Abstract
Agricultural workers, especially those who work in swine confinement facilities, are at increased risk for developing pulmonary diseases including asthma, chronic obstructive pulmonary disease, and chronic bronchitis due to exposures to fumes, vapors, and organic dust. Repetitive exposure to agricultural dust leads to unresolved inflammation, a common underlying mechanism that worsens lung disease. Besides occupational exposure to dusts, diet also significantly contributes to inflammation and disease progression. Since DHA (docosahexaenoic acid), a polyunsaturated omega-3 fatty acid and its bioactive metabolites have key roles in inflammation resolution, we rationalized that individuals chronically exposed to organic dusts can benefit from dietary modifications. Here, we evaluated the role of DHA in modifying airway inflammation in a murine model of repetitive exposure to an aqueous extract of agricultural dust (three-week exposure to swine confinement dust extract, HDE) and after a one-week resolution/recovery period. We found that mice fed a high DHA diet had significantly increased bronchoalveolar lavage fluid (BALF) levels of DHA-derived resolvins and lower TNFα along with altered plasma levels of endocannabinoids and related lipid mediators. Following the one-week recovery we identified significantly reduced BALF cellularity and cytokine/chemokine release along with increased BALF amphiregulin and resolvins in DHA diet-fed versus control diet-fed mice challenged with HDE. We further report observations on the effects of repetitive HDE exposure on lung Ym1+ and Arg-1+ macrophages. Overall, our findings support a protective role for DHA and identify DHA-derived resolvins and endocannabinoids among the potential mediators of DHA in altering airway inflammation in chronic agricultural dust exposure.
Keywords: Omega-3 polyunsaturated fatty acids, docosahexaenoic acid (DHA), agricultural dust, specialized pro-resolving mediators, resolvins, endocannabinoids
1. Introduction
As per the USDA, agriculture is a major and vital US industry involving about 2.05 million operating farms (totaling about 910 million acres in 2017) [1]. Agricultural workers, especially livestock farmers, are at increased risk for developing pulmonary diseases including asthma, chronic obstructive pulmonary disease (COPD), and chronic bronchitis due to exposure to fumes, vapors, and agricultural dust [2-5]. Dusts from swine confinement facilities cause severe inflammation in swine confinement workers, which is mainly characterized by a large increase in lung neutrophils [3,6]. There is no cure for COPD or asthma, and management of disease consists of medications (i.e., bronchodilators, corticosteroids etc.), oxygen therapy and life-style changes; however, these treatments are not without side effects and they treat only the symptoms of disease rather than the cause [7].
An alternative approach to the management of lung disease is modification of risk factors such as diet. Dietary omega-3 and omega-6 polyunsaturated fatty acids (PUFAs), such as those found in fatty fish, EPA (eicosapentaenoic acid), DHA (docosahexaenoic acid), and DPA (docosapentaenoic acid) have the ability to modulate inflammation [8,9] and immune cells [10,11]. Because unresolved inflammation is a common underlying mechanism that significantly contributes to lung disease progression, beneficial effects of omega-3 PUFAs have been explored previously in airway inflammation including asthma, COPD, cystic fibrosis and ARDS [12-16]. While these studies suggest DHA over EPA or the combination of EPA and DHA to be the most beneficial to reduce symptoms, the benefits of a DHA-rich diet or mechanism of action of DHA in enhancing resolution of lung inflammation and improving recovery in agricultural dust-induced lung inflammation are largely unknown.
The murine model of agricultural dust exposure is well-established and it reproduces key pathologic outcomes observed in swine farm workers, where both a single and repetitive exposure to swine confinement dust extract (hog dust extract, HDE) lead to significantly increased neutrophil influx and elevated levels of pro-inflammatory cytokines in the bronchoalveolar lavage fluid (BALF) [3,17,18]. We have previously reported that DHA pretreatment in vitro (precision-cut murine lung slices, human bronchial epithelial cells, lung fibroblasts and monocyte cell cultures) or in an acute exposure in vivo (single intranasal HDE exposure) leads to reduced inflammatory cytokine/chemokine production following inflammatory insult [17,19]. However, in these studies we did not address the effects of DHA in the resolution of inflammation in a repetitive exposure model, which is most relevant to agricultural workers who are exposed to dust daily. Given the duration and frequency of agricultural dust exposure are important factors defining the type of inflammation and disease experienced by those exposed [4,5], and beneficial effects attributed to DHA in suppressing airway inflammation, we hypothesized that dietary supplementation of DHA would be effective in reducing airway inflammation in a chronic HDE exposure setting. To address this hypothesis, we performed a set of experiments in which mice were fed a high DHA diet versus control (no DHA) diet, and changes in airway inflammation were determined after a 3-week repetitive HDE exposure, and after cessation of the exposure (resolution/recovery period, one week). We found that mice fed a high DHA diet exhibited significant alterations in HDE-induced lung inflammation both after 3 weeks and after the resolution period. We also tested additional hypotheses that DHA-derived bioactive metabolites that are involved in the resolution of inflammation might mediate the beneficial effects of DHA in airway inflammation and report further observations on the effects of DHA on lung Ym1+ and Arg-1+ macrophages following repetitive HDE exposure. Overall, our results reported here are consistent with our hypothesis that DHA and DHA-derived bioactive metabolites significantly contribute to the resolution of airway inflammation following repetitive agricultural dust exposure.
2. Materials and methods
2.1. Preparation of aqueous dust extract
Dust extracts were prepared as previously described [20]. Settled dusts in swine concentrated animal feeding operations were collected and kept at −20°C. To prepare the 100% aqueous extract, 5 g dust was suspended in 50 mL Hank’s Balanced Salt Solution for 1 hour. The solution was then centrifuged to remove all large particulates and was sterile filtered through a 0.22 μm filter. Extracts were frozen in aliquots until used. A 12.5% HDE solution was prepared for instillations by diluting the 100% HDE in saline to produce a 12.5% solution. The HDE has been previously characterized for major components [21-23].
2.2. Animals and diet
Male 6-8-week-old C57Bl/6 mice were purchased from Jackson Laboratories. After a 1-week acclimation period, mice were placed on a high DHA diet or a control diet lacking DHA, prepared as we have previously described [19,24]. Both the high DHA and standard modified diet were prepared by ENVIGO (Madison, WI, USA). Both diets have been utilized in a previous study where we showed the effectiveness of the high DHA diet in altering the lung inflammatory response to a single exposure to HDE [24]. Briefly, in our study the AIN-93G diet was modified to replace soybean oil with high oleic safflower oil. In the DHA diet only a portion of the high oleic safflower oil was replaced with DHASCO oil (DSM Nutritional Products, Kingstree, SC) containing 39.2% DHA as shown in Table S1. The detailed fatty acid analysis of the DHASCO oil (certificate of analysis) and the detailed composition of the diets prepared by Envigo are also given in the ‘Supplementary Materials’. The modified high DHA diet contained approximately 5.88 g DHA/kg, or 1.4% of kcal from DHA. This corresponds to a person taking approximately 3.11 g/day based on a 2000 kcal diet [25]. This dose was chosen based on the evidence that the anti–inflammatory and cardioprotective effects attributed to omega-3 fatty acids are within the range of 1-5 g omega-3 fatty acids [26]. Also, recommendations from the U.S. FDA suggests that there are no adverse health effects of omega-3 fatty acids up to 3 g/day [27]. Despite this suggested maximal dosing, others reported that 3-5 g/day dose of omega-3 fatty acids are routinely consumed by Eskimos and Japanese people [28]. Moreover, omega-3 fatty acid supplementation between 6 g/day (EPA, 3.7 g + DHA, 2.5 g) versus 3 g/day (EPA, 1.8 g + DHA, 1.5 g) reduces exercise-induced bronchoconstriction and airway inflammation markers [29]. We have shown that airway inflammation induced after a single exposure to agricultural dust is largely inhibited by the high dose DHA supplementation for 4-weeks [24]. Mice had ad libitum access to food and water and were housed 5 mice/microisolator cage with cages changed every two weeks. Food was replenished weekly. Mice were initiated on the DHA/control diets for 4 weeks before the start of HDE exposure protocols. Animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
2.3. Murine model of dust exposure and lung histopathology
The repetitive model of HDE-induced airway inflammation was performed similarly to previously described methods [18,30,31]. Mice were challenged with 15 HDE instillations (given as 50 μl 12.5% HDE or saline intranasally under light anesthesia) over 3 weeks. Mice were then euthanized 5 hours following the last instillation or allowed to recover for 7 days following the final HDE instillation (recovery phase). Following euthanasia, blood was collected in K2 EDTA tubes (BD, Franklin Lakes, NJ) and frozen as whole (un-separated) blood. Bronchoalveolar lavage was performed 3 times with 1 mL PBS each time. The 3 fractions were centrifuged, and the first 1 mL fraction obtained was saved for cytokine/mediator analyses. The cells pelleted from all three fractions were counted, cytocentrifuge slides were prepared and stained with Diff-Quik (Siemens, Newark, DE), and differential cell counts enumerated. Lungs were then inflated with formalin at 15 cm pressure. Fixed lungs were paraffin embedded, sectioned, and stained with Hematoxylin and Eosin to visualized pathological signs of inflammation. Images were obtained from hematoxylin and eosin-stained (H&E) slides with 10X (for lymphoid aggregates) and 20X (alveolar cellularity and epithelial hyperplasia) objectives using an Echo Revolve brightfield microscope (San Diego, CA, USA). Stained whole-lung sections were assessed for alveolar cellularity, epithelial hyperplasia, and lymphoid aggregates. The observer was blinded to the identity of the H & E slides. To assess alveolar cellularity, objectives 4x and 10x both with 150% optical zoom were utilized to scan entire lung sections, with the number of lymphoid aggregates present recorded for each lung section. A lymphoid aggregate was defined as greater than 20 lymphocytes closely aggregated. For epithelial hyperplasia, a total of 10 images of bronchioles within each tissue section were obtained with 20x objective with 150% optical zoom. Utilizing measurement and annotation software available on the Echo Revolve, epithelial cell nuclei were quantified, and airway areas were measured to determine their size (μm2). These two values produced a ratio of nuclei/ μm2 for each airway and this was repeated for 10 airways imaged per lung section. These ratios were then averaged to obtain a single value for each tissue section. Finally, alveolar cellularity was assessed based on five images with 40x objective with 150% optical zoom randomly throughout each lung tissue section parenchyma. Cells within alveolar spaces were manually quantified for each image, which produced five values per section. These values were averaged to obtain a single value per tissue section. Column statistical analysis was performed using these averaged values obtained for epithelial hyperplasia and alveolar cellularity, along with the number of lymphoid aggregates quantified for each tissue section. Scores (0-4) for each parameter were assigned based on the percentile values obtained.
