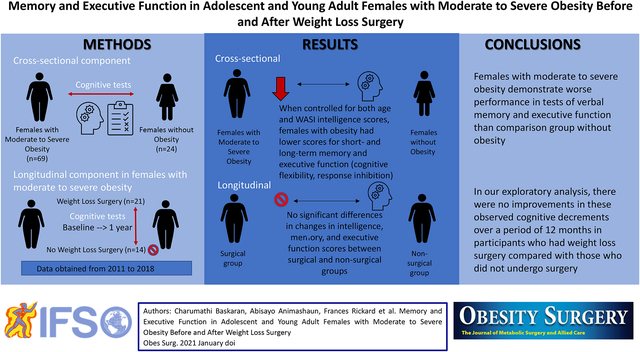
Abstract
There is a global increase in the prevalence of severe obesity in females during adolescence, which is a critical period for neurocognitive development. An increasing number of adolescents and young adults are now undergoing weight loss surgery as a treatment strategy for obesity. In addition to metabolic complications, obesity has been linked to neurocognitive comorbidity, and studies exploring cognitive performance in adolescents with severe obesity and the impact of bariatric surgery on cognitive abilities are limited. Verbal memory and executive function were assessed cross-sectionally in 69 females with moderate to severe obesity and 24 females without obesity, 13–24 years old. In an exploratory analysis, cognitive changes were also assessed longitudinally over 12 months in a subset of 35 females with moderate to severe obesity following weight loss surgery (n = 21) or following usual care without surgery (n = 14). In cross-sectional analysis, females with moderate to severe obesity showed lower scores for short-term and long-term recall (verbal memory) and response inhibition and cognitive flexibility (executive function) than the comparison group, when adjusted for age and baseline intelligence. Females with moderate to severe obesity who underwent surgery showed significant weight loss but no improvement in verbal memory and executive function scores over 12 months compared with those who did not have surgery. In conclusion, females with moderate to severe obesity demonstrate worse performance in tests of verbal memory and executive function than the comparison group without obesity. In addition, exploratory analyses provide no indication that weight loss surgery improves these observed cognitive decrements over a period of 12 months. Further studies are necessary to comprehensively evaluate changes in cognitive function following bariatric surgery.
Keywords: Moderate to severe obesity, Memory, Executive function, Bariatric surgery, Adolescents, Young adults
Graphical Abstract
Introduction
Recent studies indicate increasing rates of severe obesity both in female adolescents aged 16–19 years [1] and in adult women compared with men [2]. As a consequence, weight loss surgery (WLS) in this population has also been on the rise [3–6]. In addition to metabolic complications, severe obesity is associated with a broad range of mood [7, 8], emotional [8, 9], and cognitive alterations [10, 11]. Multiple authors have examined the relationship between pediatric obesity and neurocognitive outcomes and have reported a negative correlation between body weight and cognitive indices [11–13]. These deficits were mainly noted in the areas of language [11, 14], memory [11, 14], and executive function aspects of cognitive function [12, 13]. Adolescents with obesity have also been shown to have structural and functional changes in brain regions that regulate these cognitive functions [12, 15].
Recent metanalyses exploring the cognitive impact of WLS in adults indicate positive effects of WLS on neurocognitive measures [16]. Possible improvements in associated comorbidities such as hypertension and insulin resistance, changes in adipokines/inflammatory cytokines, and in the gut microbiome have been cited as possible mechanisms underlying the structural and functional brain changes thought to mediate neurocognitive effects [16]. Despite the abundance of studies in adults, literature examining cognitive endpoints after WLS in adolescents and young adults is sparse. In a small pilot study, researchers examined memory and executive function and its associated neural correlates in adolescents and young adults aged 14–21 years. The authors found no improvements in assessed cognitive functions 4 months after surgery [17]. However, in a second-related manuscript, the authors reported reduced neural activation with fMRI tasks indicating improvement in neural performance to memory and reward-based tasks [18]. Adolescence and young adulthood mark a crucial phase of brain maturation and behavioral development; however, data are limited regarding cognitive changes in adolescents and young adults undergoing WLS. It is therefore important to evaluate the impact of WLS on cognitive indices during this important period of neurobehavioral development.
