Abstract
Members of the kinesin II family are thought to play essential roles in many types of intracellular transport. One distinguishing feature of kinesin II is that it generally contains two different motor subunits from the Kif3 family. Three Kif3 family members (Kif3A, Kif3B, and Kif3C) have been identified and characterized in mice. Intracellular localization and biochemical studies previously suggested that Kif3C is an anterograde motor involved in anterograde axonal transport. To understand the in vivo function of the Kif3C gene, we used homologous recombination in embryonic stem cells to construct two different knockout mouse strains for the Kif3C gene. Both homozygous Kif3C mutants are viable, reproduce normally, and apparently develop normally. These results suggest that Kif3C is dispensable for normal neural development and behavior in the mouse.
All cells require protein synthesis followed by transport and correct targeting of these proteins to their proper destinations. Biochemical, genetic, and intracellular localization studies of kinesin motors have suggested that some of these motor proteins may power intracellular transport events in neurons (1, 6, 7). As microtubule-dependent motors, members of the kinesin superfamily share extensive sequence similarity within the motor domain but display diversification in their tail domains. The motor domain is composed of a catalytic domain that hydrolyzes ATP and interacts with the microtubule track and a short neck domain important for processive movement and control of direction. The tail domains have been suggested to provide different cargo-binding or regulatory partners and to confer the ability to form different types of oligomers (21).
The kinesin II holoenzyme was first identified in sea urchins and subsequently identified in most species and found to be composed of two different motor subunits from the Kif3 family (3). Genetic and localization experiments in Chlamydomonas, Tetrahymena thermophila (4, 22), Caenorhabditis elegans (19), sea urchins (5), and mice (9, 23, 24) suggest that kinesin II in many cases is essential for the construction and maintenance of motile and nonmotile cilia and flagella (11, 18). In Chlamydomonas, kinesin II appears to transport a large protein complex, termed a raft, possibly with protein cargoes attached, from the sites of synthesis in the cell body to the sites of utilization at the tip of the flagellum (4, 10, 16).
Three members (Kif3A, Kif3B, and Kif3C) of the Kif3 family have been characterized in mice (9, 23, 24). Kif3A was previously reported to form a heterodimer with Kif3B or Kif3C, but Kif3B and Kif3C cannot associate with each other (14, 24). Mouse mutants lacking either the Kif3A or Kif3B gene resulted in embryonic lethality and embryonic ciliary morphogenesis defects (13, 15, 20), suggesting that they also play roles in ciliary morphogenesis in mammals. When Kif3A was specifically deleted from retinal photoreceptors using the Cre-loxP system, complete loss of Kif3A caused large accumulations of opsin, arrestin, and membranes within the photoreceptor inner segment, which suggests that kinesin II is required to transport opsin and arrestin from the inner to the outer segments (12). In contrast to Kif3A and Kif3B, whose expression is ubiquitous, Kif3C expression is highly enriched in both the central and peripheral nervous systems. Intracellular localization and biochemical studies suggest that Kif3C is an anterograde motor which may be involved in anterograde axonal transport (14, 24). Nevertheless, the precise in vivo functions of Kif3C remain unknown.
To understand the in vivo function of the Kif3C gene, we used homologous recombination in embryonic stem cells to construct two types (Kif3CtypeI and Kif3Cnull) of knockout mouse strain for the Kif3C gene. In the Kif3CtypeI mice, the motor region and half of the α-helical coiled-coil domain of the Kif3C protein were removed, but the rest of the α-helical coiled-coil domain and the tail of the protein still remained. In the Kif3Cnull mice, no Kif3C mRNA or protein can be detected by Northern and Western blotting, which suggests that homozygous Kif3Cnull mice are null mutants. Both homozygous Kif3CtypeI and Kif3Cnull mutants are viable, reproduce normally, and apparently develop normally. These results suggest that Kif3C is dispensable for normal neural development and behavior in the mouse.
MATERIALS AND METHODS
Cloning and mapping of the Kif3C gene.
