Abstract
Many promising advances in precision health and other Big Data research rely on large data sets to analyze correlations among genetic variants, behavior, environment, and outcomes to improve population health. But these data sets are generally populated with demographically homogeneous cohorts. We conducted a retrospective cohort study of patients at a major academic medical center during 2012–19 to explore how recruitment and enrollment approaches affected the demographic diversity of participants in its research biospecimen and data bank. We found that compared with the overall clinical population, patients who consented to enroll in the research data bank were significantly less diverse in terms of age, sex, race, ethnicity, and socioeconomic status. Compared with patients who were recruited for the data bank, patients who enrolled were younger and less likely to be Black or African American, Asian, or Hispanic. The overall demographic diversity of the data bank was affected as much (and in some cases more) by which patients were considered eligible for recruitment as by which patients consented to enroll. Our work underscores the need for systemic commitment to diversify data banks so that different communities can benefit from research.
Introduction
Large-scale collections of health data and biospecimens allow researchers to analyze correlations among genetic variants, behavior, environment, and outcomes to improve population health. But the data sets needed to support such efforts often fail to reflect the demographic distribution of disease. For example, 95 percent of data used in genomewide association studies worldwide are derived from participants who are of European or Asian descent.1,2 This lack of ancestral diversity holds true across both exome (focusing on protein-coding regions) and whole genome (mapping all nucleotides) sequencing studies and biorepositories in general.3,4 The disconnect between the diversity of research participants and the overall US clinical population can lead to misleading results, incorrect diagnoses, or findings that are not socially contextualized for historically excluded patients.5–7 Lack of demographic diversity in the participants represented in research data banks can arise because of differences in access to clinical care and diagnostic testing, as well as the types of participants who are generally recruited for and consent to enroll in research data banks.8,9
To explore how the recruitment and enrollment approaches at a major academic medical center (Michigan Medicine) affected the demographic diversity of the participants in its research biospecimen and data bank, we compared the age, sex, race, ethnicity, and socioeconomic status of patients automatically entered into the medical center’s clinical electronic health record database with those of the patients who were recruited to participate in the data bank and those of the patients who consented to enroll.
In contrast to prior work that has focused on enrollment in clinical research, hypothetical differences in data bank consent rates, and reviews of observational studies not designed to capture consent,10–12 here we used a retrospective cohort analysis to quantify such differences in a specific population. Our findings have important policy implications for the appropriate balance between respect for participant autonomy in research and justice considerations for the types of communities that should contribute to and can benefit from such work. Our findings also raise critical considerations regarding ethical obligations to design recruitment and enrollment methods to increase the demographic diversity of research data banks and bio banks as well as a novel methodology to measure the impact of those efforts.
Study Data And Methods
Recruitment
All patients receiving clinical care at Michigan Medicine must sign a general consent for health care services and receive a notice of privacy practices.13 These notices acknowledge that entities governed by the Privacy Rule of the Health Insurance Portability and Accountability Act of 1996 “may use or provide [protected health information] to conduct research…generally…subject to oversight by an institutional review board.” This kind of consent process is often called “notice and consent” by privacy scholars because it involves a disclosure of terms and a binary choice: Patients can either receive clinical care (and agree to the above conditions) or not. Identifiable electronic health record data generated in the process of clinical care are then available to researchers for low-risk research with a waiver of research consent.
Michigan Medicine also supports a research biospecimen and data bank, the Michigan Genomics Initiative (MGI), which deploys an additional broad research consent for participation.14,15 Trained MGI recruiters aim to approach all adult surgical patients having blood drawn in the preoperative setting during typical surgical hours. The research consent process includes a handout that explains details of the study, using bulleted conceptual text and corresponding visuals, as well as links to further information.16 Recruiters review the pamphlet and consent form with prospective participants and confirm understanding before signature.17 Materials are available in English only. At the time of this analysis more than 65,000 MGI participants had provided broad consent for use of their electronic health record data and biospecimens in this way.18
Sample
We included all adult patients (ages eighteen and older) with an inpatient or outpatient visit to Michigan Medicine over a span of approximately 7.5 years (April 29, 2012–December 11, 2019). We used the following definitions for the three comparison cohorts: The Michigan Medicine cohort is all patients with at least one in- or outpatient visit at Michigan Medicine, the MGI Recruited cohort is the subset of Michigan Medicine patients who were eligible to participate in MGI, and the MGI Enrolled cohort is the subset of MGI Recruited patients who consented to enroll in MGI.
