Abstract
Background
Exit strategy after natalizumab cessation in multiple sclerosis (MS) is a crucial point because the risk of disease reactivation is high during this period. The objective of this observational study was to compare ocrelizumab to fingolimod after natalizumab cessation in patients with relapsing–remitting multiple sclerosis (RRMS).
Methods
All RRMS patients starting fingolimod or ocrelizumab within 6 weeks after natalizumab cessation were included. The primary endpoint was the annualized relapse rate (ARR) at 1 year.
Results
We included 54 patients receiving fingolimod and 48 patients receiving ocrelizumab after natalizumab cessation. In multivariate analysis, ARR at 1 year was significantly lower in the ocrelizumab group than in the fingolimod group (0.12 ± 0.39 versus 0.41 ± 0.71, p = 0.026), i.e. a 70.7% lower relapse rate. The cumulative probability of relapses at 1 year was 31.5% (17/54 patients) with fingolimod and 10.4% (5/48 patients) with ocrelizumab, corresponding to a hazard ratio of 3.4 (95% confidence interval: 1.1–11, p = 0.04).
Conclusions
Our results suggest ocrelizumab is potentially a better exit strategy than fingolimod after natalizumab cessation.
Keywords: Multiple sclerosis, Ocrelizumab, Fingolimod, Natalizumab, Switch
Introduction
Currently, more than ten drugs are available for patients with relapsing–remitting multiple sclerosis (RRMS). Thus, the number of patients who have experienced a switch of treatment is increasing. In the case of natalizumab (NTZ), around a third of patients are reported to have clinical activity after NTZ cessation [1]. Since 2010, fingolimod was the most frequently used option in this case, with fewer relapses than with interferon beta or glatiramer acetate [2]. Ocrelizumab is a recent high-efficacy treatment for RRMS and was approved by the FDA in 2017 and by the EMA in 2018. This new drug may be a very good alternative to fingolimod but comparative data are needed on the treatment strategy after NTZ cessation. The objective of the study was to compare disease activity with ocrelizumab and fingolimod after natalizumab cessation in patients with RRMS.
Methods
Patients
In this observational retrospective study from the Alsace region of France, we included all RRMS patients starting fingolimod or ocrelizumab after NTZ cessation between January 2011 and December 2019. All patients were diagnosed according to the 2010 McDonald criteria. Treatment with NTZ was every 4 weeks without extended dosing interval. The choice between the two treatments was at the discretion of the physician. Patients with a progressive form of MS or with a washout time of more than 42 days were excluded because a longer washout time is associated with MS reactivation after NTZ cessation [3]. Indeed, after 2013–2014, the practice on the washout period before fingolimod initiation changed according to the results of Cohen et al. and was close to 1 month as usually done with ocrelizumab [3].
Outcome measures and assessments
We used the European Database for Multiple Sclerosis (EDMUS) software for the collection of data [4]. Clinical visits were scheduled every 6 months. The following data were collected: the number of relapses in the year before NTZ cessation, the reason for NTZ discontinuation, the number of relapses at 1 year after the introduction of fingolimod or ocrelizumab, the EDSS score every 6 months, the last brain MRI performed before NTZ cessation, and the rebaseline brain MRI (performed in the 3–6 months after treatment initiation).
The primary endpoint was the annualized relapse rate at 1 year. Secondary endpoints were the time free of relapses, the EDSS score at 1 year, the radiological activity on rebaseline brain MRI, and the EDA (evidence of disease activity) at 1 year.
A clinical relapse was defined as new or recurrent neurological symptoms without fever, lasting at least 24 h, and followed by a period of 30 days of improvement or stability.
Radiological activity was defined as new T2 hyperintense lesion or the presence of T1 gadolinium-enhancing lesion on brain MRI.
EDA corresponded to the occurrence of at least one following criteria: relapse, progression (defined as ≥ 1.5-point EDSS if EDSS = 0, ≥ 1-point EDSS if EDSS ≤ 5.5, or ≥ 0.5-point EDSS if EDSS ≥ 6.0), or new T2 hyperintense lesion on brain MRI.
