Abstract
Pelvic venous disorders (PeVD) also known as Pelvic Congestion Syndrome (PCS) affect a great number of women worldwide and often remain undiagnosed. Gynecological symptoms caused by vascular background demand a holistic approach for appropriate diagnosis. This is a relevant cause of chronic pelvic pain and atypical varicose veins. The diagnosis is based on imaging studies and their correlation with clinical presentation. Although the aetiology of PCS still remains unclear, it may result from a combination of factors including genetic predisposition, anatomical abnormalities, hormonal factors, damage to the vein wall, valve dysfunction, reverse blood flow, hypertension and dilatation. The following paper describes an in-depth overview of anatomy, pathophysiology, symptoms, diagnosis and treatment of PCS. In recent years, minimally invasive interventions have become the method of first choice for the treatment of this condition. The efficacy of a percutaneous approach is high and it is rarely associated with serious complications.
Key Messages
Pelvic venous disorders demand a holistic approach for appropriate diagnosis.
This article takes an in-depth look at existing therapies of Pelvic Congestion Syndrome and pathophysiology of this condition.
Embolisation is an effective and safe treatment option.
Keywords: Interventional radiology, pelvic venous disorders, pelvic congestion syndrome, chronic pelvic pain
1. Introduction
Pelvic venous disorders (PeVD) manifests as a spectrum of signs and symptoms from the abdomen, pelvis and legs [1]. The relationship between venous pathology in the pelvis and perceived complaints is highly complicated and difficult. The underlying cause of this disease is pelvic venous insufficiency (PVI), which is indicated by dilation and dysfunction of the ovarian or internal iliac veins with characteristic slow flow and reflux [2]. Richet first identified the presence of pelvic varicose veins in 1857 [3].
In 2021 American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders published a report with a new Symptoms-Varices-Pathophysiology classification [4]. Papers published before this year have mostly not approached the subject broadly. PeVD can manifest simultaneously in many areas, and similar venous disorders can produce completely different symptoms in different patients. This article will focus more on symptoms described previously as Pelvic Congestion Syndrome (PCS). The existence of PCS has been questioned over the years and this condition has only recently been accepted [5,6]. The name PCS is used in this article customarily and bearing in mind that PCS manifests as a broader spectrum of symptoms than previously thought and pelvic pain is not necessary for the diagnosis as in many patients the predominant symptoms are atypical superficial varices.
Chronic pelvic pain (CPP) is characterised as more than 6 months of persistent or intermittent pain localised in the pelvis [7]. CPP is a common condition in women and puts a significant economic burden on health budgets [8]. According to the systematic review published by Ahangari, its prevalence ranges from 6% to 27% worldwide [9]. CPP may be caused by PCS, according to some authors in even up to 30% of women [2,3,10–12].
Women affected by PCS, motivated by a deterioration in the quality of life and pelvic pain sometimes spend months seeking help from doctors of various specialties [13]. Many cases of PCS are undiagnosed, most likely due to the fact that physicians are unfamiliar with the disease and due to its vascular background, manifested by, among other things, gynecological symptoms [14–16]. Because of the wide spectrum of symptoms, a comprehensive approach is needed to make an appropriate diagnosis [17]. An additional problem is the readability and quality of online information for patients regarding treatment for PCS, which was evaluated by Lee et al. [18]. Poor quality and difficult-to-read content may block patients from accessing information about their condition.
Patients with PCS are in premenopausal age and typically multiparous. The high number of pregnancies, anomalies in pelvic venous anatomy, history of pelvic pain in family, hormonal disorders like increased levels of oestrogens, polycystic ovary syndrome, oestrogen therapy, as well as varices of the lower limbs, phlebitis, prolapsed uterus, previous pelvic surgery, heavy lifting or prolonged standing are the risk factors for PCS [10,19,20]. Nanavati et al. showed that women with PCS are more likely to have normal BMI than to be obese [21]. Genetic or ethnic predisposition is still uncertain, although a family history of pelvic pain is a risk factor. In pathogenesis, mutations of FOXC2, TIE2, NOTCH3, type 2 transforming growth factor–β and thrombomodulin genes may play a role [10,12]. Congenital disposition appears to be important, as only some women with PVI present with symptoms [10].
2. Anatomy
Blood from the uterus is drained through the interconnecting uterine plexus mainly by four veins. The lower section of the plexus is directed to the left and right internal iliac veins (IIV). Each IIV is led to the common iliac vein and then to the inferior vena cava (IVC). Occasionally IIV can drain straight into IVC. The upper part of the uterus is drained on each side through the uterine or the ovarian plexus to the ovarian veins [12,22].
The pelvic venous network has many interconnections between different regions of the pelvis. PCS manifests with a broad spectrum of symptoms mainly because there are connections to the haemorrhoidal, ovarian, wall and sacral venous plexuses. Additionally, there are numerous connections between the pelvic veins and the superficial veins in the intimate region and on the legs. Hypertension caused by incompetent drainage can result in varicosity formation in the pelvis and cause recurrent symptoms [12,22,23].
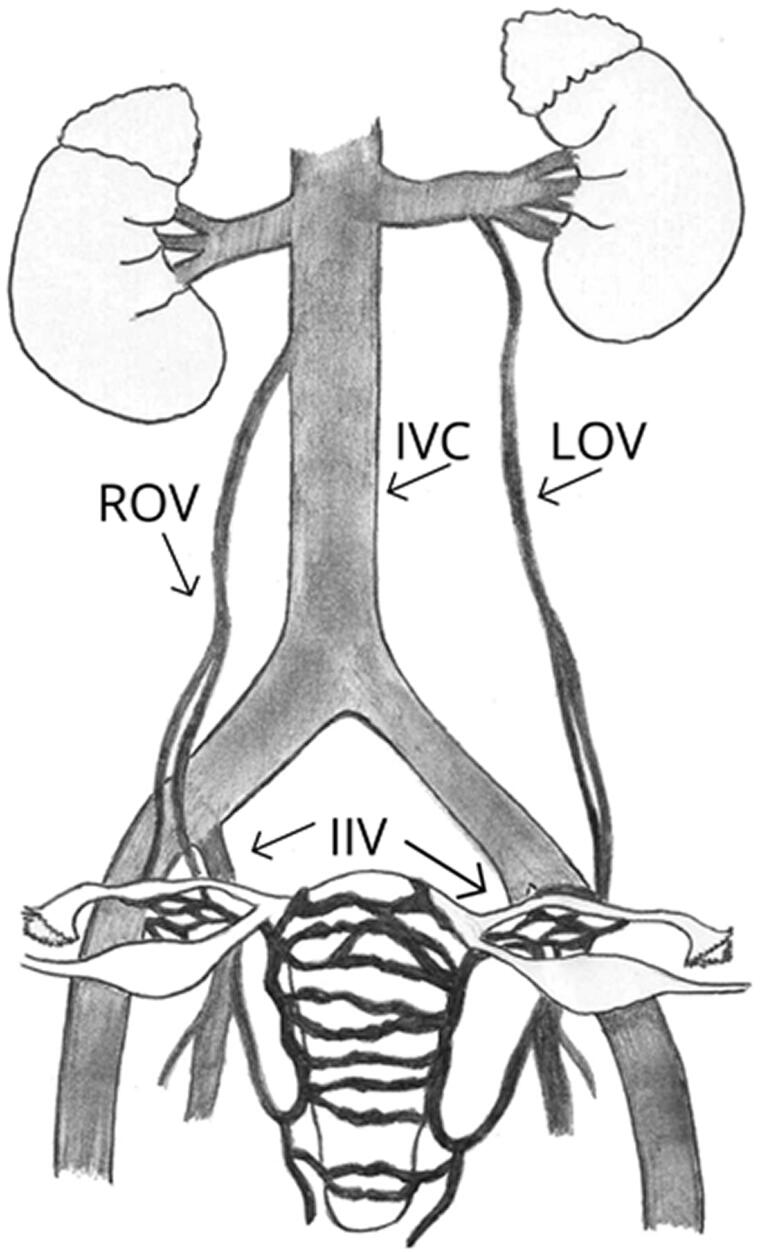
The left-sided dominance of PCS may be explained by anatomical consideration. The left ovarian vein (LOV) is longer than the right ovarian vein (ROV), which makes it more difficult to drain in the upright position. Additionally, the LOV may be compressed by the sigmoid colon located on the left side during constipation. LOV typically drains directly into the left renal vein (LRV), while ROV in most cases (about 60%) drains at a sharp angle into the antero-lateral wall of the IVC, below the right renal vein (RRV) between T12 and L2 [23–25]. In about 10% of females, ROV is led into the right renal vein (RRV) [12] (Figure 1). Nevertheless, it should be noted that pelvic venous drainage is complex and venous anatomy can vary between patients [26].
Figure 1.
Usual anatomy of venous drainage. Inferior vena cava (IVC), left ovarian vein (LOV), right ovarian vein (ROV), internal iliac veins (IIV) are presented.
The valves usually occur within the main trunks of ovarian veins, however, much less frequently are present within the IIVs (only about 10% of cases). In ovarian veins, valves are not present in 15% of women, more frequent on the left side. When valves do exist they are usually found in the distal portion of the vein, near the junction with the renal veins. However, when the ovarian vein valves are present, they are incompetent on the left and right sides in 40% and 35%, respectively [12].
Venous congestion can develop secondary to venous drainage obstruction. The presence of left common iliac vein (CIV) compression caused by the location between the right common iliac artery and the lumbar spine is a condition known as May-Thurner Syndrome (MTS) may contribute to a secondary PCS development [27]. However, Costa et al. reported in 2020 in the study using Magnetic Resonance Imaging, that the degree of stenosis is not related to symptoms, and a large percentage of both asymptomatic and symptomatic patient population show left CIV compression [28]. Similarly, Aurshina et al. also found no correlation between the degree of CIV stenosis seen on MRI and venous symptoms [29].
