Abstract
Osteoarthritis (OA) is the most common condition affecting human joints. Along with mechanical and genetic factors, low-grade inflammation is increasingly supported as a causal factor in the development of OA. Gut microbiota and intestinal permeability, via the disruption of tight junction competency, are proposed to explain a gut-joint axis through the interaction with the host immune system. Since previous studies and methods have underestimated the role of the gut-joint axis in OA and have only focussed on the characterisation of microbiota phenotypes, this systematic review aims to appraise the current evidence concerning the influence of gut permeability in the pathogenesis of OA. We propose that the tight junction disruption may be due to an increase in zonulin activity as already demonstrated for many other chronic inflammatory disorders. After years of unreliable quantification, one study optimised the methodology, showing a positive validated correlation between plasma lipopolysaccharide (LPS), obesity, joint inflammation, and OA severity. Chemokines show a prominent role in pain development. Our systematic review confirms preliminary evidence supporting a gut-joint axis in OA pathogenesis and progression. Being modifiable by several factors, the gut microbiota is a promising target for treatment. We propose a pathogenetic model in which dysbiosis is correlated to the bipartite graph of tight junctions and bacterially-produced products, aiming to direct future studies in the search of other bacterial products and tight junction disassembly regulators.
KEY MESSAGES
Previous studies and methods have underestimated the impact of the gut-joint axis in osteoarthritis and have focussed on the characterisation of microbiota phenotypes rather than clear molecular mediators of disease.
Gut dysbiosis is related to higher levels of bacterial toxins that elicit cartilage and synovium inflammatory pathways.
Future research may benefit from focussing on both tight junctions and bacterially-produced products.
Keywords: Osteoarthritis, tight junctions, gut microbiota, microbiome, intestinal permeability, joint inflammation
Introduction
Osteoarthritis (OA) is the most common condition affecting human joints and the 15th highest cause of years lived with disability worldwide [1]. Though it may develop in any joint, it predominantly affects diarthrodial joints such as the hip and, following disease progression, it ultimately leads to joint failure [2]. As our understanding of the pathophysiology continues to evolve, we have come to realise that OA is a complex disease with multifactorial aetiology, where an interplay between host and environmental factors instigate the disease [3,4]. However, it is not known why the progression of the disease is highly variable in the individuals who develop this condition. Despite traditionally considered “non-inflammatory” arthritis, low-grade inflammation seems to have a causal role in OA, and new studies suggest that it may be triggered by the complex interplay between the gastrointestinal microbiome [5], its products, and the immune system.
The gut microbiome comprises more than 3 million genes, and each person features a unique microbiome composition [6]. Taxonomic studies report that Firmicutes (including Lactobacillus, Bacillus, Clostridium, Enterococcus, and Ruminicoccus genera) and Bacteroidetes (including Sphingobacterium, Bacteroides, Alistipes, Prevotella genera) are the main phyla, representative of 90% of the total gut microbiome [7]. Many studies suggest that several intestinal [8] and extra-intestinal diseases [9] are associated with specific bacterial motifs and dysbiosis, that is a reduction in microbial diversity and functional imbalance in microbial communities. Notwithstanding the absence of proof for a causal relationship between dysbiosis and pathophysiology, for several diseases, a role in clinical severity is demonstrated [10]. Gut permeability is an emerging area of microbiome research since has been shown as pivotal in non-gut disorders [11], and diseases traditionally considered on a purely autoimmune basis. In particular, evidence for a significant role of the microbiota in the modulation of tolerogenic mechanisms has been reported [12,13].
Traditionally, low-vascularized tissues like articular cartilage were considered the core of OA pathogenesis. Thus, the joint environment seemed to be influenced by systemic factors either indirectly through the subchondral bone or in the neo-angiogenesis phase of OA pathogenesis [14] when a multitude of blood molecules, including bacteria and bacterial products, have a gate from the blood to the cartilage [15–17]. However, on a more accurate investigation, findings suggest that the synovium is part of a dynamic interplay inside the joint in OA development. Altogether, these findings cannot be dismissed as pathological [18], bacteria and bacterial products could affect the epigenetic landscape of chondrocytes [19] and prime the innate immune response in the joint via Toll-like receptors signalling [5]. In addition, a recent study [20] suggests that a microbiome exists inside the OA knee and hip joints. The overall ecological interactions among these microbial patterns are still mainly unknown.
How the gut influences OA pathogenesis is not clear. Limited attention has been paid to the role of intestinal permeability as a mediator of the effects of the microbiome on the joint. Previous studies and methods have underestimated the impact of microbiome products and intestinal permeability in the pathogenesis of OA [21]. Given the lack of mechanistic insight and the absence of a clear definition of the molecular actors involved in the pathogenesis of OA, in this review, we aim to appraise the direct and indirect effects of gut dysbiosis on the affected joint, focussing on clear molecular mediators emerging from related literature, to help direct future studies.
