Abstract
Background
Moderately post-operative hypofractionated radiotherapy (HYPO-RT) for breast cancer is a safe and effective strategy as seen in large prospective trials. This study aimed to assess overall and disease-free survivals, local control, and acute and late toxicities in patients treated with HYPO-RT.
Materials and methods
Data from patients submitted to post-operative HYPO-RT, with or without boost, were evaluated retrospectively. Demographic, disease, and treatment characteristics were collected.
Results
From March 2009 to December 2016, 393 patients were treated. Breast-conserving surgery was performed in 94.7%, immediate reconstruction after mastectomy in 6 (1.5%). Most patients (91.2%) had initial stage (0 to IIA), and chemotherapy was performed in 42.0%, HYPO-RT was mainly performed in 15 or 16 daily fractions of 267 cGy and 265 cGy, respectively. The median follow-up was 5.7 years. There were 25 deaths (6.4%) and 17 (4.3%) local recurrences. At 5 and 10 years, the overall survival, local control, and disease-free survival were, respectively, 96.0% and 79.3%, 99.2% and 94.9%, 96.6%, and 91.9%. Acute grade 3 or 4 dermatitis was observed in 0.9%. Late grade 1 or 2 occurred in less than 3% of the patients.
Conclusion
HYPO-RT is a safe and effective radiotherapy regimen with excellent disease control and overall survival rates, with low acute and late toxicity rates.
Keywords: breast cancer, local treatment, hypofractionated radiotherapy
Introduction
Moderately hypofractionated radiotherapy (HYPO-RT) for breast cancer is a safe and effective strategy as seen in European, North American, and Chinese randomized trials [1–10]. When compared to conventional 50–50.4 Gy in 25 to 28 fractions, moderate hypofractionation in 15 or 16 fractions has been found to reach similar survival outcomes, with potentially fewer side effects [1–10]. However, this experience should be validated in countries with different cultures and resource availability. No reports have been identified so far regarding HYPO-RT results in a middle or low-income country environment.
Our hospital is a university general hospital dedicated to the public health system assistance. The Radiotherapy Department is the largest in the country, with 10 linear accelerators and one high dose-rate brachytherapy equipment. Around 500 patients come every day for treatment and breast cancer patients comprise about 25% of them. Since 2009, we have been using HYPO-RT for the treatment of breast cancer in a consistent way.
Therefore, this study had the aim to report the 10-year experience of our institution with HYPO-RT regarding local control, overall and disease-free survivals, and acute and late toxicities.
Materials and methods
This is a single institution retrospective cohort study of non-metastatic breast cancer patients submitted to post-operative HYPO-RT, with or without boost. The study was performed in conformity with the Declaration of Helsinki standards, was registered under the number CAAE 08622119.5.0000.0065 and approved by the local ethical committee (NP 3.196.063).
To be eligible for HYPO-RT, patients needed to be at least 50 years old and have been submitted to breast-conserving surgery or mastectomy. Any combination of chemotherapy was allowed, as was the use of hormone therapy and/or anti-Her2 treatment.
Patients with an indication of regional nodal irradiation, alloplastic breast reconstruction, connective tissue diseases, such as systemic lupus erythematosus and scleroderma, bilateral tumors, or large breasts (breast planning target volume, PTV > 1000 cc or tangential fields separation > 20 cm) were to be excluded.
The radiotherapy protocol started with 42.5 Gy in 17 fractions, with or without a boost of 10 Gy in five fractions. After a short period of experience, the protocol was changed and followed the regimens used in the Canadian and START B trials [6, 7]: 40 Gy or 42.5 Gy total dose in 15 or 16 daily fractions, respectively; with or without boost. The boost dose was delivered in five (total dose 10 Gy) or three (total dose 8 Gy) fractions. Indication of boost followed the institutional protocol: patients younger than 60 years, or margins closer than 5 mm.
Demographic data, disease, and treatment characteristics were collected from patients’ charts. Three-year minimum follow-up or until death was required to be included.
