Abstract
Immunotherapy has revolutionized cancer therapy, largely attributed to the success of immune-checkpoint blockade. However, there are subsets of patients across multiple cancers who have not shown robust responses to these agents. A major impediment to progress in the field is the availability of faithful mouse models that recapitulate the complexity of human malignancy and immune contexture within the tumor microenvironment. These models are urgently needed across all malignancies to interrogate and predict antitumor immune responses and therapeutic efficacy in clinical trials. Herein, we seek to review pros and cons of different cancer mouse models, and how they can be used as platforms to predict efficacy and resistance to cancer immunotherapies.
Significance:
Although immunotherapy has shown substantial benefit in the treatment of a variety of malignancies, a key hurdle toward the advancement of these therapies is the availability of immunocompetent preclinical mouse models that recapitulate human disease. Here, we review the evolution of preclinical mouse models and their utility as coclinical platforms for mechanistic interrogation of cancer immunotherapies.
Recent years have seen dramatic advances in our understanding of the interactions between tumor development and the immune system. In parallel with these efforts, there has been a revolution in the development of therapeutics aimed at harnessing the immune system to mount effective antitumor responses. This is most clearly exemplified by the success of immune-checkpoint blockade (ICB) across a variety of malignancies (1). In addition, vaccine, engineered T-cell transfer, and immunomodulatory strategies hold promise for the future of cancer therapy (2). With the increased focus on the development of effective immunotherapies, a critical challenge is the development of immunocompetent mouse models that replicate human disease and can be utilized to coclinically test novel cancer immunotherapies in parallel with early-phase human investigation.
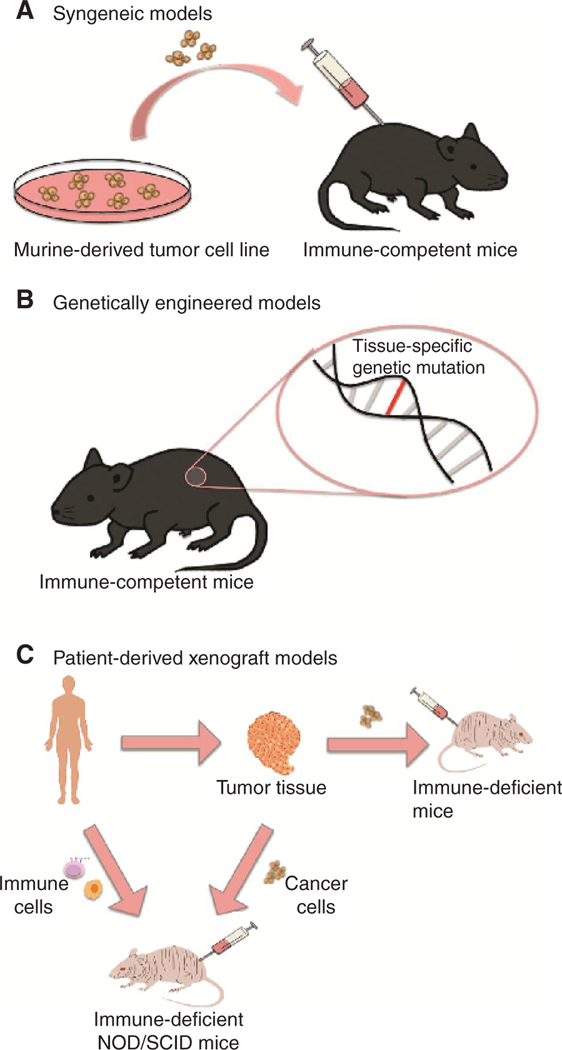
A crucial aspect of any preclinical model system is that it mimics human cancer development—this includes models that faithfully reproduce the genomic heterogeneity of human cancer, as well as develop a milieu that incorporates the multitude of immune and stromal cell populations that make up the complex tumor microenvironment. However, the development of preclinical tumor models to study immunotherapeutics presents a unique set of considerations. First, the industry gold standard for the development of standard cytotoxic cancer therapies is to utilize xenograft models of human cancer cell lines engrafted into immunocompromised mice to evaluate pharmacology, efficacy, and safety profiles of these agents (3). However, the development of immunotherapy requires a model system with a functionally intact immune system. Second, the inherent heterogeneity and adaptability of the immune system likely explains the relative success of immunotherapy, in that it is able to constantly adapt and evolve along with the tumor. However, finding preclinical models that can recapitulate this adaptability in the face of tumor heterogeneity remains a major impediment in the development of cancer immunotherapies. In this review, we discuss the current landscape of preclinical models available for coclinical immunotherapeutic evaluation (Fig. 1) and the pros and cons associated with each model in this context (Table 1).
