Abstract
Background
Serotonin reuptake inhibitor (SRI) antidepressants are implicated in increasing the risk of bleeding among users; however, the comparative increase in bleeding risk with concurrent antithrombotic therapy (anticoagulant or antiplatelet) remains unclear. As such, we performed a systematic review and meta-analysis of all available evidence to evaluate the effects of SRI and the risk of bleeding complications among patients receiving antithrombotic therapy.
Methods
We searched Medline, Embase, PubMed, PsycINFO, Cochrane Library, Web of Science, Scopus, CINAHL, and grey literature (Google Scholar and preprint reports) up to 26 November, 2020, with no language restrictions (updated on 31 July 2021). The primary outcome of interest was major bleeding. Secondary outcomes included intracranial haemorrhage, gastrointestinal bleeding, and any bleeding events. We used a random-effects model meta-analysis to estimate the odds ratios (ORs) and 95% confidence intervals (CIs).
Results
We did not identify any randomised studies but found 32 non-randomized studies (cohort or case–control) with 1,848,285 patients that fulfilled the study selection criteria and were included in the meta-analysis. Among individuals receiving anticoagulants (13 studies), SRI users experienced a statistically higher risk of major bleeding compared to non-SRI users: pooled OR was 1.39 (95% CI, 1.23–1.58; p < .001; moderate heterogeneity). Among individuals receiving antiplatelet therapy (2 studies), SRI users were associated with an increased risk of major bleeding: pooled OR was 1.45 (95% CI, 1.17–1.80; p = .001; low heterogeneity). For secondary outcomes, the use of SRI among individuals treated with antithrombotic therapy revealed a higher risk of gastrointestinal bleeding or any bleeding events, whereas only anticoagulant use was illustrated an increased risk of intracranial haemorrhage.
Conclusions
The use of SRI antidepressants among patients treated with antithrombotic therapy (either anticoagulant or antiplatelet) is associated with a higher risk of bleeding complications, suggesting that caution is warranted in co-prescription.
PROSPERO Registration
CRD42018083917
KEY MESSAGES
In this meta-analysis of 32 non-randomized studies, SRI users were associated with the risk of bleeding complications compared to non-SRI users, with concurrent antithrombotic use (either anticoagulant or antiplatelet).
The risk was consistently elevated across types of bleeding events (major bleeding, gastrointestinal bleeding, or any bleeding events), whereas only anticoagulant use was associated with intracranial haemorrhage.
To promote the rational use of medicines, our findings suggest that the risk-benefit ratio must account for the clear efficacy of SRI against safety concerns in terms of bleeding risks.
Keywords: Anticoagulation, antidepressant, antiplatelet, bleeding complications, meta-analysis, serotonin-reuptake inhibitors
Introduction
Serotonin reuptake inhibitors (SRIs), including selective serotonin reuptake inhibitors (SSRI) and serotonin-norepinephrine reuptake inhibitors (SNRI) are the most widely prescribed antidepressants that are used in various psychiatric settings including cardiac patients [1]. With respect to the favourable safety profiles compared to older generations of antidepressants, SRI antidepressants and antithrombotic agents (anticoagulants and antiplatelet) are often prescribed together as depression and anxiety often coexist with cardiovascular/cerebrovascular diseases, atrial fibrillation, myocardial infarction, and other thromboembolic disorders [2,3]. Besides the risk of bleeding complications among antithrombotic therapy, recent accumulating evidence suggests that SRI use may be associated with an increased risk of bleeding, intracranial haemorrhage, and in particular, gastrointestinal tract bleeding [4–7]. In addition, concurrent use of SRI may potentiate this risk of bleeding complications further via pharmacokinetics or pharmacodynamics drug interactions. Specifically, concurrent use of SRI and antithrombotic appear to have the potential to inhibit cytochrome P450 (CYP) isoforms metabolism and impair serotonin platelet function [8].
Although several existing epidemiological studies have recognized the increased risk of bleeding complications among patients who received SRI, the safety of their use concomitant with antithrombotic therapy has not been fully elucidated. Moreover, previous systematic reviews have focussed on the use of SRI concomitant with non-steroidal anti-inflammatory drugs (NSAIDs), with the majority of those studies investigating gastrointestinal tract bleeding risk [6,7,9]. To the best of our knowledge, no comprehensive systematic review and meta-analysis has yet been conducted to quantify the effects of SRI use concomitantly with antithrombotic therapy and the risk of bleeding complications. To address this knowledge gap, we aimed to systematically review and summarize all available evidence to evaluate the effects of SRI use and the risk of bleeding complications among patients who received antithrombotic anticoagulants or antiplatelet therapy.
Materials and methods
This systematic review and meta-analysis were performed and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [10] and the Meta-analysis of Observational Studies in Epidemiology statement [11]. The pre-specified protocol was registered in the PROSPERO International prospective register of systematic reviews (CRD42018083917).
Data sources and search strategy
In collaboration with an experienced medical librarian, we searched electronic databases, including Medline, Embase, PubMed, PsycINFO, Cochrane Library (CENTRAL), Web of Science, Scopus, and CINAHL from inception to 26 November 2020 with no language restrictions. Grey literature from Google Scholar and the preprint reports (medRxiv, bioRxiv, and PsyArXiv) were supplemented to the electronic database searches to identify all relevant articles. We used combinations of Medical Subject Headings and search terms including pharmacological class or individual drugs (i.e. “antithrombotic” or “anticoagulant” or “antiplatelet”, AND “serotonin uptake inhibitor” or “SSRI” or “SNRI”) and bleeding complications (i.e. “bleeding” or “haemorrhage” or “blood transfusion”). The full search strategy for each database is available in the Supplementary, eTable 1. Relevant articles were also browsed from the reference lists of the included studies, previous systematic reviews, and major international pharmacoepidemiology/cardiology/psychiatry congresses. To update the search, a targeted manual search of relevant articles was performed through to 31 July 2021.
Study selection and outcomes
Eligible titles and abstracts of articles identified were screened independently by two reviewers (SN and CR). Then, potentially relevant full-text articles were assessed against the selection criteria for the final set of included studies. Potentially eligible articles that were not written in English were translated before the full-text appraisal. Any disagreement was resolved by a team discussion.
We included both randomized controlled trials (RCTs) and non-randomized studies (cohort or case-control) that (i) investigated the association between the use of SRI and risk of bleeding complications among adult patients (aged 18 years or more) receiving antithrombotic therapy (anticoagulant or antiplatelet agents) for any indications; (ii) consisted of two or more groups in which one group represented the use of SRI concomitant with antithrombotic therapy; (iii) consisted of SRI users including SSRI (i.e. citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline), SNRI (i.e. desvenlafaxine, duloxetine, milnacipran, and venlafaxine), or mixed action antidepressant agents (i.e. bupropion, mirtazapine, and trazodone); (iv) reported bleeding complications or provided sufficient data to calculate the risk estimate. We excluded studies that (i) were case series/case reports, N-of-one, cross-sectional, reviews, or studies with small sample sizes (less than 50 patients); and (ii) had no control group. Details of the selection criteria are provided in the Supplementary, eTable 2. For the companion study that included overlapping patients and study periods, the study with the most detailed and relevant information was included.
The primary outcome of interest was major bleeding, defined according to the International Society on Thrombosis and Haemostasis [12,13]. Secondary outcomes of interest included intracranial haemorrhage, gastrointestinal bleeding, and any bleeding events. Additional secondary outcomes included blood transfusion, endoscopy-refractory bleeding, rebleeding, and bleeding-related mortality. Based on clinical relevance, we defined the outcomes according to each included study. For instance, gastrointestinal bleeding events that required hospitalisation or related to mortality were considered major bleeding events.
Data extraction and quality assessment
Two reviewers (SN and RA) independently extracted the following pre-specified data using a standardized approach to gather information on (i) the study characteristics (the first author’s name, study design [RCTs, cohort, case–control], study population, sample size, study country, study period, analysis method, and factors controlled for analysis); (ii) patient characteristics (mean or median age of study population, the proportion of females, and comorbid conditions); (iii) specific exposure and control groups (definition of SRI users and non-SRI users, SRI dosage, and concomitant medications); and (iv) predefined outcomes of interest (including assessment outcome definitions and outcome measurements). Studies with incomplete data or unclear information were clarified by the corresponding author. In cases where authors did not respond after two attempts of contact, we used information reported to calculate the required data or excluded the study in the analyses. The final set of data was cross-checked independently by one reviewer (CP and WC).