Primary outcomes were local control (local disease-free survival), overall (OS) and disease-free survivals (DFS), analyzed from the date of diagnosis. Local control was defined as the absence of disease in the irradiated areas. Recurrences were categorized as local (breast or surgical bed), regional (lymphatic drainage of the axillary, supraclavicular fossa or internal mammary chain), or distant (metastases), and any association of these.
Acute toxicities were considered during and up to 3 months after the end of radiotherapy, and late, thereafter. Toxicities were assessed according to the RTOG criteria [11]. The most severe toxicity presented was reported.
Statistical analysis
Descriptive and frequency analyses were performed with calculation of mean, standard deviation, and median values. Correlations were evaluated by Pearson’s chi-square method. The cumulative incidence of local recurrences, OS and DFS were calculated using the Kaplan-Meier method with comparisons between variables made by the Log-rank test. IBM SPSS Statistics Software v.20 (Chicago, Illinois) was used for the analysis with the significance level set at 5% (p ≤ 0.05).
Results
From March 2009 to December 2015, 393 patients (396 breasts) were treated. Data of demographic and clinical characteristics, as well as systemic treatment, were based on the 393 evaluated patients. All variables related to tumor characteristics and local treatment are described regarding the 396 treated breasts (Tab. 1).
Table 1.
Characteristics of the 393 evaluated patients and 396 treated breasts
| Characteristic | N | % |
|---|---|---|
|
| ||
| Age (years) | ||
| Mean (± standard deviation) | 63.9 (± 10.8) | |
| Median | 63.7 | |
| Range | 34 to 91 | |
|
| ||
| BMI [kg/m 2 ] | ||
| Mean (± standard deviation) | 28.2 (± 5.3) | |
| Median | 27.6 | |
| Range | 15.4 to 56.0 | |
|
| ||
| Comorbidities | ||
| Diabetes mellitus | 85 | 21.6 |
| Arterial hypertension | 215 | 54.7 |
| Smoker | 90 | 22.9 |
|
| ||
| Breast side | ||
| Right | 213 | 54.2 |
| Left | 177 | 45.0 |
| Bilateral | 3 | 0.8 |
|
| ||
| Histology * | ||
| Ductal carcinoma in situ | 20 | 5.1 |
| Invasive carcinoma | 324 | 81.8 |
| Invasive lobular carcinoma | 20 | 5.1 |
| Others | 32 | 8.1 |
|
| ||
| Estrogen receptor positive* | 349 | 88.1 |
|
| ||
| Progesterone receptor positive* | 306 | 77.3 |
|
| ||
| HER-2 Positive* | 22 | 5.6 |
|
| ||
| Luminal A or B “like”* | 338 | 85.4 |
|
| ||
| Triple negative* | 36 | 9.1 |
|
| ||
| Clinical stage (AJCC 7 th ed) * | ||
| 0 | 23 | 5.8 |
| I–IIA | 235 | 59.4 |
| IIB–IIIB | 138 | 34.8 |
|
| ||
| Surgery * | ||
| Conservative | 375 | 94.7 |
| Nipple-sparing mastectomy | 4 | 1.0 |
| Modified radical mastectomy | 17 | 4.3 |
| Breast reconstruction | 6 | 1.5 |
|
| ||
| Pathological stage (AJCC 7 th ed) * | ||
| 0 | 22 | 5.6 |
| I–IIA | 339 | 85.6 |
| IIB–IIIB | 35 | 8.8 |
|
| ||
| pT stage * | ||
| pTis | 22 | 5.6 |