A 1.5-kb Kif3C cDNA fragment encoding the motor domain of the Kif3C protein was used for isolating Kif3C genomic clones from a mouse 129/SV/J genomic phage library (a gift from the lab of Jamey Marth). The genomic clones we isolated from the library were then cloned into the vector Bluescript (Stratagene, La Jolla, Calif.). The map of the Kif3C gene was obtained by digestion of the genomic clones using different restriction enzymes and probing with different regions of the Kif3C cDNA. Southern blotting and other molecular biological techniques were performed according to standard methods.
Generation of targeting vectors and ES cells.
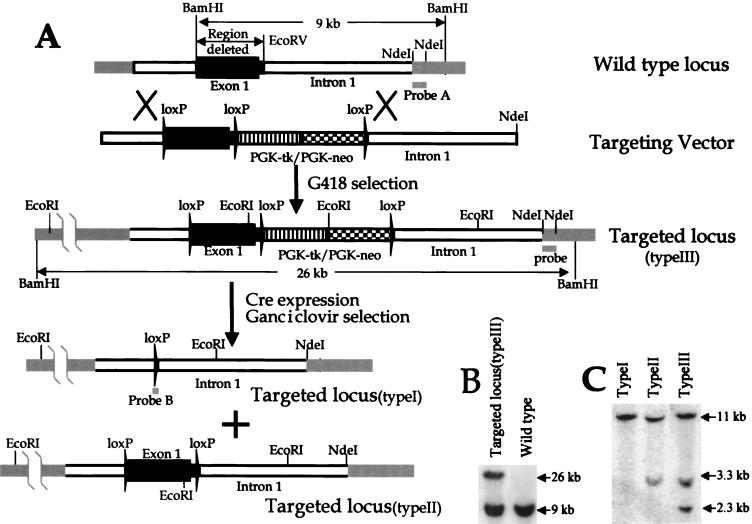
We made two targeting vectors. One vector was built using pflox (a gift from the lab of Jamey Marth) (2). In this vector we deleted a 2.1-kb DNA fragment between BamHI and EcoRV of the Kif3C gene (Fig. 1A). The DNA fragment contains the first exon (2.0 kb) and part of the first intron (0.1 kb) of the Kif3C gene and encodes amino acid residues 1 to 518 of the Kif3C protein. The linearized targeting construct with NdeI was introduced into R1 embryonic stem (ES) cells via electroporation (8) prior to selection with 250 μg of active G418/ml for 7 to 9 days. ES clones resistant to G418 were analyzed by Southern analysis. When the ES cell genomic DNA was digested with BamHI and probed with a 600-bp DNA fragment between two NdeI cut sites (Fig. 1A), the wild-type and targeted Kif3C alleles (type III) were detected as 9- and 26-kb bands, respectively (Fig. 1B). The targeted Kif3C allele was confirmed by Southern blotting with different restriction enzymes and probes. ES cell clones bearing a targeted Kif3C allele (type III) were electroporated with Cre expression plasmid pCre-Hygro (a gift from the lab of Jamey Marth) to excise the DNA fragment flanked by loxP sites (2). ES clones resistant to ganciclovir (2 μM) were analyzed by Southern analysis in which the genomic DNA was digested with EcoRI and probed with a loxP fragment (a gift from the lab of Jamey Marth). Two types of ES cells were obtained (Fig. 1A and C). One, bearing only one copy of the loxP sequence, is called Kif3CtypeI, in which the 2.1-kb Kif3C DNA fragment was deleted. The other, containing two copies of the loxP sequence, is called Kif3CtypeII, in which the Kif3C gene should function normally because the two loxP fragments were inserted in an intron and the 5′ nontranslated region and did not alter other aspects of the Kif3C gene.
FIG. 1.
Generation and analysis of Kif3CtypeI and Kif3CtypeII mutants in ES cells. (A) Strategy for generating Kif3CtypeI and Kif3CtypeII mutants. A 2.1-kb DNA fragment between BamHI and EcoRV of the Kif3C gene was flanked with two loxP fragments. A PGK-neo and PGK-tk selection marker with a third loxP fragment was added into the vector. (B) Southern analysis of targeted ES cells (Kif3CtypeIII). ES cell genomic DNA was cut with BamHI and probed with a 600-bp NdeI DNA fragment (probe A). The wild-type and targeted Kif3CtypeIII alleles were detected as 9- and 26-kb bands, respectively. (C) Southern analysis of Cre-transfected ES cells. Kif3CtypeIII (+/−) ES cells were transfected with the Cre plasmid to excise the DNA fragment flanked by loxP sites. ES clones resistant to ganciclovir were analyzed by Southern analysis, in which the genomic DNA was digested with EcoRI and probed with a loxP fragment (probe B). Each band represents a loxP fragment. Two types (Kif3CtypeI and Kif3CtypeII) of ES cells were obtained.
