treatment of focal cartilage lesions and to foster the consecutive formation of, ideally, hyaline cartilage. It consists of polyethylene glycol diacrylate (PEG-DA) and denatured human fibrinogen. GelrinC is applied in its liquid form to the defect immediately after MFX. Following a 90-second exposure to ultraviolet-A light, a cross-linked network is created and a soft, elastomeric implant is formed that closely follows the borders of the defect and thus has the ability to completely fill any defect geometry. Unlike the fibrin clot that is formed after conventional MFX, GelrinC creates a cell-impermeable barrier within the defect with a nonadhesive surface due to PEG. The surface properties of this PEG modified hydrogel have been recently shown to support cell-cell aggregation and in-turn chondrogenic differentiation of bone marrow mesenchymal stem cells. At the same time it minimizes hypertrophy, when compared with fibrin matrices. 10 In addition, it has been shown that adding fibrinogen to the PEG hydrogel enables the hydrogel to degrade via gradual surface erosion mechanisms rather than bulk degradation as observed for pure PEG controls. 11 In that context, tunable erosion mechanism of polymeric implants where tissue regeneration occurs in a manner inversely proportional to material erosion was suggested to be ideal for tissue regeneration. 12
Objective and reproducible tools are a prerequisite for the longitudinal monitoring and assessment of cartilage repair in study settings. The clinical outcome can be assessed using patient-reported outcome measures such as the International Knee Documentation Committee (IKDC) Score 13 or the overall Knee Injury and Osteoarthritis Outcome Score (KOOS). 14 These scores provide valuable insight into the patients’ function, pain, and symptoms, however, lack information specific to the local condition of the cartilage repair tissue.
Histology is still considered the aspired gold standard to assess repair tissue quality. However, due to its invasiveness, histology is rarely available in clinical studies. Magnetic resonance imaging (MRI) increasingly caters to this need and has been widely used for the noninvasive evaluation of cartilage repair in vivo after various techniques.15,16 Via assessment of the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score,17-19 morphological MR sequences allow for a semiquantitative assessment of the morphological appearance of the repair tissue and surrounding structures. For the ultrastructural and compositional assessment, a number of MR techniques are available. Sodium imaging20,21 and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC)22,23 have been shown to allow for the assessment of cartilage glycosaminoglycan (GAG) content. T2 mapping, on the other hand, has been shown to be sensitive to water content and the condition of the collagen network. 24 Whereas the GAG specific techniques are still restricted to specialized research centers, T2 mapping is widely available and ready to be used in a multicenter setting.
The purpose of this study was to prospectively assess the efficacy of GelrinC in the treatment of femoral cartilage lesions using morphological (MOCART) and quantitative (T2 mapping) MRI. Furthermore, potential differences between patients with chondral and osteochondral lesions, as well as longitudinal changes regarding the MOCART score and T2-mapping should be evaluated.
Methods
Study Design
This study was a pilot interventional, single-arm, open label, multicenter study conducted in Europe and Israel. It was carried out in accordance with the ethical standards of the institutional review boards of all participating medical centers as well as with the Declaration of Helsinki, including current revisions. Positive ethics votes were obtained from all responsible institutional review boards. Written informed consent was obtained from all patients. An interim analysis was already published by Trattnig et al. 25
Patients
Eligible patients had to be 18 to 65 years of age and had to have 1 or 2 symptomatic femoral cartilage lesions rated as International Cartilage Repair Society (ICRS) III or IVa with less than 6-mm deep affection of the subchondral bone, individually 1 to 6 cm2 in size after arthroscopic debridement. In addition, willingness to follow a standardized rehabilitation protocol was demanded. The following were defined as exclusion criteria: age <18 or >65 years, a body mass index (BMI) >32 kg/m2, diffuse degenerative joint disease, osteoarthritis or avascular necrosis, untreatable posterior lesions, a lesion size <1 cm2 or >6 cm2, cartilage lesions rated as ICRS grade larger than grade II on a surface that directly opposed the defect, patellar or trochlear cartilage lesions, prior total or subtotal meniscectomy, meniscus repair, MFX or prior tendon repair in the past 12 months, ligament repair or realignment surgery in the past 6 months, and contraindications to perform an MRI examination.
Surgical Technique
GelrinC implantation was performed as a 1-step procedure. After arthroscopic debridement of the cartilage defect and complete removal of the calcified layer, MFX was performed. Then the GelrinC procedure was performed through a mini-arthrotomy. The liquid GelrinC was applied into the debrided defect area using a standard syringe, with a proprietary accessory kit to aid in sealing the lesion during application and curing of GelrinC. Once the lesion was completely filled, the hydrogel was exposed to ultraviolet-A light (100 mW/cm2) for 90 seconds creating a soft, elastomeric implant, occupying the entire volume of the defect. After removing the GelrinC accessory kit and ensuring the integrity and retention of the implant, the incision was closed using standard techniques.
Following the surgical procedure, all patients followed a standardized rehabilitation protocol.
Magnetic Resonance Imaging
Sixteen imaging sites located in Israel and Europe carried out all MR examinations on 1.5 and 3 T MRI scanners from three vendors (Siemens Healthineers, GE Healthcare and Philips). All scanners used a standardized imaging protocol that was set up specifically for this study. Furthermore, dedicated knee coils (mostly 8-channel phased array coils) were used at all imaging sites. Patients were examined repeatedly using MRI at set follow-up intervals at baseline (1 week after surgery) and 6, 12, 18, and 24 months after surgery.
The morphological imaging protocol was designed to allow for the assessment of the semiquantitative MOCART scoring and consisted of a sagittal proton density (PD) fast spin echo (FSE) sequence, a sagittal dual PD and T2 FSE sequence, a coronal PD FSE sequence with fat suppression (fs), a sagittal T1 SE sequence and a three-dimensional gradient echo sequence (GRE; not available at all sites) at both 1.5 and 3 T systems.
T2-mapping was restricted to examinations on 3 T scanners and was acquired using a sagittal multi-echo spin-echo (MESE) sequence with an echo train length (ETL) consisting of 8 echoes, ranging from 12.5 to 87.5 ms and a repetition time (TR) of 2640 ms without fat suppression. In a total acquisition time (TA) of 5 minutes and 10 seconds, 15 slices with a slice thickness of 3 mm and a field of view (FOV) of 160 × 160 mm at a matrix size of 256 × 225 pixels were acquired. T2 maps were calculated using a pixel-wise, mono-exponential, nonnegative least-squares (NNLS) fit analysis (IDL 6.3, Interactive Data Language, RSI, Inc., Boulder, CO, USA). T2 mapping was restricted to the following time points: baseline, 12, 18, and 24 months after surgery.
