Abstract
Background: Surgical resection is the only possible choice of treatment in several pancreatic disorders that included periampullar neoplasms. The development of a postoperative pancreatic fistula (POPF) is the main complication. Despite three different surgical strategies that have been proposed–pancreatojejunostomy (PJ), pancreatogastrostomy (PG), and pancreatic duct occlusion (DO)–none of them has been clearly validated to be superior. The aim of this study was to analyse the postoperative outcomes after DO.
Methods: We retrospectively reviewed 56 consecutive patients who underwent Whipple's procedure from January 2007 to December 2014 in a tertiary Hepatobiliary Surgery and Liver Transplant Unit. After pancreatic resection in open surgery, we performed DO of the Wirsung duct with Cyanoacrylate glue independently from the stump characteristics. The mean follow-up was 24.5 months.
Results: In total, 29 (60.4%) were men and 19 were (39.6%) women with a mean age of 62.79 (SD ± 10.02) years. Surgical indications were in 95% of cases malignant diseases. The incidence of POPF after DO was 31 (64.5%): 10 (20.8%) patients had a Grade A fistula, 18 (37.5%) Grade B fistula, and 3 (6.2%) Grade C fistula. No statistical differences were demonstrated in the development of POPF according to pancreatic duct diameter groups (p = 0.2145). Nevertheless, the POPF rate was significantly higher in the soft pancreatic group (p = 0.0164). The mean operative time was 358.12 min (SD ± 77.03, range: 221–480 min). Hospital stay was significantly longer in patients who developed POPF (p < 0.001). According to the Clavien-Dindo (CD) classification, seven of 48 (14.58%) patients were classified as CD III–IV. At the last follow-up, 27 of the 31 (87%) patients were alive.
Conclusions: Duct occlusion could be proposed as a safe alternative to pancreatic anastomosis especially in low-/medium-volume centers in selected cases at higher risk of clinically relevant POPF.
Keywords: pancreatic surgery, pancreatic cancer, low-volume center, pancreatic stump, duct occlusion, COVID-19 pandemic, POPF
Introduction
Surgical resection is the only possible choice of treatment in several pancreatic disorders, such as malignancies, adenomas, traumas, and severe acute and/or chronic pancreatitis (1). Radical resection is the single most important factor in determining outcomes in patients with pancreatic adenocarcinoma (1–3). Although the surgical context has radically changed in the last 20 years with the advent of new technologies and surgical approaches improving the short-term outcomes in several abdominal surgical fields (4–8), however, the morbidity rate following pancreaticoduodenectomy (PD) remains high, ranging from 30 to 50%, with a mortality rate of 3–5% (9–12). Morbidity in pancreatic surgery is mainly related to the development of a postoperative pancreatic fistula (POPF) (13). According to the International Study Group on Pancreatic Fistula (ISGPF), it is possible to grade POPF based on clinical variables (14). “A grade” fistulas, as called a “biochemical leak” (BL) in update classification, do not need any treatment (currently it is not considered a true pancreatic fistula) and imply no clinical impact. “B grade” fistulas can be managed with medications and only prolong the length of hospital stay in association with a clinically relevant condition. “C grade” fistulas need operative treatment and might be life threatening (12). In high-volume centers for pancreatic surgery, the overall POPF incidence is around 20% (12, 14, 15). Intra-abdominal abscesses, delayed gastric emptying, postpancreatectomy hemorrhage, and sepsis represent additional sources of morbidity. In most cases, however, they occur in association or as a consequence of POPF (16, 17). Advanced age (>75 years), pancreas texture, pancreatic duct diameter, comorbidities, previous endoscopic retrograde cholangiopancreatography (ERCP), duct obstruction, and surgical technique are known risk factors for postoperative morbidity (12, 14, 15, 18–21). The incidence of postoperative complications has a significant impact on the length of hospital stay, costs, quality of life, and chance to start chemotherapy (22, 23). Several different surgical and pharmacological approaches have been proposed to avoid POPF, which might be different depending on the experience and preferences at each center (13, 24). Three main different surgical strategies have been proposed to deal with the pancreatic stump following PD—pancreatojejunostomy (PJ), pancreatogastrostomy (PG) and pancreatic duct occlusion (DO)—but none of them has been clearly demonstrated to be superior to the others (25). Despite such detailed reporting of morbidity and mortality following PD, it is still not clear whether is surgeon's experience or hospital volume to rescue patients when a complication occurs (25). If PJ is the procedure of choice in medium-/high-volume centers, DO could be proposed as a safer alternative in medium-/low-volume centers, to reduce the risk of major postoperative complications (26). We decided to review our previous experience in the light of the recent Covid pandemic where, in our country, it has been forced in many regions to displace treatment of oncological patients in low-volume hospitals with limited experience (27, 28). The encouraging results of DO in terms of overall survival, POPF, and “brittle diabetes” are here presented.
Materials and Methods
Study Design
We retrospectively reviewed 56 consecutive patients who underwent Whipple's procedure from January 2007 to December 2014 in a tertiary Hepatobiliary Surgery and Liver Transplant Unit with a low volume of pancreatic resections.
All data were obtained from a prospectively maintained database and analyzed retrospectively. All patients signed a proper informed consent for the scientific anonymous use of clinical data. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of Molise (protocol number 10/21, approved date: 12 May 2021).
The follow-up program was performed by clinical exam, CEA, CA19.9 levels, and CT scan every 3 of 6 months after surgery according to Italian guidelines (29).
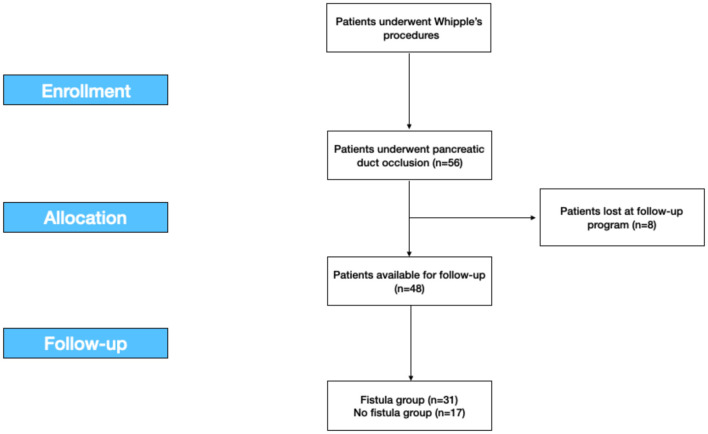
Eight patients were lost at follow-up, so the analysis on morbidity was conducted on the 48 patients available with a mean follow-up of 25.4 months (Figure 1).
Figure 1.
