Abstract
Background
Several interventions have been developed to promote informed consent for participants in clinical trials. However, many of these interventions focus on the content and structure of information (e.g. enhanced information or changes to the presentation format) rather than the process of decision making. Patient decision aids support a decision making process about medical options. Decision aids support the decision process by providing information about available options and their associated outcomes, alongside information that enables patients to consider what value they place on particular outcomes, and provide structured guidance on steps of decision making. They have been shown to be effective for treatment and screening decisions but evidence on their effectiveness in the context of informed consent for clinical trials has not been synthesised.
Objectives
To assess the effectiveness of decision aids for clinical trial informed consent compared to no intervention, standard information (i.e. usual practice) or an alternative intervention on the decision making process.
Search methods
We searched the following databases and to March 2015: Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library; MEDLINE (OvidSP) (from 1950); EMBASE (OvidSP) (from 1980); PsycINFO (OvidSP) (from 1806); ASSIA (ProQuest) (from 1987); WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/); ClinicalTrials.gov; ISRCTN Register (http://www.controlled‐trials.com/isrctn/). We also searched reference lists of included studies and relevant reviews. We contacted study authors and other experts. There were no language restrictions.
Selection criteria
We included randomised and quasi‐randomised controlled trials comparing decision aids in the informed consent process for clinical trials alone, or in conjunction with standard information (such as written or verbal) or alongside alternative interventions (e.g. paper‐based versus web‐based decision aids). Included trials involved potential trial participants, or their guardians, being asked to consider participating in a real or hypothetical clinical trial.
Data collection and analysis
At least two authors independently assessed studies for inclusion, extracted reported data and assessed risk of bias. Findings were pooled where appropriate. We used GRADE to assess the quality of the evidence for each outcome.
Main results
We identified one study (290 randomised participants) that investigated the effectiveness of decision aids compared to standard information in the informed consent process for clinical trials. This study reported two separate decision aid randomised controlled trials (RCTs). The decision aid trials were nested within two different parent trials focusing on breast cancer in postmenopausal women. One trial focused on informed consent for treatment in women who had previously had surgery for ductal carcinoma in situ (DCIS), the other on informed consent for prevention in women at high risk for breast cancer. Two different decision aids were used in these RCTs, and were compared with standard information.
The pooled findings highlight the uncertainty surrounding most reported outcomes, including knowledge, decisional conflict, anxiety, trial participation and attrition. There was very low quality evidence that decision aids lower levels of decisional regret to a small degree (MD ‐5.53, 95% CI ‐10.29 to ‐0.76). No data were identified on several prespecified primary outcomes, including accurate risk perception, values‐based decision, or whether potential participants recognised that a decision needed to be made, were able to identify features of options that matter most to individuals, or were involved in the decision.
Authors' conclusions
There was insufficient evidence to determine whether decision aids to support the informed consent process for clinical trials are more effective than standard information. Additional well designed, adequately powered clinical trials in more diverse clinical and social populations are needed to strengthen the results of this review. More generally, future research on which outcomes are most relevant for assessment in this context would be helpful.
Plain language summary
Decision aids for people deciding about taking part in clinical trials
We reviewed the evidence about the effect of specific tools, called decision aids, which aim to improve decision making in the informed consent process for people who are considering participating in a clinical trial. These tools were compared to the standard process used for informed consent in clinical trials. There is currently not enough evidence to draw conclusions about the effectiveness of decision aids in the informed consent process for clinical trials.
In clinical trials, one healthcare treatment is compared to another treatment or to no treatment. Before potential participants sign a consent form where they agree to take part in a clinical trial they must be given information about what will be expected of them and what they can expect. Research has shown that this information is often not as good as it could be. For example, people often misunderstand the information they have been given. Decision aids, which are tools that assist people to think about what matters most to them, support decision making for treatment and screening. Presenting information about trial participation through decision aids might improve the informed consent process by improving participants' knowledge, certainty with the decision and enabling them to consider what matters most to them personally.
We searched the literature for studies where potential trial participants were randomly allocated to receive decision aids, compared to no decision aids or to other types of information for informed consent. We found one study, which reported data from two separate decision aid trials, where people who were given a decision aid alongside standard information were compared to people who were given standard information alone. When data from these two trials were combined, the results were inconclusive and not able to show whether people given the decision aid had any more or less knowledge or uncertainty about their decision, or were more or less likely to participate in a trial, than the people who were only given standard information. However, people who used the decision aid may have felt less regret about their decision. Overall there was very low quality evidence to support these findings, which means that there may be uncertainty around the results, and therefore, further research is required.
Summary of findings
Summary of findings for the main comparison. Decision aids for informed consent versus standard informed consent for people considering taking part in clinical trials.
| Comparison 1: Decision aids for informed consent versus standard informed consent | ||||||
|
Patient or population: people considering taking part in clinical trials
Intervention: decision aid for informed consent Comparison: standard informed consent | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Comparison 1: Decision aid for informed consent versus standard informed consent | |||||
| Knowledge Quality of Informed Consent (QuIC) Follow‐up: post decision | The mean knowledge in the control group was 87.6¹ | The mean knowledge in the intervention groups was 1.68 higher (1.91 lower to 5.26 higher) | 146 (2 studies) | ⊕⊝⊝⊝ very low²,³,⁴ | ||
| Accurate risk perception | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome |
| Values based decision | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome |
| Recognition that a decision needs to be made | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome |
| Involvement in decision | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome |
| Decisional conflict Decision Conflict Scale Follow‐up: post decision | The mean decisional conflict score in the control group was 12.55¹ | The mean decisional conflict in the intervention groups was 3.47 higher (1.51 lower to 8.45 higher) | 146 (2 studies) | ⊕⊝⊝⊝ very low²,³,⁴ | ||
| Decisional regret Decision Regret Scale Follow‐up: 3 months | The mean decisional regret score in the control group was 18.25¹ | The mean decisional regret in the intervention groups was 5.53 lower (10.29 to 0.76 lower) | 119 (2 studies) | ⊕⊝⊝⊝ very low²,³,⁵ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ Control group scores were used to calculate the mean score across studies.
² Studies were considered at risk of bias due to a lack of blinding (participants, personnel and outcome assessors) and incomplete outcome data (30% of randomised sample were excluded from the analysis post‐randomisation)
³ Included trials were conducted in one population only (i.e. selected groups of postmenopausal women)
⁴ The mean effect estimate crosses the line of no effect and the CI is very wide. That is, at least 25% favoured either the intervention or the control.
⁵ The mean effect estimate does not cross the line of no effect but the CI is very wide.
Background
Description of the condition
A clinical trial is an experiment in which two or more interventions, possibly including a control intervention or no intervention, are compared by being (often randomly) allocated to participants. Clinical trials, and RCTs in particular, are considered the gold standard research methodology for rigorously evaluating the effectiveness of healthcare interventions (Pocock 1983). Increasingly, clinical trials are used to inform and direct clinical practice, and they constitute a significant component of publicly‐funded research. However, evidence from publicly‐funded trials has shown that approximately 70% of clinical trials fail to recruit their desired number of participants (Campbell 2007). Failure to recruit the required sample size can lead to trials being underpowered which may not allow the effects of different interventions to be detected or accurately determined. Various studies have developed different strategies that aim to improve participant recruitment to clinical trials (Treweek 2010).
There are several reasons for poor recruitment to clinical trials at the patient, clinician, and organisational level (Prescott 1999; Campbell 2007). Often, through good trial design and trial management processes, barriers with clinicians and organisational factors may be overcome (Campbell 2007). However, other patient factors can play a role in a patient's decision to participate in a clinical trial, or not, and many of these directly influence the individual's decision about participation (Prescott 1999; McCann 2013).
Patients cite many reasons for not participating in clinical trials, including: lack of knowledge about the trial's rationale; lack of understanding of the methodological processes of clinical trials, such as randomisation of treatment allocation; fears about treatment efficacy; misunderstanding the concept of equipoise; and a dislike of discussions with clinicians about treatment uncertainty (Prescott 1999; Jenkins 2000; Featherstone 2002; Abraham 2006; Fayter 2007; Mangset 2008). Such misunderstandings may result in poor quality decisions about both participation and non‐participation. There may be other influences on a person's decision to participate in a clinical trial, such as whether the treatment options are consistent with their personal values, whether the clinical trial includes outcomes that a participant considers important, and whether participation is convenient for other reasons such as cost, transportation, or the additional demands of trial participation. Many of the influences on people's decisions to participate in clinical trials may also be related to the phase of the trial. For example, in early phase trials, people may have misconceptions about potential benefits and risks, while in later phase trials issues such as randomisation and equipoise may be more important (Cox 2003; Jenkins 2010). Furthermore, participating in a clinical trial removes the decision about treatment from participants' control, which can affect their feelings of autonomy (Madsen 2002).
In response to many of the concerns about participants' lack of understanding of clinical trials, investigators have sought to improve the informed consent process. Patients and clinicians have identified concerns about the consent procedure and information provided during the consent process as a barrier to participation in clinical trials (Prescott 1999). Informed consent is a cornerstone of ethical healthcare research and is a requirement for most clinical research studies and clinical trials in particular. Ethical guidelines suggest that prospective clinical trial participants should understand a minimum amount of information about the trial in which they are invited to participate to be able to provide valid informed consent. However, poor participant understanding of the research processes, a lack of knowledge about the expectations and demands of trials, and insufficient support when faced with the decision, have been demonstrated across a range of clinical areas (Prescott 1999; Jenkins 2000; Flory 2004; Nishimura 2013). Existing approaches to obtaining informed consent for research purposes are therefore not optimal and could be improved.
Several strategies have been adopted in an attempt to improve informed consent for clinical trials. These include: written information (e.g. enhanced consent documents, simplifying language, using illustrations and altering layout); detailed verbal information; test‐feedback interventions; telephone‐based interventions; computer‐assisted programs; audio‐visual interventions; and physician‐based communication training (Ellis 2002; Coyne 2003; Angiolillo 2004; Flory 2004; Hietanen 2007; Synnot 2014; Sand 2008; Yap 2009). However, much of this empirical work has focused on the structural documents or components by aiming to improve presentation of information, or mode of delivery, rather than the process of decision making itself. This focus on improving information provision is further reflected in the results of these studies, which show few significant improvements in knowledge and understanding among trial participants when analysed together (Flory 2004; Nishimura 2013; Synnot 2014). Interestingly, a review of these studies concluded that increasing discussion during the informed consent process is one of the most successful types of intervention to improve knowledge and understanding (Flory 2004; Nishimura 2013). However, whilst knowledge and understanding are important for decision making, they are not the only important factors. Therefore, interventions which aim to support the process of decision making, as well as improving knowledge, may hold additional benefit for participants considering clinical trial participation.
It is important to reiterate that there are a range of reasons for poor recruitment to RCTs and this review does not aim to address all of these. This review focuses on interventions that aim to improve the decision making process for potential trial participants.
Description of the intervention
This review considered the effectiveness of decision aids (also called decision support tools/systems/technologies/interventions, interactive health communication applications, interactive health communication systems, shared decision making programs or risk communication tools). These decision aids are complex interventions designed to help people make specific, deliberative choices among healthcare options, by providing information about the options and outcomes that are relevant to the decision (Stacey 2014). They provide detailed information on all aspects of the decision and include exercises to help patients clarify what values are important to them, and being supported to be involved (or participate) in the decision (Stacey 2014). Specifically, decision aids have been shown to improve knowledge of key aspects of the decision when faced with options where there is no objectively correct answer (clinical equipoise), promote accurate perceptions of probabilities of outcomes, and align preferred outcomes with the choice made (Stacey 2014).
The mode of delivery for these interventions varies, and includes: pamphlets and booklets; audiotapes; audio‐guided workbooks; computer or web‐based formats; interactive videodiscs; decision boards and group presentations (Stacey 2014). The mode used to deliver the decision support is often determined during the intervention development stage by piloting with patients. Moreover, these interventions are also used in varying contexts, which can be categorised as those that are used by clinicians in face‐to‐face consultations; those that can be used independently of the clinical consultation; and those that are delivered using more interactive technologies to supplement information given during consultations (Elwyn 2010a). The target population for these interventions can be virtually any clinical population that needs patient involvement in decision making. Within a clinical area, different decision aids have been developed to target specific groups, such as adults with low literacy (Clement 2009; Smith 2010). Similarly, the decision maker in some treatment or screening decisions may be a proxy decision maker, such as the guardian for a child or dependent adult (Wallace 2006).
To determine whether an intervention meets minimum criteria for classification as a decision aid, we assessed all interventions from potentially eligible studies using the International Patient Decision Aid Standards instrument (IPDASi) (Elwyn 2009b). This enabled us to determine whether the identified interventions could be considered decision aids (by containing all of the qualifying content items) rather than other educational interventions (which do not meet the minimum content requirements).The IPDASi instrument was developed to assess the quality of decision aids and contains a checklist of key qualifying items, under broad domains, to be included in such an intervention, that is, assesses key quality requirements and creates a minimum criteria threshold. For example, some of these domains cover provision of information about options in sufficient detail for making a specific decision; presentation of outcome probabilities; ways to clarify and express values; and structured guidance in deliberation and communication. The original application of this tool was to assess the quality of decision aids (through generation of a scoring system). However, in this instance we used the IPDASi tool to evaluate the qualifying items of interventions. The IPDASi tool has been updated recently, and now contains a cut‐off score for determining whether or not an intervention is a decision support intervention that assesses key qualifying requirements and creates a minimum criteria threshold (Joseph‐Williams 2013).
Evaluations of different methods of trial recruitment almost invariably occur as subsidiary studies to larger clinical trials. For example, investigators conducting a trial of two different surgical procedures for a given condition may be interested in understanding whether a decision aid helps to inform participation in said surgical trial. For clarity, throughout this review we used the term decision aid trial to refer to these subsidiary nested studies that were the focus of this review, and used the term parent trial to refer to the (often clinically focused, such as the comparison of surgical techniques in the above example) clinical trial in which they are set.
How the intervention might work
Preference sensitive decisions require the patient to make a best choice when there is uncertain or no clear evidence to support one option over another, the options have different inherent benefits and risks, and the patient's values are important in optimising the decision (Elwyn 2009a). The decision to participate in an RCT is a preference sensitive decision. Decision aids have been shown to be particularly effective for preference sensitive treatment and screening decisions (Stacey 2014). In addition, existing patient information leaflets for clinical trials are often lacking in information deemed important for good decision making (Gillies 2014a; Brehaut 2012).