For each patient in each cohort, we assessed demographic characteristics including age, sex, race, ethnicity, and an index for socioeconomic status. All information for these three cohorts was extracted from the research data warehouse where Michigan Medicine captures patient data.19 We conducted statistical analyses to quantify the demographic differences within and across these three cohorts to understand whether there were demographic differences and, if so, the relative magnitude of the effect of the recruitment/eligibility stage versus the enrollment stage.
For patients in the Michigan Medicine cohort, demographic information was based on the latest encounter within the time frame of this study (by selecting the latest encounter, we skewed the Michigan Medicine cohort to be slightly older). For patients in the MGI Recruited and MGI Enrolled cohorts, we used the demographic information of the corresponding inpatient or outpatient preoperative visit when the patient was asked to enroll in MGI (see “Supplementary Methods” in the online appendix).20 Sex, race, and ethnicity were self-identified by each patient or their guardian. The options for sex were male, female, or unknown. The options for race were American Indian and Alaska Native, Asian, Black or African American, Native Hawaiian and other Pacific Islander, White or Caucasian, other, patient refused, or unknown. Patients who selected White or Caucasian are reported here as White in accordance with updated standards.21 The options for ethnicity were Hispanic, non-Hispanic, patient refused, and unknown. For both race and ethnicity, if these fields contain no data, race and ethnicity are reported as unknown. We used the neighborhood socioeconomic disadvantage index from the National Neighborhood Data Archive, a publicly available archival resource containing spatially referenced measures of physical and social environment, as a proxy for socioeconomic status.22 For all cohorts, the most recent patient addresses available in the research data warehouse were geocoded and mapped to a census block group and tract and then further mapped to the index, using the National Neighborhood Data Archive for 2008–17.22 Patients without a known address or whose address could not be geocoded were assigned unknown socioeconomic status.
Statistical Analysis
We first quantified the differences in demographics between pairs of cohorts. We compared the proportions of categories of discrete variables (sex, race, ethnicity), using a two-tailed z-test, with a null hypothesis that there was no difference in proportions. We also compared the distributions of continuous variables (age and disadvantage index), using a Mann-Whitney U test,23 with a null hypothesis that there was no difference. We determined the statistical significance of the difference in proportions and distributions with a Bonferroni correction for multiple hypotheses (α1 = 0.003).24 For the disadvantage index, we also performed subgroup comparisons by racial and ethnic groups.
To understand the relationship between different subgroups and the likelihood of enrolling in MGI, we calculated the consent and decline rates of each subgroup, using the cohort counts of the MGI Recruited and MGI Enrolled cohorts. This calculation also measures the effect of the opt-in consent process on demographic changes between the MGI Recruited and MGI Enrolled cohorts.
Finally, we calculated relative risks to quantify the difference in participation rates between a specific subgroup (for example, Black or African American patients) and those not in that subgroup (for example, non–Black or African American patients) as well as the overall population.25 We also compared each non-White racial subgroup’s participation rate with White patients’ participation rate (see “Supplementary Methods” in the appendix).20 For the disadvantage index, we binarized the values with respect to the median of the Michigan Medicine cohort (for race and ethnicity subgroup analyses, the median values were for the corresponding subgroup in the Michigan Medicine cohort). A relative risk of 1 corresponds to no difference in the rates of participation between the two groups. A relative risk less than 1 or greater than 1 suggests that those in the target subgroup participate at a relatively lower or higher rate, respectively. To quantify the extent to which the point of recruitment and the point of enrollment independently contributed to differences between the two cohorts, we measured the relative risk of each stage while controlling for the impact of the other,26 using a resampling-based approach (see the appendix).20 We then compared the relative risks, using a two-tailed z-test with a Bonferroni correction for multiple hypotheses (α2 = 0.001).
Limitations
Our analysis was limited in several ways. We were not able to compare patients who were eligible to enroll in MGI with those who were actually asked to enroll. Although this would have allowed us to further study potential biases in individual recruitment practices, we lacked sufficient data on which to base this assessment. As MGI recruiters aim to approach all adult surgical patients undergoing blood draws, we assumed for the purposes of this analysis that the MGI Recruited cohort was an accurate representation of those who were eligible. Our results also could not capture additional biases arising from limiting the study to the context of a single academic medical center. Our research warrants further analyses to establish the generalizability of results.