Another anatomic variant that can result in a secondary PCS is called a Nutcracker syndrome (NCS), where the LRV is compressed between the superior mesenteric artery and the aorta in the anterior type and between the aorta and a vertebral body in the posterior type [30–33]. However, in the author's opinion, it is an over-diagnosed cause, as a result of the patient's position on her back during the imaging. This was also noted in a newly published study where posture significantly affected the degree of stenosis in intravascular ultrasound (55% significantly stenosed LRV in the supine position vs. 10% when lying on the left side) [34].
3. Pathophysiology & hormones
The aetiology of PCS remains poorly understood, and probably many factors contribute to the pathogenesis. PCS can be caused by a combination of factors: genetic predisposition, anatomical abnormalities, hormonal factors, damage of the vein's wall, dysfunctional valves, reverse blood flow, hypertension and dilatation [14].
Studies show the presence of a family history of varicose veins, and up to half of the varicose veins can be related to genetics. There might exist some congenital wall abnormalities in the veins causing their dilatation, which leads to dysfunction of valves. Prolonged venous dilatation in varicose veins in the PVI causes inflammation that further damages walls of the vessel causing the growth of reflux and pressure gradient between veins [10]. However, PVI is not the only factor leading to the PCS diagnosis. Studies show that ovarian varices are symptomatic in only up to 59% of patients and dilated pelvic veins are a frequent symptomless phenomenon in women [13,14,35].
Hormonal factors also play an important role in PCS development. In addition, symptoms usually completely resolve after menopause [19]. The correlation between PCS and ovarian activity can be explained by the physiological effects of female sex hormones. Oestrogen causes increased nitric oxide secretion. This results in increased dilatation and weakening of veins, which causes greater stress on the valves [14]. Studies show that oestrogen levels are significantly higher in painful varicose veins than in unaffected veins and fluctuations in oestrogen levels affect nociceptive sensitivity [36]. Progesterone, by its activity, also weakens venous valves in the pelvic veins [19].
Pregnancy is considered to be one of the main hazards for PCS. During pregnancy, an increased volume of circulation is put on the ovarian and pelvic veins, and flow through the ovarian veins may increase up to 60 times. The increased requirement for venous return as an outcome of the pregnancy and altered hormonal environment leads to chronic venous insufficiency [22,37]. Oestradiol causing vein dilatation during pregnancy leads to higher stress on the valves and increased intrauterine pressure caused by the effect of the pregnant uterus can further increase the reflux through the ovarian veins [3,38].
Vasoconstrictors have shown some effectiveness in relieving PCS symptoms by increasing venous flow through compression, which supports the hormonal theory [12]. Some previous reviews suggested that over 50% of patients with diagnosed PCS have polycystic ovaries identified by echography [3,10,19,39]. However, we found only one article in the literature based on a study conducted in 1990 on 55 patients that maintains this theory [40].
The final result of venous outflow obstruction, irrespective of the aetiology, is the production of multiple varicose veins and painful venous congestion. Pelvic varicose vein histology is identical to varicose vein histology in other areas [22]. Venous hypertension enhances matrix metalloproteinases expression which promotes degradation of collagen, elastin, and endothelium, impairing control of vascular tension [41]. This promotes further damage to the endothelial cell and inflammation. Dilatation of veins may activate selective pain receptors in the walls of vessels. Blood stasis in the capillaries causes local hypoxia [42].
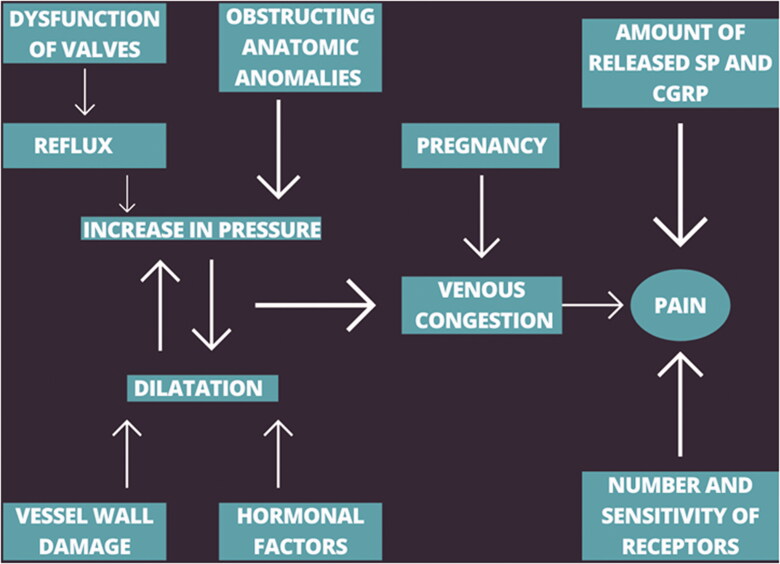
Pain sensation is based on nociceptor activation. C-afferents associated with nociceptors have a low conduction speed and mediate dull/burning pain. Some release neurotransmitters such as substance P and calcitonin gene-related peptide (CGRP), others have receptors on the membrane surface that can be activated by molecules released from damaged cells. Venous dilation and inflammation causes the release of substance P and CGRP, which further dilates the vessel and increases the permeability of the vascular wall to plasma algogens. At the same time, cytokines are released that increase inflammation and nociceptor activity [43]. Our suggested simplified scheme of the pathogenesis of PeVD-induced pain is presented in Figure 2.
Figure 2.
The simplified diagram of the pathogenesis of PeVD-induced pain.
Studies show that both CGRP and substance P are related to pelvic pain [10]. A study published in 2019 by Gavrilov et al. proved that CGRP and SP levels are tightly correlated with pelvic pain. The mean CGRP level was 0.71 ± 0.11 in the group with PVI and pelvic pain and 0.26 ± 0.02 in the group with pelvic varicosities with no pain. The mean substance P levels were 0.42 ± 0.18 and 0.15 ± 0.06 respectively [44]. A role for vascular dysfunction and increased CGRP expression has been shown in the pathogenesis of migraine. Monoclonal antibodies that block CGRP activity are now recognised as an effective treatment for migraine [45,46]. It is possible that this could be one of the future directions of development in the treatment of CPP resulting from venous disorders.
Mechanical compression effects may be another source of pain in PCS. Dilatation of pelvic veins can compress nearby nerves leading to worsening pelvic pain [47]. Interestingly, studies show that patients with lower limb varicosities and PVI have greater levels of pain relative to patients with isolated lower limb varicosities [48].
4. Symptoms
Previous concepts of PCS described the pain as a typical symptom related to PCS, which was characterised as chronic, dull, unilateral or bilateral [20,49]. Nowadays the concept of PeVD, proposed by Meissner et al. notices the complexity of the problem, as a similar degree of venous insufficiency may produce different symptoms, as well as identical symptoms may have different underlying pathophysiology in different patients [4]. Superficial varicose veins may occur without any pelvic pain and be the only symptom of PVI [4,10].
Pelvic pain-enhancing factors described in the literature are long periods of standing, walking or sitting and factors increasing abdominal pressure such as lifting, pregnancy [10,14,24,47]. Pain also increases during and after intercourse. Osman et al. published that dyspareunia due to endometriosis is typically associated with deep penetration, while pain due to PCS is typically exacerbated by intercourse, causing a throbbing ache after [13]. However, there is a strong need for research and data to help distinguish between types of dyspareunia in patients suspected of having PCS. Younger women are more likely than older women to report dyspareunia or gynecological disorders [50]. Pain generally worsen throughout the day and also before and in the first days of menstruation. Symptoms are usually reduced by lying down [26]. Atypically, pain can also be acute or may occur in the abdomen, lower back, hips or legs [10].
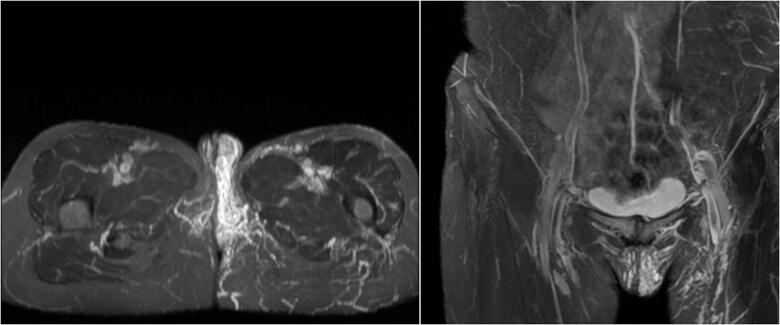
Because the pelvic veins form a network around the organs and many connections are present between the veins draining different pelvic regions patients with PVI often present with atypical varicose veins of the upper inner and back thigh of the lower limb and vulvovaginal, glutaeal, suprapubic perineal varices [19] (Figures 3 and 4). The prevalence of vulvar varices in patients with PCS is as high as 24–40% [51,52] (Figure 3). When the pelvic inflow is not treated, the varicose veins managed surgically often return [19]. In up to 80% of patients with pelvic venous dilatation different degrees of associated lower limb venous insufficiency can be observed [53,54]. The frequency of reporting leg symptoms such as pain, edoema, heaviness increases with age [50]. PCS is also suspected as a cause of venous leg ulcers and infertility [55,56]. In the course of the PCS, urinary symptoms may occur due to perivesical varicosities such as bladder irritability and urgency or dysuria. PCS can also mimic mons pubis abscess or osteoarthritis of the hip [57,58]. Other manifestations of PCS may also include headache, dysmenorrhoea, lumbosacral neuropathy, leg heaviness, rectal discomfort, swollen vulva, vaginal discharge, persistent genital arousal and non-specific gastrointestinal symptoms such as bloating and nausea [10,12,14,26,39,49].
Figure 3.
Vulvar varices in patients with PCS in MRI.
Figure 4.