Decision aids may enhance the informed consent process by improving people's knowledge and understanding of the decision to participate and enabling them to reflect on what matters most to them. Preliminary exploratory studies have shown that decision aids to inform participation in cancer trials aid understanding about the trial without increasing patients' anxiety (Juraskova 2008; Sundaresan 2011). Another study showed that explicit values clarification techniques resulted in potential trial participants evaluating more information in accordance with personal values, and exhibiting less decisional conflict than the control group (Abhyankar 2011). These studies provide some evidence that decision aids could be useful in this context. Better informed participants may be more likely to make improved decisions (whether consent or refusal) about trial participation (Juraskova 2008; Sundaresan 2011), and be more aware of the expectations on them as a trial participant throughout the study.
Research regulatory guidelines refer to informed consent within clinical trials as a process (ICH GCP 1996), yet many efforts to improve the informed consent process to date have focused on improving the information delivery at the point of a decision about participation. The decision to participate in a clinical trial extends beyond the signing of the consent form and continues throughout the duration of the trial. Often this continued consent is implied by a participant adhering to the trial protocol's follow‐up procedures, yet this is not always driven by an informed choice at the outset (Flory 2004; Nishimura 2013). It could be hypothesised that a good decision about trial participation may also result in some instances in participants completing all trial follow‐up and thus improving retention rates. Decision aids are designed to support the process that surrounds decision making, and in some cases may provide ongoing support by acting as a point of reference for people to refer back to. Therefore, they may also provide ongoing support for people throughout the decision making process when considering clinical trial participation. As such, other models to improve decision making at the point of participation are being considered by researchers (Juraskova 2008; Sundaresan 2011; Brehaut 2010; Gillies 2012b).
A tension exists in clinical trials between ensuring potential participants are adequately informed, and ensuring that recruitment and retention are maximised. The evidence on whether informed‐ness is correlated with recruitment is equivocal, but it could be hypothesised as both a positive or a negative relationship (Flory 2004; Nishimura 2013). The use of decision aids in this context may result in reduced rates of participation for some trials, as evidence suggests similar interventions promote more conservative decisions for treatment or screening (Stacey 2014). This may be considered a negative outcome as it could result in trials taking longer to recruit their desired sample size, and impact on cost and time to report, which may mean that implementation of more effective interventions takes longer. However, inadequately informed participation in a clinical trial may result in participants dropping out of the trial at a later stage, or worse, participating in a trial that they might not have chosen had they been better prepared for the trial decision. Therefore, both outcomes of participation and withdrawal could be beneficial or harmful to a participant, depending on the specific trial.
Why it is important to do this review
Whilst use of decision aids within a trial context is relatively novel, the decision support literature is more mature, with international standards on best practice for use of decision aids (Elwyn 2006). Furthermore, there is now increased discussion in the ethics literature about the outcome of consent for research in the face of increasing regulatory requirements. Consideration is being given to other models for informed consent for research, of which decision aids are one. Therefore, it is timely to review these interventions in this context.
Objectives
To assess the effects of decision aids compared with no intervention, usual care, alternative interventions or a combination of these in people making decisions about participation in RCTs.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs of decision aids (i.e. decision aid trials) for informed consent for participation in a parent RCT were eligible for inclusion. We used the terms decision aid trial to refer to the subsidiary nested studies that were the focus of this review, and parent trial to refer to the (often clinically focused) clinical trial in which they are set. The decision may relate to participating in a real or hypothetical parent trial. We investigated studies in which the decision to participate in the trial was a hypothetical decision, so as to provide a comparison between real and hypothetical decisions in this context. Quasi‐RCTs were defined as trials where randomisation was attempted but subject to potential manipulation or confounding, for example using day of week, date of birth or sequence of entry into trial.
Types of participants
We included potential clinical trial participants, or guardians of or proxy decision makers for potential trial participants. The term guardian in this review was used to mean parents or other guardians acting on behalf of their children, and guardians of adults who were unable to consent for themselves. There were no restrictions by age, gender, ethnicity or health condition of participants.
Types of interventions
Adhering to our protocol (Gillies 2012a), included studies evaluated the use of decision aids in the informed consent process for clinical trials. Decision aids may vary in the type of support they provide and their specific aims. However, in general they are tools designed to prepare patients to participate in making specific and deliberative informed choices about their health care, including participation in clinical trials. These decision aids differ from standard patient information leaflets used in trial contexts, as in addition to providing evidence‐based information about a health condition and identifying the options and outcomes, associated benefits, harms, probabilities, and scientific uncertainties, they:
help potential RCT participants to identify the values‐sensitive nature of the decision and to clarify, either implicitly or explicitly, the value they place on the benefits, harms, and scientific uncertainties;
provide structured guidance in the steps of decision making; and
assist potential RCT participants in communicating about the decision and their values with others involved (e.g. clinician, family, friends) (Stacey 2014).
We assessed all interventions from included studies for inclusion using the IPDASi (Elwyn 2009b). Two authors independently assessed the content of interventions from included studies using the IPDASi. The authors discussed their results and, if required, a third author repeated the process to enable consensus to be reached.
The following interventions were excluded from this review:
decision aids about screening or treatment decisions that were not set within the context of making a decision about participating in a parent RCT;
any interventions that were not decision aids (as determined by the IPDASi) that aimed to enhance the informed consent process;
any interventions designed only to improve communication (i.e. not focus on the decision process) about trial participation between health professionals and patients;
studies that did not meet the minimum criteria for the intervention to be defined as a decision aid (Elwyn 2009b).
Included studies compared an intervention to: no intervention; standard information (usual care); alternative interventions (an adapted version of the intervention, such as a more concise version of the comparator intervention, or a change in mode of delivery (audio versus paper)); or a combination of these.
Types of outcome measures
In line with previously published reviews of treatment and screening decision aids, and reviews of information considered important for participation in RCTs (Flory 2004; Synnot 2014Stacey 2014), we considered the outcomes listed below as important.
We also included any relevant studies that met the inclusion criteria but that included outcomes other than those specified.
Primary outcomes
1. Evaluation of informed choice
Knowledge or understanding;
Accurate risk perception;
Values‐based decision;
Recognition that a decision needs to be made;
Ability to identify features of options that matter most to individuals;
Involvement in decision.
2. Decision‐making process measures
Decisional conflict: personal uncertainty about which course of action to take when faced with a choice between competing options. Conflict can be measured using the Decision Conflict Scale (DCS) and is most often measured at the point of decision making i.e. contemporaneously (O'Connor 1995);
Decision regret: healthcare decisions that result in bad outcomes can lead to regret, which can subsequently affect decision making. Regret can be measured using the Decision Regret Scale and is most often measured after a decision has been made i.e. retrospectively (Brehaut 2003).
Secondary outcomes
We collected data on the following secondary outcomes relating to the parent RCT that people were being recruited to:
Participation (willingness to participate, or participation rate);
Attrition.
Other secondary outcomes related to the decision support RCT were:
Anxiety;
Cost of intervention;
Patient‐recruiter communication.
Search methods for identification of studies
Electronic searches
In March 2015 we searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL);
The Cochrane Library, March 2015);
MEDLINE (OvidSP) (1950 to March Week 1 2015);
EMBASE (OvidSP) (1980 to 2015 Week 09);
PsycINFO (OvidSP) (1806 to 9 March 2015);
ASSIA (ProQuest) (1987 to 9 March 2015).
The strategies for each of the databases are presented in Appendix 1. There were no language or date restrictions.
To identify ongoing clinical trials, the following registers were also searched:
WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/);
ClinicalTrials.gov;
ISRCTN Register (http://www.controlled‐trials.com/isrctn/).
Searching other resources
Grey literature
We searched grey literature using ProQuest Dissertations to access digital dissertations and theses that were relevant to this review.
Reference lists
We searched reference lists of included studies and relevant review articles.
Handsearching
We handsearched journals that frequently publish articles on decision aids, such as Medical Decision Making, Health Expectations and Patient Education and Counseling, along with specific health services research journals such as Trials; Clinical Trials; BMC Health Services Research; BMC Medical Research Methodology; Research Ethics; American Journal of Bioethics and Journal of Empirical Research on Human Research Ethics.
Correspondence
We contacted the shared decision making community through social media to identify any additional new or ongoing studies. We also contacted Directors of UKCRC Clinical Trials Units to identify any new or ongoing studies. In addition, when authors of included studies were contacted for further details of interventions we also asked if they were aware of any additional studies in this area. No studies were identified through these routes. Two ongoing studies (see Characteristics of ongoing studies) were identified.
Data collection and analysis
Selection of studies
Stage 1
We conducted searches for relevant studies on the prespecified databases. We combined results and removed duplicates.
Stage 2
All review authors independently screened titles and abstracts of identified articles for relevance. Sets of abstracts were created such that all identified articles were screened by two authors.
Stage 3
Four authors (KG, ZS, SC, JB) further assessed the set of potentially relevant abstracts identified from the initial full screen and discussed any disagreements. We retrieved full text copies for all potentially relevant papers, including those where the description (usually relating to the intervention) was insufficient to make a decision about inclusion.
Stage 4
Two authors independently screened the full text articles against eligibility criteria for inclusion or exclusion. Where interventions were deemed eligible, authors assessed interventions using the IPDASi, as per the process outlined by Elwyn 2009b and Joseph‐Williams 2013. Two review authors discussed results, and if required, a third researcher repeated the process to enable consensus to be reached. See Characteristics of excluded studies for details on reasons for exclusion of full text articles.
We provided citation details and any available information about ongoing studies (see Characteristics of ongoing studies), and collated and reported details of duplicate publications, so that each study was the unit of interest in the review. We reported the screening and selection process in an adapted PRISMA flow chart (Liberati 2009); see Figure 1.
1.
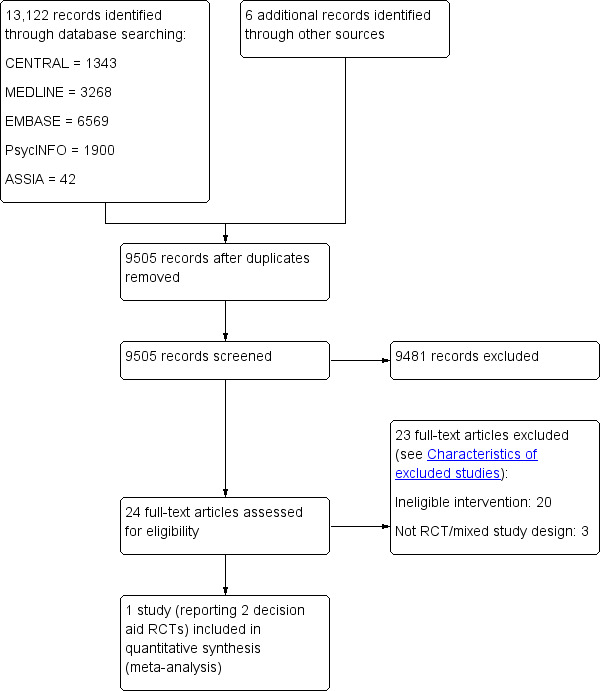
Study flow diagram
Data extraction and management
Four authors (KG, ZS, SC, JB) independently extracted data from each included study. Any discrepancies were resolved by discussion to reach consensus, or through consultation with a third author where necessary. We based data extraction categories on the Cochrane Consumers and Communication Group Data Extraction Template but supplemented those with additional important categories for this context (relating to parent RCT), and included the following categories: features of the parent RCT; decision support RCT methods; intervention and comparator features; outcomes; data and results; conclusions and limitations.
All extracted data were entered into RevMan (RevMan 5.3) by one review author, and checked for accuracy against the data extraction sheets by a second review author working independently.
Information is presented in Characteristics of included studies.
When more than one primary outcome was available from an included study (e.g. when multiple outcomes contribute to a single category, such as knowledge and understanding) we used the following process for selecting a single outcome (Brennan 2009):
Select the primary outcome that was identified by the authors of the included study;
When no primary outcome was identified, select the outcome specified in the sample size calculation;
If there were no sample size calculations, we ranked the effect estimates and used the median effect estimate.
Assessment of risk of bias in included studies
We assessed and reported the risk of bias of included studies in accordance with the Cochrane Handbook (Higgins 2011) and the guidelines of the Cochrane Consumers and Communication Group (Ryan 2011), which recommends reporting the following items for RCTs based on the risk of bias tool:
random sequence generation;
allocation concealment;
blinding (participants, personnel);
blinding (outcome assessors);
completeness of outcome data;
selective outcome reporting;
baseline comparability (for quasi‐randomised studies).
Four authors (KG, ZS, SC, JB) independently assessed risk of bias of the included study, rating each of the domains as high risk, unclear or low risk (as detailed in the Cochrane Handbook), with any disagreements being resolved through discussion and consensus.
Our assessment is reported in Risk of bias in included studies, along with a justification for the ratings given. Whilst we had planned to consider the results from the risk of bias assessment of included studies when performing and presenting analyses, and restricting the primary analysis to studies at low risk of bias, this was not appropriate due to inclusion of a small number of studies in the analysis.
The results of the risk of bias assessment have been incorporated into the review through standard tables, and systematic narrative description and commentary about each of the elements, leading to an overall assessment the risk of bias of included studies and a judgement about the internal validity of the reviews results.
Studies were deemed to be at the highest risk of bias if they are scored as at high or unclear risk of bias for either the sequence generation or allocation concealment domains, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011).
Measures of treatment effect
A meta‐analysis was conducted. It should be highlighted that whilst only one study was included, it reported data from two decision aid trials (Juraskova 2014 (Prevention); Juraskova 2014 (DCIS)). The data were presented separately in the original publication (patients were being recruited to two separate parent trials and as such generated two separate decision aid trials) but it was felt to be appropriate to pool the results for this review (see justification in Included studies). The included study had several continuous outcomes. Therefore, we analysed data based on the mean, standard deviation (SD) and number of people assessed for both the intervention and comparison groups to calculate a mean difference (MD), with 95% confidence interval (CI), between the post‐intervention values of the randomised groups. For dichotomous outcomes we analysed data based on numbers of events and numbers of people assessed in the intervention and comparison groups, and used these to calculate a risk ratio (RR) with 95% CI.
Unit of analysis issues
We analysed both the mean and SD provided in the included study. We calculated the mean and SD according to the overall numbers within each arm using established approaches (Higgins 2011).
If cluster‐RCTs were included we would have checked for unit‐of‐analysis errors. If errors were found, and sufficient information was available, we would have re‐analysed the data using the appropriate unit of analysis, by taking account of the intra‐cluster correlation (ICC). We would have obtained estimates of the ICC by contacting authors of included studies, or imputing them by using estimates from external sources. If it was not possible to obtain sufficient information to re‐analyse the data we would report effect estimates and annotate unit‐of‐analysis error.
Dealing with missing data
We contacted study authors to obtain missing data (participant, outcome, or summary data). For participant data, we analysed outcomes as reported as no information on intention‐to‐treat was available within the study report or was available from authors of the study. We reported on the levels of loss to follow‐up and assessed this as a source of potential bias.
