Abstract
Background:
During bone fracture repair, resident mesenchymal stem cells (MSCs) differentiate into chondrocytes, to form a cartilaginous fracture callus, and osteoblasts, to ossify the collagen matrix. our laboratory previously reported that alcohol administration led to decreased cartilage formation within the fracture callus of rodents and this effect was mitigated by postfracture antioxidant treatment. Forkhead box protein O (FoxO) transcription factors are activated in response to intracellular reactive oxygen species (ROS), and alcohol has been shown to increase ROS. Activation of FoxOs has also been shown to inhibit canonical Wnt signaling, a necessary pathway for MSC differentiation. These findings have led to our hypothesis that alcohol exposure decreases osteochondrogenic differentiation of MSCs through the activation of FoxOs.
Methods:
Primary rat MSCs were treated with ethanol (EtOH) and assayed for FoxO expression, FoxO activation, and downstream target expression. Next, MSCs were differentiated toward osteogenic or chondrogenic lineages in the presence of 50 mM EtOH and alterations in osteochondral lineage marker expression were determined. Lastly, osteochondral differentiation experiments were repeated with FoxO1/3 knockdown or with FoxO1/3 inhibitor AS1842856 and osteochondral lineage marker expression was determined.
Results:
EtOH increased the expression of FoxO3a at mRNA and protein levels in primary cultured MSCs. This was accompanied by an increase in FoxO1 nuclear localization, FoxO1 activation, and downstream catalase expression. Moreover, EtOH exposure decreased expression of osteogenic and chondrogenic lineage markers. FoxO1/3 knockdown restored proosteogenic and prochondrogenic lineage marker expression in the presence of 50 mM EtOH. However, FoxO1/3 inhibitor only restored proosteogenic lineage marker expression.
Conclusions:
These data show that EtOH has the ability to inhibit MSC differentiation, and this ability may rely, at least partially, on the activation of FoxO transcription factors.
Keywords: fracture healing, MSC, differentiation, alcohol, FoxO, AS1842856
BONE FRACTURE HEALING is the result of a complex series of molecular and cellular events in and around the fracture. Although most fractures heal without complications, fracture nonunion still remains a significant health problem. Up to 10% of all long bone fractures progress to a fracture nonunion, costing nearly 1 billion dollars annually in healthcare costs in the United States (Hak et al., 2014; Tzioupis and Giannoudis, 2007; Zimmermann and Moghaddam, 2010). Moreover, there are several conditions that contribute to improper, delayed, or incomplete healing. These range from medical conditions like diabetes to behavioral conditions such as alcohol abuse (Chakkalakal, 2005; Nyquist et al., 1997; Zura et al., 2016). Alcohol abuse has a significant detrimental effect on many organs and body tissues, which includes significant effects to the skeletal system. Alcohol abusers have an increased risk of developing osteopenia and have a fracture rate 4 times higher than that of nonabusers (Bikle et al., 1985; Chakkalakal, 2005; Savola et al., 2004, 2005). In an acute setting, up to 40% of orthopedic trauma patients present with a positive blood alcohol content at the time of hospital admission (Blake et al., 1997; Levy et al., 1996). Furthermore, alcohol consumption significantly raises the risk of healing complications leading to nonunion and increased fracture healing time (Zura et al., 2016). A recent epidemiological study of over 300,000 primary fracture patients found that alcohol use increased the risk of nonunion by 67% (Zura et al., 2016). There is also a substantial amount of basic science evidence that shows both acute and chronic alcohol administration have deleterious effects on bone health and proper fracture healing (Bikle et al., 1985; Callaci et al., 2009; Elmali et al., 2002; Janicke-Lorenz and Lorenz, 1984; Lauing et al., 2008; Volkmer et al., 2011).
The underlying factors contributing to nonunion are not well understood, and the mechanisms behind alcohol-induced deficient fracture healing remain largely unknown. However, it is known that mesenchymal stem cells (MSCs) are indispensable to both bone health and proper fracture healing, while ambiguity remains about the ability to truly identify a single-cell population as MSCs. For the purposes of this paper, we use MSCs to refer to cells residing within the bone marrow and the periosteum with the ability to differentiate into a variety of cell types including osteoblasts, chondrocytes, and adipocytes (Caplan, 2017; Chang and Knothe Tate, 2012; Colnot et al., 2004; Park et al., 2012). Proper differentiation of MSCs is critical for normal fracture healing (Bielby et al., 2007; Colnot, 2009). During the repair process, MSCs in the periosteum differentiate into chondrocytes that form the majority of the external callus through the deposition of cartilaginous matrix (Colnot, 2009). This matrix is then ossified by osteoblasts, also of MSC origin, in order to heal the fracture (Colnot, 2009; Colnot et al., 2006; Obermeyer et al., 2012; Park et al., 2012). This process is the primary cellular mechanism by which most fractures heal.
There is evidence that alcohol negatively affects the osteogenic potential of MSCs. MSCs isolated from the femoral head of alcohol-induced osteonecrosis patients have a reduced potential for osteogenic differentiation compared to MSCs isolated from nonalcoholic femoral heads (Bielby et al., 2007; Suh et al., 2005). Another study found that MSCs isolated from non–alcohol-abusing patients had suppressed osteogenic differentiation and increased adipogenic differentiation when cultured ex vivo in the presence of ethanol (EtO; Wezeman and Gong, 2004).
While multiple cellular signaling pathways regulate MSC differentiation potential, canonical Wnt signaling has been identified as indispensable for osteochondral commitment, osteoblast differentiation, and chondrocyte hypertrophy, which are all critical processes for proper fracture repair (Chen et al., 2007; Dong et al., 2006; Glass and Karsenty, 2007; Lauing et al., 2012; Ling et al., 2009; Liu et al., 2008). Our laboratory has previously shown that episodic alcohol treatment disrupts canonical Wnt signaling within the fracture callus of rodents (Callaci et al., 2010; Lauing et al., 2012, 2014). We have also shown that episodic alcohol exposure leads to an increase in expression of activated Forkhead box protein O (FoxO) transcription factors within the fracture callus during healing. These changes are associated with an inhibition of endochondral ossification and are prevented by the administration of antioxidant N-acetylcysteine (NAC) during healing (Roper et al., 2016). FoxO transcription factors are activated by oxidative stress and bind to β-catenin as a necessary cofactor in order to promote the expression of specific target genes (Essers et al., 2005; Hoogeboom and Burgering, 2009; Hoogeboom et al., 2008).
In summation, these findings begin to delineate a potential mechanism behind alcohol-induced inhibition of fracture repair. Once FoxO transcription factors are activated by oxidative stress, they directly inhibit Wnt signaling by diverting β-catenin away from proosteochondrogenic gene transcription (Almeida et al., 2007; Iyer et al., 2013). Therefore, we presently hypothesize that alcohol is able to induce activation of FoxOs in cultured MSCs, and this activation will lead to decreased MSC osteochondrogenic differentiation potential. By showing alcohol-induced activation of FoxOs in MSCs, we stand to further elucidate a mechanism behind alcohol-induced bone loss and deficit fracture healing.
MATERIALS AND METHODS
Rat MSC Isolation and Culture
MSCs were either purchased (Cyagen US Inc., Santa Clara, CA) or isolated from 6- to 7-week-old male Lewis rats using a modified protocol as described previously (Einhorn, 2005; Obermeyer et al., 2012; Soleimani and Nadri, 2009). MSCs were cultured in Dul-becco’s modified Eagle medium (DMEM; Gibco, Thermo Fisher Scientific, Rockford, IL) supplemented with 10% FBS (Gibco, Thermo Fisher Scientific), 1% penicillin–streptomycin (Thermo Fisher Scientific), and 1 g/l glucose, L-glutamine, and sodium pyruvate in a humidified chamber at 5% CO2 and 37°C. MSCs were grown to approximately 80% confluency before administering treatments. Molecular-grade 100% EtOH (Sigma-Aldrich, St Louis, MO) and 30% (w/w) hydrogen peroxide (H2O2; Sigma-Aldrich) were diluted to final concentrations in DMEM. In order to mitigate EtOH loss by evaporation, cell cultures containing EtOH were incubated in a sealed system at standard culture conditions with excess EtOH equal to treatment concentrations added to the chamber water bath.