The study flow-chart according to the STROBE statements. STROBE, the Strengthening the Reporting of Observational Studies in Epidemiology.
In all cases, DO was performed with Cyanoacrylate glue injection.
We recorded data about medical history, body mass index (BMI), American Society of Anaesthesiologists' (ASA) score, preoperative CA19.9, survival, mean operative time, incidence of POPF, the incidence of sepsis, the incidence of postoperative hemorrhage, re-laparotomy rate, hospital stay, incidence of preoperative and postoperative diabetes, 30-day and 90-day postoperative mortality, oncological recurrence, and pancreatic exocrine function.
The pancreatic exocrine function was evaluated by personal or telephonic interviews assessing any substitutive pancreatic enzyme therapy (yes/no) related to steatorrhea/diarrhea since surgery.
This retrospective study was developed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for cohort studies (Figure 1) (30).
Preoperative Workup
Our preoperative workup consisted of total body CT and/or MRI scan for oncological staging and for the exact determination of tumor size and resectability. If total bilirubin was higher than 20 mg/dl, biliary drainage was placed via ERCP in patients whose surgery was not scheduled within 2 wk. A cephalosporin + metronidazole was used as infection prophylactic treatment. No patient was allergic to this regimen.
Surgical Technique
We performed a Whipple procedure with an open approach. Gastrectomy was performed using GIA 90 without pylorus preservation.
After pancreatic resection, we performed DO of the Wirsung duct with Cyanoacrylate glue independently from the stump characteristics. In detail, the pancreatic stump was closed with 3/0 polypropylene stitches during glue polymerization while the catheter inserted in the main pancreatic duct for glue injection was simultaneously removed to obtain a complete duct closure (Figure 2). No patients underwent vascular resection. We finally performed biliary reconstruction with a Roux-en-Y anastomosis. We always performed a mechanical gastro-jejunal anastomosis.
Figure 2.
Cyanoacrylate glue injection in Wirsung duct to obtain pancreatic duct occlusion.
Two abdominal drainages were placed (one close to the pancreatic remnant and one in the pelvis).
Postoperative Care
All patients stayed at least 1 day in the intensive care unit (range: 1–3 days) and then returned to the ward. Amylase and lipase were routinely monitored in serum starting from postoperative day 3. POPF was defined according to the 2016 update of the International Study Group (ISGPS) (14, 25).
A cephalosporin + metronidazole regimen was adopted when needed. No patient was allergic to this antibiotic regimen and/or presented resistant bacteria. Octreotide 0.1 ml was administered subcutaneously three times a day. In the absence of POPF, patients were allowed oral intake on postoperative day 5.
Complications were graded according to Clavien-Dindo (CD) classification (31).
Statistical Analysis
Descriptive statistics were collected and reported as a whole number (percentage) and mean or median (range). Chi-square test and Fisher exact test including or not Yates' continuity correction, two-by-two cross tables, Student's t-test, and ANOVA test were used to compare categorical data and to analyse normally distributed quantitative data.
Differences were statistically significant when p-values were <0.05. Statistical analysis was carried out using IBM SPSS Statistics for Macintosh, Version 27.0.
Results
For 8 years, from January 2007 to December 2014, we retrospectively collected data of 56 patients who underwent Whipple's procedure for benign and malignant diseases in a Tertiary Hepatobiliary Surgery and Liver Transplant Unit with a low volume of pancreatic resections. Eight patients (8) were excluded upon they were lost at the follow-up program. Total 48 patients were included (Figure 1).
In total, 29 (60.4%) were men and 19 were (39.6%) women with a mean age of 62.79 (SD ± 10.02) years. Thirty-one (64.58%) developed POPF. Figure 3 shows POPF grade in detail.
Figure 3.
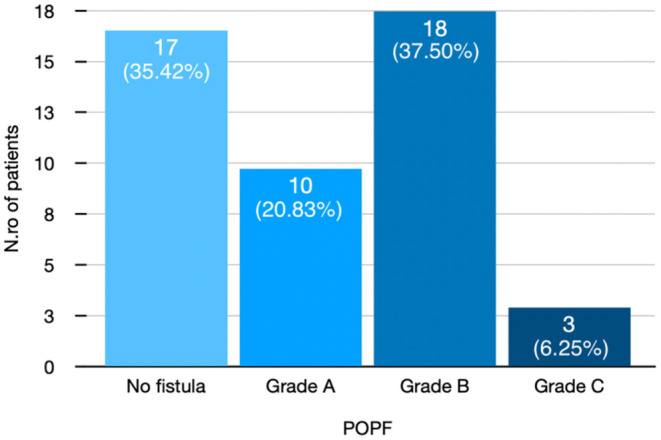
Distribution of patients after pancreatic duct occlusion according to POPF grade. POPF, postoperative pancreatic fistula.
Body mass index, ASA score, and other baseline characteristics of patients according to the development of pancreatic fistula are shown in Table 1.
Table 1.
Baseline characteristics of patients who underwent pancreatic duct occlusion.
| Duct occlusion, =48 | Fistula, n = 31 | No fistula, n = 17 | p -value | |
|---|---|---|---|---|
| Age (yrs) | ||||
| Mean (± SD) | 62.79 (± 10.02) | 62.87 (± 8.23) | 62.65 (± 12.96) | 0.9429 |
| Median | 66.00 | 66.00 | 66.00 | |
| Range | (34–78) | (44–78) | (34–78) | |
| Gender, n (%) | ||||
| Male | 29 (60.4) | 22 (70.97) | 7 (41.18) | 0.0651 |
| Female | 19 (39.6) | 9 (29.03) | 10 (58.82) | |
| BMI | ||||
| Mean (± SD) | 25.27 (± 1.64) | 25 (± 1.54) | 25.51 (± 1.71) | 0.2968 |
| Median | 25 | 25 | 25 | |
| Range | (21–28) | 23–28 | 21–28 | |
| ASA, n (%) | ||||
| I | 1 (2.1) | 1 (3.24) | 0 (0) | 1.0000 |
| II | 16 (33.3) | 12 (38.71) | 4 (23.53) | 0.5316 |
| III | 19 (39.6) | 12 (38.71) | 7 (41.18) | 1.0000 |
| IV | 12 (25.0) | 6 (19.34) | 6 (35.29) | 0.3002 |
| Previous procedures, n (%) | ||||
| ERCP | 16 (33.3) | 14 (45.16) | 2 (11.76) | 0.486 |
| PTC stent | 2 (4.2) | 1 (3.22) | 1 (5.88) | 1.0000 |
| Colecistectomy | 1 (2.1) | 0 (0) | 1 (5.88) | 0.3673 |
| Comorbidities, n (%) | ||||
| Arterial hypertension Diabetes mellitus Atrial fibrillation HCV positive COPD Liver transplantation Cerebral ischemia |
16 (33.3) 10 (20.8) 6 (12.5) 3 (6.3) 3 (6.3) 1 (2.1) 1 (2.1) |
10 (32.26) 5 (16.13) 4 (12.90) 3 (9.68) 2 (6.45) 1 (3.22) 1 (3.22) |
6 (35.29) 5 (29.41) 2 (6.45) 0 (0) 1 (5.88) 0 (0) 0 (0) |
1.0000
0.2947 1.0000 0.5430 1.0000 1.0000 1.0000 |
| Pre-operative Ca19.9, U/ml | ||||
| Mean, (± SD) Median Range |
285.14 (± 660.83) 80.45 (1–2734.10) |
117.79 (±85.29) 80.45 (22.4–2431) |
787.2 (±1307) 206.85 (1–2734.10) |
0.0062 |
BMI, Body Mass Index; ERCP, Endoscopic Retrograde Cholangiopancreatography; PTC, Percutaneous Transhepatic Cholangiography; COPD, Chronic Obstructive Pulmonary Disease; Ca19.9, carbohydrate antigen 19–9 or cancer antigen 19–9 or sialylated Lewis.