Where possible, missing standard deviations were calculated from other reported statistics. Specifically, this was the case for percentage enrolled and percentage who dropped out. We discussed any impact of missing data on the findings of the review in the main text and the Risk of bias in included studies table.
Assessment of heterogeneity
Where studies were considered similar enough (based on consideration of populations and/or interventions) to enable pooling of data using meta‐analysis, we assessed the degree of heterogeneity by visual inspection of forest plots. We assessed heterogeneity between the decision aid trials from the included study using the Chi² statistic, to provide evidence of heterogeneity, and the I² statistic, to quantify the degree of heterogeneity (a Chi² P value of less than 0.10 or an I² value equal to or more than 50% was considered to indicate substantial heterogeneity).
Where heterogeneity was present in pooled effect estimates we planned to explore possible reasons for variability by conducting subgroup analysis.
We planned that where we detected substantial clinical, methodological or statistical heterogeneity across included studies we would not report pooled results from meta‐analysis but instead use a narrative approach to data synthesis. In this event, we planned to attempt to explore possible clinical or methodological reasons for this variation by grouping studies that were similar in terms of populations, interventions and methodological differences (such as real or hypothetical decision) to explore differences in intervention effects. However, the small number of included studies meant that subgroup analyses could not be conducted.
Assessment of reporting biases
We planned to assess reporting bias qualitatively based on the characteristics of the included studies (such as if only small studies indicating positive findings were identified for inclusion), and if information that we could obtain from contacting experts and authors of studies suggested that there were relevant unpublished studies.
If we had identified sufficient studies (at least 10) for inclusion we would have constructed a funnel plot to investigate small study effects, which may have indicated the presence of publication bias. We would have formally tested for funnel plot asymmetry, with the choice of test made based on advice in Higgins 2011, and bearing in mind that there may be several reasons for funnel plot asymmetry when interpreting the results.
However, we could not conduct assessment of reporting bias due to only one study being eligible for inclusion, but this was not deemed to be a considerable risk.
Data synthesis
There was only one included study, presented as two separate decision aid trials, with no difference in the comparator groups. In future updates we will analyse studies according to comparison groups, specifically:
Decision aid versus no intervention;
Decision aid versus usual practice;
Decision aid versus alternative interventions.
We conducted a meta‐analysis where trial data were sufficiently similar (in intervention, outcome measure, length of follow‐up and type of analysis). We present results for each of the reported outcomes, organised by the comparison intervention.
Due to the variability in both the populations and interventions of the included study, we used a random‐effects model for meta‐analysis.
We did not use narrative synthesis, but would do in future updates where studies are not suitable for meta‐analysis. For example, if mean and SD cannot be extracted for continuous outcomes, we will present the summary statistic and measure of variance at follow‐up available in the text of the included studies, and if there is more than one study, we will present these data in additional tables.
Subgroup analysis and investigation of heterogeneity
We found there were insufficient data to conduct subgroup analysis. In future updates we will conduct subgroup analyses where data are available, according to:
Decision regarding trial participation: real versus hypothetical;
Mode of delivery (e.g. video/computer versus audio/pamphlet). Mode of delivery may make a difference to the effectiveness of decision support tools. For example, an RCT that compared a paper‐based decision aid versus an Internet‐based version for prostatic specific antigen (PSA) screening showed that participants randomised to the Internet version had different levels of screening uptake (Evans 2010). This may translate to different modes of delivery affecting uptake to clinical trials, and as such, this would be explored in subgroup analysis;
-
Context of intervention delivery. Context of intervention delivery is also linked to mode of delivery but may impact more on cost effectiveness of the intervention (Belkora 2010). Moreover, context has been proposed by other researchers in the decision aid literature as being an important variable for consideration during decision aid development, delivery and evaluation (Elwyn 2010a, Thomson 2010). The following contexts will be explored in subgroup analysis:
used in face‐to‐face clinical encounters;
used independently from the clinical encounter;
Quality of intervention as measured by the IPDASi (dichotomised by score: 0 to 50 and 51 to 100);
Participant characteristics. Various participant characteristics may have an impact on the effectiveness of decision aids. Age and gender have been shown to have a significant effect on participants' perception of the factors that determine decision processes (Sanz de Acedo Lizararraga 2007). Also, decision aids designed specifically for use with low level literacy groups have been shown to be effective in supporting informed choices and greater participant involvement in some screening decisions (Smith 2010). As such, the following would be explored in subgroup analysis:
age (categorised as under 18, 18 to 65, over 65 years);
gender (male versus female);
education (no formal education and higher education).
Sensitivity analysis
Because there was only one included study, which included two methodologically similar decision aid trials which did not fulfil the high/unclear risk of bias requirements outlined below, we were unable to conduct a sensitivity analysis. In future updates, we will group studies according to whether they are at high/unclear risk of bias or low risk of bias to investigate the effect of trial quality on meta‐analysis results. We will categorise studies at overall high or unclear risk of bias if rated as being at high or unclear in one or more of the following domains: sequence generation, allocation concealment or selective outcome reporting. The remaining studies would be considered at low risk of bias. These three domains were selected because limited, but growing, empirical evidence from methodological studies suggests they can most strongly influence intervention effect estimates (Higgins 2011). We will exclude studies at high/unclear risk of bias in a sensitivity analysis to determine whether the risk of bias influenced review findings.
Assessing the overall quality of the evidence
We used the GRADE approach to make assessments of the overall quality of the evidence for each outcome on each of the following domains: risk of bias, inconsistency, imprecision, indirectness and publication bias. We downgraded a starting rating of high quality evidence by one level for serious concerns (or by two levels for very serious concerns) about each of these domains. We considered the impact of the following factors (as specified by Higgins 2011) on the quality of the evidence:
risk of bias: limitations in the design (e.g. lack of allocation concealment, lack of blinding, large loss to follow up, etc.) and implementation of included studies;
inconsistency: unexplained heterogeneity or inconsistency of results;
imprecision: imprecise results, that is, wide confidence intervals generated from small samples and few events;
indirectness: where the included evidence is from indirect populations, interventions, controls or outcomes;
publication bias: probability of publication bias.
Each quality domain was assessed and where there was a low risk of bias the quality rating remained high, an unclear risk of bias resulted in a downgrading of the evidence by one level and a high risk of bias by two levels for very serious concerns. The judgements regarding downgrading were guided by Table12.2d in the Cochrane Handbook (Higgins 2011).
Two authors independently assessed the quality of the evidence as implemented and described in the GRADEprofiler (GRADEpro) software (Schünemann 2011).
Summary of findings table
We used the GRADE criteria to evaluate quality of evidence using GRADEprofiler (GRADEPro) software (Schünemann 2011) before presenting data in a summary of findings table and taking into account the quality of the evidence, magnitude of the effect of the intervention and the sum of the available data on the primary rather than main outcomes, as outlined in Types of outcome measures. We presented outcomes in the summary of findings table in terms of:
Evaluation of informed choice (knowledge; accurate risk perception; values‐based decision; recognition that a decision needs to be made; involvement in decision); and
Decision making process measures (decisional conflict and decisional regret). We provide a source and rationale for each assumed risk cited in the table. Because GRADE allows only seven outcomes to be listed in the summary of findings table, a primary outcome (ability to identify features of options that matter most to individuals) was not included. Our justification was that we felt this outcome may overlap with 'values based decision' outcome. It should be noted that this was a post‐hoc decision.
Consumer participation
As part of a larger project (led by KG), a survey and interviews with RCT stakeholders, which included potential participants, clinicians, and trialists, was conducted to determine key information to include in, and perceptions of, a decision aid to inform RCT participation (Gillies 2013; Gillies 2014b). This stakeholder consultation stage helped to identify consumer‐relevant content of these interventions and relevant outcomes, which mapped on to the decision aids identified in this review. The first author (KG) is leading additional work to identify a core outcome set for evaluation of interventions to improve informed consent (Gillies 2014e). This work will inform future updates of this review.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies.
Results of the search
We conducted electronic searches in March 2015 and identified 13,122 references and we identified six references from other sources. Following de‐duplication, we screened 9505 records for eligible studies. From the initial screen, we further assessed 32 abstracts for eligibility, with and requested full text for 24 records to provide more detail about interventions under investigation. Of these 24 full text papers, we assessed six for inclusion using the IPDASi to assess the reported intervention (see Types of interventions) and subsequently excluded five of these (see Characteristics of excluded studies and Excluded studies) . There was one paper that reported two decision aid trials which met the inclusion criteria (Juraskova 2014 (Prevention); Juraskova 2014 (DCIS)). Figure 1 illustrates the searching and screening process.
Included studies
We included one study that presented data from 290 women who participated in two separate decision aid trials (Juraskova 2014 (DCIS); Juraskova 2014 (Prevention)). These women were considering participation in one of two parent RCTs ‐ either the International Breast Cancer Intervention Study II (IBIS‐II) Prevention Trial (a primary prevention trial comparing anastrozole or placebo in postmenopausal women at high risk of breast cancer) or the IBIS‐II Ductal Carcinoma in Situ (DCIS) Trial (a treatment trial comparing anastrozole or tamoxifen in postmenopausal women who had previous surgery for DCIS) (Cuzick 2008). The authors of the decision aid trials reported the nested setting as being within two separate parent RCTs and presented the data analysis separately for each decision aid trial. Outcomes measured (both content and timing) were identical across both decision aid trials. For the purposes of this review we have also treated the decision aid trials as two individual trials. This is for several reasons, not least because the study authors treated the decision aid trials as two separate clinical trials, but in addition:
the parent trials to which participants were being recruited were testing different clinical interventions in different populations and addressed different decisions about trial participation;
the recruitment methods for each decision aid trial differed (e.g. the parent primary prevention trial identified women using media advertisements who were telephoned by clinical staff and invited to join the relevant decision aid trial; whereas women eligible for the parent treatment DCIS trial were approached directly by their surgeon and invited to join the relevant decision aid trial);
the eligible populations included in the decision aid trials differed;
the decision aid interventions tested in each decision aid trial differed.
For clarity, throughout the remainder of this review when referring to the decision aid trials collectively we refer to them as the decision aid trials and separately as:
prevention decision aid trial when referring to the nested decision aid trial that recruited women to the IBIS‐II parent primary prevention trial;
and
DCIS decision aid trial when referring to the nested decision aid trial that recruited women to the IBIS‐II parent DCIS treatment trial.
Study design
Both decision aid trials nested in the included study were two‐arm parallel RCTs.
Sample size
The randomised sample for the DCIS decision aid trial was 67 and 223 for the prevention decision aid trial. In total 290 people participated in the included study.
Setting
Participants were recruited via the parent trial sites which were largely based in high‐income countries (Australia, New Zealand, and United Kingdom).
Participants
The women who participated in the decision aid trials were eligible for the parent trial, the IBIS‐II, specifically, either the Prevention or DCIS trials. The population of participants recruited to both decision aid trials were very similar and included postmenopausal women with a mean age of 59 years. As per the parent trial, the recruitment approach differed by decision aid trial; the prevention decision aid trial women were invited by clinical staff over the telephone and the DCIS decision aid trial women were invited by their surgeon.
It was explicit in the report of the included study that the interventions in both decision aid trials were conducted in English, with most (94%) women specifying English as their spoken language. Further information about the participants is presented in Characteristics of included studies.
Interventions
The included study reported two decision aid trials that were both two‐arm parallel RCTs of a single comparison: decision aids versus standard informed consent procedures. The interventions in the decision aid trials were paper‐based decision aids. These were compared to standard informed consent procedures, which included a patient information leaflet, but no further details about the control intervention were available.
Two decision aids were tested, one specific to each of the clinical trials (one each for the prevention and DCIS decision aids trials). The delivery of the interventions was not clear but likely was without direct supervision and may have differed by decision aid trial (the prevention decision aid trial participants may have received the decision aid by post and the DCIS decision aid trial participants in a face‐to‐face setting). The decision aid booklets were designed to include evidence‐based representation of breast cancer risk, the parent trial rationale, explanation of management options available on and off the trial, a comparison of the risks and benefits of each option, and values clarification worksheets. The decision aids were designed to meet IPDAS guidelines for content development.
Outcomes
Primary outcomes
Knowledge and understanding
Evaluation of informed choice
Knowledge or understanding;
Accurate risk perception;
Values‐based decision;
Recognition that a decision needs to be made;
Ability to identify features of options that matter most to individuals;
Involvement in decision.
Decision‐making process measures
Decisional conflict: personal uncertainty about which course of action to take when faced with a choice between competing options. Conflict can be measured using the Decision Conflict Scale (DCS) and is most often measured at the point of decision making i.e. contemporaneously (O'Connor 1995);
Decision regret: healthcare decisions that result in bad outcomes can lead to regret, which can subsequently affect decision making. Regret can be measured using the Decision Regret Scale and is most often measured after a decision has been made i.e. retrospectively (Brehaut 2003).
The included study reported data for three different measures of knowledge:
Knowledge of clinical trials (study specific seven item measure of general trial related knowledge);
Objective knowledge (assessed using a 12 and 16 item study specific knowledge scale in the Prevention and DCIS cohorts respectively); and
Subjective knowledge (assessed using Part B (14 items assessing subjective/perceived understanding) of the Quality of Informed Consent (QuIC) scale (Joffe 2001)).
Knowledge was measured at baseline (post‐randomisation after a trial participation decision had been made ‐ post‐decision). As the decision aid trials did not rank knowledge outcomes, we chose to include only data collected with a validated tool, i.e. the QuIC measure of subjective/perceived understanding . This judgement was based on a hierarchy of measures which assumed validated objective measures to be superior to study‐specific non‐validated measures This hierarchical judgement relating to multiple measures of the same outcome was a post‐hoc decision made during the review process.
Decisional conflict was measured at baseline in the decision aid trials using the Decisional Conflict Scale (DCS), which contains 16 items that measure the amount of uncertainty an individual has about a course of action (O'Connor 1995). This was the primary outcome for the decision aid trials.
Decision regret was measured at three months using the Decisional Regret Scale, a five‐item scale with good internal consistency that measures regret associated with a decision made in the past (Brehaut 2003).
The decision aid trials did not report data for the following primary outcomes:
accurate risk perception;
values‐based decision;
or if potential participants had:
recognised that a decision needed to be made;
ability to identify features of options that matter most to individuals;
involvement in decision making.
Secondary outcomes
Data on secondary outcomes relating to the decision about entry into the parent trial were collected in the decision aid trials:
participation (presented as both intention to participate and percentage actually enrolled in IBIS‐II (parent trial), we included data captured using the second measure (% enrolled) as it is a more definitive measure of participation);
attrition (as percentage who dropped out from IBIS‐II (parent trial)); and
anxiety (measured at baseline using a six‐item short form of the State‐Trait Anxiety Inventory (STAI‐S) scale (Marteau 1992)).