| pT0–T2 | 367 | 92.6 |
| pT3–T4 | 7 | 1.8 |
|
| ||
| pN stage * | ||
| pN0 | 331 | 83.6 |
| pN1mi | 26 | 6.6 |
| pN1a | 34 | 8.6 |
| pN2a | 4 | 1.0 |
| pN3a | 1 | 0.2 |
|
| ||
| Chemotherapy | ||
| None | 228 | 58.0 |
| Neoadjuvant | 16 | 4.1 |
| Adjuvant | 149 | 37.9 |
|
| ||
| Hormone therapy | 344 | 87.5 |
|
| ||
| Radiotherapy fractionation | ||
| 15 fractions | 161 | 40.6 |
| 16 fractions | 147 | 37.1 |
| 17 fractions | 88 | 22.3 |
|
| ||
| Additional radiotherapy fields * | ||
| Supraclavicular/axillary | 13 | 3.3 |
| Internal mammary | 1 | 0.3 |
| Boost | 110 | 27.8 |
|
| ||
| Tangential fields separation [cm] * | ||
| Mean (± standard deviation) | 18.0 (± 2.9) | |
| Median | 17.8 | |
| Range | 8.5 to 27.0 | |
|
| ||
| PTV [cc] * | ||
| Mean (± standard deviation) | 915.6 (± 368.5) | |
| Median | 868.8 | |
| Range | 211.9 to 2402.8 | |
N — number; BMI — body mass index; PTV — planning target volume;
Data regarding the number of treated breasts
The mean age was 63.9 years (range 34 to 91 years). There were 5.8% ductal carcinoma in situ and all the others comprised invasive tumors. Breast-conserving surgery was performed in 95.7% and immediate reconstruction after mastectomy in six (1.5%) patients, three with autologous and three with alloplastic implants. Most tumors were stage pT1 (60.0%), pN0 or pN1mi (90.2%), “luminal A or B like” (85.4%) pattern. Neoadjuvant or adjuvant chemotherapy was administered in 42.0%, of which 73.3% were anthracycline-based, 35.8% combined with taxanes, and 20.6% with an anti-Her2 drug. Adjuvant hormone therapy was prescribed for 87.5% of the patients. HYPO-RT was delivered in 15 or 16 fractions in 77.3%, with boost indicated in 110 (27.8%) breasts. The boost was prescribed mostly in five fractions of 2 Gy, and a small percentage (7.3%) in 3 fractions of 2.67 Gy. Fourteen (3.5%) cases received irradiation of the lymphatic drainage, only one including the internal mammary chain (first three intercostal spaces).
Dosimetric data could be retrieved for 343 patients treated with three-dimensional conformal radiotherapy. The others were treated with a conventional two-dimensional technique. The tangential fields separation ranged from 8.5 to 27 cm (mean = 18.0 ± 2.9 cm; median = 17.7 cm), and the breast PTV from 211.9 to 2402.8 cc (mean = 915.6 cc and median = 868.8 cc). The institutional protocol defines the optimal coverage as 95% of the volume receiving at least 95% of the prescribed dose. However, 90% coverage of the PTV with 90% of the prescribed dose is accepted as a small protocol deviation. Hot spots of 115% are allowed with less than 50% of the PTV receiving more than 110% of the prescribed dose. All patients achieved the dosimetric goals while complying with the organs at risk dose constraints, with three-dimensional conformal radiotherapy using a forward-planned modulated field-in-field technique to homogenize the dose distribution.