Materials and methods
Study search strategy
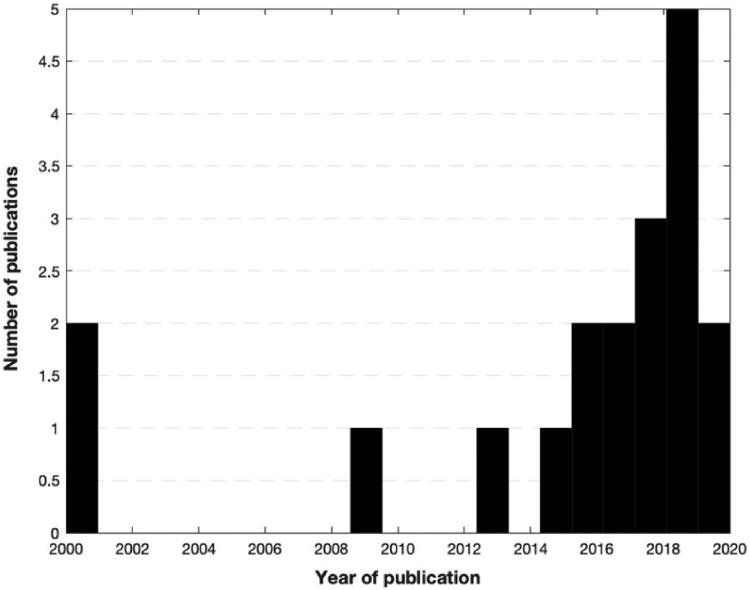
This systematic review was conducted according to the 2021 guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Figure 2) [22]. A comprehensive search was performed using three electronic medical databases (PubMed, EMBASE, and Cochrane Library) by two independent authors (GA and VI) from January 2020 to April 2020. To achieve maximum sensitivity we combined the terms “gut microbio* OR gut OR streptococcus OR lactobacillus OR bifidobacterium OR clostridium OR microbiome” and typical anatomical landmarks of disease “hip OR knee” with some terms related to osteoarthritis and inflammation (arthritis, osteoarthritis, inflammation, synovial, synovitis, cytokines, chemokines, molecular mediators) as either keywords or Medical Subject Heading (MeSH) terms. To elucidate the growing interest in the review topic in the medical literature, we graphically summarised the number of articles per year (Figure 1). The research was then repeated in February 2021 but did not retrieve new results.
Figure 2.
PRISMA flow diagram of study selection.
Figure 1.
A number of publications about microbiome and osteoarthritis selected per year.
References of all included articles, previous reviews, and Google Scholar top results were reviewed to identify further relevant studies. Moreover, intending to avoid overlap with other ongoing systematic reviews, we searched PROSPERO for any similar work.
Selection criteria
Selected studies included those investigating the influence of microbiome composition on osteoarthritis, the role of diet and dietary interventions on the inflammatory state, and the molecular mediators involved. Both clinical and preclinical studies published in English in peer-reviewed journals were screened. We excluded studies with missing or not accessible data. We excluded studies for which a full-text article was not available, as well as not well-reported studies. We excluded duplicates and studies with a poor or unclear methodology. Finally, we excluded reviews, case reports, conference presentations, and articles only containing opinions. Three authors (GA, GG and VI) searched and evaluated the articles independently. An experienced researcher (EC) resolved cases of doubt. All abstracts were read and, according to inclusion and exclusion criteria, relevant articles were selected. A month later, the rereading of the same studies ensured agreement among the investigators. One investigator (GA) extracted data from full-texts into Excel to analyse each study and data were double-checked by the other two investigators (GG, VI).
Data extraction and criteria appraisal
Data were extracted from article texts, tables, and figures using the Population, Intervention, Comparison, Outcome framework [23]. Title, year of publication, study design, number and characteristics of the subjects involved were considered together with study outcomes and conclusions. Three investigators (GA, GG, and VI) independently reviewed each article. Discrepancies were resolved by discussion.
Risk of bias assessment
The risk of bias of 4 non-randomized clinical studies was assessed according to the ROBINS risk of bias tool [24]. This tool used “low,” “moderate,” and “high” to describe the risk of bias. The assessment was performed by two authors (GA and VI) independently, with an inter-rater agreement of 90%. Any discrepancy was solved by consensus. 3 studies had a moderate risk of bias, and 2 studies had a low risk of bias. One article was excluded because of a serious risk of bias. The risk of bias assessment for pre-clinical studies was instead performed using the SYRCLE’s tool [25]. A score ranging from 0 (lowest) to 10 (highest) was used. Two investigators evaluated the studies independently (GA and VI) with 95% agreement. The risk of bias for the included studies was low to moderate (mean SYRCLE score 4.4, range 3–6) and no studies were excluded following this assessment.