No data were reported for:
cost of intervention;
patient‐recruiter communication.
Consumer involvement
Although the decision aid trials did not report consumer involvement, an earlier linked publication described consumer involvement in the development of the decision aids tested in the prevention decision aid trial (Juraskova 2008).
Funding sources
The included study was supported by Susan G. Komen for the Cure (grant number BCTR0503961) and discretionary funding from the Breast Cancer Institute of Australia, which is the fundraising and education department of the Australia and New Zealand Breast Cancer Trials Group (ANZBCTG; no grant number).
Excluded studies
We excluded 23 studies following assessment of full text articles. The reasons for excluding papers that went through full text review are outlined in the Characteristics of excluded studies. There were four reasons that contributed to studies being excluded. The most prevalent reason was the intervention not being a decision aid (determined by assessment of qualifying items using IPDASi) (n = 8), followed by the intervention not being a decision aid for trial participation (i.e. a decision aid for treatment or screening) (n = 5), or the intervention ineligibility was decided based on published report and/or information present in a similar Cochrane review (Synnot 2014) and/or discussion with study author (n = 7) and ineligible study design (n = 3).
Risk of bias in included studies
We assessed the two decision aid trials from one included study for risk of bias and assessed them to be at moderate to high risk of bias overall (Risk of bias in included studies; Figure 2; Figure 3). The included study provided insufficient information in the published paper about some aspects of study design and conduct and was assessed as unclear on a number of domains. Risk of bias was highest in the domains relating to blinding of participants and outcome assessors which reflects the inherent difficulties of blinding in trials testing information provision in this context.
2.
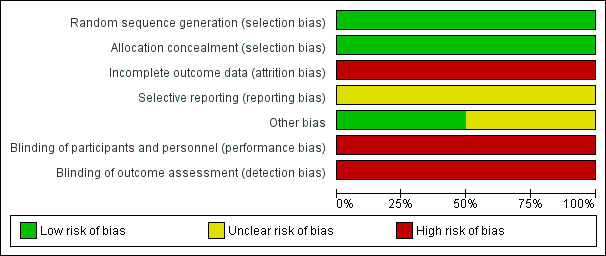
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
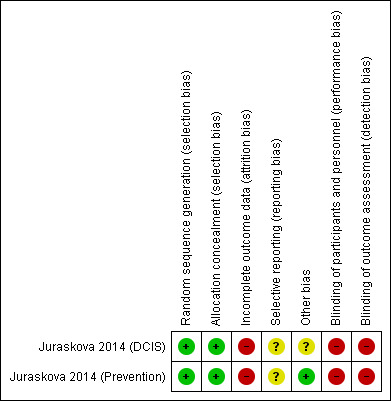
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
As all outcomes (for which data were identified) included pooled data from RCTs only, the evidence was downgraded from high to low quality for risk of bias, indirectness, imprecision or publication bias for all outcomes with the exception of attrition, which also included downgrading of the evidence (from high to low quality for inconsistency).
Allocation
Random sequence generation
The authors of the included study reported using a randomisation sequence that was generated using a web‐based random number generator (www.randomizer.org) with randomisation performed in blocks of 10 according to centre and was rated as low risk of bias.
Allocation concealment
A pre‐randomised, sequentially numbered system using sealed envelopes was reported by the authors and assessed as being adequate allocation concealment at low risk of bias.
Blinding
Most outcomes measured in RCTs of this type capture self‐reported outcomes often relating to knowledge and other aspects of decision making. As the participants are not blinded to their allocation, and they are the outcome assessors, it is indeed difficult for investigators to blind outcome assessment. Of all outcomes reported, only two could be measured objectively: actual enrolment and drop out from the parent RCT. The included study was assessed at being of high risk of bias for blinding of both participants and outcome assessment.
Incomplete outcome data
With regard to completeness of outcome data, the authors reported that 66 participants (23% of those randomised) were not included in the analysis (a post‐randomisation decision) due to previous participation in a clinical trial which they hypothesise may have resulted in ceiling effects for several of the measures. In addition, data were only reported on 146 of the 290 randomised (50%) participants, bringing into question whether the participants for whom data were missing differed from those who did not. However, the authors reported that there were no differences in rates or reasons for dropout across the arms.
Selective reporting
The study did not refer to a published protocol against which the published report could be assessed. Risk of bias was therefore assessed to be unclear. However, of those outcomes listed in the methods all were presented in the results.
Other potential sources of bias
There were recruitment problems reported for the DCIS decision aid trial, which resulted in that trial being underpowered. Each of the reported decision aid trials required a sample size of 128 (64 per arm) to detect an effect size of 0.5 with 80% power. The DCIS decision aid trial randomised 67 participants and analysed data on 24, and the Prevention decision aid trial randomised 223 and analysed data on 95 participants (see Incomplete outcome data (attrition bias) for more information).
Effects of interventions
See: Table 1
Decision aid for informed consent versus standard informed consent
Primary outcomes
Knowledge
The included study reported three separate measures of knowledge for both decision aid trials (see Included studies). The data on subjective knowledge was selected for analysis of knowledge due to the fact that these data were collected using a validated tool (QuIC; Joffe 2001). The pooled intervention arms from the decision aid trials highlighted that effects on knowledge are uncertain, compared with standard informed consent procedures, given the wide confidence intervals and small sample sizes (MD 1.68, 95% CI ‐1.91 to 5.26; Analysis 1.1). There was no indication of heterogeneity in these results. The quality of the evidence was rated as very low due to risk of bias (a lack of blinding and incomplete outcome data), indirectness of populations studied, and the wide confidence intervals around the effect estimate, with at least 25% variation in both control and intervention groups.
1.1. Analysis.
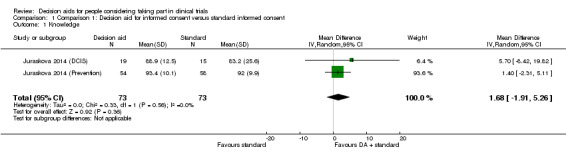
Comparison 1 Comparison 1: Decision aid for informed consent versus standard informed consent, Outcome 1 Knowledge.
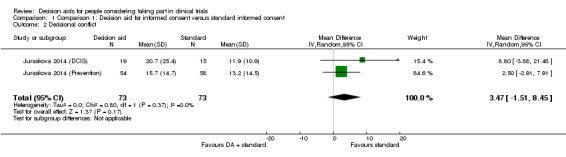
Decision conflict
Pooling the decision conflict scores (a measure of uncertainty) for both decision aid trials also showed uncertain effects on decision conflict, compared with standard informed consent procedures, due to the wide confidence intervals and small sample size (MD 3.47, 95% CI ‐1.51 to 8.45; Analysis 1.2). Again there was no indication of heterogeneity in the results for this outcome.
1.2. Analysis.
Comparison 1 Comparison 1: Decision aid for informed consent versus standard informed consent, Outcome 2 Decisional conflict.
As with the knowledge outcome, the quality of the evidence for decisional conflict was downgraded from high to very low due to risk of bias (a lack of blinding and incomplete outcome data), indirectness of populations studied, and the wide confidence intervals around the effect estimate, with at least 25% variation in both control and intervention groups.
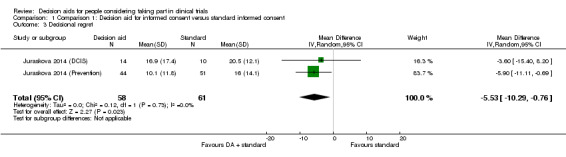
Decision regret
The pooled intervention arms showed evidence of a small effect in favour of the decision aids on decisional regret, compared with standard consent procedures, when combining the results from both decision aid trials (MD ‐5.53, 95% CI ‐10.29 to ‐0.76; Analysis 1.3), again with no evidence of heterogeneity in the results. However, the data were from two relatively small decision aid trials, with the weighting of the evidence in favour of the larger prevention decision aid trial which had a significant effect estimate.
1.3. Analysis.
Comparison 1 Comparison 1: Decision aid for informed consent versus standard informed consent, Outcome 3 Decisional regret.
The quality of the evidence was rated as very low due to risk of bias (a lack of blinding and incomplete outcome data), indirectness of populations studied, and the confidence intervals around the effect estimate being relatively large in relation to the effect size.
Secondary outcomes
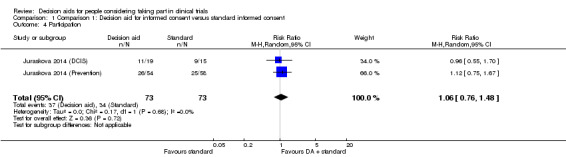
Participation
Actual enrolment was measured as a percentage of those who participated in the parent trial. Following pooling of results across both decision aid trials there was uncertainty around any effect on enrolment, compared with standard consent procedures (RR 1.06, 95% CI 0.76 to 1.48; Analysis 1.4). There was no indication of heterogeneity in the results; but the quality of the evidence was rated as very low due to risk of bias (a lack of blinding and incomplete outcome data), indirectness of populations studied, and wide confidence intervals around the effect estimate.
1.4. Analysis.
Comparison 1 Comparison 1: Decision aid for informed consent versus standard informed consent, Outcome 4 Participation.
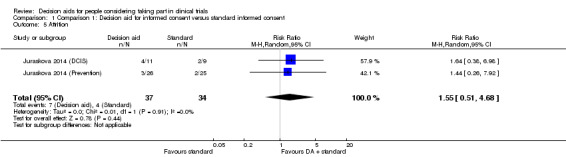
Attrition
Attrition was reported as the number of participants who dropped out of the parent RCT who were enrolled in the decision aid trial. Results from the decision aid trials were pooled and showed uncertain effects on attrition, compared with standard consent procedures (RR 1.55, 95% CI 0.51 to 4.68; Analysis 1.5). As for other outcomes, there was no evidence of heterogeneity in this result; but the quality of the evidence was rated as very low due to risk of bias (a lack of blinding and incomplete outcome data), indirectness of populations studied, and wide confidence intervals around the effect estimate, with at least 25% variation of the events in both the control and intervention groups.
1.5. Analysis.
Comparison 1 Comparison 1: Decision aid for informed consent versus standard informed consent, Outcome 5 Attrition.
Anxiety
Uncertain effects were observed on anxiety when the results from the decision aid trials were pooled (MD ‐2.38, 95% CI ‐10.65 to 5.90; Analysis 1.6). There was substantial heterogeneity (I² = 78%) in this result, with both positive and negative effects on anxiety for the two different trials (but with confidence intervals that passed through the line of no effect). The quality of the evidence was again rated as very low due to risk of bias (a lack of blinding and incomplete outcome data), indirectness of populations studied, and wide confidence intervals around the effect estimate.
1.6. Analysis.
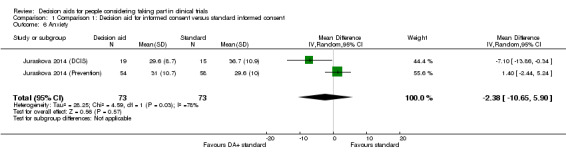
Comparison 1 Comparison 1: Decision aid for informed consent versus standard informed consent, Outcome 6 Anxiety.
No data were reported for the following prespecified primary outcomes: accurate risk perception; values based decision; recognition that a decision needs to be made; ability to identify features of options that matter most to individuals; or involvement in decision; or secondary outcomes of intervention cost or patient‐recruiter communication.
Discussion
Summary of main results
We identified one study reporting two decision aid trials recruiting with a total of 290 participants that investigated the effectiveness of decision aids (compared to standard information) in the informed consent process for RCTs (Juraskova 2014 (Prevention); Juraskova 2014 (DCIS)). This study report included postmenopausal women being recruited to one of two decision aid trials, each nested within the context of a larger parent RCT, either the IBIS‐II Prevention Trial (a prevention RCT comparing anastrozole or placebo in postmenopausal women at high risk of breast cancer) or the IBIS‐II DCIS Trial (a treatment RCT comparing anastrozole or tamoxifen in postmenopausal women who had previous surgery for DCIS) (Cuzick 2008).
When the results from each of the individual decision aid trials were pooled, there was considerable uncertainty about the effects of the intervention, compared with standard information, on most of the outcomes reported: knowledge, decisional conflict, anxiety, trial participation and trial attrition, due to wide confidence intervals and small sample sizes. There was very low quality evidence that decision aids may decrease decisional regret to a small degree (i.e. less regret amongst those exposed to the intervention), when compared with standard information. Additional outcomes we identified as being of potential importance were not reported. These included accurate expectations about benefits and harms; reaching choices that are consistent with personal values; recognition that a decision needs to be made; and involvement in the decision.
Overall completeness and applicability of evidence
Population and setting
Our conclusions were limited because only one study was identified (albeit including two decision aid trials) that met our inclusion criteria. The interventions investigated were delivered in high income countries to mainly English speaking postmenopausal women who had existing breast cancer or who were at high risk of developing breast cancer.
Control intervention
It was noteworthy that the included study compared decision aids with existing, written, consent documents and the intervention group received both. Other reviews of informed consent interventions (for trials (Synnot 2014) and treatment (Kinnersley 2013)) have highlighted the potential for both the intervention and control groups to benefit in studies of this type. We believe the results of the included study to be consistent with this finding for a number of reasons. Firstly, the authors note that the trial recruiters involved in the parent trial (IBIS‐II) had received communication skills training, which may have influenced the results of the decision aid trials. Moreover, trial recruiters may have changed their behaviour during the decision aid trials, optimising their informed consent practice. Another potential limitation of the included study is the lack of detail about the fidelity of both the intervention and standard information. In other words, no information was recorded about whether the decision aids or the standard information was in fact read by participants. Trials of this kind may benefit from process evaluations to explore how trial processes and interventions are delivered in context.
Study design
Several full reviews (both published and in progress) have investigated the effectiveness of a variety of interventions in the informed consent process for clinical trials (Flory 2004; Hon 2012; Nishimura 2013; Synnot 2014).