Atypical varicose veins of two patients due to chronic pelvic venous insufficiency.
Chronic pelvic pain and additional symptoms negatively affect the quality of life of the patients, which translates into significantly more frequent depression, anxiety and generalised lethargy in this group [49]. Additionally, neurotransmitters released from varicose veins such as substance P and the neurokinins A and B are known to be involved in the regulation of emotions and psychological stress pathways [25].
5. Diagnosis
Certain recognition of the PCS is very difficult, due to its multiformity. In addition, many medical conditions have similar manifestations, see (Table 1). Additionally demonstrating PVI in imaging studies supports the diagnosis however, it cannot define it.
Table 1.
| Gastroenterology | Gynecology | Musculoskeletal | Neurology & psychiatry | Urology |
|---|---|---|---|---|
| Chronic constipation | Adenomyosis | Fibromyalgia | Abdominal epilepsy/migraine | Interstitial cystitis |
| Diverticular disease | Adhesions | Fractured coccyx | Herniated nucleus pulposus | Recurrent urinary tract infections |
| Hernia | Cancer or metastases | Hip joint pathology | Major depression | Urethral diverticulum |
| Inflammatory bowel disease | Chronic pelvic inflammatory disease | Myofascial pain | Neuralgia of ilioinguinal, genitofemoral, or pudendal nerves | |
| Irritable bowel syndrome | Endometriosis | Pelvic floor myalgia | Neuropathic pain | |
| Porphyria | Fibroids | Piriformis syndrome | Physical, sexual, or substance abuse | |
| Ovarian cysts | Psoas inflammation | Sleep disorders | ||
| Uterine prolapse | Sacroiliac joint inflammation | Somatization |
New Symptoms-Varices-Pathophysiology (SVP) classification covers three areas: Symptoms (S), Varices (V), and Pathophysiology (P). The pathophysiology domain includes Anatomical (A), hemodynamic (H), and etiologic (E) aspects of the patient's disease. Despite its complexity, it allows for uniformity of naming [4].
Ultrasound (US) remains the first line, screening imaging study. Conventional B-mode assesses pelvic anatomy and excludes masses, while color-Doppler measures flow. Ultrasound has the advantage of allowing dynamic examination with provocative Valsalva manoeuvres [60,61]. The ultrasound can be either transvaginal, transabdominal or transperineal [62]. Transvaginal ultrasound (TVU) may better rule out other gynecological problems, but transabdominal and transperineal US allows to visualise the vessel on a longer course [62,63]. Findings suggesting the diagnosis are tortuous pelvic veins with a diameter more than 4 mm, slow (≤ 3 cm/s) blood flow and dilated arcuate veins in the myometrium, communicating with the pelvic varicosities [63]. According to Park et al. positive predictive value as a cut-off diameter of a left ovarian vein in the US was 71.2% at 5 mm, 83.3% at 6 mm, 81.8% at 7 mm, and 75.8% at 8 mm [64].
Computer tomography (CT) provides cross-sectional imaging and accurate anatomical visualisation. The diagnostic criteria for both CT and MRI recommended by Coakley et al. include at least four ipsilateral parauterine veins of varying calibre, at least one measuring more than 4 mm in diameter, or the diameter of the ovarian vein over 8 mm, but cut-off diameters vary between studies [16,35,65]. However, according to Dos Santos et al. vessel diameter alone is only 56% accurate in reflux identification [66]. Hiromura et al., divided the degree of reflux into three grades. Grade I, when the retrograde flow is limited to the ovarian vein, grade II, when the retrograde flow is present in parauterine veins and grade III- retrograde flow crossing midline, passing to parauterine plexus on the other side [67] (Figure 5). A rather similar classification containing 4 grades was recently proposed by Szary et al. [68].
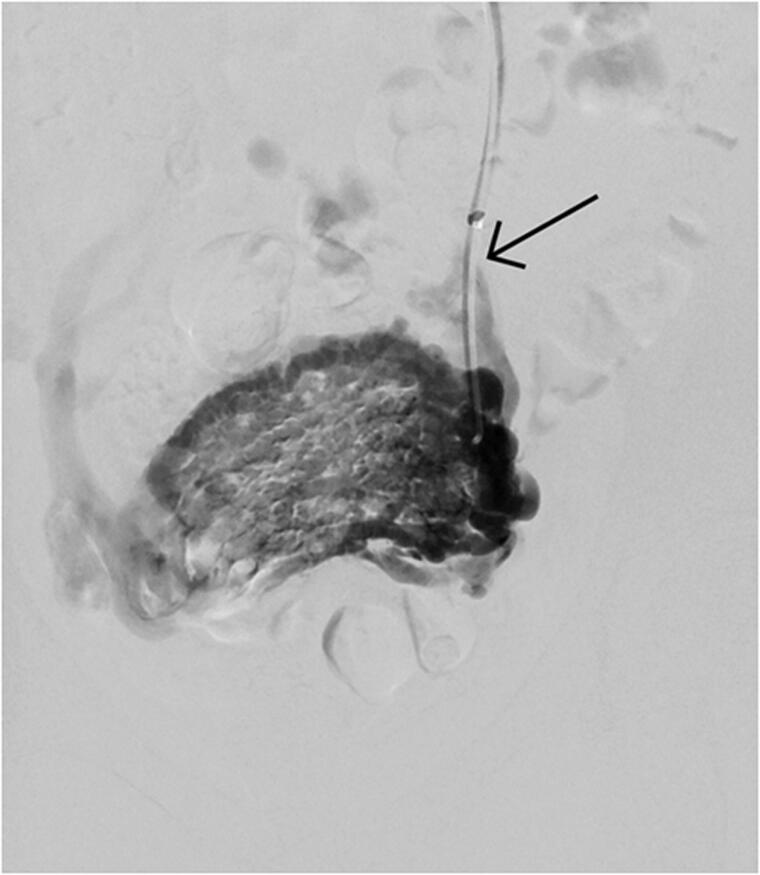
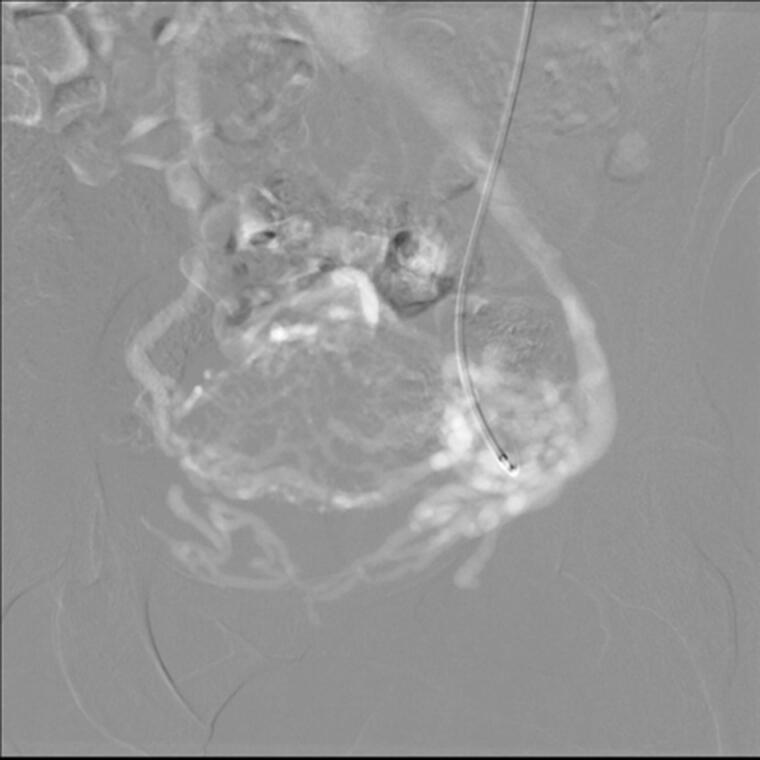
Figure 5.
Grade III reflux in venography. Retrograde flow crossing midline, passing to parauterine plexus on the other side. Catheter in the left ovarian vein indicated by an arrow.
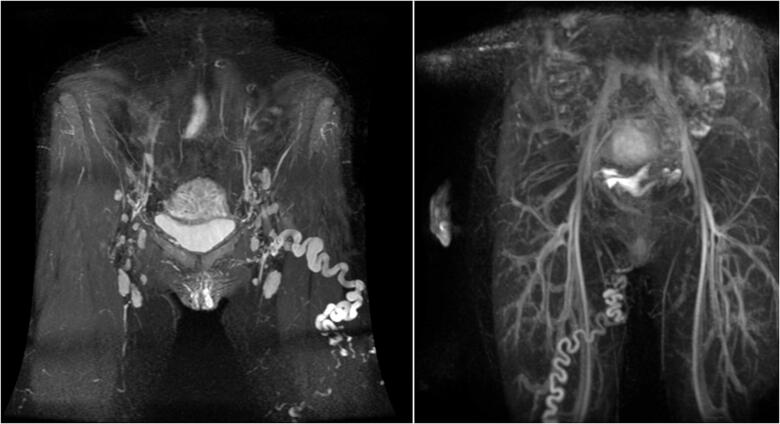
Magnetic Resonance Imaging (MRI) of the pelvis can provide excellent image quality and high spatial resolution in imaging anatomical details and pelvic vessels [47]. Unlike CT, MRI does not involve the use of radiation and can be used more safely in women of child-bearing age. Cross-sectional imaging allows detection of other pathologies such as abnormal uterine, endometriosis, gastrointestinal or musculoskeletal pathology or tumours [47,69]. Both contrast-enhanced MRA and non-contrast MRA sequences provide good sensitivity in diagnosing venous insufficiency [70] (Figures 6 and 7). Information regarding flow through the veins in MRI can be provided using phase-contrast velocity mapping (PCVM) or Time-Resolved Imaging (TRI) [71]. Magnetic Resonance Venography with TRI provides accurate information, whether flow present in the ovarian vein is anterograde or retrograde [72–74]. Yang et al. compared TRI with conventional venography. Results showed that TRI is an excellent non-invasive diagnostic tool for PVI, as there was no significant difference between conventional venography and TRI for determining the level of ovarian venous reflux [75].
