Abstract
Introduction:
This systematic review provides supporting evidence for the accompanying clinical practice guideline on the referral of adults with obstructive sleep apnea (OSA) for surgical consultation.
Methods:
The American Academy of Sleep Medicine commissioned a task force of experts in sleep medicine. A systematic review was conducted to identify studies that compared the use of upper airway sleep apnea surgery or bariatric surgery to no treatment as well as studies that reported on patient-important and physiologic outcomes pre- and postoperatively. Statistical analyses were performed to determine the clinical significance of using surgery to treat obstructive sleep apnea in adults. Finally, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) process was used to assess the evidence for making recommendations.
Results:
The literature search resulted in 274 studies that provided data suitable for statistical analyses. The analyses demonstrated that surgery as a rescue therapy results in a clinically significant reduction in excessive sleepiness, snoring, blood pressure (BP), apnea-hypopnea index (AHI), respiratory disturbance index (RDI), and oxygen desaturation index (ODI); an increase in lowest oxygen saturation (LSAT) and sleep quality; and an improvement in quality of life in adults with OSA who are intolerant or unaccepting of positive airway pressure (PAP) therapy. The analyses demonstrated that surgery as an adjunctive therapy results in a clinically significant reduction in optimal PAP pressure and improvement in PAP adherence in adults with OSA who are intolerant or unaccepting of PAP due to side effects associated with high pressure requirements. The analyses also demonstrated that surgery as an initial treatment results in a clinically significant reduction in AHI/RDI, sleepiness, snoring, BP, and ODI and an increase in LSAT in adults with OSA and major anatomical obstruction. Analysis of bariatric surgery data showed a clinically significant reduction in BP, AHI/RDI, sleepiness, snoring, optimal PAP level, BMI, and ODI and an increase in LSAT in adults with OSA and obesity. Analyses of very limited evidence suggested that upper airway surgery does not result in a clinically significant increase in risk of serious persistent adverse events and suggested that bariatric surgery may result in a clinically significant risk of iron malabsorption that may be managed with iron supplements. The task force provided a detailed summary of the evidence along with the quality of evidence, the balance of benefits and harms, patient values and preferences, and resource use considerations.
Citation:
Kent D, Stanley J, Aurora RN, et al. Referral of adults with obstructive sleep apnea for surgical consultation: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17(12):2507–2531.
Keywords: upper airway surgery, hypoglossal nerve stimulation, bariatric surgery, maxillomandibular advancement, obstructive sleep apnea
INTRODUCTION
This systematic review provides supporting evidence for the accompanying clinical practice guideline1 on the referral of adults with obstructive sleep apnea (OSA) for surgical consultation to treat OSA in adults and update the evidence review conducted for the previously published American Academy of Sleep Medicine (AASM) guideline.2 Surgical consultation for upper airway sleep apnea surgery or bariatric surgery was considered. The 2010 systematic review3 compared the efficacy of different surgical procedures to inform recommendations for specific surgical procedures. This review was designed to determine if surgical therapies for OSA are effective for improving outcomes of interest when analyzed collectively, which will inform recommendations for when health care providers treating OSA (subsequently referred to as “sleep clinicians” in this document) should discuss referral for upper airway or bariatric surgery evaluation with adults with OSA. For this document, a “sleep surgeon” refers to an otolaryngologist or oral and maxillofacial surgeon with training and expertise in upper airway surgery who has an appropriate understanding of sleep medicine and modern surgical techniques for the treatment of OSA.
BACKGROUND
OSA is a common chronic disease characterized by repetitive upper airway collapse, with resultant oxyhemoglobin desaturations and arousals. The prevalence of OSA is high and is expected to continue to rise in tandem with the obesity epidemic. Based on data from the Wisconsin Sleep Cohort, it is estimated that 34% of men and 17% of women aged 30–70 years have at least mild OSA, while 13% of men and 6% of women in this age range have moderate-to-severe OSA, with prevalence increasing with age.4 The adverse consequences of untreated OSA can be seen at many levels. Untreated OSA is associated with cardiometabolic consequences such as hypertension, atrial fibrillation, heart failure, ischemic heart disease, and type 2 diabetes, although the causal nature of these associations has yet to be conclusively established.4,5 Untreated OSA has a negative impact on patient-centered outcomes, with reduced quality of life (QOL) observed on both generic and disease-specific health questionnaires. The reduction in QOL is mediated primarily by excessive daytime sleepiness,6 which is also implicated as the cause of workplace absenteeism and decreased productivity7 and motor vehicle crashes8 seen in individuals with OSA.
Positive airway pressure (PAP) has remained first-line therapy for all severities of symptomatic OSA since its initial description as a treatment for OSA in 1981.9 Extensive evidence from randomized clinical trials has demonstrated a beneficial effect of PAP therapy on sleepiness, QOL, and blood pressure (BP);10,11 however, adherence to PAP therapy is difficult for many patients, with an overall reported nonadherence rate ranging from 20%–40%.12–15 Evidence suggests that patients with moderate to severe OSA and only partial nightly adherence to PAP therapy may continue to experience moderate to severe disease burden, even when meeting Centers for Medicare & Medicaid Services (CMS) requirements for adherence.16 Other therapeutic medical options for OSA include lifestyle modifications, such as exercise, weight loss, and avoidance of agents that can affect upper airway patency, like alcohol. Mandibular repositioning appliances17 and positional therapy18 are also effective treatment modalities in appropriate patient subsets. For many patients with OSA, a more definitive treatment that does not involve ongoing external equipment use may be preferable. Surgical modifications of the upper airway have been a part of the armamentarium for OSA treatment since the 1970s. Initially, tracheostomy was the sole surgical option available, although acceptance was limited due to associated social and lifestyle challenges. In 1981, Fujita introduced uvulopalatopharyngoplasty (UPPP) in the United States, the first specialized surgical procedure specifically designed to treat OSA.19
By 1996, several additional surgical procedures for treatment of OSA were available, and the initial systematic review and practice parameter on surgical modifications of the upper airway for adults with OSA was published by the American Sleep Disorders Association, now known as the American Academy of Sleep Medicine (AASM). The AASM updated the original systematic review3 and original practice parameters2 in 2010. The review focused on individual, classic surgical interventions, and their available data such as UPPP, modified UPPP, other pharyngeal procedures, laser-assisted uvulopalatoplasty, upper airway radio frequency treatment, soft palatal implants, multilevel simultaneous surgeries, and multilevel phased surgeries. Advanced pharyngoplasty approaches were not considered in the 2010 systematic review and practice parameters due to the paucity of published evidence. The primary outcome was the apnea-hypopnea index (AHI), as many study investigators defined surgical success as a 50% reduction in AHI to a level less than 20 events/h (ie, definition of mild OSA prior to 1999). While these previous systematic reviews and guidelines recognized the evolution in surgical techniques, the role of the surgeon in identifying appropriate interventions and providing in-depth patient counseling in their area of expertise was not explicitly addressed. The task force additionally sought to evaluate patient-centered outcomes more formally than had been done in prior systematic reviews. Looking beyond upper airway surgery, the amassing evidence surrounding the impact of weight loss surgery on OSA also necessitated review of bariatric surgery as a potential OSA treatment option.
The AASM recognized that current management guidelines do not address the critical question of when to consider discussing surgical treatment options with adults with OSA. The AASM chose to focus the current systematic review and accompanying recommendations on when to discuss referral to a sleep or bariatric surgeon with adults with OSA rather than evaluating specific surgical procedures. The purpose of the current systematic review is to inform clinical care by considering specific, commonly encountered clinical scenarios in which discussion of a referral for sleep or bariatric surgery consultation may provide patient benefit, while acknowledging that the training and depth of surgical knowledge needed for appropriate anatomic evaluation and patient counseling are outside the practice boundaries of most sleep medicine providers.
METHODS
Expert task force
The AASM commissioned a task force (TF) comprising both board-certified sleep medicine specialists and experts with proficiency in the use of surgery in adults with OSA to develop this systematic review. The TF was required to disclose all potential conflicts of interest (COI) per the AASM’s COI policy prior to being appointed to the TF, and throughout the research and writing of this paper. In accordance with the AASM’s COI policy, TF members with a Level 1 conflict were not allowed to participate. Members of the TF with a Level 2 conflict were required to recuse themselves from any related discussion or writing responsibilities. All relevant COI are listed in the Disclosures section.
PICO questions
PICO (Patient, Intervention, Comparison, and Outcomes) questions were developed based on a review of the existing AASM practice parameters on the use of surgery and a review of systematic reviews, meta-analyses, and guidelines published since 2010. The AASM Board of Directors (BOD) approved the final list of PICO questions presented in Table 1 before the literature search was performed. To develop the PICO questions, the TF identified patient populations that could benefit from surgery as well as a list of patient-oriented, clinically relevant outcomes to determine if, and when, referral of adults with OSA for surgical consultation should be discussed by the sleep clinician. The TF rated the relative importance of each outcome to determine which outcomes were critical vs important for decision-making. A summary of these outcomes by PICO is presented in Table 2.
Table 1.
PICO questions.
| 1 | Population: Adult patients with OSA who are intolerant or unaccepting of PAP therapy |
| Intervention: Upper airway surgery as a salvage treatment | |
| Comparison: No surgery | |
| Outcomes: Excessive sleepiness, snoring, sleep-related quality of life (QOL), sleep quality, motor vehicle accident (MVA) risk, AHI/RDI, oxygen desaturation index (ODI), lowest oxygen saturation (LSAT), PAP adherence/acceptance, perioperative death, permanent dysphagia | |
| 2 | Population: Adult patients with OSA and obesity who are intolerant or unaccepting of PAP therapy |
| Intervention: Bariatric surgery | |
| Comparison: No bariatric surgery or best medical care | |
| Outcomes: Excessive sleepiness, snoring, sleep-related QOL, sleep quality, MVA risk, AHI/RDI, PAP adherence/acceptance, optimal PAP level, BP, ODI, LSAT, perioperative death, permanent dysphagia, body mass index (BMI) | |
| 3 | Population: Adult patients with OSA who have persistent suboptimal PAP adherence due to pressure-related side effects |
| Intervention: Upper airway surgery as an adjunctive treatment to PAP | |
| Comparison: No adjunctive surgery | |
| Outcomes: Excessive sleepiness, snoring, sleep-related QOL, sleep quality, PAP adherence/acceptance, optimal PAP level, perioperative death, permanent dysphagia | |
| 4 | Population: Adult patients with OSA and tonsillar hypertrophy and/or maxillomandibular abnormalities |
| Intervention: Upper airway surgery as an initial treatment | |
| Comparison: No surgery | |
| Outcomes: Excessive sleepiness, snoring, sleep-related QOL, sleep quality, MVA risk, blood pressure (BP), AHI/RDI, ODI, LSAT, perioperative death, permanent dysphagia |
Table 2.
Outcomes by PICO question.
| Outcomes | PICO Question | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Excessive sleepiness | ✓ | ✓ | ✓ | ✓ |
| Adherence to PAP therapy | – | – | ✓ | – |
| Optimal PAP level | – | ✓* | ✓ | – |
| Quality of life | ✓ | ✓ | ✓ | ✓ |
| Sleep quality | ✓ | ✓ | ✓ | ✓ |
| AHI/RDI | ✓ | ✓ | ✓* | ✓ |
| Lowest oxygen saturation | ✓* | ✓* | ✓* | ✓ |
| Oxygen desaturation index | ✓* | ✓* | – | ✓ |
| Snoring | ✓ | ✓* | ✓ | ✓ |
| Blood pressure | ✓ | ✓ | – | ✓* |
| Perioperative death | ✓ | ✓ | – | ✓ |
| Permanent dysphagia | ✓ | – | – | ✓ |
| BMI | – | ✓* | – | – |
| Motor vehicle accidents | – | ✓* | – | ✓* |
*Outcomes considered important but not critical for decision-making.
The TF set a clinical significance threshold (CST) for each outcome to determine whether the mean changes in the outcomes assessed were clinically significant based on their clinical expertise, other AASM guidelines, and available literature. The CST was defined as the minimum level of improvement in the outcome of interest that would be considered clinically important to clinicians and patients. A summary of the CSTs for the clinical outcome measures is presented in Table 3. CSTs were determined based on a TF literature review of commonly used thresholds. When no clearly established threshold values could be determined, the TF used their clinical judgment and experience to establish a CST based on consensus.
Table 3.
Summary of clinical significance thresholds for outcome measures.
| Outcome Measure | Clinical Significance Threshold*,† |
|---|---|
| AHI/RDI | –10% |
| Adherence to PAP therapy | +0.5 h/night; +10% patient use > 4 h/night10,20,21 |
| Excessive sleepiness | — |
| ESS | –2 points22–24 |
| Quality of life | — |
| FOSQ | +1 point10,21 |
| SAQLI | +1 point10,21 |
| SF-36 | — |
| Physical Component Summary | +3 points25 |
| Mental Component Summary | +3 points25 |
| Vitality Summary | +12.5 points26 |
| Sleep quality | — |
| PSQI | –3 points27 |
| Blood pressure | — |
| SBP | –2 mm Hg28,29 |
| DBP | –1 mm Hg28,29 |
| Snoring | — |
| VAS | –25% |
| Frequency | –10% |
| Lowest oxygen saturation | –5% |
| Oxygen desaturation index | –5 events/h |
| Perioperative death | Any reduction |
| Permanent dysphagia | — |
| Risk difference | +5% |
| MD Anderson score | +10 points30 |
| Obesity | — |
| BMI | –2 kg/m2 |
| Optimal PAP level | –1 cm H2O |
| PAP adherence | +0.5 h/night10,20,21 |
| PAP acceptance | +10% patients used |
| Motor vehicle crashes | Risk ratio of 0.9 (–10%)31–33 |
*References used to inform task force consensus. †The clinical significance thresholds are for comparison of pre- vs posttreatment effects as well as between surgery and control. AHI = apnea-hypopnea index, BMI = body mass index, DBP = diastolic blood pressure, ESS = Epworth Sleepiness Scale, FOSQ = Functional Outcome of Sleep Questionnaire, PSQI = Pittsburgh Sleep Quality Index, RDI = respiratory disturbance index, SAQLI = Calgary Sleep Apnea Quality of Life Index, SBP = systolic blood pressure; SF-36 = Short Form-36-item, VAS = visual analog scale. PAP – positive airway pressure.
Literature searches, evidence review, and data extraction
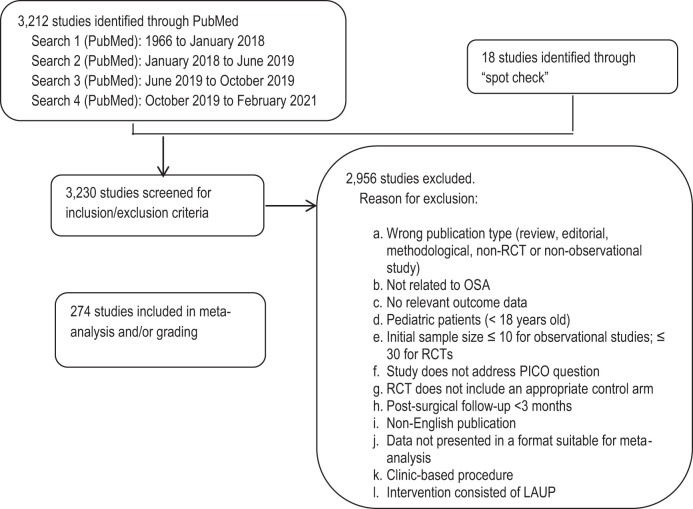
The TF performed an extensive review of the scientific literature to retrieve articles that addressed the PICO questions. Separate literature searches were performed by the TF for each PICO question using the PubMed database (see Figure 1). The key terms, search limits, and inclusion/exclusion criteria specified by the TF are detailed in the supplemental material.
Figure 1. Evidence base flow diagram.
The initial literature search in PubMed was performed in January 2018. In June 2019, the TF performed a second literature search specifically targeting the use of hypoglossal nerve stimulation to treat adults with OSA. A third search was performed in October 2019 to update the evidence during completion of the draft. A fourth search was performed in February 2021 to update the evidence prior to publication. These searches identified a total of 3,212 articles. Lastly, the TF reviewed previously published guidelines, systematic reviews, and meta-analyses to spot-check for references that may have been missed during the prior searches. The TF identified 18 additional articles for a total of 3,230 articles that were screened for inclusion/exclusion in the guideline.
The TF set inclusion and exclusion criteria, which are presented in the supplemental material and summarized in Figure 1. All studies were reviewed based on inclusion/exclusion criteria by 2 TF members. Any discrepancies between the reviewers were discussed and resolved by the 2 reviewers or a third TF member. A total of 274 studies were determined to be suitable for meta-analysis and/or grading.
Meta-analysis
Meta-analysis was performed on outcomes of interest, when possible, for each PICO question. Comparisons of surgery to no treatment and/or assessment of efficacy before and after surgery to treat OSA in adult patients were performed. For the purposes of this review, meta-analyses were only performed on operating room–based surgical procedures. These procedures included tonsillectomy, adenoidectomy, uvulopalatopharyngoplasty (UPPP), modified UPPP, maxillomandibular advancement (MMA), anterior palatoplasty, rhinoplasty, z-palatoplasty, z-palatopharyngoplasty, expansion sphincter pharyngoplasty, transoral robotic surgery, tongue base reduction, tongue base suspension, hyoid myotomy, hyoid suspension, lingual suspension, lingualplasty, hyoidthyroidpexia, genioglossal advancement, bimaxillary osteotomy, glossectomy, pharyngoplasty, endoscopic sinus surgery, septal surgery, septorhinoplasty, turbinate surgery, nasal surgery, oropharyngeal surgery, velopharyngeal surgery, multilevel surgery, bilateral endoscopic total ethmoidectomy, bilateral endoscopic middle meatal antrostomy, hypoglossal nerve stimulation, gastric bypass, gastric banding, and sleeve gastrectomy. Clinic-based procedures were excluded from meta-analysis.
Meta-analysis was performed using Review Manager 5.3 software (developed by The Cochrane Collaboration) by pooling data across studies for each outcome measure. Posttreatment data were used for meta-analysis of RCTs, except where change values were reported. Pre- and postsurgical treatment data were used for meta-analyses of observational studies. The pooled results for each continuous outcome measure were expressed as the mean difference between the intervention and control for RCTs or presurgery vs postsurgery for observational studies. The pooled results for dichotomous outcome measures were expressed as the odds ratio or risk difference between the intervention and comparator or presurgery vs postsurgery. All analyses were performed using a random-effects model with results displayed as a forest plot. Interpretation of clinical significance for the outcomes of interest was conducted by comparing the mean difference in effect of each treatment approach to the CST (see Table 3). Meta-analyses performed for PICOs 1–3 included any operating room–based procedure. Meta-analyses performed for PICO 4 included 2 subgroups consisting of craniofacial and oropharyngeal surgical procedures. This analysis was performed to determine if specific subgroups (ie, patients with craniofacial abnormalities vs tonsillar hypertrophy) would respond more favorably to surgery as an initial therapy. Studies of participants with prior CPAP use were not excluded from analysis for PICO 4 since prior CPAP exposure was not expected to reasonably affect surgical outcomes and was dependent on the presence of specific anatomic abnormalities. Therefore, studies included for analysis for PICO 1 could also be included for PICO 4.
GRADE assessment for developing recommendations
The assessment of evidence quality was performed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) process.34,35 The TF assessed the following 4 components to determine the direction and strength of a recommendation: quality of evidence, balance of beneficial and harmful effects, patient values and preferences, and resource use, as described below.
Quality of evidence—Based on an assessment of the overall risk of bias (randomization, blinding, allocation concealment, selective reporting), imprecision (95% confidence interval crosses the CST and/or sample size < 100 participants), inconsistency (I2 ≥ 50%), indirectness (study population vs target patient population), and risk of publication bias, the TF determined their overall confidence that the estimated effect found in the body of evidence was representative of the true treatment effect that typical adult patients with OSA would see. The quality of the evidence was based on outcomes that the TF deemed critical for decision-making; important outcomes are not considered when determining the overall quality of evidence. The TF was not able to identify studies that evaluated the benefit of a sleep clinician having a discussion vs no discussion about referral for surgical consultation. Therefore, the TF evaluated the efficacy of surgery to address the PICO questions with consideration for the indirectness of the evidence.
Benefits vs harms—Based on the meta-analysis of harmful outcomes (if data were available), analysis of any harms/side effects reported within the accepted literature and the clinical expertise of the TF, the TF determined if the beneficial outcomes of the intervention outweighed any harmful side effects.
Patient values and preferences—Based on the clinical expertise of the TF members and any data published on the topic relevant to patient preferences, the TF determined if patient values and preferences would be generally consistent across most patients, and if patients would use the intervention based on the relative harms and benefits identified.
Resource use—Based on the clinical expertise of the TF members, the TF judged resource use to be important for determining whether to recommend the use of surgery for the treatment of adults with OSA.
A summary of each GRADE domain is provided after the detailed evidence review.
Public comment and final approval
A draft of the guideline and systematic review was made available for public comment for a 4-week period on the AASM website. The TF took into consideration all the comments received and made decisions about whether to revise the draft based on the comments. The revised guideline and systematic review were submitted to the AASM BOD for subsequent approval. This review reflects the state of knowledge at the time of publication and will be reviewed and updated as new information becomes available.
THE USE OF SURGICAL INTERVENTION
The aims of the current literature reviews and data analyses were focused on addressing 4 questions pertaining to the use of surgery to treat OSA in adults. Below are detailed summaries of the evidence identified in the literature searches and the statistical analyses performed by the TF. Each evidence summary is accompanied by a discussion of the quality of evidence, balance of benefits and harms, patient values and preferences, and resource use considerations that contributed to the development of the recommendations provided in the accompanying clinical practice guideline.