Whilst some of these studies focus on specific types of interventions (such as audio‐visual interventions, Synnot 2014), others have reviewed all interventions and grouped them accordingly (Nishimura 2013). Interventions for informed consent vary significantly, from simplified consent forms to enhanced discussions involving directed training for staff (Nishimura 2013). However, many of these interventions focus more on the structure and content of the presented information rather than the process of decision making. Therefore, to focus our review we only included interventions that aimed to support an informed decision making process and these interventions were defined according to the IPDASi (Joseph‐Williams 2013). This definition deviates from the method used in the review of treatment and screening decision aids, which defined interventions as decision aids if they were "designed to help people make specific and deliberative choices among options (including the status quo), by making the decision explicit and by providing (at the minimum) a) information on the options and outcomes relevant to a person's health status and b) implicit methods to clarify values" (Stacey 2014). Some of the interventions we excluded from our review (Abhyankar 2011; Meropol 2013; Tait 2010) contained components of decision aids but did not meet minimum criteria we defined as a requirement for inclusion based on those described by the IPDASi (Joseph‐Williams 2013). In addition, some of these interventions were treatment decision aids being used alongside patient information leaflets (i.e. trial participation was not the index decision); and rather the decision to have treatment or not was the index decision (Eccles 2013). Future updates may wish to consider the inclusion of these studies (and others which may fit the definition used by Stacey 2014 in specific subgroups comparisons (i.e. values clarification exercises) or comparisons (i.e. treatment decision aid + patient information leaflet vs. patient information leaflet). The effect of excluding these studies on the results of this review is unclear.
Outcomes
As noted by other authors, the assessment and measurement of outcomes associated with informed consent is problematic (Kinnersley 2013; Synnot 2014; Nishimura 2013).The heterogeneity of outcome measures used to assess knowledge or understanding has significant implications for systematic reviews and meta‐analysis of these types of outcome data. There is currently no standardised validated measure for knowledge or understanding as an outcome (Nishimura 2013). Neither is there consensus on whether this is an adequate measure of being informed, and when it should be measured in relation to the decision. This is an area requiring further research (Gillies 2012b; Gillies 2014c). We are currently conducting a systematic review of existing validated measures of informed consent, but again many of these measures focus on knowledge and understanding and are largely assessed through recall (Gillies 2014d). There has also been debate in the literature about the adequacy of decisional regret as a measure of the decision making process, both in the context of treatment (Elwyn 2010b) and trial decisions (Gillies 2014c). Some opponents have argued that regret can be biased by decision outcomes (due to the timing of outcome measurement i.e. post‐decision) and may not offer a measured representation of the decision process but more a judgement related to outcomes (Elwyn 2010b).
When considering interventions aimed to improve the decision making process it is also important to consider outcomes in addition to knowledge and understanding. Whilst these outcomes are important building blocks for informed decision making they are not the only components that can help support patients' decisions (Stacey 2014). When determining our specified outcomes for this review we were informed by the Cochrane review on decision aids for treatment and screening, which considered decision making as two components: evaluation of informed choice (encompassing attributes of the decision and its process); and decision making process measures (Stacey 2014). Other authors of similar reviews have also highlighted the need for outcome measures that capture informed consent "as a unified concept" (Kinnersley 2013) and that more research is needed to gain consensus on defining the range of potentially relevant outcomes in this context, not just from a researcher's perspective, but also from patients (Synnot 2014). We are conducting research with a range of stakeholders (including patients) aimed to develop a core outcome set for the evaluation of interventions intended to improve informed consent for clinical trials (Gillies 2014e).
Lastly, process measures associated with delivery of the intervention were prespecified in this review (e.g. patient‐recruiter communication and costs of intervention) but not reported in the included study. Data from process measures would be helpful to make decisions about implementation of decision aids, especially where the evidence is equivocal.
Quality of the evidence
The evidence for each reported outcome was assessed as very low quality according to the GRADE assessment. This was primarily due to the small sample sizes and wide confidence intervals, which in all but one case, crossed the line of no effect. This significant uncertainty surrounding most of the outcomes, together with a lack of data for many other potentially important outcomes, supports the case for additional research.
When assessing the standard Cochrane risk of bias domains it was noted that the included study did not provide sufficient information about study design and conduct, and as such, a number of domains were judged as unclear. Risk of bias was highest in domains relating to blinding of participants and outcome assessors which reflects the inherent difficulties of blinding in trials that test information provision.
Synnot 2014 and others suggest that in addition to the standard Cochrane risk of bias domains, consumer health information interventions might also consider whether interventions are developed using a theoretical framework and if consumers are involved in intervention development (Sheridan 2011; Synnot 2014). The authors of the included study in this review published an earlier paper that reports the piloting of the interventions tested in the decision aid trials (Juraskova 2008). They reported that they included consumers in the development of the intervention and it was informed by the theoretically‐based Ottawa decision support framework (O'Connor 1998). Future updates of this review may wish to consider subgroup analyses based on presence and absence of consumer involvement, and theoretically informed versus theoretical decision aids.
Potential biases in the review process
We applied standard Cochrane review methodology with the aim of minimising bias. At least two authors were involved in all critical stages of the review process. Where appropriate, we contacted authors of included and potentially eligible studies, largely to determine eligibility of interventions, and access additional data to support the published report. Several potentially eligible studies were excluded based on inability of the study authors to provide copies of the original interventions tested. However, through email discussion it often became apparent that the reported interventions were not formal decision aids or could be excluded based on the published information. Like the review by Synnot 2014, some outcomes reported in the included study were not assessed in this review because they were not prespecified outcomes, such as decisional satisfaction. Similarly, we collected only one outcome for each prespecified outcome category, and made this judgement on a hierarchy of measures (such as from validated objective measure to study specific non‐validated measures).
The included study reported three different measures of knowledge (two developed for use in the study with no validation data reported and one validated subjective measure (Joffe 2001)). As the authors did not specify whether they ranked the knowledge outcomes, we chose to report the more robust data collected using a validated measure. This was also the case for recruitment to the parent trial, which was also reported three ways (attitude towards parent trial (IBIS‐II), intention to participate, and percentage actually enrolled), of which we chose percentage actually enrolled. Further consideration and revision of the outcomes reported in this review may be required in future updates.
The biggest potential threat of bias to this review arose from the possibility that we did not identify all relevant published and unpublished studies. As part of a larger program of work, a formal survey of the Directors of UK registered Clinical Trials Units, and informal assessment of decision aid researchers through social media was conducted to determine if any work was being carried out in this area. No reports were identified. The lead researchers of those studies included in the Characteristics of ongoing studies were also asked whether they were aware of any other research in this area, of which none was reported.
Agreements and disagreements with other studies or reviews
Due to the inclusion of only one study (reporting two decision aid trials) with relatively small sample sizes, and the inconclusive findings of the review, we were unable to make clear comparisons with other studies or reviews. If future updates of this review include more studies, there may be areas for direct comparison. Specifically, it would be interesting to draw comparisons between this review and others investigating the effectiveness of different types of interventions for informed consent to clinical trials (Flory 2004; Hon 2012; Nishimura 2013; Synnot 2014). Due to the lack of research comparing informed consent for research and informed consent for treatment, it would also be interesting to compare the findings from this review with the Cochrane review of decision aids for treatment and screening decisions, which have been shown to have an effect across several outcome domains such as knowledge, decisional conflict and values clarification (Stacey 2014).
Authors' conclusions
Implications for practice.
There was insufficient evidence to determine if decision aids to support the informed consent process for clinical trials participants are more effective than existing approaches. The pooled findings from the included decision aid trials highlight considerable uncertainty surrounding most of the outcomes reported (knowledge, decisional conflict, anxiety, trial participation and trial attrition). There was very low quality evidence indicating that decision aids may lower levels of decisional regret. In addition, several primary outcomes (important for the decision process) identified as being of importance were not reported. These included accurate risk expectations; reaching choices that are consistent with personal values; or whether potential participants recognised that a decision needed to be made, were able to identify features of options that matter most to individuals, or were involved in the decision. Although the findings from Stacey 2014 suggest that decision aids are more effective than standard consent processes for treatment and screening, the applicability of these findings in the context of consent for clinical trials remains equivocal and requires more research.
Implications for research.
The findings from this review highlight a gap in the evidence base. More high quality RCTs of decision aids to support the informed consent process for clinical trials are needed. Evidence is needed from sufficiently powered (in terms of recruitment and analysis) trials across various trial populations (both in terms of clinical conditions, but also with regard to low literacy etc.), considering different interventions (e.g. comparison of drug versus surgery; behavioural intervention versus standard care), with measurement of relevant outcomes at appropriate time points.
The outcomes that should be assessed in future trials in this context requires further work (Gillies 2014e) but as a start could consider knowledge; decision conflict; satisfaction with the decision making process; values congruence; anxiety; and trial specific measures (recruitment and retention). Where possible, validated objective measures should be used to increase the study's validity but also to enable meaningful comparison across studies when combined in systematic reviews. In addition, due to the problems with accessing and locating interventions from published studies, researchers should make efforts for interventions (or intervention manuals) to be accessible post‐publication, for example, various formats of interventions stored securely or accessible through web links in published article. This reporting and availability of interventions should also be extended to include a description of comparators, especially when describing standard or usual information.
Through both design and reporting, trialists should strive to minimise bias through and refer to the CONSORT statement and TIDieR checklist (a template for the reporting of interventions to enable better replication) for guidance on the issue (www.consort‐statement.org; Hoffman 2014). Moreover, trialists should recognise the potential difficulties associated with conducting trials within trials (irrespective of focus) and ensure these are considered when designing nested RCTs (Graffy 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 15 April 2015 | Amended | Since protocol publication the references have been updated throughout |
| 14 June 2014 | Amended | Amendment to title |
Acknowledgements
The authors would like to thank the Cochrane Consumers and Communication Group editors and staff, in particular Sandy Oliver and John Kis‐Rigo, for their help and assistance with this review. We would also like to thank Cynthia Fraser, based at the Health Services Research Unit (University of Aberdeen), for help with development, refinement and running of the search strategies and Graeme MacLennan (also based at the Health Services Research Unit) for statistical advice. We would like to thank Muhammad Omar (Managing Editor of the Cochrane Incontinence Group) and Steven MacLennan (Academic Urology Unit, University of Aberdeen) for advice regarding RevMan and GRADE software.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials
#1 MeSH descriptor: [Clinical Trials as Topic] explode all trees
#2 MeSH descriptor: [Longitudinal Studies] explode all trees
#3 MeSH descriptor: [Evaluation Studies as Topic] this term only
#4 MeSH descriptor: [Pilot Projects] explode all trees
#5 MeSH descriptor: [Research Subjects] this term only
#6 MeSH descriptor: [Informed Consent] this term only
#7 MeSH descriptor: [Patient Participation] this term only
#8 MeSH descriptor: [Refusal to Participate] this term only
#9 (clinical or intervention or evaluation or comparative) next stud*:ti,ab,kw
#10 (longitudinal or follow‐up or followup or prospective) next stud*:ti,ab,kw
#11 (multi‐center or multicenter or multi‐centre or multicentre) next stud*:ti,ab,kw
#12 (research or study) next (subject* or participant*):ti,ab,kw
#13 ((particpa* or (tak* next part) or enrol* or recruit*) near/6 (research or stud* or experiment*)):ti,ab,kw
#14 (informed near/2 (consent or decision* or choice)) .ti,ab,kw
#15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 in Trials
#16 MeSH descriptor: [Decision Support Techniques] this term only
#17 MeSH descriptor: [Decision Support Systems, Clinical] this term only
#18 MeSH descriptor: [Decision Trees] this term only
#19 MeSH descriptor: [Decision Making, Computer‐Assisted] this term only
#20 MeSH descriptor: [Decision Making] this term only
#21 MeSH descriptor: [Choice Behavior] this term only
#22 computer* near/1 "decision making":ti,ab,kw
#23 "risk communication" near/3 tool*:ti,ab,kw
#24 (decision next (board* or guide* or counseling)):ti,ab,kw
#25 (decision* or decid*) near/3 (support* or aid* or tool* or instrument*):ti,ab,kw
#26 (decision* or decid*) near/3 (technolog* or technique* or system* or program* or algorithm*):ti,ab,kw
#27 (decision* or decid*) near/3 (process* or method* or intervention* or material*):ti,ab,kw
#28 (interacti* near/3 tool*):ti,ab,kw
#29 (interactive next health next communication*):ti,ab,kw
#30 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 in Trials
#31 #15 and #30 in Trials (988)
MEDLINE
exp Clinical Trials as topic/ (296396)
exp Longitudinal Studies/ (883743)
Evaluation Studies as Topic/ (121291)
Pilot Projects/ (84444)
Research Subjects/ (5215)
((particpa$ or tak$ part or enrol$ or recruit$) adj7 (research or stud$ or experiment$ or trial?)).tw. (111720)
Informed Consent/ (31662)
(informed adj3 (consent or decision? or choice)).tw. (30027)
Patient Participation/ (18146)
Refusal to Participate/ (537)
OR/1‐10 (1471406)
Decision Support Techniques/ (12281)
Decision Support Systems Clinical/ (5166)
Decision Trees/ (9021)
Decision Making/ (69125)
Choice Behavior/ (22303)
Decision‐making Computer Assisted/ (2561)
((decision$ or decid$) adj4 (support$ or aid$ or tool$ or instrument$ or technolog$ or technique$ or system$ or program$ or algorithm$ or process$ or method$ or intervention$ or material$)).tw. (45833)
(decision adj (board$ or guide$ or counseling)).tw.(125)
(risk communication adj4 tool$).tw. (51)
(computer$ adj2 decision making).tw. (151)
interactive health communication$.tw. (60)
(interacti$ adj4 tool$).tw. (2387)
OR/12‐23 (146628)
11 AND 24 ( 22784)
Randomized Controlled Trial.pt. (390288)
Controlled Clinical Trial.pt. (89931)
randomi?ed.ab (372420)
placebo.ab. (164048)
randomly.ab. (216716)
trial.ti. (132126)
exp animals/ not humans/ (8160129)
OR 26‐31 (838352)
33 NOT 32 (767535)
25 AND 34 (3268)
EMBASE
exp "Clinical Trial (topic)"/ (82009)
Longitudinal Study/ (65940)
Prospective Study/ (254724)
Intervention Study/ (17697)
Follow Up/ (760000)
Evaluation/ (190672)
clinical trial?.tw. (272070)
((clinical or intervention or experimental or follow‐up or followup or prospective or multi‐center or multicenter or double blind or pilot or random$ or control$ or crossover or cross‐over) adj2 stud$).tw. (887369)
Research Subject/ (5272)
((particpa$ or tak$ part or enrol$ or recruit$) adj7 (research or stud$ or experiment$ or trial?)).tw. (151174)
Informed Consent/ (61112)
(informed adj3 (consent or decision? or choice)).tw. (44998)
Patient Participation/ (16718)
Refusal to Participate/ (763)
OR/1‐14 (2271179)
Decision Support System/ (12875)
Decision Tree/ (5735)
Decision Making/ (136260)
((decision$ or decid$) adj4 (support$ or aid$ or tool$ or instrument$ or technolog$ or technique$ or system$ or program$ or algorithm$ or process$ or method$ or intervention$ or material$)).tw. (57075)
(decision adj (board$ or guide$ or counseling)).tw. (145)
(risk communication adj4 tool$).tw. (55)
(computer$ adj2 decision making).tw. (177)
interactive health communication$.tw. (55)
(interacti$ adj4 tool$).tw. (2548)
OR/16‐24 (191815)
15 AND 25 (31136)
exp Controlled Clinical Trial/ (494677)
Randomization/ (63887)
Crossover Procedure/ (38971)
Single Blind Procedure/ (18506)
Double Blind Procedure/ (118651)
randomi?ed.ab. (445792)
placebo.ab. (189562)
randomly.ab. (245999)
((singl$ or doubl$) adj (blind$ or mask$)).tw.(157344)
random$.tw.(857492)
(assign* or allocat* or volunteer* or crossover or cross over or factorial* or latin square).tw. (553648)
nonhuman/ not human/ (3340797)
OR/27‐37 (1500828)
39 not 38 (1303193)
26 and 40 (5713)
ASSIA