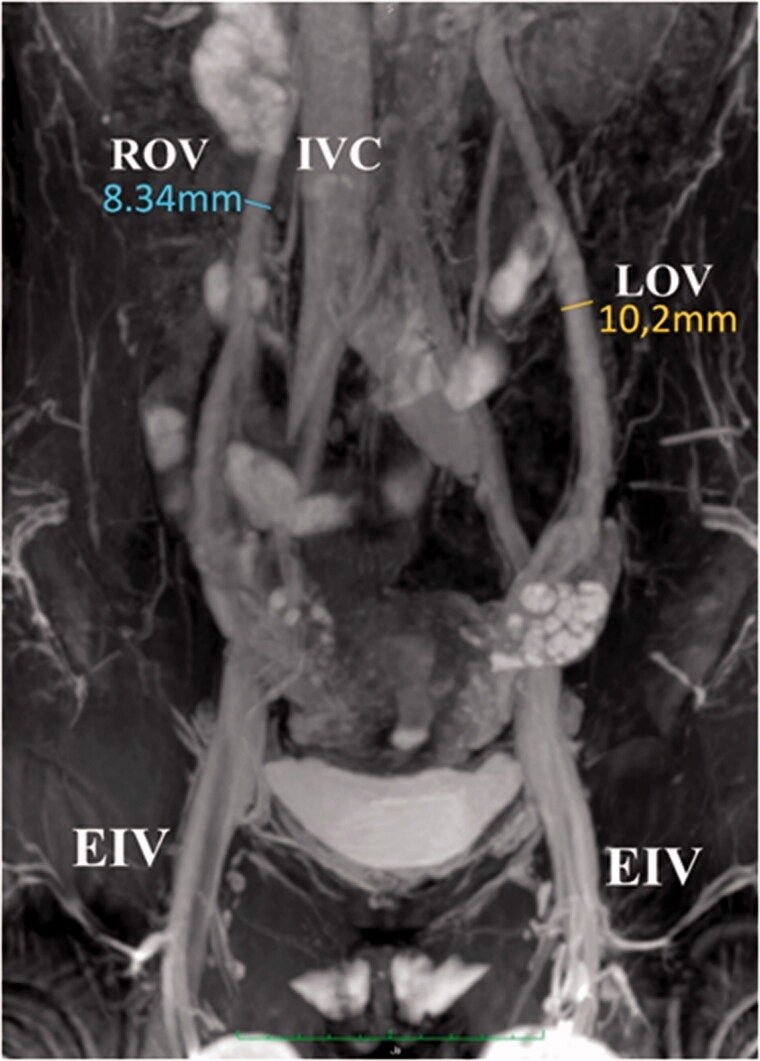
Figure 6.
MRI,3D IFIR without contrast agent. Frontal section. Inferior vena cava (IVC), left ovarian vein (LOV) and right ovarian vein (ROV), common iliac vein, external iliac vein (EIV), internal iliac vein and its branches are visible.
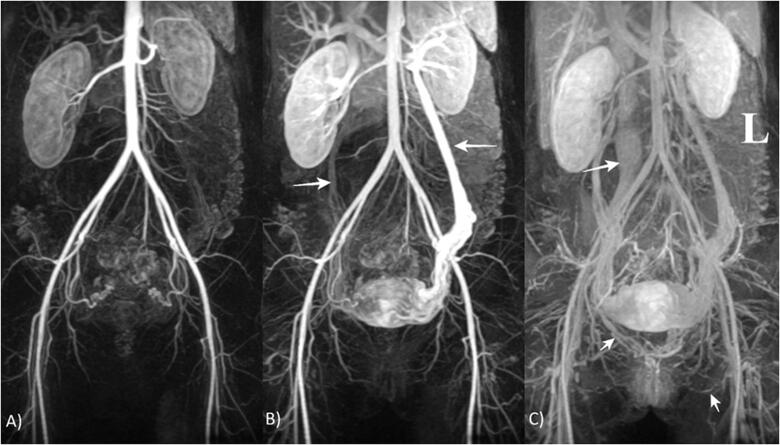
Figure 7.
MRI, TRICKS with intravenous administration of contrast agent. Dynamic imaging of the abdominal and pelvic vascular system. (A) Arterial phase; (B) late venous phase with ovarian veins indicated by arrows; and (C) very late venous phases with IVC, right internal vulvar vein and left obturator vein indicated by arrows.
It is reported that in about 40% of CPP cases diagnostic laparoscopy is performed. This procedure can miss up to 80-90% of PCS cases due to the routine use of Trendelenburg's position as well as due to vein compression by CO2 pressure. Diagnostic value can be slightly improved when using low-pressure laparoscopy and the lower degree of Trendelenburg's position [76,77]. Sometimes, however, laparoscopy enables the visualisation of other causes of chronic pelvic pain [20].
Another diagnostic tool frequently cited in previous reviews was ovarian point tenderness [10,12,14,19,22,23,25,26,38,39,47,49,78]. The clinical examination showed that ovarian point tenderness and the history of postcoital pain was 94% sensitive and 77% specific for PCS diagnosis. However, this is based on the study published in 1988 on 35 patients [79]. A more recent study conducted by Herrera-Betancourt et al. showed 87% sensitivity and only 37% specificity of ovarian point tenderness [80].
Venography still remains the gold standard for diagnosis of PCS. Since it is an invasive examination it should be reserved for patients who had prior non-invasive imaging, while interventional therapy is planned [60,69,81]. Selective ovarian and iliac catheter venography can be performed under local anaesthesia. Another way of venographic diagnostics is the direct injection of contrast to the uterine fundus through a needle inserted into the myometrium and evaluating venous flow under fluoroscopy [80]. Evaluation can be based on Beard’s criteria, which consists of three components: maximum diameter of the ovarian vein (<5mm considered normal, 5–8 mm moderate, >8mm severe), time to the disappearance of contrast material (0, 20, and 40 s), and degree of congestion (normal when veins are small and straight, moderate when tortuous and severe if veins are highly tortuous and wide). Each component is scored from 1 to 3 and the final sum of 5 or more is considered to fulfil the diagnostic criteria, which are believed to have 91% sensitivity and 89% specificity [82].
6. Treatment
Pharmacological treatment for PCS is limited due to the lack of data determining long-term efficacy [26]. Hormonal therapies that inhibit ovarian function, such as medroxyprogesterone acetate (MPA) and gonadotropin-releasing hormone (GnRH) agonists have shown some efficacy, but the therapy was accompanied by numerous side effects. In addition, stable results 9 months after treatment with MPA were obtained only in combination with psychotherapy [12]. Implanon, a subcutaneous implant containing the desogestrel metabolite etonogestrel was associated with improvements in symptom relief and venographic findings 1 year after treatment. However, the implant was used in only 12 cases and no data regarding long-term results are available [83]. The study published by Reginald et al. in 1987 showed some effectiveness of dihydroergotamine in reducing pain scores. However, the treatment showed only transient efficacy, side effects, and was carried out in only 12 patients [84]. In the short term, nonsteroidal anti-inflammatory drugs may reduce symptoms, while the patient is undergoing further care, but they do not contribute to curing the problem [19]. In a study published in 2019 Tadalafil improved bladder function in rats with pelvic venous congestion. However, this requires further study [85].
Micronized purified flavonoid fraction (MPFF), a venoactive drug has been investigated by Simsek et al., Tsukanov et al. and Gavrilov et al. All researchers demonstrated that 1000 mg of MPFF per day reduced the severity of pelvic symptoms such as pain, heaviness and labia majora swelling secondary to pelvic varicose veins [52,86–88]. Additionally, Gavrilov et al. showed that a double dose of MPFF (1000 mg twice daily) in the first month of treatment provides a quicker resolution of symptoms [89]. Interestingly, MPFF also reduces CPP caused by prostatitis occurring as a result of increased venous return through the perineum [90].
Compression is a conservative treatment used to treat varicose veins [91]. In a study conducted by Gavrilov et al. class II compression shorts used for 2 weeks reduced chronic pelvic pain, dyspareunia, and discomfort in 81.3% of patients. They also decreased leg heaviness and swelling. However, they had no effect on clinical symptoms of vulvar varicose veins. No clinical improvement or improvement in venous drainage was observed in the group using elastic stockings [92].
Surgery is associated with a longer hospital stay and greater mortality when compared to endovascular therapy [3]. Rundqvist et al. first reported extraperitoneal resection of the left ovarian vein to treat PCS in 1984 [93]. Studies show a 20% recurrence rate after hysterectomy, and residual pain occurs in 33% of patients [39]. There is a lack of randomised controlled trials [94]. In a randomised control study conducted by Chung et al. ovarian vein embolisation was significantly more effective than hysterectomy with unilateral or bilateral salpingo-oophorectomy 12 months following treatment. The average visual analogue pain scale (VAS) in the embolisation group decreased from 7.8 to 3.2, compared to a decrease in VAS scale from 7.7 to 4.6 and from 7.8 to 5.6 in other groups [95].
The first description of embolisation as a treatment for PCS was published in 1993 by Edwards [96]. According to the Society for Vascular Surgery and American Venous Forum embolisation is recommended with a 2B level of evidence for the treatment of PCS [97].
Due to the poor recognition of this disease, there is no definitive endovascular treatment protocol for PCS. Both the technique like the intravascular access site and the materials used for embolisation like sclerosants, coils, plugs vary in publications on this topic [98]. Studies comparing multiple embolisation agents are still lacking. There have been no trials that have found large differences in clinical outcomes between agents [99,100]. In our opinion, the best embolisation devices are platinum coils, preferably detachable and packable (Figure 8).
