Abstract
Ischemic stroke is one of the major causes of severe disability and death worldwide. It is mainly caused by a sudden reduction in cerebral blood flow due to obstruction of the supplying vessel by thrombi and subsequent initiation of a complex cascade of pathophysiological changes, which ultimately lead to brain ischemia and even irreversible infarction. Thus, timely and effective thrombolysis therapy remains a mainstay for acute ischemic stroke treatment. Tissue plasminogen activator (tPA), the only thrombolytic agent approved globally, provides substantial benefits by exerting a fibrinolysis effect, recovering the blood supply in occluded vessels and, thereby, salvaging the ischemic tissue. However, the clinical application of tPA was limited because of a few unsolved issues, such as a narrow therapeutic window, hemorrhagic complications, and limited thrombolytic efficacy, especially, for large thrombi. With the prosperous development of nanotechnology, a series of targeted delivery strategies and nanocomposites have been extensively investigated for delivering thrombolytic agents to facilitate thrombolysis treatment. Excitingly, numerous novel attempts have been reported to be effective in extending the half-life, targeting the thrombus site, and improving the thrombolytic efficacy in preclinical models. This article begins with a brief introduction to ischemic stroke, then describes the current state of thrombolysis treatment and, finally, introduces the application of various nanotechnology-based strategies for targeted delivery of thrombolytic agents. Representative studies are reviewed according to diverse strategies and nano-formulations, with the aim of providing integrated and up-to-date information and to improve the development of thrombolysis treatment for stroke patients.
Keywords: Targeted drug delivery, thrombolysis, ischemic stroke, nanothrombolysis, tissue plasminogen activator
1. Introduction
Stroke is the second-most leading cause of death and a major cause of severe disability in adults worldwide (Global Burden of Diseases, 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Stroke poses a severe public health burden and huge consumption of public resources for the community, as well as great expense and loss of labor for families. An increasingly large amount of financial expenditure is devoted to drug development and research for stroke treatment every year, yet the progress remains sluggish. Acute ischemic stroke (AIS), the major proportion of all stroke types (accounting for approximately 87%) (Benjamin et al., 2018), is characterized by a sudden disruption of cerebral blood flow due to the obstruction of the supplying vessel. AIS leads to ischemia or even irreversible cell death in certain brain regions, and manifests as transient or permanent neurological functional disorders ranging from motor impairments, aphasia, coma, and even death (Dirnagl et al., 1999).
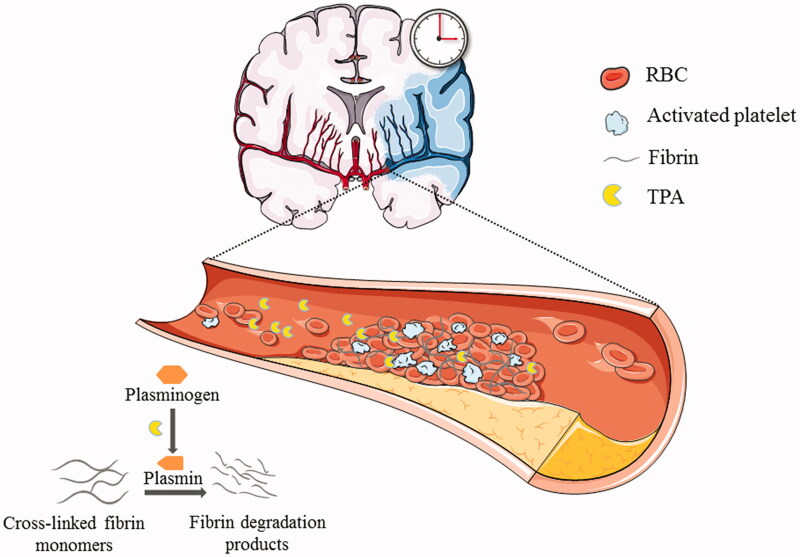
According to the varied pathogenesis of stroke, obstruction of the supplying vessel can be mainly blocked by thrombosis in situ or embolism (mainly thrombus) from the heart, carotid arteries, aortic arch, or other systemic circulation. Although intra-arterial thrombi may vary greatly in proportion and structure, they generally contain aggregation of activated platelets, red blood cells, and cross-linked fibrin monomers, which lay a theoretical foundation for thrombolytic treatment of AIS (Singh et al., 2013; De Meyer et al., 2017; Henderson et al., 2018). Since brain tissue is extremely sensitive to the ischemia insult, once the blood vessel is occluded, without timely recanalization, an infarct core will inevitably form, surrounded by a salvageable ischemic penumbra, and a complex series of pathophysiological events will evolve over time and space. Thus, efficient and prompt recanalization of the supplying vessel, as one of the most effective strategies for treatment of early-stage AIS, has a positive effect on salvaging the ischemic penumbra tissue; consequently, neurological functions can be regained and clinical outcomes can be improved. However, alternative drugs for thrombolysis are extremely limited. As a serine protease with a molecular weight of 63 kDa, tissue plasminogen activator (tPA) physiologically functions as a pivotal thrombolytic agent that can convert plasminogen to plasmin to exert a fibrinolytic effect and dissolve the main structure of the thrombus (Figure 1). Since 1996, tPA is the only US Food and Drug Administration (FDA)-approved treatment for patients with AIS. Intravenous administration of recombinant human tPA (rtPA), Alteplase, a representative of second-generation thrombolytics, revolutionized acute stroke treatment and is also highly recommended in clinical practice by current management guidelines (Powers et al., 2018; Marshall, 2015). Nevertheless, rigorous clinical indications limit its practical application. Of these limitations, the major restraint is the narrow therapeutic window which is usually less than 4.5 h from symptom onset to treatment (Powers et al., 2018). In addition, current rtPA medication has an extremely short half-life of approximately 4–6 min, mainly due to the fast-acting endogenous inhibitors in circulation, such as plasminogen activator inhibitor-1 (Colucci et al., 1986). Therefore, to achieve effective thrombolysis it is necessary to administer a bolus injection followed by continuous intravenous maintenance and this poses a risk of hemorrhagic complications which to some extent are inevitable. Although the indications are strictly applied, there is still a 2–7% risk of fatal intracranial hemorrhage to be considerate of (Yaghi et al., 2017). Moreover, rtPA merely functions on the surface of the thrombus and fails to break down some large embolisms occluding the proximal cerebral arteries, derived from the heart or systemic circulation, as well as older artery atherosclerotic plaque and clots (Singh et al., 2013). Even if temporarily dissolved by tPA, re-occlusion of the artery and dissolved components of the emboli spread to the distal arterioles, which may contribute to the fluctuation of symptoms and the deterioration of functional outcomes (Heo et al., 2003). Furthermore, the neurotoxic effects of exogenous tPA cannot be ignored. It has been revealed that tPA upregulates various metalloproteases (MMPs), leading to matrix degradation and blood-brain-barrier (BBB) breakdown (Kelly et al., 2006). In addition, tPA activates N-methyl-D-aspartate (NMDA) receptors and results in an influx of calcium and NMDA receptor-mediated neural apoptosis, augmented dilation of the cerebral vasculature of the healthy brain, and thus aggregated ischemia of the stroke lesion (Yepes et al., 2009; Abu Fanne et al., 2010). Although the molecular bases by which tPA causes these neurotoxic effects has not been fully elucidated, they may serve as potential targets for the combined delivery strategy of tPA and neuroprotectants.
Figure 1.
Schematic illustration of acute ischemic stroke and the function of tissue plasminogen activator (tPA) for thrombolysis treatment. The supplying vessel (middle cerebral artery [MCA]) was blocked by thrombosis in situ and the blood supply is halted within the MCA territory. The brain tissue of this region will experience oxygen and glucose deprivation and, ultimately, an irreversible infarct will develop if the blood supply is not restored. Timely recanalization of the supplying vessel is one of the most effective strategies for treatment, at early stages of acute ischemic stroke. The thrombus is generally comprised of aggregated activated platelets, red blood cells, and cross-linked fibrin monomers. tPA physiologically functions as a pivotal thrombolytic agent which could convert plasminogen to plasmin, and the latter could degenerate the cross-linked fibrin monomers to fibrin degradation products and thus dissolve the main structure of the thrombus.
To seek optimal therapy and clinical benefits, as well as compensate for the limitations of intravenous administration of rtPA alone, the increasingly matured technique of endovascular thrombectomy has gradually become an essential part of AIS treatment, especially for patients suffering from large vessel occlusions and treated within a specific time window (Powers et al., 2018). It has been consistently verified that as compared to rtPA administration alone, the combination of rtPA followed by endovascular therapy, improves long-term outcomes with comparable mortality and rates of symptomatic intracranial hemorrhage (Berkhemer et al., 2015; Saver et al., 2015). However, only a minority of patients with large vessel occlusion have the chance to undergo the operation due to the complex screening process, and the fact that the endovascular operation is highly specialized and demanding, which requires it to be performed in comprehensive stroke centers.
Overall, despite the limitations for widespread application and even potential detrimental risks, the intravenous administration of rtPA for thrombolysis is still the primary and most highly recommended option for AIS treatment. Therefore, the pursuit of feasible strategies for enhancing thrombolysis efficacy remains an unsettled task for researchers.