A pair reviewer (SN and CR) independently assessed and appraised the methodological quality of each included study using the Cochrane revised tool for assessing the risk of bias in randomised trials (RoB 2) [14] and the Newcastle–Ottawa Scale (NOS) for assessing the quality of included non-randomised studies [15]. The overall risk of bias of included studies was then classified into low, high, or some concerns for randomized trials (RoB 2), and the highest quality, if the summary score of the NOS was 8 or more points, for non-randomized studies. Moreover, we also used the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool to assess the risk of bias and categorized the overall judgement as low, moderate, serious, or critical risk of bias [16]. To interpret our findings, the strength of evidence for each outcome was critically appraised independently by a pair of reviewers (SN and CR) using the Grading of Recommended Assessment, Development, and Evaluation (GRADE) guidelines [17]. The strength of a body of evidence findings was then classified into very low-, low-, moderate-, or high-quality. Any discrepancies were addressed by team discussion.
Statistical analysis
Two-tailed with a P-value of less than .05 was considered statistically significant. All analyses and generated forest plots of the summary pooled effects estimate were performed using Stata software version 16.0 (StataCorp, College Station, TX, USA). Inter-rater agreements were tested using kappa (κ) statistics to assess the agreement between reviewers in the study selection and risk of bias assessment processes. Based on the common risk estimates across the included studies, we used the aggregate odds ratios (ORs) with the greatest degree of adjustment for potential confounding factors as the summary effect estimates of association for each outcome of interest. As the methodological approach varied across included studies, we employed the random-effects models using the DerSimonion–Laird method for estimating the pooled ORs with corresponding 95% confidence intervals (CIs) to account for heterogeneity between studies [18].
Heterogeneity was assessed using the Cochran Q test, with a P-value of less than 0.10. The degree of inconsistency was investigated using I2 and tau-squared (τ2) statistics, [19,20] in which the heterogeneity was estimated as low (I2=25.0%, τ2=0.01), moderate (I2=50.0%, τ2=0.06), and high (I2=75.0%, τ2=0.16). We tested publication bias using Begg’s and Egger’s tests for each specific outcome of interest (P-value of less than .10 indicated statistical publication bias) [21,22]. The visual inspection of funnel plots was also performed where there was sufficient data to explore for asymmetry of the funnel graph. Moreover, the trim and fill method was then performed to calibrate for publication bias and account for the number of studies with null effects which were missing from the meta-analysis [23].
Pre-planned subgroup analyses were conducted based on (i) patient characteristics (i.e. age, proportion of males, history of bleeding events, comorbid conditions [atrial fibrillation, diabetes, chronic heart failure, coronary artery disease, renal failure, cancer, and Helicobacter pylori infection], and concomitant medications [use of NSAIDs, corticosteroids, and gastroprotective agents]); and (ii) study characteristics (sample size [less than 5000 vs. 5000 or more], study design (RCTs, cohort, or case-control), and study location (North America vs. non-North America). If data were available, individual SRI use and dosage were also assessed to establish the evidence of a dose–response and duration–response relationship.
A set of sensitivity analyses were conducted to assess the robustness of primary findings, including (i) restricting analysis to studies that adjusted for key confounding factors (age, sex, and history of bleeding); (ii) restricting the analysis to studies with high quality; (iii) limiting the analysis to studies with the directness of effect estimates; (iv) removing unpublished studies; (v) removing individual study approaches (leave one out analysis); and (vi) using the fixed-effects models if the I2 index less than 25.0%. Additionally, a random-effects univariate meta-regression was also performed according to the level of risk of bias, study characteristics, and baseline patient characteristics to explore the pre-specified effects on the meta-analytic estimates.
Results
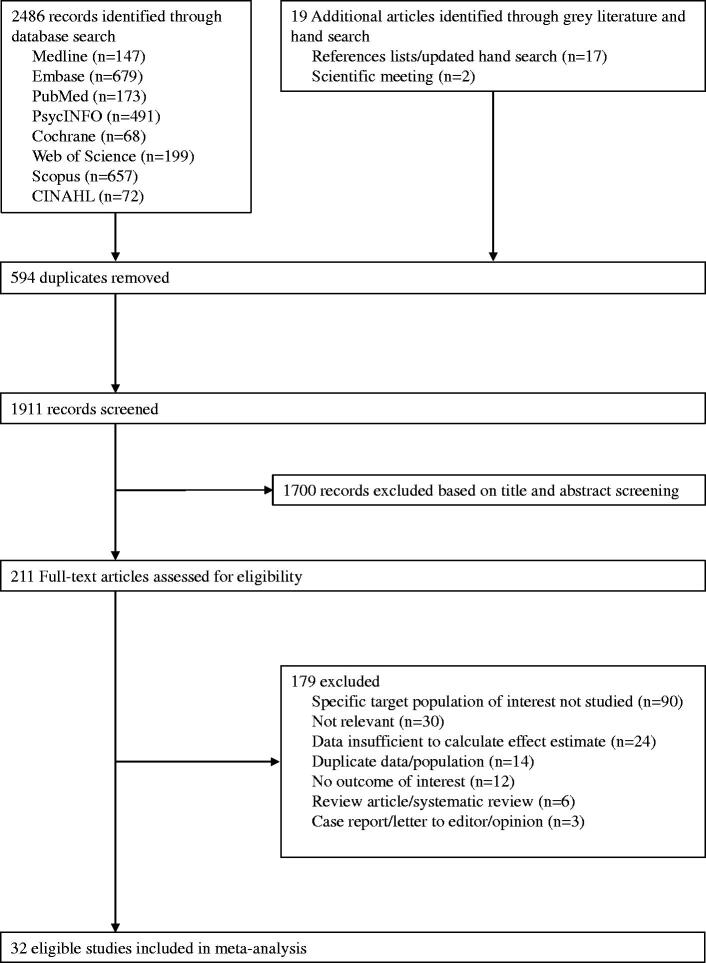
The search strategy retrieved 2505 records. From these, 594 duplicate records were removed, and 1911 records remained. Based on the title and abstract screening, we identified 211 articles that seemed to be relevant to the study question. Of these, 32 non-randomized studies fulfilled the study selection criteria and were included in the meta-analysis, while we did not identify any clinical randomised trials (Figure 1). The inter-rater agreement between reviewers on the study selection and data extraction was 0.87 and 0.79, respectively.
Figure 1.
Study selection flowchart.
Table 1 summarizes the characteristics of all the included studies. In total, 1,848,285 patients were identified with a mean age ranging from 52.4 to 82.4 years, proportion of male sex ranging from 22.5% to 79.0%, and most of the included studies not providing a specific indication of the use of SRI and antithrombotic therapy. Detailed measurement and definition of bleeding events, methodology for the study, comorbid conditions, and concomitant medications of the included studies are described in Supplementary, eTables 3, 4, and 5, respectively. According to the risk of bias assessed in 32 non-randomised included studies (Supplementary, eTable 6), summary scores ranged from 3 to 9 points, with 19 studies (59.4%) having the highest quality (NOS of 8 or more). Based on the ROBINS-I tool, we found that most included studies had a moderate risk of bias (21 studies, 65.6%); however, no study with critical risk of bias was observed (Supplementary, eTable 6). The summary results and strength of evidence findings are provided in Table 2. Details of evidence synthesis by the GRADE system are provided in Supplementary, eTable 7.
Table 1.
