Abstract
Purpose:
Anti-GD2 mAbs, acting via antibody-dependent cell-mediated cytotoxicity, may enhance the effects of chemotherapy. This pilot trial investigated a fixed dose of a unique anti-GD2 mAb, hu14.18K322A, combined with chemotherapy, cytokines, and haploidentical natural killer (NK) cells.
Experimental Design:
Children with recurrent/refractory neuroblastoma received up to six courses of hu14.18K322A (40 mg/m2/dose, days 2–5), GM-CSF, and IL2 with chemotherapy: cyclophosphamide/topotecan (courses 1,2), irinotecan/temozolomide (courses 3,4), and ifosfamide/carboplatin/etoposide (courses 5,6). Parentally derived NK cells were administered with courses 2, 4, and 6. Serum for pharmacokinetic studies of hu14.18K322A, soluble IL2 receptor alpha (sIL2Rα) levels, and human antihuman antibodies (HAHA) were obtained.
Results:
Thirteen heavily pretreated patients (9 with prior anti-GD2 therapy) completed 65 courses. One patient developed an unacceptable toxicity (grade 4 thrombocytopenia >35 days). Four patients discontinued treatment for adverse events (hu14.18K322A allergic reaction, viral infection, surgical death, second malignancy). Common toxicities included grade 3/4 myelosuppression (13/13 patients) and grade 1/2 pain (13/13 patients). Eleven patients received 29 NK-cell infusions. The response rate was 61.5% (4 complete responses, 1 very good partial response, 3 partial responses) and five had stable disease. The median time to progression was 274 days (range, 239–568 days); 10 of 13 patients (77%) survived 1 year. Hu14.18K322A pharmacokinetics was not affected by chemotherapy or HAHA. All patients had increased sIL2Rα levels, indicating immune activation.
Conclusions:
Chemotherapy plus hu14.18K322A, cytokines, and NK cells is feasible and resulted in clinically meaningful responses in patients with refractory/recurrent neuroblastoma. Further studies of this approach are warranted in patients with relapsed and newly diagnosed neuroblastoma.
Introduction
High-risk neuroblastoma is an aggressive pediatric malignancy with a poor outcome (1). The addition of chimeric 14.18 (ch14.18, dinutuximab), a mAb that binds to disialoganglioside (GD2) expressed on neuroblasts, given with granulocyte macrophage colony stimulating factor (GM-CSF), IL2, and isotretinoin improves survival and is now included as part of standard treatment for patients with high-risk neuroblastoma (2). Dinutuximab induces cell lysis through antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC; ref. 3).
In the treatment of neuroblastoma, anti-GD2 mAbs have been administered at the end of therapy in the setting of minimal disease to avoid concurrent chemotherapy-induced immunosuppression and an adverse effect on ADCC. However, limited preclinical studies performed in neuroblastoma and clinical studies in adult malignancies have demonstrated synergistic effects of concurrent mAb therapy with chemotherapy, even in bulky disease (4–11). These reports support the evaluation of concurrent chemotherapy with anti-GD2 mAb therapy in the treatment of patients with high-risk neuroblastoma.
Toxicities including pain, hypotension, capillary leak, and hypersensitivity related to murine and chimeric anti-GD2 mAbs have limited their clinical use in a subset of patients (2, 12, 13). In this study we used a humanized anti-GD2 mAb, hu14.18K322A, manufactured by Children’s GMP, LLC at St. Jude Children’s Research Hospital (Memphis, TN). Hu14.18K322A is an anti-GD2 mAb that retains the binding specificity of dinutuximab but is 98% human to reduce allergic reactions, has a single-point mutation designed to reduce pain associated with complement activation and is produced in a YB2/0 rat myeloma cell line which reduces fucosylation, thereby potentially enhancing ADCC (14).
Here we describe the results of a pilot trial, which is the first to evaluate the novel combination of a humanized anti-GD2 mAb (hu14.18K322A) with cytotoxic chemotherapy in patients with recurrent or refractory neuroblastoma. IL2 and GM-CSF were administered with each cycle of therapy to augment hu14.18K322A-mediated ADCC (15–21). In addition, we assessed the feasibility and tolerability of administering haploidentical natural killer (NK) cells, the primary effector cells for anti-GD2 mAb killing. Finally, we evaluated the pharmacokinetics of hu14.18K322A, and assessed for immune activation and the development of human antihuman antibodies (HAHA).
Patients and Methods
Patients
This study (GD2NK, NCT01576692) was approved by the Institutional Review Board in accordance with the U.S. Common Rule. Written informed consent was obtained from patients and/or parents/legal guardians. All patients were treated at St. Jude Children’s Research Hospital.
Patients were eligible for the trial if they were age ≤21 years at the time of enrollment, had recurrent or refractory neuroblastoma, and had evaluable disease by bone marrow morphology, computed tomography, magnetic resonance imaging, and/or iodine-123 metaiodobenzylguanidine (mIBG) scans. Patients were required to have an adequate performance score (Lansky or Karnofsky performance score ≥50), and were a minimum 2 weeks from last systemic therapy, 1 week from hematopoietic growth factors, 2 weeks from immunotherapy, 12 weeks for previous myeloablative therapy, and 2 weeks from radiation therapy. Patients were eligible to participate even if they were previously treated with anti-GD2 mAb or any of the chemotherapeutic combinations administered in the trial.
In addition, eligible patients required: absolute neutrophil count ≥750/mm3, unsupported platelet count ≥75,000 mm3, total bilirubin ≤2 times the upper limit of age-adjusted normal value (ULN), ALT ≤2.5× ULN, and serum creatinine ≤1.5 times the ULN and a cardiac shortening fraction ≥ 27%prior to enrollment.
Patients were excluded if they were pregnant, breastfeeding, had an uncontrolled infection, prior severe allergic reaction to anti-GD2 mAb therapy, or a known hypersensitivity to other recombinant human antibodies.