Standard protocol approvals, registrations, and patient consent
This study was approved by local ethics committee. All patients gave written informed consent to be included in EDMUS.
Statistical analysis
Descriptive analyses were performed with mean values (standard deviations) and medians (inter-quartiles ranges [IQR]).
The primary endpoint was analyzed in modified intention-to-treat including patients stopping treatment for clinical activity in the analysis and excluding patients stopping treatment for another reason. We performed a multivariate logistic regression analysis adjusted for age, disease duration, sex, initial EDSS, number of relapses the year before natalizumab cessation, the time with natalizumab, and the presence of infratentorial lesions on brain MRI. The lesions load on brain MRI was not added in the multivariate analysis because all patients had at least 9 T2-lesions except two patients with fingolimod. More precise data on brain MRI were not added because the exact lesions load is not a minimal data in EDMUS. Because data were not normally distributed, we performed statistical inference by bootstrap (1000 iterations) using R software [5]. The cumulative probability of relapse during the first year was calculated using a Cox model adjusted for age, disease duration, sex, initial EDSS, number of relapses the year before natalizumab cessation, the time with natalizumab, washout period, and the presence of infratentorial lesions on brain MRI. Other comparisons between the two groups were performed using a Mann–Whitney test for quantitative variables and a Chi-square test or Fisher’s exact test for qualitative variables.
Missing data were managed as follows: if the percentage was < 5% we performed an imputation by median (quantitative variables) or mode (qualitative variables) and if it was 5–20% we performed a multivariate imputation by chained equations.
Data availability
Anonymized data will be shared on request from any qualified investigator.
Results
Descriptive analysis
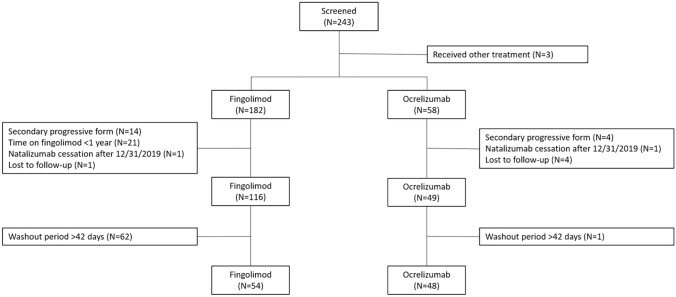
Two hundred forty-three patients were screened; we included 102 patients, 48 (47%) with ocrelizumab and 54 (53%) with fingolimod, in the analysis (Fig. 1). According to ocrelizumab availability in France, all included patients started ocrelizumab from 2019. Only one patient started fingolimod after 2018. Baseline characteristics are summarized in Table 1. The two groups were well balanced. No progressive multifocal leukoencephalopathy or other serious infections during the follow-up were observed in either group.
Fig. 1.
Flowchart of the study
Table 1.
Baseline and 1-year characteristics in fingolimod and ocrelizumab groups
| Characteristic | Fingolimod (n = 54) | Ocrelizumab (n = 48) | p value |
|---|---|---|---|
| Baseline | |||
| Age, y, mean ± SD | 39.5 ± 9.3 | 40.6 ± 10.3 | 0.6 |
| Female, n (%) | 35 (65%) | 35 (73%) | 0.4 |
| MS duration, m, mean ± SD | 138 ± 79.9 | 162 ± 81.7 | 0.1 |
| Oligoclonal bands, n (%) | 31/34 (91%) | 33/36 (92%) | 0.999 |
| Number of relapses per year before NTZ cessation, mean ± SD | 0.29 ± 0.62 | 0.15 ± 0.36 | 0.1 |