Surgical indications were in 95% of cases malignant diseases. Pathological findings according to POPF are depicted in Table 2.
Table 2.
Clinico-pathological data of patients who underwent pancreas duct occlusion included in follow-up program.
| Duct occlusion, n = 48 | Fistula, n = 31 | No fistula, n = 17 | P -value | |
|---|---|---|---|---|
| Histological findings, n (%) | ||||
| Pancreatic adenocarcinoma Ampullary adenocarcinoma Bile duct cancer Neuroendocrin carcinoma Mucinous cystadenoma Gallbladder cancer Choronic pancreatitis |
24 (50) 10 (20.84) 6 (12.50) 3 (6.25) 3 (6.25) 1 (2.08) 1 (2.08) |
14 (45.16) 6 (19.35) 5 (16.14) 2 (6.45) 3 (9.68) 1 (3.22) - |
10 (58.83) 4 (23.53) 1 (5.88) 1 (5.88) - - 1 (5.88) |
0.5469 0.7266 0.4022 1.0000 0.5430 1.0000 0.3542 |
| Pancreatic texture, n (%) | ||||
| Soft Hard Normal |
33 (68.75) 8 (16.67) 7 (14.58) |
25 (80.65) 5 (16.13) 1 (3.22) |
8 (47.06) 3 (16.65) 6 (35.39) |
0.0164 1.0000 0.0055 |
| Pancreatic duct diameter | ||||
| Mean, mm Range, mm ≤ 3 mm, n (%) > 3mm, n (%) |
3.98 (± 2.18) 1–10 19 (39.58) 29 (60.42) |
4.25 (± 1.88) 3–10 |
5.00 (± 2.14) 1–8 |
0.2145 |
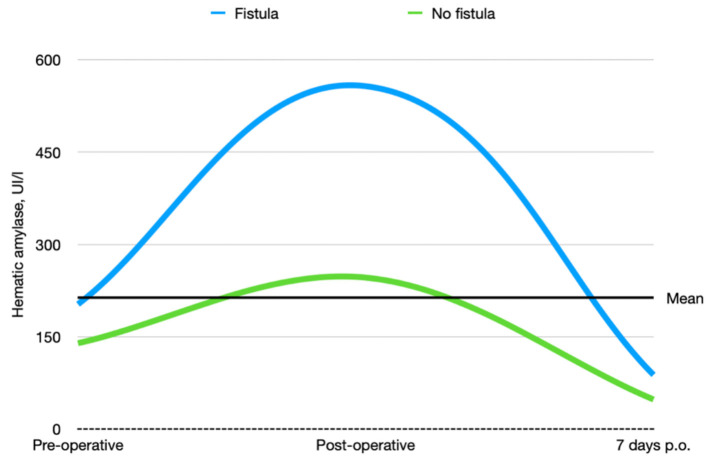
| Hematic amylase, UI/l | ||||
| Pre-operative mean (± SD) Post-operative, mean (± SD) 7 days p.o., mean (± SD) |
178.41 (± 201.37) 451.31 (± 510.78) 74.10 (± 57.44) |
202.75 (±236.89) 557.37 (±567.52) 88.10 (±60.90) |
139.47 (±123.41) 246.33 (±298.06) 47.93 (±40.21) |
0.3110 0.0413 0.0187 |
Biliary drainage was performed before surgery in 16 (33.3%) patients who underwent ERCP, in one patient (4.2%) who underwent PTC. The incidence of pancreatic fistula after biliary drainage is shown in Table 1.
Duct diameter was reported larger than 3 mm in 60% of patients. As depicted in Table 2, no statistical differences were demonstrated in the development of POPF according to pancreatic duct diameter groups (p = 0.2145).
The soft pancreatic texture was recorded in 68% of cases. As shown in Table 2, the POPF rate was significantly higher in the soft pancreatic group (p = 0.0164).
The mean operative time was 358.12 min (SD ± 77.03, range: 221–480 min). Six (12.5%) patients needed intraoperative blood cells transfusions (Table 3).
Table 3.
Perioperative data.
| Operative time, min | |
| Mean (± SD) Median Range |
358.12 (± 77.03) 360 221–480 |
| Procedures, n (%) | |
| Glubran | 48 (100) |
| Blood trasfusion | |
|
n (%) packed red blood cells, mean (range) |
6 (12.5) 1.5 (1–4) |
| Hospital stay, days, mean (± SD) | |
| Fistula group No fistula group p-value |
38 (± 22), (r.:13–115) 17.37 (± 9), (r.:3–45) < 0.001 |
Hospital stay was significantly longer in patients who developed POPF (p < 0.001) as described in Table 3.
According to the CD classification (31), seven of 48 (14.58%) patients were classified as CD III–IV. Complications, reoperation rate, and whole short-term outcomes that include 30- and 90-day mortality according to pancreatic fistula are extensively described in Tables 4–6 and Figure 4.
Table 4.
