Abstract
The TSH receptor (TSHR) is the major regulator of thyroid hormone biosynthesis in human thyrocytes by regulating the transcription of a number of genes including thyroglobulin (TG) and thyroperoxidase (TPO). Until recently, it was thought that TSHR initiated signal transduction pathways only at the cell-surface and that internalization was primarily involved in TSHR desensitization and downregulation. Studies primarily in mouse cells showed that TSHR internalization regulates gene transcription at an intracellular site also. However, this has not been shown for genes involved in thyroid hormone biosynthesis in human thyrocytes. We used human thyrocytes in primary culture. In these cells, the dose-response to TSH for gene expression is biphasic with low doses upregulating gene expression and higher doses decreasing gene expression. We used two approaches to inhibit internalization. In the first, we used inhibitors of dynamins, dynasore and dyngo-4a. Pretreatment with dynasore or dyngo-4a markedly inhibited TSH upregulation of TG and TPO mRNAs, as well as TG secretion. In the second, we used knockdown of dynamin 2, which is the most abundant dynamin in human thyrocytes. We showed that dynamin 2 knockdown inhibited TSHR internalization and decreased the TSH-stimulated levels of TG and TPO mRNAs and proteins. Lastly, we showed that the level of the activatory transcription factor phosphorylated cAMP response element binding protein (pCREB) in the cell nuclei was reduced by 68% when internalization was inhibited. We conclude that upregulation of genes involved in thyroid hormone synthesis in human thyrocytes is, in part, dependent on internalization leading to nuclear localization of an activated transcription factor(s).
Keywords: TSH, receptor internalization, gene transcription, human thyrocytes, dynamin 2, phospho-CREB
1. Introduction
A previous consensus was that all G protein-coupled receptors (GPCRs), including TSH receptors (TSHRs), initiated their signaling only at the cell-surface and that internalization was primarily involved in receptor desensitization and downregulation [1-3]. It had been thought that TSHR regulation of gene transcription was primarily via the cAMP-protein kinase A pathway [4] but that this pathway was activated solely at the cell surface. However, studies performed primarily in mouse thyroid cells showed that TSHR regulates gene transcription at an intracellular site also [5]. In mouse thyrocytes, the TSHR co-internalizes with TSH, and a protein complex containing TSHRs, Gs proteins, and protein kinase A at a site in the trans-Golgi network near the cell nucleus induces a second, persistent phase of cAMP production [5, 6]. cAMP generated in the second signaling phase at a critical position near the nucleus, like cAMP generated near the cell surface, binds to and activates protein kinase A that then enters the nucleus and phosphorylates/activates the transcription factor cAMP response element binding protein (CREB). Phosphorylated CREB (pCREB) interacts with a number of co-activators to form activatory or inhibitory complexes that regulate gene transcription [7]. In contrast to mouse thyrocytes, generation of cAMP near the nucleus, which appears to be required for efficient CREB phosphorylation and gene transcription in response to TSH, has not been shown in human thyrocytes, especially not for genes involved in thyroid hormone biosynthesis.
We have shown previously that TSH regulation of genes involved in thyroid hormone biosynthesis in human thyrocytes is biphasic [8, 9]. Low doses of TSH upregulate and higher TSH doses decrease the levels of mRNAs of thyroglobulin (TG), thyroperoxidase (TPO) and other thyroid specific genes leading to the exposition of a biphasic dose-response curve that has been termed an “inverted U-shaped dose-response curve” (IUDRC). This type of regulation has been found in a number of cell systems and has been postulated to be a more sensitive system for regulation of cell function [10]. We have suggested that this type of response prevents overstimulation of thyroid hormone biosynthesis by TSH in human thyrocytes.
In this report, we determined whether internalization was involved in TSH regulation of TG and TPO in primary cultures of human thyroid cells. Specifically, we measured the effect of inhibiting internalization on TSH stimulation on the TG and TPO mRNA and protein levels and on the localization of pCREB in the nucleus. We found that TSH upregulation of these genes is, in part, dependent on internalization.