Study Results
Unless otherwise specified, all differences in cohort characteristics (appendix tables S1 and S2) reported are statistically significant (appendix table S3).20 Extended comparisons are reported in the “Supplementary Results” and tables of the appendix.20
Study Population
During the study period, 1,242,826 unique adult patients were seen as inpatients or outpatients at Michigan Medicine: 95,206 (7.7 percent) of these patients were eligible for enrollment in MGI, and 67,687 (71.1 percent) of those recruited consented to enroll (exhibit 1; appendix table S1).20 Race and ethnicity data were more likely to be captured for surgical patients than for people who were patients of Michigan Medicine in general.
Exhibit 1.
Number of patients and race breakdown of each cohort in the study of patients at Michigan Medicine, 2012–19
| Cohorts | Number | Percenta | White | Unknownb | Otherc |
|---|---|---|---|---|---|
| Michigan Medicined | 1,242,826 | 100.0 | 70% | 18% | 12% |
| MGI Recruitede | 95,206 | 7.7 | 87 | 4 | 10 |
| MGI Enrolledf | 67,687 | 71.1 | 90 | 3 | 7 |
• SOURCE Authors’ analysis of data from the Michigan Medicine Research Data Warehouse and Michigan Genomics Initiative (MGI). NOTES Data shown are from April 29, 2012, to December 11, 2019, accessed January 1, 2020. Percentages may sum to greater than 100 percent because of rounding.
Percent for MGI Recruited uses Michigan Medicine as the denominator; percent for MGI Enrolled uses MGI Recruited as the denominator.
The race category of “unknown” includes unknown, other, and patient refused.
The race category of “other” reported here includes Asian, American Indian and Alaska Native, Black or African American, and Native Hawaiian and other Pacific Islander. For race, if these fields contained no data, race is reported as “unknown.”
All adult patients with at least one in- or outpatient visit at Michigan Medicine.
Subset of the Michigan Medicine cohort who were eligible to participate in MGI.
Subset of the MGI Recruited cohort who consented to enroll in MGI.
SOURCE Authors’ analysis of data from the Michigan Medicine Research Data Warehouse and Michigan Genomics Initiative (MGI). NOTES Data shown are from April 29, 2012, to December 11, 2019, accessed January 1, 2020. Percentages may sum to greater than 100 percent because of rounding.
Percent for MGI Recruited uses Michigan Medicine as the denominator; percent for MGI Enrolled uses MGI Recruited as the denominator.
The race category of “unknown” includes unknown, other, and patient refused.
The race category of “other” reported here includes Asian, American Indian and Alaska Native, Black or African American, and Native Hawaiian and other Pacific Islander. For race, if these fields contained no data, race is reported as “unknown.”
All adult patients with at least one in- or outpatient visit at Michigan Medicine.
Subset of the Michigan Medicine cohort who were eligible to participate in MGI.
Subset of the MGI Recruited cohort who consented to enroll in MGI.
Age
Within the MGI Recruited cohort, younger patients (younger than age 50.7, the median age of the Michigan Medicine cohort) were slightly more likely to consent (71.3 percent versus 70.9 percent) (appendix table S4).20 However, the median age of the MGI Enrolled cohort was greater than that of the Michigan Medicine cohort (56.0 versus 50.7 years) (exhibit 2). This difference was created at the eligibility stage: The median age of the patients in the MGI Recruited cohort was greater than that of the Michigan Medicine cohort (56.2 versus 50.7 years).
Exhibitt 2.
Characteristics of patients at Michigan Medicine, by cohort, 2012–19
| Cohort | |||
|---|---|---|---|
| Characteristics | Michigan Medicine | MGI Recruited | MGI Enrolled |
| Number | 1,242,826 | 95,206 | 67,687 |
| Age (years) | |||
| Median | 50.7 | 56.2 | 56.0 |
| 25th percentile | 32.3 | 42.2 | 42.5 |
| 75th percentile | 65.2 | 66.9 | 66.4 |
| Sex (%) | |||
| Female | 56.0 | 53.7 | 53.7 |
| Male | 44.0 | 46.3 | 46.3 |
| Unknown | 0.011 | 0.003 | 0.003 |
| Race (%) | |||
| White | 69.8 | 86.7 | 89.7 |
| Black or African American | 7.3 | 7.0 | 5.1 |
| Asian | 4.7 | 2.1 | 1.5 |
| Othera | 18.2 | 4.2 | 3.6 |
| Ethnicity (%) | |||
| Hispanic | 2.3 | 2.2 | 1.9 |
| Non-Hispanic | 77.1 | 94.3 | 94.7 |
| Otherb | 20.6 | 3.5 | 3.3 |
• SOURCE Authors’ analysis of data from the Michigan Medicine Research Data Warehouse and Michigan Genomics Initiative (MGI). NOTES Data shown are from April 29, 2012, to December 11, 2019, accessed January 1, 2020. The three cohorts are described in the notes to exhibit 1.