Certainty assessment
The certainty of clinical studies was assessed using GRADE [26]. According to this method, each study was classified as “very low”, “low”, “moderate” or “high”. With the exception of one study that featured low confidence of evidence, all the studies ranked either “high” (3 studies) or “moderate” (2 studies).
The Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist [27,28] was used to assess the certainty of the pre-clinical studies (n = 13). The assessment was performed independently by two authors (GA, VI) with 94% agreement. Each study was assessed and scored on a scale from 0 (lowest) to 10 (highest) points. The overall certainty was moderate among the included studies (mean CAMARADES score 4.3, range 3–6).
Results
Study characteristics
We included results from 18 studies. 13 were performed on animal models [18,29–40] and 5 were human observational studies [5,21,41–43]. The models investigated were adult mice [21,29,30,32,33,36,38] and adult rats [18,34,37]. In the clinical studies included, the population was aged between 50 and 65 years.
Most of the considered studies analysed the relationship between OA and the microbial populations’ variations in the gastrointestinal tract or investigated cytokines with a mostly documented role in OA pathogenesis [30–33,39–43]. Metabolic alterations of the microbiome and their correlation to gut permeability were investigated through the serum concentration of LPS [18,21,34,35,38]. Diet and obesity were analysed concomitantly to the microbiome in mice [18,34,35]. Some studies analysed the role in OA severity of physical exercise [34] and other dietary aspects: the administration of nutritional supplementation [37] and/or prebiotics in rodents [34–37] and humans.
Dysbiosis-related gut permeability
When gut dysbiosis was induced by a high-fat diet (HFD), high-sugar diet, faecal transplant or sex steroid deficiency, higher levels of bacterial toxins and gut bacterial translocation to the blood were retrieved [34,35]. One study transplanted the faecal microbiota of patients with metabolic syndrome in mice, and assessed gut permeability, detecting lower mRNA levels of tight junction proteins (TJs), zonulin-1 (ZO-1) and occludin, and higher LPS plasma levels. Bacterial translocation via the intestinal endothelium was also confirmed using FISH methodology [29].
It was hypothesised that oligofructose could prevent gut dysbiosis and potentially reduce the OA of the obesity retrieved in previous animal studies [35]. While oligofructose supplementation in mice did not prevent the onset of obesity, it was shown that the gut had improved epithelial function, and bacterial products levels, such as LPS serum levels, were found to be lower [35]. This was then found to be associated with lower cartilage degeneration in the interventional group [35]. These effects seem to be mediated by the ability of oligofructose to partially alter the gut microbiome relative abundance avoiding the total loss of Bifidobacterium, which is commonly observed in mice obesity models [18,34,35]. Similar results were replicated by other pre- and probiotics [34,36,37]. Moreover, oligofructose administration was found to upregulate Cdx2, a transcription factor regulating cell adhesion, and increase Grp (a stimulator of epithelial proliferation in the intestine) and Aqp4 (involved in water reabsorption), all related to the maintenance of normal intestinal permeability [35].
Bacterial products contribute to low grade inflammation in the joint
Germ-free mice with destabilised medial meniscus showed a reduction in lipopolysaccharide-binding protein (LBP) but not LPS due to difficulty in blood LPS quantification [38]. When optimised methodology to assess LPS was performed, a positive association of synovial LPS with inflammation and disease severity was reported [21]. Articular chondrocytes mount an LPS-induced stress response via Toll-like receptors (TLRs) (especially TLR4) and secrete matrix metalloproteinases as well as innate immune mediators [46]. Fibroblast-like synoviocytes from OA patients upregulate their expression of the inflammasome receptors NLRP1/3 upon in vitro stimulation with LPS [47].
Firmicutes/Bacteroides (F/B) phyla ratio increase in gut dysbiosis models has shown to be mostly lead by a decrease in Bacteroidetes phylum population, whereas the absolute Firmicutes abundance resulted almost unchanged, but showed significant qualitative changes, particularly a decrease in Lactobacillus spp. and an increase in Clostridiales order [18]. In addition, Streptococcus spp., have shown to represent 20% of total Firmicutes in OA patients of a large population cohort study [5] and resulted positively correlated with higher OA pain scores and lower functionality [5]. Bacterial toxins may therefore connect the increased gut permeability to low-grade inflammation in OA.
Molecular mediators of inflammation and joint pain
Obese mice displayed a 5-fold increase in the number of infiltrating macrophages [35] and increased analytes: CXC-family chemokines (KC, MIG), adiponectin, leptin, IL-1Ra [44], IP-10, MCP-1, MIP-2 and MIP-1alfa [18]. A role for IL-18 [39] and IL-12 [40] was identified. From several studies balance between pro- and anti-inflammatory mediators emerges as key [36,37,39,40].