2. Materials and Methods
2.1. Materials
Dulbecco’s modified Eagles’ medium (DMEM), 1 M HEPES buffer, 100-fold penicillin-streptomycin solution, phosphate-buffered saline (PBS), and Hanks’ balanced salt solution (HBSS) were obtained from Mediatech Inc. (Manassas, VA). HyClone-fetal bovine serum (FBS) was purchased from GE Healthcare (Logan, UT). Dynasore, Dyngo-4a, bovine TSH (bTSH), cycloheximide, and Human Thyroglobulin (TG) ELISA kit were obtained from Millipore-Sigma (St. Louis, MO). ON-TARGET plus Dynamin 2 (DNM2) siRNA, ON-TARGET plus non-targeting pool siRNA (scrambled siRNA), and DharmaFECT1 transfection reagent were purchased from Dharmacon, Inc. (Lafayette, CO). pCREB, TGF-β1, Hyaluronan (HA) ELISA kits were obtained from R&D Systems (Minneapolis, MN). RNeasy Mini Kit was obtained from Qiagen (Germantown, MD). High capacity cDNA Archive Kit was purchased from Applied Biosystems (Foster City, CA). iTaq™ Universal Probe Supermix was obtained from Bio-Rad Laboratories (Hercules, Ca). TaqMan probes for TG (TaqMan assay ID: Hs00174974_m1), TPO (TaqMan assay ID: Hs00892519_m1), DNM2 (TaqMan assay ID: Hs00974704_m1), and GAPDH (TaqMan assay ID: Hs99999905_m1) were purchased from Thermo Fisher Scientific (Waltham, MA). Bovine serum albumin (BSA) was obtained from MP Biomedicals (Santa Ana, CA). Rabbit polyclonal anti-DNM2, Rabbit polyclonal anti-TPO antibodies, Alexa Fluor 647 Protein Labeling Kit, SYTO-9, YOYO-1, goat serum, NuPAGE 4-12% Bis-Tris Gel, Nitrocellulose Membrane, collagenase type IV, RIPA buffer, Protease & Phosphatase Inhibitor Cocktail (EDTA-free), and Tween 20 were purchased from Invitrogen by Thermo Fisher Scientific (Carlsbad, CA). Rabbit monoclonal anti-TG antibody was obtained from Abcam (Cambridge, MA). Rabbit monoclonal anti-phospho CREB antibody was obtained from Cell Signaling (Danvers, MA). Odyssey blocking buffer, IRDye secondary antibodies, and Alexa fluor 633-conjugated anti-rabbit antibody were purchased from LI-COR Biotechnology (Lincoln, NE).
2.2. Primary culture of human thyrocytes
Isolation and primary culture of human thyrocytes were described in our previous publications [11, 12]. The National Institutes of Health Clinical Center provided us normal thyroid tissues from patients undergoing surgery for thyroid tumors. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Institutional Review Board reviewed and approved the studies involving human participants. Normal thyroid tissues were kept in HBSS on ice after the surgery followed by isolation of thyrocytes within 4 hours under sterile conditions. Tissues were minced into small pieces in a 3 cm culture dish containing ice-cold HBSS and centrifuged (150 x g for 5 min). The minced tissues were digested with 3 mg/ml of collagenase type IV solution at 37°C for 30 min, and centrifuged (150 x g for 5 min). The isolated cells were resuspended in DMEM with 10% FBS, 1% penicillin-streptomycin, and 10 mM HEPES and plated in culture flasks. After 5-7 days of culture at 37°C in a humidified 5% CO2 incubator, primary thyrocytes formed a confluent monolayer. They maintained measurable levels of thyroid-specific genes and responded to TSH for up to 12 passages; we used only passages 2 to 6 for the experiments in this report.
2.3. Dynamin 2 (DNM2) knockdown with small interfering RNA (siRNA)
Primary human thyrocytes were seeded into 12-well plates with 1 x 105 cells per well or MatTek dish with 0.25 x 105 cells per dish in DMEM with 10% FBS, 1% penicillin-streptomycin, and 10 mM HEPES. After 1 day, the cells were transfected with 20 nM of ON-TARGET plus non-targeting pool siRNA (scrambled) or DNM2 siRNA using DarmaFECT transfection reagent 1 according to the manufacturer’s directions. After 48 hours of incubation, the transfection medium was changed to DMEM containing 0.1% bovine serum albumin (BSA) followed by incubation overnight. For measuring thyroid-specific gene expressions, the cells were stimulated with 0-1.8 μM (0-100 mU/ml) bovine TSH (bTSH) for 48 hours, lysed, and subjected to quantitative reverse transcriptase PCR (RT-PCR). The conditional medium was collected for measuring secreted TG. The knockdown efficiency of DNM2 determined by Western blot was > 90%.
2.4. Quantitative reverse transcriptase PCR (RT-PCR)
Cells were lysed, and total RNA was purified using RNeasy Mini Kits. First-strand cDNA was synthesized using High Capacity cDNA Archive kit. RT-PCR was performed in 25 μl reaction volumes with the synthesized cDNA, iTaq™ Universal Probe Supermix and probes for TG and TPO. For measuring thyroid-specific gene expressions with dynamin inhibitors (dynasore and dyngo-4a), the cells were pre-treated with 200 uM of dynasore or dyngo-4a for 30 min followed by stimulation with 0, 0.018, and 1.8 μM (0, 1, and 100 mU/ml) bTSH for 48 hours, and the conditional medium was collected for measuring secreted TG. mRNA results were normalized to GAPDH to correct for differences in RNA input. There was no effect of inhibition of internalization on the level of GAPDH within a given experiment.