NK-cell donors and collection
NK-cell donors were eligible if they were a biologic parent, ≥18 years, and HIV negative. NK-cell donors were excluded if they were pregnant or had a medical condition that, in the opinion of an independent non-study team physician, precluded performance of an apheresis procedure. In the event that there were two eligible donors, the donor was selected based on the killer immunoglobulin receptor (KIR) status, with the maximum number of donor KIR–recipient KIR-ligand mismatches. Donors underwent leukapheresis. The mononuclear cells were depleted of T cells by CD3+ depletion with the ClinicMACS system then purified for CD56+ NK cells as previously described (22). Chimerism studies were performed by standard variable number of tandem repeats techniques (23) and engraftment was assessed by NK-cell phenotyping and direct measurement of surface expression of KIRs by flow cytometry.
Protocol therapy
Protocol therapy consisted of six courses of chemotherapy with concurrent hu14.18K322A, GM-CSF, and IL2. In courses 2, 4, and 6, patients could also receive a haploidentical NK-cell infusion (administered on day 7 or 8). Table 1 describes the doses and schedule of the courses including: cyclophosphamide/topotecan (courses 1, 2), irinotecan/temozolomide (courses 3, 4), and ifosfamide/carboplatin/etoposide (courses 5, 6). Hu14.18K322A was initiated on day 2 of each 21-day cycle and was given intravenously (over 4 hours), for 4 consecutive days. The dosage of hu14.18K322A was fixed at 40 mg/m2/dose, two dose levels below the single agent MTD (14). All patients received premedications with acetaminophen and diphenhydramine. Patients were also started on a morphine (or equivalent) patient controlled analgesia pump prior to the mAb infusion. GM-CSF (250 mcg/m2/dose) was given subcutaneously starting 24 to 36 hours following chemotherapy until the ANC was >2,000 mm3 or the patient met criteria to start the next course. Low-dose IL2 (1 million units/m2) was administered subcutaneously every other day for six doses starting 1 day after completion of chemotherapy. One patient with SD as best response had significant clinical benefit (improved pain and quality of life) and the IRB approved for her to receive two additional courses of cyclophosphamide and topotecan with hu14.18K322A and cytokines (for a total of eight courses).
Table 1.
Trial agents, doses, and schedule
| Course | Chemotherapy | Immunotherapy |
|---|---|---|
|
| ||
| 1 | Cyclophosphamide 400 mg/m2 i.v., days 1–5; Topotecan 1.2 mg/m2/d 1–5 i.v., days 1–5 |
hu14.18K322A 40 mg/m2 i.v., days 2–5 GM-CSF and IL2a |
| 2 | Cyclophosphamide 400 mg/m2 i.v., days 1–5; Topotecan 1.2 mg/m2/d 1–5 i.v., days 1–5 |
hu14.18K322A 40 mg/m2 i.v., days 2–5 GM-CSF and IL2a NK cells |
| 3 | Irinotecan 50 mg/m2 i.v., days 1–5 Temozolomide 150 mg/m2 p.o., days 1–5 |
hu14.18K322A 40 mg/m2 i.v., days 2–5 GM-CSF and IL2b |
| 4 | Irinotecan 50 mg/m2 i.v., days 1–5 Temozolomide 150 mg/m2 p.o., days 1–5 |
hu14.18K322A 40 mg/m2 i.v., days 2–5 GM-CSF and IL2b NK cells |
| 5 | Ifosfamide 2 g/m2 i.v., days 1–3 Carboplatin AUC-guided of 8 i.v., day 1 Etoposide 100 mg/m2 i.v., days 1–3 |
hu14.18K322A 40 mg/m2 i.v., days 2–5 GM-CSF and IL2a |
| 6 | Ifosfamide 2 g/m2 i.v., days 1–3 Carboplatin AUC-guided of 8 i.v., day 1 Etoposide 100 mg/m2 i.v., days 1–3 |
hu14.18K322A 40 mg/m2 i.v., days 2–5 GM-CSF and IL2a NK cells |
NOTE: hu14.18K322A infusion over 4 hours unless rate decreased secondary to toxicity.
GM-CSF 250 mcg/m2/day s.q., day 7 through the nadir until ANC > 2,000/mm3; IL2 1 million units/m2 s.q. every other day for six doses (day 6, 8, 10, 12, 14, and 16).
GM-CSF 250 mcg/m2/day s.q., day 8 through the nadir until ANC > 2,000/mm3; IL2 1million units/m2 s.q. every other day for six doses (day 7, 9, 11, 13, 15, and 17). GM-CSF and IL2 start 1 day later in cycles 3 and 4 due to a delay in the NK-cell infusion to ensure that at least two half-lives of the active metabolite of irinotecan, SN-38, have elapsed.
Toxicity assessment
Toxicity was graded according to the Common Terminology Criteria for Adverse Event v3.0. In this trial, unacceptable toxicities were defined as: (i) all grade 4 toxicities that did not return to baseline by day 35, (ii) use of pressors, (iii) need for mechanical ventilation, and (iv) death from toxicity. Dose modifications of hu14.18K322A were allowed for: (i) hypotension during the infusion of hu14.18K322A (without concurrent hypersensitivity) and (ii) grade 1 or 2 hypersensitivity reaction.
If a patient developed hypotension, the hu14.18K322A infusion was stopped and fluid resuscitation was initiated. If blood pressure normalized, the hu14.18K322A infusion was resumed at 50% of the initial rate. For a grade 1 hypersensitivity reaction, the rate of the hu14.18K322A infusion was decreased to 50% and antihistamines were administered. For grade 2 hypersensitivity, the hu14.18K322A infusion was stopped and upon recovery was restarted at 50% rate. If the symptoms persisted, antihistamines were scheduled and treatment was delayed for 24 hours. If symptoms resolved within 24 hours, then antihistamines were continued and hu14.18K322A could resume at 50% rate.
All patients with grade 3 or 4 hypersensitivity were removed from the protocol.