For MSC Differentiation
MSCs were seeded at 10,000 cells/cm2 and grown to approximately 80% confluency before replacing growth media with either StemPro Osteogenesis Differentiation or StemPro Chondrogenesis Differentiation media (Gibco, Thermo Fisher Scientific).
RNA Isolation and qRT-PCR
TRIzol reagent (Thermo Fisher Scientific) was applied directly to cell monolayer. RNA was isolated and purified using Ambion Ribo-Pure RNA Purification Kit (Thermo Fisher Scientific). RNA was quantified using a NanoDrop ND-2000 spectrophotometer, and RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). cDNA was amplified using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific), and changes in mRNA transcription were determined using TaqMan Fast Advanced Master Mix and TaqMan FAM primer probes specific (Thermo Fisher Scientific) for each target gene. Quantitative real-time PCRs (qRT-PCRs) were carried out using an Applied Biosystems 7500 Fast RT-PCR system. The resulting data were analyzed by the ΔΔCt method compared to endogenous control, β2M. Osteogenic and chondrogenic markers were selected at various stages of differentiation to determine the effect of EtOH on lineage differentiation. Osterix (OSX) and SRY-box transcription factor 9 (SOX9) are important transcription factors for osteogenesis and chondrogenesis, respectively, and were selected for further analysis in FoxO1/3 knockdown and inhibition experiments.
Protein Isolation, Western Blotting, and DNA-Binding Assay
MSCs were harvested using 0.25% trypsin-EDTA and pelleted by centrifugation at 500×g for 5 minutes. Cell pellets were either lysed in radioimmunoprecipitation assay buffer (Thermo Fisher Scientific) for total protein or processed using NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Thermo Fisher Scientific) to isolate proteins localized within nuclear and cytoplasmic fractions. Protein concentrations were measured by BCA assay (Thermo Fisher Scientific). For Western blotting, equal amounts of protein were loaded into 4 to 20% SDS-PAGE gel, electrotransferred to a PVDF membrane, and probed with either a Rb pAB FoxO3a antibody (Abcam, Cambridge, MA), total Rb pAb FoxO1 antibody (Abcam), or Rb pAb PGK1 antibody (Abcam). Coomassie Blue was used to stain total protein on PVDF membranes (Blot Bands) for protein loading normalization. Densitometric analysis was carried out utilizing Image Lab software (Bio-Rad, Hercules, CA), and Western blot data were presented as the densitometric ratio of target protein: loading protein bands. For FoxO1 DNA-binding assays, nuclear fractions from MSCs were assayed for FoxO-specific DNA element binding using TransAM FKHR (FOXO1) Transcription Factor Activation Assay (Active Motif, Carlsbad, CA).
FoxO1/3 Knockdown
Nontargeting, FoxO1, and FoxO3a ON-TARGETplus siRNA was obtained from Dharmacon (Lafayette, CO). MSCs were transfected using Lipofectamine RNAiMAX siRNA Transfection Reagent (Thermo Fisher Scientific) and incubated for 24 hours before splitting into treatment groups. The following day, growth media were replaced with osteogenic or chondrogenic differentiation media with and without 50 mM EtOH. mRNA was isolated as described above at 24 and 48 hours to assess knockdown and expression of OSX and SOX9 transcription factors.
FoxO Pharmacological Inhibition
FoxO1/3-specific inhibitor AS1842856 (Millipore Sigma, St Louis, MO) was reconstituted in DMSO to a superstock concentration of 27 mM and stored at −20°C. AS1842856 was diluted to final concentrations of 0.1 and 1 μM in differentiation media with and without EtOH. MSCs were treated with the above at approximately 80% confluency. mRNA was isolated at 48 and 72 hours later as outlined above to assess the expression of OSX and SOX9 transcription factors.
Statistical Analysis
All data are expressed as the mean ± standard error of the mean of 3 or more separate experiments per group. Due to large interexperimental variation in expression of early osteogenic and chondrogenic transcription factors, differentiation controls were assigned a value of 1 for each experiment and fold changes were determined for the remaining treatment groups in Figs 4 and 5. Experiments were repeated at least 4 independent times. Data were analyzed using GraphPad Prism 7. p values were determined by the statistical tests defined in the figure legends, with post hoc analysis where appropriate.
Fig. 4.
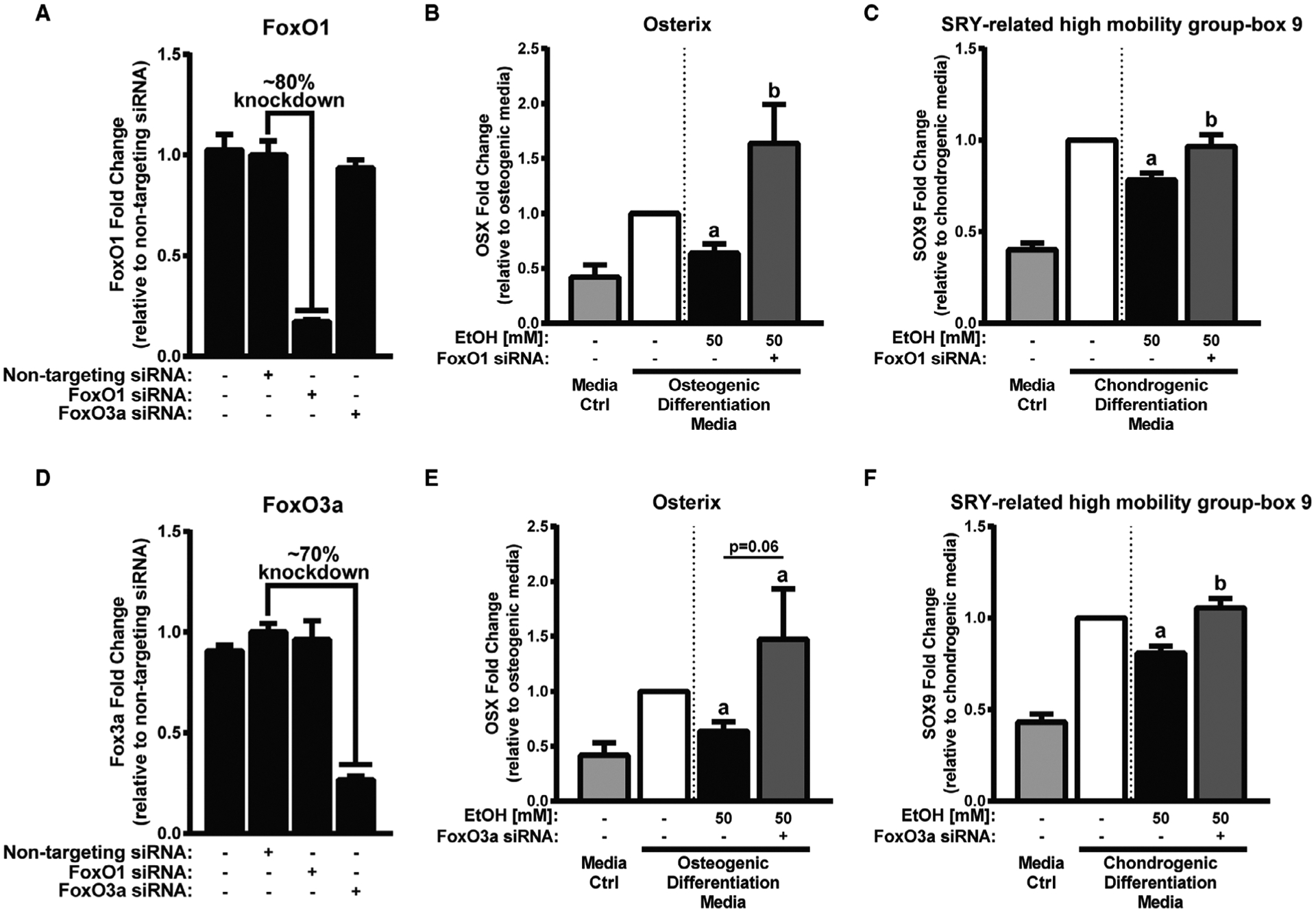
FoxO1 and FoxO3a knockdown partially restores osteogenic transcription factor OSX and chondrogenic transcription factor SOX9 expression. A, Relative FoxO1 mRNA expression in mesenchymal stem cells (MSCs) following nontargeting, FoxO1, or FoxO3a siRNA knockdown. B, Relative osterix (OSX) mRNA expression in differentiating MSCs treated with 50 mM EtOH or media alone for 24 hours. C, Relative SRY-related high-mobility group-box 9 (SOX9) mRNA expression in differentiating MSCs treated with 50 mM EtOH or media alone for 48 hours. D, Relative FoxO3a mRNA expression in MSCs following nontargeting, FoxO1, or FoxO3a siRNA knockdown. E, Relative OSX mRNA expression in differentiating MSCs treated with 50 mM EtOH or media alone for 24 hours. F, Relative SOX9 mRNA expression in differentiating MSCs treated with 50 mM EtOH or media alone for 48 hours. Media control and differentiation control (first and second columns in panels B, C, E, and F) are shown to confirm differentiation marker expression and are not for statistical purposes. Different letters demonstrate significance, n = 4, p < 0.05,1-tailed Student’s t-test.