2.5. Secreted thyroglobulin (TG) measurement
The conditioned media collected from the experiments for quantitative RT-PCR were used to measure TG secretion using a human Thyroglobulin ELISA kit according to the manufacturer’s instructions. For measurement of other secreted factors, we used TGF-β1 and Hyaluronan (HA) ELISA kits to measure the level of TGF-β1 and HA from the conditioned media as well.
2.6. Western blotting
Human thyrocytes were seeded into 12-well plates with 1 x 105 cells per well and cultured for one day. The cells were transfected with siRNA against DNM2 for 48 hours, and the medium was replaced with DMEM with 0.1% BSA overnight. The cells were stimulated with 0, 0.018, and 1.8 μM (0, 1, and 100 mU/ml) bTSH for 48 hours, washed with ice-cold PBS, and lysed with RIPA buffer containing protease and phosphatase inhibitor cocktail. Whole-cell lysates were electrophoresed by SDS-PAGE under reducing conditions on a NuPAGE 4 - 12% Bis-Tris Gel. The separated proteins were transferred to nitrocellulose membranes and blocked with Odyssey blocking buffer. The membranes were incubated with specific primary antibodies for DNM2, TG, and TPO overnight at 4°C, or with anti-GAPDH for 1 hour at RT, and probed with appropriate secondary antibodies conjugated with IRDye. Blots were imaged on the LI-COR Odyssey-CLX imaging system.
2.7. Analysis of internalization of TSHR by confocal microscopy
Labeling of bTSH with Alexa fluor-647 was performed using Alexa Fluor-647 Protein Labeling Kit according to the manufacturer’s directions. Human thyrocytes were seeded into MatTek dish with 0.25 x 105 cells per dish. After one day, cells were transfected with scrambled or DNM2 siRNA for 48 hours. The media were replaced with DMEM with 0.1% BSA overnight. For analysis of TSHR internalization, cells were incubated with Alexa fluor-647 labeled bTSH for one hour at 37 °C and fixed with 4% PFA for 15 min. After 3 washes with PBS, the cells were stained with SYTO-9 for 5 min before imaging by a Zeiss 510 NLO/Meta system (Zeiss, Oberkohen, Germany) with a 40x oil-immersion objective. The fluorescence signal of internalized TSHRs was quantified by ImageJ 1.53e software [13-15] as previously described [16].
2.8. Analysis of total cell and nuclear pCREB
Total pCREB was measured in lysates of cells incubated with 0 or 100 mU/ml (0 and 1.8 μM) bTSH for 30 min using ELISA kit (R&D Systems, Minneapolis, MN). For detection of nuclear pCREB, the cells were stimulated with 0 or 100 mU/ml (0 or 1.8 μM) bTSH for 30 min and fixed with 4% PFA for 15 min. After 3 washes with PBS, the cells were permeabilized with ice-cold methanol for 10 min at −20 °C and washed with PBS 3 times. After one hour of blocking with 5% goat serum, the cells were stained with pCREB anti-rabbit primary antibody overnight at 4°C. The cells were then incubated with a secondary antibody (Alexa fluor-633-conjugated anti-rabbit) for 1 hour at RT. Nucleic acid was stained with YOYO-1 for 15 min at RT prior to taking images by a Zeiss 510 NLO/Meta system with a 40x oil-immersion objective. The levels of nuclear pCREB in over 100 nuclei per experimental condition were quantified with ImageJ 1.53e software [13-15]. To study the effect of newly synthesized TSHR on pCREB, we pretreated thyrocytes after DNM2 KD with cycloheximide (50 μg/ml) for 1 hour and stimulated with 0 or 10 mU/ml (0 or 0.18 μM) bTSH for 2 hours. Subsequently, thyrocytes were lysed with RIPA buffer and subjected to Western blotting analysis with a pCREB antibody.
2.9. Statistics
Data analysis was performed with GraphPad Prism Version 8.1.0 for Windows. Data are expressed as mean ± SEM, and a Student’s t-test or two-way analysis of variance (ANOVA) among groups were used to consider statistical significance with p < 0.05. All experiments in this report were independently performed with thyrocytes from at least three different donors.