2.4. Whole blood fatty acid level measurements
Whole blood was collected from the inferior vena cava and stored at −80°C until fatty acid analysis by gas chromatography (GC). Cryopreserved whole blood samples were thawed on ice, and 25 microliters were added into a 2 mL glass vial for direct transesterification using 250 microliters of 14% boron-trifluoride methanol and 250 microliters of hexane solution at 100°C for 60 minutes as previously reported [24,32]. A quantitative internal standard,17:0 heptadecanoic acid and a quantitative external standard, 15:0) were included. Then, phase separation was performed after samples were mixed with HPLC grade water. Fatty acid methyl-esters in the hexane phase were separated on a capillary (GC) column with Agilent CP7489 Capillary Column (100m length x 0.25mm internal diameter x 0.36 mm outer diameter, 0.20μm film thickness) using an Agilent 7890A GC equipped with flame ionized detector (Agilent Technologies, Santa Clara, CA). Hydrogen was used as the carrier gas. Medium to long chain fatty acids (C10 to C24) were identified and data acquisition was performed using ChemStation interface system (Agilent Technologies). Peak identification and relative quantification were performed using Open LAB Chromatography Data System and corresponding authentic standard, 37 FAME mix (Sigma Aldrich, St. Louis, MO) and internal standard. Fatty acid composition of whole blood is reported as wt:wt% of the sample.
2.5. Plasma endocannabinoid level measurements
Blood was collected as described above in K2 EDTA tubes at the end of 3-week HDE repetitive dust exposure, and plasma was isolated following centrifugation at 1500 g for 10 min. Samples were kept at −80°C until analysis. Lipids were extracted from plasma as previously described [33]. Separation of lipids was performed using a UPLC BEH C18 column (2.1 × 50 mm i.d.; 1.7 μm, Waters Corporation, Milford, MA, USA) with an Acquity guard column (UPLC BEH C18 VanGuard Pre-column; 2.1 × 5 mm, i.d., 1.7 μm, Waters Corporation) and detection was performed using UPLC-MS/MS (ultra-performance liquid chromatography tandem mass spectrometry) [33]. Data acquisition was performed using Acquity I Class UPLC/Xevo TQ-S Micro Mass Spectrometer (Waters Corporation). UPLC and MS parameters for the detection of arachidonoylethanolamide (AEA), 2-arachidonoyl-sn-glycerol (2-AG), 2-docosahexaenoyl-sn-glycerol (2-DG), docosahexaenoylethanolamide (DHEA) and oleoylethanolamide (OEA) were reported previously [34,35].
2.6. Immunofluorescence staining
The M2 macrophage markers Ym1 and Arginase-1 (Arg-1) and a lectin recognizing both M1/M2 macrophages, GSL (Griffonia Simplicifolia Lectin-1 isolectin B4, biotinylated, Vector Laboratories, # B-1205-.5), were detected using immunofluorescence staining and microscopy. Briefly, unstained slides from 5 μm lung tissue sections were deparaffinized with xylene and rehydrated with serial ethanol washes and lastly PBS. Antigen retrieval was performed with Diva Decloaker, (Biocare Medical, Pacheco, CA), and then sections were blocked for 10 min with Avidin/Biotin blocker (for GSL) with 5 min PBST washes in between and then with 1% BSA in PBST for half an hour. After the blocking step, tissues were incubated with rabbit polyclonal antibody against mouse Ym1 (Stem Cell Technologies, Cambridge, MA, USA, catalog #: 60130, 1:200 dilution), with rabbit antibody against mouse Arg-1 (Cell Signaling, MA, USA, catalog #: 93668, 1:50 dilution) and biotinylated GSL-1 (Vector Laboratories, Burlingame, CA, USA, catalog #: B-1205-.5, 1:50 dilution) in 1% BSA in PBST (0.1% Tween-20 in PBS) overnight at 4°C. Sections were then washed with PBST for 5 min three times and incubated with the secondary antibodies, donkey anti–rabbit IgG (Dylight 550, ab98489, 1:500 dilution), goat anti–rabbit IgG (H+L), Alexa Fluor 488 (Thermo Fisher Scientific, Waltham, MA, USA, catalog #: A-11008, 1:500 dilution) or Streptavidin Cy 5 (Vector Laboratories, Burlingame, CA, USA, catalog #: SA-1500-1, 1:50 0 dilution) in 1% BSA in PBST for 2 hours at room temperature. After washing in PBST for 5 min three times, sections were mounted using Prolong Gold antifade reagent with DAPI. Tissues were visualized with Echo Revolve (San Diego, CA, USA). Secondary-only (no primary antibody) controls were included in Suppl. Figure 2. For the quantification of Ym1 images, a total of 5-7 images were obtained for each mouse lung, resulting in at least 20 images per treatment group. Integrated density/area was calculated for the whole image for each image using Fiji open-source software (Image J version 1.52p, imagej.nih.gov). ‘Plot Profile’ function (https://imagej.nih.gov/ij/docs/menus/analyze.html#plot) was applied on the whole image after background subtraction which was applied using the same parameter (rolling ball radius, 50 pixels) for each image (Suppl. Fig. 1. For the quantification of Arg-1 staining, 5-7 images for airways and parenchyma were obtained in each mouse lung, and 5-7 images were obtained for the bone marrow macrophages (Suppl. Fig.3). Similar to Ym1 quantification, integrated density/ area and number of cells showing positive staining for Arg-1 and GSL were determined for each image to obtain fluorescence intensity and Arg-1/GSL+ cell counts using Fiji software.
2.7. Cytokine/mediator measurements
TNF-α, IL-6, CXCL1, MIF, MPO, and AREG were detected using Duoset enzyme-linked immunosorbent assay (ELISA) kits from R&D systems (Minneapolis, MN). Resolvin D1 (RvD1) and Resolvin D2 (RvD2) were detected using ELISAs from Cayman Chemical (Ann Arbor, MI).
2.8. Statistical analyses
GraphPad Prism software was utilized to perform two-way ANOVA tests on data to determine statistical significance of results, using the Tukey method for post-hoc comparisons amongst groups. Differences between groups were considered significant if the P value ≤ 0.05. Data are shown as means ± standard error of the mean. N values for each experimental group are provided within the bar of each group. In the experimental design, we performed power calculations that estimated that 8-10 mice/ group would be necessary to detect 20% difference with 85-90% power. We based these calculations on detecting 20% difference in cytokine levels between saline- versus. dust-exposed animals.
3. Results
3.1. Effects of a high DHA diet on blood PUFA levels
To assess the efficacy of our dietary DHA supplementation in leading to increased DHA plasma membrane incorporation, we analyzed the whole blood levels of medium- and long-chain PUFAs. As anticipated, mice fed the high DHA diet exhibited significantly increased DHA, EPA, and DPA levels in their whole blood as compared to mice on the control diet (Fig. 1 A-C, left panels, P<.0001). Specifically, two-way ANOVA analysis revealed a significant main effect of dietary DHA supplementation on whole blood levels of DHA (F=53.34, P<.0001), EPA (F=21.66, P<.001) and DPA (F=25, P<.0001) levels. In contrast, alpha-linolenic acid (ALA) levels were decreased in HDE-challenged mice fed the high DHA diet when compared to saline controls, with a 2-way ANOVA analysis showing a significant main effect of DHA diet on whole blood ALA levels (F=9.069, P=.0047) (Fig. 1 D, left panel). These alterations in blood omega-3 fatty acid levels were coincident with significantly decreased whole blood levels of the omega-6 PUFA arachidonic acid (ARA) in mice fed the high DHA diet compared to mice fed the control diet (Fig. 1E, left panel, significant main effect of DHA diet, F=98.84, P<.0001). Levels of the omega-6 fatty acid linoleic acid (LA) were unchanged in control diet- versus DHA diet-fed mice (Fig. 1F, left panel). Of note, HDE exposure did not result in any significant alterations in DHA, EPA, DPA, ALA, ARA, upon sacrifice at 5 hours post exposure in mice on the control diet. However, when mice were allowed to recover for 7 days following the final HDE exposure (Fig. 1A-F, right panels), we identified a significant decrease in ARA levels in control diet-fed mice with a significant main effect of HDE (F=10.45, P=.0027), DHA diet (F=81.16, P<.0001) and significant interaction between exposure and diet (F=5.28, P=.027) (Fig. 2E, right panel), while also finding a statistically significant main effect of HDE exposure (F=5.05, P=.031) and DHA diet (F=7.605, P=.0091) in mediating ALA whole blood levels (Fig. 2D, right panel). In contrast, HDE exposure did not significantly impact DHA, EPA, DPA, or LA during the recovery phase (Fig. 2A-C, F, right panels). Additional measured PUFA are shown in Table S2. Overall, we found an approximately 6-8-fold decrease in the omega-6-to-omega-3 PUFA ratio with dietary DHA supplementation as detailed in the Table S3. These findings confirm the success of our feeding strategy to increase omega-3 fatty acid levels in mice fed the high DHA diet and identify alterations in whole blood ARA and ALA fatty acids during recovery following chronic HDE exposure.
Fig. 1.
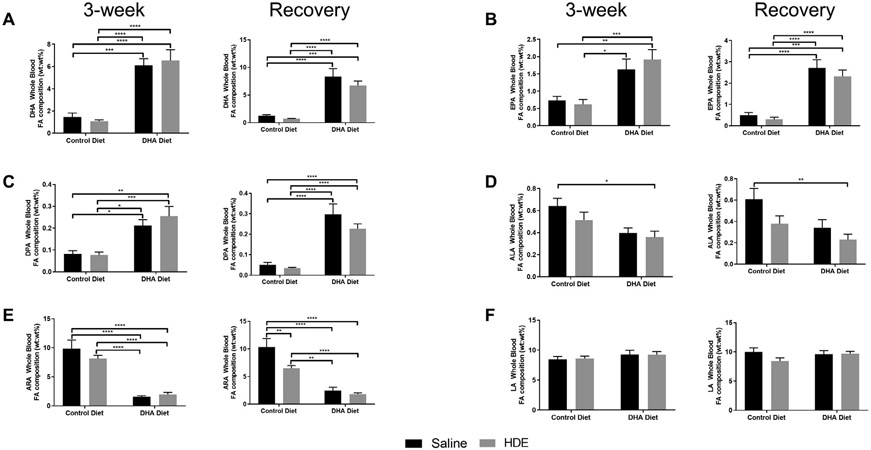
Changes in whole blood levels of omega-3 and omega-6 polyunsaturated fatty acids after high DHA diet and three-week repetitive HDE exposure at three weeks and after the recovery period. Mice were fed DHA or control diets for four weeks before commencing three-week repetitive 12.5% HDE exposure. At the end of three-week exposure, blood was collected, and fatty acid levels of (A) DHA, (B) EPA, (C) DPA, (D) ALA, (E) ARA and (F) LA were determined by GC-FID as described in Methods. Left and right panels show data for 3-week and recovery periods, respectively. Data are mean ± standard error of the mean, n=7-8 for saline control and DHA alone groups (no HDE exposure) and n=11-13 mice for HDE and DHA+HDE groups. * P<.05, ** P<.01, *** P<.001, **** P<.0001.