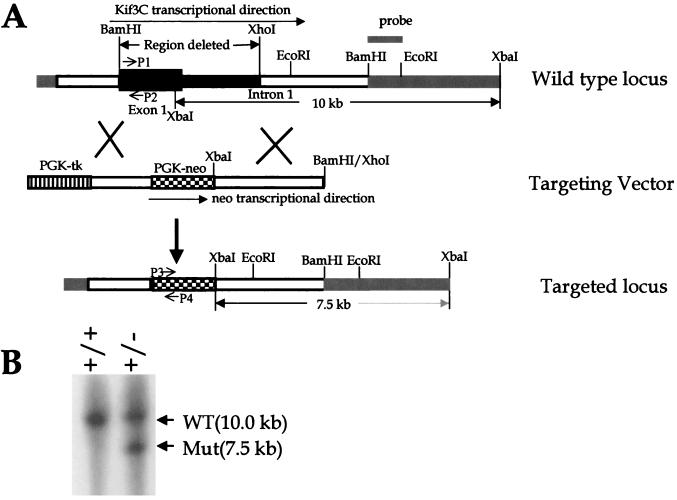
Using a traditional strategy, we also made a second targeting vector to delete a 4.5-kb DNA fragment between BamHI and XhoI of the Kif3C gene (see Fig. 3A). This DNA fragment contains the first exon (2.0 kb) and part of the first intron (2.5 kb) of the Kif3C gene and also encodes amino acid residues 1 to 518 of the Kif3C protein. In the targeting vector, the deleted region was replaced with a 1.7-kb phosphoglycerine kinase promoter (PGK)-driven neo cassette which contains a poly(A) signal and should mediate termination of transcription next to the deleted region. To increase the frequency of homologous recombination in ES cells, the targeting vector also included a negative selection marker, PGK-tk (see Fig. 3A). The vector was linearized with XhoI (see Fig. 3A) and introduced into R1 ES cells by electroporation as was done for the first targeting vector (8). ES clones resistant to both G418 and ganciclovir were analyzed by Southern analysis. The wild-type and targeted Kif3C alleles (Kif3Cnull) were detected as 10- and 7.5-kb bands (see Fig. 3B), respectively, by digesting the ES cell genomic DNA with XbaI and probing with a 1.5-kb BamHI and EcoRI DNA fragment (see Fig. 3A). The targeted Kif3C allele was confirmed by Southern blotting with different restriction enzymes and probes.
FIG. 3.
Generation and analysis of Kif3Cnull mutants in ES cells. (A) Strategy for generating Kif3Cnull mutants. One 4.5-kb DNA fragment between BamHI and XhoI of the Kif3C gene was replaced with a 1.7-kb PGK-neo cassette. The targeting vector included a negative selection marker, PGK-tk. The targeting vector was linearized with XhoI and introduced into R1 ES cells. (B) Southern analysis of ES cells. ES cell genomic DNA was cut with XbaI and probed with a 1.5-kb BamHI/EcoRI DNA fragment. The wild-type (WT) and targeted Kif3C (Mut) alleles were detected as 10.0- and 7.5-kb bands, respectively. P1, P2, P3, and P4 indicate the locations of primers used for genotyping Kif3Cnull mice (Fig. 4).
Generation of Kif3C targeted mice.