Figure 1.
Preclinical murine models for evaluation of immuno-oncology agents. A, Syngeneic tumor models utilize murine tumor cell lines that are grown and expanded in vitro and then injected into immunocompetent hosts (commonly either subcutaneously or orthotopically). B, Genetically engineered mouse models incorporate tissue-specific expression of oncogenes or tissue-specific deletion of tumor suppressors, which drives autochthonous tumor cell growth. C, Patient-derived xenografts involve collecting tumor tissue from patients and then injecting into immunodeficient murine hosts and further propagation in vivo. Tumor cells can be injected alone or can be injected along with immune reconstitution using autologous immune cells (from peripheral blood, bone marrow, or selected progenitor populations).
Table 1.
Preclinical models for cancer immunotherapy
| Model | Advantages | Disadvantages |
|---|---|---|
|
| ||
| Syngeneic tumor cells | • Reproducible and rapid growth • Easily manipulated • No host breeding requirements |
• Lack of native tumor microenvironment • Variability of phenotype depending on the site of engraftment • Relatively few transplantable cell lines • Lack of heterogeneity • Relatively few host strains |
| Genetically engineered | • Autochthonous growth provides native microenvironment • Tumor development driven by relevant genetic alterations • Can incorporate genomic instability |
• Breeding challenges • Variability in penetrance and latency • Low immunogenicity due to defined perturbations |
| Patient-derived xenograft | • Reproduces complexity of human disease (genomic heterogeneity, cell types) • Does not require immune reconstitution |
• Conducted in immune-deficient host (rely on human immune cells transferred in xenograft) • Murine stroma • Low implantation rate |
| Humanized patient-derived xenograft | • Reproduces complexity of human disease and immune system • Can utilize engineered hosts to increase immune reconstitution |
• Requires autologous immune reconstitution • Low rates and duration of immune reconstitution |
MURINE TUMOR MODELS
Syngeneic Tumor Cell Lines
Syngeneic tumor models are the oldest and most heavily utilized preclinical models to evaluate anticancer therapeutics. Using inbred strains such as C57BL/6, BALB/c, and FVB mice, it is possible to isolate spontaneous, carcinogen-induced, or transgenic tumor cell lines that can be expanded in vitro, and then used to inoculate wild-type hosts to establish a tumor-bearing system. As these models are fully immunocompetent, they are particularly useful in the evaluation of immuno-oncology agents, as they can be used to study the generation of de novo antitumor immune responses and do not require the adoptive transfer of immune populations.
One of the most common uses of syngeneic tumor models involves using carcinogens to induce tumor formation in mice and directly evaluating these tumor-bearing mice for antitumor efficacy of tumor immunotherapies. These models were used in the groundbreaking work by Schreiber and others to identify and characterize the process of immunoediting (4), using agents such as methylcholanthrene (MCA). Carcinogen-induced models can be interrogated for their impact on tumor development and anticancer immune responses, as well as the evaluation of immunotherapies (5). In contrast to genetically defined cancer models, carcinogen-induced models provide a higher level of genomic instability, resulting in the development of a more “physiologically relevant” tumor microenvironment. However, this complexity also comes with challenges, including issues regarding tumor penetrance and latency, as well as a lack of shared tumor antigens.
Many of these carcinogen-derived tumors were used to generate cancer cell lines, which are commonly utilized to develop syngeneic murine tumor models. One of the central benefits of syngeneic tumor cell lines is their ease of use. Using tumor cell lines that can be rapidly and reproducibly expanded in large numbers prior to implantation into hosts, these syngeneic models can be used for studies that require large group numbers that are difficult to obtain using genetically engineered models or patient-derived xenografts (PDX). Another logistical advantage of using syngeneic tumor models is that they can be genetically manipulated to evaluate specific tumor cell–intrinsic sensitivity or resistance biomarkers of response to immunotherapy. For example, studies evaluating antigen-specific immunization approaches can utilize tumor cells that are engineered to express the target antigen and can be used for in vitro and in vivo analysis of antitumor effector responses. This can be challenging when targeting antigens whose expression is normally restricted to tissues that lack effective preclinical models. Additionally, it is possible to evaluate the relative contribution of various factors that may affect immunotherapeutic efficacy. This is particularly relevant to checkpoint blockade, where checkpoint ligands can be removed or altered to evaluate their contribution to the antitumor response.