| EDSS score, mean ± SD | 2.8 ± 1.7 | 2.4 ± 1.7 | 0.2 |
| Last brain MRI with NTZ, n (%) | |||
| ≥ 9 T2-lesions | 48/50 (96%) | 40/40 (100%) | 0.5 |
| ≥ 1 GdE lesion | 1/42 (2.4%) | 0/28 (0%) | 0.999 |
| ≥ 1 new T2-lesion | 1/43 (2.3%) | 2/41 (4.9%) | 0.6 |
| ≥ 1 infratentorial lesion | 35/49 (71%) | 31/40 (78%) | 0.5 |
| Reason for NTZ cessation, n (%) | 0.2 | ||
| PML risk* | 39 (72.2%) | 42 (87.5%) | |
| Intolerance | 4 (7.4%) | 1 (2.1%) | |
| Lack of efficacy | 2 (3.7%) | 2 (4.2%) | |
| Anti-NTZ ab | 1 (1.9%) | 0 (0%) | |
| Other | 4 (7.4%) | 0 (0%) | |
| Missing data | 4 (7.1%) | 3 (6.2%) | |
| Time with natalizumab, d, mean ± SD | 1257 ± 842 | 2063 ± 1486 | < 0.01 |
| Washout period, d, mean ± SD | 30.1 ± 9.2 | 30.5 ± 3.9 | 0.8 |
| One-year | |||
| Number of relapses at one year, mean ± SD | 0.41 ± 0.71 | 0.12 ± 0.39 | 0.026† |
| Time before the first relapse, d, median (IQR) | 160 (121–192) | 125 (46–275) | 0.4 |
| Number of relapses at 1 year, n (%) | 0.05 | ||
| 0 | 37 (68.5%) | 43 (89.6%) | |
| 1 | 14 (25.9%) | 4 (8.3%) | |
| 2 | 1 (1.9%) | 1 (2.1%) | |
| 3 | 2 (3.7%) | 0 | |
| EDSS score, mean ± SD | 2.8 ± 1.8 | 2.3 ± 1.7 | 0.6 |
| First brain MRI after NTZ cessation | |||
| ≥ 1 GdE lesion, n (%) | 2/33 (6.1%) | 0/31 (0%) | 0.5 |
| ≥ 1 new T2-lesion, n (%) | 5/30 (16.7%) | 0/33 (0%) | 0.02 |
EDSS Expanded Disability Status Scale, GdE gadolinium-enhanced, IQR interquartiles ranges, MS multiple sclerosis, NTZ natalizumab, PML progressive multifocal leukoencephalopathy
*Before 2014, if JCV serology was positive, and from 2014, if JCV index was > 0.9 or 1.5 according to the threshold choose by the physician
†Multivariate linear regression adjusted for age, disease duration, sex, initial EDSS, number of relapses the year before natalizumab cessation, the time with natalizumab, washout period, and the presence of infratentorial lesions on brain MRI
Endpoints
Endpoint characteristics are summarized in Table 1.
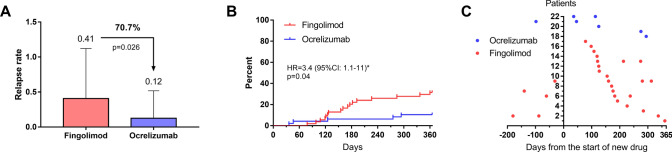
In the multivariate analysis, patients with ocrelizumab had fewer relapses during the first year than patients with fingolimod (0.12 ± 0.39 versus 0.41 ± 0.71, respectively, p = 0.035; Fig. 2A, Table 2), corresponding to a 70.7% lower relapse rate. In the Cox model, the cumulative probability of relapses at 1 year was 31.5% (17/54 patients) with fingolimod and 10.4% (5/48 patients) with ocrelizumab, corresponding to a hazard ratio of 3.4 (95% confidence interval: 1.1–11, p = 0.04; Fig. 2B). For patients with relapses, the median time before the first relapse was 160 days (121–192) with fingolimod and 125 days (46–275) with ocrelizumab (p = 0.4). No correlation was observed between the time before the first relapse and the washout time for either group (fingolimod: Spearman coefficient = 0.139, p = 0.59; ocrelizumab: Spearman coefficient = 0.359, p = 0.55). One patient (1.8%) with fingolimod had a relapse within the washout period (at day 27) and was treatment with corticosteroid boluses; no patients with ocrelizumab had a relapse within this period.
Fig. 2.