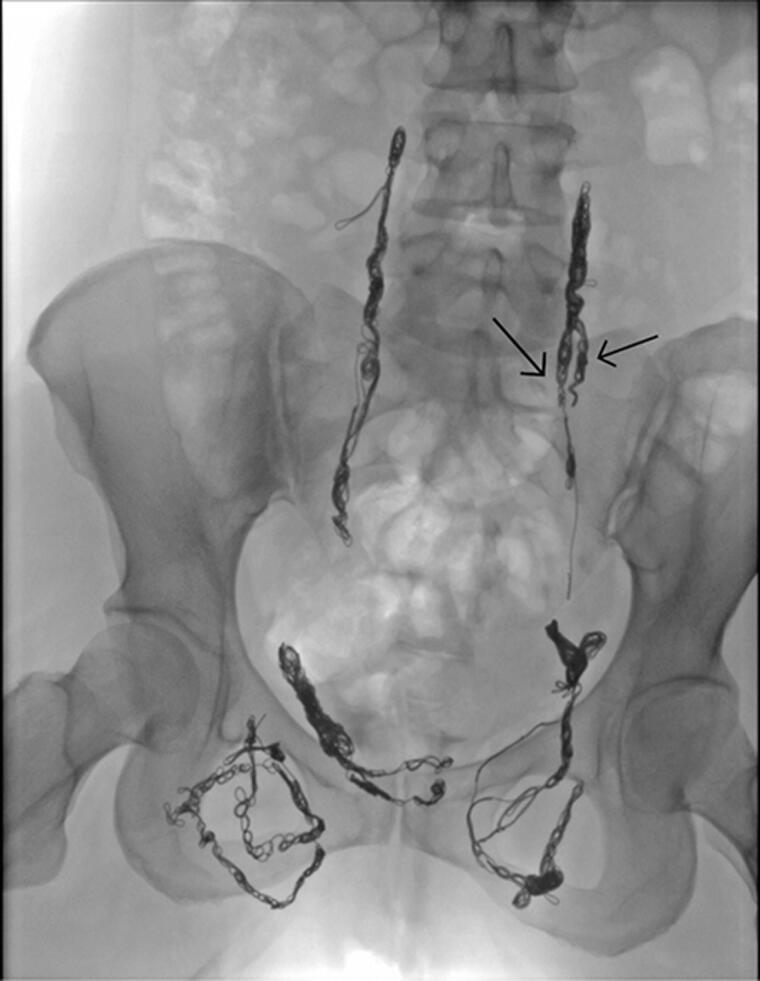
Figure 8.
Patient after embolisation of the insufficient pelvic veins. Duplication of the left ovarian vein indicated by arrows.
Sustained clinical improvement after embolisation ranges in various studies from 47 to 100% [101]. There is no consensus on how to report results, and the outcomes are very heterogeneous, which is one of the reasons why it is difficult to compare trials. The systematic review by Brown et al. covering 14 studies and 828 women found an improvement in clinical symptoms after endovascular treatment ranging from 68.3 to 100% [102]. In another systematic review, a statistically significant decrease in pelvic pain was reported in 9 of 13 studies [103]. Daniels et al. analysed 22 cohorts involving 1,308 patients. The average rate of symptom improvement within the first 3 months was 75% [104]. Patients in their 20 s usually have a shorter duration of improvement than older women [50]. Interestingly, smaller ovarian vein diameter may be associated with better clinical improvement [105]. In a study conducted by Chung et al. women who had higher stress scores achieved in standardised stress questionnaire (Revised Social Readjustment Rating Scale) had significantly less improvement after embolisation compared to women with lower perceived stress. The authors suggest a tendency in women who have low-stress tolerance to pay more attention to their physical complaints, such as pain, and a tendency for stress to manifest in autonomic innervated sites such as vascular smooth muscle [95].
There is still debate about how many veins should be embolised [99]. The difference is not statistically significant in the comparison of unilateral and bilateral embolisation [69]. Maleux et al. found no statistically significant difference in clinical results in patients with LOV and ROV insufficiency treated with bilateral embolisation versus patients with LOV insufficiency treated with unilateral embolisation only [106]. Some clinicians perform only unilateral ovarian vein embolisation while others perform complete embolisation.
Laborda et al. reported the results of coil embolisation of both gonadal veins and both hypogastric veins in patients with PCS with a 5-year follow-up after the procedure. Clinical success was obtained in 93.85%, with a decrease in pain perceived on the VAS scale from 7.34 ± 0.7 before the procedure to 0.78 ± 1.2 [107]. Similarly, De Gregorio et al. reported good results of embolisation of all four veins with a reduction in pain reported on the VAS scale from 7.63 ± 0.9 points before treatment to 0.91 ± 1.5 with a mean follow-up of almost 5 years [99]. In our opinion, because of the interconnection between the ovarian veins and the branches of the IIVs, all insufficient venous outlets should be closed and when multiplication of the ovarian vein is present, embolisation of each trunk should be performed (Figure 8).
Embolisation can also be performed without the use of iodine-containing contrast, as in our centre, a procedure of successful ovarian vein embolisation using CO2 was performed in a patient allergic to contrast (Figure 9). CO2 is classified as a negative contrast agent because the density of CO2 is lower than the surrounding tissue. To image the vessels with CO2 contrast it is necessary to use equipment with appropriate software dedicated to negative contrast media. The biggest advantage of CO2 is the absence of adverse effects typical for iodine agents, primarily kidney damage and allergic reactions. However, the patient may experience discomfort or pain when injecting CO2, and the vein may also react with spasm [108].
Figure 9.
Digital Subtraction Angiography was performed using CO2 before embolisation in a patient allergic to contrast.
The effect of embolisation on fertility is not precisely known. It appears that embolisation may treat infertility caused by venous congestion. The largest study focussing on this issue was published by Liu et al. and included only 12 female patients. Ovarian varices were suspected to be the only factor of infertility and other factors that may possibly cause infertility were excluded. Overall, 8 of the women had a subsequent pregnancy after the procedure, of which six had intrauterine pregnancies leading to live births [56]. Several other studies have reported single cases of pregnancy after embolisation [109–112].
When stenosis is present, representing a hemodynamically significant problem, removal of the obstruction should be undertaken [113]. However, stenting of the left CIV without embolisation of gonadal veins relieves symptoms of PCS due to May-Thurner Syndrome in only 16.6% of patients [114]. Different results were published by Lakhanpal et al. A total of 56% of women with PVI caused by CIV stenosis experienced complete resolution of symptoms after iliac vein stenting alone [115]. The major risk of endovascular treatment failure is stent occlusion, and the duration of antithrombotic therapy post-procedure varies between studies [116]. In a systematic review published by Padrnos et al. CIV stent patency rate ranged from 60% to 100% [117].
Left renal vein stenting in the management of the NCS has shown some efficacy in the treatment of PCS caused by this syndrome. However, there are few studies with small numbers of participants [118,119]. Stenting of the left renal vein is associated with a high risk of migration to the vena cava and the heart due to short vein length and change in vein diameter when the patient changes position or performs the Valsalva manoeuvre [120]. Left renal vein transposition is not always successful and it is correlated with serious complications like bleeding, thrombosis, kidney injury or infection [42,118]. In 2020 Gilmore et al. reported gonadal vein transposition in 18 patients, with complete symptom relief in 11 patients (61.1%) after a median follow-up of 178 days [121]. Complications of percutaneous embolisation are usually rare and harmless. These include recurrence of symptoms, haematoma at the puncture site, allergic reaction, embolic agent migration or coil erosion [78,122,123].
PCS symptoms may reoccur after ovarian vein embolisation from other tributaries in the venous network. Hasjim et al. reported recurrence of PCS symptoms four years after embolisation. Although the gonadal vein remained embolised, the recurrence was through the median sacral vein. Coil embolisation of the incompetent median sacral vein caused the resolution of symptoms [124].
Postembolization syndrome may occur in 20% of patients. It is characterised by increased pelvic pain, hyperthermia and tenderness around the embolised vein, and usually resolves with NSAIDs [125]. A potentially dangerous complication may be coil or vascular plug migration to the pulmonary artery. However, it is typically successfully removed endovascularly [99,126,127].
7. Challenges
The management of PeVD continues to present many challenges for investigators [128]. The creation of the new Symptoms-Varices-Pathophysiology classification instrument has allowed for the systematisation of nomenclature and is the first step towards the further systematisation of disease management [4]. Due to this classification, finally, it is possible to create a homogeneous group of patients in trials. However, there is still a lack of commonly used, globally accepted diagnostic algorithms that allow objective diagnosis and differentiation from diseases with similar symptoms [101]. There is a need for validated clinical and imaging diagnostic criteria that take into account the patient's position during imaging. Significant variability in how the diagnosis is made affects the assessment of treatment outcomes. Endovascular therapy seems to be an effective method of treatment, but its efficacy is difficult to compare between studies because diagnostic algorithms, embolisation of different numbers of veins, embolisation materials and post-procedure follow-up vary between studies. There is a great need for randomised, large clinical trials comparing the results of treatment with different embolic agents and embolisation techniques in specific groups of patients in the SVP scale [4,19]. Finally, determining the degree of clinical improvement is difficult because it is highly subjective and the symptoms experienced by patients may overlap with other coexisting conditions, and the reporting of outcomes varies considerably between studies. It is important to develop a validated questionnaire that will standardise the reporting of treatment outcomes, and enable their proper comparison across studies [128].