The innate immune pro-inflammatory cytokine IL-1beta was shown to up-regulate aquaporins AQP1 and AQP3 in an OA model. These molecules explain the joint swelling in early OA, and the subsequent loss of proteoglycans and chondrocytes apoptosis that accelerate the progression of the disease [30].
Among all the molecular mediators, chemokines have been shown to have a prominent role in the establishment of pain. While many chemokines show a mixed role in pain, chemoattraction and disease progression [31,41–43], the CCL2/CCR2 axis plays a predominant role in the development of pain, as confirmed by many studies [32,33,45].
Discussion
Gut permeability as a foundational element of the gut-joint axis
Gut microbiome changes are acute and precede obesity in mouse models. Indeed, obesity is associated with impairment of gut mucosa and microbiome translocation [46]. Interestingly, partaking in exercise, together with weight loss, is among the strong recommendations based on sound evidence for OA control [47]. Both happen to be associated with reduced pain and disease progression [48,49], and along with nutraceutical use [36,50], these interventions have been shown effective in reverting the acute microbiome compositional changes associated with OA.
However, when gut dysbiosis occurs either for chronic disease (e.g. inflammatory bowel disease, immunosuppression), chronic antibiotic treatment, or lifestyle modification (e.g. obesity and metabolic disease), gut permeability is significantly affected [51–56]. HFD modulates tight junctions, their expression and distribution, directly through dietary fats or indirectly via the increase in cytokines release [57]. HFD not only reduces TJ molecules but also depletes eosinophils associated with correct gut permeability [58], reduces mucus and antimicrobial peptides production [59]. Moreover, microbes are spatially redistributed in the intestine, mainly occupying intervillous/cryptal spaces [60].
If such changes do occur, progressively, innate immune receptors in the gut get activated by microbial products and stimulate pro-inflammatory mediators production. Pro-inflammatory cytokines, in turn, dysregulate TJs formation creating a vicious cycle. TNFα, for instance, is known to be involved in occludin internalisation, while IFNγ reduces both ZO-1 and occludin expression. Myosin light chain kinase (MLCK) seems to be important in the cytokine-mediated regulation of TJ complexes [61]. As a general view, TJ dysregulation may be induced by cytokines, by immune cells, by NSAIDs, or alcohol chronic use, as well as by pathogens in the context of a dysbiotic microbiome [62].
It is indeed a well-known fact that many enteric pathogens produce toxins that affect the host’s tight junctions [63]. In particular, given that TJ competency was found to be affected via a decrease in ZO-1 and occludin mRNA levels in the analysed studies, we propose that this tight junction disruption may be in part due to an increase in zonulin activity, already demonstrated for many chronic inflammatory and autoimmune disorders [64]. Zonulin is a physiologic regulator of intestinal permeability but is known to be pathologically triggered by gliadin and by bacteria in the gut. Zonulin release seems to be dependent on MyD-88 [65]. Inappropriate zonulin activity has been shown for diseases as varied as coeliac disease, ankylosing spondylitis, anxiety and depression (in a dysbiosis-mediated manner) and multiple sclerosis, in which zonulin may explain increased permeability to both the gut and the blood-brain barrier [66–68]. Similarly, we propose that zonulin may be involved in the gut-joint axis of osteoarthritis. TJ disruption provides a framework to unify several inflammatory diseases under the common denominator of permeability, then interact with the patient genetic background [69].
Dysbiosis perturbates the delicate balance between pro- and anti-inflammatory mediators
Our main findings support an increase in Firmicutes/Bacteroidetes (F/B) phyla ratio [18,34,35]. Given the decrease in Bacteroidetes and their ability to produce high levels of short-chain fatty acids, which regulate the differentiation of Treg cells [70], and the role of butyrate, which has been shown to be able to regulate zonulin [71,72], this particular enteric phenotype may further influence intestinal permeability, summing to the above mechanisms.
Another key feature of the OA dysbiotic enterotype is Streptococcus spp. the abundance which seems to be critical to prime both local and systemic inflammation through LPS-induced macrophage activation [5] via the NFkB or MAPK pathways [73], or by forming complexes with LBP and CD14 [21]. Mechanistic evidence was reported with increased CD14 levels in the synovium of OA patients compared to healthy controls [74]. Macrophages can be detected not only in the synovium but also in the synovial fluid and in the peripheral blood [75]. CX3CR1-expressing macrophages have been found to form a highly dynamic barrier (expressing epithelial genes and tight junctions) in the lining layer of the synovium, adjacent to fibroblasts [76]. This barrier undergoes remodelling in arthritis models, thus single-cell studies of macrophage populations are warranted in OA-specific models. Moreover, the M1/M2 macrophages ratio determines the severity of the disease. While the former induces chondrocyte apoptosis and decreases the synthesis of extracellular matrix components, the latter, through TGFβ expression, stimulates chondrogenesis and collagen II/proteoglycan formation [77].