Surgical treatment of patients who are intolerant or unaccepting of PAP
A total of 4 RCTs and 239 observational studies36–279 investigated the use of surgery as rescue therapy for participants who were intolerant or unaccepting of PAP to improve 1 or more of the following outcomes: excessive sleepiness, quality of life (QOL), sleep quality, snoring, blood pressure (BP), perioperative death, permanent dysphagia, apnea-hypopnea index (AHI), respiratory disturbance index (RDI), lowest oxygen saturation (LSAT), and oxygen desaturation index (ODI). Participants included in the studies had a BMI <40 kg/m2. Participants in the RCTs had moderate to severe OSA and received UPPP with or without tonsillectomy. Participants in the control group originally received no treatment but were eventually treated with the same procedure(s). Participants in the observational studies represented a broad population of adults undergoing a wide variety of surgical interventions for OSA including palatal modification, tongue base resection, multilevel pharyngeal airway surgery, nasal surgery, maxillomandibular advancement, and hypoglossal nerve stimulation. Most observational studies were retrospective cohort studies with reassessment of participants at approximately 6 months postoperatively, though some followed participants out to about 1 year. A large range of sleep apnea severity was represented with most participants having moderate to severe OSA. Participants were primarily middle-aged or older adults, and most cohorts were composed of predominantly men. Participants tended to be overweight or mildly obese. A variety of upper airway surgical procedures including palatal modification, base-of-tongue reduction, skeletal modification, nasal surgeries, multilevel surgeries, tracheostomy, and hypoglossal nerve stimulation were performed in an operating room setting. All participants were evaluated for improvement in outcomes after 3 months and up to 1 year after surgery except for those receiving an upper airway stimulator. For this procedure shorter follow-up periods were permitted for inclusion in the meta-analyses. Meta-analyses were performed to assess the efficacy of surgery as a rescue therapy for adults with OSA. The meta-analyses are provided in Figure S1 (1.2MB, pdf) through Figure S34 (1.2MB, pdf) in the supplemental material. A summary of findings is provided in Table S1 (1.2MB, pdf) in the supplemental material. A summary of the evidence for each outcome is provided below.
Critical outcomes
The following outcomes were determined by the TF to be critical for evaluating the efficacy of surgery as a rescue therapy: excessive sleepiness, QOL, sleep quality, snoring, BP, AHI/RDI, perioperative death, and permanent dysphagia. Meta-analyses for AHI/RDI included all definitions as reported in the studies. None of the studies identified in our literature review reported data for perioperative death.
Excessive sleepiness
The efficacy of rescue surgery to reduce excessive sleepiness was evaluated using a meta-analysis of 3 RCTs.37–39 Most of the participants were male, aged 18–70 years, with moderate to severe OSA and BMI < 36 kg/m2, who underwent either palatal modification surgery36,39 or multilevel upper airway surgery.38 The duration of patient follow-up after surgery ranged from 3–15 months. Participants in the control group originally received ongoing medical management or no treatment but were eventually treated with the same procedure(s). The meta-analysis demonstrated a clinically significant reduction in excessive sleepiness of –5.6 points (95% CI, –7.3 to –4.0 points) as measured by the ESS (see Figure S1 (1.2MB, pdf) ). The quality of evidence was high.
The efficacy of rescue surgery to reduce excessive sleepiness was also evaluated using a meta-analysis of 145 observational studies.39–41, 43–48,50–53,55,57–59,62,64,68–71,73–75,77,79,80,87–92,94–97,99–101,104,106,109,111,114,116,117,120,122,124–127,129,131–133, 138,139,143,145,146,150–152,154–159,162,168,170,171,174–184,186–188,190,196–199,201–206,209–213,215,219,220,222,225,228–230,232,234–239,241,242,246–248,252–259,261,264,265,270–273,278,280 Participants represented a broad population of PAP-intolerant adults, primarily middle-aged or older adults, of predominantly male sex with moderate to severe OSA, who underwent a wide variety of surgical interventions as listed above. The meta-analysis demonstrated a clinically significant reduction in excessive sleepiness of –5.8 points (95% CI, –6.3 to –5.4 points) as measured by the ESS (see Figure S2 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
QOL
The efficacy of rescue surgery to improve sleep-related QOL was also evaluated from an analysis of 1 RCT38 that reported on the Functional Outcome of Sleep Questionnaire (FOSQ). The participants were primarily middle-aged, of men with moderate to severe OSA who underwent multilevel upper airway surgery or medical management. The analysis demonstrated a clinically significant improvement in sleep quality of 3.5 points (95% CI, 2.6–4.4) as measured by the FOSQ (see Figure S3 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size.
The efficacy of rescue surgery to improve sleep-related QOL was also evaluated using a meta-analysis of 11 observational studies67,88,89,114,140,185,237,239,248,253,265 that reported on the Functional Outcome of Sleep Questionnaire (FOSQ). Most of the participants were older men with moderate to severe OSA who refused or were intolerant to PAP therapy and underwent a variety of surgical interventions, the majority of which consisted of hypoglossal nerve stimulation (HNS). The meta-analysis demonstrated a clinically significant improvement in sleep related QOL of 3.5 points (95% CI, 2.9–4.0 points) with rescue surgery as measured by the FOSQ (see Figure S4 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
The efficacy of rescue therapy to improve sleep apnea–related QOL was evaluated based on a meta-analysis of 3 observational studies88,104,248 as measured by the Sleep Apnea-Related QOL Index (SAQLI). The participants, who were mostly male, aged 21 to 73 years, with moderate to severe OSA and intolerant to CPAP,88,104 underwent HNS and were followed for 6 months. Meta-analysis demonstrated an improvement in sleep apnea–related QOL as measured by the SAQLI that was not clinically significant (see Figure S5 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size and a wide 95% confidence interval that crossed the clinical significance threshold (CST).
The efficacy of rescue therapy to improve general QOL was evaluated based on an analysis of 1 RCT37 that reported on the Short Form 36-item (SF-36) component summary scores. The participants in the RCT37 that reported on the SF-36 component summary scores included older men who were CPAP-intolerant with moderate to severe OSA and significant daytime sleepiness undergoing palatal surgery or observation. Participants were followed for approximately 7 months. The efficacy of rescue therapy to improve general QOL was also evaluated based on a meta-analysis of 2 observational studies155,248 that reported on the SF-36 component summary scores. The participants included mostly men of varying age with moderate to severe OSA who underwent palatal modification surgery and were followed for up to 2 years after surgery.
An analysis of 1 RCT37 demonstrated an improvement in general QOL that was not clinically significant as measured by the SF-36 physical component score (see Figure S6 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
A meta-analysis of 2 observational studies155,248 demonstrated a clinically significant improvement in general QOL of 6.3 points (95% CI, –0.4 to 13.0 points) with rescue therapy as measured by the SF-36 physical component summary score (see Figure S7 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
An analysis of 1 RCT37 demonstrated a clinically significant improvement in general QOL of 5.4 points (95% CI, 0.1–10.7 points) with rescue therapy as measured by the SF-36 mental component score (see Figure S8 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
A meta-analysis of 2 observational studies155,248 demonstrated a clinically significant improvement in general QOL of 9.5 points (95% CI, –2.0 to 20.9 points) with rescue therapy as measured by the SF-36 mental component score (see Figure S9 (1.2MB, pdf) . The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
An analysis of 1 RCT37 demonstrated a clinically significant decrease in general QOL of –21.1 points (95% CI, –32.7 to –9.5 points) with rescue therapy as measured by the SF-36 vitality score (see Figure S10 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
An analysis of 2 observational studies155,248 demonstrated a clinically significant improvement in general QOL of 14.4 points (95% CI, 9.0–19.8 points) with rescue therapy as measured by the SF-36 vitality score (see Figure S11 (1.2MB, pdf) . The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
Sleep quality
The efficacy of rescue therapy to improve sleep quality was evaluated based on a meta-analysis of 4 observational studies88,142,210,269 that reported on the Pittsburgh Sleep Quality Index (PSQI). The participants were mostly male and aged 19–66 years with mild to moderate OSA who underwent either oropharyngeal surgery,143,210 nasal surgery,269 or HNS88 and were followed from 3–39 months. The meta-analysis demonstrated an improvement that was not clinically significant (see Figure S12 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
Snoring
The efficacy of rescue surgery to improve snoring was evaluated using an analysis of 1 RCT39 that reported on snoring as measured on a 1–10 visual analog scale (VAS). Most of the participants were male and aged 18–65 years with a baseline BMI < 35 kg/m2 and moderate to severe OSA. All patients were CPAP intolerant and had an oropharyngeal obstruction. All patients had follow-up at 3 months after surgery. The analysis demonstrated a clinically significant decrease in snoring of –3.7 points (95% CI, –5.3 to –2.1 points) with rescue surgery as measured on a 1–10 VAS (see Figure S13 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
The efficacy of rescue surgery to improve snoring was also evaluated using a meta-analysis of 36 observational studies40,43,44,47,59,73,75,90,91,93,101–103,109,120,125,143,154,159,171, 174–177,181,203–206,222,247,264,265,271,272,274 that reported on snoring as measured on a 1–10 VAS in adults with OSA. Most of the participants were male, aged 18–69 years, with a baseline BMI < 33 kg/m2. While several of the studies included participants with mild and moderate OSA, most of the participants had severe OSA. The duration of patient follow-up after surgery ranged from 3–62 months. The meta-analysis demonstrated a clinically significant reduction in snoring of –5.2 points (95% CI, –5.9 to –4.6 points) with rescue surgery as measured by the 1–10 VAS in adults with OSA (see Figure S14 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
Blood pressure
The efficacy of rescue surgery to reduce BP was evaluated using an analysis of 1 RCT38 and a meta-analysis of 10 observational studies.86,124,144,151,152,190,206,212,219,262 The RCT38 included mostly middle-aged participants with moderate to severe OSA who had failed conventional treatment and were allocated to multilevel upper airway surgery or medical management and followed for 6 months. The observational studies included retrospective and prospective cohort and case-control designs. Most of the participants were male, aged 18–69 years, with a baseline BMI < 35 kg/m2 and moderate to severe OSA. The duration of patient follow-up after surgery ranged from 3–12 months.
The efficacy of rescue surgery to reduce systolic blood pressure (SBP) was evaluated from an analysis of 1 RCT.38 The analysis demonstrated a reduction in SBP that was not clinically significant (see Figure S15 (1.2MB, pdf) ). The quality of evidence was low due to serious imprecision associated with a wide 95% confidence interval that crossed both sides of the CST.
The efficacy of rescue surgery to reduce SBP was also evaluated using a meta-analysis of 10 observational studies.86,124,144,151,152,190,206,212,219,262 The meta-analysis demonstrated a clinically significant reduction in SBP of –6.3 mm Hg (95% CI, –11.6 to –0.9 mm Hg) with rescue therapy in adult patients with OSA (see Figure S16 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a wide 95% confidence interval that crossed the CST.
The efficacy of rescue surgery to reduce diastolic blood pressure (DBP) was evaluated from an analysis of 1 RCT.38 The analysis demonstrated a reduction in DBP that was not clinically significant (see Figure S17 (1.2MB, pdf) ). The quality of evidence was low due to serious imprecision associated with a wide 95% confidence interval that crossed both sides of the CST.
The efficacy of rescue surgery to reduce DBP was also evaluated using a meta-analysis of 9 observational studies.86,124,144,151, 152,190,206,212,262 The meta-analysis demonstrated a clinically significant reduction in DBP of –2.7 mm Hg (95% CI, –7.9 to 2.5 mm Hg) with rescue therapy in adults with OSA (see Figure S18 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
AHI/RDI
The AHI and RDI are commonly reported as measures of OSA severity. The efficacy of rescue surgery to reduce the AHI was evaluated using a meta-analysis of 3 RCTs.36,38,39 Most of the participants were male, aged 18–70 years, with moderate to severe OSA and BMI < 36 kg/m2 who underwent either palatal modification surgery36,39 or multilevel upper airway surgery.38 The duration of patient follow-up after surgery ranged from 3–15 months. The meta-analysis demonstrated a clinically significant reduction in OSA severity as measured by the AHI of –18.4 events/h (95% CI, –26.4 to –10.5 events/h) with rescue surgery (see Figure S19 (1.2MB, pdf) ). The quality of evidence was high.
The efficacy of rescue surgery to reduce the AHI was evaluated using a meta-analysis of 194 observational studies.39,40,42–53, 55–66,68–81,84,86–99,102–111,113–117,119–124,126,128–131,133–142,144–146,149–151,154,156–158,162,164,168–173,175–178, 180–183,185,187–192,195–201,203,205,207,209–213,215,218–220,222–230,232,233,235–243,245–247,249–261,263,264,266–278,281 Most of the participants were male, aged 19–78 years, with a baseline BMI < 42–kg/m2. The duration of patient follow-up after surgery ranged from 3–60 months. While several of the studies included participants with mild OSA, most of the participants had moderate to severe OSA. The meta-analysis demonstrated a clinically significant reduction in the AHI of –24.0 events/h (95% CI, –25.6 to –22.4 events/h), representing a 58% reduction with rescue surgery (see Figure S20 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and large effect size.
The efficacy of rescue surgery to reduce the RDI was evaluated using a meta-analysis of 2 RCTs.36,39 Most of the participants were male, aged 18–70 years, with moderate to severe OSA and BMI < 36 kg/m2 who underwent palatal modification surgery. The duration of patient follow-up after surgery ranged from 3–15 months. While several of the studies included participants with moderate OSA, most of the participants had severe OSA. The meta-analysis demonstrated a clinically significant reduction in the RDI of –16.4 events/h (95% CI, –33.3 to 0.6 events/h) with rescue surgery (see Figure S21 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
The efficacy of rescue surgery to reduce the RDI was evaluated using a meta-analysis of 27 observational studies.51,82,105,118,125, 134,152,153, 160,161, 163, 166, 167, 179, 193, 194, 202, 204,205,210,216,217,231, 234,242,244,247 The observational studies included retrospective and prospective cohort and case-control designs. Most of the participants were male, aged 21–61 years with a baseline BMI < 50 kg/m2. The duration of patient follow-up after surgery ranged from 3–50 months. While several of the studies included participants with mild OSA, most of the participants had moderate to severe OSA. The meta-analysis demonstrated a clinically significant reduction in the RDI of –31.0 events/h (95% CI, –35.7 to –26.3 events/h), representing a 64% reduction with rescue surgery (see Figure S22 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and large effect size.
Permanent dysphagia
The risk of permanent dysphagia from upper airway surgery was evaluated using a meta-analysis of 10 observational studies48,112,122,145,181,204,208,217,246,266 that reported on persistent long-term dysphagia. The observational studies included retrospective and prospective cohort designs. The participants were mostly male, ranging from 18–73 years of age, with moderate to severe OSA who underwent a variety of surgical procedures including palatal modification, tonsillectomy, multilevel upper airway surgery, maxillomandibular advancement, and tongue base suspension. The meta-analysis demonstrated that the risk of permanent dysphagia was not clinically significant (see Figure S23 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a wide 95% confidence interval that crossed the CST.
The risk of permanent dysphagia was also evaluated from an analysis of 1 observational study259 that reported on the MD Anderson dysphagia score after upper airway surgery. The participants were mostly male, ranged from 29–65 years of age, had moderate to severe OSA and underwent either transoral robotic surgery with expansion sphincter pharyngoplasty or transoral robotic surgery with UPPP. The analysis demonstrated a change in the MD Anderson dysphagia score after surgery that was not clinically significant (see Figure S24 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for evaluating the efficacy of this intervention: LSAT and ODI.
Lowest oxygen saturation
The efficacy of rescue surgery to increase the LSAT was evaluated using a meta-analysis of 3 RCTs.36,38,39 Most of the participants were male, aged 18–70 years with moderate to severe OSA with BMI < 36 kg/m2 who underwent either palatal modification surgery36,39 or multilevel upper airway surgery.38 The duration of patient follow-up after surgery ranged from 3–15 months. The meta-analysis demonstrated an increase in the LSAT that was not clinically significant with rescue surgery (see Figure S25 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a wide 95% confidence interval that crossed the CST.
The efficacy of rescue surgery to increase the LSAT was also evaluated using a meta-analysis of 133 observational studies43–46,49–51,57–60,63–65,69,73,75,77–79,83–85,90–97,103,107,109,111,116,118–120,124–127,129–135,138–144,146,147,150–152,156,157,162,165–171,173–178,180–183,185,187–191,193–195,201–203,205,207,210–212,214,216–218,221–225,230–232,234,237,240–242,244,245,247,251,252,254,256, 258,261,263,264,266,267,269,272,273,279,282 that reported on the LSAT. The observational studies included retrospective and prospective cohort and case-control designs. Most of the participants were male, aged 19–73 years, with a baseline BMI < 40 kg/m2. The duration of participant follow-up ranged from 3–36 months. While several of the studies included participants with mild OSA, most of the participants had moderate to severe OSA. The meta-analysis demonstrated a clinically significant increase in the LSAT of 7.2% (95% CI, 6.4%–8.0%), resulting in an 8.2% improvement with rescue surgery (see Figure S26 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
Oxygen desaturation index
The efficacy of rescue surgery to decrease the ODI was evaluated using a meta-analysis of 2 RCTs.36,38 Most of the participants were middle-aged males with moderate to severe OSA and a baseline BMI < 36 kg/m2 who underwent palatal modification surgery. The mean duration of participant follow-up was 7 months. Meta-analysis demonstrated a clinically significant decrease in the ODI of –17.0 events/h (95% CI, –24.9 to –9.2 events/h) with rescue surgery (see Figure S27 (1.2MB, pdf) ). The quality of evidence was high.
The efficacy of rescue surgery to decrease the ODI was also evaluated using a meta-analysis of 48 observational studies.46,52,54,58,59,63,68,69,85,88,89,104–106,109,115,116,134,135,142, 146–148,151,152,182–184,196–198,200,210,213,220,235–239,242,251–253,255, 257,266,278 The observational studies included retrospective and prospective cohort and case-control designs. Most of the participants were male, ranged from 20–80 years of age, and had a BMI < 40 kg/m2 with mild to severe OSA who underwent a variety of surgical procedures. The duration of participant follow-up ranged from 3 months–4 years. The meta-analysis demonstrated a clinically significant decrease in the ODI of –16.9 units (95% CI, –19.4 to –14.4 units), representing a 54% reduction with rescue surgery (see Figure S28 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and large effect size.
Overall quality of evidence
The TF determined that the overall quality of evidence for the use of surgical treatments in patients who are intolerant or unaccepting of PAP was low based on the critical outcomes and downgrading of the evidence due to risk of bias associated with observational studies and imprecision within the RCTs (see Table S1 (1.2MB, pdf) ).
Benefits vs harms
The potential benefits of upper airway surgery as a rescue therapy include a reduction in excessive sleepiness, snoring, blood pressure, and AHI/RDI, and an improvement in QOL in patients intolerant to PAP therapy. Benefits demonstrated in literature are limited to patients appropriate for surgery. This may not be representative of all patients with OSA. The potential harms of upper airway surgery include short-term discomfort that is expected during postoperative recovery and is discussed during the preoperative informed consent process between the surgeon and patient. Additionally, potential persistent long-term side effects have been reported including dysphagia, taste alteration, mandibular paresthesia, perceived worsening of facial appearance, aspiration, hemorrhage, and globus pharyngeus, but the incidence of these is low. The incidence of perioperative death was not reported in the studies. A meta-analysis of 5 observational studies41,112,157,178,217 demonstrated a risk in persistent taste alteration that was not clinically significant (see Figure S29 (1.2MB, pdf) ). A meta-analysis of 7 observational studies67,107,166,181,193,216,217 demonstrated a clinically significant risk of persistent mandibular paresthesia of 0.11 (95% CI, 0.03–0.19) with surgery (see Figure S30 (1.2MB, pdf) ). An analysis of 1 prospective cohort study67 demonstrated a risk in persistent perceived worsening of facial appearance that was not clinically significant (see Figure S31 (1.2MB, pdf) ). An analysis of 1 prospective cohort study217 demonstrated a clinically significant risk in persistent aspiration of 0.05 (95% CI, –0.01 to 0.11) with surgery (see Figure S32 (1.2MB, pdf) ). An analysis of 1 retrospective study112 demonstrated a risk of persistent hemorrhage that was not clinically significant (see Figure S33 (1.2MB, pdf) ). An analysis of 1 retrospective study120 demonstrated a risk in persistent globus pharyngeus that was not clinically significant (see Figure S34 (1.2MB, pdf) ). Based on their combined clinical experience and the substantial effects of surgery on objective and subjective measures of disease, the TF judged that the potential benefits of a discussion regarding referral to a sleep surgeon with patients intolerant or unaccepting of PAP therapy outweigh the potential harms of untreated OSA. The TF observed that the balance of risks vs benefits for upper airway surgery is variable and dependent upon an individual patient’s OSA severity, symptoms, medical comorbidities, and selected surgical therapy but noted that a discussion of individualized risks and benefits is a standard component of the preoperative informed consent process.
Resource use
There are insufficient data to assess differences in resource requirements for surgery vs PAP therapy or no treatment.
Patient values and preferences
Because acceptability of surgical interventions varies and there is little harm in discussing a referral for consultation, based on their combined clinical experience, the TF judged that most patients would generally be accepting of a discussion regarding referral. The choice to pursue referral is expected to vary between patients based on personal values, beliefs, and expectations for recovery time or pain with surgery.
Surgical treatment of patients with obesity with bariatric surgery
A total of 2 RCTs283,284 and 28 observational studies285–313 investigated the use of bariatric surgery to improve 1 or more of the following outcomes: blood pressure, AHI/RDI, excessive sleepiness, BMI, ODI, LSAT, optimal PAP pressure, PAP adherence, snoring, motor vehicle accident risk, and perioperative death. For the RCTs,283,284 participants were randomized to either bariatric surgery or nutritional care. Participants ranged from 18–65 years of age with a BMI > 35 kg/m2 and severe OSA who were treated with laparoscopic adjustable gastric banding (LAGB) and followed for a period ranging from 2–3 years. All participants received CPAP therapy prior to surgery. For the observational studies,285–291,293–313 comparisons between pre- and posttreatment were made. Participants were mostly female, 20–66 years of age, and obese, with a mean BMI > 30 kg/m2 and mild to severe OSA. The participants underwent a variety of bariatric procedures including gastric banding, gastric bypass, and sleeve gastrectomy and were typically followed for 1 year (range: 6 months–5 years) after surgery. The observational studies included retrospective and prospective cohort and case-control designs. All procedures were performed in an operating room setting. Several meta-analyses were performed to assess the efficacy of bariatric surgery to treat OSA in adults. The meta-analyses are provided in Figure S35 (1.2MB, pdf) through Figure S50 (1.2MB, pdf) in the supplemental material. A summary of findings table is provided in Table S2 (1.2MB, pdf) in the supplemental material. A summary of the evidence for each outcome is provided below.