2. Strategies of targeted nano-delivery for facilitating thrombolysis treatment
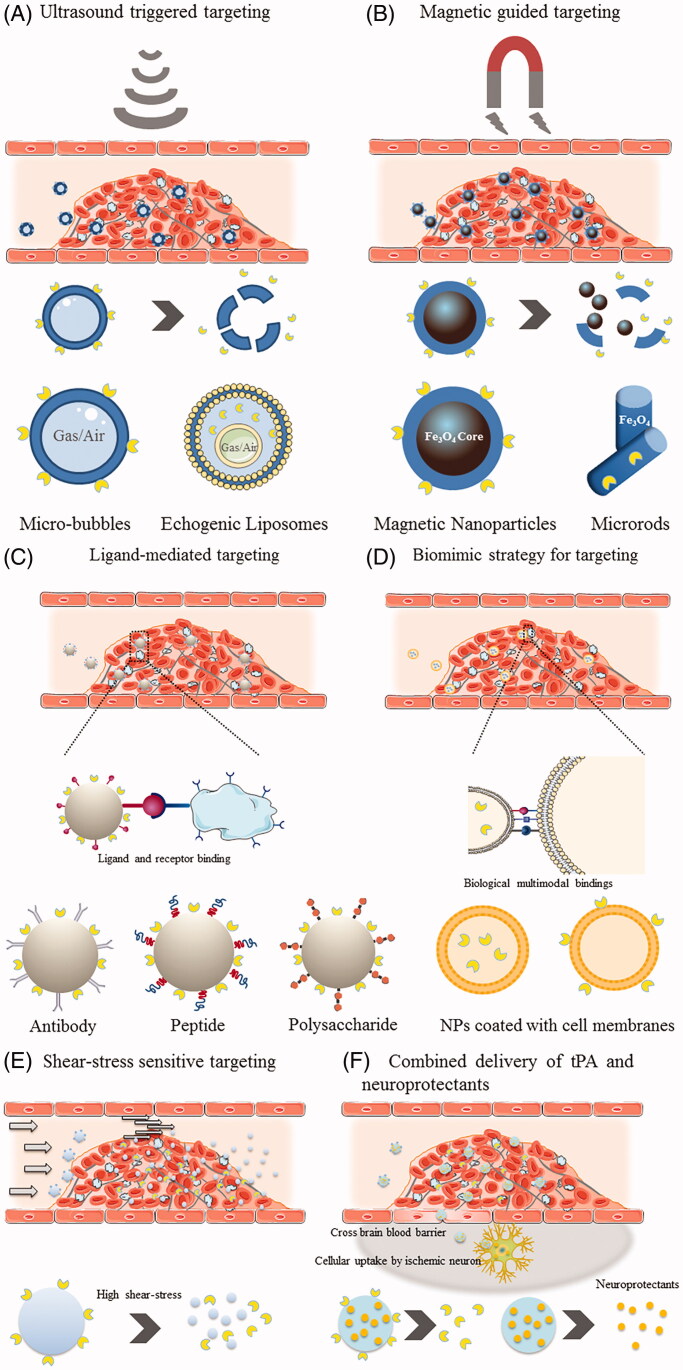
One of the most desirable innovations required for tPA to exert its thrombolytic effect more efficiently is the modification and optimization of drug delivery. To this end, nanotechnology has proved to be a promising strategy. Therefore, to further overcome the drawbacks involved in the traditional delivery of tPA, drug delivery utilizing various vehicles and stimuli for facilitating thrombolysis treatment has aroused a great interest in researchers and has emerged in the field of nanothrombolysis. The aim of nanothrombolysis is to protect and prolong the circulating time of tPA to reduce the total dosage, to precisely target the site of thrombus, and to improve thrombolytic efficacy in a controlled manner to minimize off-target effects and hemorrhagic complications. Additionally, reduced toxicity, superior biocompatibility, non-immunogenicity, and biodegradability of the applied materials should also be a consideration. It is worth noting that because the thrombus is located in the systematic circulation, thrombolytic agents only need to reach the targeted site of the occlusive thrombus, without the need to overcome or circumvent the BBB and reach the brain parenchyma. Instead, confining tPA crossing of the BBB and appropriate protection for brain cells should be considered when developing new tPA delivery strategies/formulations (Abu Fanne et al., 2010). With regard to the use of nanomaterials for carrying tPA, various polymeric materials have been employed, such as poly (lactide-co-glycolide) (PLGA), poly (lactic acid) (PLA), poly (alkyl cyanoacrylate) (PACA), poly (acrylic acid) (PAA), and natural polymers like chitosan, gelatin, etc. Additionally, liposomes as phospholipid-based vesicles with high resemblance to cell membranes, are also preferred, owing to their outstanding biocompatibility and free of immunogenicity. To accommodate the demand for thrombus targeting, surface modification with diverse moieties can be easily achieved using these materials. Some specific formulations such as microbubbles (MBs), echogenic liposomes (ELIPs), and magnetic nanoparticles (MNPs) have been frequently adopted in combination with ultrasound or magnetic force, for passive physical targeting and penetration capacity into clots. In particular, breakthroughs in biogenetic and biomimic nanomaterials have enriched the options for targeted tPA delivery, and currently predominate the new generation of nano-based thrombolysis. In the following section, we provide readers with a comprehensive cataloging of studies that utilized various targeted nano-delivery strategies for facilitating thrombolysis treatment with representative studies. Schematic illustrations of various targeted nano-delivery strategies for facilitating thrombolysis treatment are depicted in Figure 2.
Figure 2.
Schematic illustration of various targeting strategies for facilitating thrombolysis treatment. (A) Ultrasound triggered targeting. (B) Magnetic guided targeting. (C) Ligand-mediated targeting. (D) Biomimetic strategy (Cell-membrane camouflage) for targeting. (E) Shear-stress sensitive targeting. (F) Combined delivery of thrombolytics and neuroprotectants.
2.1. Ultrasound triggered targeting
As a regular imaging tool in clinical practice, ultrasound has the advantages of being noninvasive, convenient, and safety for diagnosis. In the field of ultrasound-based thrombolysis, that is, sonothrombolysis, ultrasound could be used to exert a thrombolytic effect itself or can be applied as an adjunctive therapy combined with tPA or other tPA delivery strategies (Eggers et al., 2003; Alexandrov et al., 2004; Rubiera & Alexandrov, 2010). The mechanisms of ultrasound for thrombolysis are generally proposed as directly inducing mechanical disruption and accelerating the transport of thrombolytic agents, promoting a deeper penetration into the thrombus by acoustic cavitation, microstreaming, and radiation force (Miller, 1987, 1988; Francis et al., 1995; Everbach & Francis, 2000; Sakharov et al., 2000; Polak, 2004). To make the most of the thrombolytic activity of ultrasound, contrast agents, for example MBs and ELIPs, have been widely explored to trigger site-specific drug release and enhance thrombolytic effect synergistically by cavitation-related mechanisms when exposed to ultrasound, thereby increasing the concentration of tPA locally, effectively reducing the therapeutic dose, and minimizing the risk of hemorrhage side effects (Perren et al., 2008; Smith et al., 2010; de Saint Victor et al., 2014). To date, multiple randomized clinical trials focusing on human stroke have been carried out to investigate the safety and efficiency of sonothrombolysis combined with tPA, as well as with the assistance of contrast agents (Alexandrov et al., 2004; Perren et al., 2008; Saqqur et al., 2014; Nacu et al., 2015; Nacu et al., 2017; Kvistad et al., 2018; Alexandrov et al., 2019). The CLOTBUST trial, the first international prospective phase II clinical trial to examine ultrasound in combination with tPA, verified that ultrasound could augment tPA-induced recanalization, with a non-significant trend toward a higher rate of recovery (Alexandrov et al., 2004). Recently, the phase III trial of CLOTBUST-ER, the largest trial conducted to date validating the safety and efficacy of a novel operator-independent ultrasound in patients treated with rtPA after AIS. This study demonstrated comparable data between the sonothrombolysis group and the sham ultrasound group in both safety indicators and a higher rate of complete recanalization, however in indicators of functional outcome improvement, no significant differences were detected (Alexandrov et al., 2019). Of note, in contrast to ultrasound frequencies aimed at temporarily opening the BBB, relatively high frequencies are usually used for sonothrombolysis in animal (27–200 kHz) and clinical (2 MHz) studies, to avoid breakdown of the BBB and minimize the occurrence of intracranial hemorrhage (Daffertshofer & Hennerici, 2003; Tsivgoulis et al., 2010; Ricci et al., 2012). MBs are gas- or air-filled microspheres ranging from hundreds of nanometers to one micron in diameter, and can also be applied alone or loaded with tPA for thrombolysis (Hua et al., 2010; Hua et al., 2014; Yan et al., 2017) (Figure 2(A)). In vitro and in vivo studies have suggested that MBs carrying tPA and Arg-Gly-Asp-Ser tetrapeptide could be prepared by lyophilization, and these tPA-loaded MBs plus ultrasound, showed superior thrombolytic efficacy, lowered the tPA dosage, and potentially decreased hemorrhagic risk (Hua et al., 2010; Hua et al., 2014). ELIPs are multifunctional phospholipid-bilayer encapsulated carriers, which could be used as contrast agents and also deliver and trigger site-specific release of tPA under ultrasound guidance (Smith et al., 2010) (Figure 2(A)). Various ELIPs have been investigated and been shown to be capable of specific delivery to the thrombus site and improved the ultrasound-mediated thrombolytic efficacy (Shaw et al., 2009; Laing et al., 2012; Hagisawa et al., 2013; Shekhar et al., 2017). Laing et al. explored the thrombolytic efficacy of a tPA-loaded ELIP formulation, in an in vivo rabbit model with an abdominal aortic thrombus. The results showed that tPA-loaded ELIP was the most efficacious regimen for thrombolysis than tPA alone and tPA mixed with MBs (Laing et al., 2012). In Kandadai’s work, a novel monodisperse rtPA-loaded ELIP (µtELIP) loaded with perfluorocarbon gas, was generated using a microfluidic flow-focusing device. This technique could effectively improve the encapsulation of both rtPA and perfluorocarbon MBs within µtELIP (Kandadai et al., 2016). Besides MBs and ELIPs, other ultrasound responsive nanomaterials for delivering tPA have also been investigated. In Uesugi’s study, a cationic tPA–gelatin complex was incorporated with polyethylene glycol (PEG) on the surface. The PEG-modified complexes were shown to significantly suppress to 45% of the original tPA activity and could fully recover after ultrasound irradiation. In a rabbit model of thrombosis, the intravenous administration of the complexes followed by ultrasound resulted in complete recanalization (Uesugi et al., 2010). Recently, a urokinase-type plasminogen activator (uPA) loaded with hollow nanogels (nUK) was synthesized and explored in a rat model of stroke. The nUK was synthesized by a one-step reaction of glycol chitosan and aldehyde-capped poly (ethylene oxide). The results proved a controlled release triggered by ultrasound and enhanced thrombolysis efficiency of nUK with a smaller infarct volume and better clinical scores. Furthermore, the nUK formulation also prolonged the circulation duration time and potentially protected the BBB without increasing hemorrhagic risk (Teng et al., 2018). Recently, Correa-Paz et al. produced sub-micrometric CaCO3-templated polymer capsules using the layer-by-layer method for ultrasound-controlled delivery of rtPA. It was demonstrated that encapsulation of rtPA prevented endogenous biological inactivation without interfering with the thrombolytic activity, and the efficacy for breaking down blood clots was improved upon ultrasound stimulation, in vitro. Additionally, the ultrasound triggered delivery of rtPA from capsules was demonstrated in vivo, and the encapsulation greatly extended the half-life and activity of rtPA in mice compared with the non-encapsulated one (Correa-Paz et al., 2019).