Although their ease of use and experimental reproducibility has led syngeneic tumor models to become the most commonly used preclinical model for the evaluation of immunotherapies, these pragmatic benefits also highlight one of the detrimental aspects of this system: namely, that these models lack the genomic and microenvironmental heterogeneity that defines cancer. Tumor heterogeneity results in not just interpatient heterogeneity that makes every patient’s cancer unique, but also intrapatient heterogeneity (6). This is one of the major challenges for the development of effective cancer therapies, and thus an ideal preclinical system to interrogate immunotherapeutics would also effectively model this heterogeneity. However, syngeneic tumor models are largely deficient on both accounts—the cell lines lack mutational patterns that recapitulate human intrapatient genomic heterogeneity and are implanted into a limited number of inbred strains of mice that lack the interpatient heterogeneity. The lack of mutational heterogeneity within the syngeneic xenograft tumors is due in part to the lack of cancer stem cells and other progenitor populations that are present in the tumor microenvironment, which can provide a constant source of tumor mutational evolution (7). In addition, mutational heterogeneity requires clonal evolution of differentiated cancer cells, which can be challenging with many syngeneic murine models given their overall lower levels of genomic instability compared with humans (8). Moreover, syngeneic tumor models often have undergone significant selection through adaptation to stringent in vitro or in vivo conditions, the effect of which is to limit clonal diversity. Although implanted syngeneic tumor cells can certainly behave differently during the outgrowth of these tumors in vivo, they often lack the intrinsic characteristics that contribute to genomically heterogeneous tumors. To overcome these challenges, it is possible to inject multiple lineages to result in tumors that are comprised of multiple populations (9); however, this artificial heterogeneity does not impart the tumor cell–intrinsic functional plasticity that helps tumors constantly adapt and evolve to the immune response during the immunoediting process (4).
Another challenge associated with syngeneic models is that the implanted tumors develop as de novo poorly differentiated malignancies and do not undergo the natural steps of tumor evolution that can be observed with genetically engineered models, which include premalignant transformation, tumor development, and progression (10). This results in abbreviated tumor growth in most syngeneic tumor models, resulting in tumor outgrowth over the span of weeks. In contrast, there is usually a latency period prior to development and amplification of antitumor immune responses elicited by immunotherapy (as opposed to cytotoxic agents), so clinical benefit is often observed as increases in overall survival compared with objective clinical responses (11). Therefore, the rapid kinetics for tumor growth in syngeneic models often provides an inadequate time window to evaluate immunotherapy efficacy. In addition, it does not permit the evaluation of immunotherapeutics in earlier stages of disease, which have been suggested as a potentially optimal time point to initiate immunotherapeutic interventions in certain cancers to maximize clinical benefit (12).
A pivotal consideration for preclinical models evaluating immunotherapies is the role of the tumor microenvironment in the generation of antitumor responses. The various populations that come together to form a malignant lesion—not just the tumor cells, but also the surrounding stroma, vasculature, and immune populations themselves—are all critical components in determining whether immune-based therapies will be able to successfully infiltrate and eliminate tumor cells. However, most syngeneic tumor models utilize subcutaneous implantation, which (although convenient for measuring tumor shrinkage) lacks the complex architecture that is associated with de novo tumor growth. Although orthotopic injections have attempted to circumvent this limitation, this can be technically demanding depending on the tumor site and also relies on injecting cancer cell lines that are already poorly differentiated into a healthy organ. As a result, although the tumor may grow at the organ site, the lack of autochthonous disease does not allow for the natural development of the complex tumor microenvironment. Additionally, the process of injecting syngeneic tumor cells itself can promote inflammatory immune responses, which can vary depending on the site of injection, further confounding the interpretation of immunotherapeutic efficacy (13). As a result, the identification of reliable autochthonous genetically engineered mouse models (GEMM) for tumor development and therapeutic evaluation has been a focus of intense research over the past two decades.