Critical outcomes
The following outcomes were determined by the TF to be critical for evaluating the efficacy of bariatric surgery to treat OSA in adults with obesity: excessive sleepiness, QOL, sleep quality, BP, AHI/RDI, and perioperative death. Meta-analyses for AHI/RDI included all definitions as reported in the studies. None of the studies identified in our literature review reported data for sleep quality and perioperative death.
Excessive sleepiness
The efficacy of bariatric surgery in reducing excessive sleepiness in adults with obesity and OSA was evaluated using a meta-analysis of 9 observational studies290,291,296,302,303,306,311–313 that reported on the ESS. The observational studies included retrospective and prospective cohort designs. Participants were mostly female, 20–66 years of age, with mean BMI > 31 kg/m2 and moderate to severe OSA. The meta-analysis demonstrated a clinically significant reduction in excessive sleepiness as measured by the ESS of –5.6 points (95% CI, –7.3 to –3.9 points) with bariatric surgery (see Figure S35 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and a large effect size.
The efficacy of bariatric surgery in reducing excessive sleepiness in adults with obesity and OSA was also evaluated using an analysis of 1 very large case-control study295 that reported on the frequency of daytime sleepiness. Participants in the study were mostly female, and had a mean age of 47 years, with a mean BMI < 43 kg/m2 and OSA of unknown severity. Participants in the surgery group underwent a variety of procedures including gastric bypass, vertical banded gastroplasty, or gastric banding and were followed for 2 years. Participants in the control group underwent a conservative weight loss program. The analysis demonstrated a clinically significant reduction in the odds of experiencing daytime sleepiness of 0.4 (95% CI, 0.4–0.5) with bariatric surgery as compared with a conservative weight loss program (see Figure S36 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
Blood pressure
The efficacy of bariatric surgery to lower BP was evaluated using a meta-analysis of 5 observational studies.287,288,291,299,312 The studies included both prospective cohort and case-control designs. Participants were mostly female, 26–60 years of age, with a BMI > 31 kg/m2 and moderate to severe OSA. The participants underwent either gastric banding or gastric bypass and were followed from a range of 6 months–2 years after surgery. The meta-analyses demonstrated a clinically significant decrease in SBP and DBP of –9.3 mm Hg (95% CI, –14.3 to –4.2 mm Hg) and –6.9 mm Hg (95% CI, –10.2 to –3.6 mm Hg), respectively, with bariatric surgery (see Figure S37 (1.2MB, pdf) and Figure S38 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and a large effect size.
AHI/RDI
The AHI and RDI are commonly reported as measures of OSA severity. The efficacy of bariatric surgery in reducing the AHI in adults with obesity was evaluated using a meta-analysis of 2 RCTs.283,284 Participants were randomized to bariatric surgery or nutritional care. Participants were mostly male, obese, and ranged from 18–65 years of age, with a BMI >35 kg/m2 and severe OSA, who were treated with LAGB and followed for a period ranging from 2–3 years. All participants received CPAP therapy prior to surgery. The meta-analysis demonstrated a clinically significant mean difference in the AHI of –12.5 events/h (95% CI, –23.0 to –2.0 events/h) with bariatric surgery compared with conservative nutritional care (see Figure S39 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a wide 95% confidence interval that crossed the CST.
The efficacy of bariatric surgery in reducing the AHI in adults with obesity was also evaluated using a meta-analysis of 20 observational studies.285–287,289–291,294,298–300,302,303,305–310,312,313 Participants were mostly female, 20–66 years of age, with a mean BMI > 35 kg/m2 and moderate to severe OSA. The participants were treated with a variety of procedures including gastric banding, gastric bypass, or sleeve gastrectomy and followed for a period ranging from 6 months–5 years. This meta-analysis of 20 observational studies285–287,289–291,294,298–300,302,303,305 –310,312,313 demonstrated a clinically significant mean difference in the AHI of –23.1 events/h (95% CI, –29.0 to –17.2 events/h), representing a 66% reduction with bariatric surgery as measured by the AHI (see Figure S40 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and a large effect size.
The efficacy of bariatric surgery in reducing the RDI in adults with obesity was evaluated using a meta-analysis of 2 observational studies.286,296 Participants were mostly female and middle-aged with a BMI > 35 kg/m2 who underwent gastric bypass surgery and were followed for 6–42 months. The meta-analyses demonstrated a clinically significant difference in the RDI of –36.3 events/h (95% CI, –40.6 to –32.0 events/h) for a reduction of 71% with bariatric surgery (see Figure S41 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and a large effect size.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for evaluating the efficacy of bariatric surgery to treat OSA in adults with obesity: LSAT, ODI, snoring, BMI, optimal PAP level, and motor vehicle accidents. None of the studies identified in our literature review reported data for motor vehicle accidents.
Lowest oxygen saturation
The efficacy of bariatric surgery in increasing the LSAT was evaluated using a meta-analysis of 9 observational studies.286,294,296,298,299,302,305,312,313 The studies included retrospective and prospective cohort designs. Participants were mostly female, aged 20–66 years with moderate to severe OSA and a BMI > 25 kg/m2. The participants underwent LAGB or gastric bypass and were followed for 6 months–2 years. The meta-analysis demonstrated a clinically significant increase in LSAT of 7.8% (95% CI, 6.0%–9.6%) with bariatric surgery (see Figure S42 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
Oxygen desaturation index
The efficacy of bariatric surgery in reducing the ODI was evaluated using a meta-analysis of 5 observational studies.286,300,305,312,313 The observational studies included retrospective and prospective cohort designs. Participants ranged in age from 20–66 years with mild to severe OSA and a BMI > 30 kg/m2. Both sexes were nearly equally represented across all studies. The participants underwent LAGB or gastric bypass and were followed for 6 months–2 years. The meta-analysis demonstrated a clinically significant reduction in the ODI of –19.1 events/h (95% CI, –25.0 to –13.3 events/h), representing a 73% reduction with bariatric surgery (see Figure S43 (1.2MB, pdf) ). The quality of evidence for ODI was moderate due to risk of bias associated with observational studies and a large effect size.
Snoring
The efficacy of bariatric surgery to decrease snoring was evaluated using an analysis of 1 prospective observational cohort study286 that reported on the percentage of patients snoring before and after surgery. Participants had a mean age of 39 ± 10 years and a BMI > 35 kg/m2 with moderate to severe OSA who underwent gastric bypass surgery and were followed for mean of 14 months. Both sexes were equally represented. Analysis demonstrated a clinically significant reduction in the percentage of patients snoring of –37.8% (95% CI, –60.9% to –14.7%) with bariatric surgery (see Figure S44 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size.
The efficacy of bariatric surgery to decrease snoring was also evaluated using a meta-analysis of 2 observational studies295,302 that reported on snoring frequency. Participants were mostly female, 30–60 years of age, with a BMI > 35 kg/m2 and moderate to severe OSA who underwent gastric bypass, vertical banded gastroplasty, or gastric banding and were followed for 1–2 years. The meta-analysis demonstrated a clinically significant decrease in the odds of snoring of 0.4 (95% CI, 0.03–5.10) with bariatric surgery (see Figure S45 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a wide 95% confidence interval that crossed the CST.
BMI
The efficacy of bariatric surgery in reducing BMI in adults with obesity and OSA was evaluated using an analysis of 1 RCT284 that reported on BMI. Participants were randomized to LAGB or nutritional care. The RCT included participants (21 Male:16 Female) with OSA ranging from moderate to severe, a BMI > 35 kg/m2, and no significant comorbidities who used PAP therapy prior to surgery. The duration of follow-up after surgery was 2 years. The analysis demonstrated a clinically significant reduction in BMI of –10.4 kg/m2 (95% CI, –15.3 to –5.5 kg/m2) with bariatric surgery compared with conservative nutritional care (see Figure S46 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size.
The efficacy of bariatric surgery in reducing BMI in adults with obesity and OSA was also evaluated using 25 observational studies285–291,293,294,296–309,312,313 that reported on BMI. The observational studies included retrospective and prospective cohort and case-control designs. Participants were mostly female, 20–66 years of age, with a mean BMI > 30 kg/m2 and mild to severe OSA. The participants underwent a variety of bariatric procedures including gastric banding, gastric bypass, and sleeve gastrectomy and were typically followed for 1 year (range: 6 months–5 years) after surgery. The meta-analysis demonstrated a clinically significant reduction in BMI of –12.8 kg/m2 (95% CI, –14.3 to –11.4 kg/m2) with bariatric surgery (see Figure S47 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and a large effect size.
Optimal PAP level
The efficacy of bariatric surgery to lower PAP level requirements to facilitate future PAP use was evaluated using a meta-analysis of 3 observational studies.296,301,302 The studies included retrospective and prospective cohort designs. Participants were mostly female with mild to severe OSA and a BMI > 40 kg/m2 who were prescribed CPAP prior to surgery. Participants underwent either LAGB or gastric bypass and were followed for 1–2 years. The meta-analysis demonstrated a clinically significant decrease in optimal CPAP level of –3.1 cm H2O (95% CI, –4.2 to –1.9 cm H2O) with bariatric surgery (see Figure S48 (1.2MB, pdf) ). The quality of evidence for optimal CPAP level was very low due to risk of bias and imprecision associated with a small sample size.
Overall quality of evidence
The TF determined that the overall quality of evidence for the use of bariatric surgery in patients with obesity and OSA was moderate due to risk of bias and large effect size associated with the observational studies, and imprecision within the RCTs (see Table S2 (1.2MB, pdf) ).
Benefits vs harms
The benefits of bariatric surgery in patients with obesity and OSA include a reduction in AHI/RDI, BP, ODI, excessive sleepiness, BMI, snoring, and optimal CPAP level, and an increase in the LSAT. Benefits demonstrated in literature are limited to patients considered appropriate for bariatric surgery by the treating surgeon and may not be representative of all patients with OSA and obesity. While the benefits of bariatric surgery are clinically significant, the surgeon needs to consider factors that would make a patient at higher risk of surgical intervention, which are not captured by this analysis. Selection bias may be present in the observed outcomes as compliance with lifestyle changes is required of patients undergoing bariatric surgery. It is difficult to determine whether the effects of bariatric surgery on BP and ESS are directly attributed to weight loss from surgery or the lowering of AHI. Bariatric surgery is therefore not considered a cure for OSA. Potential harms of bariatric surgery include short-term perioperative discomfort, and this should be discussed as part of the preoperative informed consent process between the surgeon and patient. Additionally, iron malabsorption, gastric ulcer, vitamin deficiency, bowel obstruction or leak, gastrointestinal reflux disorder, and gastric band slippage have been reported but the incidence of these is low. However, 1 observational study294 demonstrated a clinically significant increase in the risk difference in iron malabsorption of 0.1 (95% CI, –0.1 to 0.3) after bariatric surgery (see Figure S49 (1.2MB, pdf) ). Analysis of 1 RCT283 demonstrated a risk difference in incidence of gastric ulcer that was not clinically significant after bariatric surgery compared with conservative weight loss (see Figure S50 (1.2MB, pdf) ). Based on their combined clinical experience and the substantial effects of bariatric surgery on objective and subjective measures of disease, the TF judged that the potential benefits of a discussion regarding referral to a bariatric surgeon with patients who are intolerant or unaccepting of PAP therapy outweigh the potential harms of untreated OSA. The TF observed that the balance of risks vs benefits for bariatric surgery is highly dependent upon an individual patient’s OSA severity, symptoms, medical comorbidities, and selected surgical therapy but noted that a discussion of individualized risks and benefits is a standard component of the preoperative informed consent process.
Resource use
There is insufficient evidence in the literature to compare the costs of bariatric surgery to nutritional care or untreated OSA.
Patient values and preferences
Because acceptability of surgical interventions varies and there is little harm in discussing referral, based on their combined clinical experience the TF judged that most patients would generally be accepting of a discussion regarding referral. The choice to pursue referral is expected to vary between patients based on personal values, beliefs, and expectations for recovery time or pain with surgery.
Surgical treatment of patients to facilitate PAP use
A total of 7 observational studies46,57,141,201,250,279,314 investigated the use of surgery as an adjunctive procedure to facilitate the use of PAP by improving 1 or more of the following outcomes: optimal PAP level, excessive sleepiness, adherence, AHI/RDI, sleep-related QOL, sleep quality, and LSAT. Three of the studies46,141,279 were retrospective and 4 of the studies57,201,250,314 were prospective cohorts. Participants in the studies were mostly male, 23–66 years of age, with a mean BMI < 32 kg/m2 and moderate to severe OSA, who underwent a variety of surgical procedures including nasal, tonsil, and palatal modification procedures and were offered CPAP after surgery. Most of the participants were intolerant to CPAP prior to surgery. In all studies CPAP titration was performed before and after surgery. All procedures were performed in an operating room and patients were followed for a period ranging from 3–12 months. Meta-analyses were performed to assess the efficacy of surgery as an adjunctive treatment of OSA in adults. The meta-analyses are provided in Figure S51 (1.2MB, pdf) through Figure S55 (1.2MB, pdf) in the supplemental material. A summary of findings is provided in Table S3 (1.2MB, pdf) in the supplemental material. A summary of the evidence for each outcome is provided below.
Critical outcomes
The following outcomes were determined by the TF to be critical for evaluating the efficacy of surgery as an adjunctive procedure to facilitate the use of PAP by improving 1 or more of the following outcomes: excessive sleepiness, QOL, sleep quality, snoring, optimal PAP level, and adherence to PAP therapy. None of the studies identified in our literature review reported data for QOL or snoring.
Excessive sleepiness
The efficacy of adjunctive surgery to reduce excessive sleepiness was evaluated using a meta-analysis of 3 observational studies.46,57,201 The observational studies included retrospective and prospective cohort designs. Participants were mostly male, 29–63 years of age, with a mean BMI < 32 kg/m2 and moderate to severe OSA, who were intolerant to CPAP prior to multilevel upper airway surgery,46 tonsillectomy,201 and nasal57 surgery. Participants were followed for a range of 3–6 months after surgery. The meta-analysis demonstrated a clinically significant decrease in excessive sleepiness as measured by a change in ESS of –6.0 points (95% CI, –7.2 to –4.7 points) with adjunctive surgery (see Figure 51). The quality of evidence was low due to risk of bias associated with observational studies.
Optimal PAP level
The efficacy of adjunctive surgery to reduce the optimal PAP level was evaluated using a meta-analysis of 6 observational studies.46,141,201,250,279,314 Participants were mostly male, 18–68 years of age, who had a BMI of <40 kg/m2 and moderate to severe OSA who underwent a variety of surgical procedures and were followed for 6 months. The meta-analysis demonstrated a clinically significant reduction in optimal CPAP level of –2.5 cm H2O (95% CI, –3.5 to –1.4 cm H2O) with adjunctive surgery (see Figure S52 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
PAP adherence
The efficacy of adjunctive surgery to improve PAP adherence was evaluated using a meta-analysis of 2 prospective cohort studies.250,314 Participants were mostly male, 31–66 years of age with severe OSA and underwent modified tongue-base suspension250 or multilevel surgery314 to facilitate CPAP use and were followed for a range of 3–6 months. One study250 included participants with no prior CPAP use while the other study314 included participants who were intolerant to CPAP. The meta-analysis demonstrated a clinically significant increase in CPAP adherence of 2.2 h/night (95% CI, 0.2–4.1 h/night) with adjunctive surgery (see Figure S53 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for evaluating the efficacy of surgery as an adjunctive procedure to facilitate the use of PAP by improving 1 or more of the following outcomes: AHI/RDI and LSAT. Meta-analyses for AHI/RDI included all definitions as reported in the studies.
AHI/RDI
The efficacy of adjunctive surgery to reduce the AHI severity was evaluated using a meta-analysis of 5 observational studies.46,141,201,250,314 Participants were mostly male and 31–66 years of age with a mean BMI < 32 kg/m2 and moderate to severe OSA who underwent multilevel46,314 or palatal modification141,201,250 surgery and were followed for a range of 3–12 months. The meta-analysis demonstrated a clinically significant reduction in the AHI of –22.9 events/h (95% CI, –33.9 to –11.9 events/h) for a 41% reduction (see Figure S54 (1.2MB, pdf) ). None of the studies reported on the RDI. The quality of evidence was low due to risk of bias associated with observational studies.
Lowest oxygen saturation
The efficacy of adjunctive surgery to increase the LSAT was evaluated using a meta-analysis of 2 prospective cohort studies.201,314 Participants in the studies were mostly male and 23–54 years of age with severe OSA who underwent multilevel314 or palatal modification201 surgery and were followed for 3–4 months and 6 months, respectively.
Meta-analysis of 2 observational studies demonstrated a clinically significant increase in the LSAT of 10.4% (95% CI, 7.0%–13.8%) as measured by PSG (see Figure S55 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
Overall quality of evidence
The TF determined that the overall quality of evidence for the use of surgical treatments to facilitate PAP use was very low based on the critical outcomes and downgrading of the evidence due to risk of bias associated with observational studies and imprecision within the RCTs (see Table S3 (1.2MB, pdf) ).
Benefits vs harms
The potential benefits of upper airway surgery as an adjunctive procedure to facilitate effective PAP therapy include a reduction in optimal PAP level, excessive sleepiness, and AHI/RDI, as well as an increase in PAP adherence and LSAT. Benefits demonstrated in literature were limited to patients considered appropriate for surgery by the treating surgeon and may not be representative of all patients with PAP-related side effects or suboptimal use. The potential harms of upper airway surgery include short-term discomfort that is expected during postoperative recovery and is discussed during the informed preoperative consent process between the surgeon and patient. Surgery carries inherent risks but based on their combined clinical experience and the moderate effects of surgery on PAP pressure requirements and adherence, the TF judged that the potential benefits of a discussion regarding referral to a sleep surgeon for consideration of surgery as an adjunctive procedure to facilitate PAP use may, in some patients, outweigh the potential harms of suboptimal PAP-related side effects and adherence depending on their severity. Regarding whether referral is discussed, the TF observed that the balance of risks vs benefits for upper airway is highly dependent upon an individual patient’s OSA severity, symptoms, medical comorbidities, and selected surgical therapy but noted that a discussion of individualized risks and benefits is a standard component of the preoperative informed consent process.
Resource use
There are insufficient data to assess differences in resource requirements for surgical referral vs suboptimal PAP use.
Patient values and preferences
Because acceptability of surgical interventions varies and there is little harm in offering referral, based on their combined clinical experience the TF judged that most patients would generally be accepting of a discussion regarding referral but that the clinical utility of it may be more limited in patients who are partially PAP compliant as opposed to those who are completely untreated. The choice to pursue referral is expected to vary between patients based on personal values, beliefs, and expectations for recovery time or pain with surgery.
Surgical treatment as an initial therapy in patients with a major upper airway anatomical abnormality
Two RCTs36,39 and 15 observational studies60,81,82,102,103,121,122, 145,173,193,204,217,219,244,266 investigated the use of surgery to improve 1 or more of the following outcomes: AHI/RDI, excessive sleepiness, LSAT, sleep-related QOL, snoring, ODI, SBP, optimal PAP pressure, motor vehicle accidents, perioperative death, permanent dysphagia, and other serious persistent side effects. For the RCTs,36,39 participants were randomized to surgery or no treatment. Participants were mostly male, 18–65 years of age, with a mean BMI < 30 kg/m2, moderate to severe OSA, and tonsillar hypertrophy with velopharyngeal obstruction who were intolerant or unaccepting of CPAP therapy. The participants underwent palatal modification surgery and were followed for 4–7 months. For the observational studies,60,81,82,102,103,121, 122,145,173,193,204,217,219,244,266 comparisons between pretreatment and posttreatment were made. The studies included retrospective and prospective cohort and case-control designs. Participants were mostly male, 21–67 years of age, with a mean BMI < 35 kg/m2, moderate to severe OSA, and a major upper airway anatomic abnormality. These abnormalities included tonsillar hypertrophy, class II occlusion (Angle classification), retrognathia, or maxillary hypoplasia. Participants underwent either tonsillectomy or maxillomandibular surgery and were followed for 3 months–3 years. Several meta-analyses were performed to assess the efficacy of surgery as an initial therapy to treat OSA in adults. The meta-analyses are provided in Figure S56 (1.2MB, pdf) through Figure S71 (1.2MB, pdf) in the supplemental material. A summary of findings table is provided in Table S4 (1.2MB, pdf) in the supplemental material. A summary of the evidence for each outcome is provided below.
Critical outcomes
The following outcomes were determined by the TF to be critical for evaluating the efficacy of surgery as an initial therapy: excessive sleepiness, QOL, sleep quality, snoring, AHI/RDI, LSAT, ODI, perioperative death, and permanent dysphagia. Meta-analyses for AHI/RDI included all definitions as reported in the studies. None of the studies identified in our literature review reported data for QOL, sleep quality, or perioperative death.
Excessive sleepiness
The efficacy of surgery as an initial therapy to reduce excessive sleepiness was evaluated using an analysis of 1 RCT39 that reported on the ESS. Participants were mostly male, 18–65 years of age, with a mean BMI < 30 kg/m2, moderate to severe OSA, tonsillar hypertrophy with velopharyngeal obstruction, and intolerance to CPAP. Participants underwent palatal modification surgery and were followed for a mean of 4.4 ± 1 months. The analysis demonstrated a clinically significant reduction in excessive sleepiness of –3.4 points (95% CI, -6.3 to –0.5 points) as measured by the ESS (see Figure S56 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
The efficacy of surgery as an initial therapy to reduce excessive sleepiness was evaluated using a meta-analysis of 2 prospective cohort studies122,145 that reported on the ESS. Participants were mostly male, 21–67 years of age, with a mean BMI < 30 kg/m2, moderate to severe OSA, and tonsillar hypertrophy. Participants underwent tonsillectomy122 or palatal modification surgery145 and were followed for 3–6 months. The meta-analysis demonstrated a clinically significant reduction in excessive sleepiness of –6.0 points (95% CI, –8.4 to –3.6 points) as measured by the ESS (see Figure S57 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size.