2.2. Magnetic guided targeting
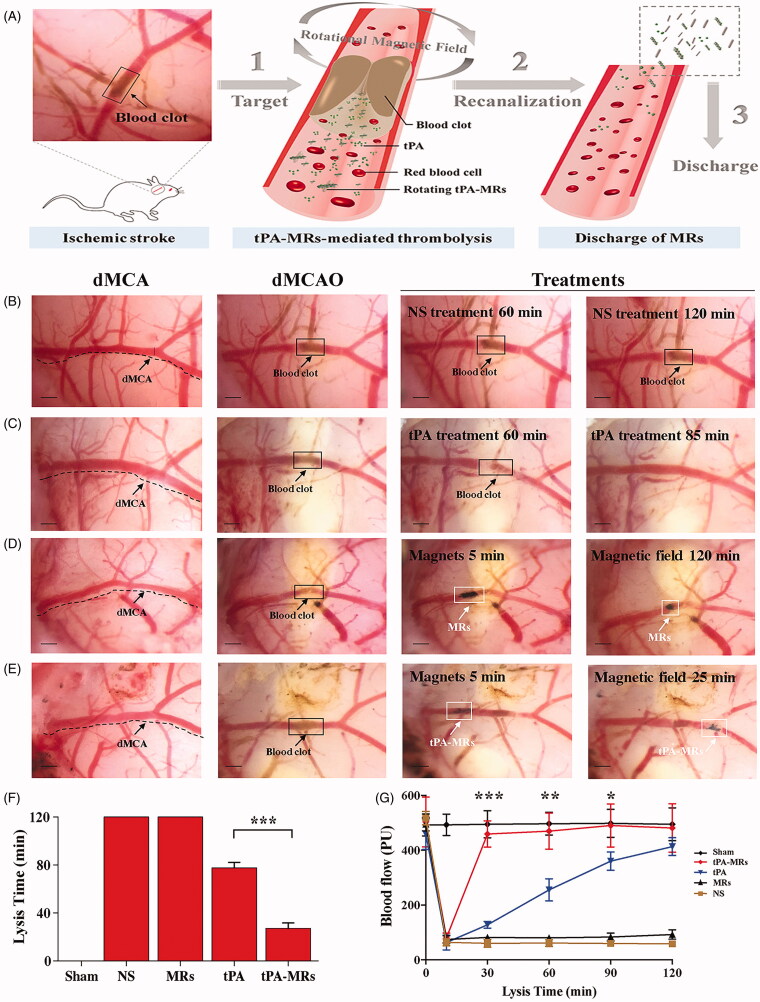
Magnetic guided targeting is another potential physical strategy which functions by applying an external magnetic field to manipulate magnetic nanoparticles (MNPs) carrying drugs to achieve site-specific targeting, in a controlled method (Ma et al., 2009; Yang et al., 2012; Chen et al., 2016; Tadayon et al., 2016; Hu et al., 2018; de Saint Victor et al., 2019; Liu et al., 2019; Zhang et al., 2019). MNPs are suitable candidates for a multitude of biomedical applications not limited to drug delivery including imaging and biosensing, and the combination of these functions makes it possible to realize the diagnosis and treatment of diseases simultaneously, making it a popular clinical pattern of theranostics. MNPs are usually composed of an iron oxide core (Fe3O4 or γ-Fe2O3) and an organic or inorganic shell as a surface coating (Figure 2(B)). The aim of surface coating is to encapsulate the magnetic core to decrease hematological toxicity, minimize particle aggregation, improve biodegradability and biocompatibility, and add functionalized modification. In the pioneering work of Ma and colleagues, who produced a PAA MNP and covalently binded rtPA on the particle and investigated the feasibility and efficacy of targeting thrombolysis. They found that intra-arterial administration of MNP-rtPA restored the iliac blood flow to 82% of that before the clot lodging, within 75 min, and targeted thrombolysis was achieved with <20% of the regular dose of rtPA (Ma et al., 2009). Yang et al. conjugated rtPA to Fe3O4 MNPs coated with poly [aniline-co-N-(1-one-butyric acid) aniline] for targeted thrombolysis. With this nano-formulation, improved thrombolysis efficacy and reduced clot lysis time (from 39.2 ± 3.2 min to 10.8 ± 4.2 min) were proved in vitro. In addition, 20% of the regular rtPA dose was used for magnetic nanocarriers to restore blood flow without triggering hematological toxicity (Yang et al., 2012). A similar thrombolysis efficacy (with only 20% of regular dose of rtPA) also confirmed with the chitosan MNP nanocomposites (Chen et al., 2016). Besides improving the targeting capability, as a useful adjuvant approach for thrombolysis, an external rotation field could be utilized to induce a magnetic force which subjects the magnetic nanocomposites to a mechanical dragging force and helps to penetrate into clots, therefore enhances clot lysis efficiency (Hu et al., 2018; Huang et al., 2019; Zhang et al., 2019). Recently, Huang et al. confirmed the value of PAA MNPs as a feasible and effective carrier for targeted tPA delivery with a rotating magnetic field in a rat model of distal middle cerebral artery occlusion, evidenced by an increased lysis efficiency and a reduced infarct area of the brain (Huang et al., 2019). Hu et al. incorporated tPA into porous magnetic Fe3O4-microrods (tPA-MRs) to facilitate targeted thrombolysis in a mouse model of ischemic stroke. They demonstrated that with the aid of an external magnetic field, tPA-MRs could target the distal cerebral artery and dissolve clots via both tPA (chemical lysis) and rotating MRs (mechanical lysis) and significantly improved tPA-mediated thrombolysis at lower concentrations and in less than 1/3 of the time required to lyse the blood clot (Figure 3) (Hu et al., 2018). In addition to traditional Fe3O4 MNPs carrying rtPA, other novel magnetic nanocomposites have also been explored by researchers owing to their more strategic advantages for drug delivery. Tadayon et al. applied an extracellular biological synthesis of nanoparticles (CuNP) with optimized a Streptococcus equi supernatant and immobilized tPA and streptokinase (SK) to the MNP. They showed that SiO2-MNP-tPA-SK targeted site-specific thrombolysis, under the guidance of an external magnet field by using a rat model of embolic stroke (Tadayon et al., 2016). In Liu et al.’s investigation, a thermosensitive magnetoliposome was produced and optimized. This study demonstrated, for the first time, that intravenous administration of magnetoliposomes with thermal controlled release of rtPA, induced effective thrombolysis in a rat model of embolic stroke, thus achieving dual targeting by both magnetic and thermal manipulation (Liu et al., 2019). The efficacy of sonothrombolysis with MBs was limited due to the hydrodynamic conditions in the occluded vessels. By taking advantage of its magnetic properties, magnetic MBs can be retained by an outer magnetic field against blood flow, and studies have shown that the use of magnetic MBs significantly increases the lysis rate of blood clots in vitro both under a single permanent and a rotational magnetic field (de Saint Victor et al., 2019; Zhang et al., 2019). Recently, a precision delivery strategy was adopted by combining magnetic targeting and ultrasound-triggered release to accelerate thrombolysis. In an investigation by Wang et al., a porous magnetic microbubble was developed to formulate tPA, and this nanoparticle-shelled microbubble (MMB-SiO2-tPA) was fabricated through self-assembly at the liquid-air interface. This nano-formulation satisfied the criteria for activity maintenance in circulation, targeting clots, and penetration into clots. In a mouse model of venous thrombosis, the residual thrombus was dramatically decreased by 67.5% with MMB-SiO2-tPA, as compared with conventional injection of tPA (Wang et al., 2020).
Figure 3.
Tissue plasminogen activator-porous magnetic microrods (tPA-MR) for targeted thrombolytic therapy. (A) Illustration of a new strategy for targeted thrombolytic therapy by tPA-MR with a rotational magnetic field using a mouse model. (B)–(E) The tPA-MRs-mediated thrombolysis effect in a mouse model. (B) The representative images of thrombolysis in a distal middle cerebral artery occlusion (dMCAO) mouse, treated with normal saline. (C) With the treatment of tPA (10 mg/kg), the blood clot was lysed 85 min after injection. (D) The representative images of thrombolysis in the dMCAO mouse treated with MRs (1 mg/kg) under a 5 rotational magnetic field (RMF, 20 Hz, 40 mT). (E) In the tPA-MRs-treated (1 mg/kg) group, thrombus could be lysed in 25 min under a rotational magnetic field (RMF, 20 Hz, 40 mT). (F)-(G) The lysis time for recanalization is significantly shortened with tPA-MRs. Adapted with permission from Ref. (Hu et al., 2018). Copyright 2018 American Chemical Society.