3. Results
3.1. Dynasore, a dynamin inhibitor, reduces expression of TG and TPO mRNA stimulated by TSH
The internalization of TSHRs and retrograde trafficking to the trans-Golgi network (TGN), and the induction of a second phase of local cAMP production near the nucleus appears to be needed for fully efficacious nuclear signaling by TSH in mouse models [5]. However, TSH regulation by internalized TSHRs of genes involved in thyroid hormone biosynthesis had not been studied in human thyrocytes. We pharmacologically inhibited internalization using two dynamin inhibitors, dynasore and dyngo-4a, in normal human thyrocytes in primary culture and measured gene expression as mRNA levels of TG and TPO after stimulation with increasing doses of TSH (Fig. 1A, B). It is of note that in vitro cell systems like primary cultures of human thyrocytes are less sensitive to TSH than thyrocytes in humans, and the potency of TSH is shifted to higher doses since the levels of TSHR expression in cultured human thyrocytes are 100-fold lower than in freshly isolated thyroid tissues [17]. Nevertheless, in vitro studies using higher doses of TSH have been found to be good models of TSH action in humans.
Fig. 1. Dynasore, a dynamin inhibitor, reduces expression of TG and TPO mRNA stimulated by TSH.

Primary human thyrocytes were pretreated with dynasore (200 μM) or Control (DMSO) for 30 min followed by stimulation with the indicated doses of TSH for 48 hr. Thereafter, total RNAs were isolated, subjected to synthesize cDNAs, and mRNAs for TG and TPO were measured by quantitative RT-PCR. Data represent the mean ± SEM in thyrocytes from three different donors; ****, p < 0.0001.
We observed an IUDRC for TG and TPO mRNA levels stimulated by TSH without inhibitors [8, 18]. TSH stimulation dose-dependently upregulated TG and TPO at lower doses (< 0.018 μM or < 1 mU/ml) whereas gene expression was decreased at higher doses (> 0.018 μM or > 1 mU/ml) of TSH. Upregulation by TSH was markedly diminished by pretreating the cells with 200 μM dynasore.
3.2. Dynamin inhibitors dose-dependently downregulate TSH-stimulated TG and TPO mRNAs as well as TG secretion
We then treated the thyrocytes with varying doses of dynasore in the absence or presence of TSH (0.018 and 1.8 μM or 1 and 100 mU/ml) to establish the dose-response characteristics of inhibition (Fig. 2A). We used these two doses since 0.018 μM TSH maximally upregulated TG and TPO mRNA expression and 1.8 μM decreased these mRNAs by more than 50% from the maximum levels. TG and TPO mRNAs were dose-dependently decreased by dynasore with a half-maximal inhibitory concentration (IC50) of 1-3 μM. To support the conclusion that the effect of dynasore was due to its effect to inhibit internalization, we examined another dynamin inhibitor analogue, dyngo-4a. Dyngo-4a showed similar inhibition of gene expression as dynasore with an IC50 of 1 μM (Fig. 2B).
Fig. 2. Dynamin inhibitors, dynasore and dyngo-4a, dose-dependently downregulate TG and TPO mRNAs stimulated by TSH.

Primary human thyrocytes were pre-incubated with the indicated doses of (A) dynasore or (B) dyngo-4a for 30 min followed by stimulation without or with TSH (0.018 and 1.8 μM (1, and 100 mU/ml)) for 48 hr. Thereafter, quantitative RT-PCR was used to measure TG and TPO mRNAs. Data represent the mean ± SEM in thyrocytes from three different donors. The IC50s for inhibitors of between1 and 3 uM were significant at all doses of TSH; ****, p < 0.0001.
To confirm the effects of dynasore and dyngo-4a on TG mRNAs at the protein level, we measured the effects of the dynamin inhibitors on the levels of TG secretion. We measured TSH-stimulated TG secretion in the presence of dynasore (Fig. 3A) or dyngo-4a (Fig. 3B). TG secretion was dose-dependently decreased by these inhibitors, which is consistent with the effects on TG mRNA levels.
Fig. 3. TSH-stimulated TG secretion is downregulated by dynamin inhibitors dynasore and dyngo-4a.

Primary human thyrocytes were pretreated with the serial concentrations of (A) dynasore or (B) dyngo-4a, subsequently stimulated without or with TSH (0.018, and 1.8 μM (1, and 100 mU/ml)) for 48 hr. Secreted TG protein was measured in the conditioned media by TG ELISA. Data represent the mean ± SEM in thyrocytes from three different donors. The IC50s for inhibitors of approximately 10 uM were significant at all doses of TSH; *, p < 0.05; **, p < 0.01, ****, p < 0.0001.