Three types (Kif3CtypeI, Kif3CtypeII, and Kif3Cnull) of ES cells were used for generating chimeric mice (129/SvJ-derived ES cells in blastocysts of C57BL/6J mice) as previously described (8). When the chimeras were backcrossed to C57BL/6J mice, heterozygous Kif3CtypeI (+/−), Kif3CtypeII (+/−), and Kif3Cnull (+/−) mice were obtained. These heterozygous mice were used for interbreeding to produce homozygous Kif3CtypeI (−/−), Kif3CtypeII (−/−), and Kif3Cnull (−/−) mice, respectively. The genotypes of animals were obtained by Southern blotting or PCR as indicated.
Analysis of Kif3C targeted mice.
Gross and histopathological analyses employed standard techniques. Littermate Kif3C (+/+) and either Kif3CtypeI (−/−) or Kif3Cnull (−/−) mice were examined for appearance, posture, circadian activity, home cage assessment, rotating rod task performance, and balance. These behavioral tests were carried out using standard protocols.
Western analysis and immunoprecipitation.
Monoclonal anti-Kif3A and polyclonal anti-Kif3B antibodies were from Babco. Monoclonal anti-actin antibody was from Boeihringer Mannheim. Monoclonal antibody SUK4 is directed against the kinesin heavy chain. Affinity-purified rabbit antibodies against the C terminus of mouse Kif3C were prepared and Western analysis and immunoprecipitation of mouse brain lysates were performed as described previously (24).
RESULTS AND DISCUSSION
Generation and analysis of Kif3CtypeI and Kif3CtypeII mice.
To explore the in vivo function of the Kif3C gene, we first made a targeting vector to delete a 2.1-kb DNA fragment of the Kif3C gene in ES cells. Because we were concerned that Kif3CtypeI (−/−) mice might be inviable, Kif3CtypeII (−/−) mice, which are conditional (tissue specific) knockout mice, would serve for functional analysis of the Kif3C gene. As described in Materials and Methods, we obtained both Kif3CtypeI (+/−) and Kif3CtypeII (+/−) ES cells (Fig. 1A). When both Kif3CtypeI (+/−) and Kif3CtypeII (+/−) ES cells were injected into the blastocysts of C57BL/6J mice, several chimeras were generated. These chimeras were successfully used to generate homozygous Kif3CtypeI (−/−) and Kif3CtypeII (−/−) mice, respectively. Both homozygous Kif3CtypeI (−/−) and Kif3CtypeII (−/−) mice were normal and healthy, just like their wild-type littermates. The ratios of wild-type Kif3C (+/+), heterozygous Kif3CtypeI (+/−) or Kif3CtypeII (+/−), and homozygous Kif3CtypeI (−/−) or Kif3CtypeII (−/−) mice from the heterozygous parents yielded the predicted Mendelian ratios of 1:2:1 expected for nonlethal alleles. Thus, the Kif3CtypeI (−/−) and Kif3CtypeII (−/−) pups were no less viable than their wild-type and heterozygous littermates.
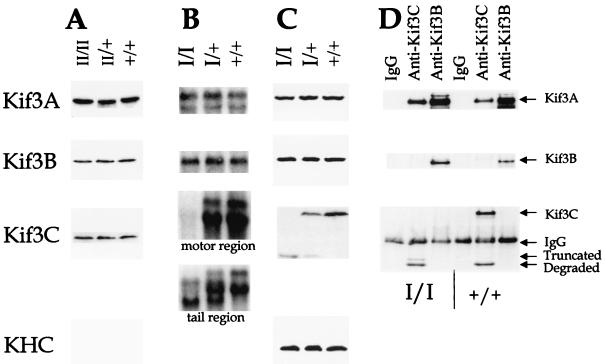
For the Kif3CtypeII (−/−) mice, it is expected that the two loxP fragments were inserted into an intron and nontranslated region without altering other aspects of the Kif3C gene, and thus this Kif3C allele should function normally. In fact, when Western blotting was used to analyze brain lysates from Kif3CtypeII mice, the amount of the Kif3C protein in the Kif3CtypeII (−/−) mice was normal compared to that of their heterozygous Kif3CtypeII (+/−) and wild-type littermates (Fig. 2A). As controls, the Kif3A and Kif3B protein levels also did not change.
FIG. 2.