Baseline characteristics of the included studies in the meta-analysis.
| Author, year | Study design | Country | Sample size | Population with antithrombotic therapy | Study period | Age in years, mean ± SD | Male sex, n (%) | Exposure: SRI antidepressants | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Kurdyak et al. [43], 2005 | Nested case-control | Canada | 16734 | Elderly patients (>65 years) treated with warfarin for ≥ 1 years | January 1994– December 2002 | 80.8 ± 6.6 | NR | SSRIs: citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Gastrointestinal bleeding (UGIB) |
| Kharofa et al. [39], 2007† | Case–control | United States | 2692 | Treated with aspirin 2 weeks before index date | May 1997– October 2005 | Range: 50.9–70.2 | NR | SSRIs: citalopram, escitalopram, fluoxetine, paroxetine, sertraline | Brain haemorrhage (ICH and SAH) |
| de Abajo et al. [44], 2008† | Nested case–control | United Kingdom | 11321 | Current use of antiplatelet agents (primary low-dose aspirin) or oral anticoagulants (within 0–30 days of index date) | January 2000– December 2005 | 40–59 (27.2%) 60–69 (21.6%) 70–79 (34.7%) 80–84 (16.5%) |
6446 (56.9%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline SNRIs: duloxetine, venlafaxine |
Gastrointestinal bleeding (UGIB) |
| Schalekamp et al. [24], 2008 | Nested case–control | The Netherlands | 7666 | New users of coumarin: acenocoumarol (90.4%) and phenprocoumon (9.6%) | January 1991– December 2004 | 72.8 ± 9.8 | 4166 (54.3%) | SSRIs: citalopram, escitalopram, fluvoxamine, fluoxetine, paroxetine, sertraline | Major bleeding, Brain haemorrhage (ICH), Gastrointestinal bleeding |
| Dall et al. [49], 2009† | Case–control | Denmark | 40154 | Current use of aspirin (within the past 90 days) | August 1995– July 2006 | 72.1 ± 14.1 | 20541 (51.2%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Gastrointestinal bleeding (UGIB) |
| Wallerstedt et al. [25], 2009 | Retrospective cohort | Sweden | 234 | Treated with warfarin due to AF | January 1999– September 2005 | 72.0 ± 7.0 | 122 (52.1%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Major bleeding |
| Cochran et al. [26], 2011† | Retrospective cohort | United States | 100 | Treated with warfarin in an outpatients for ≥ 6 months | January 2007– November 2009 | 58.5 ± 16.0 | 25 (25.0%) | SSRIs: citalopram, escitalopram, fluoxetine, paroxetine, sertraline | Major bleeding, any bleeding |
| Labos et al. [37], 2011 | Retrospective cohort | Canada | 27058 | ACS with antiplatelet therapy: aspirin, clopidogrel, DAPT (aspirin and clopidogrel) | January 1998– March 2007 | 72.7 ± 10.6 | 19087 (70.5%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Major bleeding, Gastrointestinal bleeding |
| Schelleman et al. [45], 2011 | Nested case–control | United States | 666235 | Treated with warfarin | January 1999 – December 2005 | 18–50 (12.9%) 51–60 (12.2%) 60–70 (18.9%) 70–80 (27.5%) ≥81 (28.5%) |
242984 (36.5%) | SSRIs: citalopram, escitalopram, fluoxetine, paroxetine, sertraline SNRIs: venlafaxine |
Gastrointestinal bleeding |
| Vitry et al. [27], 2011 | Retrospective cohort | Australia | 17661 | Veterans who were new users of warfarin | July 2002– June 2006 | 81.8 ± 4.4 | 11277 (63.8%) | SSRIs: not specified | Major bleeding |
| Baillargeon et al. [28], 2012 | Nested case–control | United States | 3192 | Treated with warfarin for at least 180 days | January 2007 – December 2008 | 66–70 (10.4%) 71–75 (19.3%) 76–80 (22.2%) 81–85 (22.6%) ≥86 (25.5%) |
1116 (35.0%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline SNRIs: desvenlafaxine, duloxetine, milnacipran, venlafaxine |
Major bleeding |
| Lin et al. [50], 2013 | Retrospective cohort | Taiwan | 3238 | Treated with clopidogrel with an average dose of > 150 DDD per one-half year | January 2001 – December 2010 | 68.6 ± 11.6 | 1899 (58.6%) | SSRIs: not specified | Gastrointestinal bleeding (UGIB, LGIB) |
| Mosholder et al. [29], 2013 | Retrospective cohort | United States | 324356 | Treated with warfarin for at least 1 months | June 2006– October 2010 | <65 (13.0%) 65–74 (28.2%) 75–84 (37.4%) >84 (21.4%) |
213803 (65.9%) | SSRIs: not specified | Major bleeding, Brain haemorrhage (ICH), Gastrointestinal bleeding |
| Seitz et al. [54], 2013‡ | Retrospective cohort | Canada | 8568 | Treated with antiplatelet agents or warfarin in the 120 days preceding index | April 2003 – December 2009 | 82.4 ± 7.0 | 1927 (22.5%) | Current users of high-affinity SRIs: citalopram, escitalopram, clomipramine, duloxetine, fluoxetine, fluvoxamine, paroxetine, sertraline, venlafaxine | Perioperative blood transfusion |
| Giang et al. [52], 2014 | Retrospective cohort | United States | 162 | Treated with DAPT (aspirin and P2Y12 inhibitors) following coronary stent placement | October 2010 – January 2012 | NR | NR | SSRIs: citalopram, fluoxetine, sertraline | Any bleeding |
| Nguyen et al. [48], 2014 | Retrospective cohort | United States | 3153 | Veterans who were prescribed warfarin | October 2009 – September 2011 | NR | NR | SSRIs: not specified | Any bleeding |
| Quinn et al. [30], 2014† | Retrospective cohort | United States | 9186 | Treated AF with warfarin among the ATRIA study | Diagnosed AF from Jul 1996 – Dec 1997, and followed up to 6 years | ≥75 (53.3%) | 5337 (58.1%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline SNRIs: venlafaxine |
Major bleeding, Brain haemorrhage |
| Rashid et al. [38], 2016 | Retrospective cohort | Australia | 839 | ACS underwent angioplasty and received DAPT | January 2014 – December 2015 | 61.8 ± 12.5 | 663 (79.0%) | SSRIs: not specified | Major bleeding, any bleeding |
| Lai et al. [46], 2017 | Retrospective cohort | United States | 21503 | Treated with DOACs: apixaban (25.3%), dabigatran (25.9%), rivaroxaban (48.5%) | November 2010 – December 2015 | 18–64 (22.5%) 65–74 (30.7%) ≥75 (46.8%) |
11597 (53.9%) | SSRIs: not specified | Gastrointestinal bleeding |
| Laursen et al. [55], 2017† | Prospective registry | Denmark | 14343 | Treated with low-dose aspirin (≤150 mg/d) | August 2006 – August 2014 | 75.0 ± 27.6 | 7727 (53.9%) | SSRIs: not specified | Endoscopy-refractory bleeding, re-bleeding in peptic ulcer bleeding |
| Renoux et al. [40], 2017† | Nested case–control | United Kingdom | 92738 | Current use of antiplatelet agents or oral anticoagulants ( within 1 month before index date) | January 1995– June 2014 | 66.6 ± 16.6 | 36305 (39.1%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Brain haemorrhage (ICH) |
| Samuel et al. [31], 2017 | Retrospective cohort | United States | 575 | Primary or secondary diagnosis of an VTE and treated with full dose enoxaparin | October 2009 – October 2014 | 59.0 ± 38.3 | 310 (53.9%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Major bleeding |
| Scheitz et al. [41], 2017 | Prospective registries | Finland, France, Germany, Netherlands, Switzerland | 6242 | Preadmission with anticoagulants among acute ischaemic stroke patients treated by thrombolysis | June 1998–August 2016 | 70.1 ± 14.0 | 3501 (56.1%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Brain haemorrhage (post-thrombolysis symptomatic ICH) |
| Quinn et al. [32], 2018§ | Prospective cohort: using data from the ROCKET AF trial | International collaboration | 1474 | AF patients treated with rivaroxaban or warfarin for the prevention of stroke/systematic embolism | December 2006– June 2009 | 73.8 ± 8.5 | 703 (47.7%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline SNRIs: desvenlafaxine, duloxetine, venlafaxine |
Major bleeding, any bleeding |
| Iasella et al. [53], 2019 | Retrospective cohort | United States | 6819 | Treated with DAPT (clopidogrel-based) after PCI | July 2010– December 2014 | 66.8 ± NR | 4516 (66.2%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Any bleeding |
| Luo et al. [51], 2019 | Retrospective cohort | Taiwan | 11105 | Treated with aspirin with an average dose of > 14 DDD per month | January 2001 – December 2010 | 64.0 ± 12.7 | 5864 (52.8%) | SSRIs: not specified | Gastrointestinal bleeding (UGIB) |
| Gaist et al. [42], 2020† | Case–control | Denmark | 446264 | Current use (supply with grace period extended [60 days] up to cover index date) of antiplatelet agents (low-dose aspirin, clopidogrel) or oral anticoagulants (phenprocoumon, warfarin, apixaban, dabigatran, rivaroxaban) | January 2000– December 2016 | 71.3 ± 14.8 | 290280 (65.0%) | SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Brain haemorrhage (SDH) |
| Komen et al. [33], 2020 | Retrospective cohort | Sweden | 30595 | AF patients with a new prescription of warfarin or DOACs | July 2011– December 2017 | 74.4 ± 11.0 | 17139 (56.0%) | Antidepressant: SSRIs (61.0%), TCA (11.3%), other (27.7%) | Major bleeding, Gastrointestinal bleeding, brain haemorrhage |
| Lee et al. [34], 2020 | Nested case–control | Korea | 25893 | AF patients with a new prescription of DOACs (apixaban, rivaroxaban, edoxaban, dabigatran) | January 2013 – December 2017 | 76.3 ± 9.1 | 11949 (46.1%) | SSRIs: escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Major bleeding, Gastrointestinal bleeding (UGIB, LGIB), brain haemorrhage (ICH) |
| Marchena et al. [35], 2020 | Prospective registry | Spain | 47050 | Adult patients receiving anticoagulant therapy for VTE (VKAs, LMWH, DOACs) | February 2009– September 2019 | 66.5 ± 17.8 | 23999 (51.0%) | SSRIs: citalopram, escitalopram, paroxetine, sertraline, SNRIs: duloxetine, venlafaxine Mixed: mirtazapine, trazodone |
Major bleeding, brain haemorrhage (ICH) |
| Mawardi et al. [47], 2020 | Retrospective cohort | United States | 248 | LVAD patients treated with warfarin and aspirin (81 mg or 325 mg) | January 2009 – January 2016 | 52.4 ± 8.4 | 142 (57.2%) | SSRIs: citalopram, escitalopram, fluoxetine, paroxetine, sertraline SNRIs: duloxetine, venlafaxine Mixed: bupropion, mirtazapine, trazodone |
Gastrointestinal bleeding |
| Zhang et al. [36], 2020 | Nested case–control | United Kingdom | 1887 | Adult patients with new users of DOACs (dabigatran, apixaban, rivaroxaban) | January 2008– December 2015 | 78.7 ± 10.2 | 1175 (62.3%) | SSRIs: citalopram, escitalopram, fluoxetine, nefazodone, paroxetine, sertraline, SNRIs: venlafaxine, duloxetin | Major bleeding, Gastrointestinal bleeding |
†On the basis of the entire study population.