The imaging parameters of the basic MRI protocol are displayed in Table 1 ; however, these had to be optimized and therefore slightly adapted for each scanner-coil combination.
Table 1.
Imaging Parameters at 1.5 and 3 Tesla.
| Orientation | Sequence | TR (ms) | TE (ms) | Fat Saturation | No. of Slices | Slice Thickness (mm) | FOV (mm) | Matrix | Phase Resolution (%) | Scan Time (min:s) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 Tesla | ||||||||||
| sag | PDw FSE | 2000 | 27 | No | 19 | 2 | 120 | 320 | 90 | 4:24 |
| cor | PDw FSE fs | 3430 | 31 | Yes | 25 | 3 | 160 | 384 | 100 | 4:36 |
| sag | dual PD + T2w FSE | 3480 | 13 + 94 | No | 19 | 2 | 160 | 384 | 90 | 4:16 |
| sag | T1w SE | 600 | 13 | No | 19 | 2 | 160 | 384 | 100 | 4:09 |
| 3 Tesla | ||||||||||
| sag | PDw FSE | 2000 | 37 | No | 19 | 2 | 120 | 384 | 85 | 3:20 |
| cor | PDw FSE fs | 2970 | 27 | Yes | 25 | 3 | 160 | 448 | 80 | 3:29 |
| sag | dual PD + T2w FSE | 3050 | 11 + 80 | No | 19 | 2 | 160 | 448 | 80 | 3:17 |
| sag | T1w SE | 680 | 12 | No | 19 | 2 | 160 | 384 | 100 | 2:50 |
| sag | T2 map | 2640 | 12.5-87.5 | No | 15 | 3 | 160 | 256 | 88 | 5:10 |
PDw FSE = proton-density-weighted fast spin echo sequence; fs = fat saturated; T2w = T2-weighted; T1w SE = T1-weighted spin-echo sequence; TR = repetition time; TE = echo time, FoV = field of view; sag = sagittal; cor = coronal.
MRI Evaluation
For morphological evaluation, the semiquantitative MOCART scoring was used.17,18 Based on the assessment of 9 different variables, a total score ranging from 0 to 100 points may be obtained with 0 being the worst and 100 points being the best possible outcome. The MOCART score was assessed at baseline (1 week), 6, 12, 18, and 24 months after surgery by 2 senior musculoskeletal radiologists, with 24 and 6 years of dedicated experience in musculoskeletal MRI. In some patients, the variable “signal intensity” was only assessable in 1 of the 2 demanded sequences due to a lack of GRE images. In these patients, the maximum score was 85 points. For statistical evaluation purposes, this was corrected by multiplying the score of these patients with a correction factor (1.176) thus reaching a total of 99.96 (~100) points. 25 Good interobserver variability has been previously reported for the MOCART score. 19 In this present study, rating disagreements between readers were discussed and a consensus reading was reached. Both readers were blinded to lesion location and clinical history.
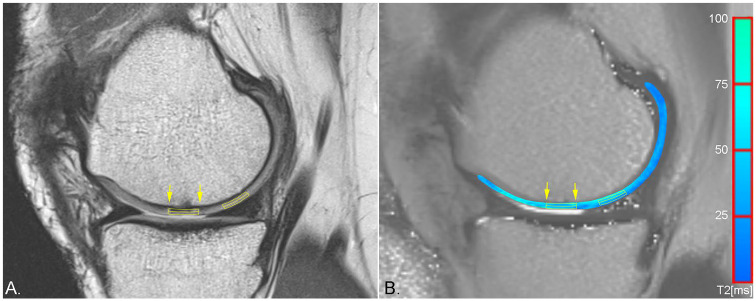
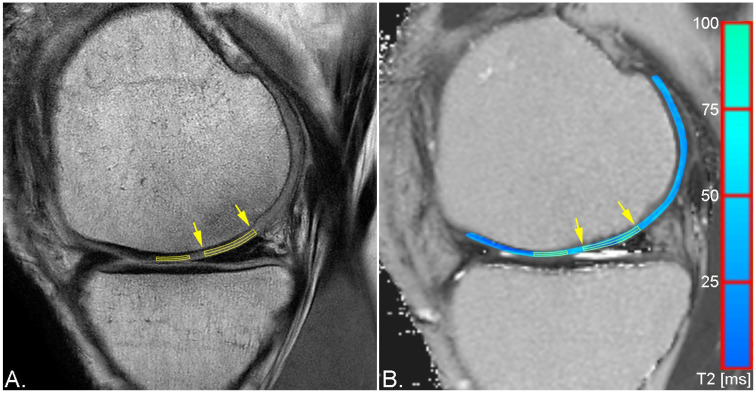
T2 mapping at baseline, 12, 18, and 24 months after surgery, was evaluated via manual region-of-interest (ROI) analysis by a single reader with 24 years of experience in musculoskeletal MRI. In addition to the repair tissue, an area of morphologically intact healthy cartilage was selected as reference for each patient on the same femoral condyle as the lesion and evaluated identically as the respective repair tissue. ROIs were placed on 1 to 4 consecutive slices, depending on the size of the repair tissue. In cases that allowed for placement of ROIs on multiple slices, the T2 values were averaged. For each lesion and healthy reference cartilage, three types of ROIs were placed: a full-thickness ROI covering the entire defect, a superficial and a deep layer ROI, subdividing the full-thickness ROI in two equally thick ROIs. Exemplary ROI placement for a chondral and an osteochondral lesion can be appreciated in Figures 1 and 2 . To diminish the variability arising from different combinations of MR systems and coils that were used for image acquisition due to the multicenter setting of this study, global and zonal T2 indices were calculated and used for statistical assessment rather than the absolute relaxation times, which are given in Table 2 . The global T2 index was calculated by dividing the mean T2 of the repair tissue by the mean T2 of healthy reference cartilage. This was performed separately for the full-thickness, the deep, and the superficial ROI.
Figure 1.
Sagittal proton density weighted (PDw) fast spin echo (FSE) sequence (A) and pseudo-color T2 map overlaid on a grayscale T2 map (B) of the same patient as in Figure 3 demonstrating region of interest (ROI) placement for zonal evaluation of repair tissue of a smaller chondral lesion (yellow arrows) and healthy reference cartilage.
Figure 2.
Sagittal proton density weighted (PDw) fast spin echo (FSE) sequence (A) and pseudo-color T2 map overlaid on a grayscale T2 map (B) of the same patient as in Figure 4 demonstrating region of interest (ROI) placement for zonal evaluation of repair tissue of a larger osteochondral lesion (yellow arrows) and healthy reference cartilage.