Fig. 5.
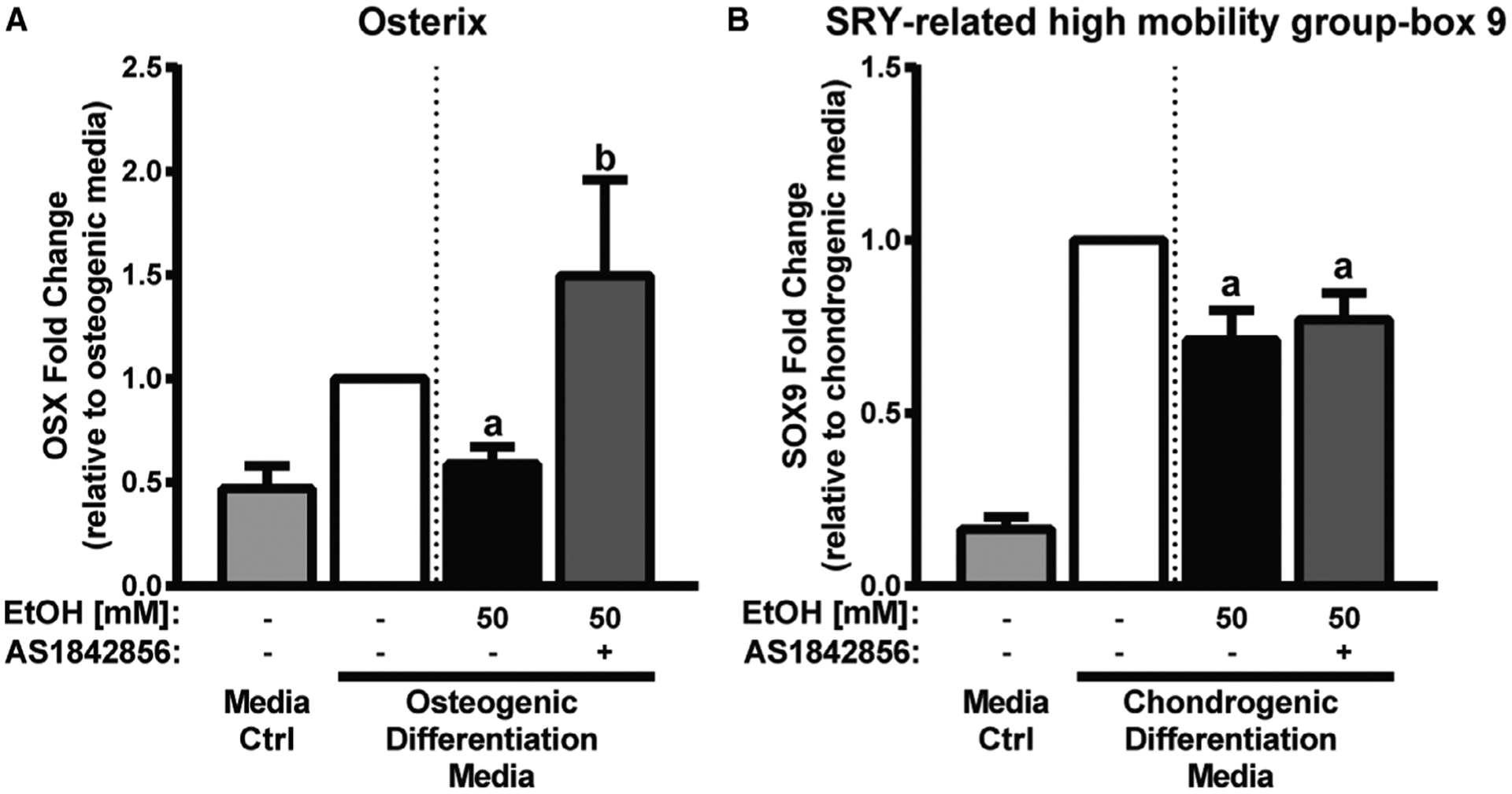
FoxO1/3 inhibition partially restores osteogenic transcription factor OSX, but does not restore chondrogenic transcription factor SOX9. A, Relative osterix (OSX) mRNA expression in differentiating mesenchymal stem cells (MSCs) treated with 50 mM EtOH or media alone for 24 hours. B, Relative SRY-related high-mobility group-box 9 (SOX9) mRNA expression in differentiating MSCs treated with 50 mM EtOH or media alone for 48 hours. Media control and differentiation control (first and second columns in panels A and B) are shown to confirm differentiation marker expression and are not for statistical purposes. Different letters demonstrate significance, n = 4 to 5, p < 0.05,1-tailed Student’s t-test.
RESULTS
Effect of EtOH Exposure on FoxO Expression and Activity in MSCs
First, we examined whether EtOH exposure was associated with elevated levels of FoxO1/3 mRNA and protein expression. mRNA expression was assessed at 3, 6, and 9 hours using qRT-PCR. At 3 hours, FoxO3a mRNA expression was significantly increased in MSCs exposed to 50 mM EtOH when compared to MSCs cultured in media alone and remained elevated throughout the course of treatment (Fig. 1A). Next, we assessed by Western blot whether treating MSCs with EtOH would alter FoxO3a protein expression. In agreement with our qRT-PCR findings, MSCs cultured with 50 mM EtOH had increased FoxO3a protein expression that peaked 6 hours post-EtOH treatment as compared to MSCs cultured in media alone (Fig. 1B). MSCs exposed to a positive control, H2O2, had increased levels of FoxO3a protein expression at 3 hours that was not observed at later time points. H2O2-mediated FoxO3a protein expression was not sustained over the course of treatment, whereas EtOH elicited a longer time period of elevated FoxO3a protein expression.
Fig. 1.