Response evaluation
Patients had disease evaluations performed at baseline and following courses 2, 4, and 6. Tumor response was assessed using the RECIST criteria in patients with measureable disease by CT and/or MRI (24). Patients with mIBG positive lesions were evaluable for mIBG response. The response of mIBG-avid lesions were reported using the Curie scale (25, 26). Imaging for each time point was centrally reviewed by two radiologists. Bone marrow involvement was assessed using routine staining; bilateral evaluations were required. All bone marrow exams were reviewed on site. Disease response was determined by the study team. Disease response was assessed using the International Response Criteria (27).
Pharmacokinetic and NK studies
All patients had blood samples (3 mL) drawn for pharmacokinetic studies of hu14.18K322A during each course on the first (day 2) and the last (day 5) day of hu14.18K322A. Levels were obtained: pre-hu14.18K332A infusion, post-infusion, and at hours 1, 8, and 20 post-infusion. A single blood sample was obtained on days 9, 12,16, and 21 of each course. Measurement of hu14.18K322A in patient’s sera was quantified using an ELISA previously described (28–31). Serum samples obtained on days 1, 2, 5, 9, 16, and 21 were also tested for soluble IL2 receptor alpha (sIL2Rα) levels. SIL2Rα was measured in the serum using a DuoSet ELISA Kit from R and D Systems Inc., as previously described (28). Patients were also monitored for the development of human antihuman antibodies (HAHA). During each course on days 1, 9, and 21, a 3 mL blood sample was obtained and processed as previously described (28, 30, 31).
NK-cell phenotyping was performed using flow cytometry to characterize the surface expression of KIR. NK-cell engraftment and phenotyping was assessed on days +7 (prior to NK-cell infusion), +14 (7 days after NK-cell infusion), and +21 (14 days after NK-cell infusion). KIR genotyping and KIR-ligand assessment was performed.
Statistical analysis
A two-stage stopping rule was used to monitor for unacceptable toxicities in participants during the first two courses of therapy. Six patients were enrolled in the first stage. If two or more patients developed an “unacceptable toxicity” during the first two courses of treatment or did not recover to baseline organ function by day 35 of a course, then the regimen would be considered too toxic and the trial would close. If the stopping rule was not triggered, then participants could be enrolled until at least 12 participants completed the first two courses of therapy. Time to progression (TTP) was evaluated and defined as the time period between the start of treatment to the time of progression. Patients were censored if they had a significant adverse event leading to discontinuation of the study or if they received other cancer-directed therapy (in the setting of nonprogressive disease).
Pharmacokinetic parameters for individual patients were summarized using standard descriptive methods. Modeling was performed using NONMEM v7.3 (ICON) software running on gfortran and incorporated Wings for NONMEM and XPOSE4 for pre- and post-run processing.
ADCC evaluation of hu14.18K322A
The ADCC Reporter Bioassay (Promega) is an ADCC mechanism of action assay that quantifies the biologic activity of pathway activation by therapeutic antibodies. The Bioassay Core Kit was used to measure the ADCC activity of six lots of hu14.18K322A and two lots of dinutuximab at 12 time points over a 10-month period. The assay has been previously described and is further described in the supplementary data (Supplementary Fig. S1; ref. 32).
Results
Patient characteristics
Between April 2012 and August 2014, 13 patients (1 refractory, 2 partial responders and 10 recurrent) were enrolled. Patient characteristics are described in Table 2. The patient population was heavily pretreated having received a median of three prior therapeutic regimens. Nine patients had prior anti-GD2 mAb therapy (6 with dinutuximab, 3 with hu14.18K322A) and 12 had prior myeloablative therapy with autologous stem cell rescue. All patients had previously received at least one of the chemotherapeutic regimens used in this trial (Table 5).
Table 2.
Patient characteristics (n = 13)
| Characteristics | Value (%) |
|---|---|
|
| |
| Median age at diagnosis | 6.4 years (1.9–13.4 years) |
| Median time from diagnosis to entry | 38 months (12.8–72.3 months) |
| Sex | |
| Male | 5 (38%) |
| Female | 8 (62%) |
| Disease status | |
| Recurrent | 10 (77%) |
| Primary refractory | 1 (8%) |
| Partial responders | 2 (15%) |
| Stage at diagnosis | |
| 3 | 1 (8%) |
| 4 | 12 (92%) |
| MYCN-amplified | 2 (15%) |
| Prior therapies | |
| Myeloablative therapy | 12 (92%) |
| Radiation | 12 (92%) |
| Anti-GD2 mAb | 9 (69%) |
| Median number of prior chemotherapy regimens | 3 (1–10) |
| Disease involvement at study entry | |
| Bone marrow | 7 (54%) |
| Bone | 11 (85%) |
| mIBG avid | 13 (100%) |
| Measureable disease | 10 (77%) |
| Median Curie score | 6 (range, 1–27) |
NOTE: Primary refractory includes progression of disease during induction chemotherapy; partial responders include patients who experienced a partial response to their primary treatment but continued to have active disease on therapy.
Abbreviation: mIBG, metaiodobenzylguandine.
Table 5.