Thus, an increase in F/B ratio and Streptococcus spp. prevalence are both able to ultimately dysregulate the delicate balance between pro-inflammatory markers (IL-1beta, IL-6, TNFα, IL-12, IL-18) at the expense of anti-inflammatory molecules (IL-4, IL-10, TGFβ) [37]. Moreover, the role of the key pro-inflammatory cytokine IL-1beta in the local deterioration of the joint via aquaporins [78] may suggest a further probable mechanism, mediated by other inflammatory mediators.
It is important to notice that studies regarding the microbiota in various diseases have shown a poor concordance of findings, mainly due to the dynamic nature of bacterial presence in the gut [79]. More than 50% of the characterised bacterial species in a recent study [80] were never described before, and this raises concerns about the possibility of spurious associations, limiting our capacity to extrapolate solid causal relationships. Therefore, we suggest focussing on small molecules, such as bacterially produced products and immune mediators, rather than singular bacterial species in order to gain useful mechanistic insight.
Endotoxemia
An evidence-based gut-joint axis clearly stems from many of the studies. Both inflammatory mediators and bacterial toxins are translocated into the circulation [81] which raises concerns in patients that have joint prosthetic implants or artificial valves at high risk of infection [82,83]. The increased leucocytes and macrophage presence [35,84] may possibly let them act as a “Trojan Horse” [85–87] from the gut to the joint. The articular cartilage does not receive nutrition from blood vessels, instead, support is provided on one side by the synovial fluid and on the other by the subchondral bone marrow. Systemic bacterial toxins, inflammatory mediators and blood microbiota [88] can thus influence the joint in two ways: via large cracks of the articular cartilage [89] or via vascular channels arising from neoangiogenesis occurring at the osteochondral junction [90]. Moreover, given the aforementioned potent effects of HFD, meals might trigger pulsatile increases in LPS or other bacterial products in the blood, contributing to chronic low-grade inflammation. Experimental support or confutation to this idea is warranted. The circadian response to endotoxemia should also be assessed in the context of OA given the interesting outcome of some preliminary animal studies [91]. This may inform lifestyle modifications in these patients. Another level of complexity is added by the finding that LPS elicits distinct immune responses based on the bacterial species of origin [92]. An analysis of LPS subtypes, rather than the mere quantification of them, may provide further insight.
Pain symptomatology and chemokines
Studies show that the microbiome also relates to pain-associated symptoms and molecules [93]. The main actors are cartilage degradation products (following mechanical and inflammatory stress on the joint) and other damage-associated molecular patterns through direct neuronal activation of dorsal root ganglia or by indirect neuro-immune signalling acting on immune cells receptors that in turn stimulate neurons amplifying the mechanism. Even if cytokines and LPS partially explain the onset of pain, chemokines are reported as critical players of chronic pain [84]. Consistent with this interpretation, in our results, chemokines were regularly increased among all the considered analytes [35].
Conclusions
Future research directions
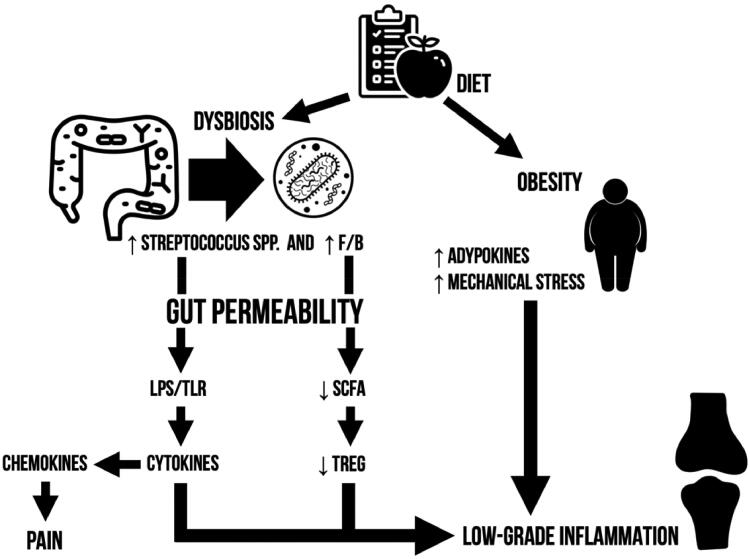
In order to be able to translate these findings, research priorities need to be identified. The pathogenetic model herein discussed correlates dysbiosis to the bipartite graph of tight junctions and bacterially produced products (Figure 3). Putting the accent on these two key features we aim to direct future studies in the search of bacterial toxins/products other than LPS and tight junction complexes disassembly regulators. However, we recommend caution since quantification assays commonly encounter reliability and reproducibility issues.