Snoring
The efficacy of surgery as an initial therapy to decrease snoring was evaluated using an analysis of 1 RCT39 that reported on snoring. Participants were mostly male, 18–65 years of age, with a mean BMI < 30 kg/m2, moderate to severe OSA, tonsillar hypertrophy with velopharyngeal obstruction, and intolerance to CPAP. Analysis demonstrated a clinically significant decrease in snoring of –3.7 points (95% CI, -5.3 to –2.1 points) as measured on a 10-point VAS with surgery (see Figure S58 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size.
The efficacy of surgery as an initial therapy to decrease snoring was also evaluated using a meta-analysis of 2 observational studies103,204 that reported on snoring. Participants were mostly male, 29–60 years of age, with a mean BMI < 40 kg/m2, moderate to severe OSA, and a major anatomical obstruction who failed CPAP therapy. Participants underwent palatal modification219 or UPPP103 procedures and were followed for 6 months–1 year. The meta-analysis demonstrated a clinically significant decrease in snoring of –5.5 points (95% CI, -5.9 to –5.1 points) as measured on a 10-point VAS after surgery (see Figure S59 (1.2MB, pdf) ). The quality of evidence was low due to risk of bias associated with observational studies.
AHI/RDI
The AHI and RDI are commonly reported as measures of OSA severity. The efficacy of surgery as an initial therapy to reduce the AHI was evaluated using a meta-analysis of 2 RCTs.36,39 Participants were mostly male, 18–65 years of age, with a mean BMI < 30 kg/m2, moderate to severe OSA, and tonsillar hypertrophy with velopharyngeal obstruction who were intolerant or unaccepting of CPAP therapy. The meta-analysis demonstrated a clinically significant reduction in AHI of –20.6 events/h (95% CI, –39.0 to –2.1 events/h) with surgery (see Figure S60 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
The efficacy of surgery as an initial therapy to reduce the AHI was also evaluated using a meta-analysis of 7 observational studies.60,81,102,121,122,145,173 Participants were mostly male, 21–67 years of age, with a mean BMI < 35 kg/m2 and moderate to severe OSA who had tonsillar hypertrophy or maxillomandibular abnormality.60,76,81,102,103,173,181,201,219,256,276,315–317 Most of them were offered but intolerant to CPAP and underwent palatal modification or maxillomandibular advancement (MMA) surgery and were followed for 3 months–2 years. The meta-analysis demonstrated a clinically significant reduction in AHI of –37.6 events/h (95% CI, –49.4 to –25.7 events/h), resulting in an 84% reduction with surgery (see Figure S61 (1.2MB, pdf) ). A subgroup analysis of 4 observational studies60,81,121,173 including participants with maxillomandibular abnormalities demonstrated a clinically significant reduction in AHI of –42.5 events/h (95% CI, –49.4 to –35.5 events/h), resulting in an 85% reduction with surgery (see Figure S61 (1.2MB, pdf) ). A subgroup analysis of 3 observational studies102,122,145 including participants with tonsillar hypertrophy demonstrated a clinically significant reduction in AHI of –31.1 events/h (95% CI, –48.7 to –13.4 events/h), resulting in an 82% reduction with surgery (see Figure S61 (1.2MB, pdf) ). The quality of evidence was moderate due to risk of bias associated with observational studies and a large effect size.
The efficacy of surgery as an initial therapy to reduce the RDI was evaluated using an analysis of 1 RCT39 that reported on RDI. Participants were randomized to tonsillectomy with UPPP or no treatment and were followed for 3 months. Participants were mostly male, middle-aged, and had a BMI < 34 kg/m2, with moderate to severe OSA, tonsillar hypertrophy, and intolerance to CPAP. The RCT39 demonstrated a clinically significant reduction in the RDI of –6.9 events/h (95% CI, –21.0 to 7.2 events/h) (see Figure S62 (1.2MB, pdf) ). The quality of evidence was moderate due to imprecision associated with a small sample size.
The efficacy of surgery as an initial therapy to reduce the RDI was also evaluated using meta-analysis of 2 observational studies.82,244 The participants underwent palatal modification surgery or no treatment and were followed for 4–7 months. The meta-analysis demonstrated a clinically significant reduction in the RDI of –41.5 events/h (95% CI, –65.6 to –17.4 events/h), resulting in an 82% reduction with surgery (see Figure S63 (1.2MB, pdf) . A subgroup analysis of 1 observational study82 that included participants with maxillomandibular abnormalities demonstrated a clinically significant reduction in the RDI of –53.7 events/h (95% CI, –64.1 to –43.3 events/h) (see Figure S63 (1.2MB, pdf) ). Another subgroup analysis of 1 observational study213 that included participants with tonsillar hypertrophy demonstrated a clinically significant reduction in the RDI of –29.1 events/h (95% CI, –40.4 to –17.8 events/h) with surgery (see Figure S63 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size.
Lowest oxygen saturation
The efficacy of surgery as an initial therapy to increase the LSAT was evaluated using an analysis of 1 RCT in participants with a major upper airway anatomical obstruction39 that reported on the LSAT. Participants were mostly male, 18–65 years of age, with a mean BMI < 30 kg/m2, moderate to severe OSA, tonsillar hypertrophy with velopharyngeal obstruction, and intolerance to CPAP. Participants underwent palatal modification surgery and were followed for a mean of 4.4 ± 1 months. Analysis demonstrated an increase in the LSAT that was not clinically significant with surgery (see Figure S64 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size and a wide 95% confidence interval that crossed the CST.
The efficacy of surgery as an initial therapy to increase the LSAT was also evaluated using a meta-analysis of 4 observational studies in participants with either tonsillar hypertrophy or maxillomandibular abnormalities60,102,173,244 that reported on the LSAT. Participants were mostly male, 24–55 years of age, with a mean BMI < 30 kg/m2 with moderate to severe OSA and a major upper airway anatomical obstruction. Participants underwent MMA,60,173 palatal modification,244 or UPPP102 procedures and were followed for 3–6 months. The meta-analysis demonstrated a clinically significant increase in the LSAT of 9.1% (95% CI, 6.4%–11.9%) that represented a 16% improvement with surgery (see Figure S65 (1.2MB, pdf) ). A subgroup analysis of 2 studies60,173 including participants with maxillomandibular abnormalities demonstrated a clinically significant increase in LSAT of 10.3% (95% CI, 4.5%–16%) with surgery (see Figure S65 (1.2MB, pdf) ). A subgroup analysis of 2 studies including participants with tonsillar hypertrophy demonstrated a clinically significant increase in LSAT of 8.3% (95% CI, 5.2%–11.4%) with surgery (see Figure S65 (1.2MB, pdf) ).102,244 The quality of evidence was low due to risk of bias associated with observational studies.
Oxygen desaturation index
The efficacy of surgery as an initial therapy to decrease the ODI was evaluated using an analysis of 1 retrospective study266 that reported on the ODI. Participants were mostly male, mean age 42 ± 11 years, and mean BMI < 30 kg/m2, with severe OSA and retroglossal obstruction. No use of CPAP was mentioned prior to surgery. Participants underwent coblation lingual tonsil removal and were followed for 6 months. Analysis demonstrated a clinically significant decrease in the ODI of –18.5 events/h (95% CI, –26.8 to –10.2 events/h) for a 56% reduction after surgery (see Figure S66 (1.2MB, pdf) ). The quality of evidence was very low based on risk of bias associated with observational studies and imprecision associated with a small sample size.
Permanent dysphagia
The risk of permanent dysphagia from upper airway surgery in patients with OSA and a major obstruction was evaluated using a meta-analysis of 5 observational studies.122,145,204,217,266 The observational studies included retrospective and prospective cohort designs. Participants were mostly male, 21–67 years of age, with a mean BMI < 35 kg/m2 and moderate to severe OSA with an oropharyngeal obstruction. Participants underwent multilevel or hypopharyngeal surgery, tonsillectomy, or palatal modification procedures and were followed for 3 months–4 years. The meta-analysis demonstrated a risk difference in permanent dysphagia before and after surgery that was not clinically significant (see Figure S67 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a wide 95% confidence interval that crossed the CST.
Important outcomes
The following outcomes were determined by the TF to be important for evaluating the efficacy of surgery as an initial therapy: BP and motor vehicle accidents. None of the studies identified in our literature review reported data for motor vehicle accidents.
Blood pressure
The efficacy of surgery as an initial therapy to decrease mean SBP was evaluated using an analysis of 1 retrospective study.219 Participants were mostly male, 29–51 years of age, with a mean BMI < 35 kg/m2 and severe OSA with tonsillar hypertrophy who failed CPAP before beginning surgery. Participants underwent tonsillectomy and simultaneous palatoplasty procedures and were followed for 1 year. Analysis demonstrated a clinically significant decrease in SBP of –8.7 mm Hg (95% CI, –11 to –6 mm Hg) after surgery (see Figure S68 (1.2MB, pdf) ). The quality of evidence was very low due to risk of bias associated with observational studies and imprecision associated with a small sample size.
None of the articles included in this review reported on DBP.
Overall quality of evidence
The TF determined that the overall quality of evidence for the use of surgical treatments as an initial therapy was low based on the critical outcomes and downgrading of the evidence due to risk of bias associated with the observational studies and imprecision within the RCTs (see Table S4 (1.2MB, pdf) ).
Benefits vs harms
The potential benefits of upper airway surgery as an initial therapy include a reduction in excessive sleepiness, snoring, SBP, AHI/RDI, and ODI, and an increase in LSAT. Benefits demonstrated in literature are limited to patients with a major upper airway anatomical obstruction considered appropriate for surgery by the treating surgeon and may not be representative of all patients with OSA with similar anatomic findings. The potential harms of surgery include short-term discomfort that is expected during postoperative recovery and is discussed during the preoperative informed consent process. Additionally, potential persistent long-term side effects including taste alteration, mandibular paresthesia, and aspiration have been reported with some surgical procedures, but the incidence of these is low. An analysis of 1 observational study217 demonstrated a risk difference in persistent taste alteration with surgery that was not clinically significant (see Figure S69 (1.2MB, pdf) ). Meta-analysis of 2 observational studies193,217 demonstrated a clinically significant risk of persistent mandibular paresthesia of 0.17 (95% CI, 0.09–0.24) with surgery (see Figure S70 (1.2MB, pdf) ). An analysis of 1 observational study217 demonstrated a clinically significant risk in persistent aspiration of 0.05 (95% CI, –0.01 to 0.11) with surgery (see Figure S71 (1.2MB, pdf) ). Given that even low surgical risks are elevated as compared to the minimal risk of initial PAP therapy, the balance of benefits to harms favors PAP therapy as initial treatment over discussion of referral for surgical evaluation. Nevertheless, the presence of major upper airway anatomical obstruction may tip the balance in favor of surgical referral discussion depending on a patient’s upper airway medical history. Despite the low risk of surgical referral discussion, there is no harm in an initial trial of PAP therapy if other surgical indications are not present. Given that the intent of discussion of sleep surgery referral in this clinical scenario is consideration of upper airway surgery prior to any PAP trial, based on their combined clinical experience the TF judged that the potential benefits of surgical referral discussion in patients with major upper airway anatomical obstruction do not exceed the potential benefits of an initial PAP trial for OSA in the absence of other medical conditions affecting upper airway patency.
Resource use
There are insufficient data to assess differences in resource requirements for surgery vs PAP therapy or no treatment.
Patient values and preferences
Because acceptability of surgical interventions varies and there is little harm in offering referral, based on their combined clinical experience the TF judged that most patients would generally be accepting of a discussion regarding referral but that its clinical utility may be more limited in patients who are partially PAP compliant as opposed to those who are completely untreated. The choice to pursue referral is expected to vary between patients based on personal values, beliefs, and expectations for recovery time or pain with surgery.
DISCUSSION AND FUTURE DIRECTIONS
OSA is a common sleep disorder with significant physical, psychological, and social impacts. PAP is a highly efficacious treatment, but adherence to therapy is variable. Alternative surgical therapies to OSA have been available for decades but are sporadically employed for patients unaccepting or intolerant of PAP, creating a large, untreated population often lost to follow-up. Previous AASM surgical guidelines for OSA focused on evaluation of specific surgical therapies with recommendations that were broadly applied across diverse populations. Nonetheless, a growing body of evidence suggests that OSA is a heterogeneous disease composed of many pathophysiologic mechanisms with varying presentations and responses to different treatments. The review of surgical literature presented here was designed to meet the needs of the sleep clinician considering a discussion of referral for surgical consultation for a patient found to be intolerant or unaccepting of PAP therapy or to have significant anatomic abnormalities. To that end, this review sought to best inform the sleep clinician’s decision-making process regarding a discussion of referral for surgery by evaluating the overall impact of surgical interventions rather than stratifying by specific intervention. Patient-specific values and preferences may affect the decision for or against a variety of surgical options. It is ultimately the purview of the consulting surgeon to assess a patient’s anatomy for potential surgical options, and to conduct a comprehensive discussion regarding potential risks and benefits so that the patient may make an informed decision. The conclusions in this review are limited to the available published data and by any inherent issues with underlying study designs. The systematic review performed by the task force identified several areas that merit further investigation to determine effects on patient outcomes and better inform clinical decision-making. Consistent limitations across the literature are outlined below.
Limitations
Several issues were noted across the studied literature.
Variability in the procedural choice and technique: A wide variety of surgical techniques was evaluated for therapeutic use in populations described using only basic demographic information. Anatomic information was rarely included to justify procedural selection. Many studies were isolated retrospective cohorts examining a unique modification of a standard surgical technique.
Nonstandardized reporting of outcomes: A portion of the studies reviewed did not define internal success or failure criteria in assessed surgical outcomes, used nonstandard criteria for evaluating outcomes of interest, or used nonvalidated internal metrics for measuring outcomes, preventing inclusion for meta-analysis.
Small and heterogeneous study populations with inherent selection bias: The surgical literature mainly comprises retrospective cohort studies without a control comparator. This is partly due to ethical considerations: randomized controlled trials with sham surgical interventions may cause significant harm without potential for benefit. Nonetheless, inclusion and exclusion criteria were often ill-defined or undocumented, limiting understanding of patient selection criteria and introducing potential selection biases. Generalizability to underrepresented groups in the extant literature may be limited.
Lack of blinding: Many of the patient-centered outcomes evaluated depend on patient-reported validated questionnaires to assess treatment response. Because there are ethical considerations surrounding sham surgery, patients in most evaluated studies were not blinded to their treatment modality, which may impact responses.
Some literature limitations are due to the historical progression of alternative surgical therapies for OSA treatment. Until the mid-2000s, surgical therapy was primarily limited to uvulopalatopharyngoplasty, maxillomandibular advancement, and tracheostomy. These procedures continue to form the bulk of the available surgical literature and in the past were applied indiscriminately across heterogeneous populations prior to the development of current diagnostic and therapeutic options. The last 15 years have seen a proliferation of new therapeutic options enabled by advances in surgical technology including pharyngeal surgeries tailored to the individual patient’s anatomy, transoral robotic surgery, and hypoglossal nerve stimulation. More recently, surgeons have begun to make a concerted effort to better understand what anatomic features best respond to selected interventions, using more complex diagnostic tools such as ultrasound, optical coherence tomography, cineMRI, and drug-induced sleep endoscopy.
Changing popularity of bariatric procedures over the last decade (from gastric banding as the most common procedure in 2010 to sleeve gastrectomy as the most common procedure in 2020) may have a different impact on OSA than observed in this analysis due to increased effectiveness on weight loss. In addition, weight loss occurs at different rates after bariatric surgery, and there was no standard time frame for postoperative outcomes assessment. During the surgical consultation, the surgeon will discuss lifestyle changes necessary to be successful with bariatric surgery. Ultimately, patients will have to agree to major lifestyle changes to be successful with bariatric surgery and some are not ready for these changes.
Future research needs
Despite the promise of these emerging diagnostic and therapeutic alternatives, more studies are needed to better characterize OSA surgery response criteria and to ensure reproducibility. Clinical decision-making and guideline recommendations are expected to improve as research efforts expand into several key areas. In general, there is a need for standardized classification schema incorporating anatomic and nonanatomic data found to predict physician and patient-centered outcomes of interest. Research has historically focused on the AHI, but it does not always correlate well with patient-reported concerns such as daytime sleepiness, socially disruptive snoring, and comorbid health risks. Validated metrics for quantifying patient-centered outcomes of interest (eg, QOL) would benefit from development through focus group and survey studies evaluating patient treatment priorities. More research on surgical outcomes beyond standard polysomnography metrics are needed to better quantify changes in patient-centered outcomes as well as long-term cardiometabolic, neurocognitive, and mortality outcomes.
A relative paucity of literature evaluates the impact of surgical interventions on PAP setting requirements, adherence, and benefit. Surgery is historically considered to be a second-line therapy option for OSA after absolute failure of PAP, but there is a growing recognition of the role for surgery to improve PAP tolerance. Subpopulation research is needed to determine which surgical therapies can benefit initial or repeat PAP exposure, or even be curative of disease. Further work is also required to better evaluate patient preferences for PAP vs surgery as a first-line therapy, and to evaluate which patient characteristics and surgical interventions most impact future PAP adherence. More comparative effectiveness trials comparing surgery to medical therapies for OSA would help clinicians better understand where different surgical therapies stand in the OSA treatment algorithm. Longitudinal cohort studies tracking long-term surgical outcomes are rare and complicated by substantial attrition rates. Further studies are required to assess the true long-term durability of surgical interventions.
Last, the large effect size of bariatric surgery on OSA requires further exploration to better understand patient-centered outcomes of interest and phenotypes best responding to surgery. The optimal timing of postoperative re-evaluation for OSA is unknown, and there is a need for studies exploring the impact of bariatric surgery on OSA in patients with a BMI < 35 mg/kg2 given the observed benefit seen in patients with other comorbidities, such as diabetes.
