ABSTRACT
Background
Hydroxychloroquine had attracted significant attention in the initial phases of the COVID-19 pandemic but current recommendations do not support its use. However, the evidence against its use as pre-exposure prophylaxis have been of low to moderate quality and have been limited by high risk of bias.
Methods
Following institutional ethics committee approval, healthcare workers (n = 1294) completing their first week-long COVID in-patient duty, subsequent institutional quarantine and RT-PCR testing for COVID-19 infection were included for this prospective cohort study. Demographic data, hydroxychloroquine usage and related adverse effects were captured through a ‘Caring for the Caregivers’ surveillance system. A chi-Square test of independence was used to determine the effect of hydroxychloroquine prophylaxis.
Results
Among the 1294 participants (age: 31 ± 7 years, 61% women), 273 (21.1%) healthcare workers used hydroxychloroquine prophylaxis as per Indian Council of Medical Research recommendations and 83/1294 (6.4%) tested positive after their duty. There was no significant difference in COVID-19 incidence between those on hydroxychloroquine prophylaxis and those not on it (5.9% vs 6.6%, χ2 = 0.177, p = 0.675; RR = 0.89, 95% CI – 0.53 to 1.52). There were no significant adverse effects to hydroxychloroquine usage.
Conclusion
This study demonstrated no benefit of hydroxychloroquine prophylaxis and provides quality evidence against its use in COVID-19 prevention.
KEYWORDS: Chemoprevention, COVID-19, drug-related side effects and adverse reactions, health personnel, hydroxychloroquine
1. Introduction
With the coronavirus disease 2019 (COVID-19) pandemic taking the country in its grip, India’s healthcare system came under a tremendous strain. In order to cater to this surge in patients, a very large healthcare workforce comprising of clinicians, nurses, laboratory, allied health, housekeeping personnel, and community health workers was deployed. Indeed, aside from the core departments of internal medicine, respiratory medicine, emergency medicine and critical care, it became necessary to avail the services of medical professionals from multiple other specialties in order to manage the barrage of COVID-19 infected patients [1].
The risk that the COVID-19 infection posed to the frontline health workers was evident from the very beginning of the pandemic [2]. Ensuring the safety of healthcare workers engaged in the management of COVID-19 patients was paramount in order for them to work selflessly and efficiently. The use of personal protective equipment (PPE) when treating COVID-19 patients had been the only proven measure for primary prevention until the arrival of vaccines [3]. Yet a number of drugs were initially claimed to be effective as chemoprophylactic agents against COVID-19 such as zinc supplements, vitamin D supplements, ivermectin, and hydroxychloroquine (HCQ) [4–6]. Considering the widespread availability, low cost and the established pharmacological profile of HCQ, it was reasonable to explore its role in the management of this health crisis. HCQ is a less toxic metabolite of the antimalarial chloroquine with a similar mechanism of action. It is thought to exert its antimicrobial properties by increasing the lysosomal pH, thereby inhibiting the fusion of the viral particle with the host cell. It has been used in the treatment and prevention of malaria. Its efficacy against COVID-19 virus was demonstrated in early in-vitro studies that provided the grounds to consider its clinical use in the prophylaxis against COVID-19 [7].
In March 2020, it received worldwide fame when the then president of the United States (U.S), Donald J Trump, endorsed its use in COVID-19 [8]. Soon afterward, the U.S Food and Drug Administration (FDA) and the Indian Council of Medical Research (ICMR) approved it for off-label use in COVID-19 infections. However, in May 2020 a reputed journal retracted the now infamous article that had dismissed the use of HCQ in COVID-19 [9]. This was shortly followed by the announcement of the preliminary results of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial that deemed HCQ to be of no benefit in the treatment of COVID-19. The World Health Organization (WHO) also cleared its stand on HCQ by suspending the HCQ arm of its solidarity trial [10].
Yet the ICMR had continued to advocate the use of HCQ prophylaxis among healthcare workers in India who were involved in the care of patients with COVID-19 infection based on in vitro experiments conducted at the city of Pune as well as a subsequent small observational study [11,12]. The WHO currently recommends against the use of HCQ prophylaxis for COVID-19 infection based on the summary of six trials [13–18]. However, the WHO indicated that the evidence against the use of pre-exposure HCQ prophylaxis was of moderate certainty due to ‘serious risk of bias.’ Hitherto, the studies that have explored the role of HCQ in the pre-exposure prophylaxis of COVID-19 among healthcare workers have had numerous shortcomings such as limited sample size, inadequate representation of healthcare workers in the overall study population, constraints in maintaining uniformity of exposure to the infection among the frontline healthcare workers and disparity in the methods used to confirm COVID-19 infections [12,15,16]. Although there has been a shift in the COVID-19 prevention strategy with the arrival of multiple vaccines, there are still many unanswered questions regarding the role of HCQ as a chemoprophylactic agent against COVID-19.