8. Conclusions
Pelvic Venous Disorders manifests in many clinical presentations. Pelvic Congestion Syndrome is a common condition occurring worldwide, in which a significant proportion of cases remain undiagnosed and symptoms reported by women are often underestimated, due to poor knowledge of the condition. It is an important cause of chronic pelvic pain in female patients. It can also present with superficial varicose veins as the only symptom as well as in combination with pain. Symptoms can be non-specific and difficult to distinguish from other diseases. Certain diagnosis of the PCS is very challenging, due to its multiformity. Determining which patients suffer from symptoms associated with PCS is hard, but also extremely important to implement appropriate and targeted treatment. Future randomised trials on embolisation management are needed. A common treatment algorithm for trials based on an understanding of the mechanisms leading to symptoms would be particularly helpful in objectively evaluating outcomes.
Author contributions
K.B, M.T. and R.P. were responsible for the conception; K.B. was responsible for the design of the study; K.B. and M.T. were responsible for the acquisition of the literature for the manuscript. K.B. wrote the original draft of the manuscript. M.T. and R.P. reviewed and edited. M.T. and R.P. supervised the paper. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
There is no raw data associated with this review. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- 1.Meissner MH, Khilnani NM, Labropoulos N, et al. The Symptoms-Varices-Pathophysiology classification of pelvic venous disorders: a report of the American Vein & Lymphatic Society International Working Group on pelvic venous disorders. J Vasc Surg Venous Lymphat Disord. 2021;9(3):568–584.[ [DOI] [PubMed] [Google Scholar]
- 2.Jurga-Karwacka A, Karwacki GM, Schoetzau A, et al. A forgotten disease: pelvic congestion syndrome as a cause of chronic lower abdominal pain. PLOS One. 2019;14(4):e0213834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrêa MP, Bianchini L, Saleh JN, et al. Pelvic congestion syndrome and embolization of pelvic varicose veins. J Vasc Bras. 2019;18:e20190061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meissner MH, Khilnani NM, Labropoulos N, et al. The Symptoms-Varices-Pathophysiology (SVP) classification of pelvic venous disorders a report of the American Vein & Lymphatic Society International Working Group on pelvic venous disorders. J Vasc Surg Venous Lymphat. Disord. 2021. May;9(3):568-584. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Vleuten CJM, Van Kempen JAL, Schultze-Kool LJ.. Embolization to treat pelvic congestion syndrome and vulval varicose veins. Int J Gynaecol Obstet. 2012;118(3):227–230. [DOI] [PubMed] [Google Scholar]
- 6.Tropeano G, Di Stasi C, Amoroso S, et al. Ovarian vein incompetence: a potential cause of chronic pelvic pain in women. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):215–221. [DOI] [PubMed] [Google Scholar]
- 7.Speer LM, Mushkbar S, Erbele T.. Chronic pelvic pain in women. 2016;93(5):380-7. [PubMed] [Google Scholar]
- 8.Riding DM, Hansrani V, McCollum C.. Pelvic vein incompetence: clinical perspectives. Vasc Health Risk Manag. 2017;13:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician. 2014;17(2):141–147. [PubMed] [Google Scholar]
- 10.Phillips D, Deipolyi AR, Hesketh RL, et al. Pelvic congestion syndrome: etiology of pain, diagnosis, and clinical management. J Vasc Interv Radiol. 2014;25(5):725–733. [DOI] [PubMed] [Google Scholar]
- 11.Soysal ME, Soysal S, Vicdan K, et al. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod. 2001;16(5):931–939. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien MT, Gillespie DL.. Diagnosis and treatment of the pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2015;3(1):96–106. [DOI] [PubMed] [Google Scholar]
- 13.Osman MW, Nikolopoulos I, Jayaprakasan K, et al. Pelvic congestion syndrome. Obstet Gynecol. 2013;15(3):151–157. [Google Scholar]
- 14.Borghi C, Dell'Atti L.. Pelvic congestion syndrome: the current state of the literature. Arch Gynecol Obstet. 2016;293(2):291–301. [DOI] [PubMed] [Google Scholar]
- 15.Lemasle P, G M.. MICHAL duplex ultrasound investigation in pelvic congestion syndrome: technique and results. Phlebolymphology. 2017;24:79–87. [Google Scholar]
- 16.Awad AS, Taha MMM, Manaf MHA, et al. Role of multi-detector CT venography in evaluation of pelvic congestion syndrome. Egypt J Radiol Nucl Med. 2020;51:159. [Google Scholar]
- 17.Cavezzi A. Medicine and phlebolymphology: time to change? JCM. 2020;9(12):4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RJ, O’Neill DC, Brassil M, et al. Pelvic vein embolization: an assessment of the readability and quality of online information for patients. CVIR Endovasc. 2020;3(1): 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoud O, Vikatmaa P, Aho P, et al. Efficacy of endovascular treatment for pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4(3):355–370. [DOI] [PubMed] [Google Scholar]
- 20.Wozniak S. Chronic pelvic pain. Ann Agric Environ Med. 2016;23(2):223–226. [DOI] [PubMed] [Google Scholar]
- 21.Nanavati R, Jasinski P, Adrahtas D, et al. Correlation between pelvic congestion syndrome and body mass index. J Vasc Surg. 2018;67(2):536–541. [DOI] [PubMed] [Google Scholar]
- 22.Durham JD, Machan L.. Pelvic congestion syndrome. Semin Intervent Radiol. 2013;30(4):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo S, Fan CM.. Pelvic congestion syndrome and pelvic varicosities. Tech Vasc Interv Radiol. 2014;17(2):90–95. [DOI] [PubMed] [Google Scholar]
- 24.Lopez AJ. Female pelvic vein embolization: indications, techniques, and outcomes. Cardiovasc Intervent Radiol. 2015;38(4):806–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson T, Basile A.. Pelvic congestion syndrome, who should we treat and how? Tech Vasc Interv Radiol. 2006;9(1):19–23. [DOI] [PubMed] [Google Scholar]
- 26.Bendek B, Afuape N, Banks E, et al. Comprehensive review of pelvic congestion syndrome: causes, symptoms, treatment options. Curr Opin Obstet Gynecol. 2020;32(4):237–242. [DOI] [PubMed] [Google Scholar]
- 27.Toh MR, Tang TY, Lim HHMN, et al. Review of imaging and endovascular intervention of iliocaval venous compression syndrome. World J Radiol. 2020;12(3):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa LMG, Tachibana A, Da Silva Magao F, et al. Magnetic resonance imaging evaluation of left common iliac vein compression in patients with and without symptoms of venous disease. Circ J. 2020;84(5):763–768. [DOI] [PubMed] [Google Scholar]
- 29.Aurshina A, Huber S, Deng Y, et al. Correlation of venous symptoms with iliac vein stenosis on magnetic resonance imaging. Proc J Vasc Surg Venous Lymphat Disord. 2021;9(5):1291–1296.e1. [DOI] [PubMed] [Google Scholar]
- 30.Al-Qaoud T, Bath N, Redfield R, et al. Salvage renal autotransplant following previous renal vein stenting in nutcracker syndrome. Exp Clin Transplant. 2020;18(3):300–305. [DOI] [PubMed] [Google Scholar]
- 31.Larkin TA, Hovav O, Dwight K, et al. Common iliac vein obstruction in a symptomatic population is associated with previous deep venous thrombosis, and with chronic pelvic pain in females. J Vasc Surg Venous Lymphat Disord. 2020;8(6):961–969. [DOI] [PubMed] [Google Scholar]
- 32.Sablón González N, Villalba NL, Parodis López Y, et al. Nutcracker syndrome. Medicina. 2019;79:150–153. [PubMed] [Google Scholar]
- 33.Gozzo C, Giambelluca D, Cannella R, et al. CT imaging findings of abdominopelvic vascular compression syndromes: what the radiologist needs to know. Insights Imaging. 2020;11(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krzanowski M, Partyka L, Drelicharz L, et al. Posture commonly and considerably modifies stenosis of left common iliac and left renal veins in women diagnosed with pelvic venous disorder. J Vasc Surg Venous Lymphat Disord. 2019;7(6):845–852.e2. [DOI] [PubMed] [Google Scholar]
- 35.Szaflarski D, Sosner E, French TD, et al. Evaluating the frequency and severity of ovarian venous congestion on adult computed tomography. Abdom Radiol. 2019;44(1):259–263. [DOI] [PubMed] [Google Scholar]
- 36.Yu JH, Fang HH, Liu SY, et al. Dual effects of a gonadotropin-releasing hormone agonist on an adolescent girl with pelvic congestion syndrome and precocious puberty: a case report. J Int Med Res. 2020;48(9):030006052095469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szary C, Wilczko J, Plucinska D, et al. The number of pregnancies and deliveries and their association with selected morphological and hemodynamic parameters of the pelvic and abdominal venous system. JCM. 2021;10(4):736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rane N, Leyon JJ, Littlehales T, et al. Pelvic congestion syndrome. Curr Probl Diagn Radiol. 2013;42(4):135–140. [DOI] [PubMed] [Google Scholar]
- 39.Ignacio EA, Dua R, Sarin S, et al. Pelvic congestion syndrome: diagnosis and treatment. Semin Intervent Radiol. 2008;25(4):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams J, Reginald PW, Franks S, et al. Uterine size and endometrial thickness and the significance of cystic ovaries in women with pelvic pain due to congestion. Br J Obstet Gynaecol. 1990;97(7):583–587. [DOI] [PubMed] [Google Scholar]