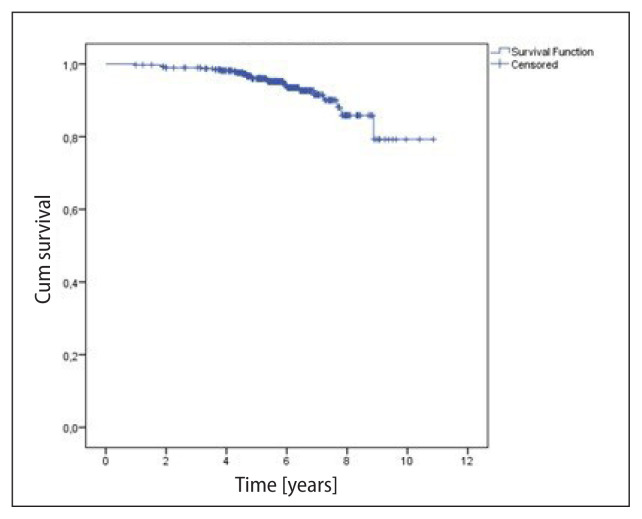
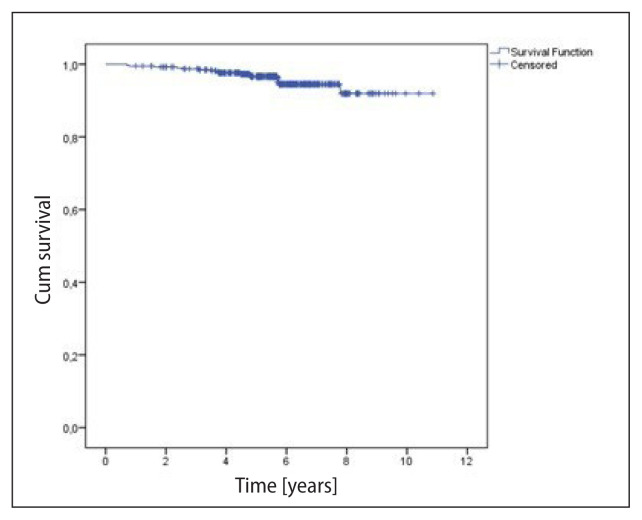
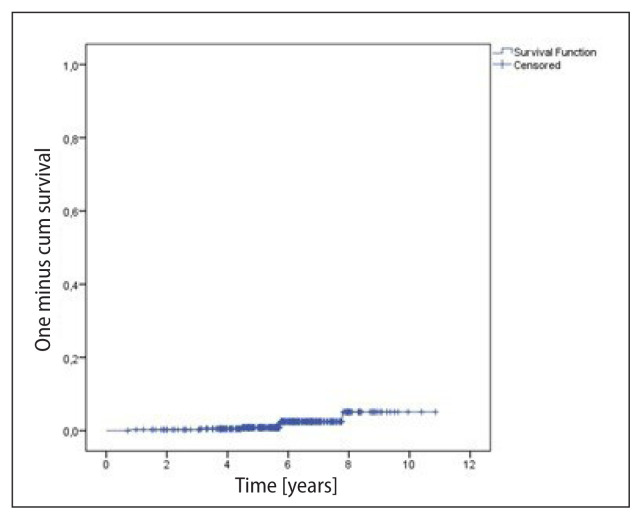
The median follow-up was 5.7 years (1 to 10.9 years). The survival analyses considered the total number of patients (393), regarding the higher risk tumor in the bilateral cases; and local control, the number of treated breasts/chest walls (396). There were 25 (6.4%) deaths and 17 (4.3%) recurrences in the period. The OS rates at 5 and 10 years were, respectively, 96.0% and 79.3% (mean = 10.0 ± 0.2 years) (Fig. 1). DFS was 96.6% and 91.9% at 5 and 10 years, respectively (Fig. 2). Local control was 99.2% and 94.9% at 5 and 10 years, respectively (Fig. 3). Recurrences were local in seven (1.8%) breasts, regional in only one (0.3%), and nine (2.3%) patients presented distant metastases. Distant metastases were more likely to occur in the more advanced stages (IIB–IIIB 8.6% vs. I–IIA 1.7%; p < 0.0001). Local recurrences occurred in a median of 5.7 years, while any recurrence in a median of 3.7 years.
Figure 1.
Overall survival (393 patients) — 25 events. Mean = 10.05 ± 0.19 years
Figure 2.
Disease-free survival (393 patients) — 17 events. Mean = 10.04 ± 0.12 years
Figure 3.
Local control (396 breasts) — seven events. Mean local disease-free survival = 10.6 ± 0.14 years
We also compared the initial HYPO-RT schedule of 17 fractions (BEDGy3 = 77.9 Gy) (88 patients, 22.3%) with the 15 or 16 fractions schedules (BEDGy3 = 76.9 Gy and 77.7 Gy, respectively) (308 patients, 77.7%). No differences were found between the schedules in all the analyzed outcomes nor between the boost and no-boost subgroups. (Supplementary File — Figure S1 and S2).