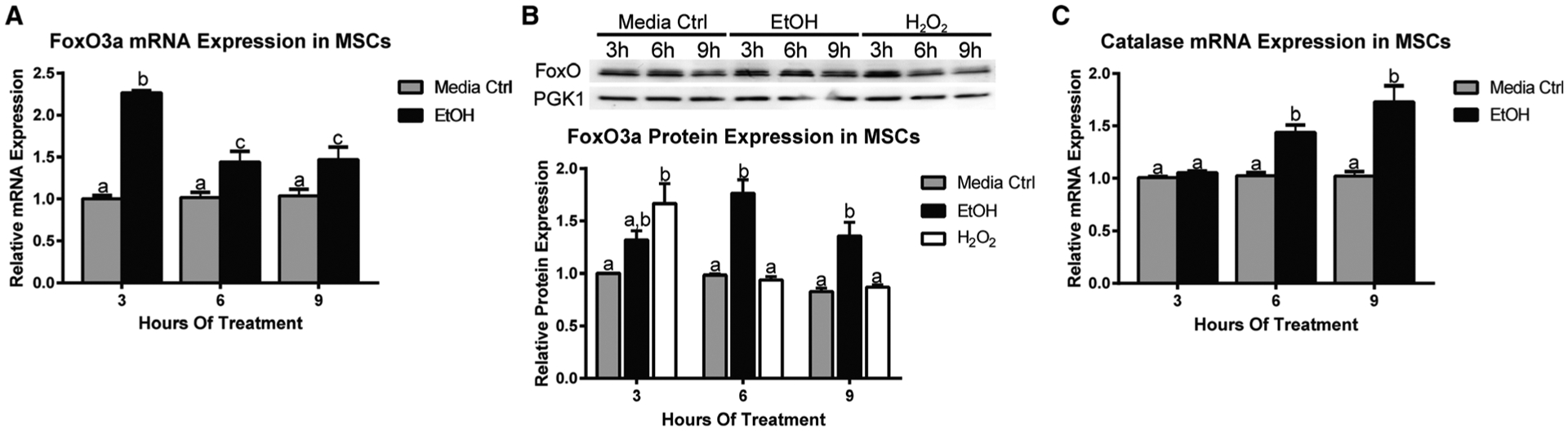
EtOH increases FoxO expression and downstream target catalase expression in MSCs. A, Relative FoxO3a mRNA expression in mesenchymal stem cells (MSCs) treated with 50 mM EtOH for 3, 6, and 9 hours. B, Western blot analysis of FoxO3a protein expression in MSCs treated with 50 mM EtOH at the same time points. Densitometry analysis is below Western blot. Hydrogen peroxide (H2O2) was used as a positive control. C, Relative catalase mRNA expression in MSCs treated with 50 mM EtOH for 3, 6, and 9 hours. Different letters demonstrate significance, n = 3, p < 0.05, 2-way ANOVA with Tukey’s post hoc test.
FoxO-Specific Target Gene Expression
To test whether EtOH activated a FoxO-specific antioxidant response in MSCs, we evaluated mRNA expression of FoxO-specific downstream target gene catalase at 3, 6, and 9 hours after EtOH exposure. 50 mM EtOH increased catalase mRNA expression when compared to media alone, reaching the highest level of expression at 9 hours posttreatment(Fig. 1C).
Effect of EtOH Exposure on FoxO Translocation and Activation
Nuclear Localization.
To determine whether EtOH exposure increased translocation and accumulation of FoxO proteins, we performed cytosolic and nuclear fractionation following 24 hours of treatment with EtOH, H2O2, or media alone. We observed a significant twofold and fourfold increase in FoxO1 nuclear translation in MSCs treated with 50 and 100 mM EtOH (Fig. 2A), respectively, when compared to media alone. Interestingly, we did not observe alterations in FoxO1 mRNA and protein expression at earlier 3-, 6-, and 9-hour time points (data not shown). MSCs treated with H2O2 had similar measured levels of FoxO1 nuclear translocation and accumulation as 100 mM EtOH.
Fig. 2.
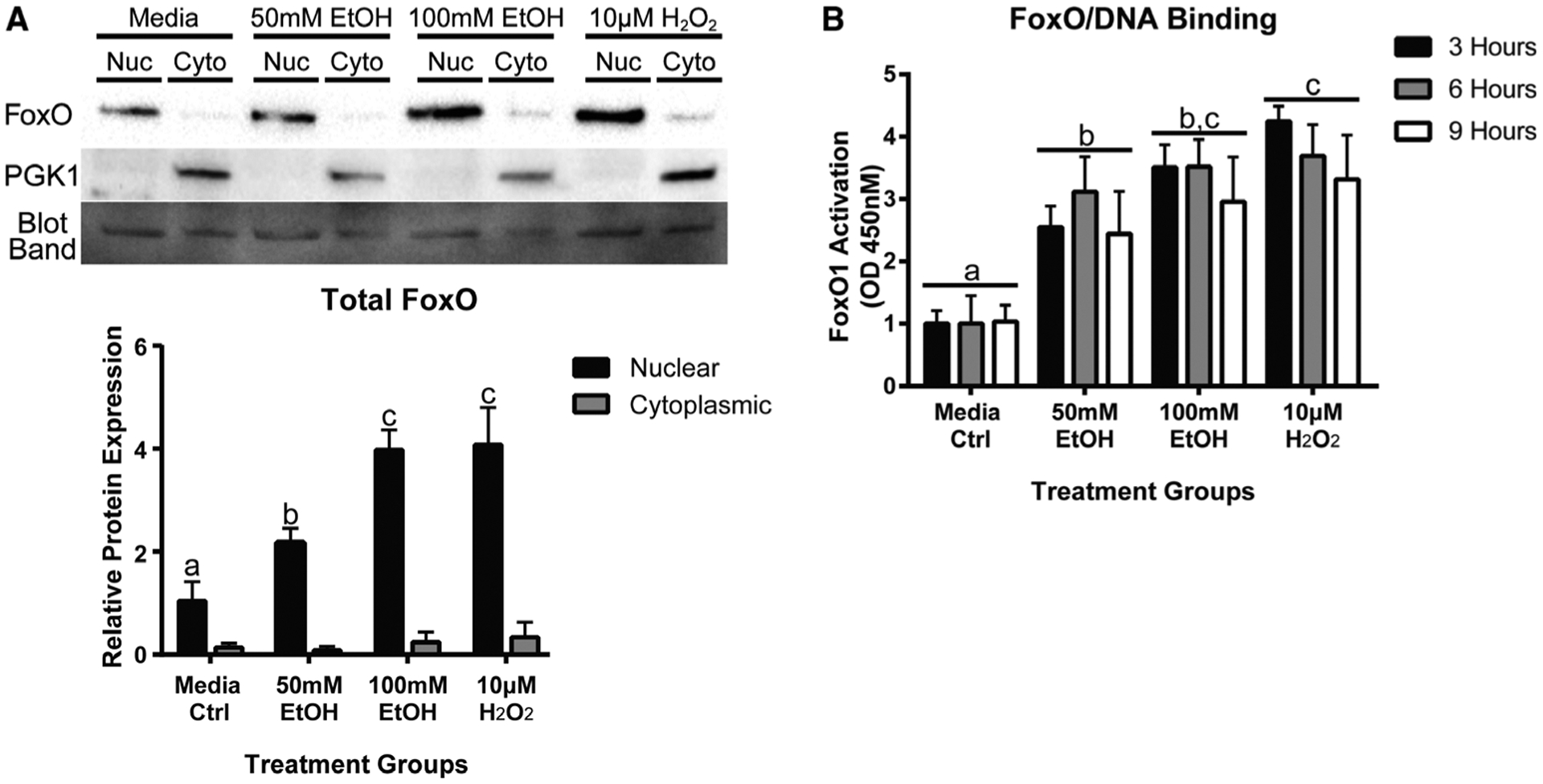
EtOH increases FoxO1 nuclear translocation and FoxO1 activation. A, Western blot analysis of FoxO1 in cytosol and nuclear fractions from mesenchymal stem cells (MSCs) treated with EtOH, hydrogen peroxide (H2O2), or media alone for 24 hours. Hydrogen peroxide (H2O2) was used as a positive control. Densitometry analysis is below Western blot. B, Quantification of FoxO1 activation in MSCs treated with EtOH, H2O2, or media alone for 3, 6, and 9 hours. FoxO1 activation was measured by nuclear FoxO1 binding to FoxO-specific DNA elements by enzyme-linked immunosorbent assay (ELISA). Different letters demonstrate significance, n = 3, p < 0.05, 2-way ANOVA with Tukey’s post hoc test.