‡On the basis of the current and former serotonergic users.
§On the basis of the propensity-score matching.
ACS: acute coronary syndrome; AF: atrial fibrillation; ATRIA: AnTicoagulation and Risk factors In Atrial fibrillation; DAPT: dual antiplatelet therapy; DDD: defined daily dose; DOACs: direct oral anticoagulants; ICH: intracerebral haemorrhage; LMWH: low-molecular weight heparin); LVAD: left ventricular assist device; NR: not reported; PCI: percutaneous coronary intervention; ROCKET AF: Rivaroxaban once daily Oral direct factor xa inhibition Compared with vitamin K antagonism for prevention of Embolism and stroke Trial in Atrial Fibrillation; SAH: subarachnoid haemorrhage; SD: standard deviation; SDH: subdural haematoma; SRIs: serotonin reuptake inhibitors; LGIB: lower gastrointestinal tract bleeding; UGIB: upper gastrointestinal tract bleeding; VKAs: vitamin K antagonists; VTE: venous thromboembolism.
Table 2.
Summary of findings and strength of evidence.
| Bleeding complication | No. of included studies (Ref.) | No. of patients | Odds ratio (95% CI) | p-Value | Heterogeneity |
Strength of evidence | |||
|---|---|---|---|---|---|---|---|---|---|
| Q statistic | p-Value | I2 index (95% CI) | τ2 | ||||||
| Major bleeding | |||||||||
| Anticoagulant therapy | 13 (24–36) | 469869 | 1.39 (1.23–1.58) | <0.001 | 31.27 | 0.005 | 55.2% (4.7–73.6) | 0.026 | Low (harm: increased risk) |
| Antiplatelet Therapy | 2 (37, 38) | 27897 | 1.45 (1.17–1.80) | 0.001 | 1.08 | 0.782 | 0.0% (0.0–67.9) | <0.001 | Very low (harm: increased risk) |
| Intracranial haemorrhage | |||||||||
| Anticoagulant therapy | 10 (24, 29, 30, 33–35, 39–42) | 443904 | 1.31 (1.02–1.68) | 0.031 | 28.48 | 0.003 | 61.4% (11.8–77.9) | 0.091 | Very low (harm: increased risk) |
| Antiplatelet Therapy | 3 (39, 40, 42) | 81173 | 1.08 (0.93–1.26) | 0.325 | 8.98 | 0.030 | 66.6% (0.0–86.4) | 0.014 | Very low (no harm) |
| Gastrointestinal bleeding | |||||||||
| Anticoagulant therapy | 10 (24, 29, 33, 34, 36, 43–47) | 1085014 | 1.34 (1.19–1.50) | <0.001 | 15.57 | 0.113 | 35.8% (0.0–67.2) | 0.010 | Low (harm: increased risk) |
| Antiplatelet Therapy | 5 (37, 44, 49–51) | 52571 | 1.30 (1.04–1.63) | 0.021 | 5.93 | 0.204 | 32.6% (0.0–74.6) | 0.021 | Very low (harm: increased risk) |
| Any bleeding events | |||||||||
| Anticoagulant therapy | 23 (24–36, 39–48) | 1209421 | 1.39 (1.24–1.55) | <0.001 | 79.03 | <0.001 | 68.4% (49.8–78.0) | 0.040 | Low (harm: increased risk) |
| Antiplatelet therapy | 11 (37–40, 42, 44, 49–53) | 153790 | 1.15 (1.06–1.25) | 0.001 | 20.08 | 0.093 | 35.3% (0.0–64.6) | 0.007 | Low (harm: increased risk) |
CI: confidence interval; NA: not applicable.
Primary outcome: major bleeding
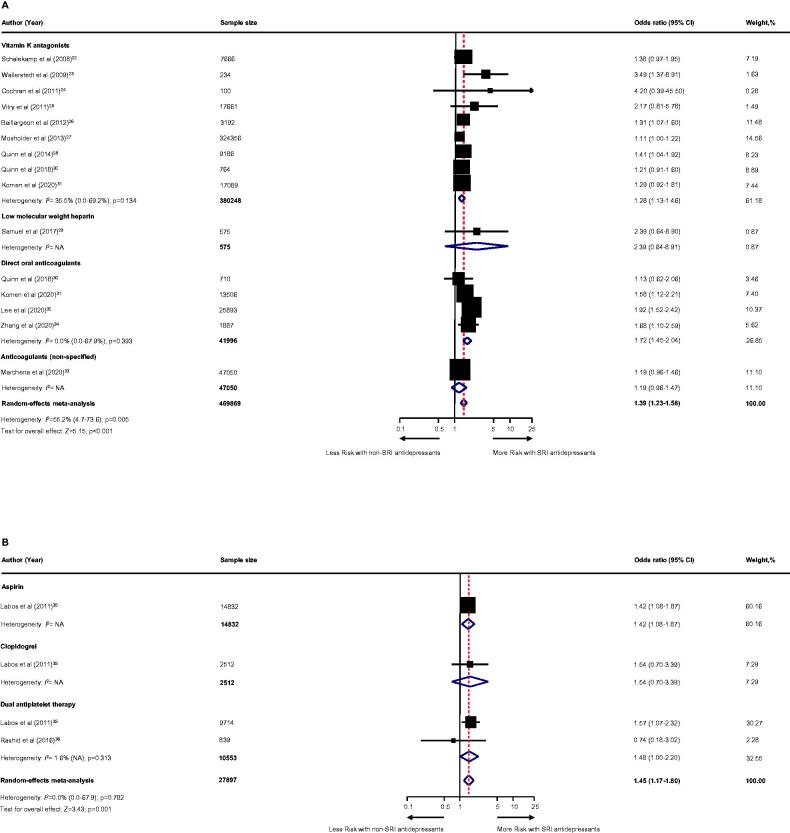