In this study, adolescent/young adult females with moderate to severe obesity were compared to adolescent/young adult females without obesity (comparison group) to examine the cognitive profile of the study cohort with obesity with respect to participants in the comparison group. Memory and executive function were chosen as study endpoints, as they represent key cognitive domains that determine academic outcomes and are reported to be impacted in individuals with obesity [13]. It was hypothesized that females with moderate to severe obesity would have lower scores for (a) short-term and long-term recall (verbal memory) and (b) response inhibition and cognitive flexibility (aspects of executive function) compared with the comparison group. In addition, an exploratory analysis was conducted to compare changes in verbal memory and executive function over 12 months in a subsample of the studied adolescent and young adult females with moderate to severe obesity who underwent WLS vs. those who did not undergo surgery and were followed with routine care.
Methods
Study Participants
Data from two previous studies from the group conducted between 2011 and 2018 (R01 HD060827 and R01 DK103946) were pooled to obtain the cognitive data. All participants were between 13 and 24 years of age. Females with moderate to severe obesity had a body mass index (BMI) > 40 kg/m2 or BMI > 35 kg/m2 with major comorbidities. The comparison group included healthy females without obesity (BMI between the 10th–90th percentiles) or other chronic medical conditions. Both groups were recruited for studies assessing bone health and metabolism; therefore, exclusion criteria included conditions other than WLS affecting bone metabolism, including primary ovarian insufficiency, hyperprolactinemia, thyroid dysfunction, and smoking [19]. Females in the comparison group were included from a study that evaluated bone outcomes in athletes with amenorrhea and controls (R01 HD060827) [20], and females in the obesity group were recruited from an ongoing trial that examines bone outcomes after WLS (R01 DK103946) and includes those undergoing surgery (surgical group) and those followed without surgery and routine care (non-surgical group) [21, 22]. Participants (including comparison group) were recruited through advertisements in the Partners HealthCare system, medical clinics, local newspapers, and colleges. In addition, participants with obesity (including those undergoing WLS) were recruited from area hospitals and weight loss centers that perform WLS in adolescents and young adults with moderate to severe obesity. Participants 18 years of age and older and parents of subjects < 18 years provided consent, and informed assent was obtained from subjects < 18 years. Changes in depressive and anxiety symptoms following WLS have been previously reported [8], and the current study is the first to analyze and report changes in cognitive indices. All studies were approved by the Institutional Review Board of Partners HealthCare.
Study Design
Cross-sectional component
Cognitive assessments in 69 females with moderate to severe obesity were compared with 24 females from comparison group of similar age without obesity.
Longitudinal component
Cognitive testing was repeated in a subset of 35 females with moderate to severe obesity 12 months after (i) undergoing WLS (surgical group; Roux-en-Y gastric bypass [RYGB]: n = 3, vertical sleeve gastrectomy [VSG]: n = 18; total n = 21) and (ii) 14 females who were being followed without surgical intervention (non-surgical group).
Neurocognitive Tests
Participants underwent three different neuropsychological tests as shown below: (i) Wechsler Abbreviated Scale of Intelligence (WASI), which was used to test for general intellectual ability and included (a) Vocabulary Test that measured indices of fluid intelligence and (b) Matrix Reasoning that measured crystallized intelligence; (ii) the California Verbal Learning Test—Second Edition (CVLT-II) that was used to test for verbal memory by means of short-term and long-term recall; and (iii) Delis-Kaplan Executive Function System Color-Word Interference Test (D-KEFS CWIT) that assessed executive function, including (a) response inhibition (participants named the color of the ink for words representing colors printed in dissonant ink color) and (b) cognitive flexibility (subjects named the color of the ink, unless words were framed in a box, where subjects were instructed to read aloud the word instead).
Statistical Analysis
JMP Pro12 was used for analysis. The Shapiro Wilk test was performed to test for normality. Mean and standard error of mean (SEM), or median and interquartile ranges (IQR) are presented for parametric and non-parametric data, respectively. Independent 2-sample t-tests or Wilcoxon rank sum tests were used to compare continuous variables (age, BMI, cognitive scores) depending on their distribution. Chi-square tests were used to compare categorical variables (race/ethnicity). Matched-pair analyses were conducted to assess cognitive score changes over 12 months. Linear regression was used to compare groups, controlling for confounding variables (age, baseline intelligence, and BMI). p values < 0.05 were considered statistically significant. Effect sizes for change in cognitive scores and BMI were calculated using formula r = Z/sqrt N for differences between surgical and non-surgical groups for Wilcoxon tests and Cohen’s d for t-tests. A posteriori power analysis for our primary confirmatory hypothesis achieved a power of 93% to reject the null hypothesis of equal means with a significance level (alpha) of 0.050 using a two-sided two-sample unequal-variance t-test.