FoxO1 Activation.
To determine whether EtOH exposure led to FoxO1 activation, we measured nuclear FoxO1 binding to a FoxO1-specific DNA element. MSCs were exposed to either 50 mM EtOH, 100 mM EtOH, 10 μM H2O2, or media alone for 3, 6, and 9 hours. We found that MSCs exposed to both 50 and 100 mM EtOH had significant increases in nuclear FoxO1 binding to a FoxO1-specific DNA element when compared to media alone (Fig. 2B). MSCs treated with H2O2 exhibited similar levels of activated FoxO1 to MSCs treated with 100 mM EtOH.
Effect of EtOH Exposure on Osteochondral Differentiation Potential
Next, we examined whether EtOH exposure had an effect on the differentiation potential of primary cultured rat MSCs. MSCs were grown in osteogenic or chondrogenic differentiation media with and without 50 mM EtOH. Alterations in osteochondrogenic lineage differentiation were measured by qRT-PCR after 48 and 72 hours of EtOH exposure. For MSC osteogenic differentiation, we found that the expression of runt-related transcription factor 2 (RUNX2) and alkaline phosphatase (Alp1) was significantly reduced in MSCs cultured in osteogenic media and EtOH when compared to MSCs cultured in osteogenic media alone (Fig. 3A,B). For MSC chondrogenic differentiation, we found that chondrogenic media with EtOH caused a significant reduction in SOX9, collagen 2 (COL2a1), and aggrecan (ACAN) expression when compared to MSCs cultured in chondrogenic media alone (Fig. 3C–E).
Fig. 3.
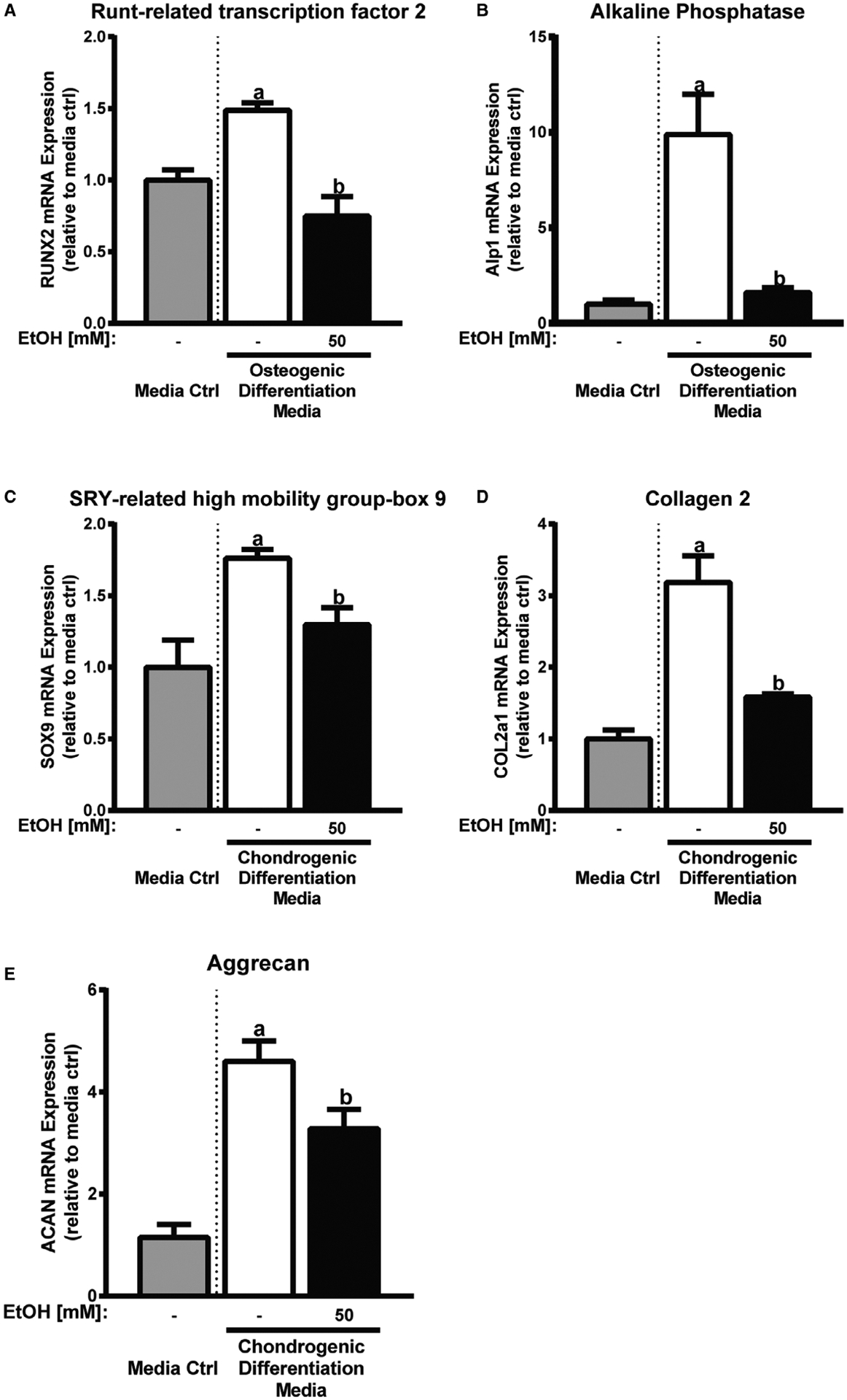
EtOH inhibits osteogenic and chondrogenic differentiation lineage markers. Relative osteogenic lineage marker RUNX2 (A) and Alp1 (B) mRNA expression in differentiating mesenchymal stem cells (MSCs) treated with 50 mM EtOH or media alone for 48 and 72 hours, respectively. Relative chondrogenic lineage marker SOX9 (C), COL2a1 (D), and aCaN (E) mRNA expression in differentiating MSCs treated with 50 mM EtOH or media alone for 48, 72, and 72 hours, respectively. Media control is shown to confirm differentiation marker expression and is not for statistical purposes. Different letters demonstrate significance, n = 3 to 5, p < 0.05, 2-tailed Student’s t-test.
Effect of FoxO1/3 Knockdown and Inhibition on MSC Differentiation Potential of MSCs Treated With EtOH
To determine whether osteochondrogenic differentiation markers suppressed with EtOH were mediated by EtOH-in-duced FoxO1/3 activation, we used a dual genetic and pharmacological approach to suppress FoxO expression and activation. The genetic approach was implemented through siRNA knockdown of FoxO1 or FoxO3a. With FoxO1 knockdown, we observed a ~80% reduction in FoxO1 expression in MSCs when compared to nontargeting siRNA (Fig. 4A). In FoxO3a knockdown experiments, we observed a ~70% reduction in FoxO3a expression in MSCs when compared to nontargeting siRNA (Fig. 4D). Forty-eight hours after knockdown, MSCs were differentiated toward osteogenic or chondrogenic lineages in the presence of 50 mM EtOH and assayed for osteochondrogenic marker expression changes 24 and 48 hours later. For MSC osteogenic differentiation, we found OSX expression was restored in MSCs with FoxO1 knockdown (Fig. 4B) and only partially restored in MSCs with FoxO3a knockdown (p = 0.062, Fig. 4E) in the presence of EtOH when compared to MSCs treated with EtOH alone. For MSC chondrogenic differentiation, we found SOX9 expression was restored in MSCs with FoxO1 (Fig. 4E) and FoxO3a (Fig. 4F) knockdown in the presence of EtOH when compared to MSCs treated with EtOH alone.