Prior therapy, response and TTP for 13 patients with recurrent and refractory neuroblastoma
| Pt. no. | MYCN amp | Recurrent or refractory | Tumor BM, B, 0 | Prior Cyclo/TPT | Prior IRN/TMZ | Prior ICE | Prior mAb (Y/N) | Courses complete | Best response | Time to best response (course) | Curie score, baseline | Curie score, best | TTP/censoredb (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 2 | N | Recurrent | B, O | Y | N | N | N | 6 | CR | 6 | 3 | 0 | 239 |
| 11 | N | Refractory | B, O | Y | Y | Y | N | 6 | CR | 4 | 8 | 0 | b197 |
| 4 | N | Recurrent | BM, B, O | Y | Y | N | Y(D) | a5 | CR | 4 | 22 | 0 | 260 |
| 8 | N | Recurrent | BM, B, O | Y | N | Y | Y(hu) | 4 | VGPR | 2 | 2 | 0 | b213 |
| 6 | N | Recurrent | O | Y | N | N | N | 6 | PR | 2 | 2 | 0 | 558 |
| 9 | N | Recurrent | BM, O | Y | N | N | Y(D) | 6 | PR | 2 | 1 | 0 | 568 |
| 10 | N | Recurrent | BM, B, O | Y | N | N | Y(D) | 6 | PR | 2 | 6 | 0 | b194 |
| 13 | Y | Refractory | B, O | Y | N | N | Y(D) | 6 | PR | 2 | 4 | 0 | b204 |
| 1 | N | Recurrent | BM, B | Y | Y | Y | Y(hu) | 6 | SD | 2 | 18 | 16 | 274 |
| 3 | N | Recurrent | BM, B | Y | N | N | Y(hu) | al | SD | 1 | 14 | 14 | b81 |
| 5 | N | Refractory | B, O | Y | Y | N | N | a3 | SD | 2 | 7 | 7 | b75 |
| 7 | Y | Recurrent | B, O | Y | N | N | Y(D) | al | SD | 1 | 3 | 3 | b42 |
| 12 | N | Refractory | BM, B, O | Y | Y | N | Y(D) | 8 | SD | 2 | 27 | 13 | b456 |
Abbreviations: B, bone; BM, bone marrow; CR, complete response; Cyclo, cyclophosphamide; D, dinutuximab; hu, hu14.18K322A; ICE, ifosfamide/carboplatin/etoposide; IRN, irinotecan; O, other; PR, partial response; SD, stable disease; TMZ, temozolomide; TPT, topotecan; VGPR, very good partial response.
Did not complete all six courses of therapy due to: pt. 4, developed BK viral infection; pt. 3, unacceptable toxicity for thrombocytopenia >35 days; pt. 5, death related to surgery; pt. 7, allergic reaction to course 2 hu14.18K322A.
TTP/censored due to: pt. 11, cis-retinoic acid following course 6; pt. 8, second malignancy; pt. 10, local consolidative radiation; pt. 13, local consolidative radiation; pt. 3, dose-limiting toxicity for thrombocytopenia >35 days; pt. 5, death related to surgery; pt. 7, allergic reaction to hu14.18K; pt. 12, mIBG therapy following course.
Toxicity
Thirteen patients received 65 courses. One heavily pretreated patient (9 prior regimens) experienced an unacceptable toxicity of delayed platelet recovery (thrombocytopenia >35 days) following the first course and was removed from the study. This patient was replaced on study. Four patients discontinued treatment for adverse events, one each with: grade 3 hu14.18K322A allergic reaction (course 2), prolonged BK viral infection (course 5), death related to a surgical complication during primary tumor resection (course 3), and a second malignancy (myelodysplastic syndrome, course 5). The most common grade 3 and 4 toxicities were hematologic (Table 3) in courses 1 and 2 (cyclophosphamide/topotecan/hu14.18K322A) and courses 5 and 6 (ifosfamide/carboplatin/etoposide/hu14.18K322A). The median duration of GMCSF administered per course was 16.6 days (courses 1, 2), 8.9 days (course 3, 4), and 24.4 days (courses 5, 6). Only half of the patients experienced grade 3 and 4 hematologic toxicities in courses 3 and 4 (irinotecan/temozolomide/hu14.18K322A). All patients had grade 1 or 2 pain with hu14.18K322A infusion. Six patients required extending the infusion of hu14.18K322A from 4 to 8 hours (2 for hypotension, 2 for grade 2 hypersensitivity, 2 for physician discretion—1 tachycardia, 1 pain).
Table 3.
Treatment-related adverse events grades 3 and 4
| Course 1 (n = 13) | Course 2 (n = 11) | Course 3 (n = 11) | Course 4 (n = 10) | Course 5 (n = 9) | Course 6 (n = 8) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| Grades | Grades | Grades | Grades | Grades | Grades | |||||||
|
|
|
|
|
|
|
|||||||
| Toxicity | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 |
|
| ||||||||||||
| Nonhematologic | ||||||||||||
| Acidosis | 1 | 1 | ||||||||||
| Alanine aminotransferase elevated | 3 | 1 | 1 | |||||||||
| Anaphylaxis | 1 | |||||||||||
| Aspartate aminotransferase elevated | 2 | 1 | ||||||||||
| Diarrhea | 2 | |||||||||||
| Febrile neutropenia | 11 | 8 | 1 | 2 | 7 | 5 | ||||||
| Fever | 2 | |||||||||||
| Increased GGT | 2 | |||||||||||
| Hypocalcemia | 1 | |||||||||||
| Hypokalemia | 3 | 1 | 3 | 2 | ||||||||
| Hyponatremia | 1 | 1 | ||||||||||
| Hypophosphatemia | 1 | 2 | 2 | |||||||||
| Infections and infestations - Other | 1 | |||||||||||
| Myelodysplastic syndrome | 1 | |||||||||||
| Nausea/vomiting | 1 | 1 | 2 | |||||||||
| Pain | 1 | 1 | 1 | |||||||||
| Weight Loss | 1 | |||||||||||
| Hematologic | ||||||||||||
| Anemia | 12 | 11 | 6 | 1 | 7 | 8 | 1 | 8 | ||||
| Leukopenia | 13 | 11 | 4 | 2 | 2 | 4 | 9 | 8 | ||||
| Lymphopenia | 3 | 10 | 11 | 3 | 3 | 2 | 3 | 9 | 8 | |||
| Neutropenia | 13 | 11 | 3 | 3 | 2 | 3 | 9 | 8 | ||||
| Thrombocytopenia | 13 | 1 | 10 | 1 | 3 | 3 | 3 | 9 | 8 | |||
Pharmacokinetics
The combined median values for hu14.18K322A PK parameters are presented in Table 4. The overall median T1/2 alpha and β were 1.6 and 9.2 days, respectively. Table 4 also includes the median data for three patients that received 40 mg/m2/dose (the same dose administered in this study) in the single agent phase I study (14).