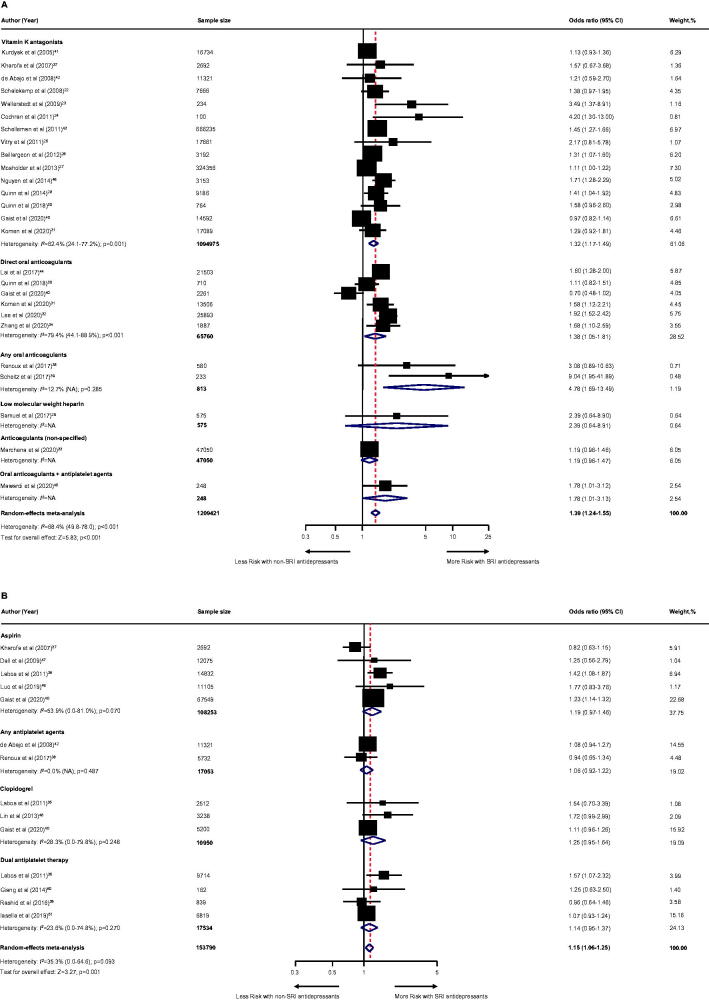
Among individuals receiving anticoagulant therapy (13 studies [24–36]; n = 469869; Figure 2(A)), SRI users experienced a statistically higher risk of major bleeding compared to non-SRI users with a moderate degree of heterogeneity: pooled OR was 1.39 (95% CI, 1.23–1.58; p < .001). With regard to anticoagulants (Figure 2(A)), the pooled OR was 1.28 (95% CI, 1.13–1.46; p < .001) for vitamin K antagonists (9 studies [24–30,32,33], n = 380,248); 2.39 (95% CI, 0.64–8.91; p = .194) for low-molecular-weight heparin (one study [31], n = 575); 1.72 (95% CI, 1.45–2.04; p < .001) for direct oral anticoagulants (4 studies [32–34,36], n = 41,996); and 1.19 (95% CI, 0.96–1.47; p = .104) for non-specified anticoagulants (one study [35]; n = 47,050).
Figure 2.
Effect of the use of SRI concomitant with antithrombotic therapy and the risk of major bleeding. Individuals treated with (A) anticoagulant therapy or (B) antiplatelet therapy. CI: confidence interval; NA: not applicable; SRI: serotonin reuptake inhibitor.
Among individuals receiving antiplatelet therapy (two studies [37,38], n = 27,897; Figure 2(B)), SRI users were associated with an increased risk of major bleeding with a low degree of heterogeneity: pooled OR was 1.45 (95% CI, 1.17–1.80; p = .001). With regard to the use of antiplatelet (Figure 2(B)), the pooled OR was 1.41 (95% CI, 1.08–1.87; p = .012) for aspirin (one study [37], n = 14,832); 1.54 (95% CI, 0.70–3.39; p = .285) for clopidogrel (one study [37], n = 2512); and 1.48 (95% CI, 1.00–2.20; p = .050) for dual antiplatelet therapy (two studies [37,38], n = 10,553).
Secondary outcomes and additional secondary outcomes
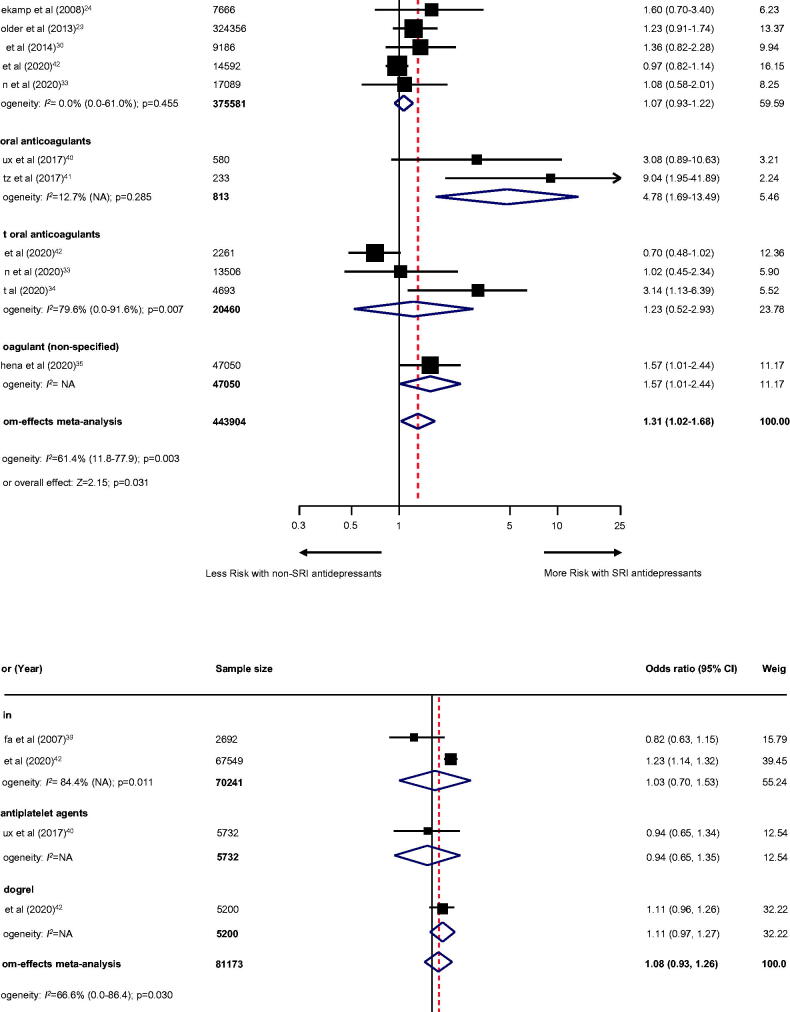
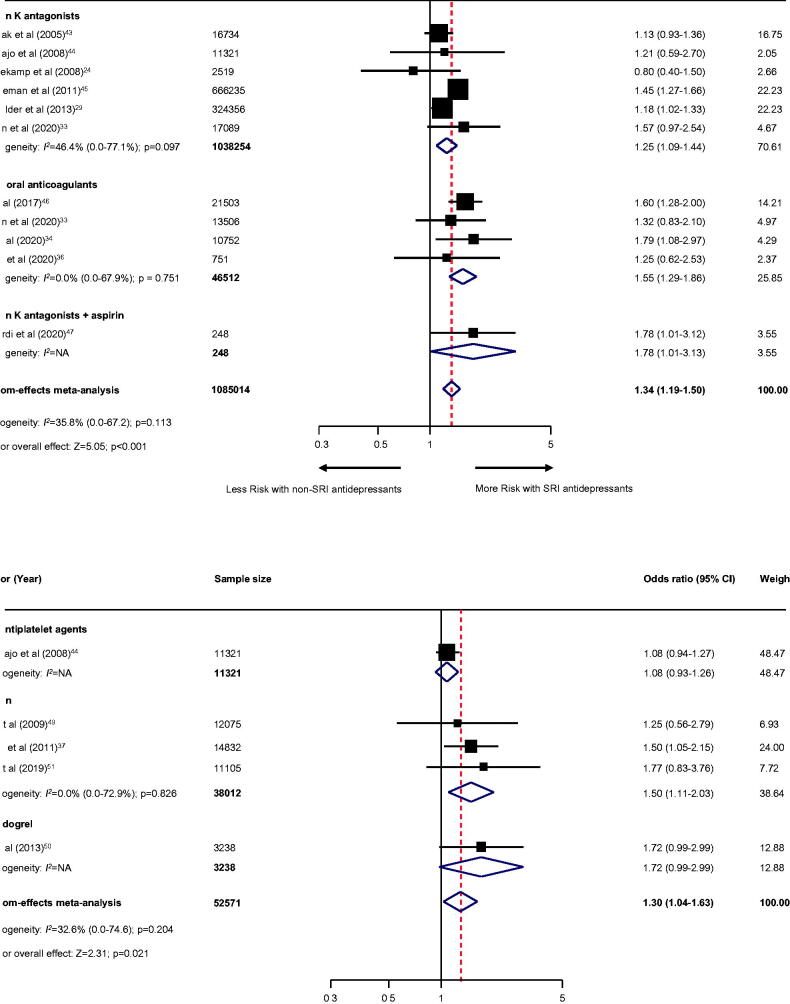
For secondary outcomes, the use of SRI among individuals treated with anticoagulant therapy revealed a higher risk of intracranial haemorrhage (10 studies [24,29,30,33–35,39–42]; n = 443,904; OR, 1.31; 95% CI, 1.02–1.68; p = .031; Figure 3(A)), gastrointestinal bleeding (10 studies [24,29,33,34,36,43–47]; n = 1085014; OR, 1.34; 95% CI, 1.19–1.50; p < .001; Figure 4(A)), and any bleeding events (23 studies [24–36,39–48]; n = 1,209,421; OR, 1.39; 95% CI, 1.24–1.55; p < .001; Figure 5(A)). Likewise, use of SRI among individuals treated with antiplatelet agents also illustrated an increased risk of gastrointestinal bleeding (five studies [37,44,49–51]; n = 52571; OR, 1.30; 95% CI, 1.04–1.63; p = .021; Figure 4(B)), any bleeding events (11 studies [37–40,42,44,49–53]; n = 153,790; OR, 1.15 (95% CI, 1.06–1.25; p = .001; Figure 5(B)), except for intracranial haemorrhage (three studies [39,40,42]; n = 81173; OR, 1.08; 95% CI, 0.93–1.26; p = .325; Figure 3(B)).