Next, we examined whether pharmacological inhibition of FoxO1/3 activation could overcome the inhibitory effect of EtOH on differentiating MSCs. To this end, MSCs were differentiated toward osteogenic and chondrogenic lineages and cultured with FoxO1/3 inhibitor AS1842856 with and without EtOH. For MSC osteogenic differentiation, we found 1 μM AS1842856 restored OSX expression in MSCs treated with EtOH compared to EtOH alone (Fig. 5A). For MSC chondrogenic differentiation, we found 1 μM AS1842856 inhibited SOX9 expression (data not shown). A lower concentration of 0.1 μM AS1842856 permitted normal SOX9 expression, but did not restore SOX9 expression in MSCs treated with EtOH compared to EtOH alone (Fig. 5B).
DISCUSSION
Alcohol abuse is a known risk factor for bone loss and bone fracture nonunion (Bikle et al., 1985; Chakkalakal, 2005; Zura et al., 2016). Our laboratory previously demonstrated that cartilaginous fracture callus formation is inhibited in rodents treated acutely with alcohol prior to tibia fracture, and this inhibition was mitigated by administration of antioxidant NAC, suggesting that oxidative stress generated from alcohol administration inhibits normal fracture callus formation (Volkmer et al., 2011). More recently, our laboratory has shown that fracture callus tissue from alcohol-treated rodents had increased protein expression of the active form of FoxO1 (Roper et al., 2016). FoxO is a group of transcription factors that are involved in many cellular responses. FoxOs, primarily FoxO1, FoxO3a, and FoxO4 family members, function as molecular responders to intracellular oxidative stress (Iyer et al., 2013; Tothova et al., 2007). In the process of their activation, FoxOs undergo posttranslational modifications that include phosphorylation and acetylation, leading to their nuclear translocation and binding of FoxO-specific DNA elements (Hedrick et al., 2012). Furthermore, activated FoxOs bind to β-catenin as a mandatory cofactor in their response to oxidative stress (Almeida et al., 2007; Essers et al., 2005; Iyer et al., 2013). β-catenin is a multifunctional protein that is crucial for signal transduction in the canonical Wnt signaling pathway, which is necessary for the initiation of MSC osteochondral lineage differentiation (Ling et al., 2009; Liu et al., 2008). Studies have demonstrated that in times of increased oxidative stress, the limited pool of cellular β-catenin are sequestered by FoxO and have limited availability for Wnt-specific signal activity (Almeida et al., 2007).
In the present study, we examined the effect of EtOH on FoxO1/3 in primary MSCs, and how EtOH affects MSC osteochondral differentiation as a potential cellular mechanism explaining our fracture callus findings. We found that FoxO3a mRNA and protein expression were both elevated within hours of EtOH administration. At later time points, we found mRNA expression of catalase, FoxO1/3 target gene, and a protective enzyme against oxidative stress (Alcendor et al., 2007; Higuchi et al., 2013) was increased with EtOH administration. Our laboratory has previously observed elevated levels of total and phosphorylated FoxO1 within the fracture callus of alcohol-exposed rodents (Roper et al., 2016). However, in our present study, we did not detect any changes in the expression of FoxO1 mRNA or protein levels in MSCs treated with EtOH. To determine whether EtOH had an effect on FoxO1 posttranslational regulation, we assessed the level of FoxO1 activation through nuclear localization and binding to a FoxO-specific DNA element following EtOH administration. EtOH significantly enhanced nuclear localization of FoxO1, suggesting EtOH was promoting activation of FoxO. We confirmed these findings by showing that nuclear lysate from EtOH-treated MSCs had higher FoxO1 binding to FoxO-specific DNA elements when compared to media controls. These data suggest that EtOH significantly enhances both the expression and the activity of FoxO1/3 transcription factors in primary MSCs.
MSC osteochondral differentiation is an essential step in the formation of the fracture callus, and previous data from our laboratory have demonstrated that alcohol inhibits cartilaginous fracture callus formation in rodents (Lauing et al., 2012, 2014; Roper et al., 2016; Volkmer et al., 2011). To determine whether alcohol inhibits early MSC differentiation, we administered EtOH to primary MSCs to determine the effect of EtOH on MSC osteochondral lineage differentiation. We found that MSCs induced toward osteogenic lineage differentiation in the presence of EtOH had decreased mRNA expression levels of RUNX2, a key transcription factor in osteoblast differentiation, and alkaline phosphatase, an important enzyme involved in osteoblast development and tissue mineralization. These findings suggest alcohol exposure inhibits early osteogenic lineage differentiation. in support of these data, a publication found that MSCs isolated from alcohol-induced femoral head osteonecrosis patients had diminished osteogenic lineage differentiation potential when cultured ex vivo (Suh et al., 2005).
When examining the effect of EtOH on MSC chondrogenic differentiation, we found SOX9, an essential transcription factor for the initiation of chondrogenic differentiation, showed diminished mRNA expression when MSCs were differentiated in the presence of EtOH. Similarly, COL2a1, a fibrillar collagen found in cartilage matrix and marker of early chondrocyte lineage commitment, and ACAN, an integral chondrogenic extracellular matrix proteoglycan, also had diminished mRNA expression in the presence of EtOH. These findings suggest EtOH may inhibit early MSC chondrogenic lineage differentiation. One study supporting this finding found that chondrocytes isolated from alcohol-induced femoral head necrosis patients had reduced SOX9 gene expression and when cultured ex vivo produced less COL2a1 when compared to healthy patient chondrocytes (Qin et al., 2018). Taken together, these findings show EtOH inhibits MSC osteochondral lineage differentiation potential, which provides evidence of one potential cellular mechanism by which alcohol impairs fracture healing.
To determine whether alcohol-induced FoxO1/3 activity was connected to the inhibition of MSC differentiation, we performed experiments to determine whether genetic and pharmacological inhibition of FoxO1/3 could restore proosteochondrogenic transcription factors in MSCs exposed to EtOH. We found that knockdown of both FoxO1 and FoxO3a increased the expression of OSX and SOX9 in the presence of EtOH. In support of our findings, 2 recent studies showed that chondrocyte-specific knockout of FoxO1 rescued fracture callus formation in diabetic rodents, improving the biomechanical strength of the callus (Alharbi et al., 2018; Lu et al., 2019). Another study found that FoxO1, FoxO3a, and FoxO4 knockout in osteoblast progenitors could attenuate the reduction of trabecular number and cortical thickness of long bone in type 1 diabetes rodents (Iyer et al., 2017). Combined with our data, these reports suggest that an inhibition of FoxO signaling in an environment of up-regulated reactive oxygen species (ROS) may restore normal MSC differentiation.
In a complementary approach, we utilized FoxO1 inhibitor AS1842856, which has been used for diabetes and adipogenesis studies (Yu et al., 2018; Zou et al., 2014). AS1842856 has been shown to directly bind and inhibit the active form of FoxO1 (He et al., 2019; Yu et al., 2018). In one study, AS1842856-mediated inhibition of FoxO showed a dose-dependent improvement on fasting-glucose levels in diabetic rodents (Nagashima et al., 2010). Moreover, FoxO1/3 inhibition by AS1842856 significantly reduced adipogenesis and lipid formation through the inhibition of mitochondrial-related proteins (Zou et al., 2014). We found that EtOH-exposed MSCs showed an significant increase in OSX expression when treated with AS1842856. The concentration of AS1842856 used in these experiments, 1 μM, has been shown to also inhibit FoxO3a activity and to a lesser degree FoxO4 (Nagashima et al., 2010). This finding suggests that FoxO1/3 inhibition can restore OSX expression in the presence of EtOH. One mechanistic explanation for these findings could be that knockdown and inhibition of FoxO1/3 activity prevents FoxO1/3 from sequestering β-catenin from the canonical Wnt signaling pathway.