Results
Cross-sectional analysis across weight groups
Participants in the comparison group had a median age of 19.5 (18.9–20.3) years and BMI of 21.7 (20.1–23.7) kg/m2, whereas females with moderate to severe obesity had a median age of 18.3 (16.4–20.4) years and median BMI of 44.8 (40.9–48.8) kg/m2 (p = 0.04 and < 0.001 for age and BMI, respectively). The comparison group was 79% Caucasian vs. the group with moderate to severe obesity, which was 61% Caucasian (p = 0.007).
Scores differed between females with moderate to severe obesity and the comparison group for all cognitive tests including WASI, CVLT-II, and D-KEFS CWIT and are shown in Table 1. Females with moderate to severe obesity consistently showed lower cognitive scores than the comparison group, including WASI vocabulary scores, CVLT-II verbal short-term recall and long-term recall scores (p < 0.01 for all), and D-KEFS CWIT response inhibition and cognitive flexibility scores (p < 0.0001 for all) after controlling for age. These differences persisted after also controlling for baseline WASI scores (p ≤ 0.01 for all).
Table 1.
WASI, CVLT-II, and D-KEFS CWIT scores by weight groups
| Females without obesity (n = 24) |
Females with moderate to severe obesity (n = 69) |
P |
P Controlled for age |
P Controlled for WASI matrix & age |
|
|---|---|---|---|---|---|
| WASI | |||||
| Matrix reasoning | 55.0 (53.0–59.0) (n = 23) |
51.0 (47.0–57.0) (n = 66) |
0.070 | 0.17 | |
| Vocabulary | 61.0 ±2.0 (n = 23) |
46.3 ±1.4 (n = 68) |
<0.0001 | < 0.0001 | |
| CVLT-II | |||||
| Short-term recall | 56.0 (51.3–60.0) | 44.0 (34.0–50.5) | <0.0001 | < 0.0001 | <0.0001 |
| Short-delay free recall | 55.0 (50.0–60.0) | 45.0 (35.0–52.5) | 0.0005 | 0.0007 | 0.002 |
| Long-delay free recall | 50.0 (45.0–60.0) | 45.0 (35.0–50.0) | 0.002 | 0.004 | 0.009 |
| D-KEFS CWIT | |||||
| Inhibition completion time | 57.0 (50.0–63.0) | 43.0 (40.0–50.0) (n = 65) |
< 0.0001 | < 0.0001 | < 0.0001 |
| Inhibition/switching completion time | 53.0 (50.0–60.0) (n = 17) |
47.0 (33.0–50.0) (n = 61) |
< 0.0001 | < 0.0001 | < 0.0001 |
Values are presented as mean ± standard error or as median (IQR). The Student t-test was used to compare groups when data were normally distributed and Wilcoxon rank sum test was used to compare groups when data were not normally distributed.
Abbreviations: WASI the Wechsler Abbreviated Scale of Intelligence, CVLT-II: California Verbal Learning Test—Second Edition, D-KEFS CWIT Delis-Kaplan Executive Function System Color-Word Interference Test
Longitudinal analysis in participants with moderate to severe obesity
Participant characteristics for surgical and non-surgical groups are presented in Table 2. BMI in the surgical group decreased by 14.1 (9.1–15.7) kg/m2 over 12 months compared with the non-surgical, whose BMI increased slightly (0.3 kg/m2 [− 1.7–1.1]) (p < 0.0001). Baseline and 12-month follow-up scores and changes in scores over 12 months are presented in Tables 3, 4, and 5, respectively. There were no significant differences in intelligence, verbal memory, and executive function scores between those who did vs. those did not undergo surgery, both for within group matched pair analysis at baseline and 12 months and for the between group comparison of changes in the scores over 12 months of follow-up. These findings persisted after adjusting for age, baseline BMI, and baseline cognitive performance scores, respectively, in separate models.