Genetically Engineered Mouse Models
Over the last two decades, there has been a remarkable growth in our understanding of the genetic basis for cancer, followed by the development of genetic engineering techniques. These advances have led to the invention of mouse models with incorporation of specific genomic alterations to provide autochthonous tumor development in a tissue-specific manner. These models predominantly utilize either tissue-specific promoters to drive either expression of an oncogene (either viral oncogenes such as the SV40 large T antigen, ref. 10; or oncogenes relevant to tumor formation, such as Kras and MYC in breast cancer, ref. 14, or BRAFV600E in melanoma, ref. 15) or tissue-specific expression of recombinase enzymes to drive deletion of tumor suppressors (such as PTEN and TP53 in prostate cancer, ref. 16; or APC in colon cancer, ref. 17). Using these genomic alterations, it is possible to not only drive autochthonous invasive cancer development but also develop precancerous lesions such as prostatic intraepithelial neoplasia in prostate cancer models (18) or pancreatic intraepithelial neoplasia (PanIN) in pancreatic cancer models (19). This extended period of tumor development and progression allows for longer windows for immunotherapeutic intervention, which are often needed to generate an effective antitumor immune response and to model immune-related adverse events (irAE; ref. 20).
As genetically engineered models drive neoplastic transformation of normal cells at the relevant organ site to drive tumor growth, this gradual development and progression of cancer allows for the autochthonous development of a complex tumor microenvironment. This is a critical advantage of GEMMs, relative to syngeneic tumor models, that makes them particularly relevant for evaluating immunotherapeutic modalities. The de novo tumor microenvironments that develop in the context of GEMMs contain native immunosuppressive stroma and vasculature, both critical variables that determine the magnitude and composition of immune cell infiltration. Additionally, it is possible to utilize models where the alteration driving tumor growth is relevant to the tumor immune microenvironment and ultimately can affect the efficacy of immunotherapy. For example, loss of PTEN in melanoma has been shown to associate with an immunosuppressive tumor microenvironment (21), and, using models in which tumor development is driven by PTEN loss, it is possible to evaluate therapeutic modalities that make these tumors more susceptible to immunotherapeutic intervention (21).
In contrast to human disease, which most often arises from the gradual accumulation of mutations in a small fraction of cells within the organ of origin that ultimately lead to transformation, GEMMs that utilize tissue-specific promoters drive oncogenesis in all cells of that lineage. In addition, by overexpression or deletion of a select number of genes, the tumor mutational burden in genetically engineered models may not replicate that seen in the corresponding human disease (22, 23). This is critical when evaluating immunotherapies, as increased mutational burden (and subsequent neoepitope generation) is an important consideration when evaluating the efficacy of ICB (24). However, by targeting genes associated with mismatch repair and genomic stability (such as MLH1, ref. 25; BRCA1/2, ref. 26; APC, ref. 17; and mTERT, ref. 27), it is possible to not only promote tumorigenesis but also drive the accumulation of additional mutations. This increased mutational rate promotes generation of neoantigens that can be recognized by CD8+ T cells (28) and facilitates immunoediting (4, 29). In addition, the increased genomic instability can facilitate coevolution of the antitumor immune response along with tumor escape mechanisms, which may ultimately lead to immunotherapy resistance.
Although GEMMs have several advantages in evaluating immunotherapeutics, these models suffer from some of the same logistical challenges that hamper their use in the evaluation of cytotoxic therapies. First, a central challenge when using GEMMs is with regard to penetrance of the tumor phenotype and latency of neoplastic development, which can vary drastically depending on the mechanisms used to induce tumor development. These limitations can be partly overcome via development of GEMMs that target multiple oncogenes or tumor suppressors that can increase penetrance and decrease latency (30). Second, it is necessary to utilize noninvasive imaging modalities, such as ultrasound or magnetic resonance imaging, to monitor tumor development, standardize treatment scheduling, and monitor the kinetics of antitumor immune responses. Third, as with syngeneic models, it is important to consider whether the murine immune target is cross-reactive with the corresponding human target. This includes antigens and surface markers that are present on human immune cells or tumors that are not present on murine cells. Cross-reactivity is particularly relevant with regard to the development of immunotherapeutic vaccines, which recognize antigens within the context of human MHC class I in patients (antigens that are not presented in the context of murine MHC class I). To evaluate these peptide-specific responses, GEMMs that incorporate human MHC class I and MHC class II have been developed that can be used to evaluate peptide-specific T-cell responses that are relevant to human antitumor immune responses (31). Additionally, expression models have been developed that can incorporate expression of target antigens into GEMMs (32), allowing for the rapid development of models that can be used to evaluate antigen-specific immunotherapies. The antigen processing differences between these mouse and human antigen-presenting cells remain, which can affect cross-reactivity with human epitopes, illustrating the underlying challenges that hamper all preclinical models that utilize murine versus human cancer cells. Therefore, an optimal model to evaluate immunotherapies would focus on responses against human tumors in immunocompetent models.