Analysis of Kif3CtypeI and Kif3CtypeII mice. (A) Western analysis of Kif3CtypeII mice. Brain lysates from Kif3CtypeII (−/−), Kif3CtypeII (+/−), and wild-type (+/+) littermates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The duplicate blots were probed with antibodies against Kif3A, Kif3B, and Kif3C. (B) Northern analysis of Kif3CtypeI mice. Total RNA was isolated from the Kif3CtypeI mouse brains and analyzed in a formaldehyde-agarose gel. The duplicate blots were probed with cDNA of Kif3A, Kif3B, and Kif3C. Two different probes, encoding either the motor region or the tail region as indicated, were used for Kif3C. (C) Western analysis of Kif3CtypeI mice. Brain lysates from Kif3CtypeI mice were analyzed by SDS-PAGE. The duplicate blots were probed with antibodies against Kif3A, Kif3B, and Kif3C and a monoclonal antibody, SUK4, for kinesin heavy chain. The antibodies against Kif3C detected a truncated Kif3C protein. (D) Immunoprecipitation-Western analysis of Kif3CtypeI mice. Brain lysates from Kif3CtypeI mice were immunoprecipitated with anti-Kif3C or anti-Kif3B antibodies or immunoglobulin G (IgG) as a control. The immunoprecipitated samples were analyzed by SDS-PAGE. The same blot was probed and reprobed with antibodies against Kif3A, Kif3B, and Kif3C. The samples in the left three lanes were from Kif3CtypeI (−/−) mice and the samples in the right three lanes were from wild-type littermates. (The truncated Kif3C protein in wild-type mice was probably generated from protein degradation.)
In Kif3CtypeI mice, we deleted a 2.1-kb DNA fragment of the Kif3C gene (Fig. 1A). The 2.1-kb DNA fragment encodes amino acid residues 1 to 518 of the Kif3C protein, which has a total of 796 amino acids (24). This deletion includes the whole motor region and half of the α-helical coiled-coil domains of the motor domain. Since the deleted motor domain contains functional ATP binding and ATPase activity sites, it is likely that the deletion will completely abolish the function of Kif3C. However, due to the design used, we did not put a transcriptional stop signal immediately after the deleted region. As a result of this, although the 2.1-kb DNA fragment was successfully deleted and no transcript containing the motor region was detected by Northern blotting, a small transcript encoding the tail region of Kif3C was detectable in the Kif3CtypeI (−/−) mice (Fig. 2B). Due to the presence of an internal ATG code in this transcript, a truncated Kif3C protein could be made in the Kif3CtypeI (−/−) mice. This fragment was indeed detected by Western blotting using antibodies against the tail region of the Kif3C proteins (Fig. 2C). The mRNA and protein levels of both the Kif3A and Kif3B genes apparently do not change in the Kif3CtypeI mice (Fig. 2B and C).
To examine whether the truncated Kif3C protein could associate with Kif3A, antibodies against Kif3B and Kif3C were used for immunoprecipitation of brain lysates from the Kif3CtypeI mice. The immunoprecipitated samples were analyzed by Western blotting by using antibodies against Kif3A, Kif3B, and Kif3C. We observed that immunoprecipitation from wild-type mice brings down not only full-length Kif3C, but also a small fragment of Kif3C; this fragment is only seen in immunoprecipitation experiments and not in Western blots (compare Fig. 2D to Fig. 2C and 4B). We think that the most likely explanation is that proteins are handled longer in the immunoprecipitation experiments and hence are more susceptible to degradation than in Western blot experiments. In support of this notion, there are two smaller bands detected in Kif3CtypeI homozygous mutants (Fig. 2D) in the immunoprecipitation experiments, and one, the upper band in Western blots (Fig. 2C), is not seen in wild-type mice. Thus, the most plausible hypothesis is that the upper band is the truncated fragment of Kif3C generated from the internal start and the lower band is derived from the upper band by degradation as in wild-type mice. Nonetheless, as shown in Fig. 2D, like the full-length Kif3C protein (14, 24), the truncated Kif3C protein also specifically associates with Kif3A but not Kif3B. It was suggested previously that opposing charge interactions within the stalk domains of the Kif3 family are important for generating heterodimers (17). This region, however, is deleted in the Kif3CtypeI (−/−) mice. The results in Fig. 2D demonstrate that the truncated Kif3C protein without the charged region still associates with Kif3A, suggesting that other interactions in addition to interactions within the stalk domains may play more important roles in selective associations among the subunits of the Kif3 family.