((SU.EXACT("Clinical randomized controlled trials") OR SU.EXACT("Double blind randomized trials") OR SU.EXACT("Clinical trials") OR SU.EXACT("Prospective controlled trials") OR SU.EXACT("Crossover trials") OR SU.EXACT("Single blind randomized controlled trials") OR SU.EXACT("Clustor randomized trials") OR SU.EXACT("Cluster randomized controlled trials") OR SU.EXACT("Randomized controlled trials") OR SU.EXACT("Trials") OR SU.EXACT("Double blind randomized controlled trials") ) OR ab((randomized or randomised or randomly OR (clinical or random*) N/3 trial)) OR ti((randomized or randomised or randomly OR (clinical or random*) N/3 trial))) AND ((SU.EXACT("Computerized decision support systems") OR SU.EXACT("Decision support systems") OR (SU.EXACT "decision making") OR SU.EXACT("Informed choice") OR TI (decision* OR decid* OR choice) OR AB (decision* OR decid* or choice))) (38)
PsycINFO
S1 ((DE "Experimentation") OR (DE "Followup Studies" OR DE "Longitudinal Studies" OR DE "Prospective Studies")) OR (DE "Experimental Subjects") (76469)
S2 TX ( (clinical or intervention or evaluation or comparative) W1 stud* ) OR TX ( (longitudinal or follow‐up or followup or prospective) w1 stud* ) OR TX ( (multi‐center or multicenter or multi‐centre or multicentre) w1 stud* ) OR TX ("double blind" or pilot) w1 stud* )) (268381)
S3 TX ( (research W1 (subject* or participant*) OR TX ( study W1 (subject* or participant*) ) (21066)
S4 TX clinical trial* OR TX experiment* (581,412)
S5 DE "Experimental Methods" (8,670)
S6 DE "Clinical Trials" (7,124)
S7 DE "Informed Consent" (3208)
S8 TX (informed N3 (consent or decision* or choice)) (9261)
S9 DE "Client Participation" (1272)
S10 TX ( ((participan* or tak* part or enrol* or recruit*) N7 research) OR TX ((participan* or tak* part or enrol* or recruit*) N7 stud*) ) OR TX ((participan* or tak* part or enrol* or recruit*) N7 experiment*)) ) (95017)
S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 (882,093)
S12 DE "Decision Support Systems" (2122)
S13 DE "Decision Making" OR DE "Choice Behavior" (57035)
S14 TX decision w1 tree? OR TX decision w1 board* OR TX decision w1 guide* OR TX decision w1 counseling (627)
S15 TX ( (decision* or decid*) N4 (support* or aid* or tool* or instrument*) ) OR TX ( (decision* or decid*) N4 (technolog* or technique* or system* or program*) ) OR TX ( (decision* or decid*) N4 (algorithm* or process* or method* or intervention* or material*) ) (34988)
S16 TX "risk communication" N4 tool* OR TX computer* N2 "decision making" OR TX interactive health communication* OR TX interacti* N4 tool* (1227)
S17 S12 OR S13 OR S14 OR S15 OR S16 (78,752)
S18 TX random* OR TX trial* OR TX controlled stud* OR TX placeb*
S19 TX ( (singl* or doubl* or trebl* or tripl*) ) AND TX ( (blind* or mask*) ) (21,472)
S20 TX cross over OR TX crossover OR TX factorial* OR TX latin square (22,200)
S21 TX assign* OR TX allocat* OR TX volunteer* (114,294)
S22 (DE "Treatment Effectiveness Evaluation") OR (DE "Mental Health Program Evaluation") (17,190)
S23 DE "Experimental Design" OR DE "Between Groups Design" OR DE "Clinical Trials" OR DE "Cohort Analysis" OR DE "Followup Studies" OR DE "Hypothesis Testing" OR DE "Longitudinal Studies" OR DE "Repeated Measures" (45,695)
S24 MR "2000" (25,297)
S25 S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 (339,493)
S32 S11 AND S17 AND S25 (1,123)
Data and analyses
Comparison 1. Comparison 1: Decision aid for informed consent versus standard informed consent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knowledge | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 1.68 [‐1.91, 5.26] |
| 2 Decisional conflict | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 3.47 [‐1.51, 8.45] |
| 3 Decisional regret | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐5.53 [‐10.29, ‐0.76] |
| 4 Participation | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.76, 1.48] |
| 5 Attrition | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.51, 4.68] |
| 6 Anxiety | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐10.65, 5.90] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Juraskova 2014 (DCIS).
| Methods | Multicentre international RCT of decision aid nested within a parent breast cancer treatment RCT, a component of the IBIS II trial (Cuzick 2008). Two armed trial (intervention plus standard information vs. standard information alone) |
|
| Participants | This nested RCT of decision aids was set within the treatment trial component of the IBIS II breast cancer trial. The parent treatment trial included postmenopausal women who had received surgery for DCIS and were subsequently randomised to anastrozole or tamoxifen for five years. A sample of participants being approached to participate in the parent trial were first randomised to a nested RCT of a decision aid for trial participation plus standard information or standard information alone before being randomised to treatment allocation. All participants were postmenopausal women recruited across sites in Australia, New Zealand and the UK. 67 participants were randomised from the DCIS parent RCT; 34 were included in T1 analysis (post‐decision) and 24 were included in the T2 analysis (3 month follow up):
Of the participants included in the T1 analysis the mean age was 58.5 years (SD 3.9) in the intervention group and 58.7 years (SD 5.0) in the control group; 22 were married (15 in intervention and 7 in control); 31 had at least high school education or higher (18 in intervention and 13 in control); 20 were in managerial or professional work roles (10 in intervention and 10 in control); and 31 only spoke English (17 in intervention and 14 in control) |
|
| Interventions | The decision aid booklet was designed to include evidence‐based representation of breast cancer risk, the IBIS‐II trial rationale, explanation of management options available on and off the trial, a comparison of the risks and benefits of each option, and values clarification worksheets. Authors state that decision aids were designed to meet IPDAS guidelines for content development. Intervention group received the standard parent RCT information sheet (no details given in publication as to content) and the decision aid booklet. Intervention development: paper states similar development process as has been published in full elsewhere (Juraskova 2008) but there were no explicit details for this intervention. Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Intervention type: booklet Time of delivery: Exact timing of delivery not clear, during recruitment consultation for parent trial |
|
| Outcomes |
At T1 (post‐decision) Primary outcome: difficulty with decision making (decisional conflict measure, which contains 16 items each rated on a 1 to 5 Likert scale). Secondary outcomes:
At T2 (3 month follow‐up)
|
|
| Notes | This study was supported by Susan G. Komen for the Cure (grant number BCTR0503961) and discretionary funding from the Breast Cancer Institute of Australia, which is the fundraising and education department of the Australia and New Zealand Breast Cancer Trials Group (ANZBCTG; no grant number). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated using a web‐based random number generator (www.randomizer.org). Randomisation was performed in blocks of 10 according to centre |
| Allocation concealment (selection bias) | Low risk | Pre‐randomised, sequentially numbered, sealed envelopes were provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors report that there were no differences (in terms of rates and reasons) for drop out in both arms. However, 66 participants (23% of those randomised) were not included in the analysis due to previous participation in a clinical trial which may result in ceiling effects for several of the measures. This was a post‐randomisation decision |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. All outcomes listed in the methods are reported in the results |
| Other bias | Unclear risk | Recruitment problems with DCIS cohort reduced power |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to intervention received, but this would not be possible. Recruiters were blinded to intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Authors state that outcome assessors were not blinded, likely because most outcomes were patient reported |
Juraskova 2014 (Prevention).
| Methods | Multicentre international RCT of decision aid nested within a parent breast cancer prevention RCT, a component of IBIS II (Cuzick 2008). Two armed trial (intervention plus standard information vs. standard information alone) |
|
| Participants | This nested RCT of decision aids was set within the prevention trial of the IBIS II breast cancer trial. The parent prevention trial aimed to randomise postmenopausal women, who were at high risk of breast cancer (based on family history, previous benign disease or mammographically dense breasts), to anastrozole or placebo for five years. A sample of participants being approached to participate in the parent prevention trial were first randomised to a nested RCT of a decision aid for trial participation plus standard information or standard information alone before randomisation to prevention allocation. All participants were postmenopausal women recruited across sites in Australia, New Zealand and the UK. 223 participants were randomised from the prevention parent trial. 112 were included in T1 analysis (post‐decision) and 95 were included in the T2 analysis (3 month follow up):
Of the participants included in the T1 analysis the mean age was 59.2 (SD 5.9) years in the intervention group and 59.2 (SD 5.3) years in the control group; 90 were married (43 in intervention and 47 in control); 111 had at least high school education or higher (52 in intervention and 59 in control); 55 were in managerial or professional work roles (28 in intervention and 27 in control); and 110 only spoke English (53 in intervention and 57 in control) |
|
| Interventions | The decision aid booklet was designed to include evidence‐based representation of breast cancer risk, the IBIS‐II trial rationale, explanation of management options available on and off the trial, a comparison of the risks and benefits of each option, and values clarification worksheets. Authors state that decision aids were designed to meet IPDAS guidelines for content development. Intervention group received the standard parent RCT information sheet (no details given in publication as to content) and the decision aid booklet. Intervention development: published in full elsewhere (Juraskova 2008) Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Intervention type: booklet Time of delivery: Exact timing of delivery not clear, during recruitment consultation for parent trial |
|
| Outcomes |
At T1 (post‐decision) Primary outcome: difficulty with decision making (decisional conflict measure, which contains 16 items each rated on a 1 to 5 Likert scale). Secondary outcomes:
At T2 (3 month follow‐up)
|
|
| Notes | This study was supported by Susan G. Komen for the Cure (grant number BCTR0503961) and discretionary funding from the Breast Cancer Institute of Australia, which is the fundraising and education department of the Australia and New Zealand Breast Cancer Trials Group (ANZBCTG; no grant number). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generated using a web‐based random number generator (www.randomizer.org). Randomisation was performed in blocks of 10 according to centre |
| Allocation concealment (selection bias) | Low risk | Pre‐randomised, sequentially numbered, sealed envelopes were provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors report that there were no differences (in terms of rates and reasons) for drop out in both arms. However, 66 participants (30% of those randomised) were not included in the analysis of the prevention trial due to previous participation in a related clinical trial (IBIS I). The authors' justification was that inclusion of these participants may result in ceiling effects for several of the measures. This was a post‐randomisation decision. Although the sample sizes were small, the proportion of excluded participants did differ (qualitatively) between arms (T1: 40% in DA group vs. 35% in control; and T3: 40% in DA group vs. 30% in control group) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. All outcomes listed in the methods are reported in the results |
| Other bias | Low risk | None reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to intervention received but this would not be possible. Recruiters were blinded to intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Authors state that outcome assessors were not blinded likely because most outcomes were patient reported |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abhyankar 2011 | Intervention is not a decision aid. Study presents a RCT of a values clarification exercise (implicit versus explicit) to support decisions about (hypothetical) trial participation. Whilst values clarification exercises are a component of a decision aid these interventions do not meet the full IPDASi criteria |
| Agre 2003 | A review paper that presents data from six studies. The interventions included are either not decision aids (e.g. enhanced information sheets, video information, computer based) or not decision aids for trial participation (present a decision aid about testing for haemophilia) |
| Agre 2003a | Intervention ineligible. Authors were contacted for intervention and responded. Authors unable to locate intervention. However, from details in published article and from a Cochrane review on a similar topic (Synnot 2014) the intervention is not likely to be a decision aid (standard consent information adjusted for reading age and then transferred to booklet, video or computer delivery) |
| Benson 1988 | Intervention ineligible. Authors were contacted and responded. Interventions could not be located. From details in published article and from a Cochrane review on a similar topic (Synnot 2014) intervention not likely to be a decision aid (e.g. video‐based 'instructional' video and 'improvements' to investigators discussions with potential participants). Content: The videotapes employed were described as 'instructional’ (standard) and 'improved’ for the two different intervention components. The standard video format involved the principal investigator or other designated project staff from the psychiatric trials describing the study as he/she chose to do so. This typically reflected the usual presentation made to subjects at the time of consent. The improved video format included feedback from the research team about areas of the disclosure that could be improved or required greater emphasis. Following this feedback, the second 'improved’ video format was produced. (taken from Synnot 2014) |
| Dear 2012 | Intervention not a decision aid. Described as "consumer‐friendly cancer clinical trials web site" that enables people to search for trials, contains general information about trials and provides a list of questions that people might want to ask if considering trial participation |
| Dunn 2002 | Intervention ineligible. Unable to assess intervention content for inclusion. Authors were contacted for intervention and responded but intervention could not be located. From details in published article and those provided by author, the intervention is not likely to be a decision aid. Intervention described as a computer based intervention that was composed of PowerPoint slides that provided a more structured review of the same material that was in the consent form |
| Eccles 2013 | Intervention not a decision aid for trial participation. Decision aid for treatment or screening |
| Foradori 2012 | Intervention not a decision aid for trial participation. Decision aid for treatment or screening |
| Hoffner 2012 | Intervention not a decision aid. Video‐based intervention developed by a US cancer centre. The aim of the video was to improve patients knowledge about trials by explaining clinical trials in a clear, simple, and balanced way |
| Hutchison 2007 | Intervention not a decision aid. Intervention delivered as an audio‐visual video based tool and covered both generic and cancer site specific information, with a particular focus on randomisation |
| Jacobsen 2012 | Intervention not a decision aid. A psycho‐educational multimedia intervention that contained generic information about clinical trials and covered "misperceptions and concerns about clinical trials" |
| Lurie 2011 | Intervention not a decision aid for trial participation. Decision aid for treatment or screening |
| Meropol 2013 | Intervention not a decision aid. Tailored videos selected by participant presented on a web‐based platform. Some components could be considered aspects of a decision aid, but as a whole, the intervention does not meet the IPDASi criteria |
| National Prescribing Centre (NHS) 2007 | Non‐eligible study design. Discussion piece |
| Norris 1990 | Intervention ineligible. No contact details for author. From details in published article and from a Cochrane review on a similar topic (Synnot 2014) the intervention is not likely to be a decision aid. Content: Information on the study protocol and adherence to the study protocol, including the following: compliance with dosing schedules; maintenance of diary cards; adherence to specific antacid limitations; presence at scheduled follow‐up visits; procedures to be used; possible adverse reactions; study consent forms; and who to call for information (taken from Synnot 2014) |
| Pinto 2008 | Intervention not a decision aid for trial participation. Study reports RCT pilot for treatment, which also includes patient preference arm, but no consent interventions |
| Saver 2007 | Intervention not a decision aid for trial participation. Decision aid for treatment or screening |
| Sundaresan 2011 | Non‐eligible study design. Mixed‐methods pilot of decision aid for trial participation. See Characteristics of ongoing studies |
| Tait 2010 | Intervention not a decision aid. RCT of different methods for presenting risk information to parents facing a hypothetical decision about their child's participation in an RCT. Components of intervention could be considered aspects of a decision aid, but as a whole, the intervention does not meet the IPDASi criteria |
| Ubel 1997 | Intervention ineligible. Unable to assess intervention content for inclusion. Authors were contacted and responded stating intervention not a decision aid. From details in published article review authors also concluded intervention not likely to be a decision aid |
| Weston 1997 | Intervention ineligible. Authors were contacted and responded. Interventions could not be located. From details in published article and from a Cochrane review on a similar topic (Synnot 2014) intervention not likely to be a decision aid. Content: Included description of the medical condition, pre labour rupture of membranes at term (Term PROM); and description of the study, including: the manoeuvre ‐ showing actual patients receiving each treatment, the risks and benefits of all study groups, the benefits of participating in clinical research and important aspects of the trial protocol, described by the principal investigator. An actual trial participant also described why she had participated in the study, the contribution she felt it made to medical science and to future women. An invitation to participate in the study and instructions on where to obtain further information on study participation were also included (taken from Synnot 2014) |
| Wragg 2000 | Intervention ineligible. Authors were contacted and responded, interventions could not be located. From details in published article and from a Cochrane review on a similar topic (Synnot 2014) intervention not likely to be a decision aid. The comparison was different framing messages used in a multi‐component intervention (taken from Synnot 2014) |
| Zwitter 1997 | Non‐eligible study design. Discussion piece |
Characteristics of ongoing studies [ordered by study ID]
Byrne.