Table 2.
T2 Relaxation Times and 95% Confidence Intervals in Milliseconds (ms) of the Global Repair Tissue (RT), Superficial RT, Deep RT, Global Reference, Superficial Reference, and Deep Reference for Osteochondral Lesions, Chondral Lesions, and All Lesions at Baseline, 12, 18, and 24 Months After Surgery.
| Visit | n | T2 Relaxation Times (ms) and 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|
| RT Global | RT Superficial | RT Deep | Reference Global | Reference Superficial | Reference Deep | |||
| Osteochondral lesions | Baseline | 2 | 119.1 (–957.4, 1195.6) | 139.95 (–1069, 1348.8) | 114.47 (–884.2, 1113.1) | 63.83 (–214.1, 341.8) | 68.59 (–136.1, 273.3) | 85.88 (–442.1, 613.9) |
| 12 Months | 6 | 52.40 (46.6, 58.2) | 53.99 (46.98, 60.99) | 80.88 (46.2, 55.5) | 47.95 (38.4, 57.5) | 48.25 (40.6, 55.9) | 45.81 (27.2, 64.4) | |
| 18 Months | 6 | 43.28 (29.8, 56.8) | 42.22 (30.1, 54.4) | 47.01 (34.7, 60.1) | 44.87 (35.1, 54.6) | 47.27 (37.4, 57.1) | 44.62 (33.3, 55.9) | |
| 24 Months | 7 | 58.48 (28.1, 88.9) | 58.72 (29.5, 88.0) | 60.06 (28.0, 92.1) | 56.80 (33.1, 80.4) | 59.54 (32.8, 86.3) | 53.19 (31.3, 75.1) | |
| Chondral lesions | Baseline | 11 | 109.01 (73.4, 144.6) | 104.98 (73.3, 136.6) | 116.27 (71.6, 160.9) | 50.98 (43.9, 58.0) | 55.66 (45.4, 66.0) | 47.42 (38.7, 56.2) |
| 12 Months | 15 | 54.94 (50.3, 59.6) | 60.45 (54.4, 66.5) | 52.58 (46.4, 58.8) | 48.78 (43.6, 53.9) | 51.04 (44.3, 57.7) | 46.41 (40.8, 52.0) | |
| 18 Months | 16 | 51.14 (45.8, 56.5) | 55.61 (47.3, 63.9) | 49.95 (42.0, 57.9) | 49.92 (43.7, 56.1) | 50.90 (44.3, 57.5) | 48.1 (40.8, 55.5) | |
| 24 Months | 18 | 53.78 (43.6, 63.9) | 56.64 (45.1, 68.1) | 51.39 (42.3, 60.5) | 51.11 (44.1, 58.3) | 52.93 (45.2, 60.7) | 48.98 (42.0, 56.0) | |
| All lesions | Baseline | 13 | 110.56 (74.5, 146.6) | 110.36 (74.5, 146.3) | 116.00 (74.5, 157.5) | 52.95 (44.5, 61.4) | 57.65 (47.86, 67.4) | 53.34 (38.1, 68.6) |
| 12 Months | 21 | 54.22 (50.7, 57.7) | 58.6 (54.0, 63.2) | 52.10 (47.7, 56.5) | 48.55 (44.4, 52.6) | 50.2 (45.3, 55.2) | 46.24 (40.7, 51.8) | |
| 18 Months | 22 | 49.0 (44.1, 53.9) | 51.96 (45.0, 58.9) | 49.25 (43.1, 55.5) | 48.54 (43.6, 53.4) | 49.91 (44.8, 55.0) | 47.17 (41.4, 52.9) | |
| 24 Months | 25 | 55.10 (45.2, 64.9) | 57.22 (46.9, 67.6) | 53.81 (44.1, 63.5) | 52.77 (45.4, 60.1) | 54.78 (46.6, 62.9) | 50.16 (43.2, 57.1) | |
To assess zonal variation, the mean T2 of the deep ROI of the repair tissue was divided by the mean T2 of the superficial ROI. This was done for healthy reference cartilage accordingly. To facilitate easy assessment, whether zonal variation is similar between repair tissue and healthy reference cartilage, the zonal T2 index was calculated according to
This dimensionless coefficient equals 1, if zonal variation in repair tissue and healthy reference cartilage are identical.
Statistical Analysis
All statistical evaluations were performed using IBM SPSS Statistics for Windows version 22.0.0.2 (IBM, Armonk, NY, USA). Metric data are described using mean ± standard deviation and 95% confidence intervals. Normal distribution was checked using Kolmogorov-Smirnov test. MOCART scoring and T2 indices between osteochondral and chondral lesions were compared using unpaired Student t tests. Changes over time between 6- and 24-month follow-up were evaluated using paired Student t tests.
A P value equal to or less than 0.05 was considered to indicate significant results.
Results
Patient Cohort
Of 88 screened patients, 56 patients (20 female) with a mean age of 38 ± 10 years and an average lesion size of 2.42 ± 1.08 cm2 were prospectively enrolled in the study from 2009 to 2014. In 4 of these 56 patients the GelrinC procedure was not completed. The remaining 52 patients were recruited by 15 institutions (4 institutions recruited 1 patient each, 4 institutions recruited 2 patients each, 2 institutions recruited 3 patients each, 3 institutions recruited 4 patients each, 2 institutions recruited 6 patients each, and 1 institution recruited 10 patients). Two patients were considered major protocol violations, 4 patients withdrew consent to continue with the follow-up examinations, and 4 patients were referred to an alternative procedure before the 24-month follow-up visit. Three patients could not be included into the MOCART and T2 mapping evaluation at one or more follow-up visits due to insufficient defect filling. Hence, MRI evaluations at 24 months were available for 39 patients. Twenty-eight of these patients had been treated for a chondral lesion ( Figure 3 ) and 11 patients for an osteochondral cartilage lesion ( Figure 4 ).