In early 2020, a large tertiary care COVID designated hospital had advocated ICMR recommendations on HCQ prophylaxis and this provided an opportunity to study its effectiveness in a controlled manner. The authors of the present study feel compelled to share the findings of the cohort study that had significant controls on the level and duration of exposure. It is believed that the findings of the present study would add robust evidence on the role of HCQ as a chemoprophylactic agent against COVID-19.
2. Participants and methods
2.1. Design
This study was planned as a prospective cohort design among healthcare workers in a 1300-bedded tertiary care medical teaching hospital. The study protocol was reviewed by the scientific committee and approved by the institutional ethics committee (XXXMC/EC/AP-12/06-2020).
2.2. Setting
In March 2020, when COVID-19 infections were beginning to rise in India, the hospital created a dedicated 200-bedded in-patient facility for treatment of patients with COVID-19 infection. The hospital started receiving patients from 20 June 2020 and subsequently the capacity was increased to a 500-bedded facility by July 2020. In preparation for the pandemic, the healthcare workers were trained for various COVID-19 related services and provisions were made for the access and availability of HCQ as a prophylactic drug as per ICMR guidelines. In addition, the institution launched a ‘Caring for the Caregivers’ surveillance system wherein a mechanism was created for daily monitoring of symptoms among healthcare workers using a web and mobile based app created specifically for this purpose. As part of the surveillance program, the healthcare workers underwent measurement of their forehead temperature and peripheral oxygen saturation on a daily basis. Using the surveillance system, they also reported the presence/absence of COVID-19 related symptoms, their previous day physical activity level, if they traveled to any containment zone and if they have been a primary contact of a known COVID-19 positive individual. In addition, they reported if they were on HCQ prophylaxis and, if yes, details such as date of initiation of the prophylactic HCQ, adherence to the prophylactic regime and self-reported adverse effects of HCQ.
2.3. Participants
The designated COVID-19 facility began receiving patients from 20 June 2020, and all healthcare workers who were to be deputed for in-patient COVID-19 care services were required to obtain medical clearance by the institutional screening committee prior to their duty. All healthcare workers above age of 55 years, and those with comorbidities such as uncontrolled diabetes mellitus, asthma, ischemic heart disease, chronic obstructive pulmonary disease, active rheumatoid arthritis, epilepsy and people living with HIV infection were exempted from being deputed for COVID in-patient services. From among the healthcare workers eligible for COVID duty, participants meeting the required criteria for this study were identified through mapping of the hospital’s COVID duty rosters with data captured through the surveillance system. Participants’ consent for using their de-identified data for education, training and research purpose was obtained as part of the surveillance system.
For the purpose of this study, physicians, nurses and housekeeping staff posted for in-patient COVID-19 care services between 20 June 2021 and 20 August 2021 were considered eligible for inclusion. In addition, they were also required to be posted once inside the COVID hospital, each one working in six-hour long daily shifts for seven consecutive days. Those who provided services in the COVID wards on any other work schedule such as on alternative days or on-call basis and the like were considered ineligible for inclusion in the study. In addition, those posted in non-inpatient COVID care services such the flu clinic, laboratory, morgue and those tested positive for COVID-19 infection prior to their in-patient duty were excluded.
All healthcare workers included in this study used the full complement of PPE viz N95 respirator, coverall, gloves, goggles and face shield during their working shifts. They were provided with accommodation within the hospital premises for the duration of their posting. Following their week-long posting, they were quarantined within a dedicated facility for one week.
2.4. Variables
COVID-19 Infection status among healthcare workers after their first posting in in-patient COVID services was the primary variable of interest. HCQ related adverse events reported by healthcare workers were the secondary variable.