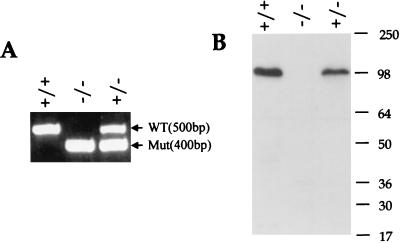
FIG. 4.
Analysis of Kif3Cnull knockout mice. (A) PCR analysis of the Kif3Cnull knockout mice. A set of four primers was used for genotyping the Kif3Cnull mice. Two primers based on Kif3C sequences (forward P1, GGT CAT GAG CAG ATT CTG AC; reverse P2, GAG AGC TGA CCT CAT TCA TG) were used for PCR to amplify a 500-bp DNA fragment which is absent from the Kif3Cnull (−/−) mutant locus. Another two primers based on the neo sequence (forward P3, GAT GGA TTG CAC GCA GGT TCT; reverse P4, AGG TAG CCG GAT CAA GCG TAT) were used for amplifying a 400-bp DNA fragment which is missing in the wild-type Kif3C locus. The positions of PCR primers are indicated in Fig. 3A. (B) Western analysis of Kif3Cnull knockout mice. Brain lysates from the Kif3Cnull mice were analyzed by SDS-PAGE. The blot was probed with antibodies against Kif3C, which was made using the C terminus of the Kif3C protein. No truncated Kif3C protein was detected. WT, wild type; Mut, targeted Kif3C alleles. Numbers to the right of panel B indicate molecular mass, in kiloDaltons.
There are two hypotheses to explain the normality of the Kif3CtypeI (−/−) mice. One is that Kif3C may not have an essential or major function and the other is that the truncated Kif3C protein associated with Kif3A may have some residual function. The second hypothesis was supported by a ligation experiment on sciatic nerves in the Kif3CtypeI (−/−) mice. The ligation experiments showed that the truncated Kif3C protein can be anterogradely transported into axons (data not shown). To further test these hypotheses, we made another knockout strain, Kif3Cnull (−/−).
Generation and analysis of Kif3Cnull mice.
Using standard methods, we used Kif3Cnull (+/−) ES cells (Fig. 3A) to generate Kif3Cnull (−/−) and wild-type littermates. In the Kif3Cnull (−/−) mice, a 4.5-kb DNA fragment of the Kif3C gene was deleted and replaced by a drug resistance cassette and a poly (A) signal. The 4.5-kb DNA fragment encodes amino acid residues 1 to 518 of the Kif3C protein. This deletion includes the whole motor region and half of the α-helical coiled-coil domains of the motor domain similar to the Kif3CtypeI (−/−) mice. The deletion was confirmed by PCR (Fig. 4A) and by Western blotting (Fig. 4B). In genomic PCR of the Kif3C mice, a 500-bp DNA fragment which represents the Kif3C wild-type allele was found for both the wild-type (+/+) and heterozygous (+/−) mice but not for the homozygous mutant Kif3Cnull (−/−) mice (Fig. 4A). In contrast, a 400-bp DNA fragment amplified from PGK-neo which represents the Kif3C mutated allele was found for both the heterozygous Kif3Cnull (+/−) and homozygous Kif3Cnull (−/−) mutants but not for the wild type (+/+) mice (Fig. 4A). The results from PCR analysis were confirmed by Southern analysis using the same enzyme and probe as described for Fig. 3B (data not shown). In Western analysis of the brain lysates from the Kif3Cnull (−/−) mice and wild-type littermates, no Kif3C-specific protein was detected in the homozygous mutants using antibodies against the C terminus of the Kif3C protein. These results clearly indicate that the mice we generated are truly null for the Kif3C gene.