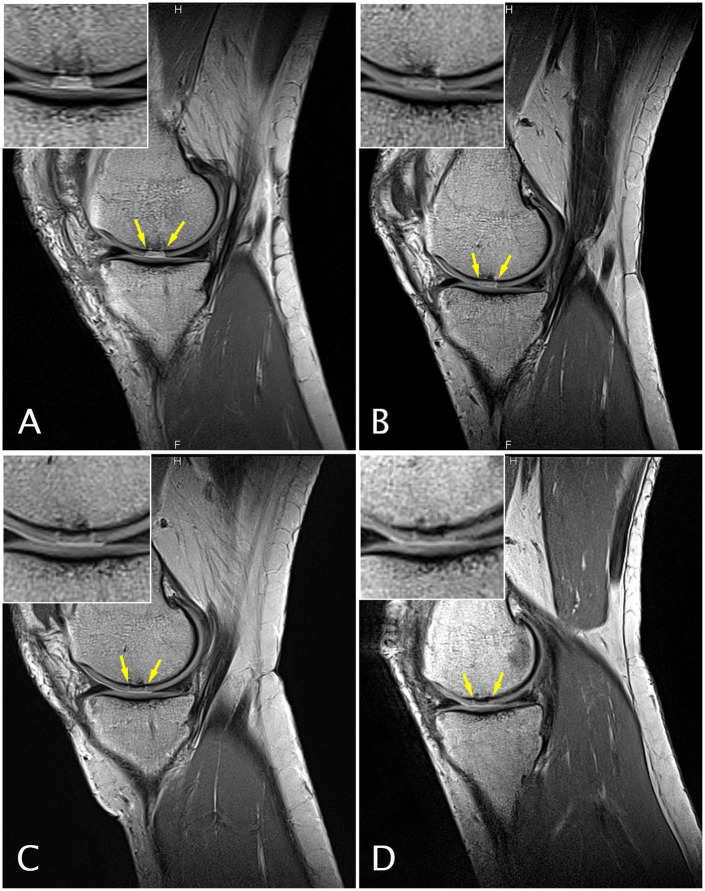
Figure 3.
Sagittal magnetic resonance (MR) images obtained with a proton density weighted (PDw) fast spin echo (FSE) sequence of a 51-year-old female patient at study entry with a chondral defect of 1 cm2 on the medial femoral condyle who underwent cartilage repair surgery with GelrinC. MR imaging was performed at baseline (A), 6 (B), 12 (C), and 24 (D) months after surgery.
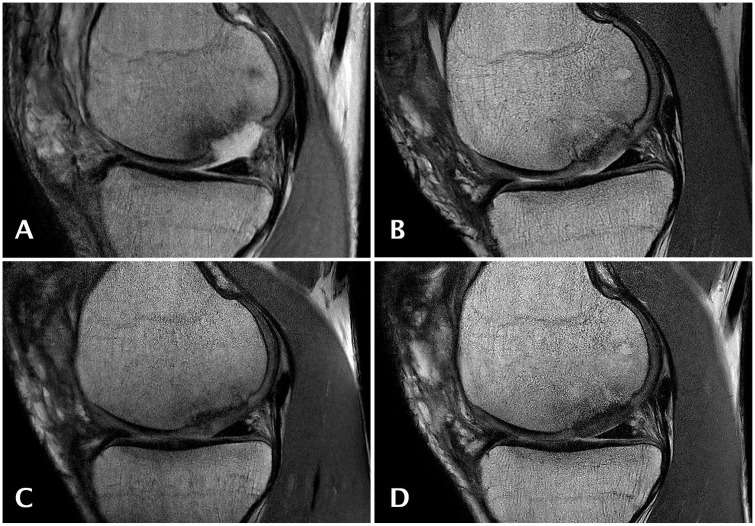
Figure 4.
Sagittal magnetic resonance (MR) images obtained with a proton density weighted (PDw) fast spin echo (FSE) sequence of an 18-year-old male patient at study entry with an osteochondral lesion of 2.8 cm2 on the medial femoral condyle who underwent cartilage repair surgery using GelrinC. MR imaging was performed at baseline (A), 6 (B), 12 (C), and 24 (D) months after surgery.
Morphological Outcome of Chondral and Osteochondral Lesions (MOCART Score)
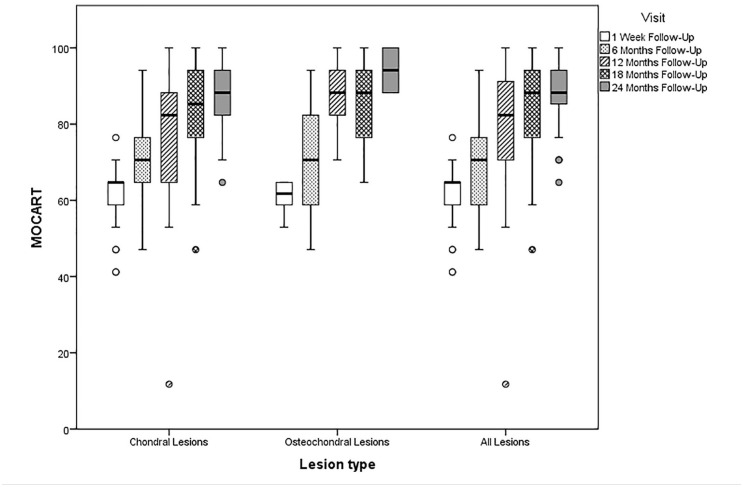
The mean overall MOCART score for all patients was 60.6 (95% CI, 58.7-62.6), 71.5 (95% CI, 67.6-75.4), 79.0 (95% CI, 73.9-84.1), 83.7 (95% CI, 79.2-88.1), and 88.8 (95% CI, 85.8-91.9) at baseline, 6, 12, 18, and 24 months respectively ( Figure 5 ). The mean overall MOCART increased significantly from baseline to the 6-month follow-up (P < 0.0001) as well as from the 6-month follow-up to the 24-month follow-up (P < 0.0001) as shown in Table 3 . At the 24-month follow-up, the average MOCART score of osteochondral lesions 94.1 (95% CI, 68.55-100) was significantly higher (P = 0.026) than that of chondral lesions with 86.8 (95% CI, 83.0-90.6). The variable “signal intensity dual T2 FSE,” which differs from adjacent healthy cartilage when the repair tissue is being fibrous or edematous, increased from a mean of 7.44 points (95% CI, 5.92-8.97) at 6 months postoperatively to 13.72 points (95% CI, 12.73-14.83) of a maximum of 15 points the at 24-month follow-up (P < 0.0001).
Figure 5.
Box plot showing the mean Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score for chondral, osteochondral and all lesion types baseline, 6-, 12-, 18-, and 24-month follow-up.
Table 3.