Includes American Indian and Alaska Native, Native Hawaiian and other Pacific Islander, unknown (including fields containing no data), other, and patient refused.
Includes unknown (including fields containing no data) and patient refused.
SOURCE Authors’ analysis of data from the Michigan Medicine Research Data Warehouse and Michigan Genomics Initiative (MGI). NOTES Data shown are from April 29, 2012, to December 11, 2019, accessed January 1, 2020. The three cohorts are described in the notes to exhibit 1.
Includes American Indian and Alaska Native, Native Hawaiian and other Pacific Islander, unknown (including fields containing no data), other, and patient refused.
Includes unknown (including fields containing no data) and patient refused.
Sex
Male and female patients consented at nearly the same rate (71.1 percent), resulting in no significant difference between the MGI Recruited and MGI Enrolled cohorts (exhibit 2; appendix table S4).20 However, the MGI Enrolled cohort had a greater proportion of male patients than the Michigan Medicine cohort (46.3 percent versus 44.0 percent) as a result of a difference that arose at the eligibility stage (appendix table S2).20
Race
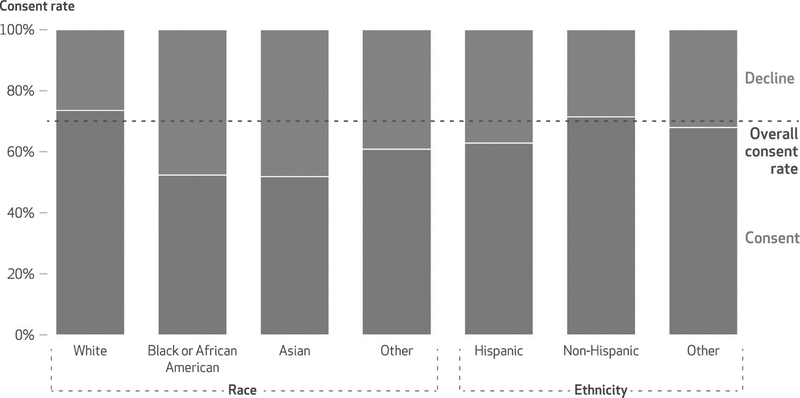
Overall, the MGI Enrolled cohort had a lower proportion of Black or African American patients compared with the Michigan Medicine cohort (5.1 percent versus 7.3 percent) (exhibit 2). This is due to two reasons. First, a lower proportion of MGI Recruited patients were Black or African American compared with Michigan Medicine patients (7.0 percent versus 7.3 percent), and a greater proportion of MGI Recruited patients were White (86.7 percent versus 69.8 percent). Second, in the MGI Recruited cohort, Black or African American patients were almost twice as likely to decline enrollment compared with White patients (47.6 percent versus 26.4 percent) (exhibit 3; appendix table S4).20 Similar trends were observed for Asian patients (48.1 percent declined; exhibit 3).
Exhibit 3.
Rates of enrollment in the Michigan Genomics Initiative, by race and ethnicity, 2012–19
SOURCE Authors’ analysis of data from the Michigan Medicine Research Data Warehouse and Michigan Genomics Initiative (MGI). NOTES Data shown are from April 29, 2012, to December 11, 2019, accessed January 1, 2020. Consent and decline rates are calculated with respect to patients in the subset of all adult patients with at least one in- or outpatient visit at Michigan Medicine who were eligible to participate in MGI (the MGI Recruited cohort). The race category of “other” reported here includes American Indian and Alaska Native, Native Hawaiian and other Pacific Islander, other, patient refused, and unknown. The ethnicity category of “other” reported here includes patient refused and unknown.
Ethnicity
The proportion of Hispanic patients in the MGI Enrolled cohort was lower than in the Michigan Medicine cohort (1.9 percent versus 2.3 percent) (exhibit 2). This was predominantly because Hispanic patients were less likely to consent to enrollment compared with non-Hispanic patients (62.9 percent versus 71.4 percent) (exhibit 3).