2.3. Ligand-mediated targeting
Conjugation with functional ligands is a highly versatile strategy to improve specific targeting between the ligands of the nanocarrier and the receptor molecules at targeted sites (Figure 2(C)). In the field of nanotechnology-assisted thrombolysis, this targeting strategy is widely applied. TPA as a fibrin-specific thrombolytic agent, has intrinsic affinity and specificity for fibrin, and this characteristics can be utilized to locate thrombi without additional binding (Tiukinhoy-Laing et al., 2007; Deng et al., 2018). Conjugation with functional ligands may theoretically offer a more specific binding property for targeting, thereby locally enhancing thrombolysis efficacy and minimizing the total dosage needed and reducing hemorrhagic risk. In view of the two main constituents of thrombus, activated platelet and fibrin-specific ligands are extensively recognized for targeted delivery strategies. One popular biomarker for targeting is platelet glycoprotein IIb/IIIa (GP IIb/IIIa). It is the most abundant receptor expressed on the platelet surface, and when platelets are activated, GPIIb/IIIa is converted from a low-affinity to a high-affinity state, allowing the binding of fibrinogen, platelet aggregation, and thrombus formation (Schwarz et al., 2006). An alternative target on platelets is P-selectin, a glycoprotein exposed on the surface of activated platelets. In addition, factor XIII, fibrin, and fibrin-fibronectin complexes, have also been explored as homing targets for thrombolytic delivery. In addition to acquiring high-binding efficacy, ligand-mediated targeting could be readily combined with other strategies to provide a multivalent design, for example, in combination with ultrasound-triggered MBs (Alonso et al., 2009; Wang et al., 2012; Wang et al., 2016), liposomal bubbles (Hagisawa et al., 2013) and magnetic-guided MNPs (Zhou et al., 2014; Chen et al. 2020). According to the various types of ligands, functional ligands could generally be classified as peptides, antibodies and polysaccharides, among which Arg-Gly-Asp (RGD) is the most frequently applied peptide modification for ligand-mediated active targeting.
2.3.1. Peptide-mediated targeting
RGD peptide, is a receptor antagonist of platelet membrane GP IIb/IIIa, and thus nanomaterial modified with a RGD motif could bind activated platelets at the thrombus site (Huang et al., 2008). For the dual purpose of both precise positioning of the thrombi and qualitative detection and dynamic monitoring of the thrombolytic efficiency, Fe3O4-based PLGA NPs with cyclic Arg-Gly-Asp (cRGD) peptide grafting onto the chitosan surface were constructed. The Fe3O4-based NPs could be imaged using a clinical MRI scanner, and the cRGD peptide possessed a higher affinity for the activated platelets at the thrombus site. In vitro and in vivo experiments confirmed that the nanocomposites had a good affinity to thrombi and exhibited strong thrombolysis and contrast-enhanced effects (Zhou et al., 2014). Such a theranostic strategy, as a potential dual function tool is helpful for managing therapeutic outcomes of thrombolysis. In another study of peptides targeting fibrin-fibronectin complexes, a clot-targeting peptide (CNAGESSKNC) was identified by phage display and combined with a newly developed direct fibrinolytic agent, microplasmin. They were fused on the surface of ferritin, constructing a double-chambered nanocage to protect the activated microplasmin from its circulating inhibitors. Moreover, the clot-targeting peptide provided superior affinity to compensate for the inefficient targeting specificity of microplasmin. This targeted ferritin-microplasmin based nanocage platform promoted a prolonged circulatory life of microplasmin and improve thrombolytic efficacy in both arteries and veins in animal models (Seo et al., 2018). A notable example involving hetero-multivalent ligand-targeted thrombolysis was shown by the work of Pawlowski and colleagues, who developed platelet microparticle-inspired nanovesicles (PMINs) based on a liposome system, which could protect encapsulated thrombolytic drugs in circulation, anchor them actively onto thrombus via ligand-mediated binding to GP IIb/IIIa and P-selectin, and trigger drug release by the clot-relevant enzyme phospholipase-A2. Based on this sophisticated design, the release of the thrombolysis agent streptokinase, could be successfully triggered and elicit a thrombolysis effect in vitro, and further intravenous delivery of PMINs can render targeted fibrinolysis without influencing systemic hemostasis in a mouse model of carotid artery thrombosis (Pawlowski et al., 2017). Recently, a PLGA MNP was formulated by conjugating rtPA and a fibrin-avid peptide (GPRPPGGSKGC) for dual targeted rtPA delivery. It was indicated that combining magnetic guidance and fibrin binding led to a higher thrombolysis rate in vitro, and in an in vivo rat model of embolic stroke, this nano-formulation used only 20% of the free rtPA dosage to restore blood flow in the targeted thrombus (Chen et al. 2020).
2.3.2. Antibody-mediated targeting
In the work by Marsh et al., fibrin-specific homing capability was achieved by loading an anti-fibrin monoclonal antibody onto the surface of a perfluorocarbon (PFC) nanoparticle. The homing capability of the thrombus was validated in dogs using fluorescent microscopy. Furthermore, it was demonstrated that one PFC nanoparticle could accommodate up to 40 anti-fibrin antibodies and 400 urokinase molecules, and therefore offered effective targeted delivery of a thrombolysis agent (Marsh et al., 2011). To enhance the specificity of targeting, Wang et al. designed an innovative theranostic MB by conjugating anti–GPIIb/IIIa single-chain antibodies (scFvs) and a novel form of thrombolytic agent, recombinant urokinase plasminogen activators (scuPA). They demonstrated that the theranostic MB could provide highly sensitive detection of thrombi in vivo and monitor the size of thrombi in real time, while also reducing thrombus size in response to the therapeutic payload, thereby simultaneously diagnosing and treating thrombosis (Wang et al., 2016).
2.3.3. Polysaccharide-mediated targeting
Fucoidan is a sulfated L-fucose-based polysaccharide mainly extracted from brown seaweed and has proved to be an efficient glycosidic ligand of P-selectin (Bachelet et al., 2009). Juenet et al. showed that the interaction of P-selectin and fucoidan was chosen as the molecular basis for promoting the specific targeting of thrombus. Polysaccharide-poly(isobutylcyanoacrylate) nanoparticles functionalized with fucoidan and loaded with rtPA were designed. The binding capability to both recombinant P-selectin and activated platelet aggregates and fibrinolytic activity were validated in vitro, and the thrombolysis efficiency was demonstrated by monitoring the platelet density via in vivo intravital microscopy (Juenet et al., 2018).
Nevertheless, synthetic nanoplatforms decorated with functional ligands are sometimes confined in further translational applications owing to their inherent drawbacks. First, the simple functional binding between nanocomposites and thrombus cannot accurately recapitulate the complex, internal interactions that naturally occur within the body. Additionally, these nanomaterials can be inevitably recognized and cleared by the immune system even with surface modifications for hiding, and this may impair the in vivo efficacy of therapeutic payloads, reduce their availability at the clot site, and hinder the efficacy of repeated administration. Finally, the process for identification of novel ligands for binding usually takes time and much effort.
2.4. Biogenic and biomimetic strategies
To overcome these drawbacks, biogenic and biomimetic drug delivery strategies have been thriving as up and coming design strategies. Taking inspiration from nature, these approaches directly leverage naturally existing biogenic material, generally the membrane or membrane fragments of cells, or imitate biological principles and systems by designing synthetic or semi-synthetic nanocarriers that navigate and interact with the complex internal environment and homeostasis more effectively (Dehaini et al., 2016; Fang et al., 2017; Chen et al., 2019). Compared to the aforementioned conventional strategies for targeted thrombolysis, the biological delivery strategies show advantages in reduced immunogenic risks, great biocompatibility, and prolonged circulation time. More importantly, some could even be endowed with superior targeting capability by providing sophisticated multimodal bindings with cellular interactions that the synthetic ones cannot compare (Figure 2(D)).
2.4.1. Natural carriers for targeting
The original natural carriers were directly making use of naturally existing materials, like cells or cell membranes. The most frequently utilized natural nanocarrier for drug delivery refers to red blood cells (RBCs) because RBCs are the most abundant blood cells with a relatively long circulation time (Villa et al., 2015; Villa et al., 2016). For instance, Danielyan et al. coupled tPA to RBCs (RBC/tPA) and evaluated the thrombo-prophylactic effect in a cerebral thromboemboli model. tPA was conjugated on the surface of RBCs via biotin-streptavidin chemistry. By applying RBC/tPA, nascent cerebral thromboemboli were rapidly lysed with durable reperfusion and reduced morbidity and mortality, whereas application of tPA failed to achieve such therapeutic effects, even at a 10-fold higher dose (Danielyan et al., 2008). Owing to the large particle size of the RBC/tPA complex, it was confined in the blood circulation and could hardly infiltrate into the brain parenchyma or firm attachment onto mature clots, neural toxicity of tPA and the risk of intracranial hemorrhage was lowered, and thus this type of RBC/tPA was generally proposed for thromboprophylaxis (Murciano et al., 2003; Murciano et al., 2009; Zaitsev et al., 2010a; Zaitsev et al., 2010b).
2.4.2. Biomimetic strategies for targeting
Inspired by the characteristics of RBCs, Colasuonno et al. conjugated tPA to the polymeric structure of porous, long-circulating discoidal nano-constructs (tPA-DPNs), to mimic RBCs. The nano-constructs are composed of a mixture of PLGA and PEG. The erythrocyte-inspired tPA-DPNs could not only preserve the lytic activity of tPA with their porous matrix and long blood circulation time, but also dissolve clots more efficiently than free tPA and comparable spherical tPA-coated nano-constructs in a mouse model of thrombosis (Colasuonno et al., 2018). In addition, erythrocyte-derived theranostic nanoparticles were intellegently devised (Vankayala et al., 2018). The nanoparticles contain fabricated constructs derived from nanosized erythrocyte ghosts. By encapsulating FDA-approved indocyanine green and through covalent attachment of tPA to its surface, the erythrocyte-derived nanoparticles offered dual functionality for both near-infrared fluorescence imaging and thrombolytic capability. Using an in vitro clot model, the dual functionality of imaging and clot lysis was demonstrated. It should be noted that RBC-based materials improved the thrombolytic capability mainly by prolonging the circulation time, but lacking clot-targeting capability without any additional targeting modifications. In addition to RBCs, considering the pivotal role of activated platelets in the pathogenesis of thrombosis (Ruggeri, 2002), circulating activated platelets could be recruited to the site of the injured vessel and become one of the major components of thrombus. Functional receptors responsible for cell attachment are abundantly expressed on the platelet membrane. Therefore, it could be suggested that platelets and their derived materials have been a potentially desirable strategy in the design of nanocarriers for drug delivery (Hu et al., 2015; Li et al., 2018; Xu et al., 2020). Li et al. fabricated platelet membrane-derived biomimetic nanobubbles (PNBs) serving as a novel theranostic nano-thrombolysis agent for both timely microvascular recanalization and ultrasound imaging in a photothrombotic ischemic stroke model. Aside from its imaging capability with real-time contrast-enhanced ultrasound, the natural lipids and protein components isolated from the platelet membrane endow the PNBs with accurate lesion-targeting, and additional microvascular bio-remodeling ability within the stroke lesion, which means PNBs could act as a potential agent for vascular recanalization and have a profound impact on extending the therapeutic time window for further treatment (Li et al., 2018). Inspired by the pathophysiological functions of platelets during thrombo-genesis, platelet membrane-camouflaged polymeric nanoparticles (nanoplatelets) were recently developed by Xu et al. for the delivery of rtPA to local thrombus sites. Nanoplatelets composed of PLGA cores coated with platelet membranes and rtPA were then chemically conjugated to the membrane. The nanoplatelets exhibited improved therapeutic efficacy with a lower risk of bleeding than free rtPA, in various thrombosis disease models. Particularly, compared to either saline or free rtPA, the therapeutic effects of nanoplatelets were demonstrated in a mouse model of ischemic stroke, including markedly lower neurological deficit scores, higher survival rate, smaller ischemic area, and less neuronal necrosis (Figure 4) (Xu et al., 2020).