MOCART Variables and Overall MOCART Scoring for All Lesions, Osteochondral Lesions, and Chondral Lesions, Respectively, Over Time. a
| Visit | n | Filling of the Defect | Integration | Surface | Structure | Signal Intensity (Dual T2-FSE) | Signal Intensity (3D-GRE) | Subchondral Lamina | Subchondral Bone | Adhesion | Effusion | MOCART Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Osteochondral lesions | Baseline | 12 | 17.5 (15.0-20.0) | 15 | 9.6 (8.7-10.5) | 3.3 (1.8-4.9) | 0 | 9.1 (8.7-9.6) | 1.3 (0.2-2.7) | 0 | 5 | 0 | 60.8 (57.9-63.7) |
| 6-Month FU | 12 | 16.3 (13.5-19.0) | 13.8 (12.3-15.2) | 8.8 (7.3-10.2) | 2.5 (0.8-4.2) | 8.3 (5.2-11.5) | 10.7 (9.3-12.0) | 2.1 (0.5-3.7) | 0.8 (0.4-2.1) | 5 | 2.9 (1.3-4.6) | 71.1 (62.0-80.2) | |
| 12-Month FU | 12 | 17.1 (15.0-19.2) | 15 | 9.6 (8.7-10.5) | 3.8 (2.3-5.2) | 13.3 (10.9-15.8) | 13.0 (12.1-13.9) | 3.8 (2.3-5.2) | 2.1 (0.5-3.7) | 5 | 4.2 (2.9-5.4) | 86.8 (80.6-93.0) | |
| 18-Month FU | 8 | 19.4 (17.9-20.9) | 15 | 7.5 (5.3-9.7) | 3.8 (1.8-5.7) | 11.9 (6.9-16.8) | 12.8 (11.3-14.3) | 4.4 (2.9-5.9) | 2.5 (0.3-4.7) | 5 | 3.1 (1.0-5.3) | 85.3 (75.1-95.5) | |
| 24-Month FU | 11 | 19.1 (17.7-20.5) | 14.6 (13.5-15.6) | 9.6 (8.5-10.6) | 5 | 15 | 14.1 (13.5-14.7) | 5 | 3.2 (1.5-4.9) | 5 | 3.6 (2.1-5.2) | 94.1 (90.2-98.1) | |
| Chondral lesions | Baseline | 40 | 17.25 (15.8-18.7) | 14.75 (14.4-15.1) | 9.0 (8.4-9.7) | 3.2 (2.3-3.9) | 1.0 (0.4-1.7) | 9.1 (8.7-9.5) | 1.3 (0.6-2.0) | 0 | 5 | 0.1 (0.1-0.4) | 60.6 (58.2-63.0 |
| 6-Month FU | 34 | 16.62 (14.9-18.3) | 14.6 (14.1-15.1) | 8.7 (7.9-9.5) | 3.2 (2.4-4.1) | 7.4 (5.5-9.2) | 10.7 (10.1-11.4) | 2.4 (1.5-3.2) | 0.9 (0.2-1.6) | 5 | 2.2 (1.3-3.1) | 71.6 (67.2-76.1) | |
| 12-Month FU | 32 | 15.9 (14.0-17.9) | 14.8 (14.5-15.2) | 8.9 (8.1-9.7) | 3.6 (2.7-4.4 | 8.6 (6.6-10.6) | 11.4 (10.5-12.4) | 4.2 (3.6-4.9) | 1.7 (0.9-2.6) | 5 | 3.0 (2.1-3.9) | 76.1 (69.6-82.6) | |
| 18-Month FU | 28 | 16.1 (14.1-18.1) | 14.8 (14.5-15.2) | 8.8 (7.9-9.6) | 3.4 (2.5-4.3) | 13.2 (11.7-14.7) | 12.5 (11.7-13.3) | 3.9 (3.1-4.7) | 2.3 (1.3-3.3) | 5 | 3.2 (2.3-4.2) | 83.2 (78.0-88.4) | |
| 24-Month FU | 28 | 17.7 (16.2-19.1) | 13.9 (12.8-15.0) | 9.6 (9.1-10.2) | 4.1 (3.4-4.9) | 13.2 (11.7-14.7) | 13.0 (12.4-13.6) | 4.3 (3.6-5.0) | 1.6 (0.7-2.5) | 5 | 4.3 (3.6-5.0) | 86.8 (83.0-90.6) | |
| All lesions | Baseline | 52 | 17.3 (16.1-18.5) | 14.8 (14.5-15.1) | 9.1 (8.6-9.7) | 3.2 (2.5-3.9) | 0.8 (0.3-1.3) | 9.1 (8.8-9.4) | 1.3 (0.6-1.9) | 0 | 5 | 0.1 (0.1-0.3) | 60.6 (58.7-62.6) |
| 6-Month FU | 46 | 16.5 (15.1-17.9) | 14.4 (13.8-14.9) | 8.7 (8.0-9.4) | 3.0 (2.3-3.8) | 7.6 (6.1-9.1) | 10.7 (10.1-11.3 | 2.9 (1.5-3.0) | 0.9 (0.3-1.4) | 5 | 2.4 (1.6-3.1) | 71.5 (67.6-75.4) | |
| 12-Month FU | 44 | 16.3 (14.8-17.7) | 14.9 (14.7-15.1) | 9.1 (8.5-8.7) | 3.6 (2.9-4.3) | 9.9 (8.2-11.6) | 11.9 (11.1-12.6) | 4.1 (3.5-4.7) | 1.8 (1.1-2.6) | 5 | 3.3 (2.6-4.0) | 79.0 (73.9-84.1) | |
| 18-Month FU | 36 | 16.8 (15.2-18.4) | 14.9 (14.6-15.1) | 8.5 (7.7-9.3) | 3.5 (2.7-4.3) | 12.9 (11.4-14.4) | 12.6 (11.9-13.2) | 4.0 (3.4-4.7) | 2.4 (1.5-3.2) | 5 | 3.2 (2.4-4.0) | 83.7 (79.2-88.1) | |
| 24-Month FU | 39 | 18.1 (17.0-19.2) | 14.1 (13.3-14.9) | 9.6 (9.2-10.1) | 4.4 (3.8-4.9) | 13.7 (12.6-14.8) | 13.3 (12.9-13.8) | 4.5 (4.0-5.0) | 2.1 (1.2-2.9) | 5 | 4.1 (3.5-4.7) | 88.8 (85.8-91.9) |
MOCART = Magnetic Resonance Observation of Cartilage Repair Tissue; T2 FSE = T2-weighted fast spin echo sequence; 3D-GRE = three-dimensional gradient echo sequence; FU = follow-up.
Values are given as means with 95% confidence intervals in parentheses.