DISCLOSURE STATEMENT
The development of this paper was funded by the American Academy of Sleep Medicine (AASM). Dr. David Kent is listed as an inventor on U.S. and international patent applications regarding surgical treatments for OSA owned and licensed by Vanderbilt University (VU) to Nyxoah SA. Dr. Kent receives a portion of any licensing revenues as per standard VU technology transfer policy. The licensed treatment methodologies do not currently exist in prototype or commercial form and are not represented in the surgical literature reviewed by the TF. The licensing agreement was completed in early 2021, after completion of the initial clinical practice guideline, supporting systematic review, and public comment period. Mr. Harrod is employed by the AASM. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The task force acknowledges the early contributions of Edward Weaver, MD, MPH who assisted with the development of the PICO questions and project charter. The task force also thanks Ofer Jacobowitz, MD, PhD; Eric Kezirian, MD, MPH; Reena Mehra, MD, MS; and Sanjay Patel, MD for serving as external reviewers of the document and providing valuable feedback.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- BP
blood pressure
- COI
conflict of interest
- CST
clinical significance threshold
- DBP
diastolic blood pressure
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcome of Sleep Questionnaire
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HNS
hypoglossal nerve stimulation
- LAGB
laparoscopic adjustable gastric banding
- LSAT
lowest oxygen saturation
- MMA
maxillomandibular advancement
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PICO
Patient, Intervention, Comparison, and Outcomes
- PSQI
Pittsburgh Sleep Quality Index
- QOL
quality of life
- RCT
randomized controlled trial
- RDI
respiratory disturbance index
- SAQLI
Calgary Sleep Apnea Quality of Life Index
- SBP
systolic blood pressure
- TF
task force
- UPPP
uvulopalatopharyngoplasty
- VAS
visual analog scale
REFERENCES
- 1.Kent D, Stanley J, Aurora RN, et al . Referral of adults with obstructive sleep apnea for surgical consultation: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med. 2021. ; 17 ( 12 ): 2499 – 2505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aurora RN, Casey KR, Kristo D, et al . American Academy of Sleep Medicine . Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults . Sleep. 2010. ; 33 ( 10 ): 1408 – 1413 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caples SM, Rowley JA, Prinsell JR, et al . Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis . Sleep. 2010. ; 33 ( 10 ): 1396 – 1407 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM . Increased prevalence of sleep-disordered breathing in adults . Am J Epidemiol. 2013. ; 177 ( 9 ): 1006 – 1014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somers VK, White DP, Amin R, et al . American College of Cardiology Foundation . Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) . Circulation. 2008. ; 118 ( 10 ): 1080 – 1111 . [DOI] [PubMed] [Google Scholar]
- 6.Waldman LT, Parthasarathy S, Villa KF, Bron M, Bujanover S, Brod M . Understanding the burden of illness of excessive daytime sleepiness associated with obstructive sleep apnea: a qualitative study . Health Qual Life Outcomes. 2020. ; 18 ( 1 ): 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurado-Gámez B, Guglielmi O, Gude F, Buela-Casal G . Workplace accidents, absenteeism and productivity in patients with sleep apnea . Arch Bronconeumol. 2015. ; 51 ( 5 ): 213 – 218 . [DOI] [PubMed] [Google Scholar]
- 8.Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J ; Cooperative Group Burgos-Santander . The association between sleep apnea and the risk of traffic accidents . N Engl J Med. 1999. ; 340 ( 11 ): 847 – 851 . [DOI] [PubMed] [Google Scholar]
- 9.Sullivan CE, Issa FG, Berthon-Jones M, Eves L . Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares . Lancet. 1981. ; 1 ( 8225 ): 862 – 865 . [DOI] [PubMed] [Google Scholar]
- 10.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG . Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment . J Clin Sleep Med. 2019. ; 15 ( 2 ): 335 – 343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javaheri S, Campos-Rodriguez F . Outcomes of positive airway pressure for sleep apnea . JAMA. 2017. ; 318 ( 20 ): 2042 – 2043 . [DOI] [PubMed] [Google Scholar]
- 12.Cistulli PA, Armitstead J, Pepin JL, et al . Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data . Sleep Med. 2019. ; 59 : 114 – 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen AR, Eriksen F, Hansen RW, et al . Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea . PLoS One. 2017. ; 12 ( 12 ): e0189614 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler M, Smith D, Tippett V, Stradling JR . Predictors of long-term compliance with continuous positive airway pressure . Thorax. 2010. ; 65 ( 9 ): 829 – 832 . [DOI] [PubMed] [Google Scholar]
- 15.Virk JS, Kotecha B . When continuous positive airway pressure (CPAP) fails . J Thorac Dis. 2016. ; 8 ( 10 ): E1112 – E1121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd SB, Upender R, Walters AS, et al . Effective apnea-hypopnea index (“effective AHI”): a new measure of effectiveness for positive airway pressure therapy . Sleep. 2016. ; 39 ( 11 ): 1961 – 1972 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaesu Y, Tsuiki S, Kobayashi M, Komada Y, Nakayama H, Inoue Y . Mandibular advancement device as a comparable treatment to nasal continuous positive airway pressure for positional obstructive sleep apnea . J Clin Sleep Med. 2016. ; 12 ( 8 ): 1113 – 1119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srijithesh PR, Aghoram R, Goel A, Dhanya J . Positional therapy for obstructive sleep apnoea . Cochrane Database Syst Rev. 2019. ; 5 ( 5 ): CD010990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita S, Conway W, Zorick F, Roth T . Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty . Otolaryngol Head Neck Surg. 1981. ; 89 ( 6 ): 923 – 934 . [DOI] [PubMed] [Google Scholar]
- 20.Young T, Peppard P, Palta M, et al . Population-based study of sleep-disordered breathing as a risk factor for hypertension . Arch Intern Med. 1997. ; 157 ( 15 ): 1746 – 1752 . [PubMed] [Google Scholar]
- 21.Kapur VK, Auckley DH, Chowdhuri S, et al . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med. 2017. ; 13 ( 3 ): 479 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antic NA, Buchan C, Esterman A, et al . A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea . Am J Respir Crit Care Med. 2009. ; 179 ( 6 ): 501 – 508 . [DOI] [PubMed] [Google Scholar]
- 23.Craig SE, Kohler M, Nicoll D, et al . Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial . Thorax. 2012. ; 67 ( 12 ): 1090 – 1096 . [DOI] [PubMed] [Google Scholar]
- 24.Patel S, Kon SSC, Nolan CM, et al . The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea . Am J Respir Crit Care Med. 2018. ; 197 ( 7 ): 961 – 963 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware JE, Kosinski M, Bjorner JB, et al . User’s Manual for the SF-36 v2 Health Survey. Lincoln, RI: : Quality Metric Incorporated; ; 2007. . [Google Scholar]
- 26.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD . A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease . Health Serv Res. 2005. ; 40 ( 2 ): 577 – 592 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buysse DJ, Germain A, Moul DE, et al . Efficacy of brief behavioral treatment for chronic insomnia in older adults . Arch Intern Med. 2011. ; 171 ( 10 ): 887 – 895 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R ; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies . Lancet. 2002. ; 360 ( 9349 ): 1903 – 1913 . [DOI] [PubMed] [Google Scholar]
- 29.Turnbull F ; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials . Lancet. 2003. ; 362 ( 9395 ): 1527 – 1535 . [DOI] [PubMed] [Google Scholar]
- 30.Hutcheson KA, Holsinger FC, Kupferman ME, Lewin JS . Functional outcomes after TORS for oropharyngeal cancer: a systematic review . Eur Arch Otorhinolaryngol. 2015. ; 272 ( 2 ): 463 – 471 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA . Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion . JAMA. 2005. ; 294 ( 9 ): 1025 – 1033 . [DOI] [PubMed] [Google Scholar]
- 32.Hingson R, Heeren T, Winter M . Effects of recent 0.08% legal blood alcohol limits on fatal crash involvement . Inj Prev. 2000. ; 6 ( 2 ): 109 – 114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tippetts AS, Voas RB, Fell JC, Nichols JL . A meta-analysis of. 08 BAC laws in 19 jurisdictions in the United States . Accid Anal Prev. 2005. ; 37 ( 1 ): 149 – 161 . [DOI] [PubMed] [Google Scholar]
- 34.Guyatt G, Oxman AD, Akl EA, et al . GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables . J Clin Epidemiol. 2011. ; 64 ( 4 ): 383 – 394 . [DOI] [PubMed] [Google Scholar]
- 35.Morgenthaler TI, Deriy L, Heald JL, Thomas SM . The evolution of the AASM Clinical Practice Guidelines: another step forward . J Clin Sleep Med. 2016. ; 12 ( 1 ): 129 – 135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Browaldh N, Nerfeldt P, Lysdahl M, Bring J, Friberg D . SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea . Thorax. 2013. ; 68 ( 9 ): 846 – 853 . [DOI] [PubMed] [Google Scholar]
- 37.Browaldh N, Bring J, Friberg D . SKUP(3) RCT; continuous study: changes in sleepiness and quality of life after modified UPPP . Laryngoscope. 2016. ; 126 ( 6 ): 1484 – 1491 . [DOI] [PubMed] [Google Scholar]
- 38.MacKay S, Carney AS, Catcheside PG, et al . Effect of multilevel upper airway surgery vs medical management on the apnea-hypopnea index and patient-reported daytime sleepiness among patients with moderate or severe obstructive sleep apnea: the SAMS randomized clinical trial . JAMA. 2020. ; 324 ( 12 ): 1168 – 1179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sommer UJ, Heiser C, Gahleitner C, et al . Tonsillectomy with uvulopalatopharyngoplasty in obstructive sleep apnea: a two-center randomized controlled trial . Dtsch Arztebl Int. 2016. ; 113 ( 1-2 ): 1 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adzreil B, Wong EHC, Saraiza AB, Raman R, Amin J . The effectiveness of combined tonsillectomy and anterior palatoplasty in the treatment of snoring and obstructive sleep apnoea (OSA) . Eur Arch Otorhinolaryngol. 2017. ; 274 ( 4 ): 2005 – 2011 . [DOI] [PubMed] [Google Scholar]
- 41.Aneeza WH, Marina MB, Razif MY, Azimatun NA, Asma A, Sani A . Effects of uvulopalatopharyngoplasty: a seven year review . Med J Malaysia. 2011. ; 66 ( 2 ): 129 – 132 . [PubMed] [Google Scholar]
- 42.Arora A, Chaidas K, Garas G, et al . Outcome of TORS to tongue base and epiglottis in patients with OSA intolerant of conventional treatment . Sleep Breath. 2016. ; 20 ( 2 ): 739 – 747 . [DOI] [PubMed] [Google Scholar]
- 43.Askar SM, El-Anwar MW . Double suspension sutures: a simple surgical technique for selected cases of obstructive sleep apnoea: our experience with twenty-two patients . Clin Otolaryngol. 2018. ; 43 ( 2 ): 753 – 757 . [DOI] [PubMed] [Google Scholar]
- 44.Askar SM, El-Anwar MW, Awad A . Modified anterior palatoplasty and double suspension sutures (with or without tonsillectomy) in selected patients with obstructive sleep apnea: a preliminary report . Sleep Breath. 2018. ; 22 ( 3 ): 789 – 795 . [DOI] [PubMed] [Google Scholar]
- 45.Aynacı E, Karaman M, Kerşin B, Fındık MO . Comparison of radiofrequency and transoral robotic surgery in obstructive sleep apnea syndrome treatment . Acta Otolaryngol. 2018. ; 138 ( 5 ): 502 – 506 . [DOI] [PubMed] [Google Scholar]
- 46.Azbay S, Bostanci A, Aysun Y, Turhan M . The influence of multilevel upper airway surgery on CPAP tolerance in non-responders to obstructive sleep apnea surgery . Eur Arch Otorhinolaryngol. 2016. ; 273 ( 9 ): 2813 – 2818 . [DOI] [PubMed] [Google Scholar]
- 47.Babademez MA, Gul F, Kale H, Sancak M . Technical update of barbed pharyngoplasty for retropalatal obstruction in obstructive sleep apnoea . J Laryngol Otol. 2019. ; 133 ( 7 ): 622 – 626 . [DOI] [PubMed] [Google Scholar]
- 48.Babademez MA, Gul F, Sancak M, Kale H . Prospective randomized comparison of tongue base resection techniques: robotic vs coblation . Clin Otolaryngol. 2019. ; 44 ( 6 ): 989 – 996 . [DOI] [PubMed] [Google Scholar]
- 49.Babademez MA, Ciftci B, Acar B, et al . Low-temperature bipolar radiofrequency ablation (coblation) of the tongue base for supine-position-associated obstructive sleep apnea . ORL J Otorhinolaryngol Relat Spec. 2010. ; 72 ( 1 ): 51 – 55 . [DOI] [PubMed] [Google Scholar]
- 50.Baisch A, Maurer JT, Hörmann K . The effect of hyoid suspension in a multilevel surgery concept for obstructive sleep apnea . Otolaryngol Head Neck Surg. 2006. ; 134 ( 5 ): 856 – 861 . [DOI] [PubMed] [Google Scholar]
- 51.Baradaranfar MH, Edalatkhah M, Dadgarnia MH, et al . The effect of uvulopalatopharyngoplasty with tonsillectomy in patients with obstructive sleep apnea . Indian J Otolaryngol Head Neck Surg. 2015. ; 67 ( Suppl 1 ): 29 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbieri M, Missale F, Incandela F, et al . Barbed suspension pharyngoplasty for treatment of lateral pharyngeal wall and palatal collapse in patients affected by OSAHS . Eur Arch Otorhinolaryngol. 2019. ; 276 ( 6 ): 1829 – 1835 . [DOI] [PubMed] [Google Scholar]
- 53.Barrera JE, Dion GR . Predicting surgical response using tensiometry in OSA patients after genioglossus advancement with uvulopalatopharyngoplasty . Otolaryngol Head Neck Surg. 2016. ; 154 ( 3 ): 558 – 563 . [DOI] [PubMed] [Google Scholar]
- 54.Bayır Ö, Acar M, Yüksel E, et al . The effects of anterior palatoplasty on floppy eyelid syndrome patients with obstructive sleep apnea . Laryngoscope. 2016. ; 126 ( 9 ): 2171 – 2175 . [DOI] [PubMed] [Google Scholar]
- 55.Benazzo M, Pagella F, Matti E, et al . Hyoidthyroidpexia as a treatment in multilevel surgery for obstructive sleep apnea . Acta Otolaryngol. 2008. ; 128 ( 6 ): 680 – 684 . [DOI] [PubMed] [Google Scholar]
- 56.Bettega G, Pépin JL, Veale D, Deschaux C, Raphaël B, Lévy P . Obstructive sleep apnea syndrome. Fifty-one consecutive patients treated by maxillofacial surgery . Am J Respir Crit Care Med. 2000. ; 162 ( 2 Pt 1 ): 641 – 649 . [DOI] [PubMed] [Google Scholar]
- 57.Bican A, Kahraman A, Bora I, Kahveci R, Hakyemez B . What is the efficacy of nasal surgery in patients with obstructive sleep apnea syndrome? J Craniofac Surg. 2010. ; 21 ( 6 ): 1801 – 1806 . [DOI] [PubMed] [Google Scholar]
- 58.Binar M, Akcam T, Karakoc O, Sagkan RI, Musabak U, Gerek M . A new surgical technique versus an old marker: can expansion sphincter pharyngoplasty reduce C-reactive protein levels in patients with obstructive sleep apnea? Eur Arch Otorhinolaryngol. 2017. ; 274 ( 2 ): 829 – 836 . [DOI] [PubMed] [Google Scholar]
- 59.Binar M, Akcam TM, Karakoc O, Sagkan RI, Musabak U, Gerek M . Effect of modern surgical treatment on the inflammatory/anti-inflammatory balance in patients with obstructive sleep apnoea . J Laryngol Otol. 2017. ; 131 ( 8 ): 719 – 727 . [DOI] [PubMed] [Google Scholar]
- 60.Blumen MB, Buchet I, Meulien P, Hausser Hauw C, Neveu H, Chabolle F . Complications/adverse effects of maxillomandibular advancement for the treatment of OSA in regard to outcome . Otolaryngol Head Neck Surg. 2009. ; 141 ( 5 ): 591 – 597 . [DOI] [PubMed] [Google Scholar]
- 61.Blumen MB, Latournerie V, Bequignon E, Guillere L, Chabolle F . Are the obstruction sites visualized on drug-induced sleep endoscopy reliable? Sleep Breath. 2015. ; 19 ( 3 ): 1021 – 1026 . [DOI] [PubMed] [Google Scholar]
- 62.Boon M, Huntley C, Steffen A, et al . ADHERE Registry Investigators . Upper airway stimulation for obstructive sleep apnea: results from the ADHERE registry . Otolaryngol Head Neck Surg. 2018. ; 159 ( 2 ): 379 – 385 . [DOI] [PubMed] [Google Scholar]
- 63.Bostanci A, Bozkurt S, Turhan M . The relationship between the duration of obstructive respiratory events and outcomes of multilevel upper airway surgery in patients with obstructive sleep apnea . Eur Arch Otorhinolaryngol. 2016. ; 273 ( 9 ): 2651 – 2657 . [DOI] [PubMed] [Google Scholar]
- 64.Bowden MT, Kezirian EJ, Utley D, Goode RL . Outcomes of hyoid suspension for the treatment of obstructive sleep apnea . Arch Otolaryngol Head Neck Surg. 2005. ; 131 ( 5 ): 440 – 445 . [DOI] [PubMed] [Google Scholar]
- 65.Bowen AJ, Nowacki AS, Kominsky AH, Trask DK, Benninger MS, Bryson PC . Voice and swallowing outcomes following hypoglossal nerve stimulation for obstructive sleep apnea . Am J Otolaryngol. 2018. ; 39 ( 2 ): 122 – 126 . [DOI] [PubMed] [Google Scholar]
- 66.Boyd SB, Walters AS, Song Y, Wang L . Comparative effectiveness of maxillomandibular advancement and uvulopalatopharyngoplasty for the treatment of moderate to severe obstructive sleep apnea . J Oral Maxillofac Surg. 2013. ; 71 ( 4 ): 743 – 751 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyd SB, Walters AS, Waite P, Harding SM, Song Y . Long-term effectiveness and safety of maxillomandibular advancement for treatment of obstructive sleep apnea . J Clin Sleep Med. 2015. ; 11 ( 7 ): 699 – 708 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brevi BC, Toma L, Pau M, Sesenna E . Counterclockwise rotation of the occlusal plane in the treatment of obstructive sleep apnea syndrome . J Oral Maxillofac Surg. 2011. ; 69 ( 3 ): 917 – 923 . [DOI] [PubMed] [Google Scholar]
- 69.Browaldh N, Bring J, Friberg D . SKUP3: 6 and 24 months follow-up of changes in respiration and sleepiness after modified UPPP . Laryngoscope. 2018. ; 128 ( 5 ): 1238 – 1244 . [DOI] [PubMed] [Google Scholar]
- 70.Cammaroto G, Montevecchi F, D’Agostino G, et al . Palatal surgery in a transoral robotic setting (TORS): preliminary results of a retrospective comparison between uvulopalatopharyngoplasty (UPPP), expansion sphincter pharyngoplasty (ESP) and barbed repositioning pharyngoplasty (BRP) . Acta Otorhinolaryngol Ital. 2017. ; 37 ( 5 ): 406 – 409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Catella C, Micoulaud-Franchi JA, Monteyrol PJ, et al . Development of a standardized evaluation of endobuccal adverse events induced by repeated tongue protrusion with both a dedicated questionnaire and an endobuccal examination . Eur Arch Otorhinolaryngol. 2019. ; 276 ( 3 ): 901 – 909 . [DOI] [PubMed] [Google Scholar]
- 72.Chen S, Shi S, Xia Y, et al . Changes in sleep characteristics and airway obstruction in OSAHS patients with multi-level obstruction following simple UPPP, UPPP-GA, or UPPP-TBA: a prospective, single-center, parallel group study . ORL J Otorhinolaryngol Relat Spec. 2014. ; 76 ( 4 ): 179 – 188 . [DOI] [PubMed] [Google Scholar]
- 73.Cho KS, Koo SK, Lee JK, Hong SL, Capasso R, Roh HJ . Limited palatal muscle resection with tonsillectomy: a novel palatopharyngoplasty technique for obstructive sleep apnea . Auris Nasus Larynx. 2014. ; 41 ( 6 ): 558 – 562 . [DOI] [PubMed] [Google Scholar]
- 74.Choi JH, Kim EJ, Cho WS, et al . Efficacy of single-staged modified uvulopalatopharyngoplasty with nasal surgery in adults with obstructive sleep apnea syndrome . Otolaryngol Head Neck Surg. 2011. ; 144 ( 6 ): 994 – 999 . [DOI] [PubMed] [Google Scholar]
- 75.Choi JH, Kim EJ, Kim YS, et al . Effectiveness of nasal surgery alone on sleep quality, architecture, position, and sleep-disordered breathing in obstructive sleep apnea syndrome with nasal obstruction . Am J Rhinol Allergy. 2011. ; 25 ( 5 ): 338 – 341 . [DOI] [PubMed] [Google Scholar]