Figure 3.
Graphical summary of microbiome-mediated osteoarthritis pathogenesis.
Future therapeutical perspectives
Current treatments for OA alleviate pain but do not target the pathogenesis of the disease [94]. Being modifiable by several factors (dietary intervention, faecal transplant, and future microbiome-targeted therapeutics), the gut microbiome is a promising target. The first line of action is exercise, diet control and if the proposed role for zonulin is confirmed, gluten-poor food. Targets such as zonulin and aquaporins as well as the pyroptosis pathways (gasdermin blockers), might be further investigated pharmacologically in OA.
Limitations
Current studies, despite the overall moderate to a high quality of evidence, still show some limitations. Most studies were conducted on animal models, not fully mimicking the complexity of the human microbiome. Notwithstanding the need for more human studies, all pieces of evidence here analysed have shown concordance of findings and show the same results of some clinical studies [5]. The heterogeneity of methods used to induce obesity and of the animal models is another limitation of the studies taken into account. However, all the validated models of obesity show the same results, and the same holds true for all the different animals used for in vivo studies. Our review is hoped to help direct future studies.
Author contributions
Study concept and design: GG and EC; data acquisition, analysis and interpretation: GG, GA, VI; Drafting of the manuscript: GG, GA, VI, EC; critical revision of the manuscript for important intellectual content: GG, GA, VI, EC. All authors read and approved the final manuscript.
Disclosure statement
The authors have nothing to disclose related to this manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author (GG), upon request.
References
- 1.Glyn-Jones S, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387. [DOI] [PubMed] [Google Scholar]
- 2.Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. . Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [DOI] [PubMed] [Google Scholar]
- 3.Guilak F. Biomechanical factors in osteoarthritis. Best Practice and Research: Clinical Rheumatology. 2011;25(6):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panoutsopoulou K, Zeggini E.. Advances in osteoarthritis genetics. J Med Genet. 2013;50(11):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boer CG, Radjabzadeh D, Medina-Gomez C, et al. . Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 2019;10:4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinninella E, Raoul P, Cintoni M, et al. . What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arumugam M, Raes J, Pelletier E, et al. . Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank DN, St. Amand AL, Feldman RA, et al. . Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104(34):13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer EA, Tillisch K, Gupta A.. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manfredo Vieira S, Hiltensperger M, Kumar V, et al. . Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnevale R, Sciarretta S, Valenti V, et al. . Low-grade endotoxaemia enhances artery thrombus growth via toll-like receptor 4: implication for myocardial infarction. Eur Heart J. 2020;41(33):3156–3165. [DOI] [PubMed] [Google Scholar]
- 12.Ruff WE, Greiling TM, Kriegel MA.. Host–microbiota interactions in immune-mediated diseases. Nat Rev Microbiol. 2020;18(9):521–538. [DOI] [PubMed] [Google Scholar]
- 13.Manfredo Vieira S, Hiltensperger M, Kumar V, et al. . Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pesesse L, Sanchez C, Henrotin Y.. Osteochondral plate angiogenesis: a new treatment target in osteoarthritis. Joint Bone Spine. 2011;78(2):144–149. [DOI] [PubMed] [Google Scholar]
- 15.Hongyi Zhu TY, Jin H, Zhang C.. Intestinal methicillin-resistant Staphylococcus aureus causes periprosthetic infection via trojan horse mechanism: Evidence from a rat model. Bone and Joint Res. 2020;9(4):152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krezalek MA, Hyoju S, Zaborin A, et al. . Can methicillin-resistant Staphylococcus aureus silently travel from the gut to the wound and cause postoperative infection? Modeling the “Trojan Horse Hypothesis”. Ann Surg. 2018;267(4):749–758. [DOI] [PubMed] [Google Scholar]
- 17.Richards RL, et al. . Persistent Staphylococcus aureus isolates from two independent cases of bacteremia display increased bacterial fitness and novel immune evasion phenotypes. Infect Immun. 2015;83(8):3311–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins KH, Paul HA, Reimer RA, et al. . Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthr Cartil. 2015;23(11):1989–1998. [DOI] [PubMed] [Google Scholar]
- 19.Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, et al. . Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. 2019;10(suppl_1):S17-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn CM, Velasco C, Rivas A, et al. . Identification of cartilage microbial DNA signatures and associations with knee and hip osteoarthritis. Arthritis Rheumatol. 2020;72(7):1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang ZY, Stabler T, Pei FX, et al. . Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr Cartil. 2016;24(10):1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson WS, Wilson MC, Nishikawa J, et al. . The well-built clinical question: a key to evidence-based decisions. ACP Journal Club. 1995;;123(3):A12-A13. [PubMed] [Google Scholar]
- 24.Sterne JA, Hernán MA, Reeves BC, et al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooijmans CR, Rovers MM, de Vries RB, et al. . SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Schünemann HJ, et al. . GRADE guidelines: a new series of articles in the journal of clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–382. [DOI] [PubMed] [Google Scholar]