Quantitative MRI (T2 Mapping)
Due to the restriction to 3 T scanners, T2 mapping was available only for 25 patients at the 24-month follow-up. The mean global T2 index for the full-thickness ROI was 2.36 (95% CI, 1.8-3.0), 1.15 (95% CI, 1.1-1.3), 1.04 (95% CI, 0.9-1.2), and 1.05 (95% CI, 0.95-1.15) at baseline, 12, 18, and 24 months, respectively ( Table 4 ). More specifically, it ranged between 0.8 and 1.2 in 7.1% (1 of 14 patients), 71.4% (15 of 21 patients), 72.7% (16 of 22 patients), and 62.5% (15 of 24 patients) at baseline, 12, 18, and 24 months, respectively ( Figure 6 ). At 24 months, chondral and osteochondral lesions showed similar mean global T2 indices for the full-thickness ROI, with 1.056 (95% CI, 0.9-1.2) and 1.028 (95% CI, 0.8-1.2) (P = 0.80), respectively.
Table 4.
Global and Zonal T2 Indices as well as 95% Confidence Intervals for Osteochondral Lesions, Chondral Lesions, and All Lesions at Baseline, 12, 18, and 24 Months after Surgery.
| Visit | n | Global T2 Indices | Zonal T2 Indices | |||||
|---|---|---|---|---|---|---|---|---|
| Deep Global T2 Index = T2 Deep RT / T2 Deep Reference | Superficial Global T2 Index = T2 Superficial RT / T2 Superficial Reference | Full-Thickness Global T2 Index = T2 Global RT / T2 Global Reference | Zonal T2 RT = T2 Deep RT / T2 Superficial RT | Zonal T2 Reference = T2 Deep Reference / T2 Superficial Reference | Zonal T2 Index = Zonal T2 RT / Zonal T2 Reference | |||
| Osteochondral lesions | Baseline | 2 | 3.97 (-1.1, 9.0) | 3.28 (-12, 18.5) | 3.68 (-11.4, 18.7) | 1.16 (-3.2, 5.5) | 0.82 (0.5, 1.1) | 1.43 (-4.3, 7.2) |
| 12-Month FU | 6 | 1.27 (0.8, 1.8) | 1.13 (1.0, 1.3) | 1.12 (0.9, 1.3) | 0.95 (0.9, 1.0) | 0.95 (0.6, 1.3) | 1.13 (0.59, 1.7) | |
| 18-Month FU | 6 | 1.04 (0.9, 1.2) | 0.88 (0.7, 1.0) | 0.96 (0.8, 1.1) | 1.14 (0.9, 1.4) | 0.98 (0.68, 1.3) | 1.21 (0.93, 1.5) | |
| 24-Month FU | 7 | 1.11 (0.9, 1.3) | 1.01 (0.8, 1.2) | 1.03 (0.8, 1.2) | 1.0 (0.9, 1.1) | 0.92 (0.8, 1.1) | 1.11 (0.9, 1.3) | |
| Chondral lesions | Baseline | 12 | 2.47 (1.9, 3.1) | 1.89 (1.5, 2.3) | 2.14 (1.6, 2.6) | 1.10 (0.9, 1.3) | 0.84 (0.7, 0.9) | 1.37 (1.0, 1.7) |
| 12-Month FU | 15 | 1.18 (1.0, 1.4) | 1.23 (1.1, 1.4) | 1.16 (1.0, 1.3) | 0.88 (0.8, 1.0) | 0.92 (0.9, 1.0) | 0.97 (0.8, 1.1) | |
| 18-Month FU | 16 | 1.11 (0.9, 1.3) | 1.13 (1.0, 1.3) | 1.08 (0.9, 1.2) | 0.92 (0.8, 1.0) | 0.95 (0.9, 1.0) | 0.99 (0.9, 1.1) | |
| 24-Month FU | 18 | 1.12 (1.0, 1.3) | 1.07 (0.9, 1.2) | 1.06 (0.9, 1.2) | 0.93 (0.9, 1.0) | 0.94 (0.9, 1.0) | 1.02 (0.9, 1.1) | |
| All lesions | Baseline | 14 | 2.68 (2.1, 3.3) | 2.09 (1.6, 2.6) | 2.36 (1.8, 3.0) | 1.11 (0.9, 1.3) | 0.84 (0.8, 0.9) | 1.38 (1.1, 1.7) |
| 12-Month FU | 21 | 1.21 (1.0, 1.4) | 1.20 (1.1, 1.3) | 1.15 (1.1, 1.3) | 0.90 (0.8, 1.0) | 0.93 (0.8, 1.0) | 1.02 (0.9, 1.2) | |
| 18-Month FU | 22 | 1.09 (1.0, 1.2) | 1.06 (0.9, 1.2) | 1.04 (0.9, 1.2) | 0.98 (0.9, 1.1) | 0.96 (0.9, 1.0) | 1.05 (1.0, 1.1) | |
| 24-Month FU | 25 | 1.12 (1.0, 1.2) | 1.05 (1.0, 1.2) | 1.05 (0.95, 1.15) | 0.95 (0.90, 1.0) | 0.93 (0.9, 1.0) | 1.04 (1.0, 1.1) | |
RT = repair tissue; FU = follow-up.
Figure 6.
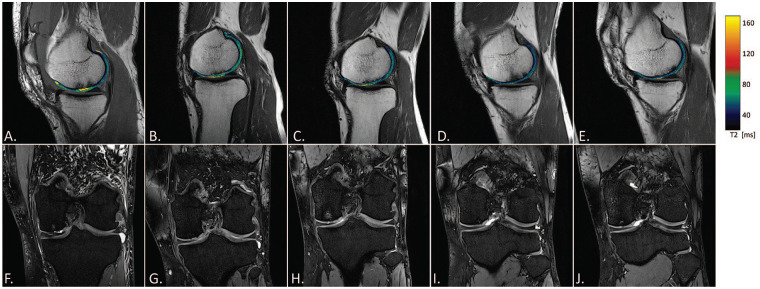
A 39-year-old male patient at study entry with a chondral defect of 2 cm2 on the medial femoral condyle without previous surgeries. Sagittal T2 maps were overlaid on a sagittal proton density weighted (PDw) fast spin echo (FSE) images (A-E). Coronal MR images obtained with a SPAIR (spectral attenuated inversion recovery) sequence of the same patient are displayed below (F-J). MR imaging was performed at baseline (A, F), 6 (B, G), 12 (C, H), 18 (D, I), and 24 (E, J) months after surgery.