Acute grade 3 or 4 radiodermatitis occurred in 0.9% (3 breasts) of the patients, with most (62.8%) presenting with grade 2. Late toxicities were reported as fibrosis (2.8%), pain (1.5%), and hyperchromia (2.8%), all grade 2 or less. No internal organ-related toxicity was observed.
Discussion
Radiotherapy is one of the cornerstones in the treatment of breast cancer, regarding both local control and survival [12, 13]. Breast cancer is the worldwide leading cancer in women and the second cause of death due to cancer in this population [14]. The classic radiotherapy schedule of 25 to 28 daily fractions has been successful over time. However, it is time-consuming and the reduction of patients’ daily visits to the hospital would be welcome. This has been achieved with the moderate hypofractionated regimens proposed by several clinical trials [1–9]. The population included in the pioneer studies [1–5] was mainly comprised of patients aged 50 years or more, with low-risk tumors and no chemotherapy combined. Also, patients with indication of regional nodal irradiation were either excluded or underrepresented in those early trials.
Therefore, in 2009, after the first five-year follow-up results of this moderately hypofractionated schedule were published, we started treating patients with this strategy in our Hospital.
This study reports our experience with median follow-up of 5.7 years in a large single-institution cohort from a Latin-American middle-income country. As can be seen in Table 1, the indication of the hypofractionated scheme became more flexible during the study period, with the inclusion of patients with bilateral tumors, and an indication of lymphatic irradiation.
When comparing the patients treated in our cohort with those included in the Canadian [6] and United Kingdom [7] trials, we observed an older population selected in our cohort, but the most relevant aspect was the use of chemotherapy, which was allowed and administered in 42% of the patients. According to more modern regimens, anthracyclines were used in most of the patients that received chemotherapy, with the use of taxanes and anti-Her2 drugs reported as well (Tab. 2). No statistically significant impact in any of the outcomes, including complications, and the use or not of chemotherapy was observed. However, the use of neoadjuvant chemotherapy, prescribed in more advanced diseases, was associated with worse survival outcomes, as expected.
Table 2.
Comparison of the present study with the trials from which we chose the HYPO-RT schedule for breast cancer [6, 7]. Comparison with a more recently published trial that aimed to evaluate the toxicity is also presented [9]. The numbers refer to the hypofractionated arm of each study
| Study Accrual period |
OCOG6 1993–96 |
START B7 1999–2001 |
DBCG9 2009–14 |
HC-FMUSP 2009–15 |
|---|---|---|---|---|
|
| ||||
| Type of study | Prospective randomized | Prospective randomized | Prospective randomized | Retrospective cohort |
|
| ||||
| N | 1234 (two arms) | 2215 (two arms) | 1854 (two arms) | 392 (single cohort) |
|
| ||||
| RT | 16 fractions | 15 fractions | 15 fractions | 15 or 16 fractions (17 fractions 23%) |
|
| ||||
| Age ≥ 50 years | 76% | 79% | 89% | 90% |
|
| ||||
| DCIS | – | – | 13% | 5.8% |
|
| ||||
| HT | 41% | 87% | 37% | 87% |
| CT | 11% | 22% | 42% | 42% |
| Anthracycline-based | 59.1% | (CMF 28.6%) | NA | 73% |
| Taxane | (9 patients) | (19 patients) | NA | 36% |
| Anti-Her2 drug | – | – | 9% | 21% |
|
| ||||
| RNI | – | 7% | 4% (IMC: 1 patient) | |
|
| ||||
| Boost | – | 43% | 23% | 28% |
|
| ||||
| Breast size | – | 74% “medium” | Median CTV 635 cc | 78% (TFS ≤ 20 cm) 64% (PTV ≤ 1000 cc) |
|
| ||||
| Median follow-up | 12 years | 9.9 years | 7.3 years | 5.7 years |
|
| ||||
| 5-year local recurrence | 2.2% | 2.8% | 1.0% | 0.8% |
|
| ||||
| 10-year local recurrence | 7.4% | 3.0% (9-year) | 5.1% | |
N —number of patients; RT — radiotherapy; HT — hormone therapy; CT — chemotherapy; CMF — cyclophosphamide, methotrexate, fluorouracil; RNI — Regional nodal irradiation; IMC — internal mammary chain; TFS — tangential fields separation; CTV — clinival target volume; PTV — breast planning target volume; NA — non-available.