ALA=alpha-linolenic acid, ARA=arachidonic acid, DHA=docosahexaenoic acid, DPA=docosapentaenoic acid, EPA=eicosapentaenoic acid, GC-FID=gas chromatography-flame ionization detector, HDE=hog dust extract, LA=linoleic acid.
Fig. 2.
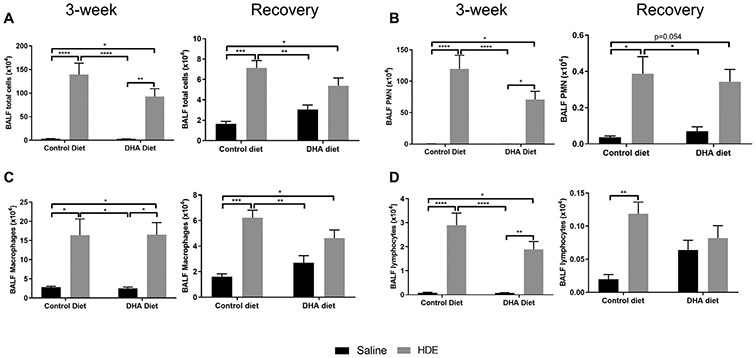
Total and differential cell counts following high DHA diet and three-week repetitive HDE exposure at three weeks and after the recovery period. Mice were fed DHA or control diets for four weeks before commencing three-week repetitive 12.5% HDE exposure. BALF was collected at the end of the three-week exposure, and cells were enumerated, (A) total cells, (B) neutrophils, (C) macrophages and (D) lymphocytes. Left and right panels show data for 3-week and recovery periods, respectively. Data are mean ± standard error of the mean, n=6-8 for saline control and DHA alone groups (no HDE exposure) and n=11-13 mice for HDE and DHA+HDE groups. * P<.05, ** P<.01, *** P<.001, **** P<.0001.
3.2. Effects of a high DHA diet on the airway inflammatory response to repetitive HDE exposure and recovery
In order to determine the effects of a high DHA diet on HDE-induced lung inflammation, we first compared total and differential cell numbers in bronchoalveolar lavage fluid (BALF) from mice provided the DHA or control diet for 4 weeks then challenged with 3 weeks of repetitive HDE exposure (Fig. 2A-D, left panels). As compared to saline controls, we observed a statistically significant increase in total cell influx (P<.0001) with the largest increase in neutrophil counts (approximately 100-fold increase, P<.0001) in response to the 3-week repetitive HDE exposure (Fig. 2A, B, left panels). We observed no significant difference in cellular influx in mice fed a high DHA diet versus control diet, with all effects driven by HDE exposure (P<.0001 by two-way ANOVA for total cells, neutrophils, and lymphocytes; P<.001 by two-way ANOVA for macrophages) (Fig. 2C, D, left panels).
In the recovery phase at 7 days following final HDE exposure, BALF total cells in control diet-fed mice challenged with HDE remained significantly elevated as compared to saline-exposed control-fed mice (Fig. 2A, right panel), including elevations in neutrophils, macrophages, and lymphocytes (Fig. 2B-D, right panels). By contrast, mice fed the high DHA diet no longer exhibited significant elevations in BALF total cells, including neutrophils, macrophages, or lymphocytes (Fig. 2). In addition, we observed a significant interaction between exposure and diet on BALF total cells (P<.05), macrophages (P=.05) and lymphocytes (P<.05).
To further evaluate the inflammatory response to HDE, we also quantified pro-inflammatory and anti–inflammatory/pro-repair mediators in BALF (Fig. 3). In mice challenged with repetitive HDE exposure, we observed significant increases in pro-inflammatory cytokines IL-6, TNF-α, CXCL1, and MPO (myeloperoxidase) (Fig. 3A-C, F, left panels), as well as the pro-repair mediator AREG (Fig. 3D, left panel). Of note, in HDE-challenged mice fed the high DHA diet, TNF-α levels were significantly blunted as compared to mice fed the control diet, with two-way ANOVA identifying a significant interaction between exposure and diet (P<.05) (Fig. 3B, left panel), while we also observed a trending reduction (P=.09) in CXCL1 release (Fig. 3C, left panel). Interestingly, MIF (macrophage migration inhibitory factor) levels were also significantly altered by diet, whereby DHA diet-fed mice exposed to HDE had elevated MIF levels compared to control diet-fed mice (Fig. 3E, left panel).
Fig. 3.
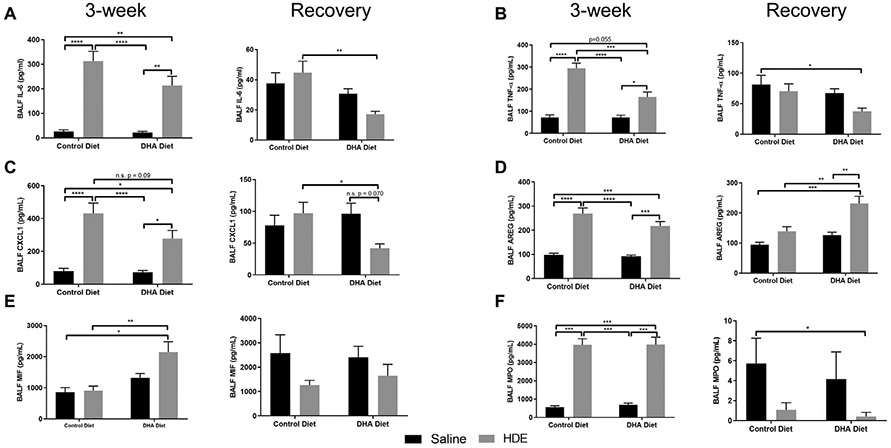
Effect of high DHA diet and three-week repetitive HDE exposure on BALF cytokine levels at three weeks and after the recovery period. Mice were fed DHA or control diets for four weeks before commencing three-week repetitive 12.5% HDE exposure. BALF was collected at the end of the three-week exposure, and BALF cytokine levels were determined as described in Methods. Left and right panels show data for three-week and recovery periods, respectively. Data are mean ± standard error of the mean. For IL-6(A), TNF-α (B), CXCL-1 (C) and AREG (D); n=6-8 for saline control and DHA alone groups (no HDE exposure) and n=11-13 mice for HDE and DHA+HDE groups. For MIF (E) and MPO (F); n=3 for control diet groups (saline control and HDE groups) and n=7 for DHA diet groups (DHA alone and DHA+HDE groups). * P<.05, ** P<.01, *** P<.001, ****P<.0001.
Following the recovery phase (after cessation of HDE for 7 days) we observed lower levels of all pro-inflammatory cytokines (Fig. 3), indicating an overall return to baseline conditions in terms of inflammatory mediator production. We found statistically significant effects for diet in altering BALF IL-6 and TNF-α levels (Fig. 3A, B, right panels) whereby DHA diet-fed mice had lower levels of these cytokines. In contrast, DHA diet and HDE exposure were also associated with significantly elevated levels of the pro-repair mediator AREG during the resolution phase (Fig. 3D, right panels). Interestingly, HDE exposure was associated with reduced MPO and MIF as compared to controls during recovery (Fig. 3F, E, right panels). We also identified a significant interaction between dust exposure and diet on CXCL1 levels (P<.05), whereby CXCL1 levels were significantly reduced in DHA diet-fed and HDE challenged mice as compared to HDE challenged mice (Fig. 3C, right panel). This decrease in CXCL-1 was a trending reduction (P=.07) in DHA diet-fed and HDE challenged mice as compared to DHA diet-fed saline controls.
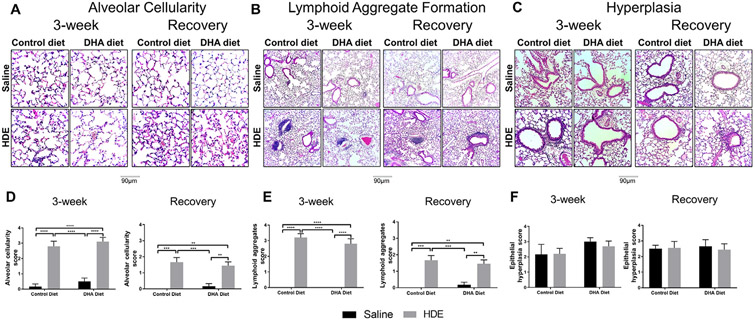
To assess for tissue inflammation, we performed blinded scoring of lung histopathology for alveolar cellularity, epithelial hyperplasia, and lymphoid aggregate formation (Fig. 4). Consistent with the increased inflammatory cell influx and cytokine levels observed with repetitive HDE exposure, we identified significantly increased alveolar cellularity in mice receiving 3 weeks repetitive HDE exposure regardless of diet (P<.0001; Fig. 4A and 4D, left panels). Despite a lower score, alveolar cellularity remained increased in these mice in the recovery period with a significant interaction between exposure and diet (P<.05) (Fig. 4A and Fig. 4D, right panels). In addition, we found a significant increase in lymphoid aggregate formation in mice receiving 3 weeks repetitive HDE exposure regardless of diet (P<.0001; Fig. 4B and 4D, left panels). Even though the scores were slightly lower in the recovery phase, they remained elevated in mice receiving 3 weeks repetitive HDE exposure (Fig. 4B and 4D, right panels). Furthermore, we did not observe any changes in epithelial hyperplasia of small bronchiole airways and those around the alveolar ducts or terminal bronchioles (Fig. 4C and F).
Fig. 4.
Representative images and scoring of lung histopathology in mice receiving DHA diet supplementation and repetitive HDE exposure at three weeks and after the recovery period. Mice were fed DHA or control diets for four weeks before commencing three-week repetitive 12.5% HDE exposure. Lung was fixed with formalin, and paraffin embedded sections were obtained for H & E staining as described in Methods. Lung histopathology was evaluated for alveolar cellularity, lymphoid aggregates, and epithelial hyperplasia both at the end of the three-week HDE exposure (A, B and C, left panels) and recovery period (A, B and C, right panels). H & E-stained lungs were visualized at 200X and 100X, lung pathology scoring was performed based on alveolar cellularity (D), lymphoid aggregates (E) and epithelial hyperplasia (F) as described in Methods. Left and right panels show data for three-week and recovery periods, respectively. Data are mean ± standard error of the mean, n=6 for saline control and DHA alone groups (no HDE exposure) and n=9-10 mice for HDE and DHA+HDE groups. * P<.05, ** P<.01, *** P<.001, **** P<.0001.