The zonal variation of the repair tissue was within <20% difference to the zonal variation of healthy reference cartilage in 57.1% (12 of 21 patients), 72.7% (16 of 22 patients), and 68% (17 of 25 patients) at 12, 18, and 24 months, respectively. Furthermore, there was a significant zonal variation of the repair tissue at 24 months (P = 0.039). Whereas the zonal variation between repair tissue 1.11 (95% CI 0.9-1.3) and healthy reference cartilage 0.84 (95% CI 0.8-0.9) significantly differed at baseline (P = 0.02), there was no significant difference (P = 0.61) at the 24-month follow-up with 0.95 (95% CI, 0.9-1.0) and 0.93 (95% CI, 0.9-1.0). In addition, zonal T2 indices of the RT did not differ significantly (P = 0.2) between chondral and osteochondral lesions at the 24-month follow-up with 1.02 (95% CI, 0.9-1.1) and 1.11 (95% CI, 0.9-1.3), respectively.
Discussion
In this study, the efficacy of GelrinC in the treatment of both chondral and osteochondral lesions was evaluated using morphological and quantitative MRI as endpoints. The main findings of the study were a continuous increase of the MOCART score throughout the follow-up period and the gradual development of a significant zonal variation of T2 relaxation times in the repair tissue, which did not significantly differ from healthy reference cartilage at the 24-month follow-up.
Good short-term outcome has been reported after MFX. 26 However, with a subsequent decline of functional scores after two years in 47% to 80% of patients and increasing failure rates after 2 to 5 years, 26 long-term treatment failures remain an issue. The assumed reason for this is that repair tissue after MFX is most commonly formed of fibrocartilage instead of the desired hyaline cartilage. 27 As fibrocartilage exhibits inferior biomechanical properties, when compared to hyaline cartilage, it is less resistant to shear stress and thus more prone to long-term failure. The significant zonal variation of T2 relaxation times of the repair tissue that was observed in this study at the 24-month follow-up, however, might be indicative of the formation of hyaline cartilage like repair tissue, since this gradient of T2 relaxation times is thought to be absent in fibrocartilage.28,29
A higher rate of hyaline-like cartilage than after MFX has also been previously reported for ACI. 27 One key disadvantage of ACI, however, is the necessity of a second procedure. Additional advantages of GelrinC include that it is easily applicable to all lesion geometries and fosters complete and mechanically stable filling of the defect immediately after the procedure.
A strength of this study is the repeated MRI follow-up in short intervals, which allows for valuable insight in morphological changes of the RT over time. A significant increase in the MOCART score was observed from baseline to the 6-month follow-up as well as from the 6-month to the 24-month follow-up. While significant remodeling and repair tissue maturation occurs within the first 6 months after surgery, it has to be acknowledged that at the baseline MRI examination, the MOCART score might have been additionally negatively affected by the immediately preceding surgery, in particular by present effusion and morphological changes to the subchondral bone after MFX. This has to be considered when interpreting the early one-week postoperative baseline examination. However, the additional increase from the 6-month to the 24-month follow-up can be interpreted as further tissue maturation. This maturation process is also reflected by the changes in the variable “signal intensity dual T2 FSE” of the MOCART score, which significantly increased from 7.44 to 13.72 points (P < 0.0001) between the 6-month and the 24-month follow-up.
Another finding of this study was the significant difference of the MOCART score between chondral and osteochondral lesions at the 24-month follow-up. However, it is worth noting that the absolute difference was only minor and might not implicate clinical relevance. Furthermore, T2 mapping showed similar outcome for both lesion types suggesting that GelrinC provides a treatment option for both chondral and osteochondral lesions.
Limitations of this study include the lack of a control group, treated with MFX only. Unfortunately, there are only very few controlled trials on surgical cartilage repair due to the high costs and difficulty to enroll a sufficient number of patients. 30 Moreover, this was the first study investigating GelrinC in humans in vivo and the presented results warrant a double-arm control trial with an MFX control group to investigate possible superiority over treatment based on MFX only. Furthermore, due to ethical considerations, no biopsies for histological analysis were obtained at follow-up. However, a comprehensive MRI protocol was performed, including quantitative imaging via T2 mapping to noninvasively assess the potential ultrastructural differences between native cartilage and repair tissue. This is particularly valuable as it has been previously shown that fibrous repair tissue exhibits lower T2 relaxation times than hyaline cartilage. 31 Furthermore, fibrocartilage after MFX lacks the typical zonal appearance of hyaline cartilage, which exhibits parallel collagen fibers in deep cartilage and more randomly organized collagen fibers in superficial cartilage. This ultrastructural difference is reflected in T2 mapping, in which the organized parallel collagen fibers of deep hyaline cartilage restrict the movement of protons and lead to shorter relaxation times than in superficial hyaline cartilage. This zonal variation in T2 mapping is absent in fibrocartilage.28,31,32 While clinical symptoms, which are reflected in a decrease of clinical scores, occur mostly later in treatment failure, quantitative MRI can depict early differences in T2 values between repair tissue and reference cartilage.29,32,33 This renders T2 mapping a noninvasive alternative for repair tissue assessment to invasive biopsy. A randomized controlled trial investigating the outcome of MFX versus BST-CarGel employed T2 mapping as well. Even though absolute T2 relaxation times in repair tissue were significantly higher than in healthy reference cartilage, the authors found a significant difference between the 2 techniques, in favor of BST-CarGel at 12 months. 34 In a multicenter study setting, however, absolute T2 relaxation times bear the risk of systematic bias due to potential systematic variability between scanners. In this study, this was diminished with the calculation of global and zonal indices. Unfortunately, it was not possible to obtain radiological follow-up from all patients at all desired time points. However, of 56 patients enrolled at baseline, 39 patients could be radiologically evaluated at the 24-month follow-up. Moreover, T2 mapping was not performed in all patients as it was restricted to 3 T systems due to the insufficient signal-to-noise ratio at 1.5 T.
In conclusion, this study showed promising results after the treatment of chondral and osteochondral femoral cartilage lesions with GelrinC. Additional studies with an MFX control group are warranted to investigate possible superiority over the treatment based on MFX only. Furthermore, a future long-term follow-up study is needed to assess, whether the observed development of zonal variation of T2 relaxation times in the repair tissue, translates into improved long-term outcome.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Regentis Biomaterials.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study was funded by Regentis Biomaterials.
Ethical Approval: This study was carried out in accordance with the ethical standards of the institutional review boards of all participating medical centers as well as with the Declaration of Helsinki, including current revisions. Positive ethics votes were obtained from all responsible institutional review boards.
Informed Consent: Written informed consent was obtained from all patients.
Trial Registration: ClinicalTrials.gov identifier: NCT00989794.