More recently, a large series of congenic C57BL/6J mouse melanoma cell lines were derived from a panel of murine melanoma GEMMs induced by combinations of genetic drivers similar to those found in the human disease (BrafV600E, and deficiencies in Pten, Trp53, Ink4a/Arf, etc.; ref. 33). Called the Yale University Mouse Melanoma (YUMM) syngeneic cell lines, several have been further mutagenized to generate greater somatic mutational loads (34). These panels of genetically defined syngeneic cell lines possess the advantage of being derived from the same tissue type, and incorporate the genetic precision of GEMMs with the ease of use of transplantable cell lines. With a sufficient number of these lines, genomic comparisons of the responders against the nonresponders may uncover genetic mechanisms of resistance to immuno-oncology therapeutics. This would require a concerted effort to collect and characterize such panels, as was done for human tumors.
HUMANIZED TUMOR MODELS
PDX Models
Human xenograft models, using human cell lines injected into immunocompromised hosts such as athymic nude or severe combined immunodeficiency (SCID) animals, are one of the oldest models used to evaluate cytotoxic therapies against cancer. These models have particular relevance for the development of chimeric antigen receptor (CAR) therapies, which can utilize either human cell lines or patient-derived samples to generate xenografts for antitumor efficacy evaluation (35). One of the critical factors that determine the utility of human xenograft models for other immunotherapeutic applications is the degree of immunodeficiency of the murine host. The classic athymic nude mice lack normal thymic development and therefore are deficient in T-cell function. However, because functional innate immune populations such as neutrophils and dendritic cells, as well as B cells and natural killer (NK) cells, remain, many aspects of the immune response, though perturbed, are present in athymic nude mice. Therefore, engraftment of human hematopoietic elements and other primary human cells is quite limited in this model. SCID mice are deficient in a DNA-dependent protein kinase required for T- and B-cell development, and Rag-deficient mice have defective Rag1 and Rag2 genes that are also deficient in T- and B-cell function. The knockout of the IL2rγ chain induces concurrent deficiencies in IL2, IL4, IL7, IL9, IL15, and IL21 receptor functions and generates mice that lack NK cells. By combining genetic mutations, the immunodeficiency in the resultant mice worsens, and with it comes an improvement in the engraftment of donor human immune cells. Thus, the best mice for the engraftment of human hematopoietic stem cells are derived from SCID, Rag1null, or Rag2null mice coupled with a targeted mutation in the IL2rγ gene (36). The NOD/SCID IL2rγ chain knockout or “NSG” mouse (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ), a preclinical model with engineered combined immunodeficiency, has been among the most frequently used host for chimeric human–mouse immune reconstitution as well as other tissue chimeras (37).
Whereas athymic nude mice were sufficient for engraftment of human cancer cell lines, the NSG mice and their equivalents are necessary for engraftment of primary human tumors (38). Using these primary tumor samples, it has been possible to create PDXs that accurately model the complexity involved in natural tumor development, including genomic heterogeneity, tumor architecture, and microenvironment factors that are critical to develop an effective in vivo preclinical tumor model for therapeutic evaluation (although these tumors are usually injected subcutaneously as opposed to orthotopically; ref. 39). Given the impact of immunotherapy across multiple human malignancies, and the limitations of both syngeneic cell line–based models and GEMMs in developing tumors that recapitulate the genotypic and phenotypic heterogeneity of human cancers, there has been a growing interest in developing methods of human immune reconstitution within PDX models in order to construct an experimental humanized model for evaluation of immunotherapy. This experimental system theoretically requires a match between the reconstituted human hematopoietic system and the tumor from the same patient. Although elegant in principle, there are impediments to the efficient generation of humanized PDXs. First, a PDX model requires high take rates for successful propagation of tumors across multiple mice within a reasonable timeframe. Second, whereas the PDX models can be generated from a single tumor that is propagated across multiple mice, the hematopoietic system cannot, so repeated invasive sampling would be necessary to develop individual humanized PDX mice. Moreover, patient survival times and resultant access to hematopoietic blood cells could be limited, thus lowering the pragmatic feasibility of this approach.