| Trial name or title | Participation in cancer clinical trials: Improving minority cancer patient informed decision making through use of a patient centred decision aid |
| Methods | Pilot RCT |
| Participants | Cancer patients who:
and
|
| Interventions | Web based decision aid |
| Outcomes |
|
| Starting date | 2014 |
| Contact information | Margaret Byrne MByrne2@med.miami.edu |
| Notes |
Politi.
| Trial name or title | A Mixed Methods Study to Reduce Disparities in Cancer Clinical Trials by Adapting a Health Literacy Intervention for Informed Consent and Comparing it to Usual Care in a Randomized Experiment |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | A targeted, web‐based decision aid focused on the topic of clinical trials in addition to usual care |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 2014 |
| Contact information | Mary C Politi PhD mpoliti@wustl.edu Hannah E Perkins, MPA perkinsh@wudosis.wustl.edu |
| Notes |
Sundaresan.
| Trial name or title | Evaluating the Utility of a Patient Decision Aid for Prospective Participants in the TROG RAVES Prostate Cancer Trial (TROG 08.03) |
| Methods | RCT |
| Participants |
|
| Interventions | Decision aid |
| Outcomes |
|
| Starting date | 2014 |
| Contact information | Dr Puma Sundaresan puma.sundaresan@sswahs.nsw.gov.au |
| Notes |
Differences between protocol and review
The title of the review was changed since the protocol was published. In the protocol the interventions being evaluated were described as "decision support interventions"; however, this has been clarified in the review to "decision aids" to accurately reflect the interventions that were searched for in included studies and the prespecified nature of the interventions in the protocol.
Contributions of authors
Katie Gillies: Did the preparatory research; wrote the title registration form, the protocol and the full review. Contributed to all stages in the review process including refining search strategy; screening abstracts; data abstraction; data analysis; and write up. Wrote the first draft of the manuscript and led the response to reviewers' comments.
Jamie Brehaut: Critically read the title registration form, protocol, and submitted review. Assessed titles and abstracts obtained from electronic searches and contributed to the assessment of methodological quality of retrieved studies and the analysis of results.
Seonaidh Cotton: Assessed titles and abstracts obtained from electronic searches and contributed to the assessment of methodological quality of retrieved studies and the analysis of results. Critically read the submitted review.
Mary Politi: Critically read the title registration form and the submitted protocol. Assessed eligibility of studies for inclusion. Critically read the submitted review.
Zoe Skea: Critically read the title registration form, protocol, and submitted review. Assessed the titles and abstracts obtained from electronic searches and contributed to the assessment of methodological quality of retrieved studies and the analysis of results. Critically read the submitted review.
Sources of support
Internal sources
-
University of Aberdeen, UK.
Infrastructure support to review authors and information scientist (C Fraser)
-
Washington University School of Medicine, USA.
Infrastructure support to review author
-
Ottawa Hospital Research Institute, Canada.
Infrastructure support to review author
-
University of Ottawa, Canada.
Infrastructure support to review author
External sources
-
Chief Scientist Office of the Scottish Government Health Directorate, UK.
Provided personal fellowship award to Dr Gillies.
-
Medical Research Council, UK.
Provided personal fellowship award to Dr Gillies
Declarations of interest
Jamie Brehaut: None known.
Seonaidh Cotton: None known.
Katie Gillies: None known.
Mary Politi: From 2011 to 2013, I was on the US Prescription Medication Adherence Advisory Board for Merck. My role on this advisory board is not related to this Cochrane review which is about patient decision aids for clinical trials, and is unrelated to medication adherence. I have a current investigator‐initiated proposal funded by Merck to examine a decision aid about cancer clinical trials. This decision aid is not included in the review and the trial is ongoing. (See Ongoing studies).
Zoe Skea: None known
New
References
References to studies included in this review
Juraskova 2014 (DCIS) {published data only}
- Juraskova I, Butow P, Bonner C, Bell ML, Smith AB, Seccombe M, et al. Improving decision making about clinical trial participation ‐ a randomised controlled trial of a decision aid for women considering participation in the IBIS‐II breast cancer prevention trial. British Journal of Cancer 2014;111(1):1‐7. [PUBMED: 24892447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Juraskova 2014 (Prevention) {published data only}
- Juraskova I, Butow P, Bonner C, Bell ML, Smith AB, Seccombe M, et al. Improving decision making about clinical trial participation ‐ a randomised controlled trial of a decision aid for women considering participation in the IBIS‐II breast cancer prevention trial. British Journal of Cancer 2014;111(1):1‐7. [PUBMED: 24892447] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abhyankar 2011 {published data only (unpublished sought but not used)}
- Abhyankar P, Bekker HL, Summers B, Velikova G. Why values elicitation techniques enable people to make informed decisions about cancer trial participation. Health Expectations 2011;14(Suppl 1):20‐32. [DOI: 10.1111/j.1369-7625.2010.00615.x; PUBMED: 20629765] [DOI] [PMC free article] [PubMed] [Google Scholar]
Agre 2003 {published data only}
- Agre P, Campbell FA, Goldman BD, Boccia ML, Kass N, McCullough LB, et al. Improving informed consent: the medium is not the message. IRB 2003;25(5):S11‐19. [PUBMED: 14870732] [PubMed] [Google Scholar]
Agre 2003a {published data only}
- Agre P, Rapkin B. Improving informed consent: a comparison of four consent tools. IRB 2003;25(6):1‐7. [PUBMED: 15035248] [PubMed] [Google Scholar]
Benson 1988 {published data only (unpublished sought but not used)}
- Benson PR, Roth LH, Appelbaum PS, Lidz CW, Winslade WJ. Information disclosure, subject understanding, and informed consent in psychiatric research. Law and Human Behavior 1988;12(4):455‐75. [PUBMED: 11659248] [DOI] [PubMed] [Google Scholar]
Dear 2012 {published data only (unpublished sought but not used)}
- Dear RF, Barratt AL, Askie LM, Butow PN, McGeechan K, Crossing S, et al. Impact of a cancer clinical trials web site on discussions about trial participation: a cluster randomized trial. Annals of Oncology 2012;23(7):1912‐8. [PUBMED: 22258366] [DOI] [PubMed] [Google Scholar]
Dunn 2002 {published data only (unpublished sought but not used)}
- Dunn LB, Lindamer LA, Palmer BW, Golshan S, Schneiderman LJ, Jeste DV. Improving understanding of research consent in middle‐aged and elderly patients with psychotic disorders. American Journal of Geriatric Psychiatry 2002;10(2):142‐50. [PUBMED: 11925275] [PubMed] [Google Scholar]
Eccles 2013 {published data only}
- Eccles BK, Cross W, Rosario DJ, Doble A, Parker C, Logue J, et al. SABRE 1 (Surgery Against Brachytherapy ‐ a Randomised Evaluation): feasibility randomised controlled trial (RCT) of brachytherapy vs radical prostatectomy in low‐intermediate risk clinically localised prostate cancer. British Journal of Urology 2013;112(3):330. [PUBMED: 23826842] [DOI] [PubMed] [Google Scholar]
Foradori 2012 {published data only}
- Foradori MA, Nolan MT. Effect of a study map intended to support informed consent in transplant research. Progress in Transplantation 2012;22(1):56‐61. [PUBMED: 22489444] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffner 2012 {published data only (unpublished sought but not used)}
- Hoffner B, Bauer‐Wu S, Hitchcock‐Bryan S, Powell M, Wolanski A, Joffe S. "Entering a clinical trial: Is it right for you?": a randomized study of The Clinical Trials Video and its impact on the informed consent process. Cancer 2012;118(7):1877‐83. [PUBMED: 22009665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutchison 2007 {published data only (unpublished sought but not used)}
- Hutchison C, Cowan C, McMahon T, Paul J. A randomised controlled study of an audiovisual patient information intervention on informed consent and recruitment to cancer clinical trials. British Journal of Cancer 2007;97(6):11. [PUBMED: 17848908] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobsen 2012 {published data only (unpublished sought but not used)}
- Jacobsen PB, Wells KJ, Meade CD, Quinn GP, Lee JH, Fulp WJ, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. Journal of Clinical Oncology 2012;30(20):2516‐21. [PUBMED: 22614993] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lurie 2011 {published data only}
- Lurie JD, Spratt KF, Blood EA, Tosteson TD, Tosteson AN, Weinstein JN. Effects of viewing an evidence‐based video decision aid on patients' treatment preferences for spine surgery. Spine 2011;36(18):1501‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meropol 2013 {published data only (unpublished sought but not used)}
- Meropol NJ, Albrecht TL, Wong Y‐N, Benson AB, Buzaglo JS, Collins M, et al. [Randomized trial of a web‐based intervention to address carriers to clinical trials]. Journal of Clinical Oncology Conference. 2013; Vol. 31:20. [DOI] [PMC free article] [PubMed]
National Prescribing Centre (NHS) 2007 {published data only}
- National Prescribing Centre (NHS). Patient decision aids: New favourable evidence. MeReC Extra 2007; Vol. 29:1P.
Norris 1990 {published data only (unpublished sought but not used)}
- Norris DR, Phillips MR. Using instructive videotapes to increase patient comprehension of informed consent. Journal of Clinical Research and Pharmacoepidemiology 1990;4(4):263‐8. [Google Scholar]
Pinto 2008 {published data only}
- Pinto H, Rumball D, Maskrey V, Holland R. A pilot study for a randomized controlled and patient preference trial of buprenorphine versus methadone maintenance treatment in the management of opiate dependent patients. Journal of Substance Use 2008;13(2):73‐82. [Google Scholar]
Saver 2007 {published data only}
- Saver BG, Gustafson D, Taylor TR, Hawkins RP, Woods NF, Dinauer S, et al. A tale of two studies: The importance of setting, subjects and context in two randomized, controlled trials of a web‐based decision support for perimenopausal and postmenopausal health decisions. Patient Education and Counseling 2007;66(2):211‐22. [PUBMED: 17317080] [DOI] [PubMed] [Google Scholar]
Sundaresan 2011 {published data only}
- Sundaresan P, Turner S, Kneebone A, Pearse M, Butow P. Evaluating the utility of a patient decision aid for potential participants of a prostate cancer trial (RAVES‐TROG 08.03). Radiotherapy and Oncology 2011;101(3):521‐4. [PUBMED: 21985948] [DOI] [PubMed] [Google Scholar]
Tait 2010 {published data only (unpublished sought but not used)}
- Tait AR, Voepel‐Lewis T, Zikmund‐Fisher BJ, Fagerlin A. The effect of format on parents' understanding of the risks and benefits of clinical research: a comparison between text, tables, and graphics. Journal of Health Communication 2010;15(5):487‐501. [PUBMED: 20677054] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ubel 1997 {published data only (unpublished sought but not used)}
- Ubel PA, Merz JF, Shea J, Asch DA. How preliminary data affect people's stated willingness to enter a hypothetical randomized controlled trial. Journal of Investigative Medicine 1997;45(9):561‐6. [PUBMED: 9444883] [PubMed] [Google Scholar]
Weston 1997 {published data only (unpublished sought but not used)}
- Weston J, Hannah M, Downes J. Evaluating the benefits of a patient information video during the informed consent process. Patient Education and Counseling 1997;30(3):239‐45. [PUBMED: 9104380] [DOI] [PubMed] [Google Scholar]
Wragg 2000 {published data only (unpublished sought but not used)}
- Wragg JA, Robinson EJ, Lilford RJ. Information presentation and decisions to enter clinical trials: a hypothetical trial of hormone replacement therapy. Social Science & Medicine 2000;51(3):453‐62. [PUBMED: 10855931] [DOI] [PubMed] [Google Scholar]
Zwitter 1997 {published data only}
- Zwitter M. Communication with the patient in clinical research. Annals of the New York Academy of Sciences 1997;809(20):83‐96. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Byrne {unpublished data only}
- Participation in cancer clinical trials: Improving minority cancer patient informed decision making through use of a patient centred decision aid. Ongoing study 2014.