Short-term and long-term outcomes.
| Duct occlusion, n = 48 | Fistula, n = 31 | No fistula, n = 17 | P -value | |
|---|---|---|---|---|
| Clavien-Dindo classification, n (%) | ||||
| I–II III–IV |
41 (85.42) 7 (14.58) |
27(87.10) 4 (12.90) |
14 (82.35) 3 (17.65) |
0.6862 |
| 30-days mortality, n (%) | 3 (6.45) | 2 (6.45) | 1 (5.88) | 1.0000 |
| 90-days mortality, n (%) | 2 (4.16) | 2 (6.45) | 0 | 1.0000 |
| Short-term outcomes, n (%) | ||||
| Sepsis Post-operative bleeding Intraddominal collection Pleura effusion Dehiscence* Hemoperitoneum Intestinal obstruction Stroke DIC |
11 (22.92) 10 (20.83) 14 (29.17) 2 (4.17) 2 (4.17) 4 (8.33) 2 (4.17) 1 (2.08) 2 (4.17) |
9 (29.03) 9 (29.03) 14(45.16) 1 (3.22) 1 (3.22) 2 (6.45) 2 (6.45) 1 (3.22) 2 (6.45) |
2 (11.76) 1 (5.88) 0 1 (5.88) 1 (5.88) 2 (11.76) 0 0 0 |
0.2840
0.0744 < 0.001 1.0000 1.0000 0.2300 0.5328 1.0000 0.5328 |
| Long-term outcomes, n (%) | ||||
| Brittle diabetes Octreotide therapy |
8 (16.67) 44 (91.67) |
3 (9.68) 31 (100) |
5 (29.41) 13 (76.47) |
0.1115 0.0122 |
| Reoperative rate, n (%) | ||||
| Total Hemostatis Total pancreasectomy GI fistula Re-anastomosis HJ Explorative laparotomy |
10(20.83) 4 (8.33) 2 (4.17) 1 (2.08) 1 (2.08) 1 (2.08) |
7 (22.58) 2 (6.45) 2 (6.45) 1 (3.22) 1 (3.22) 1 (3.22) |
2 (11.76) 2 (11.76) . . . . |
0.6073 0.5328 1.0000 1.0000 1.0000 |
| Recurrence, n (%) | 7 (14.58) | 6 (19.35) | 1 (5.88) | 0.3956 |
| Follow-up, months | ||||
| Mean Range |
24.5 (3–100) |
23.5 (3–100) |
17.7 (3–21) |
|
| Overall survival (%) | 58.3 | |||
DIC, disseminated intravascular coagulation; HJ, Hepatico-Jejunostomy;*Dehiscence: 1 Hepatico-jejunostomy; 1 wound.
Table 6.
Re-operative rate according to POPF grade and follow-up.
| POPF grade | Follow-up | |
|---|---|---|
| Hemostasis, n.ro | ||
| 1 1 1 1 |
No POPF No POPF A grade A grade |
Dead 30 days p.o. Alive 12 months p.o. Dead 7 months p.o. Alive 78 months p.o. |
| Total pancreasectomies, n.ro | ||
| 1 1 |
C grade C grade |
Dead 30 months p.o. Alive 100 months p.o. |
| GI fistula, n.ro | ||
| 1 | C grade | Alive 8 months p.o. |
| Re-anastomosis hepatico-jejunal, n.ro | ||
| 1 | C grade | Alive 27 months p.o. |
| Explorative laparotomy, n.ro | ||
| 1 | C grade | Dead 90 days p.o. |
POPF, postoperative pancreatic fistula.
Figure 4.
Preoperative, postoperative, and 7-day postoperative hematic amylase trends in patients who underwent pancreatic duct occlusion with and without fistula.
Table 5.
Mortality rate and cause of death.
| POPF grade | Cause of death | |
|---|---|---|
| 30-days mortality, n.ro | ||
| 1 1 1 |
No POPF A grade C grade |
Shock-MOFS MOFS Stroke |
| 90-days mortality, n.ro | ||
| 1 1 |
A grade B grade |
Hemorrhage-MOFS MOFS |
POPF, postoperative pancreatic fistula.
Eight (16.67%) patients developed brittle diabetes without any statistical relationship to the POPF rate (Table 4).
The mean follow-up was 24.5 months (range: 3–100; Table 4).
The overall survival at the last follow-up was 58.3% (Table 4).
Discussion
Our case series demonstrate that DO might be considered as a safe option to treat pancreatic stump after PD. Evidence supports a strong correlation between surgical outcomes and hospital volume in pancreatic surgery (32–37). Despite these findings during the Covid pandemic period, it was very difficult to provide sanitary migration to high-volume centers (38–40), so also medium- and low-volume centers, which have enough facilities and skills to provide pancreatic surgery, should perform more interventions to answer to the population needs. Our results gained in a Hepatobiliary referral center with a low-volume rate of pancreatic resections may encourage pancreatic resection allowing a reduction of patient mobility. Pedrazzoli et al. in a large systematic review on PD and pancreatic fistula analyzed 162 articles involving 54,232 patients (41). The review shows 4,813 Grade A (8.9%), 4,830 Grade B (8.9%), and 1,872 Grade C (3.5%) POPFs with a mean overall fistula rate of 21.3%. A huge variability of Grades A and B POPFs varied from <2% to more than 20% with a minimum of 0% and a maximum of 42.5% for Grade A and a minimum of 0.7% and a maximum of 33.3% for Grade B POPF. Grade C POPFs arise from 1% to more than 9% with a maximum of 13.6% (41). Di Carlo et al. showed that the DO procedure was feasible and less time-consuming than PJ, although it could be associated with higher fistula rates. However, POPF could not be clinically relevant probably due to the absence of a pancreatic enzymes activation (42). In our experience, the overall incidence of POPF was 64%. This observation is consistent with the experience of Tersigni et al. who observed a higher rate of POPF after DO (45.4%) compared to end-to-end PJ anastomosis (15.6%) and to end-to-side PJ anastomosis (11.3%), with a similar incidence of Grade C fistula in all the groups (3.1% after end-to-end PJ anastomosis, 2.3% after end-to-side anastomosis and 3.0% after DO) (43). Consistent with other reports, in our patients a soft pancreatic texture was associated with a significantly higher incidence of POPF (overall 80% of POPF with soft pancreas vs. 16% of POPF with fibrotic pancreas). Moreover, when considering only clinically relevant POPF, we had only two POPFs (4.2%) with fibrotic pancreas vs. 15 POPFs (31.4%) with the soft pancreas (p < 0.005). Our incidence of reoperation was quite high 9/48, 18.7% (Table 4). It is superimposable to Duffas et al. and Mazzaferro et al. (26, 44). In detail, if we consider patients re-operated due to POPF only in two cases the prognosis was poor. Five re-operated patients had a good prognosis, so we can consider that the stump treatment did not influence the reoperation rate. Four of our patients (8.3%) had a postoperative hemorrhage, and all of them needed to return to the operative room. Interestingly, in only two patients (50%) hemorrhage was a consequence of POPF (all grade A). In the other two cases, the bleeding originated from a small vessel from the portal vein and the gastroepiploic artery. The overall incidence of POPF-related bleeding was 