Table 4.
Median hu14.18K322A pharmacokinetic parameters for patients treated on this trial and the phase I trial at the same hu14.18K322A dose (40 mg/m2/dose)
| Study | BSA (m2) | CL (L/day)/m2 | VI (L)/m2 | AUC (mg*h/L) | AUC (ng*h/L)/(mg/m2) | Cmax (ng/mL) | Cmax (ng/mL)/(mg/m2) | Cend (ng/mL) | Cend (ng/mL)/(mg/m2) | Tl/2 Alpha (days) | Tl/2 Beta (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Combined median (n = 13) | 0.78 | 0.645 | 1.79 | 961 | 37.2 | 34,630 | 1108 | 392 | 15 | 1.6 | 9.2 |
| Range | 0.49–1.52 | 0.51–2.59 | 1.18–7.95 | 5,556–1,749 | 9.3–47.4 | 13,543–50,908 | 283–2,213 | 148–1,090 | 2.5–27.5 | 1.06–2.2 | 7.82–9.9 |
| Navid et al. median (n = 3) | 0.98 | 1.26 | 3.85 | 762 | 19.1 | 26,355 | 659 | 180 | 4.50 | 1.85 | 13.71 |
| Range | 0.76–2.02 | 1.06–1.66 | 3.81–3.92 | 580–905 | 14.5–22.6 | 20,955–27,125 | 523–678 | 162–382 | 4.05–9.55 | 1.47–2.31 | 13.50–14.61 |
Abbreviations: AUC, area under the plasma concentration–time curve; BSA, body surface area; CL, clearance; Cend, plasma concentration at the end of the infusion; Cmax, maximum plasma concentration; T1/2, half-life; Vl, volume.
PK data of three subpopulations of patients (4 with no prior anti-GD2 mAb, 6 with prior dinutuximab, and 3 with prior hu14.18K322A exposure) are presented in the supplementary data (Supplementary Table S1; Supplementary Fig. S2). There was no significant effect of prior anti-GD2 mAb exposure on either the Cmax or AUC of hu14.18K322A in patients with prior anti-GD2 mAb exposure (Supplementary Fig. S2).
HAHA response
Three patients had an increase in HAHA greater than 0.7 OD units from baseline, which was considered a positive response in prior anti-GD2 mAb studies (14, 19, 28–31). Ten patients did not have a positive HAHA response. Two (one with prior hu14.18K322A exposure and one with no prior anti-GD2 mAb exposure) of the 3 patients with a HAHA response had high baseline HAHA values prior to initiating therapy (Supplementary Table S2 and Supplementary Fig. S3). There was no association of HAHA with clearance, volume of distribution, T1/2, Cmax, and AUC using the Spearman test.
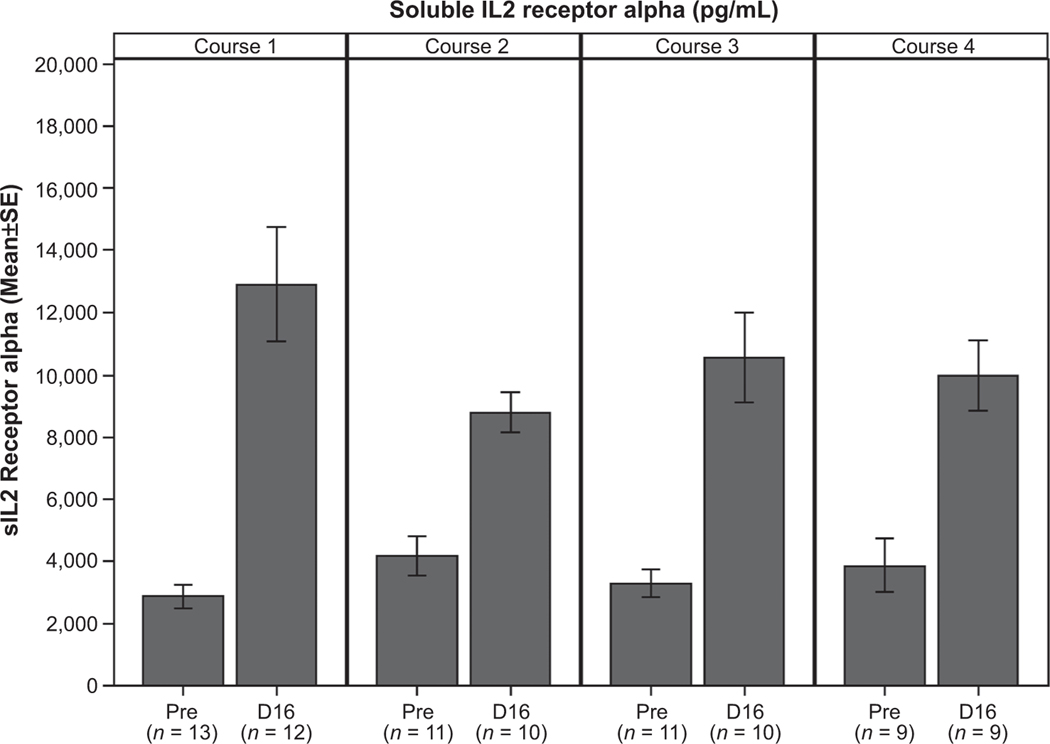
Soluble IL2R alpha
The sIL2 levels steadily increased during each course from baseline to days 5, 9, and 16 in all patients. Figure 1 shows the significant increase (P < 0.001 for each course) in sIL2 levels from baseline to day 16 for the first 4 courses. The change in sIL2 levels from baseline to day 16 was significantly larger (P = 0.029) in course 1 compared to course 2. The increase from baseline to day 16 for course 2 was not significantly different from the change in course 3 (P = 0.118) or course 4 (P = 0.160). The change in sIL2 levels from baseline to day 16 was significantly larger in course 1 when no NK cells were received compared to course 2 when NK cells were received (P = 0.029); however, there was no significant difference (P = 0.986) in the change from baseline to day 16 between course 3 (no NK cells) and course 4 (NK cells).