Table 2:
Participant characteristics for non-surgical and surgical groups
| Non-surgical (n = 14) | Surgical (n = 21) | p | |
|---|---|---|---|
| Age at baseline (years) | 16.5 (±0.57) | 18.7 (±0.49) | 0.007 |
| Ethnicity (% Non-Hispanic) | 57 | 57 | 0.59 |
| Race (% White) | 57 | 62 | 0.53 |
| BMI at baseline (kg/m2) | 40.8 (37.3–46.7) | 47.5 (42.4–54.4) | 0.004 |
| BMI at follow-up (kg/m2) | 40.3 (36.4–47.1) | 31.8 (29.3–39.3) | 0.01 |
Mean and standard errors with t-test p values and median and interquartile range with Wilcoxon rank sum test p values are presented for parametric and non-parametric values, respectively. Chi-squared test was used for race and ethnicity
Table 3:
Baseline intelligence (WASI), verbal memory (CVLT-II), and executive function (D-KEFS CWIT) scores for non-surgical and surgical groups
| Baseline | Non-surgical (n = 14) | Surgical (n = 21) | p |
|---|---|---|---|
| WASI | |||
| Matrix reasoning | 52.5 (± 1.8) (n = 13) |
50.9 (± 1.1) | 0.44 |
| Vocabulary | 48.9 (± 2.9) | 44.0 (±2.3) (n = 20) |
0.19 |
| CVLT-II | |||
| Short term recall | 42.1 (± 3.1) | 44.5 (± 1.6) | 0.47 |
| Short-delay free recall | 45.4 (± 2.6) | 48.1 (±2.3) | 0.45 |
| Long-delay free recall | 45.0 (38.8–51.3) | 50.0 (35.0–50.0) | 0.93 |
| D-KEFS CWIT | |||
| Inhibition completion time | 45.8 (± 2.7) (n = 13) |
42.1 (±2.3) (n = 20) |
0.31 |
| Inhibition/switching completion time | 47.0 (43.0–49.3) (n = 12) |
43.0 (30.0–50.0) (n = 17) |
0.41 |
Mean and standard errors with t-test p values and median and interquartile range with Wilcoxon rank sum test p values are presented for parametric and non-parametric values, respectively
Abbreviations: WASI the Wechsler Abbreviated Scale of Intelligence, CVLT-II California Verbal Learning Test—Second Edition, D-KEFS CWIT Delis-Kaplan Executive Function System Color-Word Interference Test
Table 4:
Follow-up intelligence (WASI), verbal memory (CVLT-II), and executive function (D-KEFS CWIT) scores at 12 months for non-surgical and surgical groups
| 12 months | Non-surgical (n = 14) | Surgical (n = 21) | p |
|---|---|---|---|
| WASI | |||
| Matrix reasoning | 49.5 (44.0–55.5) | 53.0 (45.5–56.5) (n = 20) |
0.65 |
| Vocabulary | 53.2 (± 3.0) (n = 13) |
45.9 (± 2.4) (n = 20) |
0.07 |
| CVLT-II | |||
| Short-term recall | 48.0 (± 4.0) | 48.2 (±2.1) | 0.96 |
| Short-delay free recall | 49.6 (± 3.3) | 48.1 (± 2.3) | 0.69 |
| Long-delay free recall | 42.5 (30.0–56.3) | 50.0 (45.0–52.5) | 0.21 |
| D-KEFS CWIT | |||
| Inhibition completion time | 51.5 (42.3–60.0) (n = 14) |
50.0 (43.0–53.0) (n = 19) |
0.58 |
| Inhibition/switching completion time | 51.5 (35.3–57.0) (n = 14) |
50.0 (43.0–53.0) (n = 14) |
0.88 |
Mean and standard errors with t-test p values and median and interquartile range with Wilcoxon rank sum test p values are presented for parametric and non-parametric values, respectively
Abbreviations: WASI the Wechsler Abbreviated Scale of Intelligence, CVLT-II California Verbal Learning Test—Second Edition, D-KEFS CWIT Delis-Kaplan Executive Function System Color-Word Interference Test
Table 5.