6%, which is in line with other experiences (25). Our length of stay was 38 days in POPF-group, higher than those observed in other experiences (45). More than 90% of patients needed pancreatic enzymes supplementation due to postoperative pancreatic insufficiency. This facet is consistent with other authors (25, 46, 47). However, Tran et al. reported that the need for enzyme supplementation 1 year after surgery was not related to the type of reconstruction (46). In addition, other authors reported that pancreatic exocrine insufficiency might be related to the pancreatic atrophy/fibrosis and preoperative texture than to DO or PJ (25, 46, 47). In our series, 16% of patients developed brittle diabetes, with only 13 patients (27.1%) developing new-onset diabetes. This might confirm that DO has a higher risk of new-onset diabetes, even if only a few patients suffer from uncontrolled diabetes (25, 46, 47). According to Tran et al., the incidence of endocrine insufficiency is significantly higher after DO compared with PJ at 3- and 12-month follow-up after surgery (p = 0.001 for both) (46). The overall mortality rate in more than 1,500 PD performed in Italy was reported to be as high as 8.1% (34). Our findings are superimposable to the literature (34), but we would clarify that only two patients who died have developed a clinically relevant fistula. On the other hand, three patients died for cardiovascular causes despite the absence of B or C POPF. We also demonstrated an overall pancreatic surgery-related mortality, which is lower than for low-volume centers (34). It has been suggested that avoiding an anastomosis of the pancreatic duct by means of duct occlusion could minimize anastomosis-related morbidity, especially in low-volume centers (43, 46–48). The aim was to obtain a “pure” pancreatic fistula with no activation by bile and/or enteric juice, thereby reducing the risk of life-threatening complications. However, in the experience of a high-volume center, postoperative mortality after PJ seemed to be higher than after DO (43). In a recent prospective randomized control study (26) compared POPF following DO in high-risk patients for pancreatic fistula vs. PJ after PD for low-risk patients for pancreatic fistula, mortality after DO was 5.9% and 2.0% after PJ anastomosis, in our serie 90-day mortality related to significant POPF was (2/48) 4%, so mortality might be considered superimposable with other authors who performed DO (Table 4) (49). He et al. (33) analyzed Randomized Controlled Trials (RCTs) and Observational Clinical Studies (OCSs), which were related to different treatments of pancreatic stump and major outcomes after PD or pylorus-preserving PD for malignant or benign pancreatic tumor, chronic pancreatitis, or extra-pancreatic tumors (periampullary, biliary or duodenal). The objective of the meta-analysis was a comparison between PJ and PG using quantitative data on POPF and overall complications. PD without anastomosis or duodenum-preserving pancreatectomy was excluded. We shall underline meta-analysis by He et al. (33) reported a lower mortality index performing PG and PJ, but these data were published by high volume and referral centers for pancreatic surgery. Nevertheless, Duffas et al. reported in their experience an incidence of death after PG and PJ of 12 and 10%, respectively (44). A summary of these findings is depicted in Tables 7–9.
Table 7.
Literature summary of pathological findings in pancreatic surgery.
| Author | Type | N.ro | Mean Operative Time, min (range) |
PA, n (%) |
Amp, n (%) |
BDC, n (%) |
Others, n (%) | Texture soft, n (%) | DD≤3mm, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Giuliani et al. | DO | 48 | 358 (r.:221–480) | 24 (50) | 10 (20.8) | 6 (12.5) | 8 (16.6) | 33 (68.7) | 19 (39.58) |
| Mazzaferro et al. (26) | DO | 51 | 480 (r.:400–533) | 33 (64.7) | 32 (65.3) | 6 (10.7) | 5 (9.8) | NA | NA |
| PJ | 49 | 490 (r.:438–540) | 32 (65.3) | 4 (8.2) | 6 (10.7) | 7 (14.3) | NA | NA | |
| Yeo (50) | PG | 73 | 444 (r.:432–456) | 40 (55) | 7 (10) | 6 (8) | 4 (5) | 16 (22) | 3,4 (mean) |
| PJ | 72 | 432 (r.:420–444) | 40 (56) | 11 (15) | 7 (10) | 7 (9.7) | 17 (24) | 2,9 (mean) | |
| Duffas (44) | PG | 81 | ≥360 54 (67%) <360 27 (33%) |
34 (42) | 17 (19) | 8 (10) | 9 (11) | 49 (60) | 32 (40) |
| PJ | 68 | ≥360 44 (65%) <360 24 (35%) |
25 (37) | 19 (28) | 11 (16) | 8 (11.7) | 41 (60) | 49 (60) | |
| Bassi (51) | PG | 69 | 337.2 (r:336-338) | 32 (46) | 13 (18.8) | 1 (1.4) | 24 (34.7) | NA | NA |
| PJ | 82 | 353.9 (r: 352-354) | 28 (34.1) | 11 (13.4) | 2 (2.4) | 43 (52.4) | NA | NA | |
| Fernàndez-Cruz (52) | PG | 53 | 300 (r.:250-350) | 26 (49) | 12 (22.6) | 8 (15) | 10 (18.8) | 24 (45) | NA |
| PJ | 55 | 310 (r.:250-370) | 28 (50.9) | 10 (18.1) | 7 (12.7) | 10 (18.1) | 25 (55) | NA |
PA, pancreatic adenocarcinoma; Amp, ampullary carcinoma; BDC, bile duct cancer; DD, duct diameter; DO, duct occlusion; PJ, pancreatic-jejunal anastomosis; PG, pancreatic-gastrostomy; NA, not available.
Table 9.
Literature summary of Clavien-Dindo classification, re-operative rate, POPF and mortality rate in pancreatic surgery.
| Author | Type | N.ro | CD I–II, n (%) | CD ≥III, n (%) | Re-operation rate, n (%) | POPF, n (%) | Mortality, n (%) |
|---|---|---|---|---|---|---|---|
| Giuliani et al. | DO | 48 | 41 (85) | 7 (14) | 10 (20.83) | 31 (64.5) | 5 (10.4) |
| Mazzaferro et al. (26) | DO | 51 | 15 (29.4) | 36 (70.6) | 9 (19) | B, C 6 (11.8) | 3 (5.9) |
| PJ | 49 | 15 (30.6) | 34 (69.4) | 8 (16.3) | B, C 8 (16.3) | 1 (2) | |
| Yeo (50) | PG | 73 | NA | NA | NA | 9 (12) | NA |
| PJ | 72 | NA | NA | NA | 8 (11) | NA | |
| Duffas (44) | PG | 81 | 44 (54.3) | 37 (45.7) | 15 (19) | 13 (16) | 10 (12) |
| PJ | 68 | 38 (55.9) | 30 (44.1) | 15 (22) | 14 (20.5) | 7 (10) | |
| Bassi (51) | PG | 69 | NA | NA | 5 (7) | 9 (15.8) | 0 |
| PJ | 82 | NA | NA | 5 (6) | 13 (15.8) | 1 (1) | |
| Fernàndez-Cruz (52) | PG | 53 | NA | NA | 1 (1.8) | A:1 (1,8) B:2 (3.7) | 0 |
| PJ | 55 | NA | NA | 1 (1.8) | B:10 (18.1) | 0 |
CD, Clavien-Dindo Classification; NA, not available.