Relapse rate at 1 year, Kaplan–Meier curves, and days between fingolimod or ocrelizumab initiation and relapses for each patient. A Relapse rate at 1 year in both groups. Analysis was performed using a multivariate logistic regression adjusted for age, disease duration, sex, initial EDSS, number of relapses the year before natalizumab cessation, the time with natalizumab, washout period, and the presence of infratentorial lesions on brain MRI. B Kaplan–Meier curves of the occurrence of relapse in both groups. Analysis was performed using a Cox model. C Days between fingolimod or ocrelizumab initiation and relapses for each patient. Relapses occurred in 17/54 (31.5%) patients with fingolimod and 5/48 (10.4%) patients with ocrelizumab. HR hazard ratio, 95% CI = 95% confidence interval. *Ocrelizumab as reference
Table 2.
Multivariate analysis of the number of relapses at 1 year
| Variable | Coefficient [95% CI]† | p value |
|---|---|---|
| Treatment (ocrelizumab versus fingolimod) | − 0.264 [− 0.510; − 0.0489] | 0.035 |
| Sex (male versus female) | − 0.128 [− 0.363; 0.109] | 0.3 |
| Age | − 0.00484 [− 0.0202; 0.0107] | 0.5 |
| Disease duration | − 0.00758 [− 0.0309; 0.0108] | 0.5 |
| Time with NTZ | 0.00150 [− 0.00721; 0.0108] | 0.8 |
| Number of relapses the year before NTZ cessation | − 0.0303 [− 0.241; 0.290] | 0.8 |
| Baseline EDSS (+ 0.1) | 0.00921 [0.00335; 0.0174] | 0.019 |
| Presence of infratentorial lesion in the last brain MRI (no versus yes) | 0.0647 [− 0.218; 0.420] | 0.6 |
CI confidence interval, EDSS Expanded Disability Status Scale, NTZ natalizumab
† Multivariate linear regression model
At 1 year, absolute EDSS scores were similar in both groups (2.8 ± 1.8 with fingolimod and 2.3 ± 1.7 with ocrelizumab, p = 0.18). In the subgroup of patients with relapses at 1 year, 5/17 patients (29.4%) treated with fingolimod had EDSS worsening and 0/5 patients treated with ocrelizumab (p = 0.29).
On the first brain MRI after NTZ cessation, 2/33 patients (6.1%) with fingolimod had a gadolinium-enhancing lesion compared to 0/31 patients with ocrelizumab (p = 0.5). New T2-lesion occurred in 5/30 patients (16.7%) with fingolimod and 0/33 patients with ocrelizumab (p = 0.02). At one year, new T2-lesion occurred in 4/24 patients (16.7%) with fingolimod and 1/19 patients (5.3%) with ocrelizumab (p = 0.36).
At one year, more patients with fingolimod (24/43, 55.8%) had at least one criteria of EDA compared to patients with ocrelizumab (5/33, 15.2%; p < 0.001).
Discussion
Our study showed a superiority of ocrelizumab to fingolimod as an exit strategy after NTZ cessation, with a 70% lower relapse rate at 1 year and threefold lower risk of relapses. Furthermore, the occurrence of EDA at 1 year is clearly in favor of ocrelizumab, with a very low level of EDA in these patients (15.2%).
The management of drug switches in MS is crucial, in particular for drugs acting on lymphocyte migration or trafficking, such as NTZ or fingolimod, because of the high risk of a return of disease activity. In our study, the proportion of patients with fingolimod who had a relapse was similar or slightly higher than that in previous observational studies, which was 16–20% within 6–18 months [3, 6]. In the same way, our results with ocrelizumab are consistent with previous studies with a proportion of patients with a clinical activity of 2–8% [6, 7]. Our results confirm those of Alping et al., who showed a superiority of rituximab compared to fingolimod in the prevention of relapses within 1.5 years [6]. This difference in efficacy could be explain by an intrinsic superiority of ocrelizumab to fingolimod on disease activity rather than a faster immunosuppression because both drugs lead to a fast lymphocyte depletion or redistribution [8, 9].
