Abstract
Background
The objective of the study was to review the outcome of patients with parotid cancer treated with postoperative radiotherapy at Complejo Hospitalario de Navarra in the last ten years.
Materials and methods
We retrospectively reviewed patients treated with adjuvant radiotherapy between January 2008 and December 2018. We analyzed demographic data, histopathologic findings, local control (LC) and overall survival (OS).
Results
A total of 40 patients received postoperative radiotherapy during the period mentioned. There were 22 men (55%) and 18 women (45%). Median age was 58 years (19–90). By tumor histology, the most common was squamous cell carcinoma (22.5%) followed by ex-pleomorphic adenoma (15%) and adenoid cystic carcinoma (10%). According to Surgery, 19 patients (47.5%) underwent a total parotidectomy, 20 (50%) partial parotidectomy, and 1 (2.5%) a radical parotidectomy. Twenty-one patients (51.2%) underwent cervical dissection, most of them being supraomohyoid (31.7%). Reasons for adjuvant RT were: R1 resection (35% of the patients), high grade tumors (27.5%) and 17.5% because R1 surgery and R1. Radiation was administered using IMRT in most patients to a total dose of 60 Gy in 30 fractions. The 5-year overall survival (OS) (Kaplan-Meier) was 81% (95% CI: 68.5–96.2%), and 10-years — 64%. The 5-year local control (LC) (Kaplan-Meier) was 82.4% (95% CI: 91.46–73.33%) and the 10-year LC — 72.2% (95% CI: 54.9–96%). To date, only 4 patients (10%) have died due to their parotid tumor.
Conclusion
The adjuvant radiotherapy added to surgery, significantly reduces the risk of recurrence in high-risk patients with a very acceptable survival rate.
Keywords: parotid cancer, adjuvant radiotherapy, external beam radiation therapy
Introduction
Malignant tumors of the salivary glands have a low annual incidence, similar in both men and women. They represent 3 to 5% of malignant head and neck tumors. Although the incidence of head and neck tumors is decreasing, the rate of parotid cancer has increased since 1973 [1]. We need more research to understand the etiology, risk factors, and pathophysiology of parotid cancer in order to decrease the incidence.
The salivary glands are divided into major (parotid, submandibular, sublingual) and minor (located in the oral cavity, pharynx, and paranasal sinuses). However, 90% of malignant salivary gland tumors are diagnosed in the parotid.
Although the histological classification of salivary gland tumors includes numerous entities [2], the most common malignant tumors are mucoepidermoid carcinomas, adenoid cystic carcinomas, and adenocarcinomas. Among the benign tumours, pleomorphic adenomas (5–10% undergo malignant transformation) and Warthin’s tumors are the most frequent.
Cytological diagnosis is usually established by FNA with ultrasound control, and its study includes CT and MRI to establish local extension. However, the definitive pathological diagnosis cannot be established until the final study of the surgical piece. PET will rule out metastatic disease and confirm lymph node involvement, if any.
The main treatment is surgery, ideally, by total resection with free margins and preservation of the facial nerve when possible. In high-grade tumors or positive neck nodes, an ipsilateral cervical lymph node dissection will be indicated [3].
Postoperative radiotherapy is indicated in cases with incomplete resections (R1 and R2), in high-grade tumors, such as cystic adenoid carcinomas, and in stages T3 and T4 [3, 4].
In this article, we focus on the role of postoperative radiotherapy in parotid carcinoma, its impact on locoregional control, and its possible prognostic factors. We report the experience of the Radiation Oncology Service (RO) of the Navarra Hospital Complex (CHN) in a postoperative treatment of parotid tumors operated in the Maxillofacial Surgery Service (MFS) during the last decade.
Materials and methods
Characteristics of the patients
Between 2008 and 2018, 40 patients with parotid carcinoma were treated with surgery and radiotherapy at the CHN. The mean follow-up was 84 months (range 6–139) for living patients.
Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Variable | Category | n (%) |
|---|---|---|
|
| ||
| Age | Mean 58.3 (19–90 y) | |
|
| ||
| PS (ECOG) | ||
|
| ||
| 0–1 | 39 (97.5%) | |
| 2 | 1 (2.5%) | |
|
| ||
| Sex | ||
| Male | 22 (55.0%) | |
|
| ||
| Female | 18 (45.0%) | |
|
| ||
| T | ||
| 1 | 12 (30.0%) | |
|
| ||
| 2 | 23 (57.5%) | |
|
| ||
| > 2 | 5 (12.5%) | |
|
| ||
| Localization | ||
|
| ||
| Superficial | 32 (80.0%) | |
|
| ||
| Deep | 4 (10.0%) | |
|
| ||
| Superficial + deep | 4 (10.0%) | |
|
| ||
| Histology | ||
|
| ||
| Epidermoid | 9 (22.5%) | |
|
| ||
| Ex pleomorphic adenoma | 6 (15.0%) | |
|
| ||
| Adenoid cystic carcinoma | 4 (10.0%) | |
|
| ||
| Secretory | 4 (10.0%) | |
|
| ||
| Mucoepidermoid | 3 (7.5%) | |
|
| ||
| Ductal | 3 (7.5%) | |
|
| ||
| Pleomorphic adenoma | 3 (7.5%) | |
|
| ||
| Sarcomatoid | 2 (5.0%) | |
|
| ||
| Others | 6 (15%) | |
|
| ||
| Type of surgery | ||
|
| ||
| Superficial parotidectomy | 20 (50.0%) | |
|
| ||
| Total parotidectomy | 19 (47.5%) | |
|
| ||
| Radical parotidectomy | 1 (2.5%) | |
|
| ||
| Indication RT | ||
|
| ||
| G3 | 11 (27.5%) | |
|
| ||
| R1 | 14 (35.0%) | |
|
| ||
| G3 + R1 | 7 (17.5%) | |
|
| ||
| Relaps | 5 (12.5%) | |
|
| ||
| Unresectable | 1 (2.5%) | |
|
| ||
| PNI, N+ | 1 (2.5%) | |
|
| ||
| Unknown | 1 (2.5%) | |
|
| ||
| Dose RT | ||
|
| ||
| Median (IQR) | 60.0 (6) | |
|
| ||
| ≤ 65 | 28 (70.0%) | |
|
| ||
| > 65 | 12 (30.0%) | |
|
| ||
| Time from surgery to RT | ||
|
| ||
| Median (IQR) | 55 (38) | |
|
| ||
| ≤ 60 | 24 (60%) | |
|
| ||
| > 60 | 16 (40%) | |
|
| ||
| Local relaps | ||
|
| ||
| No | 33 (82.5%) | |
|
| ||
| Yes | 7 (17.5%) | |
|
| ||
| Exitus | ||
|
| ||
| No | 31 (77.5%) | |
|
| ||
| Yes | 9 (22.5%) | |
RT — radiotherapy
The mean age was 58 years (range 19–90). All tumors were located in the parotid gland (20% deep lobe).
Surgery was performed by superficial (50%), total (47.5%) or radical (2.5%) parotidectomy. Surgery included some type of cervical lymph node dissection (ND) in 20 patients (51.2%). This dissection consisted of an ipsilateral SOHD in 13 patients (65%), level IIa excision in 2 patients (10%), radical ND in 2 patients (10%), and radical modified ND in 2 patients (10%). One patient underwent bilateral cervical neck dissection (5%).
The pathological anatomy showed a wide variability of histological subtypes, the most frequent being squamous cell carcinomas (22.5%), followed by ex-adenoma pleomorphic carcinoma (15%), adenoid cystic carcinoma (10%) and secretory carcinoma (10%).
After doing neck dissection, the nodal positivity rate was 40%.
All patients received radiotherapy and the main reasons were the degree of tumor differentiation G3 (27.5%), R1 resection (margin-free < 2 mm) in 35% or a combination of both (17.5%). The dose of RT was 60 Grays (Gy) in most cases and the median gap (time from surgery to application of RT) was 55 days.
Radiotherapy plan
RT was local (parotid bed) in 18 patients (44%) and loco-regional (bed and cervical lymph nodes) in 23 patients (56%).
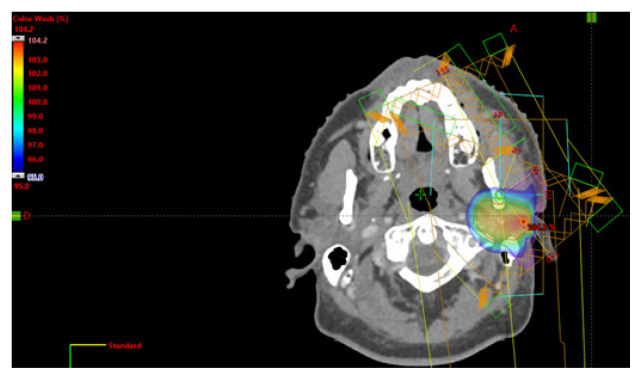
Patients were treated daily with a dose of 2 Gy up to a median dose of 60 Gy (81–50 Gys). In the case of R1surgery, the affected margin has been boosted up to 66 Gy. In the 23 patients treated with loco-regional RT, the RT doses on cervical neck nodes ranged between 63 Gy and 50 Gy. Until 2013, 19 patients received radiotherapy with a 3D technique with a combination of photons and electrons. Since 2013, 22 patients have been treated with IMRT. An example of IMRT treatment is shown in Figure 1.