- 76.Choi JH, Lee JY, Cha J, Kim K, Hong SN, Lee SH . Predictive models of objective oropharyngeal OSA surgery outcomes: success rate and AHI reduction ratio . PLoS One. 2017. ; 12 ( 9 ): e0185201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi JH, Thomas RJ, Suh SY, et al . Sleep quality change after upper airway surgery in obstructive sleep apnea: electrocardiogram-based cardiopulmonary coupling analysis . Laryngoscope. 2015. ; 125 ( 7 ): 1737 – 1742 . [DOI] [PubMed] [Google Scholar]
- 78.Choi JH, Yi JS, Lee SH, et al . Effect of upper airway surgery on heart rate variability in patients with obstructive sleep apnoea syndrome . J Sleep Res. 2012. ; 21 ( 3 ): 316 – 321 . [DOI] [PubMed] [Google Scholar]
- 79.Choi JH, Jun YJ, Kim TH, et al . Effect of isolated uvulopalatopharyngoplasty on subjective obstructive sleep apnea symptoms . Clin Exp Otorhinolaryngol. 2013. ; 6 ( 3 ): 161 – 165 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cillo JE Jr, Dalton PS, Dattilo DJ . Combined elliptical window genioglossus advancement, hyoid bone suspension, and uvulopalatopharyngoplasty decrease apnea hypopnea index and subjective daytime sleepiness in obstructive sleep apnea . J Oral Maxillofac Surg. 2013. ; 71 ( 10 ): 1729 – 1732 . [DOI] [PubMed] [Google Scholar]
- 81.Conradt R, Hochban W, Brandenburg U, Heitmann J, Peter JH . Long-term follow-up after surgical treatment of obstructive sleep apnoea by maxillomandibular advancement . Eur Respir J. 1997. ; 10 ( 1 ): 123 – 128 . [DOI] [PubMed] [Google Scholar]
- 82.Conradt R, Hochban W, Heitmann J, et al . Sleep fragmentation and daytime vigilance in patients with OSA treated by surgical maxillomandibular advancement compared to CPAP therapy . J Sleep Res. 1998. ; 7 ( 3 ): 217 – 223 . [DOI] [PubMed] [Google Scholar]
- 83.Conway W, Fujita S, Zorick F, et al . Uvulopalatopharyngoplasty. One-year followup . Chest. 1985. ; 88 ( 3 ): 385 – 387 . [DOI] [PubMed] [Google Scholar]
- 84.Cui DM, Han DM, Nicolas B, Hu CL, Wu J, Su MM . Three-dimensional evaluation of nasal surgery in patients with obstructive sleep apnea . Chin Med J. 2016. ; 129 ( 6 ): 651 – 656 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dahlöf P, Norlin-Bagge E, Hedner J, Ejnell H, Hetta J, Hällström T . Improvement in neuropsychological performance following surgical treatment for obstructive sleep apnea syndrome . Acta Otolaryngol. 2002. ; 122 ( 1 ): 86 – 91 . [DOI] [PubMed] [Google Scholar]
- 86.de Paula Soares CF, Cavichio L, Cahali MB . Lateral pharyngoplasty reduces nocturnal blood pressure in patients with obstructive sleep apnea . Laryngoscope. 2014. ; 124 ( 1 ): 311 – 316 . [DOI] [PubMed] [Google Scholar]
- 87.den Herder C, van Tinteren H, de Vries N . Hyoidthyroidpexia: a surgical treatment for sleep apnea syndrome . Laryngoscope. 2005. ; 115 ( 4 ): 740 – 745 . [DOI] [PubMed] [Google Scholar]
- 88.Eastwood PR, Barnes M, Walsh JH, et al . Treating obstructive sleep apnea with hypoglossal nerve stimulation . Sleep. 2011. ; 34 ( 11 ): 1479 – 1486 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eastwood PR, Barnes M, MacKay SG, et al . Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea . Eur Respir J. 2020. ; 55 ( 1 ): 1901320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El-Ahl MA, El-Anwar MW . Expansion pharyngoplasty by new simple suspension sutures without tonsillectomy . Otolaryngol Head Neck Surg. 2016. ; 155 ( 6 ): 1065 – 1068 . [DOI] [PubMed] [Google Scholar]
- 91.El-Anwar MW, Amer HS, Askar SM, Elsobki A, Awad A . Could nasal surgery affect multilevel surgery results for obstructive sleep apnea? J Craniofac Surg. 2018. ; 29 ( 7 ): 1897 – 1899 . [DOI] [PubMed] [Google Scholar]
- 92.El-Anwar MW, Askar S, El-Sinbawy AH, Salem AMH . Single versus double suspension sutures for selected cases of obstructive sleep apnea . Auris Nasus Larynx. 2019. ; 46 ( 5 ): 754 – 757 . [DOI] [PubMed] [Google Scholar]
- 93.Elbassiouny AM . Soft palatal webbing flap palatopharyngoplasty for both soft palatal and oropharyngeal lateral wall collapse in the treatment of snoring and obstructive sleep apnea: a new innovative technique without tonsillectomy . Sleep Breath. 2015. ; 19 ( 2 ): 481 – 487 . [DOI] [PubMed] [Google Scholar]
- 94.Emara TA, Hassan MH, Mohamad AS, Anany AM, Ebrahem AE . Anterolateral advancement pharyngoplasty: a new technique for treatment of obstructive sleep apnea . Otolaryngol Head Neck Surg. 2016. ; 155 ( 4 ): 702 – 707 . [DOI] [PubMed] [Google Scholar]
- 95.Emara TA, Omara TA, Shouman WM . Modified genioglossus advancement and uvulopalatopharyngoplasty in patients with obstructive sleep apnea . Otolaryngol Head Neck Surg. 2011. ; 145 ( 5 ): 865 – 871 . [DOI] [PubMed] [Google Scholar]
- 96.Eun YG, Kim SW, Kwon KH, Byun JY, Lee KH . Single-session radiofrequency tongue base reduction combined with uvulopalatopharyngoplasty for obstructive sleep apnea syndrome . Eur Arch Otorhinolaryngol. 2008. ; 265 ( 12 ): 1495 – 1500 . [DOI] [PubMed] [Google Scholar]
- 97.Eun YG, Kwon KH, Shin SY, Lee KH, Byun JY, Kim SW . Multilevel surgery in patients with rapid eye movement-related obstructive sleep apnea . Otolaryngol Head Neck Surg. 2009. ; 140 ( 4 ): 536 – 541 . [DOI] [PubMed] [Google Scholar]
- 98.Evans SS, Richman J, Cho DY, Withrow K . Increasing preoperative apnea severity improves upper airway stimulation response in OSA treatment . Laryngoscope. 2020. ; 130 ( 2 ): 556 – 560 . [DOI] [PubMed] [Google Scholar]
- 99.Fibbi A, Ameli F, Brocchetti F, Mignosi S, Cabano ME, Semino L . Tongue base suspension and radiofrequency volume reduction: a comparison between 2 techniques for the treatment of sleep-disordered breathing . Am J Otolaryngol. 2009. ; 30 ( 6 ): 401 – 406 . [DOI] [PubMed] [Google Scholar]
- 100.Fiorita A, Scarano E, Mastrapasqua R, et al . Moderate OSAS and turbinate decongestion: surgical efficacy in improving the quality of life and compliance of CPAP using Epworth score and SNOT-20 score . Acta Otorhinolaryngol Ital. 2018. ; 38 ( 3 ): 214 – 221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Friedman M, Duggal P, Joseph NJ . Revision uvulopalatoplasty by Z-palatoplasty . Otolaryngol Head Neck Surg. 2007. ; 136 ( 4 ): 638 – 643 . [DOI] [PubMed] [Google Scholar]
- 102.Friedman M, Ibrahim H, Joseph NJ . Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment . Laryngoscope. 2004. ; 114 ( 3 ): 454 – 459 . [DOI] [PubMed] [Google Scholar]
- 103.Friedman M, Ibrahim H, Lee G, Joseph NJ . Combined uvulopalatopharyngoplasty and radiofrequency tongue base reduction for treatment of obstructive sleep apnea/hypopnea syndrome . Otolaryngol Head Neck Surg. 2003. ; 129 ( 6 ): 611 – 621 . [DOI] [PubMed] [Google Scholar]
- 104.Friedman M, Jacobowitz O, Hwang MS, et al . Targeted hypoglossal nerve stimulation for the treatment of obstructive sleep apnea: six-month results . Laryngoscope. 2016. ; 126 ( 11 ): 2618 – 2623 . [DOI] [PubMed] [Google Scholar]
- 105.Gerbino G, Bianchi FA, Verzé L, Ramieri G . Soft tissue changes after maxillo-mandibular advancement in OSAS patients: a three-dimensional study . J Craniomaxillofac Surg. 2014. ; 42 ( 1 ): 66 – 72 . [DOI] [PubMed] [Google Scholar]
- 106.Giarda M, Brucoli M, Arcuri F, Benech R, Braghiroli A, Benech A . Efficacy and safety of maxillomandibular advancement in treatment of obstructive sleep apnoea syndrome . Acta Otorhinolaryngol Ital. 2013. ; 33 ( 1 ): 43 – 46 . [PMC free article] [PubMed] [Google Scholar]
- 107.Goh YH, Lim KA . Modified maxillomandibular advancement for the treatment of obstructive sleep apnea: a preliminary report . Laryngoscope. 2003. ; 113 ( 9 ): 1577 – 1582 . [DOI] [PubMed] [Google Scholar]
- 108.Goodday RH, Bourque SE, Edwards PB . Objective and subjective outcomes following maxillomandibular advancement surgery for treatment of patients with extremely severe obstructive sleep apnea (apnea-hypopnea index >100) . J Oral Maxillofac Surg. 2016. ; 74 ( 3 ): 583 – 589 . [DOI] [PubMed] [Google Scholar]
- 109.Gunawardena I, Robinson S, MacKay S, et al . Submucosal lingualplasty for adult obstructive sleep apnea . Otolaryngol Head Neck Surg. 2013. ; 148 ( 1 ): 157 – 165 . [DOI] [PubMed] [Google Scholar]
- 110.Günbey E, Karabulut I, Karabulut H, Zaim M . Impact of multilevel surgical treatment on mean platelet volume in patients with obstructive sleep apnea syndrome . J Craniofac Surg. 2015. ; 26 ( 4 ): 1287 – 1289 . [DOI] [PubMed] [Google Scholar]
- 111.Ha JG, Lee Y, Nam JS, et al . Can drug-induced sleep endoscopy improve the success rates of tongue base surgery? J Otolaryngol Head Neck Surg. 2020. ; 49 ( 1 ): 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haavisto L, Suonpää J . Complications of uvulopalatopharyngoplasty . Clin Otolaryngol Allied Sci. 1994. ; 19 ( 3 ): 243 – 247 . [DOI] [PubMed] [Google Scholar]
- 113.Hamans E, Boudewyns A, Stuck BA, et al . Adjustable tongue advancement for obstructive sleep apnea: a pilot study . Ann Otol Rhinol Laryngol. 2008. ; 117 ( 11 ): 815 – 823 . [DOI] [PubMed] [Google Scholar]
- 114.Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU . Patient-reported outcome: results of the multicenter German post-market study . Eur Arch Otorhinolaryngol. 2018. ; 275 ( 7 ): 1913 – 1919 . [DOI] [PubMed] [Google Scholar]
- 115.Hasselbacher K, Seitz A, Abrams N, Wollenberg B, Steffen A . Complete concentric collapse at the soft palate in sleep endoscopy: what change is possible after UPPP in patients with CPAP failure? Sleep Breath. 2018. ; 22 ( 4 ): 933 – 938 . [DOI] [PubMed] [Google Scholar]
- 116.Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B . Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience . Eur Arch Otorhinolaryngol. 2017. ; 274 ( 3 ): 1727 – 1734 . [DOI] [PubMed] [Google Scholar]
- 117.Heiser C, Steffen A, Boon M, et al . ADHERE registry investigators . Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry . Eur Respir J. 2019. ; 53 ( 1 ): 1801405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hendler BH, Costello BJ, Silverstein K, Yen D, Goldberg A . A protocol for uvulopalatopharyngoplasty, mortised genioplasty, and maxillomandibular advancement in patients with obstructive sleep apnea: an analysis of 40 cases . J Oral Maxillofac Surg. 2001. ; 59 ( 8 ): 892 – 897 . [DOI] [PubMed] [Google Scholar]
- 119.Hester TO, Phillips B, Archer SM . Surgery for obstructive sleep apnea: effects on sleep, breathing, and oxygenation . South Med J. 1995. ; 88 ( 9 ): 907 – 910 . [DOI] [PubMed] [Google Scholar]
- 120.Hobson JC, Robinson S, Antic NA, et al . What is “success” following surgery for obstructive sleep apnea? The effect of different polysomnographic scoring systems . Laryngoscope. 2012. ; 122 ( 8 ): 1878 – 1881 . [DOI] [PubMed] [Google Scholar]
- 121.Hochban W, Conradt R, Brandenburg U, Heitmann J, Peter JH . Surgical maxillofacial treatment of obstructive sleep apnea . Plast Reconstr Surg. 1997. ; 99 ( 3 ): 619 – 626 . [DOI] [PubMed] [Google Scholar]
- 122.Holmlund T, Franklin KA, Levring Jäghagen E, et al . Tonsillectomy in adults with obstructive sleep apnea . Laryngoscope. 2016. ; 126 ( 12 ): 2859 – 2862 . [DOI] [PubMed] [Google Scholar]
- 123.Hsieh YJ, Liao YF, Chen NH, Chen YR . Changes in the calibre of the upper airway and the surrounding structures after maxillomandibular advancement for obstructive sleep apnoea . Br J Oral Maxillofac Surg. 2014. ; 52 ( 5 ): 445 – 451 . [DOI] [PubMed] [Google Scholar]
- 124.Huang CC, Lin WC, Chen HL, et al . Improvement of baroreflex sensitivity in patients with obstructive sleep apnea following surgical treatment . Clin Neurophysiol. 2016. ; 127 ( 1 ): 544 – 550 . [DOI] [PubMed] [Google Scholar]
- 125.Huang TW, Cheng PW . Microdebrider-assisted extended uvulopalatoplasty: an effective and safe technique for selected patients with obstructive sleep apnea syndrome . Arch Otolaryngol Head Neck Surg. 2008. ; 134 ( 2 ): 141 – 145 . [DOI] [PubMed] [Google Scholar]
- 126.Huntley C, Chou DW, Doghramji K, Boon M . Comparing upper airway stimulation to expansion sphincter pharyngoplasty: a single university experience . Ann Otol Rhinol Laryngol. 2018. ; 127 ( 6 ): 379 – 383 . [DOI] [PubMed] [Google Scholar]
- 127.Huntley C, Steffen A, Doghramji K, Hofauer B, Heiser C, Boon M . Upper airway stimulation in patients with obstructive sleep apnea and an elevated body mass index: a multi-institutional review . Laryngoscope. 2018. ; 128 ( 10 ): 2425 – 2428 . [DOI] [PubMed] [Google Scholar]
- 128.Huntley C, Vasconcellos A, Doghramji K, Hofauer B, Heiser C, Boon M . Upper airway stimulation in patients who have undergone unsuccessful prior palate surgery: an initial evaluation . Otolaryngol Head Neck Surg. 2018. ; 159 ( 5 ): 938 – 940 . [DOI] [PubMed] [Google Scholar]
- 129.Huntley C, Topf MC, Christopher V, Doghramji K, Curry J, Boon M . Comparing upper airway stimulation to transoral robotic base of tongue resection for treatment of obstructive sleep apnea . Laryngoscope. 2019. ; 129 ( 4 ): 1010 – 1013 . [DOI] [PubMed] [Google Scholar]
- 130.Huntley C, Vasconcellos A, Mullen M, et al . The impact of upper airway stimulation on swallowing function . Ear Nose Throat J. 2019. ; 98 ( 8 ): 496 – 499 . [DOI] [PubMed] [Google Scholar]
- 131.Islam S, Selbong U, Taylor CJ, Ormiston IW . Does a patient’s Mallampati score predict outcome after maxillomandibular advancement for obstructive sleep apnoea? Br J Oral Maxillofac Surg. 2015. ; 53 ( 1 ): 23 – 27 . [DOI] [PubMed] [Google Scholar]
- 132.Islam S, Taylor CJ, Ormiston IW . The predictive value of obstructive sleep apnoea severity on clinical outcomes following maxillomandibular advancement surgery . Br J Oral Maxillofac Surg. 2015. ; 53 ( 3 ): 263 – 267 . [DOI] [PubMed] [Google Scholar]
- 133.Jacobowitz O . Palatal and tongue base surgery for surgical treatment of obstructive sleep apnea: a prospective study . Otolaryngol Head Neck Surg. 2006. ; 135 ( 2 ): 258 – 264 . [DOI] [PubMed] [Google Scholar]
- 134.Jung SY, Rhee EH, Al-Dilaijan KF, Kim SW, Min JY . Impact of AASM 2012 recommended hypopnea criteria on surgical outcomes for obstructive sleep apnea . Laryngoscope. 2020. ; 130 ( 3 ): 825 – 831 . [DOI] [PubMed] [Google Scholar]
- 135.Karakoc O, Binar M, Aydin U, Genc H, Akcam T, Gerek M . A tertiary center experience with velopharyngeal surgical techniques for treatment of snoring and obstructive sleep apnea . Auris Nasus Larynx. 2018. ; 45 ( 3 ): 492 – 498 . [DOI] [PubMed] [Google Scholar]
- 136.Karataylı-Özgürsoy S, Demireller A . Hyoid suspension surgery with UPPP for the treatment of hypopharyngeal airway obstruction in obstructive sleep apnea . Ear Nose Throat J. 2012. ; 91 ( 8 ): 358 – 364 . [PubMed] [Google Scholar]
- 137.Katsantonis GP, Miyazaki S, Walsh JK . Effects of uvulopalatopharyngoplasty on sleep architecture and patterns of obstructed breathing . Laryngoscope. 1990. ; 100 ( 10 ): 1068 – 1072 . [DOI] [PubMed] [Google Scholar]
- 138.Kayhan FT, Kaya KH, Koç AK, et al . Multilevel combined surgery with transoral robotic surgery for obstructive sleep apnea syndrome . J Craniofac Surg. 2016. ; 27 ( 4 ): 1044 – 1048 . [DOI] [PubMed] [Google Scholar]
- 139.Kent DT, Lee JJ, Strollo PJ Jr, Soose RJ . Upper airway stimulation for OSA: early adherence and outcome results of one center . Otolaryngol Head Neck Surg. 2016. ; 155 ( 1 ): 188 – 193 . [DOI] [PubMed] [Google Scholar]
- 140.Kezirian EJ, Malhotra A, Goldberg AN, White DP . Changes in obstructive sleep apnea severity, biomarkers, and quality of life after multilevel surgery . Laryngoscope. 2010. ; 120 ( 7 ): 1481 – 1488 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ . Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the Mayo Clinic experience . Mayo Clin Proc. 2009. ; 84 ( 9 ): 795 – 800 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim H, Kim MS, Lee JE, et al . Treatment outcomes and compliance according to obesity in patients with obstructive sleep apnea . Eur Arch Otorhinolaryngol. 2013. ; 270 ( 11 ): 2885 – 2890 . [DOI] [PubMed] [Google Scholar]
- 143.Kim MJ, Kim BY, Lee DC, et al . A modified uvulopalatal flap with lateral pharyngoplasty for treatment in 92 adults with obstructive sleep apnoea syndrome . Clin Otolaryngol. 2013. ; 38 ( 5 ): 415 – 419 . [DOI] [PubMed] [Google Scholar]
- 144.Kinoshita H, Shibano A, Sakoda T et al . Uvulopalatopharyngoplasty decreases levels of C-reactive protein in patients with obstructive sleep apnea syndrome . Am Heart J. 2006. ; 152 ( 4 ): 692.e1 – 692.e5 . [DOI] [PubMed] [Google Scholar]
- 145.Komada I, Miyazaki S, Okawa M, Nishikawa M, Shimizu T . A new modification of uvulopalatopharyngoplasty for the treatment of obstructive sleep apnea syndrome . Auris Nasus Larynx. 2012. ; 39 ( 1 ): 84 – 89 . [DOI] [PubMed] [Google Scholar]
- 146.Lai CC, Lin PW, Lin HC, et al . Effects of upper airway surgery on daytime sleepiness in nonobese patients with obstructive sleep apnea/hypopnea syndrome . Ann Otol Rhinol Laryngol. 2018. ; 127 ( 12 ): 912 – 918 . [DOI] [PubMed] [Google Scholar]
- 147.Larsson H, Carlsson-Nordlander B, Svanborg E . Long-time follow-up after UPPP for obstructive sleep apnea syndrome. Results of sleep apnea recordings and subjective evaluation 6 months and 2 years after surgery . Acta Otolaryngol. 1991. ; 111 ( 3 ): 582 – 590 . [DOI] [PubMed] [Google Scholar]
- 148.Larsson LH, Carlsson-Nordlander B, Svanborg E . Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome . Laryngoscope. 1994. ; 104 ( 11 Pt 1 ): 1362 – 1368 . [DOI] [PubMed] [Google Scholar]
- 149.Lee CH, Mo JH, Seo BS, Kim DY, Yoon IY, Kim JW . Mouth opening during sleep may be a critical predictor of surgical outcome after uvulopalatopharyngoplasty for obstructive sleep apnea . J Clin Sleep Med. 2010. ; 6 ( 2 ): 157 – 162 . [PMC free article] [PubMed] [Google Scholar]
- 150.Lee JM, Weinstein GS, O’Malley BW Jr, Thaler ER . Transoral robot-assisted lingual tonsillectomy and uvulopalatopharyngoplasty for obstructive sleep apnea . Ann Otol Rhinol Laryngol. 2012. ; 121 ( 10 ): 635 – 639 . [DOI] [PubMed] [Google Scholar]
- 151.Lee LA, Huang CG, Chen NH, Wang CL, Fang TJ, Li HY . Severity of obstructive sleep apnea syndrome and high-sensitivity C-reactive protein reduced after relocation pharyngoplasty . Otolaryngol Head Neck Surg. 2011. ; 144 ( 4 ): 632 – 638 . [DOI] [PubMed] [Google Scholar]
- 152.Lee MY, Lin CC, Lee KS, et al . Effect of uvulopalatopharyngoplasty on endothelial function in obstructive sleep apnea . Otolaryngol Head Neck Surg. 2009. ; 140 ( 3 ): 369 – 374 . [DOI] [PubMed] [Google Scholar]
- 153.Lee NR, Givens CD Jr, Wilson J, Robins RB . Staged surgical treatment of obstructive sleep apnea syndrome: a review of 35 patients . J Oral Maxillofac Surg. 1999. ; 57 ( 4 ): 382 – 385 . [DOI] [PubMed] [Google Scholar]
- 154.Lee YC, Lee LA, Li HY . The palatal septal cartilage implantation for snoring and obstructive sleep apnea . Auris Nasus Larynx. 2018. ; 45 ( 6 ): 1199 – 1205 . [DOI] [PubMed] [Google Scholar]
- 155.Li HY, Chen NH, Shu YH, Wang PC . Changes in quality of life and respiratory disturbance after extended uvulopalatal flap surgery in patients with obstructive sleep apnea . Arch Otolaryngol Head Neck Surg. 2004. ; 130 ( 2 ): 195 – 200 . [DOI] [PubMed] [Google Scholar]
- 156.Li HY, Cheng WN, Chuang LP, et al . Positional dependency and surgical success of relocation pharyngoplasty among patients with severe obstructive sleep apnea . Otolaryngol Head Neck Surg. 2013. ; 149 ( 3 ): 506 – 512 . [DOI] [PubMed] [Google Scholar]
- 157.Li HY, Lee LA, Kezirian EJ . Coblation endoscopic lingual lightening (CELL) for obstructive sleep apnea . Eur Arch Otorhinolaryngol. 2016. ; 273 ( 1 ): 231 – 236 . [DOI] [PubMed] [Google Scholar]