The washout period before ocrelizumab initiation was particularly short in our study, namely a mean 30 days, with only one patient excluded for a washout period > 6 weeks (47 days). This is shorter than other studies with a median time of 44–75 days [7, 10]. We reported no serious infection and especially no PML, suggesting a safety of this switch even with a washout < 6 weeks. To do a short washout period, the switch phase needs to be prepared particularly when the cessation of NTZ is not urgent, as for example a high JCV index. Thus, vaccinations with inactivated vaccines (pneumococcal or hepatitis B) or COVID-19 vaccines should be done under NTZ so as not to prolong the washout period and to have better immunization than under fingolimod or ocrelizumab. In the same way, immunization against germs requiring vaccination by live attenuated vaccines, as VZV, should be checked early in the management of MS.
Our study has several limitations. Although we made an adjustment with classic confounders, the first limitation is the retrospective design which exposes to potential selection bias. The second is a potential information bias because several patients were treated with fingolimod before the availability of ocrelizumab. Indeed, the reason for NTZ cessation maybe differs between groups in particular for the lack of efficacy of NTZ which could be stricter for more recent patients because more disease-modifying treatments were available. However, lack of efficacy was a reason for only two patients in both groups. The third is that the time with NTZ was longer in ocrelizumab group probably due to physicians or patients waited for the availability of the treatment. Although NTZ acts on lymphocytes trafficking and has a limited duration of action, a remanent effect is theoretically possible and could influence relapse rate. To mitigate that, analyses were adjusted with this variable.
In conclusion, patients with ocrelizumab had fewer relapses at 1 year compared to patients with fingolimod, suggesting that ocrelizumab is potentially a better exit strategy than fingolimod after NTZ cessation, with an impressive reduction risk of relapse (70%). These results will need to be confirmed in larger and longer studies.
Acknowledgements
The authors thank Carole Berthe and Thomas Senger for their assistance in collecting the clinical data.
Declarations
Conflicts of interest
LK, TF, MF, CG, and NC declare that they have no conflict of interest. KB and JDS report fees as consultant or for presentations in partnership with Biogen, Roche, Novartis and BMS/Celgene. MG reports fees as consultant or for presentations in partnership with Biogen. GA reports grant travel in partnership with Biogen, Novartis, Roche, Sanofi, Abbvie, and Pfizer. SC reports fees for presentations in partnership with Biogen, and Novartis.
Ethical approval
This study was approved by local ethics committee. All patients gave written informed consent to be included in EDMUS.
References
- 1.Bigaut K, Fabacher T, Kremer L, et al. Long-term effect of natalizumab in patients with RRMS: TYSTEN cohort. Mult Scler. 2021;27:729–741. doi: 10.1177/1352458520936239. [DOI] [PubMed] [Google Scholar]
- 2.Iaffaldano P, Lucisano G, Pozzilli C, et al. Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain. 2015;138:3275–3286. doi: 10.1093/brain/awv260. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M, Maillart E, Tourbah A, et al. Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol. 2014;71:436–441. doi: 10.1001/jamaneurol.2013.6240. [DOI] [PubMed] [Google Scholar]
- 4.Confavreux C, Compston DA, Hommes OR, et al. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry. 1992;55:671–676. doi: 10.1136/jnnp.55.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medistica (2020) pvalue.io, a graphic user interface to the R statistical analysis software for scientific medical publications. Available on: https://www.pvalue.io
- 6.Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79:950–958. doi: 10.1002/ana.24651. [DOI] [PubMed] [Google Scholar]
- 7.Ellwardt E, Rolfes L, Klein J, et al. Ocrelizumab initiation in patients with MS: a multicenter observational study. Neurol Neuroimmunol Neuroinflamm. 2020 doi: 10.1212/NXI.0000000000000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese MC, Kaine JL, Lowenstein MB, et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:2652–2661. doi: 10.1002/art.23732. [DOI] [PubMed] [Google Scholar]
- 9.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 10.Levin SN, Ezuma C, Levine L, et al. Switching from natalizumab to ocrelizumab in patients with multiple sclerosis. Mult Scler. 2020;26:1964–1965. doi: 10.1177/1352458520927631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request from any qualified investigator.