Table 8.
Literature summary of complications in pancreatic surgery.
| Author | Type | N.ro | P.O. haemorrhage, n (%) | SI, n (%) | Pneumonia, n (%) | Bleeding, n (%) | BF, n (%) | IA, n (%) | DGE, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Giuliani et al. | DO | 48 | 8 (16.67) | 4 (8.3) | 3 (6.2) | 11 (22.9) | 1 (2) | 14 (29.17) | NA |
| Mazzaferro et al. (26) | DO | 51 | 7 (13.7) | 5 (9.8) | 8 (15.7) | 7 (13.7) | 4 (7.8) | 4 (7.8) | 8 (15.7) |
| PJ | 49 | 5 (10.2) | 2 (4.1) | 7 (14.3) | 5 (10) | 7 (14.3) | 2 (4.1) | 9 (18.4) | |
| Yeo (50) | PG | 73 | NA | 14 (19) | 5 (7) | NA | 1 (1) | 4 (5) | 16 (22) |
| PJ | 72 | NA | 11 (15) | 2 (3) | NA | 3 (4) | 2 (3) | 16 (22) | |
| Duffas (44) | PG | 81 | 13 (16) | NA | NA | 13 (16) | 6 (7) | 11 (14) | NA |
| PJ | 68 | 9 (13) | NA | NA | 9 (13) | 2 (3) | 16 (23) | NA | |
| Bassi (51) | PG | 69 | 3 (4) | NA | NA | 3 (4) | 0 | 7 (10) | 2 (3) |
| PJ | 82 | 6 (7) | NA | NA | 6 (7) | 7 (8.5) | 22 (27) | 10 (12) | |
| Fernàndez-Cruz (52) | PG | 53 | 1 (2) | 3 (8) | 2 (4) | 1 (2) | 0 | 2 (4) | 2 (4) |
| PJ | 55 | 1(2) | 2 (4) | 4 (7) | 1 (2) | 1 (2) | 8 (14) | 8 (14) |
SI, surgical infection; BF, biliary fistula; IA, intra-abdominal abscess; DO, duct occlusion; DGE, Delayed Gastric Emptying; PJ, pancreatic-jejunal anastomosis; PG, pancreatic-gastrostomy; NA, not available.
It is clear that the outcome of complex surgical procedures may not only rely on technical aspects of surgery but is also affected by resource availability (53, 54). However, some technical aspects can be modified and reduce the risk of life-threatening postoperative complications even in low-/medium-volume centers. Pancreaticoduodenectomy can be safely performed in low-volume centers if amenities and processes typical of high-volume centers can be replicated in specialized units (55, 56). Of note, we represent the only referral center for HPB in a huge geographical region of southern Italy, so the availability of postdischarge home management, financial problems, low human resources and patients wish could affect this outcome. In our opinion, in patients with a higher risk for POPF (soft pancreas, dilated pancreatic duct), DO could be a safer option, ideally suitable in low-volume centers. The ideal concept of reserving pancreatic surgery only to highly specialized centers is probably utopian. Geographical limitations, elevated costs for the patients and their relatives, political issues, different regional healthcare systems, and the opposition by medical and surgical staff determine the need to perform this surgery even in academic or tertiary referral hospitals with a limited experience in HPB surgery, but with all the amenities required for very complex surgery (57, 58). So, considering criteria published in the literature (32, 34–36), pancreatic surgery should be centralized, this implies unavoidably an increase of interregional mobility and related healthcare costs, especially for patients from the region of southern Italy. During the Covid-19 pandemic, as we know from the survey written by Aldrighetti et al. on HPB surgery in Italy (27), 72.8% of HPB centers showed a reduction of routine elective operations ≥50%, if we combine effects of centralization to the effects of the Covid-19 pandemic we understand how difficult it would be for patients to undergo pancreatic surgery in a quite fast, safe, and effective way (59). In this situation, we decided to analyse our outcomes from a low volume center for pancreatic surgery to overcome the impossibility to send patients to pancreatic surgery referral centers, considering their overload, ensuring to patients a high-quality service at the same time. Our approach led us to guarantee effective treatment and safety procedures during the critical pandemic period. Probably, a surgical alternative such as DO during the phase of PD at higher risk of complications, i.e., the pancreatic anastomosis, could reduce the rates of subsequent morbidity and mortality with similar oncological results.
Limitations
Our study is a retrospective, single-center analysis, we considered consecutive patients who underwent PD and were registered in a prospectively maintained database. We can consider our center as low volume due to the number of PD per year, but we can be supported by high-volume center facilities, including a) being a referral center for hepatobiliary surgery, liver transplantation, advanced colorectal surgery, b) having a dedicated intensive care unit, and c) having interventional radiology and endoscopy available 24 h.
Conclusions
In conclusion, DO could be proposed as an alternative option to pancreatic anastomosis especially in low-/medium-volume centers. A comparison of DO with other types of pancreatic duct reconstructions should be advisable to draw definitive conclusions, ideally by means of an adequately designed RCT.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, with undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Università degli Studi del Molise. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AG, FC, and AR: conceptualization. AG and AR: methodology. PA: software. AG, PA, FC, and AR: validation, writing—original draft preparation, and visualization. PA, ALS, MI, AB, MCo, MCa, MB, RV, and AS: formal analysis and investigation, resources, and data curation. PA, FC, and AR: writing—review and editing. GG, BA, and AR: supervision. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This work was written thanks to the collected data in the Antonio Cardarelli Hospital, General Surgery Unit, Italy.