Socioeconomic Status
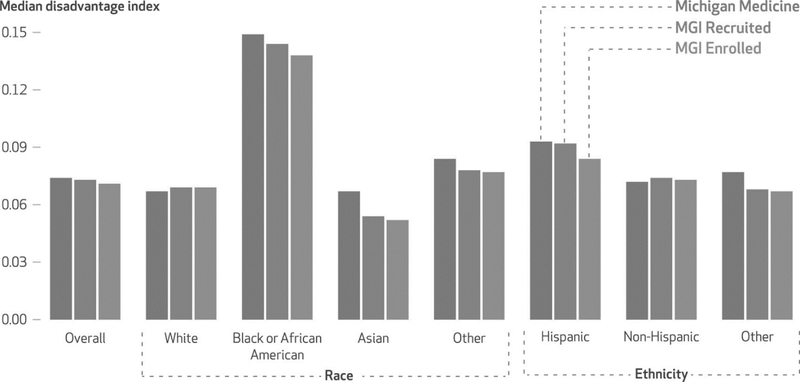
Black or African American patients in the MGI Enrolled cohort were more likely to live in socioeconomically advantaged neighborhoods compared with Black or African American patients in the Michigan Medicine cohort (median disadvantage index: 0.138 versus 0.148) (exhibit 4; appendix table S2).20 In contrast, White patients in the MGI Enrolled cohort lived in more disadvantaged neighborhoods compared with White patients in the Michigan Medicine cohort (median disadvantage index: 0.069 versus 0.067). However, compared with patients of all other races but Asian in the MGI Enrolled cohort, White patients in the MGI Enrolled cohort were more advantaged. Therefore, patients in the MGI Enrolled cohort overall were more likely to live in socioeconomically advantaged neighborhoods compared with patients in the Michigan Medicine cohort (median disadvantage index: 0.071 versus 0.074).
Exhibit 4.
Comparison of neighborhood socioeconomic disadvantage index scores in the study of patients at Michigan Medicine, by race and ethnicity and by cohort, 2012–19
SOURCE Authors’ analysis of data from the Michigan Medicine Research Data Warehouse and Michigan Genomics Initiative (MGI). NOTES Data shown are from April 29, 2012, to December 11, 2019, accessed January 1, 2020. The median values of the disadvantage index for each cohort overall as well as racial and ethnic subgroups are shown. The race and ethnicity categories of “other” are defined in the notes to exhibit 3. The three cohorts are described in the notes to exhibit 1.
Differences Due to Recruitment Versus Enrollment
On the basis of relative risks for age, sex, race, ethnicity, and socioeconomic status, the differences between the Michigan Medicine and MGI Enrolled cohorts were introduced during both the recruitment and enrollment stages (appendix tables S5–S7).20 The differences were almost always in the same direction (that is, those less likely to be recruited were also less likely to enroll) apart from socioeconomic status. Other than American Indian and Alaska Native patients, non-White patients were less likely to be eligible for recruitment and less likely to enroll in MGI compared with White patients (appendix table S8).20
Discussion
Although the demographic characteristics of research biospecimen and data banks have been documented, researchers have not fully explored the mechanics of how research data sets become homogenized. We found that patients who were eligible to enroll in the Michigan Genomics Initiative were more likely to be older, White, and male and live in socioeconomically advantaged neighborhoods in comparison with the broader Michigan Medicine population. This is because Michigan Medicine surgical patients from which MGI recruited are more likely to be older, White, socioeconomically advantaged men. This is consistent with other research that has found persistent racial disparities in access to surgical care,27,28 implying that focusing on other kinds of blood draw procedures (as opposed to surgery) may be helpful in increasing racial and ethnic diversity in research data sets. In addition, a major reason that Black and African American patients report elsewhere that they have not enrolled in research is that they have not been asked.4 Using different methods and locations for recruitment can be critical to capturing demographic diversity.29
There were several reasons why MGI designed recruitment in this fashion. Perioperative patients have time to engage in recruitment and enrollment procedures without disruption to clinical workflow. In addition, increasing convenience (for example, patients already undergoing a clinical blood draw) is an established method to engage diverse participants.30 This recruitment approach, however, resulted in only 7.7 percent of the total Michigan Medicine population being in the MGI Recruited cohort and excluded many demographically diverse patients.
Second, patients who enrolled in MGI were younger and less likely to be Black or African American, Asian, or Hispanic. This is mainly because Black or African American and Asian patients were almost twice as likely to decline enrollment compared with White patients; and Hispanic patients were more likely to decline enrollment compared with non-Hispanic patients. The work of others has highlighted the importance of enrollment processes being responsive to patient communities who may evaluate the risks and benefits of research in systematically different ways, in addition to the importance of presenting informed consent materials in several different languages.3,31–34 Unfortunately, because of our retrospective design, we could not assess why Michigan Medicine patients who were recruited for MGI did not enroll, but this is a critical area for future research.