- 158.Li HY, Lee LA, Wang PC, Fang TJ, Chen NH . Can nasal surgery improve obstructive sleep apnea: subjective or objective? Am J Rhinol Allergy. 2009. ; 23 ( 6 ): e51 – e55 . [DOI] [PubMed] [Google Scholar]
- 159.Li HY, Lee LA, Yu JF, et al . Changes of snoring sound after relocation pharyngoplasty for obstructive sleep apnoea: the surgery reduces mean intensity in snoring which correlates well with apnoea-hypopnoea index . Clin Otolaryngol. 2015. ; 40 ( 2 ): 98 – 105 . [DOI] [PubMed] [Google Scholar]
- 160.Li HY, Li KK, Chen NH, Wang CJ, Liao YF, Wang PC . Three-dimensional computed tomography and polysomnography findings after extended uvulopalatal flap surgery for obstructive sleep apnea . Am J Otolaryngol. 2005. ; 26 ( 1 ): 7 – 11 . [DOI] [PubMed] [Google Scholar]
- 161.Li HY, Li KK, Chen NH, Wang PC . Modified uvulopalatopharyngoplasty: the extended uvulopalatal flap . Am J Otolaryngol. 2003. ; 24 ( 5 ): 311 – 316 . [DOI] [PubMed] [Google Scholar]
- 162.Li HY, Lin Y, Chen NH, Lee LA, Fang TJ, Wang PC . Improvement in quality of life after nasal surgery alone for patients with obstructive sleep apnea and nasal obstruction . Arch Otolaryngol Head Neck Surg. 2008. ; 134 ( 4 ): 429 – 433 . [DOI] [PubMed] [Google Scholar]
- 163.Li HY, Wang PC, Hsu CY, Lee SW, Chen NH, Liu SA . Combined nasal-palatopharyngeal surgery for obstructive sleep apnea: simultaneous or staged? Acta Otolaryngol. 2005. ; 125 ( 3 ): 298 – 303 . [DOI] [PubMed] [Google Scholar]
- 164.Li HY, Wang PC, Lee LA, Chen NH, Fang TJ . Prediction of uvulopalatopharyngoplasty outcome: anatomy-based staging system versus severity-based staging system . Sleep. 2006. ; 29 ( 12 ): 1537 – 1541 . [DOI] [PubMed] [Google Scholar]
- 165.Li KK, Powell NB, Riley RW, Zonato A, Gervacio L, Guilleminault C . Morbidly obese patients with severe obstructive sleep apnea: is airway reconstructive surgery a viable treatment option? Laryngoscope. 2000. ; 110 ( 6 ): 982 – 987 . [DOI] [PubMed] [Google Scholar]
- 166.Li KK, Riley RW, Powell NB, Gervacio L, Troell RJ, Guilleminault C . Obstructive sleep apnea surgery: patient perspective and polysomnographic results . Otolaryngol Head Neck Surg. 2000. ; 123 ( 5 ): 572 – 575 . [DOI] [PubMed] [Google Scholar]
- 167.Li KK, Riley RW, Powell NB, Guilleminault C . Maxillomandibular advancement for persistent obstructive sleep apnea after phase I surgery in patients without maxillomandibular deficiency . Laryngoscope. 2000. ; 110 ( 10 Pt 1 ): 1684 – 1688 . [DOI] [PubMed] [Google Scholar]
- 168.Li S, Shi H . Lingual artery CTA-guided midline partial glossectomy for treatment of obstructive sleep apnea hypopnea syndrome . Acta Otolaryngol. 2013. ; 133 ( 7 ): 749 – 754 . [DOI] [PubMed] [Google Scholar]
- 169.Li S, Wu D, Bao J, Shi H . The nasopharyngeal tube: a simple and effective tool to indicate the need for uvulopalatopharyngoplasty . Laryngoscope. 2014. ; 124 ( 4 ): 1023 – 1028 . [DOI] [PubMed] [Google Scholar]
- 170.Li S, Wu D, Shi H . Treatment of obstructive sleep apnea hypopnea syndrome caused by glossoptosis with tongue-base suspension . Eur Arch Otorhinolaryngol. 2013. ; 270 ( 11 ): 2915 – 2920 . [DOI] [PubMed] [Google Scholar]
- 171.Li HY, Lee LA, Kezirian EJ, Nakayama M . Suspension palatoplasty for obstructive sleep apnea—a preliminary study . Sci Rep. 2018. ; 8 ( 1 ): 4224 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lim JH, Park P, Wee JH, et al . Evaluation of the success of obstructive sleep apnea surgery using criteria based on long-term symptoms and incident hypertension . Eur Arch Otorhinolaryngol. 2018. ; 275 ( 4 ): 1015 – 1022 . [DOI] [PubMed] [Google Scholar]
- 173.Lin CH, Liao YF, Chen NH, Lo LJ, Chen YR . Three-dimensional computed tomography in obstructive sleep apneics treated by maxillomandibular advancement . Laryngoscope. 2011. ; 121 ( 6 ): 1336 – 1347 . [DOI] [PubMed] [Google Scholar]
- 174.Lin HC, Friedman M, Chang HW, et al . Minimally invasive, single-stage, multilevel surgery for obstructive sleep apnea in Asian patients . JAMA Otolaryngol Head Neck Surg. 2017. ; 143 ( 2 ): 147 – 154 . [DOI] [PubMed] [Google Scholar]
- 175.Lin HC, Friedman M, Chang HW, Su MC, Wilson M . Z-palatopharyngoplasty plus radiofrequency tongue base reduction for moderate/severe obstructive sleep apnea/hypopnea syndrome . Acta Otolaryngol. 2010. ; 130 ( 9 ): 1070 – 1076 . [DOI] [PubMed] [Google Scholar]
- 176.Lin HC, Friedman M, Chang HW, Yalamanchali S . Z-palatopharyngoplasty combined with endoscopic coblator open tongue base resection for severe obstructive sleep apnea/hypopnea syndrome . Otolaryngol Head Neck Surg. 2014. ; 150 ( 6 ): 1078 – 1085 . [DOI] [PubMed] [Google Scholar]
- 177.Lin HS, Rowley JA, Badr MS, et al . Transoral robotic surgery for treatment of obstructive sleep apnea-hypopnea syndrome . Laryngoscope. 2013. ; 123 ( 7 ): 1811 – 1816 . [DOI] [PubMed] [Google Scholar]
- 178.Lin HS, Rowley JA, Folbe AJ, Yoo GH, Badr MS, Chen W . Transoral robotic surgery for treatment of obstructive sleep apnea: factors predicting surgical response . Laryngoscope. 2015. ; 125 ( 4 ): 1013 – 1020 . [DOI] [PubMed] [Google Scholar]
- 179.Lin SW, Chen NH, Li HY, et al . A comparison of the long-term outcome and effects of surgery or continuous positive airway pressure on patients with obstructive sleep apnea syndrome . Laryngoscope. 2006. ; 116 ( 6 ): 1012 – 1016 . [DOI] [PubMed] [Google Scholar]
- 180.Liu SR, Yi HL, Yin SK, et al . Associated predictors of therapeutic response to uvulopharyngopalatoplasty for severe obstructive sleep apnea hypopnea syndrome . Eur Arch Otorhinolaryngol. 2013. ; 270 ( 4 ): 1411 – 1417 . [DOI] [PubMed] [Google Scholar]
- 181.Liu SR, Yi HL, Yin SK, et al . Primary maxillomandibular advancement with concomitant revised uvulopalatopharyngoplasty with uvula preservation for severe obstructive sleep apnea-hypopnea syndrome . J Craniofac Surg. 2012. ; 23 ( 6 ): 1649 – 1653 . [DOI] [PubMed] [Google Scholar]
- 182.Liu SY, Huon LK, Iwasaki T, et al . Efficacy of maxillomandibular advancement examined with drug-induced sleep endoscopy and computational fluid dynamics airflow modeling . Otolaryngol Head Neck Surg. 2016. ; 154 ( 1 ): 189 – 195 . [DOI] [PubMed] [Google Scholar]
- 183.Liu SY, Huon LK, Powell NB, et al . Lateral pharyngeal wall tension after maxillomandibular advancement for obstructive sleep apnea is a marker for surgical success: observations from drug-induced sleep endoscopy . J Oral Maxillofac Surg. 2015. ; 73 ( 8 ): 1575 – 1582 . [DOI] [PubMed] [Google Scholar]
- 184.Lundkvist K, Januszkiewicz A, Friberg D . Uvulopalatopharyngoplasty in 158 OSAS patients failing non-surgical treatment . Acta Otolaryngol. 2009. ; 129 ( 11 ): 1280 – 1286 . [DOI] [PubMed] [Google Scholar]
- 185.Lye KW, Waite PD, Meara D, Wang D . Quality of life evaluation of maxillomandibular advancement surgery for treatment of obstructive sleep apnea . J Oral Maxillofac Surg. 2008. ; 66 ( 5 ): 968 – 972 . [DOI] [PubMed] [Google Scholar]
- 186.MacKay SG, Carney AS, Woods C, et al . Modified uvulopalatopharyngoplasty and coblation channeling of the tongue for obstructive sleep apnea: a multi-centre Australian trial . J Clin Sleep Med. 2013. ; 9 ( 2 ): 117 – 124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.MacKay SG, Jefferson N, Marshall NS . Beyond uvulopalatopharyngoplasty for obstructive sleep apnoea: single surgeon case series of contemporary airway reconstruction . J Laryngol Otol. 2013. ; 127 ( 12 ): 1184 – 1189 . [DOI] [PubMed] [Google Scholar]
- 188.Mahmoud AF, Thaler ER . Upper airway stimulation therapy and prior airway surgery for obstructive sleep apnea . Laryngoscope. 2018. ; 128 ( 6 ): 1486 – 1489 . [DOI] [PubMed] [Google Scholar]
- 189.Mahmoud AF, Thaler ER . Outcomes of hypoglossal nerve upper airway stimulation among patients with isolated retropalatal collapse . Otolaryngol Head Neck Surg. 2019. ; 160 ( 6 ): 1124 – 1129 . [DOI] [PubMed] [Google Scholar]
- 190.Makovey I, Shelgikar AV, Stanley JJ, Robinson A, Aronovich S . Maxillomandibular advancement surgery for patients who are refractory to continuous positive airway pressure: are there predictors of success? J Oral Maxillofac Surg. 2017. ; 75 ( 2 ): 363 – 370 . [DOI] [PubMed] [Google Scholar]
- 191.Manikandhan R, Lakshminarayana G, Sneha P, Ananthnarayanan P, Naveen J, Sailer HF . Impact of mandibular distraction osteogenesis on the oropharyngeal airway in adult patients with obstructive sleep apnea secondary to retroglossal airway obstruction . J Maxillofac Oral Surg. 2014. ; 13 ( 2 ): 92 – 98 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Meraj TS, Muenz DG, Glazer TA, Harvey RS, Spector ME, Hoff PT . Does drug-induced sleep endoscopy predict surgical success in transoral robotic multilevel surgery in obstructive sleep apnea? Laryngoscope. 2017. ; 127 ( 4 ): 971 – 976 . [DOI] [PubMed] [Google Scholar]
- 193.Miller FR, Watson D, Boseley M . The role of the Genial Bone Advancement Trephine system in conjunction with uvulopalatopharyngoplasty in the multilevel management of obstructive sleep apnea . Otolaryngol Head Neck Surg. 2004. ; 130 ( 1 ): 73 – 79 . [DOI] [PubMed] [Google Scholar]
- 194.Miller FR, Watson D, Malis D . Role of the tongue base suspension suture with The Repose System bone screw in the multilevel surgical management of obstructive sleep apnea . Otolaryngol Head Neck Surg. 2002. ; 126 ( 4 ): 392 – 398 . [DOI] [PubMed] [Google Scholar]
- 195.Miyazaki S, Itasaka Y, Tada H, Ishikawa K, Togawa K . Effectiveness of tonsillectomy in adult sleep apnea syndrome . Psychiatry Clin Neurosci. 1998. ; 52 ( 2 ): 222 – 223 . [DOI] [PubMed] [Google Scholar]
- 196.Montevecchi F, Meccariello G, Firinu E, et al . Prospective multicentre study on barbed reposition pharyngoplasty standing alone or as a part of multilevel surgery for sleep apnoea . Clin Otolaryngol. 2018. ; 43 ( 2 ): 483 – 488 . [DOI] [PubMed] [Google Scholar]
- 197.Mora R, Salzano FA, Mora F, Guastini L . Outcomes of uvulopalatopharyngoplasty with harmonic scalpel after failure of continuous positive airway pressure in sleep apnea syndrome . Acta Otolaryngol. 2012. ; 132 ( 3 ): 299 – 304 . [DOI] [PubMed] [Google Scholar]
- 198.Moxness MH, Nordgard S . An observational cohort study of the effects of septoplasty with or without inferior turbinate reduction in patients with obstructive sleep apnea . BMC Ear Nose Throat Disord. 2014. ; 14 : 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mure C, Blumen M, Alali A, Page L, Chabolle F . Surgical ablation of lingual tonsils in the treatment of obstructive sleep apnea . Eur Ann Otorhinolaryngol Head Neck Dis. 2019. ; 136 ( 1 ): 19 – 23 . [DOI] [PubMed] [Google Scholar]
- 200.Mutlu M, Vuralkan E, Akin I, et al . Alteration of serum levels of inflammatory cytokines and polysomnographic indices after uvulopalatal flap surgery in obstructive sleep apnea . Ear Nose Throat J. 2017. ; 96 ( 2 ): 65 – 68 . [DOI] [PubMed] [Google Scholar]
- 201.Nakata S, Noda A, Yanagi E, Suzuki K, Yamamoto H, Nakashima T . Tonsil size and body mass index are important factors for efficacy of simple tonsillectomy in obstructive sleep apnoea syndrome . Clin Otolaryngol. 2006. ; 31 ( 1 ): 41 – 45 . [DOI] [PubMed] [Google Scholar]
- 202.Neruntarat C . Genioglossus advancement and hyoid myotomy under local anesthesia . Otolaryngol Head Neck Surg. 2003. ; 129 ( 1 ): 85 – 91 . [DOI] [PubMed] [Google Scholar]
- 203.Neruntarat C . Uvulopalatal flap for obstructive sleep apnea: short-term and long-term results . Laryngoscope. 2011. ; 121 ( 3 ): 683 – 687 . [DOI] [PubMed] [Google Scholar]
- 204.Omur M, Ozturan D, Elez F, Unver C, Derman S . Tongue base suspension combined with UPPP in severe OSA patients . Otolaryngol Head Neck Surg. 2005. ; 133 ( 2 ): 218 – 223 . [DOI] [PubMed] [Google Scholar]
- 205.Pang KP, Piccin O, Pang EB, Pang KA, Chan YH, Rotenberg BW . Combined expansion pharyngoplasty and anterior palatoplasty for the treatment of OSA . Indian J Otolaryngol Head Neck Surg. 2016. ; 68 ( 4 ): 528 – 533 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pang KP, Pang EB, Pang KA, Vicini C, Chan YH, Rotenberg BW . Upper airway surgery for obstructive sleep apnea reduces blood pressure . Laryngoscope. 2018. ; 128 ( 2 ): 523 – 527 . [DOI] [PubMed] [Google Scholar]
- 207.Pang KP, Baptista PM, Olszewska E, et al . Does drug-induced sleep endoscopy affect surgical outcome? A multicenter study of 326 obstructive sleep apnea patients . Laryngoscope. 2020. ; 130 ( 2 ): 551 – 555 . [DOI] [PubMed] [Google Scholar]
- 208.Pang KP, Vicini C, Montevecchi F, et al . Long-term complications of palate surgery: a multicenter study of 217 patients . Laryngoscope. 2020. ; 130 ( 9 ): 2281 – 2284 . [DOI] [PubMed] [Google Scholar]
- 209.Parikh V, Thaler E, Kato M, et al . Early feasibility of hypoglossal nerve upper airway stimulator in patients with cardiac implantable electronic devices and continuous positive airway pressure-intolerant severe obstructive sleep apnea . Heart Rhythm. 2018. ; 15 ( 8 ): 1165 – 1170 . [DOI] [PubMed] [Google Scholar]
- 210.Park DY, Chung HJ, Park SC, et al . Surgical outcomes of overlapping lateral pharyngoplasty with or without coblator tongue base resection for obstructive sleep apnea . Eur Arch Otorhinolaryngol. 2018. ; 275 ( 5 ): 1189 – 1196 . [DOI] [PubMed] [Google Scholar]
- 211.Peng BG, Lai YQ, Lei HJ, Zhang N, Wang X . Strategies in the clinical diagnosis and surgical treatment of OSAHS with multilevel obstruction . J Int Med Res. 2019. ; 47 ( 4 ): 1533 – 1543 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Peng Y, Zhang L, Hu D, et al . Reduction of internal carotid artery intima-media thickness in patients with moderate-to-severe obstructive sleep apnea syndrome after nasal surgery and uvulopalatopharyngoplasty . Acta Otolaryngol. 2016. ; 136 ( 5 ): 514 – 521 . [DOI] [PubMed] [Google Scholar]
- 213.Philip P, Heiser C, Bioulac S, et al . Hypoglossal nerve stimulation on sleep and level of alertness in OSA: a preliminary study . Neurology. 2018. ; 91 ( 7 ): e615 – e619 . [DOI] [PubMed] [Google Scholar]
- 214.Philip-Joet F, Rey M, Triglia JM, et al . Uvulopalatopharyngoplasty in snorers with sleep apneas: predictive value of presurgical polysomnography . Respiration. 1991. ; 58 ( 2 ): 100 – 105 . [DOI] [PubMed] [Google Scholar]
- 215.Plaza G, Baptista P, O’Connor-Reina C, Bosco G, Pérez-Martín N, Pang KP . Prospective multi-center study on expansion sphincter pharyngoplasty . Acta Otolaryngol. 2019. ; 139 ( 2 ): 219 – 222 . [DOI] [PubMed] [Google Scholar]
- 216.Riley RW, Powell NB, Li KK, Troell RJ, Guilleminault C . Surgery and obstructive sleep apnea: long-term clinical outcomes . Otolaryngol Head Neck Surg. 2000. ; 122 ( 3 ): 415 – 421 . [DOI] [PubMed] [Google Scholar]
- 217.Robinson S, Chia M, Carney AS, Chawla S, Harris P, Esterman A . Upper airway reconstructive surgery long-term quality-of-life outcomes compared with CPAP for adult obstructive sleep apnea . Otolaryngol Head Neck Surg. 2009. ; 141 ( 2 ): 257 – 263 . [DOI] [PubMed] [Google Scholar]
- 218.Ronchi P, Cinquini V, Ambrosoli A, Caprioglio A . Maxillomandibular advancement in obstructive sleep apnea syndrome patients: a restrospective study on the sagittal cephalometric variables . J Oral Maxillofac Res. 2013. ; 4 ( 2 ): e5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rotenberg BW, Theriault J, Gottesman S . Redefining the timing of surgery for obstructive sleep apnea in anatomically favorable patients . Laryngoscope. 2014. ; 124 ( Suppl 4 ): S1 – 9 . [DOI] [PubMed] [Google Scholar]
- 220.Roustan V, Barbieri M, Incandela F, et al . Transoral glossoepiglottopexy in the treatment of adult obstructive sleep apnoea: a surgical approach . Acta Otorhinolaryngol Ital. 2018. ; 38 ( 1 ): 38 – 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ryan CF, Dickson RI, Lowe AA, Blokmanis A, Fleetham JA . Upper airway measurements predict response to uvulopalatopharyngoplasty in obstructive sleep apnea . Laryngoscope. 1990. ; 100 ( 3 ): 248 – 253 . [DOI] [PubMed] [Google Scholar]
- 222.Salapatas AM, Bonzelaar LB, Hwang MS, et al . Impact of minimally invasive multilevel surgery on mild/moderate OSA . Otolaryngol Head Neck Surg. 2016. ; 155 ( 4 ): 695 – 701 . [DOI] [PubMed] [Google Scholar]
- 223.Sanders MH, Costantino JP, Johnson JT . Polysomnography early after uvulopalatopharyngoplasty as a predictor of late postoperative results . Chest. 1990. ; 97 ( 4 ): 913 – 919 . [DOI] [PubMed] [Google Scholar]
- 224.dos Santos Junior JF, Abrahão M, Gregório LC, Zonato AI, Gumieiro EH. Genioplasty for genioglossus muscle advancement in patients with obstructive sleep apnea-hypopnea syndrome and mandibular retrognathia . Braz J Otorhinolaryngol. 2007. ; 73 ( 4 ): 480 – 486 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sarber KM, Chang KW, Epperson MV, et al . Hypoglossal nerve stimulation in veterans with obstructive sleep apnea . Laryngoscope. 2020. ; 130 ( 9 ): 2275 – 2280 . [DOI] [PubMed] [Google Scholar]
- 226.Schendel SA, Broujerdi JA, Jacobson RL . Three-dimensional upper-airway changes with maxillomandibular advancement for obstructive sleep apnea treatment . Am J Orthod Dentofacial Orthop. 2014. ; 146 ( 3 ): 385 – 393 . [DOI] [PubMed] [Google Scholar]
- 227.Schwab RJ, Wang SH, Verbraecken J, et al . Anatomic predictors of response and mechanism of action of upper airway stimulation therapy in patients with obstructive sleep apnea . Sleep. 2018. ; 41 ( 4 ): zsy021 . [DOI] [PubMed] [Google Scholar]
- 228.Sezen OS, Aydin E, Eraslan G, Haytoglu S, Coskuner T, Unver S . Modified tongue base suspension for multilevel or single level obstructions in sleep apnea: clinical and radiologic results . Auris Nasus Larynx. 2011. ; 38 ( 4 ): 487 – 494 . [DOI] [PubMed] [Google Scholar]
- 229.Shah J, Russell JO, Waters T, Kominsky AH, Trask D . Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience . Am J Otolaryngol. 2018. ; 39 ( 3 ): 266 – 270 . [DOI] [PubMed] [Google Scholar]
- 230.Shin HW, Park JH, Park JW, et al . Effects of surgical vs. nonsurgical therapy on erectile dysfunction and quality of life in obstructive sleep apnea syndrome: a pilot study . J Sex Med. 2013. ; 10 ( 8 ): 2053 – 2059 . [DOI] [PubMed] [Google Scholar]
- 231.Shine NP, Lewis RH . Transpalatal advancement pharyngoplasty for obstructive sleep apnea syndrome: results and analysis of failures . Arch Otolaryngol Head Neck Surg. 2009. ; 135 ( 5 ): 434 – 438 . [DOI] [PubMed] [Google Scholar]
- 232.Shuaib SW, Undavia S, Lin J, Johnson CM Jr, Stupak HD . Can functional septorhinoplasty independently treat obstructive sleep apnea? Plast Reconstr Surg. 2015. ; 135 ( 6 ): 1554 – 1565 . [DOI] [PubMed] [Google Scholar]
- 233.Simsek G, Haytoglu S, Muluk NB, Arikan OK, Cortuk M, Kiraz K . Mean platelet volume decreases in adult patients with obstructive sleep apnea after uvulopalatal flap surgery . J Craniofac Surg. 2015. ; 26 ( 7 ): 2152 – 2154 . [DOI] [PubMed] [Google Scholar]
- 234.Sorrenti G, Piccin O, Latini G, Scaramuzzino G, Mondini S, Rinaldi Ceroni A . Tongue base suspension technique in obstructive sleep apnea: personal experience . Acta Otorhinolaryngol Ital. 2003. ; 23 ( 4 ): 274 – 280 . [PubMed] [Google Scholar]
- 235.Steffen A, Hartmann JT, König IR, Ravesloot MJL, Hofauer B, Heiser C . Evaluation of body position in upper airway stimulation for obstructive sleep apnea—is continuous voltage sufficient enough? Sleep Breath. 2018. ; 22 ( 4 ): 1207 – 1212 . [DOI] [PubMed] [Google Scholar]