References
- 1.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. (2004) 91:586–94. 10.1002/bjs.4484 [DOI] [PubMed] [Google Scholar]
- 2.Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. (2005) 92:547–56. 10.1002/bjs.4881 [DOI] [PubMed] [Google Scholar]
- 3.D'Souza MA, Shrikhande SV. Pancreatic resectional surgery: an evidence-based perspective. J Cancer Res Ther. (2008) 4:77–83. 10.4103/0973-1482.42253 [DOI] [PubMed] [Google Scholar]
- 4.Aldrighetti L, Guzzetti E, Pulitanò C, Cipriani F, Catena M, Paganelli M, et al. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol. (2010) 102:82–6. 10.1002/jso.21541 [DOI] [PubMed] [Google Scholar]
- 5.Marte G, Scuderi V, Rocca A, Surfaro G, Migliaccio C, Ceriello A. Laparoscopic splenectomy: a single center experience. Unusual cases and expanded inclusion criteria for laparoscopic approach. Updates Surg. (2013) 65:115–9. 10.1007/s13304-013-0197-0 [DOI] [PubMed] [Google Scholar]
- 6.Casadei R, Ricci C, D'Ambra M, Marrano N, Alagna V, Rega D, et al. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: a case–control study. Updates Surg. (2010) 62:171–4. 10.1007/s13304-010-0027-6 [DOI] [PubMed] [Google Scholar]
- 7.Mege D, Frontali A, Pellino G, Adegbola S, Maggiori L, Warusavitarne J, et al. Laparoscopic subtotal colectomy with double-end ileosigmoidostomy in right iliac fossa facilitates second-stage surgery in patients with inflammatory bowel disease. Surg Endosc. (2020) 34:186–91. 10.1007/s00464-019-06749-3 [DOI] [PubMed] [Google Scholar]
- 8.Rocca A, Scacchi A, Cappuccio M, Avella P, Bugiantella W, de Rosa B, et al. Robotic surgery for colorectal liver metastases resection: a systematic review. (2021) Int J Med Robot. 17:e2330. 10.1002/rcs.2330 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. (2004) 139:718–25. 10.1001/archsurg.139.7.718 [DOI] [PubMed] [Google Scholar]
- 10.Kuhlmann K, de Castro SD, Wesseling J, ten Kate FT, Offerhaus G, Busch O, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. (2004) 40:549–58. 10.1016/j.ejca.2003.10.026 [DOI] [PubMed] [Google Scholar]
- 11.Stojadinovic A, Brooks A, Hoos A, Jaques DP, Conlon KC, Brennan MF. An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. J Am Coll Surg. (2003) 196:954–64. 10.1016/S1072-7515(03)00010-3 [DOI] [PubMed] [Google Scholar]
- 12.Bassi C, Falconi M, Salvia R, Mascetta G, Molinari E, Pederzoli P. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. (2001) 18:453–7. 10.1159/000050193 [DOI] [PubMed] [Google Scholar]
- 13.Salvia R, Lionetto G, Perri G, Malleo G, Marchegiani G. Total pancreatectomy and pancreatic fistula: friend or foe? Updates Surg. 73:1231–6. 10.1007/s13304-021-01130-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. (2005) 138:8–13. 10.1016/j.surg.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 15.Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions Dig Surg. (2004) 21:54–9. 10.1159/000075943 [DOI] [PubMed] [Google Scholar]
- 16.Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z'Graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. (2003) 138:1310–4. 10.1001/archsurg.138.12.1310 [DOI] [PubMed] [Google Scholar]
- 17.Fahy BN, Frey CF, Ho HS, Beckett L, Bold RJ. Morbidity, mortality, and technical factors of distal pancreatectomy. Am J Surg. (2002) 183:237–41. 10.1016/S0002-9610(02)00790-0 [DOI] [PubMed] [Google Scholar]
- 18.Ceccarelli G, Andolfi E, Biancafarina A, Rocca A, Amato M, Milone M, et al. Robot-assisted surgery in elderly and very elderly population: our experience in oncologic and general surgery with literature review. Aging Clin Exp Res. (2017) 29:55–63. 10.1007/s40520-016-0676-5 [DOI] [PubMed] [Google Scholar]
- 19.Rocca A, Brunese MC, Cappuccio M, Scacchi A, Martucci G, Buondonno A, et al. Impact of physical activity on disability risk in elderly patients hospitalized for mild acute diverticulitis and diverticular bleeding undergone conservative management. Medicina. (2021) 57. 10.3390/medicina57040360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aprea G., Rosa D. e., Milone D., Rocca M., Bianco A., Massa T., et al. (2017). Laparoscopic distal pancreatectomy in elderly patients: is it safe? Aging Clin Exp Res. 29:41–5. 10.1007/s40520-016-0677-4 [DOI] [PubMed] [Google Scholar]
- 21.Costa G, Frezza B, Fransvea P, Massa G, Ferri M, Mercantini P, et al. Clinico-pathological features of colon cancer patients undergoing emergency surgery: a comparison between elderly and non-elderly patients. Open Med. (2019) 14:726–34. 10.1515/med-2019-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balcom JH, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. (2001) 136:391–8. 10.1001/archsurg.136.4.391 [DOI] [PubMed] [Google Scholar]
- 23.Büchler MW, Friess H, Wagner M, Kulli C, Wagener V. Z'Graggen, K. Pancreatic fistula after pancreatic head resection. Br J Surg. (2000) 87:883–9. 10.1046/j.1365-2168.2000.01465.x [DOI] [PubMed] [Google Scholar]
- 24.Søreide K, Labori KJ. Risk factors and preventive strategies for post-operative pancreatic fistula after pancreatic surgery: a comprehensive review. Scand J Gastroenterol. (2016) 51:1147–54. 10.3109/00365521.2016.1169317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfieri S, Quero G, Rosa F, Miceli Di, Tortorelli D, Doglietto AP, et al. and results of pancreatic stump duct occlusion after duodenopancreatectomy . Updates Surg. (2016) 68:287–93. 10.1007/s13304-016-0384-x [DOI] [PubMed] [Google Scholar]
- 26.Mazzaferro V, Virdis M, Sposito C, Cotsoglou C, Droz Dit Busset M, Bongini M, et al. Permanent pancreatic duct occlusion with neoprene-based glue injection after pancreatoduodenectomy at high risk of pancreatic fistula: a prospective clinical study. Ann Surg. (2019) 270:791–8. 10.1097/SLA.0000000000003514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldrighetti L, Boggi U, Falconi M, Giuliante F, Cipriani F, Ratti F, et al. Perspectives from Italy during the COVID-19 pandemic: nationwide survey-based focus on minimally invasive HPB surgery. Updates Surg. (2020) 72:241–7. 10.1007/s13304-020-00815-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchegiani G, Perri G, Bianchi B, Esposito A, Landoni L, Casetti L, et al. Pancreatic surgery during COVID-19 pandemic: major activity disruption of a third-level referral center during 2020. Updates Surg. (2021) 1–9. 10.1007/s13304-021-01197-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colucci GFDC, Falconi M, Giuliani F, Parisi S, Reni M. Linee guida del Carcinoma del Pancreas. AIOM: (2009). p. 1. [Google Scholar]