3.3. Dynamin 2 knockdown inhibits the internalization of TSHR
As dynasore and dyngo-4a have been shown to inhibit TSHR internalization by inhibiting the action of dynamins (DNMs) in other cell types [19], we confirmed the involvement of DNMs in TSHR internalization in human thyrocytes. We found that all three DNMs are expressed in human thyrocytes but that the predominant DNM is DNM2 (23-fold higher than DNM1 and 12-fold higher than DNM3 at the mRNA level). In order to visualize inhibition of TSHR internalization, we used TSH labeled with the Alexa-647 fluorophore and measured internalization of the TSHR/Alexa-647-TSH complex without or with knockdown of DNM2 using siRNA (Fig. 4A). Unconjugated Alexa-647 fluorophore is too highly charged to permeate cell membranes. Because quantitation of fluorescence in cell monolayers may be difficult, we measured cell-associated Alexa-647 fluorescence as total fluorescence (Fig. 4B) and as the number of fluorescent dots per cell (Fig. 4C). Figure 4 shows that the internalized Alexa-647 labeled TSH was decreased by 75% by DNM2 knockdown compared to scrambled siRNA (Control) by both methods of measurement.
Fig. 4. Dynamin 2 knockdown inhibits the internalization of TSHR.
Primary human thyrocytes were transfected with 20 nM siRNA against dynamin 2 (DNM2) or Control (scrambled) for 48 hr followed by stimulation with Alexa fluor-647-labeled TSH for 1 h. (A) Visualization of internalized TSH/TSHR complexes (red dots) in the cytoplasm and SYTO-9 staining (green) for nucleic acids. (B) Quantification showing the fluorescence of Alexa fluor-647 and (C) fluorescent dots per cell analyzed in 165 cells on average for control and for DNM2 KD. Data represent the mean ± SEM of thyrocytes from three different donors; ***, p < 0.001.
3.4. Knockdown of dynamin 2 decreases TSH-stimulated TG and TPO mRNAs and their proteins
To further support the idea that the inhibition of TSH-stimulated TG and TPO expressions by DNM inhibitors was caused by inhibition of internalization, we used DNM2 knockdown and measured the mRNA levels of TG and TPO (Fig. 5A, B). The efficiency of DNM2 KD was > 90 % (Fig. 6A). We observed that DNM2 knockdown markedly inhibited TSH-stimulated TG and TPO upregulation. We then showed that DNM2 KD inhibited TSH-stimulated upregulation of the cellular levels of TG and TPO protein and of TG secretion (Fig. 6B, C). DNM2 knockdown was not a general inhibitor of secretion as it did not inhibit secretion of hyaluronan, a glycosaminoglycan involved in the pathogenesis of GO that is increased by TSHR activation [20], and had a very small effect on secretion of TGF-β1 (Supplemental Fig. 1).
Fig. 5. TG and TPO mRNAs are downregulated by dynamin 2 knockdown.

Primary human thyrocytes were transfected with 20 nM siRNA against dynamin 2 (DNM2) or Control (scrambled) for 48 hr and stimulated with the indicated doses of TSH for 48 hr. (A) TG and (B) TPO mRNAs were measured using quantitative RT-PCR. Data represent the mean ± SEM of thyrocytes from three different donors; ****, p < 0.0001.
Fig. 6. Knockdown of dynamin 2 decreases TG and TPO proteins.
Primary human thyrocytes were transfected with 20 nM siRNA against dynamin 2 (DNM2) or Control (scrambled) for 48 hr and stimulated with the indicated doses of TSH for 48 hr. (A) Western blot analysis of TG, TPO, and DNM2. GAPDH was used as a loading control. (B) Quantification of the Westen blot analysis; *, p < 0.05; **, p < 0.01. (C) Secreted TG in response to the serial concentrations of TSH. Data represent the mean ± SEM of thyrocytes from three different donors; ****, p < 0.0001.