Last, patients in the MGI Enrolled cohort overall were from more socioeconomically advantaged neighborhoods relative to the Michigan Medicine cohort, but this trend differed across race and ethnicity. Relative to White patients in the Michigan Medicine cohort, White patients in the MGI Enrolled cohort were more likely to be from slightly more disadvantaged neighborhoods. However, the opposite was true for Black or African American patients; of the Black or African American patients in the Michigan Medicine cohort, those from more socioeconomically advantaged neighborhoods were more likely to both be eligible and enroll in MGI. Our findings are consistent with previous findings that low socioeconomic status is a barrier to research participation for underrepresented populations.35,36 Socioeconomic status is related to increased morbidity and mortality across a number of diseases.37 This is an important reminder for those developing and using clinical support tools that integrate race and ethnicity as a proxy for socioeconomic status: Such assumptions are inherently limited.38
Conclusion
The choice between research data sets such as those at Michigan Medicine—one that includes the full demographic diversity of the patient population but lacks broad consent for research in addition to the standard clinical consent and one that includes broad consent for research but reflects less demographic diversity—highlights a potential tension between the bioethical principles of respect for persons and justice in research.39 The concept of respect for persons generally compels researchers to honor the autonomy of participants by giving them a choice of whether to enroll. The principle of justice compels researchers to ensure that health advances are applicable and accessible to a diverse range of communities. Our finding that the Michigan Genomics Initiative research database is significantly less diverse than the population of Michigan Medicine patients can put these two foundational ethical principles in potential tension when both data sets are accessible to researchers.
Building research structures that are more responsive to participants’ preferences and ensuring that advances made from research are applicable to diverse communities are both equally critical goals. The solution to this problem cannot be seen as a binary choice between autonomy and justice; that which is just is adopting methodologies that do not put these principles in tension for historically excluded communities in the first place.
It is also important to note that even if demographics are consistent between a given patient population and research data banks, that alone will not ensure that data banks are adequately powered to improve health at the population level across characteristics. Racial and ethnic disparities in access to care are well established.40 Ensuring that research recruitment approaches do not simply replicate existing inequities will require more than just achieving symmetry with patient populations. Creative, dedicated, and prolonged commitment on the part of institutions and researchers will be critical to achieving equitable opportunities for diverse patients to contribute to—and benefit from—research.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded in part by Precision Health at the University of Michigan, the National Human Genome Research Institute (Grant No. K01HG010496, with Kayte Spector-Bagdady as principal investigator), the National Center for Advancing Translational Sciences (Grant No. UL1TR002240, with Spector-Bagdady and Sachin Kheterpal as coinvestigators), the University of Michigan Center for Bioethics and Social Sciences in Medicine (with Spector-Bagdady as associate director), the National Science Foundation (Grant No. IIS1553146, with Jenna Wiens as principal investigator), and Novo Nordisk Foundation (Grant No. NNF17SA0027784, with Nicholson Price as coinvestigator). The views and conclusions in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of funding institutions. Kheterpal also serves as an investigator on grants and contracts to the University of Michigan from the National Institutes of Health, Patient Centered Outcomes Research Institute, Apple Inc., Merck Inc., and Blue Cross Blue Shield of Michigan. Chad Brummett is a consultant for Heron Therapeutics, Vertex Pharmaceuticals, Alosa Health, and the Benter Foundation and provides medical expert testimony. Wiens also serves as an investigator on grants to the University of Michigan from the Juvenile Diabetes Research Fund, National Institutes of Health, Agency for Healthcare Research and Quality, Cisco, Dan D. and Betty Kahn Foundation, Michigan Department of Health and Human Services and Medicaid, Alfred P. Sloan Foundation, and Centers for Disease Control and Prevention. The authors acknowledge the Michigan Genomics Initiative participants and the University of Michigan Medical School Data Office for Clinical and Translational Research for providing data storage, management, processing, and distribution services. They also thank Shawneequa Callier and John Ayanian for their thoughtful comments on a previous version of this article and Chris Krenz for his assistance with the figures.
Contributor Information
Kayte Spector-Bagdady, assistant professor of obstetrics and gynecology and an associate director of the Center for Bioethics and Social Sciences in Medicine at the University of Michigan Medical School, in Ann Arbor, Michigan..
Shengpu Tang, PhD candidate in computer science and engineering at the University of Michigan, in Ann Arbor, Michigan..
Sarah Jabbour, PhD candidate in computer science and engineering at the University of Michigan..