The ratios of wild-type (+/+), heterozygous (Kif3Cnull [+/−]), and homozygous (Kif3Cnull [−/−]) mice from the heterozygous parents yielded the predicted Mendelian ratios of 1:2:1 expected for nonlethal alleles. Thus, the Kif3Cnull (−/−) pups were no less viable than their wild-type and heterozygous littermates. Mice that were heterozygous (Kif3Cnull [+/−]) or homozygous (Kif3Cnull [−/−]) for the Kif3C deletion appeared healthy (up to 1.5 years old), developed normally, and did not display any impairment of reproductive capacity and neonatal survival. The breeding pairs with either heterozygous × heterozygous or homozygous × homozygous animals produced litters similar in size to those produced by wild-type breeding pairs, demonstrating that the absence of the Kif3C protein does not hinder the fertility of male or female mice.
Since Kif3C is specifically expressed in the nervous system, we examined whether the deletion of the Kif3C gene affects mouse behaviors using a variety of tests. Mice were tested in a rotarod apparatus to assess their motor coordination, balance, and ataxia. No differences between the knockout mice (Kif3Cnull [−/−]) and their wild-type littermates were observed.
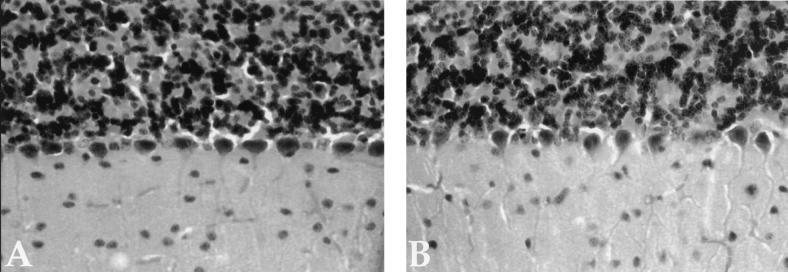
At a gross phenotypic level, the absence of Kif3C had no discernible impact. Kif3Cnull (−/−) mice were indistinguishable from their wild-type littermates with respect to body weight and body length. In addition, they exhibited no macroscopic or microscopic alterations in all organs examined (retina, brain, spinal cord, and sciatic nerve). Figure 5 shows the morphology of brain from wild type (Fig. 5A) and Kif3Cnull (−/−) knockout (Fig. 5B) mice. No differences in the morphology of the brain between the Kif3C knockout mice and their littermates were observed. We found no differences in white or red blood cell counts or in levels of hemoglobin between wild-type and Kif3Cnull (−/−) knockout mice, nor were alterations in embryonic development observed. These results suggest that Kif3C is dispensable for normal development and for many behaviors.
FIG. 5.
Morphology of brain from wild-type (A) and Kif3Cnull (−/−) (B) mice. Brains were isolated from adult animals perfused with paraformaldehyde, postfixed, and embedded in paraffin. Sections were stained with cresyl violet.
Functions of Kif3C motor.
The neural-specific expression of Kif3C had suggested that Kif3C would play an important role in the nervous system. However, when we disrupted the Kif3C gene in mice, the nervous system apparently functioned normally in the homozygous mutants. This conclusion is supported by behavioral tests and histological analyses in which we found no difference between the Kif3C knockout mice and their wild-type littermates.
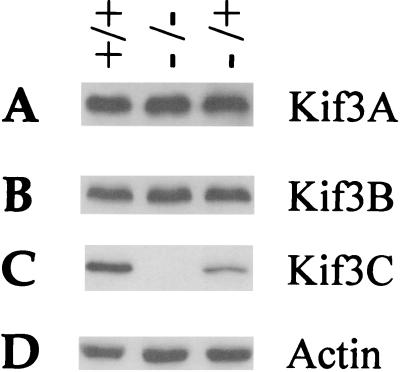
As a member of the Kif3 kinesin family, the loss of function of Kif3C in the mutants might be provided by another member of this family, such as Kif3A and Kif3B. However, when Kif3Cnull (−/−) mice were analyzed by Western blotting using anti-Kif3A and anti-Kif3B antibodies, like for actin the amount of Kif3A and Kif3B apparently did not change dramatically (Fig. 6).
FIG. 6.
Western analysis of Kif3Cnull (−/−) mice. Brain lysates from Kif3Cnull mice were analyzed by SDS-PAGE. The duplicate blots were probed with antibodies against Kif3A (A), Kif3B (B), Kif3C (C), and actin (D).