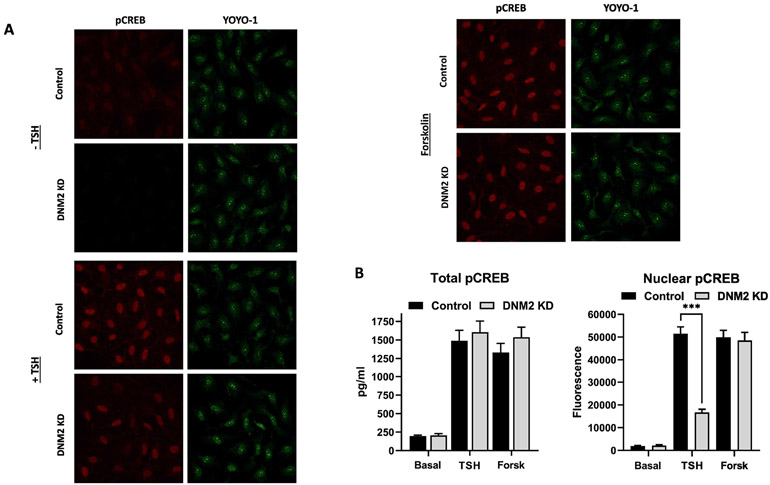
3.5. Inhibition of TSHR internalization by dynamin 2 KD reduces CREB phosphorylation (pCREB) in nuclei
It has previously been shown that activation/phosphorylation of CREB is stimulated by TSH at an intracellular site after TSHR internalization in mouse thyrocytes [21]. Based on these findings, we studied TSH-stimulated CREB phosphorylation (pCREB) in human thyrocytes. We measured pCREB levels in total cell lysates and in the nucleus. We compared pCREB stimulated by TSH to that stimulated by forskolin, an activator of adenylyl cyclase at the cell-surface that does not exhibit any dependence on internalization [22]. Figure 7A shows the photomicrographs of the control cells pretreated with scrambled siRNA and cells pretreated with DNM2 siRNA. Cells were unstimulated or stimulated by TSH or forskolin. Figure 7B illustrates the quantitation of total cell pCREB (left panel) and nuclear pCREB levels (right panel). The levels of pCREB in the total cell lysates of cells pretreated with scrambled siRNA (Control) or pretreated with DNM2 siRNA and stimulated by forskolin or TSH were not different. Moreover, we observed TSH-induced phosphorylation of CREB in the presence of cycloheximide, which is a translational inhibitor. Supplemental Fig. 2 shows that TSH upregulation of total pCREB is not dependent on de novo protein synthesis. That is, total cell pCREB levels were not affected by inhibition of internalization. The levels of nuclear pCREB in control cells stimulated by forskolin were not different than in cells with DNM2 siRNA and stimulated by forskolin. In contrast, the levels of TSH-induced nuclear pCREB in cells in which DNM2 was knocked down were decreased to a level that was 32% of those in cells treated with scrambled siRNA and stimulated by TSH. Thus, DNM2 knockdown selectively reduced nuclear pCREB stimulated by TSH by 68% compared to cells in which DNM2 was not knocked down even though DNM2 knockdown had no effect on total cell pCREB.
Fig. 7. Prevention of TSHR internalization by dynamin 2 knockdown reduces CREB phosphorylation (pCREB) in nuclei.
Primary human thyrocytes were transfected with 20 nM siRNA against dynamin 2 (DNM2) or Control (scrambled) for 48 hr and stimulated without or with TSH (1.8 μM or 100 mU/ml) or forskolin (10 μM) for 120 min. (A) Immunofluorescence of nuclear pCREB with a specific antibody (red) and nucleic acid staining with YOYO-1 (green). (B) Quantification of images of total pCREB measured by ELISA (left panel) and of nuclear pCREB (right panel) as in Figure 7A by fluorescence. Data represent the mean ± SEM of thyrocytes from three different donors; ***, p < 0.001.
4. Discussion
Our data show that TSH stimulation of TG and TPO, which are involved in thyroid hormone biosynthesis, is dependent, in part, on internalization in human thyrocytes in primary culture. Although it was proposed that this was likely, based on data in rodent thyrocytes, those studies did not show internalization-dependencies of genes involved in thyroid hormone biosynthesis. In the studies reported herein, we show that upregulation of human TG and TPO was decreased when internalization was prevented using inhibitors of DNM function or knockdown of DNM2. The mechanism of the decreased levels of TG and TPO protein at high doses of TSH (1.8 μM) in contrast to low TSH doses (0.018 μM) remains unidentified for now. However, it is unlikely that the IUDRC is linked to TSHR internalization since DNM2 knockdown does not have an effect on the IUDRC.
The association of internalization and persistent TSHR-mediated cAMP signaling is dependent on cell context. Our group showed that TSHR internalization is not associated with persistent signaling in HEK293 cells stably expressing the TSHR [19]. In contrast, the findings of Calebiro et al. in mouse thyrocytes demonstrated persistent cAMP signaling by internalized TSHRs [6] and showed co-internalization of TSH-TSHR complexes in intracellular signaling compartments causing the late-response persistent signaling [5]. The study by Wertmann et al. confirmed our data in HEK293 cells [6].