W. Nicholson Price, II, professor of law at the University of Michigan Law School, in Ann Arbor, Michigan..
Ana Bracic, assistant professor of political science and a member of the Minority Politics Initiative at Michigan State University, in East Lansing, Michigan..
Melissa S. Creary, assistant professor of health management and policy at the University of Michigan School of Public Health, in Ann Arbor, Michigan, and the senior director for the Office of Public Health Initiatives at the American Thrombosis and Hemostasis Network (ATHN), in Rochester, New York.
Sachin Kheterpal, professor of anesthesiology and the associate dean for research information technology at the University of Michigan Medical School..
Chad M. Brummett, professor of anesthesiology and senior associate chair for research at the University of Michigan Medical School.
Jenna Wiens, associate professor of computer science and engineering, associate director of the Artificial Intelligence Lab, and codirector for Precision Health at the University of Michigan..
NOTES
- 1.Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25(11):489–94. [DOI] [PubMed] [Google Scholar]
- 2.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538(7624):161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu JH, Crouch J, Jamal SM, Tabor HK, Bamshad MJ. Attitudes of African Americans toward return of results from exome and whole genome sequencing. Am J Med Genet A. 2013;161A(5):1064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millon Underwood S, Buseh AG, Kelber ST, Stevens PE, Townsend L. Enhancing the participation of African Americans in health-related genetic research: findings of a collaborative academic and community-based research study. Nurs Res Pract. 2013;2013:749563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonham VL, Callier SL, Royal CD. Will precision medicine move us beyond race? N Engl J Med. 2016;374(21):2003–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caswell-Jin JL, Gupta T, Hall E, Petrovchich IM, Mills MA, Kingham KE et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20(2):234–9. [DOI] [PubMed] [Google Scholar]
- 7.Manrai AK, Funke BH, Rehm HL, Olesen MS, Maron BA, Szolovits P et al. Genetic misdiagnoses and the potential for health disparities. N Engl J Med. 2016;375(7):655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor P Personal genomes: when consent gets in the way. Nature. 2008;456(7218):32–3. [DOI] [PubMed] [Google Scholar]
- 9.Ingelfinger JR, Drazen JM. Registry research and medical privacy. N Engl J Med. 2004;350(14):1452–3. [DOI] [PubMed] [Google Scholar]
- 10.Glickman SW, Anstrom KJ, Lin L, Chandra A, Laskowitz DT, Woods CW et al. Challenges in enrollment of minority, pediatric, and geriatric patients in emergency and acute care clinical research. Ann Emerg Med. 2008;51(6):775–780.e3. [DOI] [PubMed] [Google Scholar]
- 11.Jagsi R, Griffith KA, Sabolch A, Jones R, Spence R, De Vries R et al. Perspectives of patients with cancer on the ethics of rapid-learning health systems. J Clin Oncol. 2017;35(20):2315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McQuillan GM, Pan Q, Porter KS. Consent for genetic research in a general population: an update on the National Health and Nutrition Examination Survey experience. Genet Med. 2006;8(6):354–60. [DOI] [PubMed] [Google Scholar]
- 13.University of Michigan Health. Protecting your privacy (HIPAA) [Internet]. Ann Arbor (MI): Michigan Medicine; [cited 2021 Oct 19]. Available from: https://www.uofmhealth.org/patient-visitor-guide/protecting-your-privacy-hipaa [Google Scholar]
- 14.Michigan Genomics Initiative, University of Michigan Precision Health. About the Michigan Genomics Initiative [Internet]. Ann Arbor (MI): University of Michigan Precision Health; [cited 2021 Oct 19]. Available from: https://precisionhealth-umich-edu.proxy.lib.umich.edu/our-research/michigangenomics/ [Google Scholar]
- 15.Mikkelsen RB, Gjerris M, Waldemar G, Sandøe P. Broad consent for biobanks is best—provided it is also deep. BMC Med Ethics. 2019;20(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brummett C Donating to a University of Michigan biorepository [Internet]. Ann Arbor (MI): University of Michigan; [cited 2021 Oct 19]. Available from: https://precisionhealth-umich-edu.proxy.lib.umich.edu/wp-content/uploads/sites/67/2020/03/MGI-pamphlet_REVISED_7.24.19-1.pdf [Google Scholar]
- 17.University of Michigan Institutional Review Board. Document of consent to donate to a University of Michigan biorepository and authorization to release protected health information [Internet]. Ann Arbor (MI): University of Michigan; 2016. May [cited 2021 Oct 19]. Available from: https://research-medicine-umich-edu.proxy.lib.umich.edu/sites/default/files/resource-download/cbr_consent_text_form_template_05.2016_2.pdf [Google Scholar]
- 18.Beesley LJ, Salvatore M, Fritsche LG, Pandit A, Rao A, Brummett C et al. The emerging landscape of health research based on biobanks linked to electronic health records: existing resources, statistical challenges, and potential opportunities. Stat Med. 2020;39(6):773–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.University of Michigan Medical School, Office of Research. DataDirect: a self-serve tool for data retrieval [Internet]. Ann Arbor (MI): University of Michigan; c 2021. [cited 2021 Nov 3]. Available from: https://research-medicine-umich-edu.proxy.lib.umich.edu/our-units/data-office-clinical-translational-research/self-serve-data-tools/datadirect [Google Scholar]
- 20.To access the appendix, click on the Details tab of the article online.