Recently, it has been shown that NOD/SCID IL2Rγ (null; NSG) mice reconstituted with umbilical cord human CD34+ cells can induce regression of an engrafted human PDX tumor in response to anti–PD-1 therapeutics (40). The hematopoietic stem cells were allogeneic to the PDX tumor, and excellent antitumor responses were observed, taking advantage of the graft-versus-tumor effect. These responses were dependent on human hematopoietic engraftment, the presence of human CD8+ T cells, and the administration of an anti–PD-1 agent, and were observed regardless of the degree of HLA matching between the hematopoietic stem cells and the tumor. Importantly, some donor CD34+ cells exhibited an excellent response, whereas others were nonresponsive, revealing significant variation in the antitumor effects of T cells from different donors on the same PDX tumor. This study suggests that mice harboring human hematopoietic stem cells and a PDX tumor may have some use in deciphering the mechanisms of differential T-cell antitumor activation in human populations (40). However, this also illustrates a challenge with utilizing allogeneic immune cells to reconstitute PDX-bearing animals; as these allogeneic immune cells lack the normal immune surveillance process, tumor rejection may represent an allogeneic response rather than recognition of tumor-associated antigens that would naturally occur outside of an allogeneic system.
In an ideal humanized PDX model system, the hematopoietic stem cells would be autologous to the engrafted tumor. Reconstitution of a human immune system in an immuno compromised mouse can also be accomplished by using peripheral blood mononuclear cells (PBMC) from adults. Although the ease of collection of PBMCs from autologous patients allows for their use in autologous tumor-bearing PDX, challenges arise with regard to the length of time these cells remain viable after engraftment, which limits their utility in the evaluation of immunotherapies. Moreover, PBMCs generate a robust graft-versus-host (i.e., human vs. mouse) reaction, which also limits the window of time for observation (41). Thus, PBMCs, even from autologous human sources, have a significantly limited utility in examining in vivo immune-checkpoint inhibitor action. In contrast, it is possible to generate long-term engraftment using CD34+ cells or other hematopoietic progenitor-rich populations (42). These transferred progenitors can be further modified to incorporate chemokines and other agents that promote long-term engraftment into immunocompromised hosts, as well as other immunomodulating factors that promote the generation of stromal cells and the formation of the tumor microenvironment (42). In addition, the use of autologous tumor-infiltrating lymphocytes to reconstitute PDX-bearing animals has been shown to recapitulate antitumor responses seen in patients (43), although this technology will be limited to malignancies in which sufficient amounts of tumor-infiltrating lymphocytes can be obtained and expanded.
An alternative approach to promote expansion of human immune populations is to make changes to the host, instead of altering the transferred cells. For example, an NSG-SGM3 mouse model was generated by transgenically expressing the human stem cell factor, granulocyte macrophage colony- stimulating factor, and IL3. This model promotes long-term engraftment with expansion of T-cell (CD4+, CD8+, and Tregs), B-cell, and myeloid populations, thus reconstituting an immune system representative of natural complexity (44). Another genetically modified host for immune reconstitution is the MSTRG or MISTRG mouse, which is generated on a Rag2- and IL2rγ-deficient background and has been engineered to express macrophage colony-stimulating factor, thrombopoietin, granulocyte/macrophage colony-stimulating factor, either with or without IL3, thus enabling long-term engraftment in both secondary immune tissues and nonhematopoietic organs (45). In addition, NSG mice have been modified to incorporate expression of HLA-A2, an MHC class I haplotype particularly relevant to the development of immunotherapies and the evaluation of antigen-specific T-cell responses. How these modifications will affect antitumor response in reconstituted mice is currently unclear but brings the experimental system closer to the fully humanized state.