Figure 1.
sIL2 receptor alpha prior to each course of therapy (pre) and on day 16 (D16) of the first 4 courses.
NK cells
Eleven patients received 29 allogeneic NK-cell infusions (Supplementary Table S3). There were no complications with donor NK collection and NK administration to the patients. The median number of NK cells infused per dose was 15.5 × 106/kg (range, 4.7 × 106/kg to 59.5 × 106/kg). KIR ligand mismatch was evaluated. Mismatch was present at two ligands in four patients, one in six patients and zero ligands in two patients. There was no association between number of mismatched ligands and response to therapy. The day +14 NK chimerism had a median of 2% donor-derived cells (range, 0–50%); day +21 NK chimerism had a median of 0% donor-derived cells (range, 0–7%). The absolute NK-cell persistence per course is presented in the supplementary data (Supplementary Fig. S4).
Antitumor activity
The overall objective response rate was 61.5% (8/13 patients) with a complete response/very good partial response rate of 38.5% (5/13 patients). Table 5 describes individual patient responses. None of the patients progressed while on treatment and 10 of 10 patients with symptomatic disease at the time of enrollment had improvement in symptoms. The median TTP was 274 days (range, 239–568 days). Four patients completed therapy and received additional therapy: one with a complete response received cis-retinoic acid, two with a partial response received focal consolidative radiation, one with stable disease received mIBG therapy. Those four patients were censored at the time new therapy was initiated, median time to censoring was 201 days (range, 194–456 days). Four patients were censored due to adverse events requiring discontinuation of the trial. For all 13 patients, 10 patients survived 1-year. The 1-year survival rate was 77% (95% CI, 48–93%).
ADCC assessment
The ADCC activity of six cGMP lots of hu14.18K322A were compared to two clinical lots of dinutuximab. The average EC50 for hu14.18K322A was 2.16 ± 0.30 ng/mL (range, 1.92–2.76) versus 8.39 ± 0.30 ng/mL (range, 8.09–8.68) for dinutuximab (P = 0.0082) as shown in the supplementary data (Supplementary Fig. S1 and Supplementary Table S4).
Discussion
This trial demonstrates the feasibility, tolerability, and safety of administering an anti-GD2 mAb hu14.18K322A with chemotherapy, cytokines, and NK cells in children with recurrent/refractory neuroblastoma. The therapy was tolerated with expected pancytopenia related to the chemotherapeutic agents and mild pain related to hu14.18K322A. Clinical benefit was evident in all patients and none progressed while receiving therapy.
Historically, patients with recurrent and refractory neuroblastoma have response rates of 15% to 50% with the chemotherapeutic regimens used here (33–37), with very few patients experiencing a complete response. In this trial, the overall response rate was 61.5% with 38.5% (5/13 patients) experiencing a complete response (CR) or very good partial response (VGPR) and the majority had previously received the same chemotherapeutic combinations and anti-GD2 mAb therapy. The responses support the benefit of concurrent anti-GD2 mAb therapy plus chemotherapy in recurrent or refractory neuroblastoma.
Response to therapy in neuroblastoma is difficult to assess because disease in the bone and bone marrow is frequently not “measureable” using standard response criteria. To address this, London and colleagues reviewed 489 patient enrollments from 384patients with recurrent/refractory neuroblastoma treated with 36 phase I or II COG trials from 2002 to 2014 (38). Eleven of the 36 trials included multiagent treatment regimens. They reported that the median TTP of the historical cohort was 63 days. Fox and colleagues also validated the utility of using TTP as a valuable study endpoint to identify novel active agents in children with recurrent/refractory neuroblastoma (39). In their retrospective study of 136 patients enrolled in five phase I or II trials, the median TTP was 42 days.
In our study, patients were eligible to receive six courses of therapy and the median TTP was 274 days. This trial was limited by the small number of patients enrolled and the number of patients who were censored for toxicity or starting a new therapy. If we include the patients that were censored for toxicity related to therapy and assess the time to treatment failure (including discontinuation of the trial for toxicity or progressive disease), then the median time to treatment failure is 250 days. In either case, a TTP of 274 days or time to treatment failure/progression of 250 days is clinically significant when compared to 42 to 63 days reported using similar patient groups (38, 39), further suggesting that the combination of anti-GD2 mAbs with chemotherapy in the treatment of children with neuroblastoma warrants further study.
Because anti-GD2 mAbs mediate lysis of tumor cells primarily through ADCC, we wanted to measure the ADCC of hu14.18K322A in vitro and compare it to commercially available dinutuximab. Previously, Sorkin and colleagues reported that although hu14.18K322A retained ADCC, there was a slight decrease in the ADCC activity of hu14.18K322A compared to dinutuximab (40). In our studies using a bioreporter assay, we demonstrate that the concentration of hu14.18K322A required to lyse cells (EC50) is consistently 3.5- to 4-fold lower than that of dinutuximab. These results were confirmed using numerous lots of mAb, tested over varying time points and suggest that the ADCC of hu13.18K322A may be more robust than that of dinutuximab. Improved ADCC of hu14.18K322A may be related to decreased fucosylation of mAb as demonstrated by Gillies and colleagues (41), which suggests that there may be an optimal level of fucosylation for ADCC activity. Further studies are ongoing to optimize the mAb.