We observed a slight elevation in total cells, macrophages, and lymphocytes in the BALF as well as lung histopathology scores in mice on high DHA diet (no HDE exposure) as compared to those on control diet. Despite an apparent slight overall increase in these cellular levels, none of these observations approached significance (Fig. 2A recovery period, total BALF cells P=.68, Fig. 2C, BALF macrophages P=.75, Fig. 2D, BALF lymphocytes P=.5 and Fig. 4A, alveolar cellularity at 3-week P=.89, recovery P=.95, Fig. 4B, lymphoid aggregates in recovery period P=.97 and Fig. 4C, hyperplasia score at 3-week P=.59).
3.3. Underlying mechanisms of DHA action in resolution of inflammation: Resolvins
We next aimed to identify the mechanisms of decreased inflammation in the lung by the high DHA diet. RvD1 and RvD2 are bioactive metabolites generated from DHA by 15-LOX/ 5-LOX enzymatic activity and they have been implicated in resolution of inflammation in the lung [36]. We observed a significant increase in both RvD1 (P<.001; Fig. 5A, top panel) and RvD2 (P<.001; Fig. 5B, top panel) in BALF of DHA-fed mice receiving 3 weeks repetitive HDE exposure as compared to their corresponding DHA or HDE counterparts. The BALF levels of these DHA metabolites remained significantly elevated in the recovery phase in DHA-fed mice receiving 3 weeks repetitive exposure (P<.001; Fig. 5A, B bottom panels).
Fig. 5.
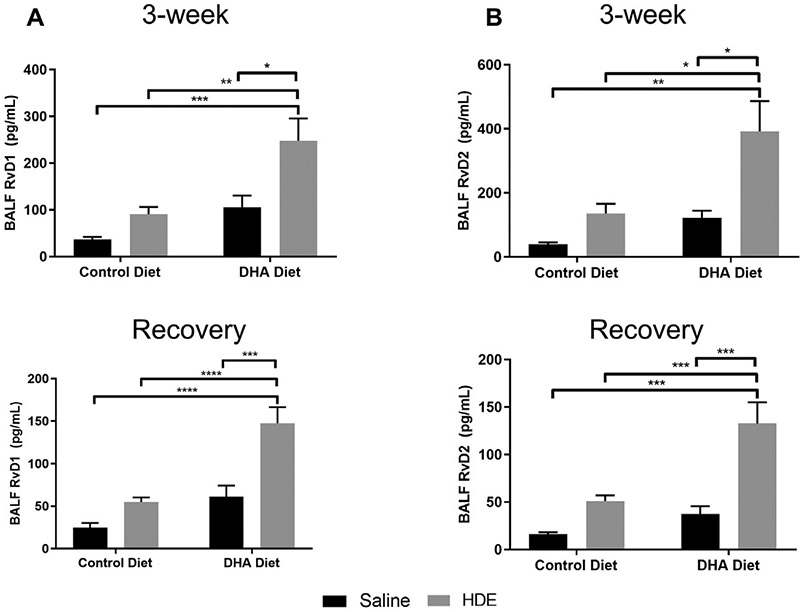
Elevated BALF levels of DHA-derived resolvin after high DHA diet and repetitive HDE exposure at three weeks and after the recovery period. Mice were fed DHA or control diets for four weeks before commencing three-week repetitive 12.5% HDE exposure. BALF was collected either at the end of the three-week exposure (top panels) or recovery period (bottom panels), and RvD1 and RvD2 were measured by ELISA as described in Methods. Data are mean ± standard error of the mean, n=6-8 for saline control and DHA alone groups (no HDE exposure) and n=12 mice for HDE and DHA+HDE groups. * P<.05, ** P<.01, *** P<.001, **** P<.0001.
3.4. Underlying mechanisms of DHA action: Endocannabinoids
To ascertain the impacts of DHA diet on changes in additional lipid metabolites outside of the specialized pro-resolving mediator (SPM) family, we next determined the circulating levels of major endocannabinoids and related lipid mediators derived from both ARA and DHA (Fig. 6). Circulating endocannabinoids are released from multiple organs and tissues and their levels are increased during systemic inflammation [37]. Regardless of the repetitive HDE exposure, we observed significant decreases in plasma endocannabinoids derived from ARA, namely AEA and 2-AG as well as oleoylethanolamide (OEA) in DHA-fed mice (P<.001; Fig. 6A-C). OEA is a non–endocannabinoid, which is a potent PPAR-alpha agonist and it is known for its fat sensing ability that mediates satiety[38]. OEA levels are also reported to be altered by inflammation and diet. In contrast, plasma levels of the DHA-derived endocannabinoid-like molecules DHEA and 2-DG were significantly increased in DHA-fed mice (P<.001; Fig. 6D, E).
Fig. 6.
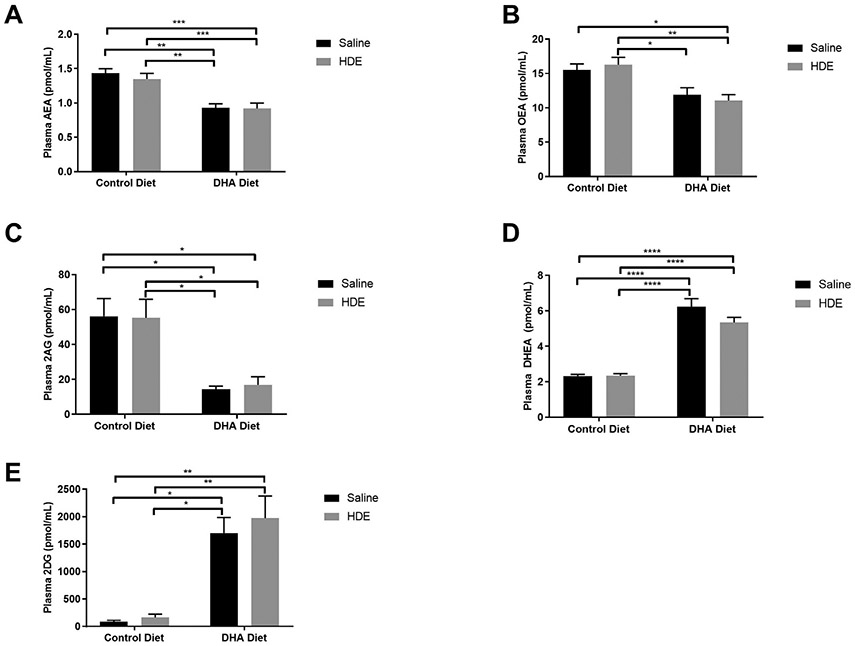
Changes in ARA and DHA-derived peripheral endocannabinoids after high DHA diet and three-week repetitive HDE exposure. Mice were fed DHA or control diets for four weeks before commencing three-week repetitive 12.5% HDE exposure. Plasma was isolated from whole bloods collected at the end of the three-week exposure and plasma levels of ARA-derived endocannabinoid AEA (A), non–endocannabinoid OEA (B), ARA-derived endocannabinoid 2-AG (C), DHA-derived endocannabinoid-like molecules DHEA (D) and 2DG (E) were determined by LC/MS as described in Methods. Data are mean ± standard error of the mean, n=5-8 for saline control and DHA alone groups (no HDE exposure) and n=12 mice for HDE and DHA+HDE groups except for 2DG (E) in the HDE alone group. * P<.05, ** P<.01, *** P<.001, **** P<.0001.
3.5. Effects of DHA diet and HDE exposure on lung macrophages
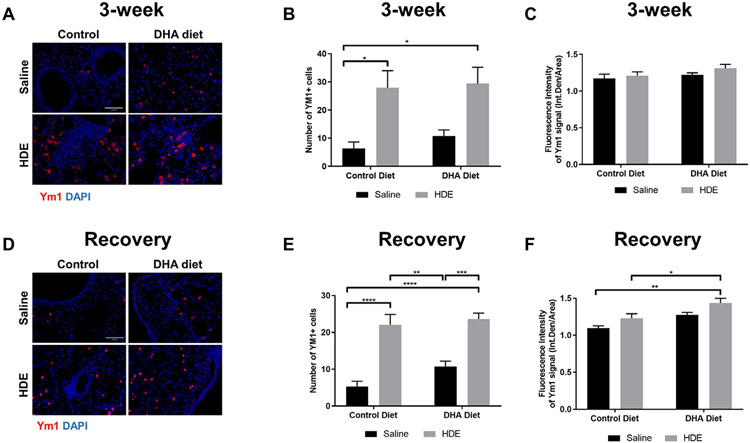
Given that macrophages are indispensable in promoting tissue repair and resolution of inflammation [39,40], we next examined tissue macrophages across all the treatment groups (Fig. 7). We determined the lung immunofluorescence signal of Ym1, a marker for M2-like macrophages associated with an anti–inflammatory, pro-repair phenotype in lung tissue sections [41]. We found a significant main effect of HDE treatment (F= 16.42, P=.0007) with post-hoc analyses revealing a significant increase in the number of Ym1 positive macrophages in HDE-challenged mice regardless of the type of diet (P<.05 as compared to saline controls) (Fig. 7A,B).
Fig. 7.
Effect of high DHA diet and repetitive HDE exposure on the number of Ym1-positive cells in the lung at three weeks (A-C) and after the recovery period (D-F). Mice were fed high DHA or control diets for four weeks before commencing three-week repetitive 12.5% HDE exposure. Lung was fixed in formalin, and paraffin embedded sections were obtained for immunofluorescence staining of Ym1 and images were obtained on Echo Revolve epifluorescence microscope with 20X objective as described in Methods. Lung representative images of Ym1+ immunofluorescence staining, number of Ym1+ macrophages and quantification of fluorescence intensity are shown on A, B and C for mice at three weeks after saline or HDE exposure, and on D, E and F for mice after the recovery period following 3-week repetitive HDE exposure. Number of Ym1+ cells were counted on ImageJ; n=5-7 for three weeks (B), and n=6 for the recovery period (E). Fluorescence intensity of the whole image was obtained by quantifying integrated density/area in Image J; n=4-6 for three weeks (C), and n=5-6 for the recovery period (F). Data are mean ± standard error of the mean. * P<.05, ** P<.01, *** P<.001, **** P<.0001.