- 30.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. (2014) 12:1500–24. 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 31.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassi C, Andrianello S. Identifying key outcome metrics in pancreatic surgery, and how to optimally achieve them. HPB. (2017) 19:178–81. 10.1016/j.hpb.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 33.He T, Zhao Y, Chen Q, Wang X, Lin H, Han W. Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: a systematic review and meta-analysis. Dig Surg. (2013) 30:56–69. 10.1159/000350901 [DOI] [PubMed] [Google Scholar]
- 34.Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Carlo Di, et al. of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. (2008) 95:357–62. 10.1002/bjs.5982 [DOI] [PubMed] [Google Scholar]
- 35.Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. (1999) 125:250–6. 10.1016/S0039-6060(99)70234-5 [DOI] [PubMed] [Google Scholar]
- 36.Simunovic M, Urbach D, Major D, Sutradhar R, Baxter N, To T, et al. Assessing the volume-outcome hypothesis and region-level quality improvement interventions: pancreas cancer surgery in two Canadian Provinces. Ann Surg Oncol. (2010) 17:2537–44. 10.1245/s10434-010-1114-0 [DOI] [PubMed] [Google Scholar]
- 37.Panni RZ, Panni UY, Liu J, Williams GA, Fields RC, Sanford DE, et al. Re-defining a high volume center for pancreaticoduodenectomy. HPB. (2021) 23:733–8. 10.1016/j.hpb.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 38.Cavaliere D, Parini D, Marano L, Cipriani F, Marzo Di, Macrì F, et al. Surgical management of oncologic patient during and after the COVID-19 outbreak: practical recommendations from the Italian society of Surgical Oncology. Updates Surg. (2021) 73:321–9. 10.1007/s13304-020-00921-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meredith JW, High KP, Freischlag JA. Preserving elective surgeries in the COVID-19 pandemic and the future. JAMA. (2020) 324:1725–6. 10.1001/jama.2020.19594 [DOI] [PubMed] [Google Scholar]
- 40.Torzilli G, Viganò L, Galvanin J, Castoro C, Quagliuolo V, Spinelli A, et al. A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann Surg. (2020) 272:e112–7. 10.1097/SLA.0000000000004081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): a systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine. (2017) 96:e6858. 10.1097/MD.0000000000006858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Carlo V, Chiesa R, Pontiroli EA, Carlucci M, Staudacher C, Zerbi A, et al. Pancreatoduodenectomy with occlusion of the residual stump by Neoprene injection. World J Surg. (1989) 13:105–10. 10.1007/BF01671167 [DOI] [PubMed] [Google Scholar]
- 43.Tersigni R, Capaldi M, Ialongo P, Grillo LR, Anselmo A. Surgical treatment of the pancreatic stump: preventive strategies of pancreatic fistula after pancreatoduodenectomy for cancer. G Chir. (2014) 35:213–22. 10.11138/gchir/2014.35.9.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duffas JP, Suc B, Msika S, Fourtanier G, Muscari F, Hay JM, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. (2005) 189:720–9. 10.1016/j.amjsurg.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 45.Ridolfini MP, Alfieri S, Gourgiotis S, Miceli Di, Rotondi D, Quero F, et al. Risk factors associated with pancreatic fistula after distal pancreatectomy, which technique of pancreatic stump closure is more beneficial? World J Gastroenterol. (2007) 13:5096–100. 10.3748/wjg.v13.i38.5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran K, Van Eijck C, Di Carlo V, Hop WC, Zerbi A, Balzano G, et al. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg. (2002) 236:422–8. 10.1097/00000658-200210000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mezza T, Clemente G, Sorice GP, Conte C, De Rose AM, Sun VA, et al. Metabolic consequences of the occlusion of the main pancreatic duct with acrylic glue after pancreaticoduodenectomy. Am J Surg. (2015) 210:783–9. 10.1016/j.amjsurg.2014.12.052 [DOI] [PubMed] [Google Scholar]
- 48.Hackert T, Hinz U, Pausch T, Fesenbeck I, Strobel O, Schneider L, et al. Postoperative pancreatic fistula: we need to redefine grades B and C. Surgery. (2016) 159:872–7. 10.1016/j.surg.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 49.Giglio MC, Cassese G, Tomassini F, Rashidian N, Montalti R, Troisi RI. Post-operative morbidity following pancreatic duct occlusion without anastomosis after pancreaticoduodenectomy: a systematic review and meta-analysis. HPB. (2020) 22:1092–101. 10.1016/j.hpb.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 50.Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. (1995) 222:580–8. 10.1097/00000658-199510000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. (2005) 242:767–71. 10.1097/01.sla.0000189124.47589.6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernández-Cruz L, Cosa R, Blanco L, López-Boado MA, Astudillo E. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study. Ann Surg. (2008) 248:930–8. 10.1097/SLA.0b013e31818fefc7 [DOI] [PubMed] [Google Scholar]
- 53.Conroy PC, Calthorpe L, Lin JA, Mohamedaly S, Kim A, Hirose K, et al. Determining hospital volume threshold for safety of minimally invasive pancreaticoduodenectomy: a contemporary cutpoint analysis. Ann Surg Oncol. (2021). 10.1245/s10434-021-10984-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acher AW, Weber SM, Pawlik TM. Does the volume-outcome association in pancreas cancer surgery justify regionalization of care? a review of current controversies. Ann Surg Oncol. (2021). 10.1245/s10434-021-10765-w [DOI] [PubMed] [Google Scholar]
- 55.Kanhere HA, Trochsler MI, Kanhere MH, Lord AN, Maddern GJ. Pancreaticoduodenectomy: outcomes in a low-volume, specialised Hepato Pancreato Biliary unit. World J Surg. (2014) 38:1484–90. 10.1007/s00268-013-2431-9 [DOI] [PubMed] [Google Scholar]
- 56.Pecorelli N, Balzano G, Capretti G, Zerbi A, Di Carlo V, Braga M. Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg. (2012) 16:518–23. 10.1007/s11605-011-1777-2 [DOI] [PubMed] [Google Scholar]
- 57.Stella M, Bissolati M, Gentile D, Arriciati A. Impact of surgical experience on management and outcome of pancreatic surgery performed in high- and low-volume centers. Updates Surg. (2017) 69:351–8. 10.1007/s13304-017-0422-3 [DOI] [PubMed] [Google Scholar]
- 58.Loffredo D, Marvaso A, Ceraso S, Cinelli N, Rocca A, Vitale M, et al. Minimal invasive surgery in treatment of liver metastases from colorectal carcinomas: case studies and survival rates. BMC Surg. (2013) 13 Suppl 2:S45. 10.1186/1471-2482-13-S2-S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bracale U, Podda M, Castiglioni S, Peltrini R, Sartori A, Arezzo A, et al. Changes in surgicaL behaviOrs dUring the CoviD-19 pandemic. The SICE CLOUD19 study. Updates Surg. (2021) 73:731–44. 10.1007/s13304-021-01010-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, with undue reservation.