2.5. Determination of COVID-19 infection status
On the fifth day of their institutional quarantine, all healthcare workers underwent mandatory testing for COVID-19 via Real Time Polymerase Chain Reaction (RT-PCR) on samples obtained from oropharyngeal and nasopharyngeal swabs. However, in case the healthcare workers developed symptoms of COVID-19 at any time during their posting or quarantine period, they were tested immediately in a similar manner. For those healthcare workers who initially tested negative after five days of quarantine but experienced symptoms of COVID-19 in the following week, a repeat RT-PCR test was performed. The RT-PCR test for COVID-19 was performed on all samples at the National Accreditation Board for Laboratories accredited laboratory attached to the designated COVID hospital. As the first step, the sample was tested for the presence of the open reading frame gene, and if this were to be detected then the final step to detect the envelop gene was run. Those who had both genes detected in their samples were deemed to have COVID-19 infection and were classified as COVID-19 positive while all others were identified as negative.
Adverse events to HCQ were primarily captured using self-reported declaration through the surveillance system. Data was also obtained from the outpatient clinic as and when healthcare workers reported with adverse events. Based on physician advice, 68 healthcare workers underwent an electrogardiogram (ECG) screening and this was included in the analysis
2.6. Bias
Efforts were made to control for known confounders. Though the surveillance system had over 2000 healthcare workers and over 400 of them were on HCQ, in an attempt to control for exposure, only those healthcare workers posted for inpatient COVID care duties during their first rotation were considered for inclusion. Variability in exposure risk such as between those posted in high aerosolization areas like intensive care units compared to those posted in general wards and potential variability in exposure between professional groups could not be controlled.
2.7. Study size
In April-May 2020, the prevailing positivity test rates in India within all healthcare workers at the beginning of the study was around 6%. For us to consider HCQ prophylaxis as effective, we anticipated a 4% lower positivity rate. For comparing proportions between two groups (RT-PCR positive rates of 2% vs 6% between HCQ and non-HCQ groups, respectively) and anticipating 20% of healthcare workers to be on HCQ prophylaxis, using an alpha of 0.05 and 80% power, the sample size derived was a minimum of 1284 participants.
2.8. Statistical methods
We used a chi-square test of independence to test the hypothesis that after their first in-patientCOVID duty, healthcare workers on HCQ prophylaxis would have significantly lower incidence of COVID-19 infection. Adverse events to HCQ prophylaxis were summarized as frequencies and proportions. Other demographic variables were summarized using appropriate descriptive statistics.
3. Results
The Caring for the Caregivers Surveillance system was launched on 3 May 2020 and on an on-going basis it had enrolled 2011 employees (319 consultants and residents, 1011 Nurses, 104 administrative staff, 360 housekeeping and support staff, and 217 employees from other categories such as engineers, technicians, allied health services) of the institution. The hospital started admitting patients from 20 June 2020 and 1294 unique employees who were posted for acute COVID in-patient services on a full-time basis and met the study inclusion criteria were considered eligible for inclusion in the analysis. Recruitments were stopped after 20 August 2020 as healthcare workers were starting to have their second round of duty in COVID wards and since the required sample size was met. The flow of participants through the study is summarized using a STROBE Flow diagram in Figure 1.