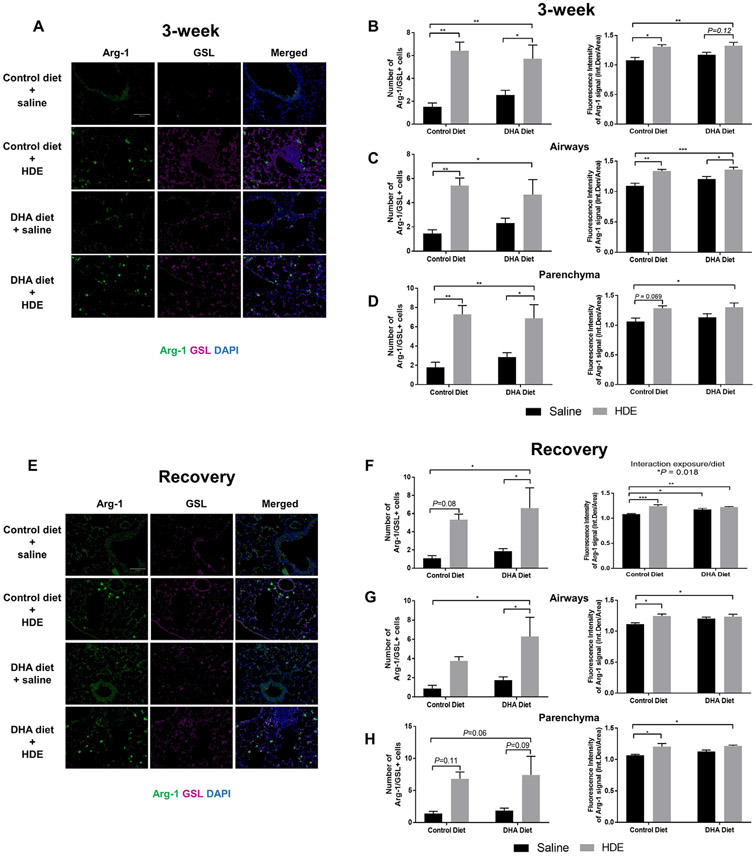
In the recovery period following 3-week HDE exposure, we also found a significant main effect of HDE (F= 59.21, P<.0001) and a trend for the main effect of DHA (F=3.291, P=.0847) on the number of Ym1 positive macrophages (Fig. 7D, E). We also evaluated the expression of Ym1 by quantifying the fluorescence intensity of the Ym1 signal. While we did not observe any significant changes in fluorescence intensity of the Ym1+ cells at 3-weeks (Fig. 7C), Ym1 intensity profiles in the recovery period were higher in the lungs of HDE-challenged mice on high DHA diet as compared to mice on control diet (Fig. 7F). Based on the YM-1+ macrophage staining, we sought to confirm the M2-like macrophage polarization using an additional M2-associated marker, arginase-1 (Arg-1). We performed an IF co-staining of Arg-1 together with a lectin that can identify both M1 and M2 macrophages, GSL (Fig. 8). Secondary-only (no primary antibody) controls are shown in Suppl. Fig. 2. When we assessed the number of cells with positive Arg-1/GSL co-staining, we found a significant main effect of HDE at 3 weeks (F=62.32, P<.0001, Fig. 8A, and 8B-D, left panel) and at the recovery period (F=46.75, P=.0014, Fig. 8E and 8F-H, left panel). Multiple comparisons showed that the number of cells with positive Arg-1 staining significantly increased with HDE at 3 weeks (P=.0015, control vs. HDE) while this increase missed significance at the recovery period (P=.0872, control vs. HDE). In additional analyses, we found that in the HDE-challenged mouse lungs there were more Arg-1 positive macrophages in the parenchyma (Fig. 8D and H) than in the airways (Fig. 8C and G). Although, the total number of Arg-1+ macrophages around the airways of DHA diet-fed mice was significantly elevated during the recovery phase (Fig. 8G, *P=.03 for DHA diet vs. DHA diet + HDE), this was not the case for airway-associated macrophages in control diet-fed mice (Fig. 8G, P=.24 for control diet vs. control diet + HDE). In considering overall Arg-1 staining intensity, we observed a significant main effect of HDE on fluorescence intensity of Arg-1 at 3 weeks (F=48.53, P=.0008, Fig. 8A, and 8B-D, right panel), and at the recovery period (F=47.91, P=.0002, with a significant interaction F=12.92, P=.0182, Fig. 8E and 8F-H, right panel). This was also reflected in multiple comparisons, a significant increase in Arg-1 immunofluorescence intensity in mouse lungs receiving the repetitive HDE exposure both at 3-weeks (P=.014 control vs. HDE, Fig. 8B, right panel) and after the recovery period (P=.0004, Fig. 8F, right panel) regardless of the diet.
Fig. 8.
Changes in the Arg-1 positive macrophages after repetitive HDE exposure and DHA diet supplementation in the lung at three weeks (A-D) and after the recovery period (E-H). Formalin fixed and paraffin embedded lung sections were stained for Arg-1 and GSL, M2 and M1/M2 macrophage marker in tissues from mice receiving control or DHA diet for four weeks prior to three-week HDE exposure. Images were obtained by epifluorescence microscope Echo Revolve with 20X objective in FITC (Arg-1) and Cy5 (GSL) channels. Between 5-7 images for airways and lung parenchyma were obtained. The fluorescence intensity of the whole image was determined by quantifying integrated density/area in Image J. Quantification of Arg-1 immunofluorescence signal around the airways and parenchyma at three-weeks (C, D) and at the recovery period (G, H) are shown as number of Arg-1/ GSL positive cells and fluorescence intensity. Data are mean ± standard error of the mean. * P<.05, ** P<.01, *** P<.001.
To further determine whether DHA diet impacted macrophage polarization, we used bone marrow-derived macrophages (BMDM) isolated from C57BL/6J male mice. After differentiating the cells for 7 days in the presence of L929 medium, cells were stimulated with M1/ M2 activators in the presence or absence of DHA at 1 μM and/or 0.5% HDE for 24 and 72 hours. We observed an increase in Arg-1 immunofluorescence signal in the presence of DHA (1 μM) with M1 activators (IFNγ and LPS) at 24 hours, which was reflected in a two-way ANOVA analysis of immunofluorescence data showing a significant main effect of DHA (F=30.57, P=.01) (Suppl. Fig. 3A and 3C). In addition, we found a significant main effect of both DHA (F=17.43, P=.0098) and HDE (F= 37.28, P=.0005) on the Arg-1 signal in the presence of the M2 activator IL-4 at 24 hours (Suppl. Fig.3A and 3D). At 72 hours, we similarly observed no changes in Arg-1 signal in the presence of 0.5% HDE and M1 activators (F=6.7, P=.26; Suppl. Fig. 3B and 3E), while DHA still trended toward increasing Arg-1 expression in the presence of M1 activators at 72 hours (Suppl. Fig. 3E); however, this missed significance (P=.07). In the presence of M2 activators, HDE had a significant effect on Arg-1 expression (P<.0001) with a trend toward an interaction between DHA & HDE at 72 hours (Suppl. Fig. 3F; P=.068). Comparing 24- vs. 72-hour timepoints, presence of DHA appears to change the kinetics and response to HDE exposure in the M1-environment.
4. Discussion
The primary goal of these studies was to elucidate the role of DHA in a murine model of repetitive agricultural dust-induced lung inflammation. While there are prevention options such as use of N95 mask respirators for individuals working in high-risk occupational environments (e.g., swine confinement facilities), compliance with requirements to wear safety masks is low among agriculture workers [3]. Therefore, we rationalized that individuals chronically exposed to agricultural dust can benefit from dietary modifications to reduce the lung inflammatory consequences of occupational dust exposure [3]. The main findings of our study are that mice fed a high DHA diet exhibit improved recovery of airway-related inflammatory markers, including significantly lower levels of inflammatory mediator TNFα at five hours following HDE exposure, as well as prevented increased levels of immune cell infiltrates at one week post-HDE exposure, a sustained increase in BALF AREG at one week post-HDE exposure, and significantly elevated SPM RvD1 and RvD2 that are elevated at both five hours following HDE exposure as well as following the recovery phase. Our results also identify significant alterations in whole blood PUFA levels that correspond with changes in PUFA-derived peripheral endocannabinoids that may be partially responsible for the beneficial effects of DHA. In addition, we identified the accumulation of Ym1+/Arg-1+ macrophages after repetitive HDE exposure, which might contribute to resolution of inflammation, where mice fed a high DHA diet also exhibit increased YM1 fluorescence intensity specifically during recovery from HDE.
Unresolved chronic lung inflammation is a common mechanism seen in respiratory diseases including asthma, COPD, chronic bronchitis and cystic fibrosis, and management of airway inflammation is critical for disease resolution [42]. Omega-3 PUFAs and their bioactive metabolites have critical roles in regulating the mitigation of inflammation and have been considered as a therapeutic approach to respiratory diseases [8,12,13,43-45]. A National Health and Nutrition Examination Survey (NHANES) study found associations of low omega-3 fatty acid intake with symptoms of COPD and found that consumption of both EPA and DHA is associated with reduced symptoms of lung disease among smokers [16]. In addition, asthma prevalence has been reported to be on the rise in Western countries, which is partly attributed to the high omega-6 fatty acid to omega-3 fatty acid ratio of ~20:1 in the Western diet [46,47]. As expected in our in vivo model, following consumption of a high DHA diet for 4 weeks, we observed significant increases in whole blood levels of the precursor omega-3 fatty acids (DHA, EPA, DPA) at the expense of omega-6 fatty acids (i.e., ARA) both at the end of the 3-week exposure and in the recovery period (Fig. 1), which corresponded to a 6-8-fold decrease in the omega-6-to-omega-3 ratio (Table S3). Indeed, DHA is efficiently incorporated into plasma phospholipids and peripheral blood mononuclear cells after a 4-week supplementation at approximately 700 mg/day dose in older healthy human subjects [48] and in mice at 45 mg DHA/kg body weight after a month of feeding [49]. Consistent with our previous results and as shown by others [17,18,23,50], repetitive HDE exposure resulted in increased total cell influx with neutrophils accounting for the bulk of the detrimental infiltration and persisting throughout the recovery period (Fig. 2). As reported by Warren et al. [50] in assessing recovery following the repetitive HDE exposure model, among the BALF immune cells, neutrophils are cleared after 1 week and lymphocytes and macrophages remain the same between 2-4 weeks post exposure. Tissue pathology was also assessed in that study, which showed persistence of lymphoid aggregates, bronchiolar and alveolar inflammation up until at least 4-weeks post exposure. The only significant difference observed between the 1-week versus 4-week time point was the pathology score for lymphoid aggregates, but even at the 4-week time point the inflammatory score for lymphoid aggregates was still significantly different than saline controls. Since HDE-induced inflammation is mostly driven by neutrophil infiltration, we focused on 1 week after 3-week HDE exposure as our ‘recovery period’.