- 27.Landis SC, Amara SG, Asadullah K, et al. . A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macleod MR, O'Collins T, Howells DW, et al. . Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–1208. [DOI] [PubMed] [Google Scholar]
- 29.Huang ZYu, Chen J, Li BLei, et al. . Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann Rheum Dis. 2020;79(5):646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan C, Zhang J, Chen W, et al. . Inflammatory cytokines via up-regulation of aquaporins deteriorated the pathogenesis of early osteoarthritis. PLOS One. 2019;14(8):e0220846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MC, et al. . CCL17 blockade as a therapy for osteoarthritis pain and disease. Arthritis Res Ther. 2018;20(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghu H, Lepus CM, Wang Q, et al. . CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76(5):914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miotla Zarebska J, Chanalaris A, Driscoll C, et al. . CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthr Cartil . 2017;25(3):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rios JL, Bomhof MR, Reimer RA, et al. . Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. 2019;9(1):3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schott EM, Farnsworth CW, Grier A, et al. . Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight. 2018;;3(8):e95997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panicker S, Borgia J, Fhied C, et al. . Oral glucosamine modulates the response of the liver and lymphocytes of the mesenteric lymph nodes in a papain-induced model of joint damage and repair. Osteoarthr Cartil. 2009;17(8):1014–1021. [DOI] [PubMed] [Google Scholar]
- 37.So J-S, Song M-K, Kwon H-K, et al. . Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011;88(7–8):358–366. [DOI] [PubMed] [Google Scholar]
- 38.Ulici V, Kelley KL, Azcarate-Peril MA, et al. . Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr Cartil. 2018;26(8):1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joosten LAB, van de Loo FAJ, Lubberts E, et al. . An IFN-γ-Independent proinflammatory role of IL-18 in murine streptococcal cell wall arthritis. J Immunol. 2000;165(11):6553–6558. [DOI] [PubMed] [Google Scholar]
- 40.Joosten LAB, Helsen MMA, Van Den Berg WB.. Blockade of endogenous interleukin 12 results in suppression of murine streptococcal cell wall arthritis by enhancement of interleukin 10 and interleukin 1 Ra. Ann Rheum Dis. 2000;59(3):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X, Gu M, Xu X, et al. . CCL3/CCR1 mediates CD14 + CD16− circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr Cartil. 2020;28(5):613–625. [DOI] [PubMed] [Google Scholar]
- 42.Yang P, Tan J, Yuan Z, et al. . Expression profile of cytokines and chemokines in osteoarthritis patients: proinflammatory roles for CXCL8 and CXCL11 to chondrocytes. Int Immunopharmacol. 2016;40:16–23. [DOI] [PubMed] [Google Scholar]
- 43.Ren G, Whittaker JL, Leonard C, et al. . CCL22 is a biomarker of cartilage injury and plays a functional role in chondrocyte apoptosis. Cytokine. 2019;115:32–44. [DOI] [PubMed] [Google Scholar]
- 44.Griffin TM, Huebner JL, Kraus VB, et al. . Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 2012;64(2):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z, Li J, Yang H, et al. . Association of CCL2 gene variants with osteoarthritis. Arch Med Res. 2019;50(3):86–90. [DOI] [PubMed] [Google Scholar]
- 46.Metcalfe D, Harte AL, Aletrari MO, et al. . Does endotoxaemia contribute to osteoarthritis in obese patients? Clin Sci. 2012;123(11):627–634. [DOI] [PubMed] [Google Scholar]
- 47.Kolasinski SL, et al. . 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheum. 2020;72(2):220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Messier SP, et al. . Intentional weight loss in overweight and obese patients with knee osteoarthritis: is more better? Arthritis Care Res. 2018.;70(11):1569-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goh S-L, Persson MSM, Stocks J, et al. . Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: systematic review and network meta-analysis. Sports Med. 2019;49(5):743–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coulson S, Butt H, Vecchio P, et al. . Green-lipped mussel extract (perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacol. 2013;21(1):79–90. [DOI] [PubMed] [Google Scholar]
- 51.Ruff WE, Kriegel MA.. Autoimmune host-microbiota interactions at barrier sites and beyond. Trends Mol Med. 2015;21(4):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee YK, Mazmanian SK.. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakaroun RM, Massier L, Kovacs P.. Gut microbiome, intestinal permeability, and tissue bacteria in metabolic disease: perpetrators or bystanders? Nutrients. 2020;12(4):1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamprecht M, Bogner S, Schippinger G, et al. . Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012; ;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aleksandrova K, Romero-Mosquera B, Hernandez V.. Diet, gut microbiome and epigenetics: emerging links with inflammatory bowel diseases and prospects for management and prevention. Nutrients. 2017;9(9):962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saltiel AR, Olefsky JM.. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, et al. . Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutrition. 2019;11(1):77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson AMF, Costanzo A, Gareau MG, et al. . High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability. PLOS One. 2015;10(4):e0122195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araújo JR, Tomas J, Brenner C, et al. . Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie. 2017;141:97–106. [DOI] [PubMed] [Google Scholar]