Change in intelligence (WASI), verbal memory (CVLT-II), and executive function (D-KEFS CWIT) scores and weight over 12-months of follow up for non-surgical and surgical groups
| 12M-BL change | Non-surgical (n = 14) | Surgical (n = 21) | p | Effect size |
|---|---|---|---|---|
| WASI | ||||
| Matrix reasoning | − 3 (− 8.0–1.5) (n = 13) |
0 (− 5.5–2.0) (n = 20) |
0.35 | r = − 0.16 |
| Vocabulary | 3.2 (± 2.0) (n = 13) |
3.2 (± 2.3) (n = 19) |
0.98 | d = 0.005 |
| CVLT-II | ||||
| Short term recall | 5.0 (0.3–14.3) | 3.0 (0–9.5) | 0.40 | r = 0.14 |
| Short-delay free recall | 4.3 (± 2.0) | 0 (± 2.2) | 0.17 | d = 0.49 |
| Long-delay free recall | 0 (− 6.3–5.0) | 5.0 (0–7.5) | 0.18 | r = − 0.23 |
| DKEFS CWIT | ||||
| Inhibition completion time | 3.0 (− 3.0–10.0) (n = 13) |
4 (2.3–7.0) (n = 18) |
0.81 | r = − 0.04 |
| Inhibition/switching completion time | 3.0 (− 3.0–6.8) (n = 12) |
3.0 (0–14.5) (n = 16) |
0.28 | r = − 0.20 |
| Weight change | ||||
| Change in BMI (kg/m2) | 0.3 (1.7–[− 1.1]) | − 14.1 (− 9.1–[− 15.7]) | <0.0001 | r = − 0.79 |
| Change in % expected BMI | − 2.7 (2.0–[− 7.8]) | − 58.8 (− 44.0–[− 73.2]) | < 0.0001 | r = − 0.71 |
Mean and standard errors with t-test p values and median and interquartile range with Wilcoxon rank sum test p values are presented for parametric and non-parametric values, respectively
Abbreviations: WASI the Wechsler Abbreviated Scale of Intelligence, CVLT-II: California Verbal Learning Test—Second Edition, D-KEFS CWIT Delis-Kaplan Executive Function System Color-Word Interference Test
Discussion
The investigation revealed two main findings. First and consistent with our hypothesis, it showed that adolescent and young adult females with moderate to severe obesity performed worse on tests engaging verbal memory and executive function compared with females without obesity. These findings are consistent with other studies in adolescents and adults [12, 13], suggesting that the current study cohort was similar to other study populations demonstrating detrimental effects of obesity on human cognition [13]. Second, in this study sample, cognitive function did not improve following WLS despite significant weight loss compared to those who did not undergo surgery.
The physiology underlying cognitive alterations in obesity is still debated. Inflammation, adipokines, alterations in gut hormones and in the microbiome, and associated metabolic/psychiatric comorbidities represent possible biological mechanisms that might predispose individuals to cognitive alterations in obesity [23]. Interestingly, most adult studies report an improvement of cognitive function following WLS [16]. However, adult data may not be reflective of the impact of obesity and subsequent weight loss in adolescents and young adults, who are at a critical period of brain maturation and development. In the study cohort of 21 participants undergoing weight loss surgery and 14 non-surgical participants, cognitive assessments were repeated 12 months post-WLS or routine care, and in contrast to data reported in adults, there were no improvements in these endpoints in the surgical vs. the non-surgical group, suggesting that cognitive indices may not necessarily improve with weight loss in this younger cohort.
To our knowledge, only one previous study in adolescents has compared memory and executive function in 10 participants who underwent VSG to 12 controls without obesity and 14 participants with obesity waitlisted for WLS, finding no group differences in changes in cognitive indices 4 months following surgery [17]. In the same sample with a smaller n ranging from 6 to 8 WLS participants with functional-MRI, the authors showed improvement in an n-back task (assessing working memory) and monetary incentive delay task (measuring reward-based decision-making) 4 months after VSG surgery [18]; however, episodic memory did not improve in this study. A higher BMI at 18 years of age was indicative of poorer recovery of cognitive function in a cohort of adults undergoing WLS, suggesting a link between adolescent weight status and future cognition [24]. Taken together, these findings bring up the important point that cognitive alterations from obesity acquired earlier in life may not improve with weight loss and may have a long-lasting impact.
The small number of participants in the study is a limitation but is reflective of the small number of adolescent participants undergoing WLS despite the recent increase in utilization rates. Our posteriori analysis had adequate power for the primary confirmed hypothesis. Measurement of adipokines, gut hormones, and the microbiome and their changes following WLS may provide additional information. However, currently these data are not available. Our exploratory analysis served to generate hypothesis for future studies which should also consider controlling for comorbidities to shed light on their role in cognitive changes.