Our results are in line with these reported findings, and in addition we found that high DHA diet is effective in decreasing BALF infiltrating cells without affecting repetitive HDE-induced changes in the lung (alveolar cellularity and lymphoid aggregate formation) (Fig. 4). Changes at the tissue level are to some extent consistent with changes in MPO (Fig. 3), which contributes to modulation of tissue damage and microbial killing by neutrophils [51]. Like MPO, increased MIF levels in HDE-challenged mice fed the high DHA diet are consistent with the recognized role of MIF in tissue repair [52]. MIF is secreted in response to tissue injury (i.e., bleomycin inducing lung fibrosis), oxidative stress, endotoxin and as a result of aging and in aging-related lung disease including COPD, bacterial infections, and pulmonary fibrosis [52-54]. Moreover, MIF has been shown to promote cell proliferation and angiogenesis and protect cells from apoptosis [55,56], all of which are also mechanisms supporting tissue repair and return to homeostasis. With regards to the lymphoid aggregates, it was reported that DHA supplementation at a minimum of 10 g DHA/kg diet delays B- and T-cell lymphoid aggregate formation at 5 weeks after silica exposure [57], suggesting that tissue level changes require a longer and higher dietary DHA dose. However, it is plausible that the persistent lymphoid aggregates identified following repetitive HDE exposure are protective, as has been shown in other models that develop induced bronchus-associated lymphoid tissue (iBALT) [58,59]. Characterized by the accumulation and persistence of leukocytes under the airway epithelium into organized tertiary lymphoid structures, iBALT formation occurs in the lungs following certain infections or inflammatory challenges and could be a protective response that leads to more effective responses during pathogen challenge [60]. Thus, additional work is warranted to explore the role of these structures following HDE exposure.
Omega-3 and omega-6 PUFAs are metabolized into different classes of bioactive lipids. There is accumulating evidence that DHA-derived active metabolites are in part responsible for their beneficial effects in suppressing airway inflammation [13,18,61,62]. DHA is mainly metabolized by cytochrome P450, COX and LOX enzymes, which generate many active eicosanoids including epoxides and prostaglandins, as well as specialized pro-resolving mediators (SPMs, leukotrienes, lipoxins, resolvins and maresins) [8]. SPMs are produced in response to a “temporal lipid mediator class switch”, which initiates the resolution phase of acute inflammation [63,64]. While dietary consumption of omega-3 PUFAs has been shown to increase SPMs in plasma, synovial fluids and lungs of patients with rheumatoid arthritis and acute respiratory distress syndrome [65-67], patients with lung disease have been reported to have decreased SPM levels [13] and reduced levels of SPM and DHA in respiratory secretions have been associated with disease severity in asthma and cystic fibrosis [68-70]. DHA-derived SPMs also exhibit beneficial effects in pre-clinical mouse models [68,71]. In particular, resolvins exhibit both anti–inflammatory and pro-resolving effects, which include interrupting PMN infiltration, promoting macrophage uptake of debris and apoptotic PMNs, enhancing bacterial killing and promoting resolution [36]. Among the resolvins, RvD1 and RvD2 are metabolites synthesized from DHA via 15-LOX/5-LOX activity on 17S-hydroperoxy-DHA[13], and both can limit pro-inflammatory cytokine release and neutrophilic inflammation in sepsis [71], and RvD1 has been shown to decrease airway hyperresponsiveness and maintain epithelial barrier integrity in acute lung injury [72,73]. Consistent with these observations, we found that a high DHA diet significantly alters the BALF levels of both RvD1 and RvD2 while reducing inflammatory cell influx into the lungs and decreasing pro-inflammatory cytokine TNFα levels in BALF during the 3-week repetitive exposure or in the resolution period (Fig. 2,3 and 5). A recent study reported that HDE-induced TNF-α release is regulated by IL-10 through an SRA (scavenger receptor A)/ Protein kinase C- ζ dependent mechanism [74]. Repetitive HDE exposure has been linked to Th1 and Th17 response in mice and both RvD1 and RvD2 have the ability to modulate Th1 and Th17 response by decreasing production of IFN-γ and IL-17 and transcription factors RORc and T-bet [61], a likely mechanism of action of resolvins that remains to be determined in our model. Strikingly, both resolvin BALF levels increased specifically in response to repetitive HDE exposure in DHA-fed mice (Fig. 5), suggesting these mediators are released primarily in response to an inflammatory insult and has a modulatory role in resolution of inflammation. Therefore, RvD1 and RvD2 might be contributing in part to the beneficial effects of DHA supplementation in repetitive HDE-induced lung inflammation; however, establishing the role of resolvins warrants further studies.
Endocannabinoids are another class of metabolites generated by various enzymatic and non–enzymatic pathways in relation to omega-6 and omega-3 PUFAs [75]. We observed significant increases in DHA-derived endocannabinoid-like lipids, DHEA and 2-DG, after dietary DHA supplementation (Fig. 6). Like SPMs, the levels of these mediators are reportedly altered by inflammation and diet [18,35,76-79]. While their specific mechanism of action and associated receptor systems are not entirely known, evidence suggests they act via cannabinoid receptors [80,81]. AEA and 2-AG are the most well-studied ligands acting on CB1 and CB2 G-protein coupled receptors and significant levels of these mediators are detected in the circulation and other organs [82-84]. The anti–inflammatory effects of peripheral endocannabinoids include regulating pro-inflammatory cytokine production, inflammatory cell recruitment, macrophage phagocytosis, T and B cell proliferation [85]. The endocannabinoid AEA reduces IL-6, IL-8 production at nanomolar concentrations and inhibits TNFα, IFN-γ, and IL-4 at higher concentrations in human peripheral blood mononuclear cells after LPS exposure [85]. The DHA-derivative DHEA has been linked directly to dietary intake [86], and in low nanomolar ranges DHEA regioisomers have been shown to alter human PMN shape and chemotaxis in response to IL-8 in microfluidic chambers, and to protect mice from second organ reperfusion injury through reducing PMN infiltration to the lung [87]. Pre-clinical studies report that plasma DHEA levels increase 2 to 6-fold in mice fed a high DHA diet [88]. Similarly, we found that the plasma DHEA levels increase approximately 3-fold in mouse fed the high DHA diet (Fig. 6D). Like DHEA, the DHA-derived 2-DG also increases with high omega-3 intake and increased levels have been associated with mature human milk as compared to transitional milk [89]. In mice, its plasma levels have been reported to increase slightly in response to a high DHA diet [79]. Unlike this study, we observed >10 times increase in plasma 2-DG in mice on high DHA diet (Fig. 6E). At low micromolar concentrations (1-3 μM), 2-DG has been shown to reduce vasoconstriction of pulmonary arteries, whereas at high micromolar concentrations (30 μM) it inhibits RhoA activity [90]. In human lung adenocarcinoma epithelial cells (Calu-3), 2-DG reduces pro-inflammatory cytokines IL-6 and IL-8 at 3 μM concentration after P. aeruginosa LPS stimulation of Calu-3 cells, a model used to address inflammation in cystic fibrosis [91]. The high plasma levels of DHEA and 2-DG we observed in HDE-challenged mice fed the high DHA diet suggest a potentially important role of the peripheral endocannabinoid system in controlling immunity; however, this needs further investigation.
Besides our findings with DHA, we report an increase in lung Ym1+ macrophages both at 3 weeks and after the recovery period following repetitive HDE exposure (Fig. 7). It has been reported that repetitive dust exposure alters in vitro differentiation of monocytes to macrophages [92]. Generally, M1 macrophages are associated with pro-inflammatory or Th1 immune response, whereas M2 macrophages are associated with anti–inflammatory, tissue repair and pro-resolving activities characterized by increased phagocytotic activity, increased anti–inflammatory cytokine and chemokine secretion or Th2 immune response; however, their roles in different disease settings are complex. Recent findings on Ym1+ macrophages have been conflicting. In a murine model of allergic airway inflammation (house dust mite), Ym1+ M2 macrophages have been reported to be the critical regulators of tipping the balance between development of eosinophilic versus neutrophilic inflammation [93], which in this case YM1+ M2 macrophages were associated with higher eosinophilia and worse outcomes. Also, chitinase like proteins such as Ym1 and Ym2 have been shown to recruit neutrophils through increasing the number of IL-17 producing γδT cells [94]. Pertinent to this point, airway inflammation in swine confinement workers differs from naïve subjects even after repetitive exposures; patients displaying neutrophilic airway inflammation in the former case, and neutrophilic & eosinophilic inflammation in the latter [95]. While the role of Ym1+ macrophages remain to be investigated in HDE-driven inflammation, M2 type macrophages are associated with resolution of inflammation and better outcomes in human asthma as compared to asthma models in mice [96]. We have previously found that M2-polarization following HDE treatment in peritoneal macrophages results in increased AREG levels and reduced inflammation [31]. In our efforts to understand whether the Ym1+ macrophages in HDE-induced inflammation are M1 or M2-like, this study did not place Ym1+ macrophages in the alternatively activated M2a category, which is in contrast to previous reports [41]. Nonetheless, we found increased Arg-1 immunofluorescence signal in mouse lungs receiving the repetitive HDE exposure (Fig. 8), suggesting a compensatory mechanism in response to HDE. This parallel increase in Ym1+ and Arg1+ macrophages, suggest an increase in the M2-like macrophage populations. The finding that Ym1+ macrophages at the end of the 3-week period is comparable to the 1-week recovery period adds further evidence that this is a compensatory mechanism that may be a response to chronic exposure or attempted resolution of inflammation across these time periods. While this increase in M2-type macrophages was independent of DHA diet supplementation in vivo, DHA appears to change the kinetics of macrophage polarization in the bone marrow-derived macrophages toward an M2-like stage in the presence of M1 activators (Suppl. Fig. 3). Furthermore, differences in Arg-1+ and/or Ym1+ staining intensity were observed based on lung location (airway- vs. parenchyma-associated) and time point following exposure (3-week vs. Recovery) that appeared to be diet-dependent based on post-hoc analyses (Fig. 7F and Fig. 8G). These results are consistent with previous reports supporting the effect of DHA on macrophage polarization [97,98].