Although we did not show directly that an intracellular site of TSHR signaling was lost by inhibiting TSHR internalization in primary cultures of human thyrocytes, we conclude, based on the previous data from the studies of Calebiro and colleagues [5], that loss of TSHR signaling in the trans-Golgi network close to the nucleus was the cause of decreased upregulation of TG and TPO genes. We acknowledge that internalization of other cell-surface proteins might also be decreased by DNM2 knockdown and they may play a role in regulation of thyroid gene expression. The conclusion that TSH stimulation of TG and TPO is dependent in part on internalization, and that intracellular second phase cAMP signaling and internalization might be linked in human thyrocytes also, was further supported by our demonstration that pCREB, an activatory transcription factor phosphorylated cAMP response element binding protein of some thyroid genes [18, 23], and a downstream target of TSHR signaling, was increased in the nucleus by TSH stimulation and that this increase was inhibited by DNM2 knockdown even though total cell pCREB stimulated by TSH was not decreased by DNM2 knockdown. Upregulation of total cell pCREB by TSH after DNM2 KD was not affected by inhibition of de novo protein synthesis. Moreover, stimulation of nuclear pCREB by forskolin, a direct activator of adenylyl cyclase that does not lead to internalization, was not affected by DNM2 knockdown thereby further supporting the idea that nuclear pCREB was in part dependent on intracellular generation of cAMP close to the nucleus.
It is noteworthy, that dynasore and dyngo-4a were more effective inhibitors of TSH-stimulated TG and TPO gene expression than was DNM2 knockdown. There are several possible reasons for these findings. 1) The DNM inhibitors may not be specific for DNM2 inhibition but have additional actions that added to the inhibition of internalization. 2) Another pathway that is not dependent on DNMs may be involved in TSHR internalization. 3) DNM1 or DNM3 may play a role in TSHR internalization. And 4), DNM2 knockdown may have been causing decreased but not complete inhibition of internalization while DNM inhibitors fully inhibited DNM-mediated internalization (Fig. 4).
To our knowledge, this is the first demonstration that internalization is involved, in part, in TSH-stimulated thyroid hormone biosynthesis in human thyrocytes. Although not proved by our findings, our data support the previous conclusion [5] that the internalized TSH/TSHR complex is a component of a signaling complex (“signalosome”) that transduces TSH signaling from the cell surface to the nucleus. This dependency on internalization appears to be a component of signaling via the cAMP-protein kinase A pathway but may not be involved in signaling by other pathways initiated by binding of TSH to TSHR.
Supplementary Material
Supplemental Fig. 1 DNM2 KD does not affect TSH-stimulated secretion of Hyaluronan (HA) or TGF-β1. Primary human thyrocytes were transfected with 20 nM siRNA against dynamin 2 (DNM2) or Control (scrambled) for 48 hr and stimulated without or with the indicated doses of TSH for 48 hrs. The conditioned media was harvested and the levels of (A) HA and (B) TGF-β1 was measured by ELISA. Data represent the mean ± SEM of thyrocytes from two different donors.
Supplemental Fig. 2 Total pCREB is not dependent on de novo protein synthesis. Primary human thyrocytes were transfected with 20 nM siRNA against dynamin 2 (DNM2) or Control (scrambled) for 48 hr and pre-treated with or without cycloheximide (50 μg/ml) for 1 hr and stimulated with the indicated doses of TSH for 2 hrs. The cell lysates were harvested and (A) total pCREB was measured by Western blot and (B) quantification.
Highlights.
Upregulation of TG and TPO is dependent on TSHR internalization in human thyrocytes
Dynamin inhibitors reduce upregulation of TG and TPO mRNAs as well as TG secretion
Dynamin 2 knockdown inhibits TSHR internalization and upregulation by TSH of TG and TPO mRNAs and proteins
TSH upregulation of nuclear pCREB is reduced by inhibition of TSHR internalization
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Z01 DK011006). We are grateful for the contribution of Shilpa Takur and Sonam Kumari of the Metabolic Disease Branch, National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, who provided us with human thyroid tissue.