- 236.Steffen A, Kilic A, König IR, Suurna MV, Hofauer B, Heiser C . Tongue motion variability with changes of upper airway stimulation electrode configuration and effects on treatment outcomes . Laryngoscope. 2018. ; 128 ( 8 ): 1970 – 1976 . [DOI] [PubMed] [Google Scholar]
- 237.Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C . Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study . Laryngoscope. 2018. ; 128 ( 2 ): 509 – 515 . [DOI] [PubMed] [Google Scholar]
- 238.Steffen A, Abrams N, Suurna MV, Wollenberg B, Hasselbacher K . Upper-airway stimulation before, after, or without uvulopalatopharyngoplasty: a two-year perspective . Laryngoscope. 2019. ; 129 ( 2 ): 514 – 518 . [DOI] [PubMed] [Google Scholar]
- 239.Strollo PJ Jr, Soose RJ, Maurer JT, et al . STAR Trial Group . Upper-airway stimulation for obstructive sleep apnea . N Engl J Med. 2014. ; 370 ( 2 ): 139 – 149 . [DOI] [PubMed] [Google Scholar]
- 240.Suh GD . Evaluation of open midline glossectomy in the multilevel surgical management of obstructive sleep apnea syndrome . Otolaryngol Head Neck Surg. 2013. ; 148 ( 1 ): 166 – 171 . [DOI] [PubMed] [Google Scholar]
- 241.Sun X, Yi H, Cao Z, Yin S . Reorganization of sleep architecture after surgery for OSAHS . Acta Otolaryngol. 2008. ; 128 ( 11 ): 1242 – 1247 . [DOI] [PubMed] [Google Scholar]
- 242.Sundman J, Browaldh N, Fehrm J, Friberg D . Eight-year follow-up of modified uvulopalatopharyngoplasty in patients with obstructive sleep apnea . Laryngoscope. 2021. ; 131 ( 1 ): E307 – E313 . [DOI] [PubMed] [Google Scholar]
- 243.Süslü AE, Pamuk G, Pamuk AE, Özer S, Jafarov S, Önerci TM . Effects of expansion sphincter pharyngoplasty on the apnea-hypopnea index and heart rate variability . J Oral Maxillofac Surg. 2017. ; 75 ( 12 ): 2650 – 2657 . [DOI] [PubMed] [Google Scholar]
- 244.Tan LT, Tan AK, Hsu PP, et al . Effects of tonsillectomy on sleep study parameters in adult patients with obstructive sleep apnea—a prospective study . Sleep Breath. 2014. ; 18 ( 2 ): 265 – 268 . [DOI] [PubMed] [Google Scholar]
- 245.Thaler ER, Rassekh CH, Lee JM, Weinstein GS, O’Malley BW Jr . Outcomes for multilevel surgery for sleep apnea: obstructive sleep apnea, transoral robotic surgery, and uvulopalatopharyngoplasty . Laryngoscope. 2016. ; 126 ( 1 ): 266 – 269 . [DOI] [PubMed] [Google Scholar]
- 246.Thaler E, Schwab R, Maurer J, et al . Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy . Laryngoscope. 2020. ; 130 ( 5 ): 1333 – 1338 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Toh ST, Han HJ, Tay HN, Kiong KL . Transoral robotic surgery for obstructive sleep apnea in Asian patients: a Singapore sleep centre experience . JAMA Otolaryngol Head Neck Surg. 2014. ; 140 ( 7 ): 624 – 629 . [DOI] [PubMed] [Google Scholar]
- 248.Tsui WK, Yang Y, McGrath C, Leung YY . Improvement in quality of life after skeletal advancement surgery in patients with moderate-to-severe obstructive sleep apnoea: a longitudinal study . Int J Oral Maxillofac Surg. 2020. ; 49 ( 3 ): 333 – 341 . [DOI] [PubMed] [Google Scholar]
- 249.Tunçel U, Inançlı HM, Kürkçüoğlu SS, Enoz M . A comparison of unilevel and multilevel surgery in obstructive sleep apnea syndrome . Ear Nose Throat J. 2012. ; 91 ( 8 ): E13 – E18 . [PubMed] [Google Scholar]
- 250.Turhan M, Bostanci A, Akdag M . The impact of modified tongue base suspension on CPAP levels in patients with severe OSA . Eur Arch Otorhinolaryngol. 2015. ; 272 ( 4 ): 995 – 1000 . [DOI] [PubMed] [Google Scholar]
- 251.Turhan M, Bostanci A, Bozkurt S . Predicting the outcome of modified tongue base suspension combined with uvulopalatopharyngoplasty . Eur Arch Otorhinolaryngol. 2015. ; 272 ( 11 ): 3411 – 3416 . [DOI] [PubMed] [Google Scholar]
- 252.Turhan M, Bostanci A . Robotic tongue-base resection combined with tongue-base suspension for obstructive sleep apnea . Laryngoscope. 2020. ; 130 ( 9 ): 2285 – 2291 . [DOI] [PubMed] [Google Scholar]
- 253.Van de Heyning PH, Badr MS, Baskin JZ, et al . Implanted upper airway stimulation device for obstructive sleep apnea . Laryngoscope. 2012. ; 122 ( 7 ): 1626 – 1633 . [DOI] [PubMed] [Google Scholar]
- 254.Varghese R, Adams NG, Slocumb NL, Viozzi CF, Ramar K, Olson EJ . Maxillomandibular advancement in the management of obstructive sleep apnea . Int J Otolaryngol. 2012. ; 2012 : 373025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Verse T, Maurer JT, Pirsig W . Effect of nasal surgery on sleep-related breathing disorders . Laryngoscope. 2002. ; 112 ( 1 ): 64 – 68 . [DOI] [PubMed] [Google Scholar]
- 256.Vicini C, Dallan I, Canzi P, Frassineti S, La Pietra MG, Montevecchi F . Transoral robotic tongue base resection in obstructive sleep apnoea-hypopnoea syndrome: a preliminary report . ORL J Otorhinolaryngol Relat Spec. 2010. ; 72 ( 1 ): 22 – 27 . [DOI] [PubMed] [Google Scholar]
- 257.Vicini C, Hendawy E, Campanini A, et al . Barbed reposition pharyngoplasty (BRP) for OSAHS: a feasibility, safety, efficacy and teachability pilot study. “We are on the giant’s shoulders.” Eur Arch Otorhinolaryngol. 2015. ; 272 ( 10 ): 3065 – 3070 . [DOI] [PubMed] [Google Scholar]
- 258.Vicini C, Montevecchi F, Campanini A, et al . Clinical outcomes and complications associated with TORS for OSAHS: a benchmark for evaluating an emerging surgical technology in a targeted application for benign disease . ORL J Otorhinolaryngol Relat Spec. 2014. ; 76 ( 2 ): 63 – 69 . [DOI] [PubMed] [Google Scholar]
- 259.Vicini C, Montevecchi F, Pang K, et al . Combined transoral robotic tongue base surgery and palate surgery in obstructive sleep apnea-hypopnea syndrome: expansion sphincter pharyngoplasty versus uvulopalatopharyngoplasty . Head Neck. 2014. ; 36 ( 1 ): 77 – 83 . [DOI] [PubMed] [Google Scholar]
- 260.Vigneron A, Tamisier R, Orset E, Pepin JL, Bettega G . Maxillomandibular advancement for obstructive sleep apnea syndrome treatment: long-term results . J Craniomaxillofac Surg. 2017. ; 45 ( 2 ): 183 – 191 . [DOI] [PubMed] [Google Scholar]
- 261.Vilaseca I, Morelló A, Montserrat JM, Santamaría J, Iranzo A . Usefulness of uvulopalatopharyngoplasty with genioglossus and hyoid advancement in the treatment of obstructive sleep apnea . Arch Otolaryngol Head Neck Surg. 2002. ; 128 ( 4 ): 435 – 440 . [DOI] [PubMed] [Google Scholar]
- 262.Walia HK, Thompson NR, Strohl KP, et al . Upper airway stimulation vs positive airway pressure impact on BP and sleepiness symptoms in OSA . Chest. 2020. ; 157 ( 1 ): 173 – 183 . [DOI] [PubMed] [Google Scholar]
- 263.Walker EB, Frith RW, Harding DA, Cant BR . Uvulopalatopharyngoplasty in severe idiopathic obstructive sleep apnoea syndrome . Thorax. 1989. ; 44 ( 3 ): 205 – 208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang L, Liu JX, Yang XL, Yang CW, Qin YX . Z-palatoplasty and tongue radiofrequency for patients with small tonsils . Otolaryngol Head Neck Surg. 2013. ; 148 ( 5 ): 873 – 877 . [DOI] [PubMed] [Google Scholar]
- 265.Weaver EM, Woodson BT, Yueh B, et al . SLEEP Study Investigators . Studying Life Effects & Effectiveness of Palatopharyngoplasty (SLEEP) study: subjective outcomes of isolated uvulopalatopharyngoplasty . Otolaryngol Head Neck Surg. 2011. ; 144 ( 4 ): 623 – 631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wee JH, Tan K, Lee WH, Rhee CS, Kim JW . Evaluation of coblation lingual tonsil removal technique for obstructive sleep apnea in Asians: preliminary results of surgical morbidity and prognosticators . Eur Arch Otorhinolaryngol. 2015. ; 272 ( 9 ): 2327 – 2333 . [DOI] [PubMed] [Google Scholar]
- 267.Wetmore SJ, Scrima L, Snyderman NL, Hiller FC . Postoperative evaluation of sleep apnea after uvulopalatopharyngoplasty . Laryngoscope. 1986. ; 96 ( 7 ): 738 – 741 . [DOI] [PubMed] [Google Scholar]
- 268.Woodson BT, Robinson S, Lim HJ . Transpalatal advancement pharyngoplasty outcomes compared with uvulopalatopharyngoplasty . Otolaryngol Head Neck Surg. 2005. ; 133 ( 2 ): 211 – 217 . [DOI] [PubMed] [Google Scholar]
- 269.Xiao Y, Han D, Zang H, Wang D . The effectiveness of nasal surgery on psychological symptoms in patients with obstructive sleep apnea and nasal obstruction . Acta Otolaryngol. 2016. ; 136 ( 6 ): 626 – 632 . [DOI] [PubMed] [Google Scholar]
- 270.Yaremchuk K, Tacia B, Peterson E, Roth T . Change in Epworth Sleepiness Scale after surgical treatment of obstructive sleep apnea . Laryngoscope. 2011. ; 121 ( 7 ): 1590 – 1593 . [DOI] [PubMed] [Google Scholar]
- 271.Yi HL, Sun XQ, Chen B, et al . Z-palatopharyngoplasty plus genioglossus advancement and hyoid suspension for obstructive sleep apnea hypopnea syndrome . Otolaryngol Head Neck Surg. 2011. ; 144 ( 3 ): 469 – 473 . [DOI] [PubMed] [Google Scholar]
- 272.Yi HL, Yin SK, Zhang YJ, et al . Z-palatopharyngoplasty for obstructive sleep apnea/hypopnea syndrome . Otolaryngol Head Neck Surg. 2009. ; 140 ( 5 ): 640 – 645 . [DOI] [PubMed] [Google Scholar]
- 273.Yin SK, Yi HL, Lu WY, Guan J, Wu HM, Cao ZY . Genioglossus advancement and hyoid suspension plus uvulopalatopharyngoplasty for severe OSAHS . Otolaryngol Head Neck Surg. 2007. ; 136 ( 4 ): 626 – 631 . [DOI] [PubMed] [Google Scholar]
- 274.Yüksel E, Mutlu M, Bayır Ö, et al . Comparison of postoperative pain scores and dysphagia between anterior palatoplasty and uvulopalatal flap surgeries . Acta Otolaryngol. 2018. ; 138 ( 12 ): 1092 – 1098 . [DOI] [PubMed] [Google Scholar]
- 275.Zhang J, Ye J, Xian J, Wang J, Dong J . Upper airway anatomical changes after velopharyngeal surgery in obstructive sleep apnea patients with small tonsils . Otolaryngol Head Neck Surg. 2013. ; 149 ( 2 ): 335 – 341 . [DOI] [PubMed] [Google Scholar]
- 276.Zhang X, Liu F, Lan X, et al . Clinical observation of submandibular gland transfer for the prevention of xerostomia after radiotherapy for nasopharyngeal carcinoma: a prospective randomized controlled study of 32 cases . Radiat Oncol. 2014. ; 9 : 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zhao D, Li Y, Xian J, Qu Y, Cao X, Ye J . The combination of anatomy and genioglossus activity in predicting the outcomes of velopharyngeal surgery . Otolaryngol Head Neck Surg. 2017. ; 156 ( 3 ): 567 – 574 . [DOI] [PubMed] [Google Scholar]
- 278.Zhu Z, Hofauer B, Wirth M, et al . Selective upper airway stimulation in older patients . Respir Med. 2018. ; 140 : 77 – 81 . [DOI] [PubMed] [Google Scholar]
- 279.Zonato AI, Bittencourt LR, Martinho FL, Gregório LC, Tufik S . Upper airway surgery: the effect on nasal continuous positive airway pressure titration on obstructive sleep apnea patients . Eur Arch Otorhinolaryngol. 2006. ; 263 ( 5 ): 481 – 486 . [DOI] [PubMed] [Google Scholar]
- 280.Yüksel A, Ugur KS, Kizilbulut G, et al . Long-term results of one staged multilevel surgery with tongue suspension surgery or one level palatal surgery for treatment of moderate and severe obstructive sleep apnea . Eur Arch Otorhinolaryngol. 2016. ; 273 ( 5 ): 1227 – 1234 . [DOI] [PubMed] [Google Scholar]
- 281.Li S, Yang J . Nasopharyngeal carcinoma metastasis to the mammary gland: a case report . Oncol Lett. 2015. ; 9 ( 1 ): 275 – 277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Li HY, Wang PC, Hsu CY, Chen NH, Lee LA, Fang TJ . Same-stage palatopharyngeal and hypopharyngeal surgery for severe obstructive sleep apnea . Acta Otolaryngol. 2004. ; 124 ( 7 ): 820 – 826 . [DOI] [PubMed] [Google Scholar]
- 283.Feigel-Guiller B, Drui D, Dimet J, et al . Laparoscopic gastric banding in obese patients with sleep apnea: A 3-year controlled study and follow-up after 10 years . Obes Surg. 2015. ; 25 ( 10 ): 1886 – 1892 . [DOI] [PubMed] [Google Scholar]
- 284.Joosten SA, Khoo JK, Edwards BA, et al . Improvement in obstructive sleep apnea with weight loss is dependent on body position during sleep . Sleep. 2017. ; 40 ( 5 ): zsx047 . [DOI] [PubMed] [Google Scholar]
- 285.Al-Jumaily AM, Ashaat S, Martin B et al . A pilot study on the biomechanical assessment of obstructive sleep apnea pre and post bariatric surgery . Respir Physiol Neurobiol. 2018. ; 250 : 1 – 6 . [DOI] [PubMed] [Google Scholar]
- 286.Bae EK, Lee YJ, Yun CH, Heo Y . Effects of surgical weight loss for treating obstructive sleep apnea . Sleep Breath. 2014. ; 18 ( 4 ): 901 – 905 . [DOI] [PubMed] [Google Scholar]
- 287.Bakker JP, Balachandran JS, Tecilazich F, et al . Pilot study of the effects of bariatric surgery and continuous positive airway pressure treatment on vascular function in obese subjects with obstructive sleep apnoea . Intern Med J. 2013. ; 43 ( 9 ): 993 – 998 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.de Assunção Machado AC, da Silva AMV, Signori LU, da Costa Alvarez G, Mottin CC . Endothelial function of patients with morbid obesity submitted to Roux-en-Y gastric bypass with and without obstructive sleep apnea-hypopnea syndrome . Obes Surg. 2018. ; 28 ( 11 ): 3595 – 3603 . [DOI] [PubMed] [Google Scholar]
- 289.de Raaff CA, Coblijn UK, Ravesloot MJ, de Vries N, de Lange-de Klerk ES, van Wagensveld BA . Persistent moderate or severe obstructive sleep apnea after laparoscopic Roux-en-Y gastric bypass: which patients? Surg Obes Relat Dis. 2016. ; 12 ( 10 ): 1866 – 1872 . [DOI] [PubMed] [Google Scholar]
- 290.del Genio G, Limongelli P, Del Genio F, Motta G, Docimo L, Testa D . Sleeve gastrectomy improves obstructive sleep apnea syndrome (OSAS): 5 year longitudinal study . Surg Obes Relat Dis. 2016. ; 12 ( 1 ): 70 – 74 . [DOI] [PubMed] [Google Scholar]
- 291.Dixon JB, Schachter LM, O’Brien PE . Polysomnography before and after weight loss in obese patients with severe sleep apnea . Int J Obes (Lond). 2005. ; 29 ( 9 ): 1048 – 1054 . [DOI] [PubMed] [Google Scholar]
- 292.Dixon JB, Schachter LM, O’Brien PE, et al . Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial . JAMA. 2012. ; 308 ( 11 ): 1142 – 1149 . [DOI] [PubMed] [Google Scholar]
- 293.Fredheim JM, Rollheim J, Sandbu R, et al . Obstructive sleep apnea after weight loss: a clinical trial comparing gastric bypass and intensive lifestyle intervention . J Clin Sleep Med. 2013. ; 9 ( 5 ): 427 – 432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Fritscher LG, Canani S, Mottin CC, et al . Bariatric surgery in the treatment of obstructive sleep apnea in morbidly obese patients . Respiration. 2007. ; 74 ( 6 ): 647 – 652 . [DOI] [PubMed] [Google Scholar]
- 295.Grunstein RR, Stenlöf K, Hedner JA, Peltonen M, Karason K, Sjöström L . Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity . Sleep. 2007. ; 30 ( 6 ): 703 – 710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Haines KL, Nelson LG, Gonzalez R, et al . Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea . Surgery. 2007. ; 141 ( 3 ): 354 – 358 . [DOI] [PubMed] [Google Scholar]
- 297.Hariri K, Guevara D, Dong M, Kini SU, Herron DM, Fernandez-Ranvier G . Is bariatric surgery effective for co-morbidity resolution in the super-obese patients? Surg Obes Relat Dis. 2018. ; 14 ( 9 ): 1261 – 1268 . [DOI] [PubMed] [Google Scholar]
- 298.Jiao X, Zou J, Zhang P, et al . Roux-en-Y gastric bypass surgery on obstructive sleep apnea-hypopnea syndrome: factors associated with postoperative efficacy . Obes Surg. 2016. ; 26 ( 12 ): 2924 – 2930 . [DOI] [PubMed] [Google Scholar]
- 299.Krieger AC, Youn H, Modersitzki F, et al . Effects of laparoscopic adjustable gastric banding on sleep and metabolism: a 12-month follow-up study . Int J Gen Med. 2012. ; 5 : 975 – 981 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lage-Hansen PR, Holm J, Gram J, Larsen K . Sleep apnoea in patients undergoing bariatric surgery . Dan Med J. 2018. ; 65 ( 2 ): A5440 . [PubMed] [Google Scholar]
- 301.Lankford DA, Proctor CD, Richard R . Continuous positive airway pressure (CPAP) changes in bariatric surgery patients undergoing rapid weight loss . Obes Surg. 2005. ; 15 ( 3 ): 336 – 341 . [DOI] [PubMed] [Google Scholar]
- 302.Lettieri CJ, Eliasson AH, Greenburg DL . Persistence of obstructive sleep apnea after surgical weight loss . J Clin Sleep Med. 2008. ; 4 ( 4 ): 333 – 338 . [PMC free article] [PubMed] [Google Scholar]
- 303.Peromaa-Haavisto P, Tuomilehto H, Kossi J, et al . Obstructive sleep apnea: the effect of bariatric surgery after 12 months. A prospective multicenter trial . Sleep Med. 2017. ; 35 : 85 – 90 . [DOI] [PubMed] [Google Scholar]
- 304.Pillar G, Peled R, Lavie P . Recurrence of sleep apnea without concomitant weight increase 7.5 years after weight reduction surgery . Chest. 1994. ; 106 ( 6 ): 1702 – 1704 . [DOI] [PubMed] [Google Scholar]
- 305.Poitou C, Coupaye M, Laaban JP, et al . Serum amyloid A and obstructive sleep apnea syndrome before and after surgically-induced weight loss in morbidly obese subjects . Obes Surg. 2006. ; 16 ( 11 ): 1475 – 1481 . [DOI] [PubMed] [Google Scholar]
- 306.Priyadarshini P, Singh VP, Aggarwal S, Garg H, Sinha S, Guleria R . Impact of bariatric surgery on obstructive sleep apnoea-hypopnea syndrome in morbidly obese patients . J Minim Access Surg. 2017. ; 13 ( 4 ): 291 – 295 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Shaarawy H, Abdelrahman Sarhan A, Hawary EL . Assessment of the effect of bariatric surgery on severe obstructive sleep apnea patients not tolerating CPAP therapy . Egypt J Chest Dis Tuberc. 2016. ; 65 ( 3 ): 661 – 666 . [Google Scholar]
- 308.Sillo TO, Lloyd-Owen S, White E, et al . The impact of bariatric surgery on the resolution of obstructive sleep apnoea . BMC Res Notes. 2018. ; 11 ( 1 ): 385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sugerman HJ, Fairman RP, Sood RK, Engle K, Wolfe L, Kellum JM . Long-term effects of gastric surgery for treating respiratory insufficiency of obesity . Am J Clin Nutr. 1992. ; 55 ( 2) : 597S – 601S . [DOI] [PubMed] [Google Scholar]
- 310.Valencia-Flores M, Orea A, Herrera M, et al . Effect of bariatric surgery on obstructive sleep apnea and hypopnea syndrome, electrocardiogram, and pulmonary arterial pressure . Obes Surg. 2004. ; 14 ( 6 ): 755 – 762 . [DOI] [PubMed] [Google Scholar]
- 311.Varela JE, Hinojosa MW, Nguyen NT . Resolution of obstructive sleep apnea after laparoscopic gastric bypass . Obes Surg. 2007. ; 17 ( 10 ): 1279 – 1282 . [DOI] [PubMed] [Google Scholar]
- 312.Xu H, Zhang P, Han X, et al . Sex effect on obesity indices and metabolic outcomes in patients with obese obstructive sleep apnea and type 2 diabetes after laparoscopic Roux-en-Y gastric bypass surgery: a preliminary study . Obes Surg. 2016. ; 26 ( 11 ): 2629 – 2639 . [DOI] [PubMed] [Google Scholar]
- 313.Zou J, Zhang P, Yu H, et al . Effect of laparoscopic Roux-en-Y gastric bypass surgery on obstructive sleep apnea in a Chinese population with obesity and T2DM . Obes Surg. 2015. ; 25 ( 8 ): 1446 – 1453 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Friedman M, Soans R, Joseph N, Kakodkar S, Friedman J . The effect of multilevel upper airway surgery on continuous positive airway pressure therapy in obstructive sleep apnea/hypopnea syndrome . Laryngoscope. 2009. ; 119 ( 1 ): 193 – 196 . [DOI] [PubMed] [Google Scholar]
- 315.Liu SA, Li HY, Tsai WC, Chang KM . Associated factors to predict outcomes of uvulopharyngopalatoplasty plus genioglossal advancement for obstructive sleep apnea . Laryngoscope. 2005. ; 115 ( 11 ): 2046 – 2050 . [DOI] [PubMed] [Google Scholar]
- 316.Ronchi P, Novelli G, Colombo L, et al . Effectiveness of maxillo-mandibular advancement in obstructive sleep apnea patients with and without skeletal anomalies . Int J Oral Maxillofac Surg. 2010. ; 39 ( 6 ): 541 – 547 . [DOI] [PubMed] [Google Scholar]
- 317.Sorrenti G, Piccin O, Mondini S, Ceroni AR . One-phase management of severe obstructive sleep apnea: tongue base reduction with hyoepiglottoplasty plus uvulopalatopharyngoplasty . Otolaryngol Head Neck Surg. 2006. ; 135 ( 6 ): 906 – 910 . [DOI] [PubMed] [Google Scholar]