Our study is not without limitations. We are not using a whole dust inhalation exposure model; however, swine farm workers chronically exposed to dust have been reported to have increased BALF neutrophils, macrophages and pro-inflammatory cytokines IL-6, IL-8, and TNFα, all of which is consistently observed in mice exposed to repetitive dust exposure for 15 days [3,20,30]. As mentioned above, naïve subjects exposed to swine barn dust once do exhibit a heightened response as compared to chronically exposed individuals. A similar pattern is also observed in mouse studies between the repetitive and single dust exposures [30,50]. Therefore, the murine model using 12.5% HDE is a well-established model replicating the inflammatory and pathological changes seen in swine farmers inhaling the dust daily, and administration of the dust extract via intranasal instillation is an appropriate and standardized model for studying the effects of agricultural dust on the lung. It is also an excellent model to understand how DHA might affect overall lung function parameters as well as lung injury during repetitive dust exposure, which are currently largely unknown. A significant correlation between dust exposure and increased BALF albumin and α2-macroglobulin levels were shown in healthy individuals exposed to swine barn environment for 3 hours [99].
In our studies we chose to use male mice, primarily because among the swine barn workers in confinement facilities there are more male workers than female workers and previously published studies using swine barn dust have routinely been performed using male mice [17,18,24,50,74,100,101]. Thus, employing male mice makes our outcomes comparable to those studies. That said, Gao et al. previously reported sex specific differences in TLR2 gene polymorphisms [102], which is a toll like receptor activated by swine barn dust [103], and lung function among swine barn workers. They found that female workers with the rs4696480 polymorphism in TLR2 gene show better lung function, while male worker gender with the rs187084 polymorphism in the TLR9 gene have a lower lung function. Similarly, another study evaluated the association between TLR4 gene polymorphisms and sex in non–smoking healthy individuals exposed swine barn environment for 5 hours [104]. While they did not observe any differences in lung function parameters (FEV1, FVC, FEV1/FVC, and FEF25-75), they found sex-specific differences in inflammatory outcomes among those exposed to swine dust, specifically males with no polymorphism and females with the TLR4 polymorphism have enhanced levels of serum TNFα. These studies provide evidence that sex-specific differences in response to HDE need further investigation.
To the best of our knowledge, this is the first study evaluating the effects of high DHA diet on inflammatory outcomes in a murine model of repetitive dust exposure, both during the active period and in the recovery phase. Repetitive exposure is most relevant to swine farm workers since they are exposed to dust daily while working in confined areas for many hours during the day [3]. We also performed an extensive assessment of the inflammatory cytokines both in the active phase of the exposure and in the recovery period. One of the major strengths of our study is that we found an association between elevated DHA-derived pro-resolving mediators (i.e., resolvins) and peripheral endocannabinoids in exposed mice receiving the high DHA diet, which should be further studied along with a larger oxylipin analysis of ARA and DHA-derived lipid mediators in the lung to further explore other oxylipin classes/metabolites [69,105].
In conclusion, our findings reported here support a role for DHA and identify novel DHA-derived bioactive metabolites as potential mediators of DHA in resolving airway inflammation following repetitive exposure to an aqueous extract of agricultural dust. Increased DHA bioavailability can be achieved by frequent dietary intake of fish or supplements; given that omega-3 PUFA intake is low in the United States based on our recent NHANES study 2003-2014 [106], increasing omega-3 PUFA intake in vulnerable populations could improve lung health outcomes. Our results also suggest that DHA-derived RvD1 and RvD2, as well as DHA-derived peripheral endocannabinoid-like lipids might be among the mediators of DHA in recovering from HDE-induced lung inflammation. Future studies are warranted to test whether DHA and its mediators can act as either stand-alone or added compounds, or as dietary supplementation to anti–inflammatory therapies (such as aspirin-triggered SPMs [107]), which would allow personalized therapies with dose adjustment and thereby lowering the likelihood of side effects.
Supplementary Material
Funding
This work was supported in part by the National Institute of Environmental Health Sciences (K99ES025819/R00ES025819 to TMN), Veterans Administration grant (1I01 CX001714-01 to DR) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK119498 and DK114978 to NVD).
Footnotes
Declaration of competing interests
None of the authors have any financial or other conflicts of interest.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jnutbio.2021.108797.
References
- [1].Agriculture USDo Ag and Food Statistics, Charting the Essentials, vol. 080: Economic Research Service. Administrative Publication; 2018. [Google Scholar]
- [2].Fontana L, Lee SJ, Capitanelli I, Re A, Maniscalco M. Mauriello MC. et al. Chronic obstructive pulmonary disease in farmers: a systematic review. J Occup Environ Med 2017;59:775–88. [DOI] [PubMed] [Google Scholar]
- [3].Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 2003;9:185–96. [DOI] [PubMed] [Google Scholar]
- [4].Nordgren TM, Bailey KL. Pulmonary health effects of agriculture. Curr Opin Pulm Med 2016;22:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nordgren TM, Charavaryamath C. Agriculture occupational exposures and factors affecting health effects. Curr Allergy Asthma Rep 2018;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spurzem JR, Romberger DJ, Von Essen SG. Agricultural lung disease. Clin Chest Med 2002;23:795–810. [DOI] [PubMed] [Google Scholar]
- [7].Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019:53. [DOI] [PubMed] [Google Scholar]
- [8].Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol 2014;7:a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res 2010;51:3481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gutiérrez S, Svahn SL, Johansson ME. Effects of omega-3 fatty acids on immune cells. Int J Mol Sci 2019;20(20):5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Calder PC, Waitzberg DL, Klek S, Martindale RG. Lipids in parenteral nutrition: biological aspects. JPEN J Parenter Enteral Nutr 2020;44(Suppl 1):S21–7. [DOI] [PubMed] [Google Scholar]
- [12].Watson H, Stackhouse C. Omega-3 fatty acid supplementation for cystic fibrosis. Cochrane Database Syst Rev 2020;4:CD002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol 2016;785:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Das UN. Combination of aspirin with essential fatty acids is superior to aspirin alone to prevent or ameliorate sepsis or ARDS. Lipids Health Dis 2016;15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cotogni P, Trombetta A, Muzio G, Maggiora M, Canuto RA. The Omega-3 fatty acid docosahexaenoic acid modulates inflammatory mediator release in human alveolar cells exposed to bronchoalveolar lavage fluid of ARDS patients. Biomed Res Int 2015;2015:642520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lemoine SCM, Brigham EP, Woo H, Hanson CK, McCormack MC, Koch A, et al. Omega-3 fatty acid intake and prevalent respiratory symptoms among U.S. adults with COPD. BMC Pulm Med 2019;19:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nordgren TM, Friemel TD, Heires AJ, Poole JA, Wyatt TA, Romberger DJ. The omega-3 fatty acid docosahexaenoic acid attenuates organic dust-induced airway inflammation. Nutrients 2014;6:5434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nordgren TM, Bauer CD, Heires AJ, Poole JA, Wyatt TA, West WW et al. Maresin-1 reduces airway inflammation associated with acute and repetitive exposures to organic dust. Transl Res 2015;166:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nordgren TM, Heires AJ, Bailey KL, Katafiasz DM, Toews ML, Wichman CS, et al. Docosahexaenoic acid enhances amphiregulin-mediated bronchial epithelial cell repair processes following organic dust exposure. Am J Physiol Lung Cell Mol Physiol 2018;314:L421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol (1985) 2002;93:289–96. [DOI] [PubMed] [Google Scholar]
- [21].Boissy RJ, Romberger DJ, Roughead WA, Weissenburger-Moser L, Poole JA, LeVan TD. Shotgun pyrosequencing metagenomic analyses of dusts from swine confinement and grain facilities. PLoS One 2014;9:e95578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, et al. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A 2010;73:684–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Romberger DJ, Heires AJ, Nordgren TM, Souder CP, West W, Liu XD, et al. Proteases in agricultural dust induce lung inflammation through PAR-1 and PAR-2 activation. Am J Physiol Lung Cell Mol Physiol 2015;309:L388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dominguez EC, Heires AJ, Pavlik J, Larsen TD, Guardado S, Sisson JH, et al. A high docosahexaenoic acid diet alters the lung inflammatory response to acute dust exposure. Nutrients 2020. August 4;12(8):2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Whelan J Conversion of dietary polyunsaturated fats between humans and rodents: A review of allometric scaling models. Prostaglandins Leukot Essent Fatty Acids 2020;158:102094. [DOI] [PubMed] [Google Scholar]
- [26].Nording ML, Yang J, Georgi K, Hegedus Karbowski C, German JB, Weiss RH, et al. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 Fatty acids. PLoS One 2013;8:e76575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].USFD A. FDA announces qualified health claims for omega-3 fatty acids; 2004. [Google Scholar]
- [28].Albracht-Schulte K, Kalupahana NS, Ramalingam L, Wang S, Rahman SM, Robert-McComb J, et al. Omega-3 fatty acids in obesity and metabolic syndrome: a mechanistic update. J Nutr Biochem 2018;58:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Williams NC, Hunter KA, Shaw DE, Jackson KG, Sharpe GR, Johnson MA. Comparable reductions in hyperpnoea-induced bronchoconstriction and markers of airway inflammation after supplementation with 6•2 and 3•1 g/d of long-chain n-3 PUFA in adults with asthma. Br J Nutr 2017;117:1379–89. [DOI] [PubMed] [Google Scholar]
- [30].Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, et al. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 2009;296:L1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nordgren TM, Heires AJ, Zempleni J, Swanson BJ, Wichman C, Romberger DJ. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J Nutr Biochem 2019;64:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Louwagie EJ, Larsen TD, Wachal AL, Baack ML. Placental lipid processing in response to a maternal high-fat diet and diabetes in rats. Pediatr Res 2018;83:712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Argueta DA, DiPatrizio NV. Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol Behav 2017;171:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Perez PA, DiPatrizio NV. Impact of maternal western diet-induced obesity on offspring mortality and peripheral endocannabinoid system in mice. PLoS One 2018;13:e0205021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Argueta DA, Perez PA, Makriyannis A, DiPatrizio NV. Cannabinoid CB 1 receptors inhibit gut-brain satiation signaling in diet-induced obesity. Front Physiol 2019;10:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018;128:2657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018;43:155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab 2008;8:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Puttur F, Gregory LG, Lloyd CM. Airway macrophages as the guardians of tissue repair in the lung. Immunol Cell Biol 2019;97:246–57. [DOI] [PubMed] [Google Scholar]