Figure 3.
Effect of the use of SRI concomitant with antithrombotic therapy and the risk of intracranial haemorrhage. Individuals treated with (A) anticoagulant therapy or (B) antiplatelet therapy. CI: confidence interval; NA: not applicable; SRI: serotonin reuptake inhibitor.
Figure 4.
Effect of the use of SRI concomitant with antithrombotic therapy and the risk of gastrointestinal bleeding. Individuals treated with (A) anticoagulant therapy or (B) antiplatelet therapy. CI: confidence interval; NA: not applicable; SRI: serotonin reuptake inhibitor.
Figure 5.
Effect of the use of SRI concomitant with antithrombotic therapy and the risk of any bleeding events. Individuals treated with (A) anticoagulant therapy or (B) antiplatelet therapy. CI: confidence interval; NA: not applicable; SRI: serotonin reuptake inhibitor.
Evidence for blood transfusion (one study [54]), endoscopy-refractory bleeding (one study [55]), and rebleeding (one study [55]) are inconclusive owing to the limited data available (Supplementary, eTable 8). However, no study has reported bleeding complications in terms of bleeding related to mortality. Moreover, risk estimates according to individual SRI use, as well as a dose- and duration-relationship cannot be established due to lack of information.
Subgroup analyses
Several pre-planned subgroup analyses according to baseline patient characteristics and secondary outcomes, could not be performed due to the small sample size and limited information on outcomes across the included studies. Based on study characteristics, among individuals receiving anticoagulant therapy, the effect estimates between SRI use and the risk of bleeding complications was no longer statistically significant when the sample size was less than 5000 (for gastrointestinal bleeding), while the risk of intracranial haemorrhage was sensitive to sample size, study design, and study location (Supplementary, eTable 9). With a small number of included studies for individuals receiving antiplatelet therapy, no further association was observed, particularly among studies with a sample size less than 5000 or case–control study design (Supplementary, eTable 9).
Sensitivity and meta-regression analyses
The set of sensitivity analyses was robust and did not change substantially from the main findings (Supplementary, eTables 10–15). However, there was no association after restricting the analysis to studies with high quality (NOS of 8 or more) for the risk of intracranial haemorrhage among individuals who received anticoagulation therapy (OR, 1.17; 95% CI, 0.91–1.51, Supplementary, eTable 11). According to the leave-one-out analysis (Supplementary, eTable 14), after the removal of individual studies by Renoux et al., 2017 [40], Scheitz et al. [41], Lee et al. [34], and Marchena et al. [35], there was no association between SRI use and the risk of intracranial haemorrhage among individuals receiving anticoagulant therapy. Meanwhile, the association between SRI users and intracranial haemorrhage among individuals receiving antiplatelet therapy became statistically significant after a study by Kharofa et al., 2007 [39] was omitted (OR, 1.16; 95% CI, 1.04–1.29). Moreover, removing the study by Labos et al., 2011 [37] and Lin et al., 2013 [50], resulted in no further association between the use of SRI and gastrointestinal bleeding risk among individuals who received antiplatelet therapy.
A univariate meta-regression was suitable for the primary outcomes (Supplementary, eTable 16). For individuals receiving anticoagulant therapy, the increased risk of bleeding complications was associated with the baseline proportion of NSAIDs use and the proportion of male sex for major bleeding and intracranial haemorrhage, respectively. Nonetheless, the heterogeneity of the included studies was not explained by any of the study characteristics, baseline patient characteristics, and the risk of bias among individuals receiving antiplatelet therapy.
Publication bias
For individuals receiving anticoagulant therapy, evidence of publication bias related to the sample size was observed in the results of major bleeding, intracranial haemorrhage, and any bleeding, with the P-values tested for asymmetry less than .10. Asymmetry tests were observed in the results of intracranial haemorrhage and gastrointestinal bleeding among those who received antiplatelet therapy (Supplementary, eTable 17). The visually inspected funnel plots for each outcome are provided in the Supplementary, eFigure 1. However, after calibration for publication bias using the trim and fill method, the main findings were not substantially different. Notably, there was no longer an association between SRI use and the risk of intracranial haemorrhage and gastrointestinal bleeding among those who received anticoagulant and antiplatelet therapy, respectively, after the analysis was calibrated for publication bias (Supplementary, eTable 17).
Discussion
This systematic review and meta-analysis of 32 included non-randomized studies showed low certainty evidence that SRI users experienced a statistically higher risk of bleeding complications compared to non-SRI users, particularly among patients treated with anticoagulant therapy. We found very low certainty of evidence on the association between SRI use and the risk of intracranial haemorrhage and gastrointestinal bleeding among patients who received anticoagulant and antiplatelet therapy, respectively.
Several mechanisms have been proposed to explain the association between SRI use and the risk of bleeding complications. Theoretically, it has been demonstrated that serotonergic antidepressants increase bleeding complications via inhibiting platelet aggregation [8]. Another possible explanation for SRI in relation to bleeding risk is increased gastric acid secretion directly by increasing the vagal tone, subsequently leading to potential ulcerogenic effects and gastrointestinal bleeding [8,56,57]. As expected, the use of SRI concomitant with antithrombotic therapy either anticoagulants or antiplatelet agents can aggravate the risk of bleeding via both pharmacokinetics or pharmacodynamics interactions [8]. Among individuals receiving SRI and warfarin, for instance, proposed drug–drug interactions that increase bleeding risk may include impairing platelet aggregation and CYP 450 inhibition of warfarin metabolism; the potency of CYP inhibition varied among SRI [8]. Furthermore, SRI may further decrease platelet or endothelial activation and reduce the efficiency of haemostasis beyond that associated with concomitant antiplatelet agents such as aspirin or clopidogrel [56].
Our findings expanded previous meta-analyses by providing insight into the impact of SRI use concomitant with antithrombotic therapy (both anticoagulants and antiplatelet agents) and the bleeding risk, which has not been fully addressed previously. With regard to the credibility of the evidence, a previous umbrella review by Dragioti et al. (2019 [58]) was based on highly suggestive evidence, which indicates an increased risk of bleeding complications among individual use of SSRI or SNRI users. The summary ORs of severe bleeding at any site and upper gastrointestinal bleeding were 1.41 (95% CI, 1.27–1.57) and 1.55 (95% CI, 1.35–1.78), respectively [58]. These findings are also supported by our results that the use of SRI among individuals who received antithrombotic therapy (particularly anticoagulation), included the risk of major bleeding, gastrointestinal bleeding, and any bleeding events. However, it is unclear whether the use of SRI among individuals receiving antithrombotic therapy may have additional intracranial haemorrhage. Several existing meta-analyses with substantial heterogeneity have illustrated an increased risk of intracranial haemorrhage among individuals receiving SRI that did not particularly focus on patients treated with antithrombotic therapy [59–61]. On the other hand, other studies have not supported this finding when restricting analyses to high-quality data [58,62]. Furthermore, our findings did not confirm this association when sensitivity analysis and publication bias were considered. Given the statistical power and the imprecision of our findings, evidence for the risk of intracranial haemorrhage among individuals’ use of SRI antidepressant concomitant antithrombotic therapy remains inconclusive.