Figure 1.
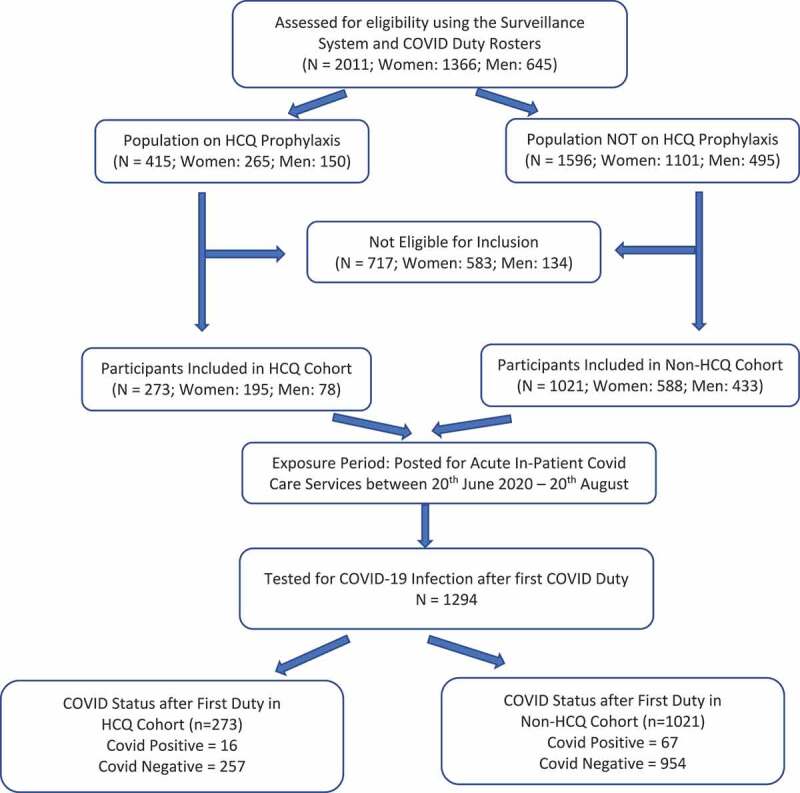
Flow diagram of participants through the study period
Among the 1294 healthcare workers (age: 31 ± 7 years, 61% women) included for this analysis, 261 were consultants and residents, 547 were nurses and 486 were housekeeping and support staff. Weekly HCQ prophylaxis in accordance with ICMR recommendations was followed by 273/1294 healthcare workers (26 consultants and residents, 200 nurses and 47 housekeeping and support staff). As reported in some of the previous studies, there was some hesitancy among healthcare workers in taking HCQ as a prophylactic agent. Only 20.6% of the healthcare workers had used HCQ as a pre-exposure prophylaxis despite the hospital notifying the ICMR recommendation. A total of 25 participants reasoned that they had not initiated the HCQ prophylaxis owing to the fear of developing side effects. Among the professional groups, nurses were observed to have greater inclination to use HCQ as prophylaxis. The distribution of HCQ prophylaxis among different category of healthcare workers is summarized in Table 1.
Table 1.
Distribution of HCQ prophylaxis among different categories of healthcare workers
| Professional Category | HCQ Prophylaxis (n = 273) |
Not on HCQ Prophylaxis (n = 1021) |
|---|---|---|
| Consultants and Residents (n = 261; 52% women) |
26 | 235 |
| Nurses (n = 547; 63% women) |
200 | 347 |
| Housekeeping and Support Staff (n = 486; 51% women) |
47 | 439 |
There was no significant difference in age (30.9 vs. 31.3 years, p = 0.096) between the HCQ and non-HCQ cohorts. In the HCQ group, 195 (71%) participants were women, while in the non-HCQ group, 588 (58%) participants were women. With respect to the duration of the HCQ prophylaxis prior to their posting inside the COVID hospital, it was found that 163 healthcare workers had used it for eight weeks or more, while the rest 110 had used it for less than eight weeks and no significant difference was found in incidence of COVID-19 infection between the two groups.
3.1. Primary outcome
Among the 1294 healthcare workers who underwent RT-PCR testing after their first acute COVID in-patient posting, 83 healthcare workers tested positive for COVID-19. This meant an overall test positivity rate of 6.4%. Among the 273 healthcare workers on HCQ prophylaxis, 16 tested positive for the presence of COVID-19 infection at the end of their posting. On the other hand, 67 healthcare workers of the remaining 1021 who were not on HCQ prophylaxis went on to contract COVID-19 infection. The association between HCQ prophylaxis and incidence of COVID-19 infection is summarized in Table 2.
Table 2.
Association between HCQ prophylaxis and incidence of COVID-19 infection
| COVID positive | COVID negative | Total | Chi Square test | |
|---|---|---|---|---|
| On HCQ Prophylaxis | 16 (5.86%) | 257 (94.13%) | 273 | X2 = 0.177 p = 0.675 RR = 0.89 95% CI 0.53–1.52 |
| Not on HCQ Prophylaxis | 67 (6.56%) | 954 (93.43%) | 1021 | |
| Total | 83 (6.41%) | 1211 (93.58%) | 1294 |
RR = Relative Risk
3.2. Adverse effects of HCQ
No major adverse effects were reported by majority of the participants on HCQ prophylaxis. During the routine screening of healthcare workers for adverse effects, 68 were recommended to undergo ECG, and among this, three healthcare workers were found to have prolonged corrected QT interval and were advised to stop using the HCQ prophylaxis. The commonest adverse effect as reported by the healthcare workers via a self-reported questionnaire is summarized in Table 3.
Table 3.