In our experiments investigating whether FoxO1/3 inhibitor AS1842856 would restore SOX9 levels in the presence of EtOH, we found 1 μM AS1842856 completely inhibited chondrogenic differentiation. Indeed, a recent study showed that FoxO1 is required for SOX9 expression (Kurakazu et al., 2019). We found that a 10-fold decrease in AS1842856 permitted chondrogenic differentiation, but did not fully restore SOX9 expression in EtOH-exposed MSCs. One possible explanation could be that the FoxO1/3 inhibitor may have off-target effects on other important pathways involved in chondrogenic differentiation such as Sirt1 (He et al., 2019), which has been shown to modulate β-catenin stability. More likely, FoxO1/3 activity may be required, in at least at some basal level of activity, for osteogenic and chondrogenic differentiation. Indeed, several studies show that FoxO1/3, as well as endogenous ROS, is required for both osteogenic and chondrogenic differentiation and normal bone turnover (Alund et al., 2016; Kurakazu et al., 2019; Teixeira et al., 2010). Presently, our findings suggest the overexpression of FoxO1/3 is responsible for the inhibition of osteogenesis and chondrogenesis in EtOH-exposed MSCs. The goal of the present study was to normalize, not eliminate, FoxO1/3 activity following EtOH exposure. This hypothesis may explain why we did not rescue chondrogenesis in our FoxO1/3 pharmacological inhibition experiments as the concentration of FoxO1/3 inhibitor used was more than double the IC50 concentration. This observation comes when comparing the pharmacological experiments to our genetic experiments, where partial knockdown of FoxO1/3 rescues MSC osteochondral lineage differentiation in the presence of EtOH. Further studies are necessary to determine whether lower concentrations of FoxO1/3 inhibitor may offer full restoration of SOX9 in the presence of EtOH.
In the present study, we described a direct connection between EtOH exposure and FoxO1/3 activation in MSCs, and EtOH exposure inhibited MSC differentiation. However, several other pathways have been shown to be important in MSC differentiation and EtOH-induced inhibition of fracture healing, suggesting FoxO1/3 activation and impaired MSC differentiation is only one part of the molecular mechanism behind inhibited fracture healing. For instance, in a model of alcohol-induced osteopenia, alcohol increased mTOR signaling in MSCs and inhibited MSC lineage differentiation, which was ameliorated by rapamycin treatment (Liu et al., 2016). Upstream of mTOR, alcohol has been shown to impair Akt phosphorylation and recruitment to the plasma membrane through PTEN up-regulation, thereby inhibiting Akt/GSK3β/β-catenin signaling and expression of osteogenic differentiation (Chen et al., 2019). To this end, a novel activator of Akt has been shown to restore osteogenic marker expression in EtOH-treated MSCs and prevents alcohol-induced osteonecrosis in rodents (Chen et al., 2017). Alcohol has also been shown to activate TNF-α signaling within the bone of rodents fed EtOH, leading to decreased bone formation and inhibited fracture healing (Perrien et al., 2004). Administering a TNF antagonist protected against the effects of EtOH feeding (Perrien et al., 2004). These findings were further supported in rodents with a TNF receptor knockout, which were also protected against the effects of EtOH feeding on bone formation and fracture healing (Wahl et al., 2012).
In summary, this study provides further evidence that EtOH exposure in primary MSCs up-regulates and activates FoxO1/3 transcription factors. This activation led to detrimental effects on MSC differentiation potential, suggesting a possible mechanism underlying poor fracture healing in alcohol-drinking patients. Additional studies are required to determine whether modulation of FoxO1/3 could be tested as an adjuvant to surgical interventions for better fracture union outcome in alcohol-drinking patients as well as patients suffering from diabetes.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R21AA025551 to JJC, and F31AA028147 and T32AA013257 to JME, and the National Institute of General Medical Sciences under award number T32GM008750 to FS.
Footnotes
CONFLICT OFINTEREST
The authors report no conflict of interest.
REFERENCES
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J (2007) Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100:1512–1521. [DOI] [PubMed] [Google Scholar]
- Alharbi MA, Zhang C, Lu C, Milovanova TN, Yi L, Ryu JD, Jiao H, Dong G, O’Connor JP, Graves DT (2018) FOXO1 deletion reverses the effect of diabetic-induced impaired fracture healing. Diabetes 67:2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC (2007) Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 282:27298–305. [DOI] [PubMed] [Google Scholar]
- Alund AW, Mercer KE, Suva LJ, Pulliam CF, Chen JR, Badger TM, van Remmen H, Ronis MJ (2016) Reactive oxygen species differentially regulate bone turnover in an age-specific manner in catalase transgenic female mice. J Pharmacol Exp Ther 358:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielby R, Jones E, McGonagle D (2007) The role of mesenchymal stem cells in maintenance and repair of bone. Injury 38(Suppl. 1):S26–S32. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Genant HK, Cann C, Recker RR, Halloran BP, Strewler GJ (1985) Bone disease in alcohol abuse. Ann Intern Med 103:42–48. [DOI] [PubMed] [Google Scholar]
- Blake RB, Brinker MR, Ursic CM, Clark JM, Cox DD (1997) Alcohol and drug use in adult patients with musculoskeletal injuries. Am J Orthop (Belle Mead NJ) 26:704–709; discussion 709–710. [PubMed] [Google Scholar]
- Callaci JJ, Himes R, Lauing K, Roper P (2010) Long-term modulations in the vertebral transcriptome of adolescent-stage rats exposed to binge alcohol. Alcohol Alcohol 45:332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaci JJ, Himes R, Lauing K, Wezeman FH, Brownson K (2009) Binge alcohol-induced bone damage is accompanied by differential expression of bone remodeling-related genes in rat vertebral bone. Calcif Tissue Int 84:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI (2017) Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal DA (2005) Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res 29:2077–2090. [DOI] [PubMed] [Google Scholar]
- Chang H, Knothe Tate ML (2012) Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med 1:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YX, Tao SC, Xu ZL, Yin WJ, Zhang YL, Yin JH, Gao YS, Zhang CQ (2017) Novel Akt activator SC-79 is a potential treatment for alcohol-induced osteonecrosis of the femoral head. Oncotarget 8:31065–31078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA (2007) Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med 4:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YX, Zhu DY, Gao J, Xu ZL, Tao SC, Yin WJ, Zhang YL, Gao YS, Zhang CQ (2019) Diminished membrane recruitment of Akt is instrumental in alcohol-associated osteopenia via the PTEN/Akt/GSK-3beta/beta-catenin axis. Febs J 286:1101–1119. [DOI] [PubMed] [Google Scholar]
- Colnot C (2009) Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 24:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, Huang S, Helms J (2006) Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun 350:557–561. [DOI] [PubMed] [Google Scholar]
- Colnot C, Lu C, Hu D, Helms JA (2004) Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol 269:55–69. [DOI] [PubMed] [Google Scholar]
- Dong YF, Soung Do Y, Schwarz EM, O’Keefe RJ, Drissi H (2006) Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol 208:77–86. [DOI] [PubMed] [Google Scholar]
- Einhorn TA (2005) The science of fracture healing. J Orthop Trauma 19:S4–S6. [DOI] [PubMed] [Google Scholar]
- Elmali N, Ertem K, Ozen S, Inan M, Baysal T, Guner G, Bora A (2002) Fracture healing and bone mass in rats fed on liquid diet containing ethanol. Alcohol Clin Exp Res 26:509–513. [PubMed] [Google Scholar]