Strengths and limitations
The strengths of this study include a large sample size. We expanded and addressed the further risk of bleeding complications associated with the use of SRI among patients who received antithrombotic therapy, which had not been investigated by previous meta-analyses. From a methodological viewpoint, we used a rigorous and comprehensive systematic review approach, as well as extensive searching without language restriction. Furthermore, according to the set of sensitivity analyses, these results were consistent with the main analysis in most cases, suggesting the robustness of our findings.
This systematic review and meta-analysis have several limitations. First, despite conducting a comprehensive search strategy, data from RCT were not identified. Our findings relied on non-randomized observational studies, confounding by indication/contraindication, and unmeasured confounders must be noted. As a result, the causality of the use of SRI among patients who received antithrombotic therapy and the subsequent risk of bleeding complications cannot be established. Most of the studies included in this review were based on routinely collected administrative data and electronic health records, which could be prone to information bias. Second, on the basis of the NOS summary score, the quality of the included studies was varied; most cases (18 studies [56.2%]) had high quality (NOS more than 8 points) and 14 studies had low quality (NOS ranged from 3 to 7). Of these, four studies [38,46,48,52] (12.5%) were reported as abstracts, which could lead to incomplete information. However, the results after restricting the analysis to studies with high-quality or removing unpublished studies, according to the mentioned sensitivity analysis methods, yielded main findings that were not substantially different. Therefore, we advocate that future studies with high methodological quality are required. Third, disparities of individual SRI exposure and bleeding outcome definitions were observed across the included studies, which could have contributed to the moderate heterogeneity of our findings. Although the degree of inconsistency improved in most cases when subgroup analysis was performed, several pre-planned subgroup analyses could not be conducted due to the small number of included studies. Fourth, data on the individual use of SRI, key patient characteristics, and several confounding factors related to bleeding complications, such as renal function, history of bleeding events, or use of NSAIDs were not gartered across all included studies. As a result, a dose- and duration-relationship and risk effects estimate, based on the different subpopulations, cannot be established due to lack of information. Fifth, information on both SRI and antithrombotic therapy in terms of treatment medication and adherence over time were also lacking; thus, misclassification bias should be stated. Moreover, the data on pre-specified additional secondary outcomes, including, blood transfusion, endoscopy-refractory bleeding, rebleeding, and bleeding-related mortality were insufficient, which is an emerging concern with respect to the increased SRI use among individuals who received antithrombotic therapy and is needed for further studies. Finally, it is possible that publication bias exists and might account for some of the effect estimates we observed. Moreover, our strength of evidence findings using the GRADE approach was based on a low or very low body of evidence. Therefore, the interpretation of our findings should be exercised.
Implications for practice and future research
Given the limited strength of the body of evidence, this systematic review and meta-analysis provides the best available evidence that can emerge as insight with respect to the use of SRI among individuals receiving antithrombotic therapy in general practice. In cardiac patients receiving antithrombotic drugs, the risk-benefit ratio must account for the clear efficacy of antidepressants against adverse health outcomes, which should be balanced with safety concerns in terms of bleeding risk. Thus far, individuals receiving combination therapy including SRI and antithrombotic therapy warrant proactive monitoring of bleeding complications, especially among individuals with a history of bleeding, or pre-existing risk of bleeding—that is, peptic ulcer disease, chronic liver disease, chronic kidney disease, or received concomitant medication that may further increase the risk of bleeding (i.e. NSAIDs). The findings from this review support the interventions or strategies that promote appropriate SRI prescriptions and minimise risk in relation to drug–drug interactions in real-world practice. In addition, patients should also be informed about the benefits and risks of concomitant SRI and antithrombotic therapy in terms of bleeding risks to promote the rational use of medicines.
Further research in RCTs alongside collaborative pharmacoepidemiology research and proactive real-world evidence surveillance systems are needed to reaffirm and clarify the potential causal association between SRI and risk of bleeding among individuals receiving antithrombotic therapy. Such research should elaborate on the use of individual SRI, clinical diagnoses and indications, pathogenesis and mechanistic processes, the severity of clinical and bleeding risk conditions, and dose–effect and duration–effect response.
Conclusions
This systematic review and meta-analysis revealed that SRI use among patients treated with antithrombotic therapy, especially anticoagulants may increase the risk of bleeding complications, including major bleeding, gastrointestinal bleeding, and any bleeding events. However, these findings were limited by the nature of non-randomised included studies and the low strength of the body of evidence. However, evidence for intracranial haemorrhage or those who received SRI concomitant with antiplatelet therapy are inconclusive. Further pharmacoepidemiologic research, including proactive longitudinal surveillance systems, is needed to clarify and confirm the safety of using SRI in concomitance with antithrombotic therapy and the subsequent risk of bleeding complications.
Ethical approval
Ethical approval was nor required as this study did not require use of patient identifiers.
Supplementary Material
Funding Statement
This work was funded by the Faculty of Pharmacy and partially supported by the Pharmacoepidemiology and Statistics Research Centre (PESRC) through the Chiang Mai University, Thailand. The funder of the study had no role in the study design collection, analysis, or interpretation of the data, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit it for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Disclosure statement
All authors declare no competing interests. All the researchers involved performed this study in the context of their research.
Data availability statement
All data generated or analysed during this study are included in this article and its supplementary information files. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Zambrano J, Celano CM, Januzzi JL, et al. Psychiatric and psychological interventions for depression in patients with heart disease: a scoping review. J Am Heart Assoc. 2020;9(22):e018686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel D, Mc Conkey ND, Sohaney R, et al. A systematic review of depression and anxiety in patients with atrial fibrillation: the mind-heart link. Cardiovasc Psychiatry Neurol. 2013;2013:159850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celano CM, Huffman JC.. Depression and cardiac disease: a review. Cardiol Rev. 2011;19(3):130–142. [DOI] [PubMed] [Google Scholar]
- 4.Laporte S, Chapelle C, Caillet P, et al. Bleeding risk under selective serotonin reuptake inhibitor (SSRI) antidepressants: a meta-analysis of observational studies. Pharmacol Res. 2017;118:19–32. [DOI] [PubMed] [Google Scholar]
- 5.Douros A, Ades M, Renoux C.. Risk of intracranial hemorrhage associated with the use of antidepressants inhibiting serotonin reuptake: a systematic review. CNS Drugs. 2018;32(4):321–334. [DOI] [PubMed] [Google Scholar]
- 6.Loke YK, Trivedi AN, Singh S.. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2007;27(1):31–40. [DOI] [PubMed] [Google Scholar]
- 7.Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(6):811–819. [DOI] [PubMed] [Google Scholar]
- 8.Andrade C, Sandarsh S, Chethan KB, et al. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565–1575. [DOI] [PubMed] [Google Scholar]
- 9.Jiang HY, Chen HZ, Hu XJ, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13(1):42–50.e43. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. [DOI] [PubMed] [Google Scholar]
- 13.Schulman S, Angerås U, Bergqvist D, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202–204. [DOI] [PubMed] [Google Scholar]
- 14.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 15.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 2, 2020.