Figure 1.
Example of radiotherapic treatment by intensity modulated radiotherapy (IMRT)
In relation to chemotherapy (CT), most of the patients did not receive concomitant CTRT due to poor evidence published on parotid cancer and, more specifically, in the postoperative setting. In this sense, only three patients were treated with weekly cisplatin (two pN1 and R1 resection, and one unresectable squamous cell carcinoma).
Statistical analysis
The characteristics of the sample are described by descriptive statistics; frequencies and percentages were used for categorical variables, and for quantitative variables, means and standard deviations (SD) or median and interquartile range (IQR), as appropriate.
Recurrence-free survival (RFS) and overall survival (OS) were measured from the date of diagnosis and the date of recurrence or death, respectively. The Kaplan-Meier method and the log-rank test were used to analyze the differences in survival time between the various factors. Cox proportional hazards regression models were used to assess the relationship between the covariates and both outcomes, which also provided an estimate of the hazard ratios (HR) and 95% confidence intervals (CI). The multivariate regression model was adjusted with those variables with HR less than 0.5 or greater than 2 and adjusted for age and sex.
Results
Local control and toxicity
Seven (17.5%) of the 40 patients included in the study, presented a local recurrence during follow-up. It should be noted that 86% were diagnosed in the first 2 years of follow-up.
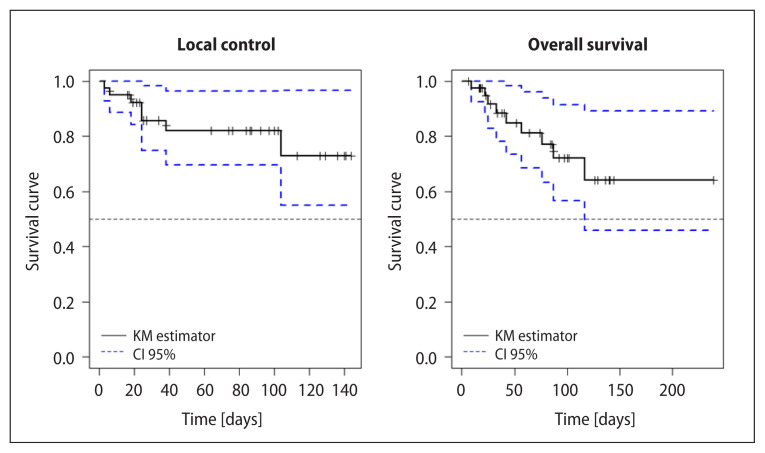
Local control at 5 years (Kaplan-Meier) was 82.4% (95% CI: 91.46–73.33%). Local control at 10 years was 72.2% (95% CI: 54.9–96%) (Fig. 2A).
Figure 2.
A. Local control (LC); B. Overall survival (OS) of the series
Acute side effects consisted only of epithelitis grade 1–2 in 80% of the patients and grade 3 in the rest. There are no grade > 2 late effects.
Overall survival
During the follow-up period, nine patients (22.5%) have died.
Overall survival at 5 years (Kaplan-Meier) was 81% (95% CI: 68.5–96.2%), and at 10 years 64% (Fig. 2B). It should be noted that of the 9 patients deceased in the series, in only 4 (44%) was the cause parotid tumor.
Prognostic factors for local control (LC)
Taking into account the size sample and a very small number of events for both LC and OS, it is unlikely to find significant associated factors. In this sense, the bivariate study (Tab. 2) shows some factors that present HR greater than 2 (grade 3, RT dose > 65 Gy and time until RT > 60 days) that could indicate a certain trend.
Table 2.