- 41.MacColl E, Khalil RA.. Matrix metalloproteinases as regulators of vein structure and function: implications in chronic venous disease. J Pharmacol Exp Ther. 2015;355(3):410–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavrilov SG, Efremova OI.. Surgical aspects of venous pelvic pain treatment. Curr Med Res Opin. 2019;35(11):1983–1989. [DOI] [PubMed] [Google Scholar]
- 43.Gavrilov SG, Vassilieva GY, Vasilev IM, et al. The role of vasoactive neuropeptides in the genesis of venous pelvic pain: a review. Phlebology. 2020;35(1):4–9. [DOI] [PubMed] [Google Scholar]
- 44.Gavrilov SG, Vasilieva GY, Vasiliev IM, et al. Calcitonin gene-related peptide and substance p as predictors of venous pelvic pain. Acta Naturae. 2019;11(4):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raffaelli B, Reuter U.. The biology of monoclonal antibodies: focus on calcitonin gene-related peptide for prophylactic migraine therapy. Neurotherapeutics. 2018;15(2):324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitsikostas DD, Reuter U.. Calcitonin gene-related peptide monoclonal antibodies for migraine prevention: Comparisons across randomized controlled studies. Curr Opin Neurol. 2017;30(3):272–280. [DOI] [PubMed] [Google Scholar]
- 47.Knuttinen MG, Xie K, Jani A, et al. Pelvic venous insufficiency: Imaging diagnosis, treatment approaches, and therapeutic issues. Am. J. Roentgenol. 2015;204(2):448–458. [DOI] [PubMed] [Google Scholar]
- 48.Asciutto G, Mumme A, Asciutto KC, et al. Pelvic vein incompetence influences pain levels in patients with lower limb varicosity. Phlebology. 2010;25(4):179–183. [DOI] [PubMed] [Google Scholar]
- 49.Ganeshan A, Upponi S, Hon LQ, et al. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol. 2007;30(6):1105–1111. [DOI] [PubMed] [Google Scholar]
- 50.Sulakvelidze L, Tran M, Kennedy R, et al. Presentation patterns in women with pelvic venous disorders differ based on age of presentation. Phlebology. 2021;36(2):135–144. [DOI] [PubMed] [Google Scholar]
- 51.Kim AS, Greyling LA, Davis LS.. Vulvar varicosities: a review. Dermatol Surg. 2017;43(3):351–356. [DOI] [PubMed] [Google Scholar]
- 52.Gavrilov SG. Vulvar varicosities: diagnosis, treatment, and prevention. Int J Womens Health. 2017;9:463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bora A, Avcu S, Arslan H, et al. The relation between pelvic varicose veins and lower extremity venous insufficiency in women with chronic pelvic pain. JBR-BTR. 2012;95(4):215–221. [DOI] [PubMed] [Google Scholar]
- 54.Gavrilov SG, Moskalenko YP.. Does pelvic congestion syndrome influence symptoms of chronic venous disease of the lower extremities? Eur J Obstet Gynecol Reprod Biol. 2019;243:83–86. [DOI] [PubMed] [Google Scholar]
- 55.Placke JM, Jockenhöfer F, Benson S, et al. Venous ulcerations occur more frequently in women on the left lower leg. Can pelvic congestion syndrome be an often undetected cause? Int Wound J. 2020;17(1):230–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Han L, Han X.. The effect of a subsequent pregnancy after ovarian vein embolization in patients with infertility caused by pelvic congestion syndrome. Acad. Radiol. 2019;26(10):1373–1377. [DOI] [PubMed] [Google Scholar]
- 57.Romero A, Hohbein J, Ross S.. Superficial thrombosis of pelvic congestion syndrome mimicking pelvic abscess. Clin Pract Cases Emerg Med. 2019;3(3):237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dos Santos SJ, Whiteley MS.. Pelvic congestion syndrome masquerading as osteoarthritis of the hip. SAGE Open Med Case Rep. 2016;4:2050313X16683630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarrell JF, Vilos GA, Allaire C, et al. No. 164-Consensus guidelines for the management of chronic pelvic pain. J Obstet Gynaecol Can. 2018;40(11):e747–e787. [DOI] [PubMed] [Google Scholar]
- 60.Steenbeek MP, van der Vleuten CJM, Schultze Kool LJ, et al. Noninvasive diagnostic tools for pelvic congestion syndrome: a systematic review. Acta Obstet Gynecol Scand. 2018;97(7):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathur M, Scoutt LM.. Nongynecologic causes of pelvic pain: ultrasound first. Obstet Gynecol Clin North Am. 2019;46(4):733–753. [DOI] [PubMed] [Google Scholar]
- 62.Baz AA. Role of trans-abdominal and trans-perineal venous duplex ultrasound in cases of pelvic congestion syndrome. Egypt J Radiol Nucl Med. 2019;50:1–11. [Google Scholar]
- 63.Patel MD, Young SW, Dahiya N.. Ultrasound of pelvic pain in the nonpregnant woman. Radiol Clin North Am. 2019;57(3):601–616. [DOI] [PubMed] [Google Scholar]
- 64.Park SJ, Lim JW, Ko YT, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182(3):683–688. [DOI] [PubMed] [Google Scholar]
- 65.Coakley FV, Varghese SL, Hricak H.. CT and MRI of pelvic varices in women. J Comput Assist Tomogr. 1999;23(3):429–434. [DOI] [PubMed] [Google Scholar]
- 66.Dos Santos SJ, Holdstock JM, Harrison CC, et al. Ovarian vein diameter cannot be used as an indicator of ovarian venous Reflux. Eur J Vasc Endovasc Surg. 2015;49(1):90–94. [DOI] [PubMed] [Google Scholar]
- 67.Hiromura T, Nishioka T, Nishioka S, et al. Reflux in the left ovarian vein: analysis of MDCT findings in asymptomatic women. Am. J. Roentgenol. 2004;183(5):1411–1415. [DOI] [PubMed] [Google Scholar]
- 68.Szary C, Wilczko J, Zawadzki M, et al. Hemodynamic and radiological classification of ovarian veins system insufficiency. JCM. 2021;10(4):646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basile A, Failla G, Gozzo C.. Pelvic congestion syndrome. Semin Ultrasound, CT MRI. 2020. Feb;42(1):3-12. [DOI] [PubMed] [Google Scholar]
- 70.Huang YK, Tseng YH, Lin CH, et al. Evaluation of venous pathology of the lower extremities with triggered angiography non-contrast-enhanced magnetic resonance imaging. BMC Med. Imaging. 2019;19,96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meneses LQ, Uribe S, Tejos C, et al. Using magnetic resonance phase-contrast velocity mapping for diagnosing pelvic congestion syndrome. Phlebology. 2011;26(4):157–161. [DOI] [PubMed] [Google Scholar]
- 72.François CJ. Abdominal magnetic resonance angiography. Magn Reson Imaging Clin N Am. 2020;28(3):395–405. [DOI] [PubMed] [Google Scholar]
- 73.Chennur VS, Nzekwu EV, Bhayana D, et al. MR venography using time-resolved imaging in interventional management of pelvic venous insufficiency. Abdom Radiol (NY)). 2019;44(6):2301–2307. [DOI] [PubMed] [Google Scholar]
- 74.Bookwalter CA, Vanburen WM, Neisen MJ, et al. Imaging appearance and nonsurgical management of pelvic venous congestion syndrome. Radiographics. 2019;39(2):596–608. [DOI] [PubMed] [Google Scholar]
- 75.Yang DM, Kim HC, Nam DH, et al. Time-resolved MR angiography for detecting and grading ovarian venous reflux: comparison with conventional venography. Br J Radiol. 2012;85(1014):e117–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radosa JC, Radosa MP, Schweitzer PA, et al. Impact of different intraoperative CO2 pressure levels (8 and 15 mmHg) during laparoscopic hysterectomy performed due to benign uterine pathologies on postoperative pain and arterial pCO2 : a prospective randomised controlled clinical trial. BJOG. 2019;126(10):1276–1285. [DOI] [PubMed] [Google Scholar]
- 77.Wagner E, Chandler JN, Mihalov LS.. Minimizing trendelenburg position for laparoscopic gynecologic surgery [6L]. Obstet. Gynecol. 2019;133(1):130S–130S. [Google Scholar]
- 78.Antignani PL, Lazarashvili Z, Monedero JL, et al. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. Int Angiol. 2019;38(4):265–283. [DOI] [PubMed] [Google Scholar]
- 79.Beard RW, Reginald PW, Wadsworth J.. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol. 1988;95(2):153–161. [DOI] [PubMed] [Google Scholar]
- 80.Herrera-Betancourt AL, Villegas-Echeverri JD, López-Jaramillo JD, et al. Sensitivity and specificity of clinical findings for the diagnosis of pelvic congestion syndrome in women with chronic pelvic pain. Phlebology. 2018;33(5):303–308. [DOI] [PubMed] [Google Scholar]
- 81.Dorobisz TA, Garcarek JS, Kurcz J, et al. Diagnosis and treatment of pelvic congestion syndrome: single-centre experiences. Adv Clin Exp Med. 2017;26(2):269–276. [DOI] [PubMed] [Google Scholar]
- 82.Beard RW, Pearce S, Highman JH, et al. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet. 1984;324(8409):946–949. [DOI] [PubMed] [Google Scholar]
- 83.Shokeir T, Amr M, Abdelshaheed M.. The efficacy of implanon for the treatment of chronic pelvic pain associated with pelvic congestion: 1-year randomized controlled pilot study. Arch Gynecol Obstet. 2009;280(3):437–443. [DOI] [PubMed] [Google Scholar]
- 84.Reginald PW, Kooner JS, Samarage SU, et al. Intravenous dihydroergotamine to relieve pelvic congestion with pain in young women. Lancet. 1987;330(8555):351–353. [DOI] [PubMed] [Google Scholar]
- 85.Nishijima S, Sugaya K, Kadekawa K, et al. Tadalafil improves bladder dysfunction and object recognition in rats with pelvic venous congestion. Int J Urol. 2019;26(5):578–585. [DOI] [PubMed] [Google Scholar]
- 86.Gavrilov SG, Moskalenko YP, Karalkin AV.. Effectiveness and safety of micronized purified flavonoid fraction for the treatment of concomitant varicose veins of the pelvis and lower extremities. Curr Med Res Opin. 2019;35(6):1019–1026. [DOI] [PubMed] [Google Scholar]