Profile of self-reported adverse effects to HCQ
| Adverse effects (N = 273) | Numbers (%) |
|---|---|
| Headache | 28 (10.4) |
| Nausea and vomiting | 12 (4.4) |
| Abdominal pain | 11 (4.1) |
| Myalgia | 7 (2.6) |
| Cough, Palpitations, Blurring of vision* | 6 (2.2) |
| Loose stools | 5 (1.8) |
| Fatigue, Itching* | 4 (1.4) |
| Dyspnea, Excess thirst, Vertigo, Menstrual abnormalities, Darkening of skin* | 3 (1.1) |
* Each of the symptoms were reported individually at the given frequencies
4. Discussion
Vaccine coverage is expanding fast across the globe and the role of pre-exposure chemoprophylactic agents is diminishing [19]. However, vaccine inequity and hesitancy are emerging as an important deterrent in controlling the spread of COVID-19 infection, and in this context, there have been calls for exploring alternative options that can be considered until universal coverage of COVID-19 vaccines is achieved [20,21]. Though multiple studies have been published on the role of HCQ as a chemoprophylactic agent for preventing COVID-19 infection and public health recommendations made against its use, they are based on studies with a high risk of bias. In this context, we believe that the present study adds quality evidence against the use of HCQ as a pre-exposure chemoprophylactic agent for preventing COVID-19 infection.
This prospective cohort study did not demonstrate any significant protective effect of pre-exposure HCQ prophylaxis on COVID-19 infection among healthcare workers. These findings strengthen the body of evidence on limited role of HCQ prophylaxis in preventing COVID-19 infection. Ever since the suspension of HCQ arm in the Solidarity Trial by the WHO in June 2020, several studies have been published on the role of HCQ either in preventing or treating COVID-19 [22]. Despite the multitude of studies that have been published on the role of HCQ in prevention and treatment of COVID-19, the evidence base on its effects has been of low quality.
A recent meta-analysis published in October 2021 indicated no clinical benefit of HCQ as pre- and post-exposure prophylaxis [23]. They had included 14 blinded, placebo-controlled trials of which four studied the effect of pre-exposure prophylaxis (1942 participants; 1271 on HCQ and 671 on placebo). Two among the four studies in the meta-analysis were cited from preprint repositories and had not undergone full peer review. The meta-analysis of the four studies found no decrease in the risk of COVID-19 infection (RR = 0.90, 95% CI: 0.46–1.77). Though the included studies were RCTs, the authors acknowledged that the included studies had a high risk of bias and the quality of evidence was deemed to be low.
In a large multi-centric study published in June 2021 from India, 12089 healthcare workers self-reported their socio-demography, comorbidities, chemoprophylaxis status, and COVID-19 status using a questionnaire [24]. The authors reported a dose–response relationship between the duration of HCQ prophylaxis and protection from COVID-19 infection. The benefits of HCQ prophylaxis were reported as proportional reduction in probability of COVID positivity in comparison to those not on prophylaxes. However, no odds ratio or other statistical inference was provided. In addition, their univariate analysis on the effect of HCQ prophylaxis on COVID-19 infection failed to demonstrate any benefit. Though, the study had a large sample size and data was collected across 44 hospitals in 17 states with varying levels of incidence, this study had a non-respondent rate of 52.3%, availability of COVID status among participants was limited to 22.5% of the respondents and the final analysis on the effect of HCQ was performed using data from 2727 participants. Despite the authors controlling for various confounders such as age, gender, comorbidities and site in their analysis; the degree of high risk COVID-19 exposure was not uniform and was not considered in the analysis of the effect of HCQ. The study design, high non-respondent rates and lack of control over exposure would significantly limit the strength of evidence of their findings.
Another preliminary meta-analysis of pre-exposure prophylaxis for COVID-19 among healthcare workers from India included 11 observational studies. The authors concluded that pre-exposure HCQ prophylaxis is safe and effective in preventing COVID-19 infection among healthcare workers. A review of included studies indicated many of them to be of low-quality evidence based on their study design, control over exposure risk and sample size. The authors acknowledged the limitations of the included studies and indicated a need for randomized controlled trials [21].
The interim guidelines published by the WHO in March 2021 dissuades the use of HCQ as a pre-exposure prophylactic agent to prevent COVID-19 infection. The evidence for this recommendation was considered to be of moderate certainty due to serious risk of bias in one of the trials [25].