- 21.Flanagin A, Frey T, Christiansen SLAMA Manual of Style Committee. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326(7):621–7. [DOI] [PubMed] [Google Scholar]
- 22.Melendez R, Clarke P, Khan A, Gomez-Lopez I, Li M, Chenoweth M. National Neighborhood Data Archive (NaNDA): socioeconomic status and demographic characteristics of ZIP Code Tabulation Areas, United States, 2008–2017 [Internet]. Ann Arbor (MI): Inter-university Consortium for Political and Social Research [distributor]; 2020. July 30 [cited 2021 Oct 19]. Available from: https://www.openicpsr.org/openicpsr/project/120462/version/V1/view [Google Scholar]
- 23.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18(1):50–60. [Google Scholar]
- 24.Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56(293):52–64. [Google Scholar]
- 25.Sistrom CL, Garvan CW. Proportions, odds, and risk. Radiology. 2004;230(1):12–9. [DOI] [PubMed] [Google Scholar]
- 26.Efron B Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7(1):1–26. [Google Scholar]
- 27.Best MJ, McFarland EG, Thakkar SC, Srikumaran U. Racial disparities in the use of surgical procedures in the US. JAMA Surg. 2021;156(3):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenfeld AJ, Sturgeon DJ, Dimick JB, Bono CM, Blucher JA, Barton LB et al. Disparities in rates of surgical intervention among racial and ethnic minorities in Medicare accountable care organizations. Ann Surg. 2019;269(3):459–64. [DOI] [PubMed] [Google Scholar]
- 29.Ochs-Balcom HM, Jandorf L, Wang Y, Johnson D, Meadows Ray V, Willis MJ et al. “It takes a village”: multilevel approaches to recruit African Americans and their families for genetic research. J Community Genet. 2015;6(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dang JH, Rodriguez EM, Luque JS, Erwin DO, Meade CD, Chen MS. Engaging diverse populations about biospecimen donation for cancer research. J Community Genet. 2014;5(4):313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin R Informed refusal: toward a justice-based bioethics. Sci Technol Human Values. 2016;41(6):967–90. [Google Scholar]
- 32.Benjamin R Race for cures: rethinking the racial logics of “trust” in biomedicine. Sociol Compass. 2014;8(6):755–69. [Google Scholar]
- 33.Boyd RW, Lindo EG, Weeks LG, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs Blog [blog on the Internet]. 2020. July 2 [cited 2021 Oct 19]. Available from: https://www-healthaffairs-org.proxy.lib.umich.edu/do/10.1377/hblog20200630.939347/full/
- 34.Isler MR, Sutton K, Cadigan RJ, Corbie-Smith G. Community perceptions of genomic research: implications for addressing health disparities. N C Med J. 2013;74(6):470–6. [PubMed] [Google Scholar]
- 35.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–42. [DOI] [PubMed] [Google Scholar]
- 36.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. [DOI] [PubMed] [Google Scholar]
- 37.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21(2):60–76. Go to the article, [DOI] [PubMed] [Google Scholar]
- 38.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874–82. [DOI] [PubMed] [Google Scholar]
- 39.Creary MS. Bounded justice and the limits of health equity. J Law Med Ethics. 2021;49(2):241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes SL, Riley P, Radley DC, McCarthy D. Reducing racial and ethnic disparities in access to care: has the Affordable Care Act made a difference? [Internet]. New York (NY): Commonwealth Fund; 2017. August 24 [cited 2021 Oct 19]. Available from: https://www.commonwealthfund.org/publications/issue-briefs/2017/aug/reducing-racial-and-ethnic-disparities-access-care-has [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.