- 87.Simsek M, Burak F, Taskin O.. Effects of micronized purified flavonoid fraction (daflon) on pelvic pain in women with laparoscopically diagnosed pelvic congestion syndrome: a randomized crossover trial. Clin Exp Obstet Gynecol. 2007;34:96–98. [PubMed] [Google Scholar]
- 88.Tsukanov YT, Tsukanov AY, Levdanskiy EG.. Secondary varicose small pelvic veins and their treatment with micronized purified flavonoid fraction. Int J Angiol. 2016;25(2):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gavrilov SG, Karalkin AV, Moskalenko YP, et al. Efficacy of two micronized purified flavonoid fraction dosing regimens in the pelvic venous pain relief. Int Angiol. 2021. Jun;40(3):180–186. [DOI] [PubMed] [Google Scholar]
- 90.Sahin A, Kutluhan MA, Yildirim C, et al. Results of purified micronized flavonoid fraction in the treatment of categorized type III chronic pelvic pain syndrome: a randomized controlled trial. Aging Male. 2019;23(5):1103–1108. [DOI] [PubMed] [Google Scholar]
- 91.Gong JM, Du JS, Han DM, et al. Reasons for patient non-compliance with compression stockings as a treatment for varicose veins in the lower limbs: a qualitative study. PLOS One. 2020;15(4):e0231218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gavrilov SG, Karalkin AV, Turischeva OO.. Compression treatment of pelvic congestion syndrome. Phlebology. 2018;33(6):418–424. [DOI] [PubMed] [Google Scholar]
- 93.Rundqvist E, Sandholm LE, Larsson G.. Treatment of pelvic varicosities causing lower abdominal pain with extraperitoneal resection of the left ovarian vein – PubMed. Ann Chir Gynaecol. 1984;73:339–341. [PubMed] [Google Scholar]
- 94.De Almeida GR, Silvinato A, Simões RS, et al. Pelvic congestion syndrome – treatment with pelvic varicose veins embolization. Rev Assoc Med Bras. 2019;65(4):518–523. [DOI] [PubMed] [Google Scholar]
- 95.Chung MH, Huh CY.. Comparison of treatments for pelvic congestion syndrome. Tohoku J Exp Med. 2003;201(3):131–138. [DOI] [PubMed] [Google Scholar]
- 96.Edwards RD, Robertson IR, MacLean AB, et al. Case report: Pelvic pain syndrome-successful treatment of a case by ovarian vein embolization. Clin Radiol. 1993;47(6):429–431. [DOI] [PubMed] [Google Scholar]
- 97.Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the society for vascular surgery and the American Venous Forum. J Vasc Surg. 2011;53(5 Suppl):2S–48S. [DOI] [PubMed] [Google Scholar]
- 98.Tiralongo F, Distefano G, Palermo M, et al. Liquid and solid embolic agents in gonadal veins. JCM. 2021;10(8):1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Gregorio MA, Guirola JA, Alvarez-Arranz E, et al. Pelvic venous disorders in women due to pelvic varices: treatment by embolization: experience in 520 patients. J Vasc Interv Radiol. 2020;31(10):1560–1569. [DOI] [PubMed] [Google Scholar]
- 100.Guirola JA, Sánchez-Ballestin M, Sierre S, et al. A randomized trial of endovascular embolization treatment in pelvic congestion syndrome: fibered platinum coils versus vascular plugs with 1-year clinical outcomes. J Vasc Interv Radiol. 2018;29(1):45–53. [DOI] [PubMed] [Google Scholar]
- 101.Champaneria R, Shah L, Moss J, et al. The relationship between pelvic vein incompetence and chronic pelvic pain in women: systematic reviews of diagnosis and treatment effectiveness. Health Technol Assess. 2016;20(5):1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown CL, Rizer M, Alexander R, et al. Pelvic congestion syndrome: systematic review of treatment success. Semin Intervent Radiol. 2018;35(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hansrani V, Abbas A, Bhandari S, et al. Trans-venous occlusion of incompetent pelvic veins for chronic pelvic pain in women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2015;185:156–163. [DOI] [PubMed] [Google Scholar]
- 104.Daniels JP, Champaneria R, Shah L, et al. Effectiveness of embolization or sclerotherapy of pelvic veins for reducing chronic pelvic pain: a systematic review. J. Vasc. Interv. Radiol. 2016;27(10):1478–1486.e8. [DOI] [PubMed] [Google Scholar]
- 105.Jambon E, Le Bras Y, Petitpierre F, et al. MRI associated factors of clinical efficacy of embolization in patients with pelvic venous insufficiency. Diagn Interv Imaging. 2020;101(10):667–676. [DOI] [PubMed] [Google Scholar]
- 106.Maleux G, Stockx L, Wilms G, et al. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical results. J Vasc Interv Radiol. 2000;11(7):859–864. [DOI] [PubMed] [Google Scholar]
- 107.Laborda A, Medrano J, De Blas I, et al. Endovascular treatment of pelvic congestion syndrome: visual analog scale (VAS) long-term follow-up clinical evaluation in 202 patients. Cardiovasc Intervent Radiol. 2013;36(4):1006–1014. [DOI] [PubMed] [Google Scholar]
- 108.Cho KJ. Carbon dioxide angiography: scientific principles and practice. Vasc Specialist Int. 2015;31(3):67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Capasso P, Simons C, Trotteur G, et al. Treatment of symptomatic pelvic varices by ovarian vein embolization. Cardiovasc Intervent Radiol. 1997;20(2):107–111. [DOI] [PubMed] [Google Scholar]
- 110.Venbrux AC, Chang AH, Kim HS, et al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain. J Vasc Interv Radiol. 2002;13(2):171–178. [DOI] [PubMed] [Google Scholar]
- 111.Kim HS, Malhotra AD, Rowe PC, et al. Embolotherapy for pelvic congestion syndrome: long-term results. J. Vasc Interv Radiol. 2006;17(2):289–297. [DOI] [PubMed] [Google Scholar]
- 112.Tarazov P, Prozorovskij K, Rumiantseva S.. Pregnancy after embolization of an ovarian varicocele associated with infertility: report of two cases. Diagn Interv Radiol. 2011;17(2):174–176. [DOI] [PubMed] [Google Scholar]
- 113.Santoshi RKN, Lakhanpal S, Satwah V, et al. Iliac vein stenosis is an underdiagnosed cause of pelvic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2018;6(2):202–211. [DOI] [PubMed] [Google Scholar]
- 114.Gavrilov SG, Vasilyev AV, Krasavin GV, et al. Endovascular interventions in the treatment of pelvic congestion syndrome caused by May-Thurner syndrome. J Vasc Surg Venous Lymphat Disord. 2020;8(6):1049–1057. [DOI] [PubMed] [Google Scholar]
- 115.Lakhanpal G, Kennedy R, Lakhanpal S, et al. J. Pelvic venous insufficiency secondary to iliac vein stenosis and ovarian vein reflux treated with iliac vein stenting alone. J Vasc Surg Venous Lymphat. Disord. 2021. Sep;9(5):1193–1198. [DOI] [PubMed] [Google Scholar]
- 116.Montes MC, Carbonell JP, Gómez-Mesa JE.. Endovascular and medical therapy of may–thurner syndrome: case series and scoping literature review. J Med Vasc. 2021;46:80–89. [DOI] [PubMed] [Google Scholar]
- 117.Padrnos LJ, Garcia D.. May-Thurner syndrome and thrombosis: a systematic review of antithrombotic use after endovascular stent placement. Res Pract Thromb Haemost. 2019;3(1):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Avgerinos ED, Saadeddin Z, Humar R, et al. Outcomes of left renal vein stenting in patients with nutcracker syndrome. Proc J Vasc Surg Venous Lymphat Disord. 2019;7:853–859. [DOI] [PubMed] [Google Scholar]
- 119.Scultetus AH, Villavicencio JL, Gillespie DL.. The nutcracker syndrome: Its role in the pelvic venous disorders. J Vasc Surg. 2001;34(5):812–819. [DOI] [PubMed] [Google Scholar]
- 120.Wu Z, Zheng X, He Y, et al. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4(2):193–199. [DOI] [PubMed] [Google Scholar]
- 121.Gilmore BF, Benrashid E, Geersen D, et al. Gonadal vein transposition is a safe and effective treatment of nutcracker syndrome. Proc J Vasc Surg Venous Lymphat Disord. 2021;9:712–719. [DOI] [PubMed] [Google Scholar]
- 122.Hamoodi I, Hawthorn R, Moss JG.. Can ovarian vein embolization cause more harm than good? J Obstet Gynaecol Res. 2015;41(12):1995–1997. [DOI] [PubMed] [Google Scholar]
- 123.Fahrni J, Gloviczki P, Friese JL, et al. Hypersensitivity to nickel in a patient treated with coil embolization for pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2015;3(3):319–321. [DOI] [PubMed] [Google Scholar]
- 124.Hasjim BJ, Fujitani RM, Kuo IJ, et al. Unique case of recurrent pelvic congestion syndrome treated with median sacral vein embolization. Ann Vasc Surg. 2020;68:569.e1–e7. [DOI] [PubMed] [Google Scholar]
- 125.Gavrilov SG, Krasavin GV, Mishakina NY, et al. Postembolization syndrome in endovascular interventions on the gonadal veins. J Vasc Surg Venous Lymphat Disord. 2021 May;9(3):697-702. [DOI] [PubMed] [Google Scholar]
- 126.Leatherby RJ, Harries P, Shah SS.. The management of pelvic congestion syndrome – a word of caution. J Obstet Gynaecol. 2020;40(2):283–284. [DOI] [PubMed] [Google Scholar]
- 127.Guerrero A, Theophanous R.. A case report of a migrated pelvic coil causing pulmonary infarct in an adult female. Clin Pract Cases Emerg Med. 2020;4(3):436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khilnani NM, Meissner MH, Learman LA, et al. Research priorities in pelvic venous disorders in women: recommendations from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2019;30(6):781–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no raw data associated with this review. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.