Because of the difference between Kif3C and Kif3B expression patterns, slightly different microtubule-binding characteristics, and selective association with Kif3A as previously reported (14, 23, 24), Kif3B and Kif3C functions could be carried out in distinct tissues and cell types, or they could selectively power different types of cargoes. Since Kif3C and Kif3B are similar in sequence and association with Kif3A, it was also suggested that the Kif3A-Kif3C and Kif3A-Kif3B heterodimers may play similar roles in anterograde transport (24). Kif3A-Kif3C may have transport activities only in neural tissues, while Kif3A-Kif3B may have a more general role in transport in neural and other tissues. Thus, it is possible that the two motors each participate in the movement of overlapping types of cargoes, in which case they might be redundant in neural tissues. The mice we report in this paper may facilitate exploration of this issue.
ACKNOWLEDGMENTS
We thank D. Chui and other members of the J. Marth lab for several plasmids and invaluable advice on ES cell work.
L.S.B.G. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Bloom G S, Endow S A. Motor proteins 1: kinesins. Protein Profile. 1995;2:1105–1171. [PubMed] [Google Scholar]
- 2.Chui D, Oh-Eda M, Liao Y F, Panneerselvam K, Lal A, Marek K W, Freeze H H, Moremen K W, Fukuda M N, Marth J D. Alpha-mannosidase-II deficiency results in dyserythropoiesis and unveils an alternate pathway in oligosaccharide biosynthesis. Cell. 1997;90:157–167. doi: 10.1016/s0092-8674(00)80322-0. [DOI] [PubMed] [Google Scholar]
- 3.Cole D G, Chinn S W, Wedaman K P, Hall K, Vuong T, Scholey J M. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 1993;366:268–270. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- 4.Cole D G, Diener D R, Himelblau A L, Beech P L, Fuster J C, Rosenbaum J L. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole D G, Scholey J M. Purification of kinesin-related protein complexes from eggs and embryos. Biophys J. 1995;68:158S–160S. [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein L S, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 8.Joyner A L. Gene targeting: a practical approach. Oxford, United Kingdom: IRL Press at Oxford University Press; 1993. [Google Scholar]
- 9.Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol. 1994;125:1095–1107. doi: 10.1083/jcb.125.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozminski K G, Beech P L, Rosenbaum J L. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marszalek J R, Goldstein L S. Understanding the functions of kinesin-II. Biochim Biophys Acta. 2000;1496:142–150. doi: 10.1016/s0167-4889(00)00015-x. [DOI] [PubMed] [Google Scholar]
- 12.Marszalek J R, Liu X, Roberts E A, Chui D, Marth J D, Williams D S, Goldstein L S. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 13.Marszalek J R, Ruiz-Lozano P, Roberts E, Chien K R, Goldstein L S. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muresan V, Abramson T, Lyass A, Winter D, Porro E, Hong F, Chamberlin N L, Schnapp B J. KIF3C and KIF3A form a novel neuronal heteromeric kinesin that associates with membrane vesicles. Mol Biol Cell. 1998;9:637–652. doi: 10.1091/mbc.9.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 16.Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci USA. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashid D J, Wedaman K P, Scholey J M. Heterodimerization of the two motor subunits of the heterotrimeric kinesin, KRP85/95. J Mol Biol. 1995;252:157–162. doi: 10.1006/jmbi.1995.0484. [DOI] [PubMed] [Google Scholar]
- 18.Scholey J M. Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles. J Cell Biol. 1996;133:1–4. doi: 10.1083/jcb.133.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signor D, Wedaman K P, Rose L S, Scholey J M. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol Biol Cell. 1999;10:345–360. doi: 10.1091/mbc.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vale R D, Fletterick R J. The design plan of kinesin motors. Annu Rev Cell Dev Biol. 1997;13:745–777. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- 22.Walther Z, Vashishtha M, Hall J L. The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J Cell Biol. 1994;126:175–188. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Goldstein L S. Characterization of the KIF3C neural kinesin-like motor from mouse. Mol Biol Cell. 1998;9:249–261. doi: 10.1091/mbc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]