FUTURE PERSPECTIVES
Given the recent FDA approvals of cancer immunotherapy across multiple malignancies, the continued development of effective preclinical models to reliably elucidate antitumor mechanism and predict antitumor efficacy in human immunotherapy clinical trials remains an area of critical unmet need. For example, the promising preclinical results observed evaluating IDO inhibitiors in combination with ICB in preclinical studies were not recapitulated in phase III melanoma clinical trials. Although current practices largely rely on syngeneic tumor models, the advent of increasingly complex GEMMs that more accurately reflect the autochthonous tumor microenvironment is valuable for predictive evaluation of tumor immunotherapies in the preclinical setting. Furthermore, the continued development of humanized PDX models has the potential of ushering in an era of personalized immunotherapy, i.e., “coclinical” approach, where a patient’s tumor is implanted into humanized immune-reconstituted preclinical models to guide therapeutic decision-making. There are several limitations across syngeneic, GEM, and humanized PDX models, thus posing a challenge for any single model type to recapitulate the heterogeneous antitumor immune responses observed in immuno-oncology clinical trials. However, the continued development of more sophisticated preclinical models will allow us to individualize their selection to most accurately represent the human malignancy being tested and assess responsiveness to immunotherapy. For example, the colorectal MC38 cell line harbors a microsatellite-unstable phenotype (MSI), resulting in a T cell–inflamed tumor microenvironment and robust responses to ICB. On the other hand, the colorectal CT26 cell line harbors a microsatellite-stable (MSS) model, resulting in a non-T cell–inflamed tumor microenvironment and poor responses to ICB. The immunophenotypic characteristics and responsiveness to ICB for MC38 and CT26 lines mirror what is observed in the clinic with patients with MSI and MSS colorectal cancer, respectively (46).
Although the generation of humanized PDX models has focused on reconstituting the patient’s immune system, a critical advance will be to incorporate microbiome analysis into the paradigm of preclinical models. Given that the microbiome within preclinical models can be influenced by several variables, which include the vendor from which animals are procured and housing conditions while on study, it is critical to incorporate these variables when assessing therapeutic responses to immunotherapy. In addition, we anticipate that therapeutic approaches designed to alter the microbiome will become increasingly incorporated into the immunotherapeutic armamentarium (47).
Another critical application of preclinical tumor models that requires further investigation is the modeling of irAEs, as preclinical models do not recapitulate the nature, kinetics, and severity of toxicity observed in patients treated with ICB. As immuno-oncology agents show an unpredictable pattern of irAEs in patients, the discovery of early biomarkers of irAEs and strategies to reverse fatal toxicities, such as myocarditis, are a critical step for the safe development of immuno-oncology combination therapies in the clinic (5). This includes utilizing models that have slower kinetics of tumor regression (allowing for a sufficient timeframe to develop irAEs), incorporating broader measures of toxicity, as well as evaluating these therapies in tumor models more susceptible to autoimmunity (such as Treg-depleted model systems; ref. 48). Because irAEs can have diverse manifestations in different tumor histologies, the inclusion of models that accurately reflect irAEs observed in the clinic is of paramount importance (49).
Footnotes
Disclosure of Potential Conflicts of Interest
E.T. Liu is President and CEO of The Jackson Laboratory. No potential conflicts of interest were disclosed by the other authors.
REFERENCES
- 1.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohmueller J, Finn OJ. Current modalities in cancer immunotherapy: immunomodulatory antibodies, CARs and vaccines. Pharmacol Ther 2017;178:31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeVita VT Jr, Chu E. A history of cancer chemotherapy. Cancer Res 2008;68:8643–53. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- 5.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med 2006;12:693–8. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 7.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 2009;138:822–9. [DOI] [PubMed] [Google Scholar]
- 8.Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci U S A 1995;92:4818–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011;19:244–56. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A 1995;92:3439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 2010;15:969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res 2011;17:3884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnotte B, Gough M, Phan V, Ahmed A, Chong H, Martin F, et al. Intradermal injection, as opposed to subcutaneous injection, enhances immunogenicity and suppresses tumorigenicity of tumor cells. Cancer Res 2003;63:2145–9. [PubMed] [Google Scholar]
- 14.Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell 1987;49:465–75. [DOI] [PubMed] [Google Scholar]
- 15.Hooijkaas AI, Gadiot J, van der Valk M, Mooi WJ, Blank CU. Targeting BRAFV600E in an inducible murine model of melanoma. Am J Pathol 2012;181:785–94. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005;436:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 1997;278:120–3. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, et al. Pathobiology of autochthonous prostate cancer in a preclinical transgenic mouse model. Prostate 2003;55:219–37. [DOI] [PubMed] [Google Scholar]