The pharmacokinetic studies confirmed that the addition of chemotherapy and/or prior anti-GD2 mAb exposure did not affect the PK of hu14.18K322A. Further, for patients with mild HAHA fluctuations, there was no pattern identified throughout treatment. These data suggest that the observed HAHA responses were different from typical “antibody immunization responses,” whereby the HAHA response increases with repeated exposure to mAb. For the three patients with an increased HAHA response >0.7 OD units, this response did not influence the PK of hu14.18K322A therapy as assessed by Cmax values. This contradicts prior studies which demonstrated declining Cmax values in subsequent treatment courses once a patient develops a strong HAHA or human anti-chimeric antibody (HACA) response (14, 30, 31). The attenuation of HAHA response and lack of HAHA influence on Cmax values is likely associated with concurrent chemotherapy administration, as demonstrated in a study using a murine anti-GD2 mAb (42).
Prior anti-GD2 antibody exposure did not influence the development of a HAHA response in this study. Three of the four patients who had never received anti-GD2 mAb therapy had detectable HAHA in their baseline serum sample, prior to receiving hu14.18K322A. These HAHA antibodies are likely anti-allotypic antibodies against alloantigens on allogeneic IgG, which developed as a result of prior blood product transfusions (43). Although the concurrent chemotherapy may have attenuated the HAHA response, this regimen which also included GM-CSF and IL2 resulted in a sustained increase in the sIL2Ra, which is a marker of immune activation (44). The change in sIL2 levels from baseline to day 16 was significantly larger in course 1 when no NK cells were received compared to course 2 when NK cells were received, however there was not a significant difference in the change between course 3 (no NK cells) and course 4 (NK cells; P = 0.986). It is likely that the concurrent chemotherapy also modulated the sIL2R response rather than the NK-cell infusions.
These data indicate that prior exposure to mAb (hu14.18K322A or dinutuximab) did not negatively affect subsequent therapy with combined hu14.18K322A and chemotherapy. Further, the HAHA responses did not lead to toxicities or change in the PK parameters. Thus, it is possible that this regimen could be appropriate as first-line therapy, without concern that it would interfere with subsequent use of hu14.18K322A in maintenance and/or it could be considered as salvage therapy for patients who failed prior anti-GD2 mAb therapy. The PK values when compared to the phase I study of single agent hu14.18K322A indicate that concurrent chemotherapy did not influence the pharmacokinetics.
In conclusion, we demonstrate that hu14.18K322A can be safely combined with three standard neuroblastoma chemotherapeutic regimens, cytokines, and NK cells in the treatment of recurrent or refractory neuroblastoma. In addition to fewer toxicities (45), hu14.18K322A may also have improved ADCC when compared to dinutuximab. The addition of chemotherapy did not alter the PK of hu14.18K322A, and the development of HAHA did not lower Cmax or change the AUC. The promising response rate and delayed TTP signal indicates that the addition of hu14.18K322A with chemotherapy and cytokines has significant clinical impact and should be evaluated further. In this limited pilot study, the therapeutic role of allogeneic NK cells cannot be determined. Based on these results, this novel approach of anti-GD2 mAb plus chemotherapy and cytokines is being evaluated in a phase II trial for patients with newly diagnosed high-risk neuroblastoma at our center (46).
Supplementary Material
Translational Relevance.
Despite dose-intensive treatment, less than half of the patients diagnosed with high-risk neuroblastoma survive and those who recur or progress during therapy often die of their disease. Over the past 7 years, the introduction of an anti-GD2 mAb in the setting of minimal residual disease and acting via antibody-dependent cell-mediated cytotoxicity (ADCC) has improved event-free survival rates of children with high-risk neuroblastoma by 20%. Preclinical studies performed in neuroblastoma and clinical studies in adult malignancies demonstrate a synergistic effect when chemotherapy is combined with mAbs. On the basis of this information, we conducted the first pilot trial that combined a humanized anti-GD2 mAb (hu14.18K322A) with chemotherapy, cytokines, and haploidentical natural killer cells for the treatment of recurrent or refractory neuroblastoma. Our results demonstrate that the therapy is feasible, has promising antitumor activity, and should be further studied in patients with relapsed and newly diagnosed neuroblastoma.
Acknowledgments
The authors thank all the patients and their families, research nurses, clinical and laboratory personnel, study coordinators, and operations staff who participated in this study.
Grant Support
Supported in part by Cancer Center GrantCA23099 and Cancer Center Support CORE GrantP30 CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med 2010; 362:2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clin Cancer Res 2012;18:2740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 2003;63:4490–6. [PubMed] [Google Scholar]
- 5.Lake RA, Robinson BW. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer 2005;5:397–405. [DOI] [PubMed] [Google Scholar]
- 6.Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, et al. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology 2011;132:226–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holden SA, Lan Y, Pardo AM, Wesolowski JS, Gillies SD. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clin Cancer Res 2001;7:2862–9. [PubMed] [Google Scholar]
- 8.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106:3725–32. [DOI] [PubMed] [Google Scholar]
- 9.Coiffier B. Diffuse large cell lymphoma. Curr Opin Oncol 2001;13:325–34. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- 12.Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol 1998;16:2169–80. [DOI] [PubMed] [Google Scholar]
- 13.Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, et al. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur J Cancer 1995; 31A:261–7. [DOI] [PubMed] [Google Scholar]
- 14.Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol 2014;32:1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batova A, Kamps A, Gillies SD, Reisfeld RA, Yu AL. The Ch14.18-GM-CSF fusion protein is effective at mediating antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in vitro. Clin Cancer Res 1999;5:4259–63. [PubMed] [Google Scholar]