- 60.Araújo JR, Tomas J, Brenner C, et al. . Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie. 2017;141:97–106. [DOI] [PubMed] [Google Scholar]
- 61.Chelakkot C, Ghim J, Ryu SH.. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50(8):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groschwitz KR, Hogan SP.. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awad WA, Hess C, Hess M.. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9(2):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sturgeon C, Fasano A.. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4(4):e1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevens BR, Goel R, Seungbum K, et al. . Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555.2–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedersen SJ, Maksymowych WP.. The pathogenesis of ankylosing spondylitis: an update. Curr Rheumatol Rep. 2019. ;21(10):58. [DOI] [PubMed] [Google Scholar]
- 68.Camara-Lemarroy CR, Silva C, Greenfield J, et al. . Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult Scler. 2020;26(11):1340–1350. [DOI] [PubMed] [Google Scholar]
- 69.Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Honda K, Littman DR.. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30(1):759–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tajik N, Frech M, Schulz O, et al. . Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;;11(1):1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fachi JL, Felipe J. D S, Pral LP, et al. . Butyrate protects mice from clostridium difficile-induced colitis through an HIF-1-Dependent mechanism. Cell Rep. 2019;27(3):750–761.e7. [DOI] [PubMed] [Google Scholar]
- 73.Lawrence T, Natoli G.. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–761. [DOI] [PubMed] [Google Scholar]
- 74.Daghestani HN, Pieper CF, Kraus VB.. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis and Rheumatology. 2015;67(4):956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller RJ, Malfait AM, Miller RE.. The innate immune response as a mediator of osteoarthritis pain. Osteoarthritis Cartilage. 2020;28(5):562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Culemann S, Grüneboom A, Nicolás-Ávila JÁ, et al. . Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature. 2019;572(7771):670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H, Cai D, Bai X.. Macrophages regulate the progression of osteoarthritis. Osteoarthr Cartil. 2020;28(5):555–561. [DOI] [PubMed] [Google Scholar]
- 78.Tan C, Zhang J, Chen W, et al. . Inflammatory cytokines via up-regulation of aquaporins deteriorated the pathogenesis of early osteoarthritis. PLOS One. 2019;14(8):e0220846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vujkovic-Cvijin I, Sklar J, Jiang L, et al. . Host variables confound gut microbiota studies of human disease. Nature. 2020;587(7834):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pasolli E, Asnicar F, Manara S, et al. . Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. 2019;176(3):649–662.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakaroun RM, Massier L, Kovacs P.. Gut microbiome, intestinal permeability, and tissue bacteria in metabolic disease: perpetrators or bystanders? Nutrients. 2020;12(4):1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alverdy JC, Hyman N, Gilbert J.. Re-examining causes of surgical site infections following elective surgery in the era of asepsis. Lancet Infect Dis. 2020;20(3):e38–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thwaites GE, Gant V.. Are bloodstream leukocytes trojan horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol. 2011;9(3):215–222. [DOI] [PubMed] [Google Scholar]
- 84.Miller RJ, Malfait AM, Miller RE.. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr Cartil. 2020;28(5):562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muraille E, Leo O, Moser M.. Th1/Th2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014;;5:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thwaites GE, Gant V.. Are bloodstream leukocytes trojan horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol. 2011;9(3):215–222. [DOI] [PubMed] [Google Scholar]
- 87.Alverdy JC, Hyman N, Gilbert J.. Re-examining causes of surgical site infections following elective surgery in the era of asepsis. Lancet Infect Dis. 2020;20(3):e38–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Païssé S, Valle C, Servant F, et al. . Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion. 2016;56(5):1138–1147. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Y, Chen B, Li S, et al. . Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci Rep.2018;8(1):14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walsh DA, Bonnet CS, Turner EL, et al. . Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthr Cartil. 2007;15(7):743–751. [DOI] [PubMed] [Google Scholar]
- 91.Moravcová S, Pačesová D, Melkes B, et al. . The day/night difference in the circadian clock’s response to acute lipopolysaccharide and the rhythmic Stat3 expression in the rat suprachiasmatic nucleus. PLoS One. 2018;13(9):e0199405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vatanen T, Kostic AD, d’Hennezel E, et al. . Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165(6):1551. [DOI] [PubMed] [Google Scholar]
- 93.Wu H-J, Ivanov II, Darce J, et al. . Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kolasinski SL, et al. . 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (GG), upon request.