Abbreviations
- GPCRs
G protein-coupled receptors
- TSH
Thyroid-stimulating hormone
- TSHR
TSH receptor
- TG
Thyroglobulin
- TPO
Thyroperoxidase
- cAMP
3’,5’-cyclic adenosine monophosphate
- pCREB
phosphorylated cAMP response element binding protein
- IUDRC
inverted U-shaped dose-response curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- [1].Baratti-Elbaz C, Ghinea N, Lahuna O, Loosfelt H, Pichon C, Milgrom E, Internalization and recycling pathways of the thyrotropin receptor, Mol Endocrinol 13(10) (1999) 1751–65. [DOI] [PubMed] [Google Scholar]
- [2].Pierce KL, Premont RT, Lefkowitz RJ, Seven-transmembrane receptors, Nat Rev Mol Cell Biol 3(9) (2002) 639–50. [DOI] [PubMed] [Google Scholar]
- [3].Löf C, Patyra K, Kero A, Kero J, Genetically modified mouse models to investigate thyroid development, function and growth, Best Practice & Research Clinical Endocrinology & Metabolism 32(3) (2018) 241–256. [DOI] [PubMed] [Google Scholar]
- [4].Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G, The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families, Proc Natl Acad Sci U S A 93(1) (1996) 116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Godbole A, Lyga S, Lohse MJ, Calebiro D, Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription, Nat Commun 8(1) (2017) 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Werthmann RC, Volpe S, Lohse MJ, Calebiro D, Persistent cAMP signaling by internalized TSH receptors occurs in thyroid but not in HEK293 cells, FASEB J 26(5) (2012) 2043–8. [DOI] [PubMed] [Google Scholar]
- [7].Chu YD, Yeh CT, The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells, Cells 9(7) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jang D, Morgan SJ, Klubo-Gwiezdzinska J, Banga JP, Neumann S, Gershengorn MC, Thyrotropin, but Not Thyroid-Stimulating Antibodies, Induces Biphasic Regulation of Gene Expression in Human Thyrocytes, Thyroid 30(2) (2020) 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jang D, Marcus-Samuels B, Morgan SJ, Klubo-Gwiezdzinska J, Neumann S, Gershengorn MC, Thyrotropin regulation of differentiated gene transcription in adult human thyrocytes in primary culture, Mol Cell Endocrinol 518 (2020) 111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Calabrese EJ, Hormetic mechanisms, Crit Rev Toxicol 43(7) (2013) 580–606. [DOI] [PubMed] [Google Scholar]
- [11].Morgan SJ, Neumann S, Gershengorn MC, Normal Human Thyrocytes in Culture, Methods Mol Biol 1817 (2018) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, Gershengorn MC, Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice, Proc Natl Acad Sci U S A 106(30) (2009) 12471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rasband WS, ImageJ, National Institutes of Health, Bethesda, MD, USA, 1997-2018. [Google Scholar]
- [14].Abramoff MD, Magalhaes PJ, Ram SJ, Image Processing with ImageJ, Biophotonics International 11(7) (2004) 36–42. [Google Scholar]
- [15].Schneider CA, Rasband WS, Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis, Nat Methods 9(7) (2012) 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krieger CC, Boutin A, Jang D, Morgan SJ, Banga JP, Kahaly GJ, Klubo-Gwiezdzinska J, Neumann S, Gershengorn MC, Arrestin-beta-1 Physically Scaffolds TSH and IGF1 Receptors to Enable Crosstalk, Endocrinology 160(6) (2019) 1468–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boutin A, Krieger CC, Marcus-Samuels B, Klubo-Gwiezdzinska J, Neumann S, Gershengorn MC, TSH Receptor Homodimerization in Regulation of cAMP Production in Human Thyrocytes in vitro, Front Endocrinol (Lausanne) 11 (2020) 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morgan SJ, Neumann S, Marcus-Samuels B, Gershengorn MC, Thyrotropin and Insulin-Like Growth Factor 1 Receptor Crosstalk Upregulates Sodium-Iodide Symporter Expression in Primary Cultures of Human Thyrocytes, Thyroid 26(12) (2016) 1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Neumann S, Geras-Raaka E, Marcus-Samuels B, Gershengorn MC, Persistent cAMP signaling by thyrotropin (TSH) receptors is not dependent on internalization, FASEB J 24(10) (2010) 3992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krieger CC, Neumann S, Place RF, Marcus-Samuels B, Gershengorn MC, Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves' disease immunoglobins, J Clin Endocrinol Metab 100(3) (2015) 1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Calebiro D, Godbole A, Internalization of G-protein-coupled receptors: Implication in receptor function, physiology and diseases, Best Pract Res Clin Endocrinol Metab 32(2) (2018) 83–91. [DOI] [PubMed] [Google Scholar]
- [22].Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ, Persistent cAMP-signals triggered by internalized G-protein-coupled receptors, PLoS Biol 7(8) (2009) e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fernandez LP, Lopez-Marquez A, Santisteban P, Thyroid transcription factors in development, differentiation and disease, Nat Rev Endocrinol 11(1) (2015) 29–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1 DNM2 KD does not affect TSH-stimulated secretion of Hyaluronan (HA) or TGF-β1. Primary human thyrocytes were transfected with 20 nM siRNA against dynamin 2 (DNM2) or Control (scrambled) for 48 hr and stimulated without or with the indicated doses of TSH for 48 hrs. The conditioned media was harvested and the levels of (A) HA and (B) TGF-β1 was measured by ELISA. Data represent the mean ± SEM of thyrocytes from two different donors.
Supplemental Fig. 2 Total pCREB is not dependent on de novo protein synthesis. Primary human thyrocytes were transfected with 20 nM siRNA against dynamin 2 (DNM2) or Control (scrambled) for 48 hr and pre-treated with or without cycloheximide (50 μg/ml) for 1 hr and stimulated with the indicated doses of TSH for 2 hrs. The cell lysates were harvested and (A) total pCREB was measured by Western blot and (B) quantification.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.