Figure 4.
Platelet membrane-camouflaged polymeric nanoparticles for targeted thrombolysis (A) Schematic illustration of the synthesis of the platelet membrane-cloaked polymeric nanoparticles (PNP) conjugated with rtPA (PNP-PA) nanoparticles. Briefly, the membrane of platelets (scale bar = 1 μm), acquired from the whole blood of mice, were used to coat the PLGA cores (scale bar = 400 nm). TPA was subsequently conjugated via the –SH groups onto the surface of the platelet membrane to form PNP-PA. (B) The proposed mechanism of action in vivo: mimicking platelets, PNP-PA are specifically targeted to the thrombus and dissolve the fibrin clot. (C)-(G) Therapeutic effects of PNP-PA in transient middle cerebral artery occlusion (MCAO) mouse model. (C) Neurological deficit scores in the indicated treatment groups of MCAO mice. (D) Survival rate of MCAO mice treated with the indicated formulations. (E) Representative 2, 3, 5-triphenyltetrazolium chloride (TTC) staining images of MCAO mouse brains, treated with the indicated formulations. (F) Histological examination staining of MCAO mouse brains. Scale bar: 200 µm. (G) Immunofluorescence staining of neurons in MCAO mice treated with the indicated treatments. Scale bar: 200 µm. Adapted with permission from Ref. (Xu et al., 2020). Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.4.3. Shear-stress sensitive targeting
Another bioinspired design for targeted tPA delivery is a biophysics-based approach that leverages high shear stress at the obstructed or stenotic blood vessels (Saxer et al., 2013; Korin et al., 2015; Epshtein&Korin, 2017). Owing to the abnormal hemodynamics at the thrombus site, platelets could sense these abnormally high shear stresses when flowing through the stenosis vessels, and then respond by activating and sticking to the vascular wall, thereby aggregating at the thrombus sites. Inspired by this natural physical mechanism of targeting, shear-activated nanotherapeutics (SA-NTs) were fabricated as aggregates of multiple smaller nanoparticles and SA-NTs remained intact when circulating under physiological flow conditions, but dispersed into small components when exposed to local high shear stress (Figure 2(E)). Initially, shear-activated nanotherapeutics focused on the delivery of thrombolytic drugs. SA-NTs coated with tPA were produced and tested in an in vivo embolism model by Korin et al. By means of shear-induced release of tPA-coated NPs, SA-NTs reopened the obstructed arteries and significantly delayed the time to vessel occlusion, with a dose of approximately 1/100th of that required for induction of similar effects by free tPA (Korin et al., 2012). Later, Marosfoi et al. assessed the efficacy and safety of SA-NTs targeted tPA delivery using a combination of the temporary endovascular bypass technique in a rabbit model of carotid vessel occlusion. Compared with various comparable control groups, this combined approach was superior, showing high rates of complete vascular recanalization. Further histological analysis showed significantly lesser vascular injury than that with the stent-retriever thrombectomy (Marosfoi et al., 2015). Since the stenosis of blood vessels is a common pathological feature associated with various diseases including ischemic stroke, cardio vascular and peripheral vascular disease, the shear-targeting strategy renders a universal platform which could be broadly applied within a range of vascular diseases.
In the addition to the above targeting approaches, other smart bioinspired strategies have been put forward and enlightened researchers about the construction of nanosysterms for targeted tPA delivery, although some of which may not be validated in the ischemic stroke models. In the work of Absar et al., they constructed an albumin-camouflaged and thrombin-triggered bio-responsive system for targeted tPA delivery and release (Absar et al., 2014). TPA was conjugated with human serum albumin by linking with a thrombin cleavable peptide linker (GFPRGFPAGGC), which was cleavable contacting with endogenous thrombin produced at the clot microenvironment for triggering the release of tPA, and albumin in this nanofomulation was used for shielding tPA and temporarily suppressing the enzymatic activity in the blood circulation. It was indicated that shielding with albumin suppressed 75% of tPA's activity and regained up to 90% of that of native tPA upon contact with 25 nM thrombin. In vivo thrombosis model also demonstrated the similar thrombolytic activity to that of native tPA and reduced circulating fibrinogen degradation. Similarly to this bio-responsive strategy, an albumin-based heparin triggered nanoconstruct has also been explored by the same team (Absar et al., 2012; Absar et al., 2013). In this nanosysterm, thrombolytic activity of tPA were reduced by conjugation with low molecular weight heparins and camouflaged with human serum albumin, and could be further provoked by site-specific delivery of heparin at therapeutic concentration.
2.5. Combination delivery of thrombolytics and neuroprotectants
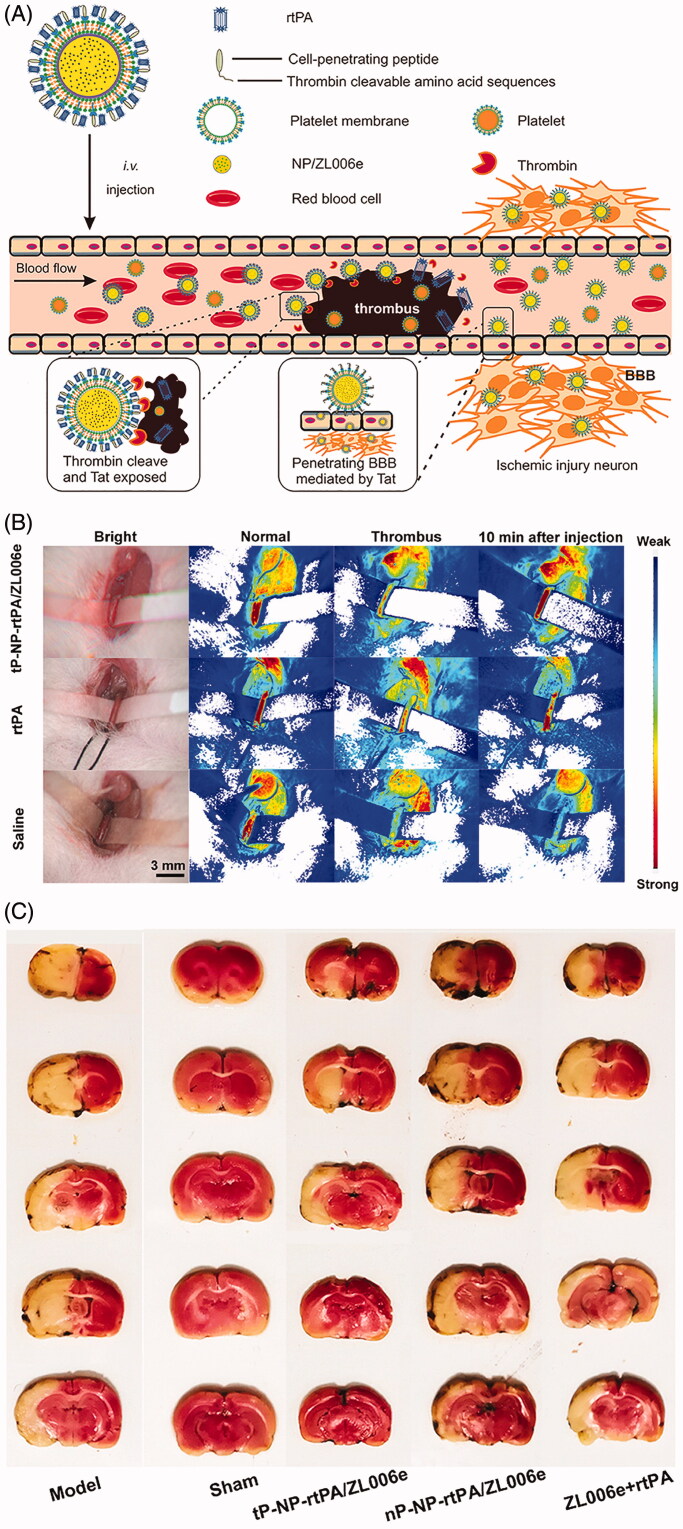
Besides the narrow therapeutic time window and hemorrhagic complications of tPA, secondary cerebral ischemia/reperfusion (I/R) injury with an explosively increasing amount of reactive oxygen species (ROS) generation is often induced by the restoration of cerebral blood flow of the ischemic brain after tPA administration, which is well known to play an essential role in the pathophysiological process of stroke (Chan, 2001; Kontos, 2001). Generally, multiple mechanisms, including oxidative stress, excitotoxicity, and inflammatory responses, are involved in the progression of I/R injury, ultimately leading to neuronal cell death and the deterioration of neurological function (Ginsberg, 2008). Therefore, revascularization of the occlusive cerebral blood vessel and protection of the survival brain tissues are two of the most fundamental strategies concerning comprehensive AIS treatment, and combination therapy has long been put forward by researchers (Lapchak & Araujo, 2007). The combination of thrombolytics and candidate neuroprotectants that function in various ischemic injury cascades theoretically represents a promising option and exhibits superiority in decreasing the risk of hemorrhagic complications, extending the therapeutic time window for tPA, and further improving neurological outcomes. According to prior investigations, tPA and neuroprotectants can be induced simultaneously or sequentially, usually with free tPA and nanocarriers loaded with specific neuroprotectants (Takamiya et al., 2012; Tiebosch et al., 2012; Petro et al., 2016; Fukuta et al., 2017; Fukuta et al., 2018), and these two agents could be intelligently incorporated into one well-designed nano-formulation, to realize a dual targeting delivery (Mei et al., 2019; Xu et al., 2019) (Figure 2(F)). Fukuta et al. investigated the usefulness of combination therapy with tPA and liposomal fasudil in a rat model of photochemically-induced ischemic stroke (Fukuta et al., 2017). Fasudil, a Rho-kinase inhibitor, has been reported to prevent BBB breakdown and MMP-9 related cerebral hemorrhagic transformation, induced by tPA (Ishiguro et al., 2012). The results indicated that intravenous administration of liposomal fasudil prior to tPA could significantly suppress BBB permeability and MMP activation. Moreover, the combined strategy exhibited significantly stronger neuroprotective effects, even in the case of delayed tPA administration, than that with each treatment alone or with the tPA/fasudil-treated group, suggesting that the combination has the potential to extend the treatment time window of tPA. Furthermore, subsequent validation confirmed the neuroprotective effect of the liposomal fasudil and tPA combination, beyond the expected treatment time window (Fukuta et al., 2018). Of note, by focusing on BBB disruption after ischemic stroke, they demonstrated that the drug permeation into the ischemic region could be realized by taking advantage of the liposomal system via crossing the disrupted BBB, similar to the passive strategy of the enhanced permeability and retention effect, which is widely used in drug delivery to tumor tissues (Maeda et al., 2013). To exert the synergistic effect of thrombolysis and antioxidants, Mei et al. designed tPA-installed, nitroxide radical-containing, poly-ion complex nanoparticles (tPA@iRNP) by self-assembly to broaden the therapeutic window of tPA and to reduce the associated oxidative stress after reperfusion. In this nanosized composite, tPA and low-molecular-weight nitroxide radicals were confined and protected in the core of tPA@iRNP, thus preventing their rapid metabolism and prolonging excretion out of the body. When exposed to the acidic ischemic penumbra region, the nanoparticles collapse to function simultaneously for thrombolysis and ROS scavenging. As a result, in a mouse model of photothrombotic middle cerebral artery occlusion (MCAO), they found that tPA@iRNP significantly decreased the cerebral infarct volume and improved neurological deficit as compared with ‘naked’ tPA, void iRNP, or tPA@niRNP (non-ROS scavenging nanoparticle as a control). Interestingly, tPA treatment-associated subarachnoid hemorrhage could be suppressed through elimination of overproduced ROS (Mei et al., 2019). Recently, Xu et al. develop a bioinspired nanoplatelet for sequential site-specific delivery of rtPA and neuroprotectant ZL006e (a selective ischemia-induced PSD-95/nNOS blocker) to thrombus and ischemic penumbra respectively. A dextran derivative polymeric nanoparticle core loaded with ZL006e and platelet membrane shell conjugated with a thrombin-cleavable Tat-peptide coupled to rtPA was uniquely designed. The nature membrane endowed the nanoplatelet with long circulation time and intrinsic binding to thrombus. After the release of rtPA was triggered by the upregulated thrombin, the Tat peptide was exposed in situ which can mediate enhanced penetration through the BBB into ischemic lesions. It was demonstrated this thrombin-responsive nanoplatelet markedly exerted protective effect in the MCAO model, with a 63% decrease in ischemic area and a 72% decrease in ROS level respectively compared with the combination of free ZL006e and rtPA (Figure 5) (Xu et al., 2019).