- 16.Barker E, Reisfeld RA. A mechanism for neutrophil-mediated lysis of human neuroblastoma cells. Cancer Res 1993;53:362–7. [PubMed] [Google Scholar]
- 17.Honsik CJ, Jung G, Reisfeld RA. Lymphokine-activated killer cells targeted by monoclonal antibodies to the disialogangliosides GD2 and GD3 specifically lyse human tumor cells of neuroectodermal origin. Proc Natl Acad Sci U S A 1986;83:7893–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munn DH, Cheung NK. Interleukin-2 enhancement of monoclonal antibody-mediated cellular cytotoxicity against human melanoma. Cancer Res 1987;47:6600–5. [PubMed] [Google Scholar]
- 19.Hank JA, Kohler PC, Weil-Hillman G, Rosenthal N, Moore KH, Storer B, et al. In vivo induction of the lymphokine-activated killer phenomenon: interleukin 2-dependent human non-major histocompatibility complex-restricted cytotoxicity generated in vivo during administration of human recombinant interleukin 2. Cancer Res 1988;48:1965–71. [PubMed] [Google Scholar]
- 20.Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res 1990;50:5234–9. [PubMed] [Google Scholar]
- 21.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982;155:1823–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyengar R, Handgretinger R, Babarin-Dorner A, Leimig T, Otto M, Geiger TL, et al. Purification of human natural killer cells using a clinical-scale immunomagnetic method. Cytotherapy 2003;5:479–84. [DOI] [PubMed] [Google Scholar]
- 23.Schichman SA, Suess P, Vertino AM, Gray PS. Comparison of short tandem repeat and variable number tandem repeat genetic markers for quantitative determination of allogeneic bone marrow transplant engraftment. Bone Marrow Transplant 2002;29:243–8. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 25.Ady N, Zucker JM, Asselain B, Edeline V, Bonnin F, Michon J, et al. A new 123I-MIBG whole body scan scoring method—application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer 1995;31A:256–61. [DOI] [PubMed] [Google Scholar]
- 26.Messina JA, Cheng SC, Franc BL, Charron M, Shulkin B, To B, et al. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer 2006;47:865–74. [DOI] [PubMed] [Google Scholar]
- 27.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11:1466–77. [DOI] [PubMed] [Google Scholar]
- 28.Albertini MR, Gan J, Jaeger P, Hank JA, Storer B, Schell K, et al. Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. J Immunother Emphasis Tumor Immunol 1996;19:278–95. [DOI] [PubMed] [Google Scholar]
- 29.Albertini MR, Hank JA, Schiller JH, Khorsand M, Borchert AA, Gan J, et al. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res 1997;3:1277–88. [PubMed] [Google Scholar]
- 30.Hank JA, Gan J, Ryu H, Ostendorf A, Stauder MC, Sternberg A, et al. Immunogenicity of the hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin Cancer Res 2009;15:5923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hank JA, Surfus JE, Gan J, Ostendorf A, Gillies SD, Sondel PM. Determination of peak serum levels and immune response to the humanized antiganglioside antibody-interleukin-2 immunocytokine. Methods Mol Med 2003;85:123–31. [DOI] [PubMed] [Google Scholar]
- 32.Cheng ZJ, Garvin D, Paguio A, Moravec R, Engel L, Fan F, et al. Development of a robust reporter-based ADCC assay with frozen, thaw-and-use cells to measure Fc effector function of therapeutic antibodies. J Immunol Methods 2014;414:69–81. [DOI] [PubMed] [Google Scholar]
- 33.London WB, Frantz CN, Campbell LA, Seeger RC, Brumback BA, Cohn SL, et al. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol 2010; 28:3808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saylors RL III, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol 2001;19:3463–9. [DOI] [PubMed] [Google Scholar]
- 35.Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol 2011;29:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marina NM, Rodman J, Shema SJ, Bowman LC, Douglass E, Furman W, et al. Phase I study of escalating targeted doses of carboplatin combined with ifosfamide and etoposide in children with relapsed solid tumors. J Clin Oncol 1993;11:554–60. [DOI] [PubMed] [Google Scholar]
- 37.Kushner BH, Modak S, Kramer K, Basu EM, Roberts SS, Cheung NK. Ifosfamide, carboplatin, and etoposide for neuroblastoma: a high-dose salvage regimen and review of the literature. Cancer 2013;119:665–71. [DOI] [PubMed] [Google Scholar]
- 38.London WB, Bagatell R, Weigel B, Fox E, VanRyn C, Naranjo A, et al. Historical gold standard for time to progression (TTP) and progression-free survival (PFS) from relapsed/refractory neuroblastoma modern era (2002–2014) patients. J Clin Oncol 2014;32:(suppl; abstr 10034). [Google Scholar]
- 39.Fox E, Mosse YP, Meany HM, Gurney JG, Khanna G, Jackson HA, et al. Time to disease progression in children with relapsed or refractory neuroblastoma treated with ABT-751: a report from the Children’s Oncology Group (ANBL0621). Pediatr Blood Cancer 2014;61:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorkin LS, Otto M, Baldwin WM III, Vail E, Gillies SD, Handgretinger R, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain 2010;149:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillies SD, inventor; Merck Patent Gmbh, assignee. Anti-cancer antibodies with reduced complement fixation. United States patent US W02005070967 A2. 2005. August 4. [Google Scholar]
- 42.Kushner BH, Cheung IY, Kramer K, Modak S, Cheung NK. High-dose cyclophosphamide inhibition of humoral immune response to murine monoclonal antibody 3F8 in neuroblastoma patients: broad implications for immunotherapy. Pediatr Blood Cancer 2007;48:430–4. [DOI] [PubMed] [Google Scholar]
- 43.Jefferis R, Lefranc MP. Human immunoglobulin allotypes: possible implications for immunogenicity. MAbs 2009;1:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogner MP, Voss SD, Bechhofer R, Hank JA, Roper M, Poplack D, et al. Serum CD25 levels during interleukin-2 therapy: dose dependence and correlations with clinical toxicity and lymphocyte surface sCD25 expression. J Immunother 1992;11:111–8. [PubMed] [Google Scholar]
- 45.Anghelescu DL, Goldberg JL, Faughnan LG, Wu J, Mao S, Furman WL, et al. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr Blood Cancer 2015;62:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furman WL, Federico SM, McCarville MB, et al. Improved clinical responses with the concomitant use of an anti-GD2 monoclonal antibody and chemotherapy in newly diagnosed children with high-risk neuroblastoma: Preliminary results of a phase II study. ASCO MEETINGS, Oral abstract 10501, Chicago, IL; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.