Politi {unpublished data only}
- A Mixed Methods Study to Reduce Disparities in Cancer Clinical Trials by Adapting a Health Literacy Intervention for Informed Consent and Comparing it to Usual Care in a Randomized Experiment. Ongoing study 2014.
Sundaresan {unpublished data only}
- Evaluating the Utility of a Patient Decision Aid for Prospective Participants in the TROG RAVES Prostate Cancer Trial (TROG 08.03). Ongoing study 2014.
Additional references
Abraham 2006
- Abraham NS, Young JM, Solomon MJ. A systematic review of reasons for non‐entry of eligible patients into surgical randomized controlled trials. Surgery 2006;139(4):469‐83. [PUBMED: 16627056] [DOI] [PubMed] [Google Scholar]
Angiolillo 2004
- Angiolillo AL, Simon C, Kodish E, Lange B, Noll RB, Ruccione K, et al. Staged informed consent for a randomized clinical trial in childhood leukemia: impact on the consent process. Pediatric Blood & Cancer 2004;42(5):433‐7. [PUBMED: 5049015] [DOI] [PubMed] [Google Scholar]
Belkora 2010
- Belkora JB, Stupar Franklin L, Wilson L, Loucks A, Moore D, Jupiter C, et al. Effectiveness and economics of telephone‐based decision support for rural patients: a randomized controlled trial. 6th International Shared Decision Making Conference, Abstract 197. 2010.
Brehaut 2003
- Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Medical Decision Making 2003;23(4):281‐92. [PUBMED: 12926578] [DOI] [PubMed] [Google Scholar]
Brehaut 2010
- Brehaut JC, Fergusson DA, Kimmelman J, Shojania KG, Saginur R, Elwyn G. Using decision aids may improve informed consent for research. Contemporary Clinical Trials 2010;31(3):218‐20. [PUBMED: 20156597] [DOI] [PubMed] [Google Scholar]
Brehaut 2012
- Brehaut JC, Carroll K, Elwyn G, Saginur R, Kimmelman J, Shojania K, et al. Informed consent documents do not encourage good‐quality decision making. Journal of Clinical Epidemiology 2012;65(7):708‐24. [PUBMED: 22537428] [DOI] [PubMed] [Google Scholar]
Brennan 2009
- Brennan S, McKenzie J, Whitty P, Buchan H, Green S. Continuous quality improvement: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003319.pub2] [DOI] [Google Scholar]
Campbell 2007
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. STEPS group. Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study. Health Technology Assessment 2007;11(48):iii, ix‐105. [PUBMED: 17999843] [DOI] [PubMed] [Google Scholar]
Clement 2009
- Clement S, Ibrahim S, Crichton N, Wolf M, Rowlands G. Complex interventions to improve the health of people with limited literacy: A systematic review. Patient Education and Counseling 2009;75(3):340‐51. [PUBMED: 19261426] [DOI] [PubMed] [Google Scholar]
Cox 2003
- Cox K, McGarry J. Why patients don’t take part in cancer clinical trials: An overview of the literature. European Journal of Cancer Care 2003;12(2):114‐22. [PUBMED: 12787008] [DOI] [PubMed] [Google Scholar]
Coyne 2003
- Coyne CA, Xu R, Raich P, Plomer K, Dignan M, Wenzel LB, et al. Eastern Cooperative Oncology Group. Randomized controlled trial of an easy‐to‐read informed consent statement for clinical trial participation: A study of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2003;21(5):836‐42. [PUBMED: 12610182] [DOI] [PubMed] [Google Scholar]
Cuzick 2008
- Cuzick J. IBIS II: a breast cancer prevention trial in postmenopausal women using the aromatase inhibitor anastrozole. Expert Review of Anticancer Therapy 2008;8(9):1377‐85. [PUBMED: 18759690] [DOI] [PubMed] [Google Scholar]
Ellis 2002
- Ellis PM, Butow PN, Tattersall MH. Informing breast cancer patients about clinical trials: a randomized clinical trial of an educational booklet. Annals of Oncology 2002;13(9):1414‐23. [DOI: 10.1093/annonc/mdf255] [DOI] [PubMed] [Google Scholar]
Elwyn 2006
- Elwyn G, O'Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. International Patient Decision Aids Standards (IPDAS) Collaboration. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ 2006;333(7565):417. [PUBMED: 16908462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2009a
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implementation Science 2009;18(4):75. [PUBMED: 19922647] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2009b
- Elwyn G, O'Connor AM, Bennett C, Newcombe RG, Politi M, Durand MA, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLoS One 2009;4(3):e4705. [PUBMED: 19259269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2010a
- Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: A definition and classification of decision support interventions for people facing difficult health decisions. Medical Decision Making 2010;30(6):701‐11. [PUBMED: 21088131] [DOI] [PubMed] [Google Scholar]
Elwyn 2010b
- Elwyn G, Miron‐Shatz T. Deliberation before determination: the definition and evaluation of good decision making. Health Expectations 2010;13(2):139‐47. [PUBMED: 19740089] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2010
- Evans R, Joseph‐Williams N, Edwards A, Newcombe RG, Wright P, Kinnersley P, et al. Supporting informed decision making for prostate specific antigen (PSA) testing on the web: an online randomized controlled trial. Journal of Medical Internet Research 2010;12(3):e27. [DOI: 10.2196/jmir.1305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fayter 2007
- Fayter D, McDaid C, Eastwood A. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. Journal of Clinical Epidemiology 2007;60(10):990‐1001. [PUBMED: 17884592] [DOI] [PubMed] [Google Scholar]
Featherstone 2002
- Featherstone K, Donovan JL. "Why don't they just tell me straight, why allocate it?" The struggle to make sense of participating in a randomised controlled trial. Social Science & Medicine 2002;55(5):709‐19. [PUBMED: 12190265] [DOI] [PubMed] [Google Scholar]
Flory 2004
- Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: A systematic review. JAMA 2004;292(13):1593‐601. [PUBMED: 15467062] [DOI] [PubMed] [Google Scholar]
Gillies 2012b
- Gillies K, Entwistle V. Supporting positive experiences and sustained participation in clinical trials: looking beyond information provision. Journal of Medical Ethics 2012;38(12):751‐6. [PUBMED: 22875981] [DOI] [PubMed] [Google Scholar]
Gillies 2013
- Gillies K, Skea ZC, MacLennan SJ, Ramsay CR, Campbell MK. Determining information for inclusion in a decision‐support intervention for clinical trial participation: a modified Delphi approach. Clinical Trials 2013;10(6):967‐76. [PUBMED: 24188963] [DOI] [PubMed] [Google Scholar]
Gillies 2014a
- Gillies K, Huang W, Skea Z, Brehaut J, Cotton S. Patient information leaflets (PILs) for UK randomised controlled trials: a feasibility study exploring whether they contain information to support decision making about trial participation. Trials 2014;15:62. [PUBMED: 24548781] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillies 2014b
- Gillies K, Skea Z, Campbell MK. Decision aids for randomised controlled trials: a qualitative exploration of stakeholders’ views. BMJ Open 2014;4(8):e005734. [PUBMED: 25138811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillies 2014c
- Gillies K, Elwyn G, Cook JC. Making a decision about trial participation: the feasibility of measuring deliberation during the informed consent process for clinical trials. Trials 2014;15(1):307. [PUBMED: 25073967] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillies 2014d
- Gillies K, Campbell MK. Measures of informed consent for clinical trials. (manuscript in preparation). [DOI] [PMC free article] [PubMed]
Gillies 2014e
- Gillies K, Entwistle V, Treweek S, Williamson P, Campbell M. Development of core outcome sets for interventions to improve informed consent for clinical trials. http://www.comet‐initiative.org/studies/details 2014. [DOI] [PMC free article] [PubMed]
Graffy 2010
- Graffy J, Bower P, Ward E, Wallace P, Delaney B, Kinmonth AL, et al. Trials within trials? Researcher, funder and ethical perspectives on the practicality and acceptability of nesting trials of recruitment methods in existing primary care trials. BMC Medical Research Methodology 2010;10:38. [PUBMED: 20433728] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hietanen 2007
- Hietanen PS, Aro AR, Holli KA, Schreck M, Peura A, Joensuu HT. A short communication course for physicians improves the quality of patient information in a clinical trial. Acta Oncologica 2007;46(1):42‐8. [PUBMED: 17438704] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available from www.cochrane‐handbook.org.
Hoffman 2014
- Hoffman TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. British Medical Journal 2014;348:g1687. [PUBMED: 24609605] [DOI] [PubMed] [Google Scholar]
Hon 2012
- Hon YK, Narayanan P, Goh PP. Extended discussion of information for informed consent for participation in clinical trials. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009835] [DOI] [Google Scholar]
ICH GCP 1996
- Guideline for Good Clinical Practice E6 (R1). ICH Harmonised Tripartite Guideline 1996.
Jenkins 2000
- Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. British Journal of Cancer 2000;82(11):1783‐8. [PUBMED: 10839291] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkins 2010
- Jenkins V, Farewell D, Batt L, Maughan T, Branston L, Langridge C, et al. The attitudes of 1066 patients with cancer towards participation in randomised clinical trials. British Journal of Cancer 2010;103(12):1801‐7. [PUBMED: 21119659] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joffe 2001
- Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. Journal of the National Cancer Institute 2001;93(2):139‐47. [PUBMED: 11208884] [DOI] [PubMed] [Google Scholar]
Joseph‐Williams 2013
- Joseph‐Williams N, Newcombe R, Politi M, Durand M‐A, Sivell S, Stacey D, et al. Toward minimum standards for the certification of patient decision aids: A correlation analysis and modified Delphi consensus process. Medical Decision Making 2013;34(6):699‐710. [PUBMED: 23963501] [DOI] [PubMed] [Google Scholar]
Juraskova 2008
- Juraskova I, Butow P, Lopez A, Seccombe M, Coates A, Boyle F, et al. Improving informed consent: pilot of a decision aid for women invited to participate in a breast cancer prevention trial (IBIS‐II DCIS). Health Expectations 2008;11(3):252‐62. [PUBMED: 18816321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinnersley 2013
- Kinnersley P, Phillips K, Savage K, Kelly MJ, Farrell E, Morgan B, et al. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009445.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6::e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madsen 2002
- Madsen SM, Mirza MR, Holm S, Hilsted KL, Kampmann K, Riis P. Attitudes towards clinical research amongst participants and non‐participants. Journal of Internal Medicine 2002;251(2):156‐68. [PUBMED: 11905591] [DOI] [PubMed] [Google Scholar]
Mangset 2008
- Mangset M, Førde R, Nessa J, Berge E, Wyller TB. I don't like that, it's tricking people too much...: acute informed consent to participation in a trial of thrombolysis for stroke. Journal of Medical Ethics 2008;34(10):751‐6. [PUBMED: 18827109] [DOI] [PubMed] [Google Scholar]
Marteau 1992
- Marteau T, Bekker H. The development of a six‐item short‐form of the state scale of the Spielberger State‐Trait Anxiety Inventory (STAI). British Journal of Clinical Psychology 1992;31(Pt 3):301‐6. [PUBMED: 1393159] [DOI] [PubMed] [Google Scholar]
McCann 2013
- McCann S, Campbell MK, Entwistle V. Recruitment to clinical trials: a meta‐ethnographic synthesis of studies of reasons for participation. Journal of Health Services Research and Policy 2013;18(4):233‐41. [PUBMED: 23986530] [DOI] [PubMed] [Google Scholar]
Nishimura 2013
- Nishimura A, Carey J, Erwin PL, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Medical Ethics 2013;14:28. [PUBMED: 23879694] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 1995
- O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making 1995;15(1):25‐30. [PUBMED: 7898294] [DOI] [PubMed] [Google Scholar]
O'Connor 1998
- O'Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Education and Counseling 1998;33(3):267‐79. [PUBMED: 9731164] [DOI] [PubMed] [Google Scholar]
Pocock 1983
- Pocock SJ. Clinical trials: a practical approach. New York: John Wiley & Sons, 1983. [Google Scholar]
Prescott 1999
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technology Assessment 1999;3(20):1‐143. [PUBMED: 10683591] [PubMed] [Google Scholar]
Ryan 2011
- Ryan R, Hill S, Prictor M, McKenzie J, Cochrane Consumers and Communication Review Group. Study Quality Guide. http://www.latrobe.edu.au/chcp/cochrane/resources.html 2011 (accessed 13 Sep 2011).
Sand 2008
- Sand K, Loge JH, Berger O, Grønberg BH, Kaasa S. Lung cancer patients' perceptions of informed consent documents. Patient Education and Counseling 2008;73(2):313‐7. [PUBMED: 18691845 ] [DOI] [PubMed] [Google Scholar]
Sanz de Acedo Lizararraga 2007
- Sanz de Acedo Lizarraga ML, Sanz de Acedo Baquedano MT, Cardelle‐Elawar M. Factors that affect decision making: gender and age differences. International Journal of Psychology and Psychological Therapy 2007;7(3):381‐91. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Sheridan 2011
- Sheridan SL, Halpern DJ, Viera AJ, Berkman ND, Donahue KE, Crotty K. Interventions for individuals with low health literacy: a systematic review. Journal of Health Communication 2011;16(Suppl 3):30‐54. [PUBMED: 21951242] [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith SK, Trevena L, Simpson JM, Barratt A, Nutbeam D, McCaffery KJ, et al. A decision aid to support informed choices about bowel cancer screening among adults with low education: Randomised controlled trial. BMJ 2010;26:341‐54. [PUBMED: 20978060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2014
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes‐Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD001431.pub4] [DOI] [PubMed] [Google Scholar]
Synnot 2014
- Synnot A, Ryan RE, Prictor MJ, Fetherstonhaugh D, Parker B. Audio‐visual presentation of information for informed consent for participation in clinical trials. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD003717.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomson 2010
- Thomson RG, Lally J, Mackintosh J, Flynn D. Decision support in complex settings: the challenge of context. 6th International Shared Decision Making Conference, Abstract no.198. 2010.
Treweek 2010
- Treweek S, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.MR000013.pub5] [DOI] [PubMed] [Google Scholar]
Wallace 2006
- Wallace C, Leask J, Trevena LJ. Effects of a web based decision aid on parental attitudes to MMR vaccination: a before and after study. BMJ 2006;332(7534):146‐9. [PUBMED: 16352657] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yap 2009
- Yap TY, Yamokoski A, Noll R, Drotar D, Zyzanski S, Kodish ED, Multi‐site Intervention Study to Improve Consent Research Team. A physician‐directed intervention: teaching and measuring better informed consent. Academic Medicine 2009;84(8):1036‐42. [PUBMED: 19638769] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gillies 2012a
- Gillies K, Skea ZC, Politi MC, Brehaut JC. Decision support interventions for people making decisions about participation in clinical trials. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009736] [DOI] [Google Scholar]