- 19.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382–99. [DOI] [PubMed] [Google Scholar]
- 21.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov 2016;6:202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yen J, White RM, Wedge DC, Van Loo P, de Ridder J, Capper A, et al. The genetic heterogeneity and mutational burden of engineered melanomas in zebrafish models. Genome Biol 2013;14:R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McFadden DG, Politi K, Bhutkar A, Chen FK, Song X, Pirun M, et al. Mutational landscape of EGFR-, MYC-, and Kras-driven genetically engineered mouse models of lung adenocarcinoma. Proc Natl Acad Sci U S A 2016;113:E6409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Germano G, Lamba S, Rospo G, Barault L, Magri A, Maione F, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017;552:116–20. [DOI] [PubMed] [Google Scholar]
- 26.Wright MH, Robles AI, Herschkowitz JI, Hollingshead MG, Anver MR, Perou CM, et al. Molecular analysis reveals heterogeneity of mouse mammary tumors conditionally mutant for Brca1. Mol Cancer 2008;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bojovic B, Crowe DL. Telomere dysfunction promotes metastasis in a TERC null mouse model of head and neck cancer. Mol Cancer Res 2011;9:901–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 2017;17:209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa K, Matsushita H, Oda K, Yamamoto S, Nishijima A, Imai Y, et al. Immunoediting, neoantigen frequency, and clinical outcome in patients with ovarian clear cell carcinoma. Gynecol Oncol 2017;145(Supplement 1):7–8. [Google Scholar]
- 30.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017;355:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pajot A, Michel ML, Fazilleau N, Pancre V, Auriault C, Ojcius DM, et al. A mouse model of human adaptive immune functions: HLA-A2.1-/HLA-DR1-transgenic H-2 class I-/class II-knockout mice. Eur J Immunol 2004;34:3060–9. [DOI] [PubMed] [Google Scholar]
- 32.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell 2011;19:72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meeth K, Wang JX, Micevic G, Damsky W, Bosenberg MW. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res 2016;29:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Perry CJ, Meeth K, Thakral D, Damsky W, Micevic G, et al. UV-induced somatic mutations elicit a functional T cell response in the YUMMER1.7 mouse melanoma model. Pigment Cell Melanoma Res 2017;30:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegler EL, Wang P. Preclinical models in chimeric antigen receptor-engineered T-cell therapy. Hum Gene Ther 2018;29:534–46. [DOI] [PubMed] [Google Scholar]
- 36.Hasgur S, Aryee KE, Shultz LD, Greiner DL, Brehm MA. Generation of immunodeficient mice bearing human immune systems by the engraftment of hematopoietic stem cells. Methods Mol Biol 2016; 1438:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, et al. Humanized mouse models of clinical disease. Annu Rev Pathol 2017;12:187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puchalapalli M, Zeng XK, Mu L, Anderson A, Glickman LH, Zhang M, et al. NSG mice provide a better spontaneous model of breast cancer metastasis than Athymic (Nude) mice. PLoS One 2016;11. e0163521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrne AT, Alferez DG, Amant F, Annibali D, Arribas J, Biankin AV, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer 2017;17:254–68. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Yao LC, Cheng M, Cai D, Martinek J, Pan CX, et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J 2018;32:1537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol 2009;157:104–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drake AC, Chen Q, Chen J. Engineering humanized mice for improved hematopoietic reconstitution. Cell Mol Immunol 2012;9:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jespersen H, Lindberg MF, Donia M, Soderberg EMV, Andersen R, Keller U, et al. Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat Commun 2017;8:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jangalwe S, Shultz LD, Mathew A, Brehm MA. Improved B cell development in humanized NOD-scid IL2Rgamma(null) mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun Inflamm Dis 2016;4:427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito Y, Ellegast JM, Rafiei A, Song Y, Kull D, Heikenwalder M, et al. Peripheral blood CD34(+) cells efficiently engraft human cytokine knock-in mice. Blood 2016;128:1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Efremova M, Rieder D, Klepsch V, Charoentong P, Finotello F, Hackl H, et al. Targeting immune checkpoints potentiates immunoediting and changes the dynamics of tumor evolution. Nat Commun 2018;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gajewski T. Manipulating the microbiome to improve the efficacy of immunotherapy. Clin Adv Hematol Oncol 2016;14:424–6. [PubMed] [Google Scholar]
- 48.Liu J, Blake SJ, Harjunpaa H, Fairfax KA, Yong MC, Allen S, et al. Assessing immune-related adverse events of efficacious combination immunotherapies in preclinical models of cancer. Cancer Res 2016;76:5288–301. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Blake SJ, Smyth MJ, Teng MWL. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin Transl Immunology 2014;3:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]