Our data demonstrated that local recurrence was low and comparable to the results of published prospective randomized clinical trials for early breast cancer [6, 7, 9] (Tab. 2). Moreover, overall complication rates were also low, as expected. At first, we were more conservative, establishing restrictions related to the breast size and tangential field separation. However, later on, with a better acquaintance with the fractionation schedule and using 3D planning, these restrictions were no longer an issue, so long as the breast dose homogeneity was kept within the institutional protocol recommendations. The same strategy was used in the Danish trial [9], where the primary endpoint was the occurrence of breast induration. There were no restrictions regarding the breast size, and the late toxicity was very low and comparable with the conventional fractionation arm. Nevertheless, a limitation of our study was the scarceness of data to evaluate grades 1 or 2 adverse effects, which are not always specified in the charts.
Consensus recommendations for the use of HYPO-RT were first published by the American Society of Therapeutic Radiation Oncology (ASTRO) in 2011 [15] when we were already using HYPO-RT for breast cancer (these recommendations were further updated in 2018 [16] with more flexible criteria).
When analyzing Table 1, most treated patients followed the 2011 guidelines, except for the chemotherapy, prescribed in almost half of the patients in our cohort. And also 21% received an anti-Her2 drug, not available at the time of recruitment of the first trials on hypofractionation and not considered in the guidelines.
Comparing our results with the long-term publications of the Canadian and START B trials [6, 7], on which we based our choice of HYPO-RT schedule, our patient selection was more restrictive than the original ones, although with a few deviations such as the amount of combined chemotherapy used, the treatment of a few patients with breast reconstruction and some with an indication of regional nodal irradiation (Tab. 2).
The local recurrence rate in our cohort was low, and comparable with that observed in those studies, with a very low rate of significant early and late complications. Probably, the use of more modern radiotherapy techniques, better chemotherapy regimens, and even the better patient staging that can be achieved with the currently available imaging exams could have contributed to our better results.
With a median follow-up of 5.7 years, the survival analyses were by what was expected for this low-risk population. There was an early death at 12 months follow-up in a 34-year-old patient with a fast-growing locally advanced disease (stage IIIC) due to clinical complications after local recurrence.
In 2018, the Brazilian Society of Radiotherapy (Sociedade Brasileira de Radioterapia — SBRT) published recommendations for hypofractionated whole-breast irradiation [17], with a high agreement (100%) among the experts on indicating this schedule for almost all patients older than 40 years, with low-risk disease, and an indication of a breast only irradiation. Despite the scarcity of evidence available regarding association with neoadjuvant chemotherapy or anti-HER2 drugs, above 90% of the experts would treat these patients with hypofractionated schedules. These recommendations came to corroborate our experience.
Existing data confirm that moderately hypofractionated RT for breast cancer is efficient, convenient, and safe for all indications, target volumes, and techniques [18], even for advanced stages as seen in the prospective randomized trial from Beijing published in 2019 [8]. The trial included 820 pT3–4 pN2–3 post-mastectomy breast-cancer patients, who were randomized to receive post-mastectomy RT of the chest wall and selected nodal irradiation (supraclavicular and level 3 axillary) with 50 Gy in 25 fractions in five weeks or 3-week hypofractionation, 43.5 Gy in 15 fractions (EDQ2 = 50 Gy). The 5-year loco-regional relapse rates were 8.1% and 8.3% for the conventional dose and hypofractionation groups, respectively, resulting in a non-significant absolute difference of 0.2% (90% CI: −3.0–2.6) and a hazard ratio of 1.10 (90%CI: 0.72–1.69; p < 0.001 for non-inferiority). There were no significant differences between the groups in acute and late toxicities, except that fewer patients in the hypofractionated radiotherapy group had grade 3 acute skin toxicity than in the conventional fractionated radiotherapy group [14 (3%) of 401 patients vs. 32 (8%) of 409 patients; p < 0·0001].