- 16.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borenstein M, Higgins JP, Hedges LV, et al. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5–18. [DOI] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R.. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 24.Schalekamp T, Klungel OH, Souverein PC, et al. Increased bleeding risk with concurrent use of selective serotonin reuptake inhibitors and coumarins. Arch Intern Med. 2008;168(2):180–185. [DOI] [PubMed] [Google Scholar]
- 25.Wallerstedt SM, Gleerup H, Sundström A, et al. Risk of clinically relevant bleeding in warfarin-treated patients-influence of SSRI treatment. Pharmacoepidemiol Drug Saf. 2009;18(5):412–416. [DOI] [PubMed] [Google Scholar]
- 26.Cochran KA, Cavallari LH, Shapiro NL, et al. Bleeding incidence with concomitant use of antidepressants and warfarin. Ther Drug Monit. 2011;33(4):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitry AI, Roughead EE, Ramsay EN, et al. Major bleeding risk associated with warfarin and co-medications in the elderly population. Pharmacoepidemiol Drug Saf. 2011;20(10):1057–1063. [DOI] [PubMed] [Google Scholar]
- 28.Baillargeon J, Holmes HM, Lin YL, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125(2):183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosholder AD, Racoosin JA, Young S, et al. Bleeding events following concurrent use of warfarin and oseltamivir by medicare beneficiaries. Ann Pharmacother. 2013;47(11):1420–1428. [DOI] [PubMed] [Google Scholar]
- 30.Quinn GR, Singer DE, Chang Y, et al. Effect of selective serotonin reuptake inhibitors on bleeding risk in patients with atrial fibrillation taking warfarin. Am J Cardiol. 2014;114(4):583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuel NG, Seifert CF.. Risk of bleeding in patients on Full-Dose enoxaparin with venous thromboembolism and selective serotonin reuptake inhibitors. Ann Pharmacother. 2017;51(3):226–231. [DOI] [PubMed] [Google Scholar]
- 32.Quinn GR, Hellkamp AS, Hankey GJ, et al. Selective serotonin reuptake inhibitors and bleeding risk in anticoagulated patients with atrial fibrillation: an analysis from the ROCKET AF trial. J Am Heart Assoc. 2018;7(15):e008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komen JJ, Hjemdahl P, Mantel-Teeuwisse AK, et al. Concomitant anticoagulant and antidepressant therapy in atrial fibrillation patients and risk of stroke and bleeding. Clin Pharmacol Ther. 2020;107(1):287–294. [DOI] [PubMed] [Google Scholar]
- 34.Lee MT, Park KY, Kim MS, et al. Concomitant use of NSAIDs or SSRIs with NOACs requires monitoring for bleeding. Yonsei Med J. 2020;61(9):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchena PJ, Tzoran I, Brenner B, RIETE Investigators, et al. Psychotropic drugs and outcome in patients receiving anticoagulant therapy for venous thromboembolism. Thromb Haemost. 2020;120(4):620–626. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Souverein PC, Gardarsdottir H, et al. Risk of major bleeding among users of direct oral anticoagulants combined with interacting drugs: a population-based nested case-control study. Br J Clin Pharmacol. 2020;86(6):1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labos C, Dasgupta K, Nedjar H, et al. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ. 2011;183(16):1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashid H, Hu J, Chan J, et al. Bleeding outcomes with selective serotonin reuptake inhibitors in combination with dual antiplatelet therapy following acute coronary syndrome. Heart Lung Circ. 2016;25:S33–S34. [Google Scholar]
- 39.Kharofa J, Sekar P, Haverbusch M, et al. Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke. 2007;38(11):3049–3051. [DOI] [PubMed] [Google Scholar]
- 40.Renoux C, Vahey S, Dell'Aniello S, et al. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. 2017;74(2):173–180. [DOI] [PubMed] [Google Scholar]
- 41.Scheitz JF, Turc G, Kujala L, et al. Intracerebral hemorrhage and outcome after thrombolysis in stroke patients using selective Serotonin-Reuptake inhibitors. Stroke. 2017;48(12):3239–3244. [DOI] [PubMed] [Google Scholar]
- 42.Gaist D, García Rodríguez LA, Hald SM, et al. Antidepressant drug use and subdural hematoma risk. J Thromb Haemost. 2020;18(2):318–327. [DOI] [PubMed] [Google Scholar]
- 43.Kurdyak PA, Juurlink DN, Kopp A, et al. Antidepressants, warfarin, and the risk of hemorrhage. J Clin Psychopharmacol. 2005;25(6):561–564. [DOI] [PubMed] [Google Scholar]
- 44.de Abajo FJ, García-Rodríguez LA.. Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents. Arch Gen Psychiatry. 2008;65(7):795–803. [DOI] [PubMed] [Google Scholar]
- 45.Schelleman H, Brensinger CM, Bilker WB, et al. Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case-control study. PLoS One. 2011;6(6):e21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai JH, Vaidya S, Sudat S, et al. Prevalence and risk factors for gastrointestinal bleeding in novel oral anticoagulant users. Gastroenterology. 2017;152(5):S475–S476. [Google Scholar]
- 47.Mawardi G, Markman TM, Muslem R, et al. SSRI/SNRI therapy is associated with a higher risk of gastrointestinal bleeding in LVAD patients. Heart Lung Circ. 2020;29(8):1241–1246. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen TN, Bird J, Furrh R, et al. Retrospective review of bleeding incidence associated with concomitant use of warfarin and selective serotonin reuptake inhibitors (SSRIs) in a veteran population. Pharmacotherapy. 2014;34(6):E86–E86. [Google Scholar]
- 49.Dall M, Schaffalitzky de Muckadell OB, Lassen AT, et al. An association between selective serotonin reuptake inhibitor use and serious upper gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2009;7(12):1314–1321. [DOI] [PubMed] [Google Scholar]
- 50.Lin CC, Hu HY, Luo JC, et al. Risk factors of gastrointestinal bleeding in clopidogrel users: a nationwide population-based study. Aliment Pharmacol Ther. 2013;38(9):1119–1128. [DOI] [PubMed] [Google Scholar]
- 51.Luo PJ, Lin XH, Lin CC, et al. Risk factors for upper gastrointestinal bleeding among aspirin users: an old issue with new findings from a population-based cohort study. J Formos Med Assoc. 2019;118(5):939–944. [DOI] [PubMed] [Google Scholar]
- 52.Giang K, Mouwakeh H, Stubbs M, et al. Assessing bleeding association with combined use of SSRI and dual antiplatelet therapy. Crit Care Med. 2014;42(12 Supple 1):A1511–A1512. [Google Scholar]
- 53.Iasella CJ, Kreider MS, Huang L, et al. Effect of selective serotonin reuptake inhibitors on cardiovascular outcomes after percutaneous coronary intervention: a retrospective cohort study. Clin Drug Investig. 2019;39(6):543–551. [DOI] [PubMed] [Google Scholar]
- 54.Seitz DP, Bell CM, Gill SS, et al. Risk of perioperative blood transfusions and postoperative complications associated with serotonergic antidepressants in older adults undergoing hip fracture surgery. J Clin Psychopharmacol. 2013;33(6):790–798. [DOI] [PubMed] [Google Scholar]
- 55.Laursen SB, Leontiadis GI, Stanley AJ, et al. The use of selective serotonin receptor inhibitors (SSRIs) is not associated with increased risk of endoscopy-refractory bleeding, rebleeding or mortality in peptic ulcer bleeding. Aliment Pharmacol Ther. 2017;46(3):355–363. [DOI] [PubMed] [Google Scholar]
- 56.Andrade C, Sharma E.. Serotonin reuptake inhibitors and risk of abnormal bleeding. Psychiatr Clin North Am. 2016;39(3):413–426. [DOI] [PubMed] [Google Scholar]
- 57.de Abajo FJ. Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging. 2011;28(5):345–367. [DOI] [PubMed] [Google Scholar]
- 58.Dragioti E, Solmi M, Favaro A, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry. 2019;76(12):1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hackam DG, Mrkobrada M.. Selective serotonin reuptake inhibitors and brain hemorrhage: a Meta-analysis. Neurology. 2012;79(18):1862–1865. [DOI] [PubMed] [Google Scholar]
- 60.Shin D, Oh YH, Eom CS, et al. Use of selective serotonin reuptake inhibitors and risk of stroke: a systematic review and Meta-analysis. J Neurol. 2014;261(4):686–695. [DOI] [PubMed] [Google Scholar]
- 61.Biffi A, Scotti L, Corrao G.. Use of antidepressants and the risk of cardiovascular and cerebrovascular disease: a Meta-analysis of observational studies. Eur J Clin Pharmacol. 2017;73(4):487–497. [DOI] [PubMed] [Google Scholar]
- 62.Jensen MP, Ziff OJ, Banerjee G, et al. The impact of selective serotonin reuptake inhibitors on the risk of intracranial haemorrhage: a systematic review and meta-analysis. Eur Stroke J. 2019;4(2):144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article and its supplementary information files. The data that support the findings of this study are available from the corresponding author upon reasonable request.