In the present study, many confounding variables were controlled by means of population selection. Only eligible healthcare workers who were medically cleared for COVID care services in an in-patient setting of a designated COVID hospital were selected for inclusion. All participants were negative for COVID-19 prior to their duty and the exposure period was limited to one week of their COVID duty schedule. During their duty, all participants were provided institutional accommodation. All participants underwent institutional quarantine for five days after the conclusion of their week-long posting followed by mandatory RT-PCR testing for COVID-19 on the 6th day. Though there are possibilities of false negative results with RT-PCR testing, it is the recommended standard for COVID-19 infection confirmation. Rates of false negative results is generally considered to be low in symptomatic individuals and if tested between 5–7 days of exposure. In our cohort, all symptomatic participants returned a COVID-19 positive result on RT-PCR testing. All these steps ensured uniformity in the exposure of all healthcare workers participating in the study to COVID-19 and these factors permitted to study the impact of HCQ prophylaxis in a much-controlled manner compared to previous studies. In addition, an a priori sample size estimate was done and participants were recruited for the study to be adequately powered.
The infectivity rate among those who were on HCQ prophylaxis was not statistically different from those who were not on it (5.8 vs. 6.6%) which is in agreement with several other studies. Based on our findings, it can be reliably inferred that HCQ did not offer any protection to healthcare workers from COVID-19 beyond what was offered by the PPE. Although all healthcare workers who turned positive on testing were either asymptomatic or mild, they were all hospitalized owing to the prevailing hospital guidelines that aimed to prevent the spread of infection to their family members. None of them progressed to moderate or severe disease and there were no fatalities.
There were no major adverse effects among healthcare workers who used HCQ as per ICMR regime. These findings are also in line with other published studies from India [24]. Notwithstanding the favorable safety profile of HCQ, its use as a pre-exposure prophylactic drug for COVID-19 is not warranted.
Beyond HCQ, there have been several attempts to repurpose existing drugs for COVID-19 prevention and one such medication that received prominence was the anti-parasite drug ivermectin. A recent systematic review based on three studies indicated beneficial effects of ivermectin as a chemoprophylactic agent, but the certainty of evidence was low [26]. In addition, two of the three studies included in the review are from pre-print repositories and one of them had raised multiple concerns with regard to potential scientific misconduct and has subsequently been withdrawn from the pre-print repository [27]. Ivermectin is not currently recommended for prevention and treatment of COVID-19 infection.
It has been reported that in comparison to general community, healthcare workers are at an increased risk of COVID-19 infection [2]. Healthcare workers were one of the first groups to be vaccinated; however, breakthrough infections among them are becoming fairly common [28]. This highlights the need for adherence to basic safe practices including appropriate PPE usage. Until failproof discoveries against the spread of COVID-19 infection are made, the hunt for panacea remains alive.
5. Conclusion
This study demonstrated that HCQ is indeed ineffective in preventing COVID-19 infections and the pursuit for a suitable prophylactic agent still continues.
Acknowledgments
The authors would like to acknowledge the support of Ms. Farah Mistry, Department of Physiotherapy, and Mr. Lokesh Karigowda, Staff Nurse, MS Ramaiah Medical College Hospital, for the support in data collection.
Funding Statement
This study was not funded.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Concept and design: The study was conceived by N Shetty, A Rao and S Kumar Veluswamy. B Gunjiganur Shankarappa, L John and L Mathew contributed towards the design of the study.
Data collection: A Rao, S Kumar Veluswamy. L John, L Mathew, N Umesh and R Manjunatha Reddy contributed towards data collection.
Analysis: A Rao and S Kumar Veluswamy analysed the data.
Writing: A Rao and S Kumar Veluswamy together wrote the first draft and all authors critically reviewed the manuscript and contributed towards the writing.
Approval: All authors read and approved the final version of the manuscript for submission
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Bourgeault IL, Maier CB, Dieleman M, et al. The COVID-19 pandemic presents an opportunity to develop more sustainable health workforces. Hum Resour Health. 2020;18(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen LH, Drew DA, Graham MS, et al. Coronavirus pandemic epidemiology consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook TM. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic – a narrative review. Anaesthesia. 2020;75(7):920–927. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Kubota Y, Chernov M, et al. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med Hypotheses. 2020;144:109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutolo M, Paolino S, Smith V. Evidences for a protective role of vitamin D in COVID-19. RMD Open. 2020;6(3):e001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade BS, Rangel FS, Santos NO, et al. Repurposing approved drugs for guiding COVID-19 prophylaxis: a systematic review. Front Pharmacol. 2020;11:590598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niburski K, Niburski O. Impact of Trump’s promotion of unproven COVID-19 treatments and subsequent internet trends: observational study. J Med Internet Res. 2020;22(11):e20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehra MR, Ruschitzka F, Patel AN. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395(10240):1820. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper started the increased scrutiny of HCQ usage.