ORCID iDs: Markus M. Schreiner  https://orcid.org/0000-0002-3163-0481
https://orcid.org/0000-0002-3163-0481
Marcus Raudner  https://orcid.org/0000-0001-7575-0090
https://orcid.org/0000-0001-7575-0090
Vladimir Mlynarik  https://orcid.org/0000-0002-5789-8179
https://orcid.org/0000-0002-5789-8179
References
- 1. Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011;19:1286-93. [DOI] [PubMed] [Google Scholar]
- 2. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 Suppl):S362-S369. [DOI] [PubMed] [Google Scholar]
- 3. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170-6. [PubMed] [Google Scholar]
- 4. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 5. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13:194-202. [DOI] [PubMed] [Google Scholar]
- 6. Frappier J, Stanish W, Brittberg M, Steinwachs M, Crowe L, Castelo D, et al. Economic evaluation of BST-CarGel as an adjunct to microfracture vs microfracture alone in knee cartilage surgery. J Med Econ. 2014;17:266-78. [DOI] [PubMed] [Google Scholar]
- 7. de Windt TS, Sorel JC, Vonk LA, Kip MM, Ijzerman MJ, Saris DBF. Early health economic modelling of single-stage cartilage repair. Guiding implementation of technologies in regenerative medicine. J Tissue Eng Regen Med. 2017;11(10):2950-9. [DOI] [PubMed] [Google Scholar]
- 8. Hunziker EB, Lippuner K, Keel MJ, Shintani N. An educational review of cartilage repair: precepts & practice—myths & misconceptions—progress & prospects. Osteoarthritis Cartilage. 2015;23:334-50. [DOI] [PubMed] [Google Scholar]
- 9. Brix M, Kaipel M, Kellner R, Schreiner M, Apprich S, Boszotta H, et al. Successful osteoconduction but limited cartilage tissue quality following osteochondral repair by a cell-free multilayered nano-composite scaffold at the knee. Int Orthop. 2016;40:625-32. [DOI] [PubMed] [Google Scholar]
- 10. Goldshmid R, Cohen S, Shachaf Y, Kupershmit I, Sarig-Nadir O, Seliktar D, et al. Steric interference of adhesion supports in-vitro chondrogenesis of mesenchymal stem cells on hydrogels for cartilage repair. Sci Rep. 2015;5:12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berdichevski A, Shachaf Y, Wechsler R, Seliktar D. Protein composition alters in vivo resorption of PEG-based hydrogels as monitored by contrast-enhanced MRI. Biomaterials. 2015;42:1-10. [DOI] [PubMed] [Google Scholar]
- 12. Ham TR, Lee RT, Han S, Haque S, Vodovotz Y, Gu J, et al. Tunable keratin hydrogels for controlled erosion and growth factor delivery. Biomacromolecules. 2016;17:225-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600-13. [DOI] [PubMed] [Google Scholar]
- 14. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88-96. [DOI] [PubMed] [Google Scholar]
- 15. Guermazi A, Roemer FW, Alizai H, Winalski CS, Welsch G, Brittberg M, et al. State of the art: MR imaging after knee cartilage repair surgery. Radiology. 2015;277:23-43. [DOI] [PubMed] [Google Scholar]
- 16. Schreiner MM, Mlynarik V, Zbýň Š, Szomolanyi P, Apprich S, Windhager R, et al. New technology in imaging cartilage of the ankle. Cartilage. 2017;8(1_suppl):31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trattnig S, Ba-Ssalamah A, Pinker K, Plank C, Vecsei V, Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging. 2005;23:779-87. [DOI] [PubMed] [Google Scholar]
- 18. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52:310-9. [DOI] [PubMed] [Google Scholar]
- 19. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16-23. [DOI] [PubMed] [Google Scholar]
- 20. Zbyn S, Stelzeneder D, Welsch GH, Negrin LL, Juras V, Mayerhoefer ME, et al. Evaluation of native hyaline cartilage and repair tissue after two cartilage repair surgery techniques with 23Na MR imaging at 7 T: initial experience. Osteoarthritis Cartilage. 2012;20:837-45. [DOI] [PubMed] [Google Scholar]
- 21. Newbould RD, Miller SR, Tielbeek JA, Toms LD, Rao AW, Gold GE, et al. Reproducibility of sodium MRI measures of articular cartilage of the knee in osteoarthritis. Osteoarthritis Cartilage. 2012;20:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watanabe A, Wada Y, Obata T, Ueda T, Tamura M, Ikehira H, et al. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: preliminary results. Radiology. 2006;239:201-8. [DOI] [PubMed] [Google Scholar]
- 23. Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205:551-8. [DOI] [PubMed] [Google Scholar]
- 24. Lusse S, Claassen H, Gehrke T, Hassenpflug J, Schunke M, Heller M, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000;18:423-30. [DOI] [PubMed] [Google Scholar]
- 25. Trattnig S, Ohel K, Mlynarik V, Juras V, Zbyn S, Korner A. Morphological and compositional monitoring of a new cell-free cartilage repair hydrogel technology—GelrinC by MR using semi-quantitative MOCART scoring and quantitative T2 index and new zonal T2 index calculation. Osteoarthritis Cartilage 2015;23(12):2224-32. [DOI] [PubMed] [Google Scholar]
- 26. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 27. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86:455-64. [DOI] [PubMed] [Google Scholar]
- 28. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407-14. [DOI] [PubMed] [Google Scholar]
- 29. Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures—initial experience. Radiology. 2008;247:154-61. [DOI] [PubMed] [Google Scholar]
- 30. Lyman S, Nakamura N, Cole BJ, Erggelet C, Gomoll AH, Farr J, 2nd. Cartilage-repair innovation at a standstill: methodologic and regulatory pathways to breaking free. J Bone Joint Surg Am. 2016;98:e63. [DOI] [PubMed] [Google Scholar]
- 31. Nieminen MT, Nissi MJ, Mattila L, Kiviranta I. Evaluation of chondral repair using quantitative MRI. J Magn Reson Imaging. 2012;36:1287-99. [DOI] [PubMed] [Google Scholar]
- 32. Welsch GH, Trattnig S, Domayer S, Marlovits S, White LM, Mamisch TC. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis Cartilage. 2009;17:1219-27. [DOI] [PubMed] [Google Scholar]
- 33. Domayer SE, Kutscha-Lissberg F, Welsch G, Dorotka R, Nehrer S, Gabler C, et al. T2 mapping in the knee after microfracture at 3.0 T: correlation of global T2 values and clinical outcome—preliminary results. Osteoarthritis Cartilage. 2008;16:903-8. [DOI] [PubMed] [Google Scholar]
- 34. Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95:1640-50. [DOI] [PubMed] [Google Scholar]