- [40].Hu G, Christman JW. Editorial. Alveolar Macrophages in Lung Inflammation and Resolution. Front Immunol 2019;10:2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rőszer T. Understanding the Mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015;2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leitch AE, Duffin R, Haslett C, Rossi AG. Relevance of granulocyte apoptosis to resolution of inflammation at the respiratory mucosa. Mucosal Immunol 2008;1:350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kumar A, Mastana SS, Lindley MR. n-3 Fatty acids and asthma. Nutr Res Rev 2016;29:1–16. [DOI] [PubMed] [Google Scholar]
- [44].Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int 2015;64:27–34. [DOI] [PubMed] [Google Scholar]
- [45].Teopompi E, Risé P, Pisi R, Buccellati C, Aiello M, Pisi G, et al. Arachidonic acid and docosahexaenoic acid metabolites in the airways of adults with cystic fibrosis: effect of docosahexaenoic acid supplementation. Front Pharmacol 2019;10:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity 2014;40:833–42. [DOI] [PubMed] [Google Scholar]
- [48].Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr 2001;131:1918–27. [DOI] [PubMed] [Google Scholar]
- [49].Sugasini D, Thomas R, Yalagala PCR, Tai LM, Subbaiah PV. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci Rep 2017;7:11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Warren KJ, Wyatt TA, Romberger DJ, Ailts I, West WW, Nelson AJ, et al. Post-injury and resolution response to repetitive inhalation exposure to agricultural organic dust in mice. Safety (Basel) 2017;3(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2013;48:531–9. [DOI] [PubMed] [Google Scholar]
- [52].Sauler M, Bucala R, Lee PJ. Role of macrophage migration inhibitory factor in age-related lung disease. Am J Physiol Lung Cell Mol Physiol 2015;309:L1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sauler M, Leng L, Trentalange M, Haslip M, Shan P, Piecychna M, et al. Macrophage migration inhibitory factor deficiency in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2014;306:L487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Florez-Sampedro L, Soto-Gamez A, Poelarends GJ, Melgert BN. The role of MIF in chronic lung diseases: looking beyond inflammation. Am J Physiol Lung Cell Mol Physiol 2020;318:L1183–97. [DOI] [PubMed] [Google Scholar]
- [55].White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, et al. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res 2003;9:853–60. [PubMed] [Google Scholar]
- [56].Arenberg D, Luckhardt TR, Carskadon S, Zhao L, Amin MA, Koch AE. Macrophage migration inhibitory factor promotes tumor growth in the context of lung injury and repair. Am J Respir Crit Care Med 2010;182:1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bates MA, Akbari P, Gilley KN, Wagner JG, Li N, Kopec AK, et al. Dietary docosahexaenoic acid prevents silica-induced development of pulmonary ectopic germinal centers and glomerulonephritis in the lupus-prone NZBWF1 mouse. Front Immunol 2018;9:2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Marin ND, Dunlap MD, Kaushal D, Khader SA. Friend or foe: the protective and pathological roles of inducible bronchus-associated lymphoid tissue in pulmonary diseases. J Immunol 2019;202:2519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Silva-Sanchez A, Randall TD. Role of iBALT in Respiratory Immunity. Curr Top Microbiol Immunol 2020;426:21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hwang JY, Randall TD, Silva-Sanchez A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front Immunol 2016;7:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chiurchiù V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med 2016;8:353ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fussbroich D, Colas RA, Eickmeier O, Trischler J, Jerkic SP, Zimmermann K, et al. A combination of LCPUFA ameliorates airway inflammation in asthmatic mice by promoting pro-resolving effects and reducing adverse effects of EPA. Mucosal Immunol 2020;13:481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2001;2:612–19. [DOI] [PubMed] [Google Scholar]
- [64].Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol 2014;76:467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tedeschi SK, Bathon JM, Giles JT, Lin TC, Yoshida K, Solomon DH. Relationship between fish consumption and disease activity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018;70:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids 2016;107:24–9. [DOI] [PubMed] [Google Scholar]
- [67].Hecker M, Linder T, Ott J, Walmrath HD, Lohmeyer J, Vadász I, et al. Immunomodulation by lipid emulsions in pulmonary inflammation: a randomized controlled trial. Crit Care 2015;19:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol 2004;5:388–92. [DOI] [PubMed] [Google Scholar]
- [69].Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med 2012;53:160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J Allergy Clin Immunol 2013;131:353–60. [DOI] [PubMed] [Google Scholar]
- [71].Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009;461:1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, et al. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol 2013;6:256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, et al. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther 2011;24:434–41. [DOI] [PubMed] [Google Scholar]
- [74].Wyatt TA, Nemecek M, Chandra D, DeVasure JM, Nelson AJ, Romberger DJ, et al. Organic dust-induced lung injury and repair: Bi-directional regulation by TNFα and IL-10. J Immunotoxicol 2020;17:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kunos G, Osei-Hyiaman D, Bátkai S, Sharkey KA, Makriyannis A. Should peripheral CB(1) cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol Sci 2009;30:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Di Paola M, Bonechi E, Provensi G, Costa A, Clarke G, Ballerini C, et al. Oleoylethanolamide treatment affects gut microbiota composition and the expression of intestinal cytokines in Peyer’s patches of mice. Sci Rep 2018;8:14881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ramsden CE, Zamora D, Makriyannis A, Wood JT, Mann JD, Faurot KR, et al. Diet-induced changes in n-3- and n-6-derived endocannabinoids and reductions in headache pain and psychological distress. J Pain 2015;16:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 2010;120:2953–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res 2010;51:1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Brown I, Cascio MG, Wahle KW, Smoum R, Mechoulam R, Ross RA, et al. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 2010;31:1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Pertwee RG. Endocannabinoids and Their Pharmacological Actions. Handb Exp Pharmacol 2015;231:1–37. [DOI] [PubMed] [Google Scholar]
- [82].Moradi H, Park C, Igarashi M, Streja E, Argueta DA, Soohoo M, et al. Serum endocannabinoid levels in patients with end-stage renal disease. J Endocr Soc 2019;3:1869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Moradi H, Park C, Streja E, Argueta DA, DiPatrizio NV, You AS, et al. Circulating endocannabinoids and mortality in hemodialysis patients. Am J Nephrol 2020;51:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 2003;4:873–84. [DOI] [PubMed] [Google Scholar]
- [85].Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol 2003;74:486–96. [DOI] [PubMed] [Google Scholar]
- [86].Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc Natl Acad Sci U S A 2001;98:6402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yang R, Fredman G, Krishnamoorthy S, Agrawal N, Irimia D, Piomelli D, et al. Decoding functional metabolomics with docosahexaenoyl ethanolamide (DHEA) identifies novel bioactive signals. J Biol Chem 2011;286:31532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rossmeisl M, Jilkova ZM, Kuda O, Jelenik T, Medrikova D, Stankova B, et al. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: possible role of endocannabinoids. PLoS One 2012;7:e38834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gaitán AV, Wood JT, Zhang F, Makriyannis A, Lammi-Keefe CJ. Endocannabinoid metabolome characterization of transitional and mature human milk. Nutrients 2018:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Morin C, Fortin S, Rousseau E. Docosahexaenoic acid monoacylglyceride decreases endothelin-1 induced Ca(2+) sensitivity and proliferation in human pulmonary arteries. Am J Hypertens 2012;25:756–63. [DOI] [PubMed] [Google Scholar]
- [91].Morin C, Cantin AM, Rousseau É, Sirois M, Sirois C, Rizcallah E, et al. Proresolving action of docosahexaenoic acid monoglyceride in lung inflammatory models related to cystic fibrosis. Am J Respir Cell Mol Biol 2015;53:574–83. [DOI] [PubMed] [Google Scholar]
- [92].Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, et al. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol 2008;122:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Draijer C, Robbe P, Boorsma CE, Hylkema MN, Melgert BN. Dual role of YM1+ M2 macrophages in allergic lung inflammation. Sci Rep 2018;8:5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sutherland TE, Logan N, Rückerl D, Humbles AA, Allan SM, Papayannopoulos V, et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol 2014;15:1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Larsson KA, Eklund AG, Hansson LO, Isaksson BM, Malmberg PO. Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med 1994;150:973–7. [DOI] [PubMed] [Google Scholar]
- [96].Draijer C, Boorsma CE, Robbe P, Timens W, Hylkema MN, Ten Hacken NH, et al. Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways. J Allergy Clin Immunol 2017;140:280–283.e283. [DOI] [PubMed] [Google Scholar]
- [97].Talamonti E, Pauter AM, Asadi A, Fischer AW, Chiurchiù V, Jacobsson A. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: implications for DHA supplementation during inflammation. Cell Mol Life Sci 2017;74:2815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chang HY, Lee HN, Kim W, Surh YJ. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor γ activation. Life Sci 2015;120:39–47. [DOI] [PubMed] [Google Scholar]
- [99].Ek A, Palmberg L, Larsson K. The effect of fluticasone on the airway inflammatory response to organic dust. Eur Respir J 2004;24:587–93. [DOI] [PubMed] [Google Scholar]
- [100].Poole JA, Nordgren TM, Heires AJ, Nelson AJ, Katafiasz D, Bailey KL, et al. Amphiregulin modulates murine lung recovery and fibroblast function following exposure to agriculture organic dust. Am J Physiol Lung Cell Mol Physiol 2020;318:L180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].LeVan TD, Romberger DJ, Siahpush M, Grimm BL, Ramos AK, Johansson PL, et al. Relationship of systemic IL-10 levels with proinflammatory cytokine responsiveness and lung function in agriculture workers. Respir Res 2018;19:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gao Z, Dosman JA, Rennie DC, Schwartz DA, Yang IV, Beach J, et al. Gender-specific associations between polymorphisms in the Toll-like receptor (TLR) genes and lung function among workers in swine operations. J Toxicol Environ Health A 2018;81:1186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, et al. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol 2011;45:711–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Senthilselvan A, Chénard L, Kirychuk S, Predicala B, Schwartz DA, Burch LH, et al. Gender-related tumor necrosis factor-alpha responses in naïve volunteers with Toll-like receptor 4 polymorphisms exposed in a swine confinement facility. J Interferon Cytokine Res 2009;29:781–90. [DOI] [PubMed] [Google Scholar]
- [105].Nording ML, Yang J, Hoang L, Zamora V, Uyeminami D, Espiritu I, et al. Bioactive lipid profiling reveals drug target engagement of a soluble epoxide hydrolase inhibitor in a murine model of tobacco smoke exposure. J Metabolomics 2015;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Thompson M, Hein N, Hanson C, Smith LM, Anderson-Berry A, Richter CK, et al. Omega-3 fatty acid intake by age, gender, and pregnancy status in the united states: national health and nutrition examination survey 2003-2014. Nutrients 2019. January 15;11(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt B, et al. Lipoxins and aspirin-triggered lipoxins in airway responses. Adv Exp Med Biol 2003;525:19–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.