At our institution, after this initial experience, HYPO-RT has been indicated since April 2019 for all patients with breast cancer who need adjuvant radiotherapy, irrespective of age, associated treatments, breast reconstruction, or regional node irradiation. We hope to report our results in a near future.
Currently, even shorter schedules are under consideration for radiotherapy for selected patients with breast cancer. The 5-year results of the FAST-FORWARD trial, delivering 26 Gy in 5 consecutive daily fractions of 5.2 Gy are promising and provocative and may be used as a treatment option for selected patients [19].
Conclusion
HYPO-RT is a safe and effective radiotherapy regimen with excellent disease control, overall survival rates, and low toxicity. We believe that our results may be translated to other centers with similar cultural, social, and economic environments. Since 2019, it has become the standard treatment schedule for all breast cancer patients in the institution in the adjuvant setting.
Supplementary Information
Acknowledgments
To Drs. Clarissa C.A. Ramos, Thalita S. Cordeiro, Flavia C.G. Gabrielli, Maria Luiza S. Figueiredo, Fabio P. Luz, Alfredo M.A. Cordeiro, Karina G.M.C. Vasconcelos, Paula P.R.F. Arruda from the Radiotherapy Department, Laura Testa from the Clinical Oncology Department, and José Roberto Filassi from the Mastology Department, for the support in patients’ treatment and follow-up.
Footnotes
Conflict of interest
None declared.
Funding
None declared.
References
- 1.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94(15):1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 2.Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75(1):9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Owen J, Ashton A, Bliss J, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial [published correction appears in Lancet Oncol. 2006 Aug;7(8):620] Lancet Oncol. 2006;7(6):467–471. doi: 10.1016/s1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen SM, Agrawal RK, Aird EG START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. The Lancet Oncology. 2008;9(4):331–341. doi: 10.1016/s1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bentzen SM, Agrawal RK, Aird EGA, et al. START Trialists’ Group. START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098–1107. doi: 10.1016/s1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 6.Haviland JS, Owen JR, Dewar JA, et al. START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20(3):352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- 8.Offersen BV, Alsner J, Nielsen HM, et al. Danish Breast Cancer Group Radiation Therapy Committee. Hypofractionated Versus Standard Fractionated Radiotherapy in Patients With Early Breast Cancer or Ductal Carcinoma In Situ in a Randomized Phase III Trial: The DBCG HYPO Trial. J Clin Oncol. 2020;38(31):3615–3625. doi: 10.1200/JCO.20.01363. [DOI] [PubMed] [Google Scholar]
- 9.Wang SL, Fang H, Hu C, et al. Hypofractionated Versus Conventional Fractionated Radiotherapy After Breast-Conserving Surgery in the Modern Treatment Era: A Multicenter, Randomized Controlled Trial From China. J Clin Oncol. 2020;38(31):3604–3614. doi: 10.1200/JCO.20.01024. [DOI] [PubMed] [Google Scholar]
- 10.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 11.Darby S, McGale P, Correa C, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGale P, Taylor C, Correa C, et al. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 14.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81(1):59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 15.Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8(3):145–152. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Freitas NM, Rosa AA, Marta GN, et al. SBRT, Brazilian Society of Radiotherapy. Recommendations for hypofractionated whole-breast irradiation. Rev Assoc Med Bras (1992) 2018;64(9):770–777. doi: 10.1590/1806-9282.64.09.770. [DOI] [PubMed] [Google Scholar]
- 17.Marta GN, Coles C, Kaidar-Person O, et al. The use of moderately hypofractionated post-operative radiation therapy for breast cancer in clinical practice: A critical review. Crit Rev Oncol Hematol. 2020;156:103090. doi: 10.1016/j.critrevonc.2020.103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray Brunt A, Haviland JS, Wheatley DA, et al. FAST-Forward Trial Management Group. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395(10237):1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.