- 10.Singh H, Chauhan P, Kakkar AK. Hydroxychloroquine for the treatment and prophylaxis of COVID-19: the journey so far and the road ahead. Eur J Pharmacol. 2021;890:173717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indian Council of Medical Research . Revised advisory on the use of Hydroxychloroquine (HCQ) as prophylaxis for SARS-CoV-2 infection. [Internet] 2020. [updated 2020 May 22; cited 2021 May 22; cited 2021 May 22; cited 2021 May 22]. Available from: https://www.icmr.gov.in/pdf/covid/techdoc/V5_Revised_advisory_on_the_use_of_HCQ_SARS_CoV2_infection.pdf; •• This advisory by ICMR highlights the positiion taken by it with regard to HCQ usage.
- 12.Bhattacharya R, Chowdhury S, Nandi A, et al. Pre-exposure hydroxychloroquine prophylaxis for covid-19 in healthcare workers: a retrospective cohort. Int J Res Med Sci. 2020;9(1):89–96. [Google Scholar]
- 13.Barnabas RV, Brown ER, Bershteyn A, et al. Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 infection: a randomized trial. Ann Intern Med. 2021;174(3):344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grau-Pujol B, Camprubí D, Marti-Soler H, et al. Pre-exposure prophylaxis with hydroxychloroquine for COVID-19: initial results of a double-blind, placebo-controlled randomized clinical trial. Res Square. 2020. DOI: 10.21203/rs.3.rs-72132/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abella BS, Jolkovsky EL, Biney BT, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 2021;181(2):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajasingham R, Bangdiwala AS, Nicol MR, et al. Hydroxychloroquine as pre-exposure prophylaxis for coronavirus disease 2019 (COVID-19) in healthcare workers: a randomized trial. Clin Infect Dis. 2021;72(11):e835–e843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitjà O, Marc CM, Ubals M, et al. A cluster-randomized trial of hydroxychloroquine for prevention of Covid-19. N Engl J Med. 2021;384:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organisation . WHO Coronavirus (COVID-19) dashboard. [Internet] 2021. cited 2021 Oct 16]. Available from: https://covid19.who.int/
- 20.Burki T. Global COVID-19 vaccine inequity. Lancet Infect Dis. 2021;21(7):922–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stricker RB, Fesler MC. Hydroxychloroquine pre-exposure prophylaxis for COVID-19 in healthcare workers from india: a meta-analysis. J Infect Public Health. 2021;14(9):1161–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study found HCQ prophylaxis among healthcare workers to be effective but highlighted that the methodological quality of the included studies were low.
- 22.World Health Organisation . Coronavirus disease (COVID-19): solidarity Trial and hydroxychloroquine. [updated 2020 Jun 19; cited 2021 Oct 16]. Available from: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-hydroxychloroquine.
- 23.Martins-Filho PR, Ferreira LC, Heimfarth L, et al. Efficacy and safety of hydroxychloroquine as pre-and post-exposure prophylaxis and treatment of COVID-19: a systematic review and meta-analysis of blinded, placebo-controlled, randomized clinical trials. Lancet Reg Health Am. 2021; 2: 100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinesh B, Cs J, Kaur CP, et al. Hydroxychloroquine for SARS CoV2 prophylaxis in healthcare workers - a multicentric cohort study assessing effectiveness and safety. J Assoc Physicians India. 2021;69(6):17–23. [PubMed] [Google Scholar]
- 25.World Health Organization . Drugs to prevent COVID-19: a WHO living guideline. [updated 2021 Mar 19; cited 2021 Oct 16]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-prophylaxes-2021-1 [PubMed]
- 26.Cruciani M, Pati I, Masiello F, et al. Ivermectin for prophylaxis and treatment of COVID-19: a systematic review and meta-analysis. Diagnostics (Basel). 2021. Sep 8;11(9):1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reardon S. Flawed ivermectin preprint highlights challenges of COVID drug studies. Nature. 2021. Aug;596(7871):173–174. [DOI] [PubMed] [Google Scholar]
- 28.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]