- Essers MA, De Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC (2005) Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 308:1181–1184. [DOI] [PubMed] [Google Scholar]
- Glass DA II, Karsenty G (2007) In vivo analysis of Wnt signaling in bone. Endocrinology 148:2630–2634. [DOI] [PubMed] [Google Scholar]
- Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, Simpson H, Alt V (2014) Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury 45(Suppl. 2), S3–S7. [DOI] [PubMed] [Google Scholar]
- He J, Zhang A, Song Z, Guo S, Chen Y, Liu Z, Zhang J, Xu X, Liu J, Chu L (2019) The resistant effect of SIRT1 in oxidative stress-induced senescence of rat nucleus pulposus cell is regulated by Akt-FoxO1 pathway. Biosci Rep 39:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick SM, Hess-Michelini R, Doedens AL, Goldrath AW, Stone EL (2012) FOXO transcription factors throughout T cell biology. Nat Rev Immunol 12:649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, Chan EC, Liu GS (2013) Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev 22:878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeboom D, Burgering BM (2009) Should I stay or should I go: beta-catenin decides under stress. Biochim Biophys Acta 1796:63–74. [DOI] [PubMed] [Google Scholar]
- Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM (2008) Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem 283:9224–9230. [DOI] [PubMed] [Google Scholar]
- Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, de Cabo R, Jilka RL, Weinstein RS, O’Brien CA, Manolagas SC, Almeida M (2013) FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest 123:3409–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Han L, Ambrogini E, Yavropoulou M, Fowlkes J, Manolagas SC, Almeida M (2017) Deletion of Fox O1, 3, and 4 in osteoblast progenitors attenuates the loss of cancellous bone mass in a mouse model of type 1 diabetes. J Bone Miner Res 32:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke-Lorenz J, Lorenz R (1984) Alcoholism and fracture healing. A radiological study in the rat. Arch Orthop Trauma Surg 103:286–289. [DOI] [PubMed] [Google Scholar]
- Kurakazu I, Akasaki Y, Hayashida M, Tsushima H, Goto N, Sueishi T, Toya M, Kuwahara M, Okazaki K, Duffy T, Lotz MK, Nakashima Y (2019) FOXO1 transcription factor regulates chondrogenic differentiation through transforming growth factor beta1 signaling. J Biol Chem 294:17555–17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ (2008) Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol 42:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauing KL, Roper PM, Nauer RK, Callaci JJ (2012) Acute alcohol exposure impairs fracture healing and deregulates beta-catenin signaling in the fracture callus. Alcohol Clin Exp Res 36:2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauing KL, Sundaramurthy S, Nauer RK, Callaci JJ (2014) Exogenous activation of Wnt/beta-catenin signaling attenuates binge alcohol-induced deficient bone fracture healing. Alcohol Alcohol 49:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RS, Hebert CK, Munn BG, Barrack RL (1996) Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma 10:21–27. [DOI] [PubMed] [Google Scholar]
- Ling L, Nurcombe V, Cool SM (2009) Wnt signaling controls the fate of mesenchymal stem cells. Gene 433:1–7. [DOI] [PubMed] [Google Scholar]
- Liu F, Kohlmeier S, Wang CY (2008) Wnt signaling and skeletal development. Cell Signal 20:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kou X, Chen C, Yu W, Su Y, Kim Y, Shi S, Liu Y (2016) Chronic high dose alcohol induces osteopenia via activation of mTOR signaling in bone marrow mesenchymal stem cells. Stem Cells 34:2157–2168. [DOI] [PubMed] [Google Scholar]
- Lu Y, Alharbi M, Zhang C, O’Connor JP, Graves DT (2019) Deletion of FOXO1 in chondrocytes rescues the effect of diabetes on mechanical strength in fracture healing. Bone 123:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima T, Shigematsu N, Maruki R, Urano Y, Tanaka H, Shimaya A, Shimokawa T, Shibasaki M (2010) Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Mol Pharmacol 78:961–970. [DOI] [PubMed] [Google Scholar]
- Nyquist F, Berglund M, Nilsson BE, Obrant KJ (1997) Nature and healing of tibial shaft fractures in alcohol abusers. Alcohol Alcohol 32:91–95. [DOI] [PubMed] [Google Scholar]
- Obermeyer TS, Yonick D, Lauing K, Stock SR, Nauer R, Strotman P, Shankar R, Gamelli R, Stover M, Callaci JJ (2012) Mesenchymal stem cells facilitate fracture repair in an alcohol-induced impaired healing model. J Orthop Trauma 26:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT (2012) Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10:259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrien DS, Wahl EC, Hogue WR, Feige U, Aronson J, Ronis MJ, Badger TM,Lumpkin CK Jr (2004) IL-1 and TNF antagonists prevent inhibition of fracture healing by ethanol in rats. Toxicol Sci 82:656–660. [DOI] [PubMed] [Google Scholar]
- Qin X, Jin P, Jiang T, Li M, Tan J, Wu H, Zheng L, Zhao J (2018) A human chondrocyte-derived in vitro model of alcohol-induced and steroid-induced femoral head necrosis. Med Sci Monit 24:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper PM, Abbasnia P, Vuchkovska A, Natoli RM, Callaci JJ (2016) Alcohol-related deficient fracture healing is associated with activation of FoxO transcription factors in mice. J Orthop Res 34:2106–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savola O, Niemela O, Hillbom M (2004) Blood alcohol is the best indicator of hazardous alcohol drinking in young adults and working-age patients with trauma. Alcohol Alcohol 39:340–345. [DOI] [PubMed] [Google Scholar]
- Savola O, Niemela O, Hillbom M (2005) Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol Alcohol 40:269–273. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Nadri S (2009) A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 4:102–106. [DOI] [PubMed] [Google Scholar]
- Suh KT, Kim SW, Roh HL, Youn MS, Jung JS (2005) Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res 431:220–225. [DOI] [PubMed] [Google Scholar]
- Teixeira CC, Liu Y, Thant LM, Pang J, Palmer G, Alikhani M (2010) Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J Biol Chem 285:31055–31065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, Depinho RA, Gilliland DG (2007) FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128:325–339. [DOI] [PubMed] [Google Scholar]
- Tzioupis C, Giannoudis PV (2007) Prevalence of long-bone non-unions. Injury 38(Suppl. 2):S3–S9. [DOI] [PubMed] [Google Scholar]
- Volkmer DL, Sears B, Lauing KL, Nauer RK, Roper PM, Yong S, Stover M, Callaci JJ (2011) Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. J Orthop Trauma 25:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl EC, Aronson J, Liu L, Skinner RA, Ronis MJ, Lumpkin CK JR. (2012) Distraction osteogenesis in TNF receptor 1 deficient mice is protected from chronic ethanol exposure. Alcohol 46:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezeman FH, Gong Z (2004) Adipogenic effect of alcohol on human bone marrow-derived mesenchymal stem cells. Alcohol Clin Exp Res 28:1091–1101. [DOI] [PubMed] [Google Scholar]
- Yu F, Wei R, Yang J, Liu J, Yang K, Wang H, Mu Y, Hong T (2018) FoxO1 inhibition promotes differentiation of human embryonic stem cells into insulin producing cells. Exp Cell Res 362:227–234. [DOI] [PubMed] [Google Scholar]
- Zimmermann G, Moghaddam A(2010) Trauma: Non-union: New trends, in European Instructional Lectures: Volume 10, 2010; 11th EFORT Congress, Madrid, Spain (Bentley G ed). Springer, Berlin, Heidelberg. [Google Scholar]
- Zou P, Liu L, Zheng L, Liu L, Stoneman RE, Cho A, Emery A, Gilbert ER, Cheng Z (2014) Targeting FoxO1 with AS1842856 suppresses adipogenesis. Cell Cycle 13:3759–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della-Rocca GJ, Mehta S, Mckinley T, Wang Z, Steen RGs (2016) Epidemiology of Fracture Nonunionin 18 Human Bones. JAMA Surg 151:e162775. [DOI] [PubMed] [Google Scholar]