Figure 5.
A sequential site-specific delivery of thrombolytics and neuroprotectant for ischemic stroke. (A) Schematic design of targeted delivery of tP-NP-rtPA/ZL006e. A core − shell structured nanocarrier was constructed where the platelet membranes were cloaking the surface of ZL006e-loaded acetal-modified dextran (m-dextran) polymer nanoparticles, and rtPA was decorated on the platelet membranes via click chemistry reaction. To achieve the stimuli-triggered rtPA release and ZL006e BBB-enhanced penetration, a thrombin-cleavable peptide with a sequence of LTPRGWRLGGC26 coupled with Tat cell-penetrating peptide, was introduced as a linker to obtain the tP-NP-rtPA/ZL006e. (B) In vivo targeted thromblysis was validated by the bloodstream recovery of common carotid artery, damaged by 10% FeCl3 for 10 min after injected with tP-NP-rtPA/ZL006e, rtPA, and saline. (C) Indication of neuroprotective efficacy by TTC-stained brain sections of the MCAO model group, sham-operated group, tP-NP-rtPA/ZL006e group, nP-NP-rtPA/ZL006e group, and free ZL006e + rtPA group. The nonischemic area is observed as red, and the infarct area is shown in white. Adapted with permission from Ref. (Xu et al., 2019). Copyright 2019 American Chemical Society.
3. Brief summary and future perspective for targeted thrombolysis therapy
According to current treatment guidelines, one of the most effective drug therapies for the AIS patients is recanalization of the occluded blood vessel with intravenous rtPA administration. However, in real-world clinical settings, a vast majority of patients could not benefit from this highly recommended treatment, mainly for the following reasons: a narrow therapeutic window for drug administration, hemorrhagic complications, and low efficacy for thrombolysis and vascular recanalization. In the past two decades, after rtPA was approved by the FDA for clinical thrombolysis, a variety of attempts were made through intensive collaborative work, to optimize the drug delivery of thrombolysis agents and try to pave the way and facilitate broad clinical application. With the emergence of nanotechnology, a series of targeted delivery strategies for facilitating thrombolysis have been constructively suggested and include, protecting and prolonging the circulating time of thrombolysis agents, precisely targeting thrombus sites, and releasing them at an appropriate rate to improve thrombolytic efficacy, reducing the total dosage and minimizing hemorrhagic complications. Ultrasound-enhanced thrombolysis combined with tPA is a feasible method that has proven to be effective and safe in clinical trials. A consensus has been reached that sonothrombolysis, with proper treatment protocols and settings, combined with tPA, is safe and has the potential for a higher recanalization rate, whereas in the aspects of the efficacy for functional outcome improvement, more evidence is still warranted for further evaluation. In the next steps, there is still space for improvement with further optimization of the ultrasound contrast agents that could facilitate targeted delivery and minimized side effects. Even so, ultrasound-enhanced thrombolysis remains one of the most promising applications and is worthy of deeper exploration in the field of targeted thrombolytic therapy. Moreover, there is a tendency to combine dual or even multiple targeting strategies for varied functional purposes, for instance, achieving superior thrombus binding affinity, exerting synergistic thrombolytic effects, and protecting from systemic clearance. In most cases, external modifications with targeting ligands could be one of the alternative candidate strategies for combining therapeutics, due to its flexible design and reliable targeting effects. In addition, novel theranostic approaches that can simultaneously deliver tPA and provide imaging information for thrombus diagnoses and even visualize thrombus in real time, have been emerging. Such pragmatic approaches would be substantially beneficial for disease diagnoses, treatment, and monitoring with the assistance of imaging tools such as MRI, ultrasound, and near-infrared spectroscopy. With respect to the nanomaterials for tPA delivery, biogenetic tools usually offer superior advantages in biocompatibility and intrinsic capability for homing compared with synthetic options, and some bioinspired and biomimetic strategies present more versatile functionality and flexibility, that could be a trend for the rising generation of tPA delivery strategies. Additionally, the nano-formulations incorporating tPA with neuroprotectants have been innovatively explored with a more elaborative strategy of sequential dual targeting for both thrombus and ischemic brain tissue, aimed at minimizing undesired side effects, broadening the therapeutic window, and achieving stronger neuroprotective effects. Moreover, instead of relying on carrying neuroprotective drugs, some nanomaterials themselves could serve as functional components with neuroprotective or antioxidative properties, and these materials may have the potential to be utilized as multifunctional nanocarriers for the delivery of thrombolytic agents (Lee et al., 2011; Mei et al., 2019). Nevertheless, it should be noted that although many of these strategies have shown promise, there has been minimal translation into clinical applications. First, most nanocomposites were only synthesized and validated at the experimental stage, largely provided with in vitro data and only a minority combined with in vivo data. Moreover, because of the sophisticated physiological construction of the brain vasculature and complex changes that occur at the physiological and molecular levels after AIS, specific elements need to be considered when designing in vivo experiments, which may not be similar to other organs under the context of ischemia and reperfusion injury. However, models that exclusively implicate the carotid or middle cerebral artery for mimicking human AIS are seldomly confirmed. Furthermore, according to diverse targeting strategies and the specific tissue to be verified, heterogeneous models are adopted, usually involving chemically-induced (usually by ferric-chloride) thrombosis with endothelial damage and embolic occlusion models induced by manufacturing or introducing preformed clots. Hence, the discrepancies among the varied models should be considered when interpreting the in vivo results. Moreover, with regard to animal selection for models, vast models have been carried out in rodents and very rarely in non-human primates, which also confine clinical translatability. Given these circumstances, more rigorous and precise experiments are still urgently needed before the clinical trial stage and ultimate translation into clinical application. Overall, the future prospects of targeted drug delivery for facilitating thrombolysis treatment are promising, and continued advancement along these trajectories will undoubtedly yield revolutionary progress and ultimately overcome barriers and provide benefits for stroke patients.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 81971105), the Jilin Province Department of Finance (JLSWSRCZX2020-0035) to Zhen-Ni Guo.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Absar S, Choi S, Ahsan F, et al. (2013). Preparation and characterization of anionic oligopeptide-modified tissue plasminogen activator for triggered delivery: an approach for localized thrombolysis. Thromb Res 131:e91–e99. [DOI] [PubMed] [Google Scholar]
- Absar S, Choi S, Yang VC, Kwon YM. (2012). Heparin-triggered release of camouflaged tissue plasminogen activator for targeted thrombolysis. J Control Release 157:46–54. [DOI] [PubMed] [Google Scholar]
- Absar S, Kwon YM, Ahsan F. (2014). Bio-responsive delivery of tissue plasminogen activator for localized thrombolysis. J Control Release 177:42–50. [DOI] [PubMed] [Google Scholar]
- Abu Fanne R, Nassar T, Yarovoi S, et al. (2010). Blood-brain barrier permeability and tPA-mediated neurotoxicity. Neuropharmacology 58:972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov AV, Köhrmann M, Soinne L, et al. (2019). Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Neurol 18:338–47. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Molina CA, Grotta JC, et al. (2004). Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 351:2170–8. [DOI] [PubMed] [Google Scholar]
- Alonso A, Dempfle CE, Della Martina A, et al. (2009). In vivo clot lysis of human thrombus with intravenous abciximab immunobubbles and ultrasound. Thromb Res 124:70–4. [DOI] [PubMed] [Google Scholar]
- Bachelet L, Bertholon I, Lavigne D, et al. (2009). Affinity of low molecular weight fucoidan for P-selectin triggers its binding to activated human platelets. Biochim Biophys Acta 1790:141–6. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, et al. (2018). Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137:e67–e492. [DOI] [PubMed] [Google Scholar]