The current study is a significant addition to the existing sparse literature on cognitive changes in adolescents following WLS, and in this study cohort, there was no evidence for improvement in memory and executive function even with significant weight loss. Further research is warranted examining the effect of age of obesity onset on cognitive alterations in a larger adolescent population. While WLS is effective for inducing weight loss, preventive strategies to avoid obesity in children should be emphasized in the first place to avoid possible detrimental effects on cognition.
Footnotes
Ethics Approval All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards
Consent to Participate Informed consent/assent was obtained from all individual participants included in the study.
Conflict of Interest Authors Charumathi Baskaran, Abisayo Animashaun, Frances Rickard, Alexander T. Toth, Kamryn T. Eddy, Franziska Plessow and Miriam A. Bredella declare that they have no conflicts of interest. Madhusmita Misra serves as a consultant for Sanofi and Abbvie and has served on the scientific advisory board for Abbvie and Ipsen. Dr. Misra also receives royalties from UpToDate.
References
- 1.Skinner AC, et al. , Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics, 2018. 141(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, et al. , Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, 2020(360): p. 1–8. [PubMed] [Google Scholar]
- 3.Zwintscher NP et al. The increasing incidence of adolescent bariatric surgery. J Pediatr Surg. 2013;48(12):2401–7. [DOI] [PubMed] [Google Scholar]
- 4.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161(3):217–21. [DOI] [PubMed] [Google Scholar]
- 5.English WJ et al. American society for metabolic and bariatric surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14(3):259–63. [DOI] [PubMed] [Google Scholar]
- 6.Surgery, ASMB. Estimate of bariatric surgery numbers, 2011–2019. 2021. [cited 2021 March 15th]; Available from: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
- 7.Zeller MH et al. Health-related quality of life and depressive symptoms in adolescents with extreme obesity presenting for bariatric surgery. Pediatrics. 2006;117(4):1155–61. [DOI] [PubMed] [Google Scholar]
- 8.Baskaran C et al. Depressive and anxiety symptoms and suicidality in adolescent and young adult females with moderate to severe obesity before and after weight loss surgery. Clin Obes. 2020;10(5):e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarwer DB, Heinberg LJ. A review of the psychosocial aspects of clinically severe obesity and bariatric surgery. Am Psychol. 2020;75(2):252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunstad J, Sanborn V, Hawkins M. Cognitive dysfunction is a risk factor for overeating and obesity. Am Psychol. 2020;75(2):219–34. [DOI] [PubMed] [Google Scholar]
- 11.Liang J et al. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes. 2014;38(4):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce AL, Leonhardt CA, Vaidya CJ. Executive and reward-related function in pediatric obesity: a meta-analysis. Child Obes. 2018;14(5):265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinert KR, Po’e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes. 2013;2013:820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holcke M et al. Paediatric obesity: a neurodevelopmental perspective. Acta Paediatr. 2008;97(6):819–21. [DOI] [PubMed] [Google Scholar]
- 15.Sweat V et al. Obese adolescents show reduced cognitive processing speed compared with healthy weight peers. Child Obes. 2017;13(3):190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiara G et al. Evidence for neurocognitive improvement after bariatric surgery: a systematic review. Psychosomatics. 2017;58(3): 217–27. [DOI] [PubMed] [Google Scholar]
- 17.Mackey ER et al. Cognitive performance as predictor and outcome of adolescent bariatric surgery: a nonrandomized pilot study. J Pediatr Psychol. 2018;43(8):916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce AL et al. Effect of Adolescent bariatric surgery on the brain and cognition: a pilot study. Obesity (Silver Spring). 2017;25(11): 1852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackerman KE et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53(4):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackerman KE et al. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc. 2015;47(8):1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra M et al. Impact of sleeve gastrectomy on hip structural analysis in adolescents and young adults with obesity. Surg Obes Relat Dis. 2020;16(12):2022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra M et al. Bone outcomes following sleeve gastrectomy in adolescents and young adults with obesity versus non-surgical controls. Bone. 2020;134:115290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller AL, Lee HJ, Lumeng JC. Obesity-associated biomarkers and executive function in children. Pediatr Res. 2015;77(1–2):143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spitznagel MB et al. Adolescent weight history and adult cognition: before and after bariatric surgery. Surg Obes Relat Dis. 2016;12(5): 1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