- Berkhemer OA, Fransen PS, Beumer D, et al. (2015). A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372:11–20. [DOI] [PubMed] [Google Scholar]
- Chan PH. (2001). Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2–14. [DOI] [PubMed] [Google Scholar]
- Chen H-A, Ma Y-H, Hsu T-Y, Chen J-P. (2020). Preparation of peptide and recombinant tissue plasminogen activator conjugated poly(Lactic-Co-Glycolic Acid) (PLGA) magnetic nanoparticles for dual targeted thrombolytic therapy. IJMS 21:2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-P, Liu C-H, Hsu H-L, et al. (2016). Magnetically controlled release of recombinant tissue plasminogen activator from chitosan nanocomposites for targeted thrombolysis. J Mater Chem B 4:2578–90. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang Z, Gu Z. (2019). Bioinspired and biomimetic nanomedicines. Acc Chem Res 52:1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasuonno M, Palange AL, Aid R, et al. (2018). Erythrocyte-inspired discoidal polymeric nanoconstructs carrying tissue plasminogen activator for the enhanced lysis of blood clots. ACS Nano 12:12224–37. [DOI] [PubMed] [Google Scholar]
- Colucci M, Paramo JA, Collen D. (1986). Inhibition of one-chain and two-chain forms of human tissue-type plasminogen activator by the fast-acting inhibitor of plasminogen activator in vitro and in vivo. J Lab Clin Med 108:53–9. [PubMed] [Google Scholar]
- Correa-Paz C, Navarro Poupard MF, Polo E, et al. (2019). In vivo ultrasound-activated delivery of recombinant tissue plasminogen activator from the cavity of sub-micrometric capsules. J Control Release 308:162–71. [DOI] [PubMed] [Google Scholar]
- Daffertshofer M, Hennerici M. (2003). Ultrasound in the treatment of ischaemic stroke. Lancet Neurol 2:283–90. [DOI] [PubMed] [Google Scholar]
- Danielyan K, Ganguly K, Ding BS, et al. (2008). Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation 118:1442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer SF, Andersson T, Baxter B, et al. (2017). Analyses of thrombi in acute ischemic stroke: a consensus statement on current knowledge and future directions. Int J Stroke 12:606–14. [DOI] [PubMed] [Google Scholar]
- De Saint Victor M, Barnsley LC, Carugo D, et al. (2019). Sonothrombolysis with magnetically targeted microbubbles. Ultrasound Med Biol 45:1151–63. [DOI] [PubMed] [Google Scholar]
- De Saint Victor M, Crake C, Coussios CC, Stride E. (2014). Properties, characteristics and applications of microbubbles for sonothrombolysis. Expert Opin Drug Deliv 11:187–209. [DOI] [PubMed] [Google Scholar]
- Dehaini D, Fang RH, Zhang L. (2016). Biomimetic strategies for targeted nanoparticle delivery. Bioeng Transl Med 1:30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Mei H, Shi W, et al. (2018). Recombinant tissue plasminogen activator-conjugated nanoparticles effectively targets thrombolysis in a rat model of middle cerebral artery occlusion. Curr Med SCI 38:427–35. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. (1999). Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391–7. [DOI] [PubMed] [Google Scholar]
- Eggers J, Koch B, Meyer K, et al. (2003). Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol 53:797–800. [DOI] [PubMed] [Google Scholar]
- Epshtein M, Korin N. (2017). Shear targeted drug delivery to stenotic blood vessels. J Biomech 50:217–21. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Francis CW. (2000). Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol 26:1153–60. [DOI] [PubMed] [Google Scholar]
- Fang RH, Jiang Y, Fang JC, Zhang L. (2017). Cell membrane-derived nanomaterials for biomedical applications. Biomaterials 128:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis CW, Blinc A, Lee S, Cox C. (1995). Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol 21:419–24. [DOI] [PubMed] [Google Scholar]
- Fukuta T, Asai T, Yanagida Y, et al. (2017). Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke. Faseb J 31:1879–90. [DOI] [PubMed] [Google Scholar]
- Fukuta T, Yanagida Y, Asai T, Oku N. (2018). Co-administration of liposomal fasudil and tissue plasminogen activator ameliorated ischemic brain damage in occlusion model rats prepared by photochemically induced thrombosis. Biochem Biophys Res Commun 495:873–7. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. (2008). Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology 55:363–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Diseases 2017 Disease and Injury Incidence and Prevalence Collaborators. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagisawa K, Nishioka T, Suzuki R, et al. (2013). Thrombus-targeted perfluorocarbon-containing liposomal bubbles for enhancement of ultrasonic thrombolysis: in vitro and in vivo study. J Thromb Haemost 11:1565–73. [DOI] [PubMed] [Google Scholar]
- Henderson SJ, Weitz JI, Kim PY. (2018). Fibrinolysis: strategies to enhance the treatment of acute ischemic stroke. J Thromb Haemost 16:1932–40. [DOI] [PubMed] [Google Scholar]
- Heo JH, Lee KY, Kim SH, Kim DI. (2003). Immediate reocclusion following a successful thrombolysis in acute stroke: a pilot study. Neurology 60:1684–7. [DOI] [PubMed] [Google Scholar]
- Hu CM, Fang RH, Wang KC, et al. (2015). Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Huang S, Zhu L, et al. (2018). Tissue plasminogen activator-porous magnetic microrods for targeted thrombolytic therapy after ischemic stroke. ACS Appl Mater Interfaces 10:32988–97. [DOI] [PubMed] [Google Scholar]
- Hua X, Liu P, Gao YH, et al. (2010). Construction of thrombus-targeted microbubbles carrying tissue plasminogen activator and their in vitro thrombolysis efficacy: a primary research. J Thromb Thrombolysis 30:29–35. [DOI] [PubMed] [Google Scholar]
- Hua X, Zhou L, Liu P, et al. (2014). In vivo thrombolysis with targeted microbubbles loading tissue plasminogen activator in a rabbit femoral artery thrombus model. J Thromb Thrombolysis 38:57–64. [DOI] [PubMed] [Google Scholar]
- Huang G, Zhou Z, Srinivasan R, et al. (2008). Affinity manipulation of surface-conjugated RGD peptide to modulate binding of liposomes to activated platelets. Biomaterials 29:1676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Wang J, Huang S, et al. (2019). Polyacrylic acid-coated nanoparticles loaded with recombinant tissue plasminogen activator for the treatment of mice with ischemic stroke. Biochem Biophys Res Commun 516:565–70. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Kawasaki K, Suzuki Y, et al. (2012). A Rho kinase (ROCK) inhibitor, fasudil, prevents matrix metalloproteinase-9-related hemorrhagic transformation in mice treated with tissue plasminogen activator. Neuroscience 220:302–12. [DOI] [PubMed] [Google Scholar]
- Juenet M, Aid-Launais R, Li B, et al. (2018). Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials 156:204–16. [DOI] [PubMed] [Google Scholar]
- Kandadai MA, Mukherjee P, Shekhar H, et al. (2016). Microfluidic manufacture of rt-PA -loaded echogenic liposomes. Biomed Microdevices 18:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MA, Shuaib A, Todd KG. (2006). Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp Neurol 200:38–49. [DOI] [PubMed] [Google Scholar]
- Kontos HA. (2001). Oxygen radicals in cerebral ischemia: the 2001 Willis lecture. Stroke 32:2712–6. [DOI] [PubMed] [Google Scholar]
- Korin N, Gounis MJ, Wakhloo AK, Ingber DE. (2015). Targeted drug delivery to flow-obstructed blood vessels using mechanically activated nanotherapeutics. JAMA Neurol 72:119–22. [DOI] [PubMed] [Google Scholar]
- Korin N, Kanapathipillai M, Matthews BD, et al. (2012). Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 337:738–42. [DOI] [PubMed] [Google Scholar]
- Kvistad CE, Nacu A, Novotny V, et al. (2018). Contrast-enhanced sonothrombolysis in acute ischemic stroke patients without intracranial large-vessel occlusion. Acta Neurol Scand 137:256–61. [DOI] [PubMed] [Google Scholar]
- Laing ST, Moody MR, Kim H, et al. (2012). Thrombolytic efficacy of tissue plasminogen activator-loaded echogenic liposomes in a rabbit thrombus model. Thromb Res 130:629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM. (2007). Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin Emerg Drugs 12:97–112. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Park J, Yoon OJ, et al. (2011). Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol 6:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Liu Y, Chen J, et al. (2018). Platelet bio-nanobubbles as microvascular recanalization nanoformulation for acute ischemic stroke lesion theranostics. Theranostics 8:4870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Hsu HL, Chen JP, et al. (2019). Thrombolysis induced by intravenous administration of plasminogen activator in magnetoliposomes: dual targeting by magnetic and thermal manipulation. Nanomedicine 20:101992. [DOI] [PubMed] [Google Scholar]
- Ma YH, Wu SY, Wu T, et al. (2009). Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials 30:3343–51. [DOI] [PubMed] [Google Scholar]
- Maeda H, Nakamura H, Fang J. (2013). The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev 65:71–9. [DOI] [PubMed] [Google Scholar]
- Marosfoi MG, Korin N, Gounis MJ, et al. (2015). Shear-activated nanoparticle aggregates combined with temporary endovascular bypass to treat large vessel occlusion. Stroke 46:3507–13. [DOI] [PubMed] [Google Scholar]
- Marsh JN, Hu G, Scott MJ, et al. (2011). A fibrin-specific thrombolytic nanomedicine approach to acute ischemic stroke. Nanomedicine (Lond) 6:605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS. (2015). Progress in intravenous thrombolytic therapy for acute stroke. JAMA Neurol 72:928–34. [DOI] [PubMed] [Google Scholar]
- Mei T, Kim A, Vong LB, et al. (2019). Encapsulation of tissue plasminogen activator in pH-sensitive self-assembled antioxidant nanoparticles for ischemic stroke treatment - synergistic effect of thrombolysis and antioxidant. Biomaterials 215:119209. [DOI] [PubMed] [Google Scholar]
- Miller DL. (1987). A review of the ultrasonic bioeffects of microsonation, gas-body activation, and related cavitation-like phenomena. Ultrasound Med Biol 13:443–70. [DOI] [PubMed] [Google Scholar]
- Miller DL. (1988). Particle gathering and microstreaming near ultrasonically activated gas-filled micropores. J Acoust Soc Am 84:1378–87. [DOI] [PubMed] [Google Scholar]
- Murciano JC, Higazi AA, Cines DB, Muzykantov VR. (2009). Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile. J Control Release 139:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murciano JC, Medinilla S, Eslin D, et al. (2003). Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol 21:891–6. [DOI] [PubMed] [Google Scholar]
- Nacu A, Kvistad CE, Logallo N, et al. (2015). A pragmatic approach to sonothrombolysis in acute ischaemic stroke: the Norwegian randomised controlled sonothrombolysis in acute stroke study (NOR-SASS). BMC Neurol 15:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacu A, Kvistad CE, Naess H, et al. (2017). NOR-SASS (Norwegian Sonothrombolysis in Acute Stroke Study): randomized controlled contrast-enhanced sonothrombolysis in an unselected acute ischemic stroke population. Stroke 48:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski CL, Li W, Sun M, et al. (2017). Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials 128:94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren F, Loulidi J, Poglia D, et al. (2008). Microbubble potentiated transcranial duplex ultrasound enhances IV thrombolysis in acute stroke. J Thromb Thrombolysis 25:219–23. [DOI] [PubMed] [Google Scholar]
- Petro M, Jaffer H, Yang J, et al. (2016). Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials 81:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak JF. (2004). Ultrasound energy and the dissolution of thrombus. N Engl J Med 351:2154–5. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, et al. (2018). 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49:e46–e110. [DOI] [PubMed] [Google Scholar]
- Ricci S, Dinia L, Del Sette M, et al. (2012). Sonothrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 6:CD008348. [DOI] [PubMed] [Google Scholar]
- Rubiera M, Alexandrov AV. (2010). Sonothrombolysis in the management of acute ischemic stroke. Am J Cardiovasc Drugs 10:5–10. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM. (2002). Platelets in atherothrombosis. Nat Med 8:1227–34. [DOI] [PubMed] [Google Scholar]
- Sakharov DV, Hekkenberg RT, Rijken DC. (2000). Acceleration of fibrinolysis by high-frequency ultrasound: the contribution of acoustic streaming and temperature rise. Thromb Res 100:333–40. [DOI] [PubMed] [Google Scholar]
- Saqqur M, Tsivgoulis G, Nicoli F, et al. (2014). The role of sonolysis and sonothrombolysis in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and case-control studies. J Neuroimaging 24:209–20. [DOI] [PubMed] [Google Scholar]
- Saver JL, Goyal M, Bonafe A, et al. (2015). Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372:2285–95. [DOI] [PubMed] [Google Scholar]
- Saxer T, Zumbuehl A, Müller B. (2013). The use of shear stress for targeted drug delivery. Cardiovasc Res 99:328–33. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Meade G, Stoll P, et al. (2006). Conformation-specific blockade of the integrin GPIIb/IIIa: a novel antiplatelet strategy that selectively targets activated platelets. Circ Res 99:25–33. [DOI] [PubMed] [Google Scholar]
- Seo J, Al-Hilal TA, Jee JG, et al. (2018). A targeted ferritin-microplasmin based thrombolytic nanocage selectively dissolves blood clots. Nanomedicine 14:633–42. [DOI] [PubMed] [Google Scholar]
- Shaw GJ, Meunier JM, Huang SL, et al. (2009). Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res 124:306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar H, Bader KB, Huang S, et al. (2017). In vitro thrombolytic efficacy of echogenic liposomes loaded with tissue plasminogen activator and octafluoropropane gas. Phys Med Biol 62:517–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Kaur R, Kaur A. (2013). Clot composition and treatment approach to acute ischemic stroke: the road so far. Ann Indian Acad Neurol 16:494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Vaidya SS, Kopechek JA, et al. (2010). Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med Biol 36:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayon A, Jamshidi R, Esmaeili A. (2016). Targeted thrombolysis of tissue plasminogen activator and streptokinase with extracellular biosynthesis nanoparticles using optimized Streptococcus equi supernatant. Int J Pharm 501:300–10. [DOI] [PubMed] [Google Scholar]
- Takamiya M, Miyamoto Y, Yamashita T, et al. (2012). Strong neuroprotection with a novel platinum nanoparticle against ischemic stroke- and tissue plasminogen activator-related brain damages in mice. Neuroscience 221:47–55. [DOI] [PubMed] [Google Scholar]
- Teng Y, Jin H, Nan D, et al. (2018). In vivo evaluation of urokinase-loaded hollow nanogels for sonothrombolysis on suture embolization-induced acute ischemic stroke rat model. Bioact Mater 3:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiebosch IA, Crielaard BJ, Bouts MJ, et al. (2012). Combined treatment with recombinant tissue plasminogen activator and dexamethasone phosphate-containing liposomes improves neurological outcome and restricts lesion progression after embolic stroke in rats. J Neurochem 123(Suppl 2):65–74. [DOI] [PubMed] [Google Scholar]
- Tiukinhoy-Laing SD, Buchanan K, Parikh D, et al. (2007). Fibrin targeting of tissue plasminogen activator-loaded echogenic liposomes. J Drug Target 15:109–14. [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G, Eggers J, Ribo M, et al. (2010). Safety and efficacy of ultrasound-enhanced thrombolysis: a comprehensive review and meta-analysis of randomized and nonrandomized studies. Stroke 41:280–7. [DOI] [PubMed] [Google Scholar]
- Uesugi Y, Kawata H, Jo J, et al. (2010). An ultrasound-responsive nano delivery system of tissue-type plasminogen activator for thrombolytic therapy. J Control Release 147:269–77. [DOI] [PubMed] [Google Scholar]
- Vankayala R, Corber SR, Mac JT, et al. (2018). Erythrocyte-derived nanoparticles as a theranostic agent for near-infrared fluorescence imaging and thrombolysis of blood clots. Macromol Biosci 18:e1700379. [DOI] [PubMed] [Google Scholar]
- Villa CH, Anselmo AC, Mitragotri S, Muzykantov V. (2016). Red blood cells: supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev 106:88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa CH, Pan DC, Zaitsev S, et al. (2015). Delivery of drugs bound to erythrocytes: new avenues for an old intravascular carrier. Ther Deliv 6:795–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Guo X, Xiu W, et al. (2020). Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci Adv 6:eaaz8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gkanatsas Y, Palasubramaniam J, et al. (2016). Thrombus-targeted theranostic microbubbles: a new technology towards concurrent rapid ultrasound diagnosis and bleeding-free fibrinolytic treatment of thrombosis. Theranostics 6:726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hagemeyer CE, Hohmann JD, et al. (2012). Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation 125:3117–26. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang X, Yin H, et al. (2019). Sequentially site-specific delivery of thrombolytics and neuroprotectant for enhanced treatment of ischemic stroke. ACS Nano 13:8577–88. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang Y, Xu J, et al. (2020). Engineered nanoplatelets for targeted delivery of plasminogen activators to reverse thrombus in multiple mouse thrombosis models. Adv Mater 32:e1905145. [DOI] [PubMed] [Google Scholar]
- Yaghi S, Willey JZ, Cucchiara B, et al. (2017). Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48:e343–e61. [DOI] [PubMed] [Google Scholar]
- Yan WC, Chua QW, Ong XJ, et al. (2017). Fabrication of ultrasound-responsive microbubbles via coaxial electrohydrodynamic atomization for triggered release of tPA. J Colloid Interface Sci 501:282–93. [DOI] [PubMed] [Google Scholar]
- Yang HW, Hua MY, Lin KJ, et al. (2012). Bioconjugation of recombinant tissue plasminogen activator to magnetic nanocarriers for targeted thrombolysis. Int J Nanomedicine 7:5159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Roussel BD, Ali C, Vivien D. (2009). Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci 32:48–55. [DOI] [PubMed] [Google Scholar]
- Zaitsev S, Spitzer D, Murciano JC, et al. (2010a). Targeting of a mutant plasminogen activator to circulating red blood cells for prophylactic fibrinolysis. J Pharmacol Exp Ther 332:1022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev S, Spitzer D, Murciano JC, et al. (2010b). Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood 115:5241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Kim H, Wu H, et al. (2019). Sonothrombolysis with magnetic microbubbles under a rotational magnetic field. Ultrasonics 98:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Guo D, Zhang Y, et al. (2014). Construction and evaluation of Fe3O4-based PLGA nanoparticles carrying rtPA used in the detection of thrombosis and in targeted thrombolysis. ACS Appl Mater Interfaces 6:5566–76. [DOI] [PubMed] [Google Scholar]