Bivariate COX regression
| Bivariate* | LC | ||
|---|---|---|---|
| Variable | Category | HR (95% CI) | p-value |
|
| |||
| Age | 1.03 (0.98, 1.07) | 0.216 | |
|
| |||
| Sex | |||
|
| |||
| Male | Reference | ||
|
| |||
| Female | 0.95 (0.21, 4.27) | 0.952 | |
|
| |||
| T | |||
|
| |||
| 1 | Reference | ||
|
| |||
| 2 | 1.42 (0.24, 8.31) | 0.700 | |
|
| |||
| > 2 | 1.37 (0.12, 15.51) | 0.800 | |
|
| |||
| Localization | |||
|
| |||
| Superficial | Reference | ||
|
| |||
| Superficial + deep | 0.61 (0.07, 5.07) | 0.647 | |
|
| |||
| Histology | |||
|
| |||
| Epidermoid | Reference | ||
|
| |||
| Others | 0.36 (0.08, 1.62) | 0.185 | |
| Dose RT | |||
|
| |||
| 1.04 (0.92, 1.17) | 0.505 | ||
|
| |||
| ≤ 65 | Reference | ||
|
| |||
| > 65 | 2.45 (0.54, 11.20) | 0.248 | |
|
| |||
| Indication RT | |||
|
| |||
| G3 | Reference | 0.468 | |
|
| |||
| R1 | 0.73 (0.12, 4.52) | 0.738 | |
|
| |||
| G3 + R1 | 2.59 (0.36, 18.77) | 0.348 | |
|
| |||
| Indication RT | |||
|
| |||
| Not G3 | Reference | ||
|
| |||
| G3 | 3.19 (0.61, 16.6) | 0.168 | |
|
| |||
| Type of surgery | |||
|
| |||
| Superficial parotidectomy | Reference | ||
|
| |||
| Total parotidectomy | 2.20 (0.40, 12.1) | 0.362 | |
|
| |||
| Time from surgery to RT | |||
|
| |||
| 1.00 (0.999, 1.001) | 0.788 | ||
|
| |||
| ≤ 60 | Reference | ||
|
| |||
| > 60 | 2.17 (0.48, 9.71) | 0.311 | |
LC — local control; HR — hazard ratio; CI — confidence interval; RT — radiotherapy.
There is no variable significantly associated with time to relapse-free.
There are some factors with a HR greater than 2 (highlighted in red) that could indicate a certain trend
Since there are no factors that are significantly associated with LC in the bivariate analysis, those variables that have a HR ≥ 2 or ≤ 0.5 were selected to be entered in the multivariate model together with the age and sex variables (Tab. 3). Indeed, no variable is independently associated with local control in the multivariate model, although it is very possible that it is due to the insufficient power of the analyzes due to few events
Table 3.
Multivariate COX regression
| Multivariate* | LC | ||
|---|---|---|---|
| Variable | Category | HR (95% CI) | p-value |
|
| |||
| Age | 1.06 (0.98, 1.14) | 0.131 | |
|
| |||
| Sex | |||
|
| |||
| Male | Reference | ||
|
| |||
| Female | 2.54 (0.29, 21.9) | 0.397 | |
|
| |||
| Dose RT | |||
|
| |||
| ≤ 65 | Reference | ||
|
| |||
| > 65 | 4.21 (0.51, 35.0) | 0.183 | |
|
| |||
| Indication RT | |||
|
| |||
| Not G3 | Reference | ||
|
| |||
| G3 | 4.84 (0.25, 91.3) | 0.292 | |
|
| |||
| Type of surgery | |||
|
| |||
| Superficial parotidectomy | Reference | ||
|
| |||
| Total parotidectomy | 2.83 (0.22, 36.1) | 0.422 | |
|
| |||
| Time from surgery to RT | |||
|
| |||
| ≤ 60 | Reference | ||
|
| |||
| > 60 | 5.67 (0.53, 61.0) | 0.152 | |
LC — local control; HR — hazard ratio; CI — confidence interval; RT — radiotherapy. No variable is independently associated with relapsefree time in the multivariate model, although it could be because of a lack of power due to the few events
Discussion
The role of postoperative radiotherapy as an independent factor in locoregional control of tumors of the salivary glands and, especially, of the parotid glands, is based on retrospective studies [3–11]. The low incidence of these tumors makes it difficult to conduct prospective randomized trials. However, all studies with a significant number of patients, such as those by Mahmood with 2,170 patients from the SEER base [3] or Terhaard [4] from the DHNOC with 498 patients, have shown a positive impact of postoperative radiotherapy on parotid tumors.
The local control of our series at 5 years was 82.4% comparable with the reported series [5, 6, 11]. In the analysis performed by Kim YH [12] in parotid carcinomas, he concluded that the degree of differentiation, histological grade, pathological T stage, and lymphovascular invasion were independent prognostic factors for local control and survival. In our series, there is no significant factor, although high-grade tumors, especially in those over 65 years of age, have shown a certain tendency to have worse LC.
The high percentage of squamous cell carcinomas (22.5% of the total) is remarkable in our series, with primary parotid tumors being histologically indistinguishable from intraparotid metastases of squamous cell carcinomas. In the latter case, it is usually associated with a history of squamous cell carcinoma in the area close to the gland [13].
On the other hand, long-term follow-up is recommended because recurrence can appear later [14]. In this sense, our data would not support this conclusion given that 86% of local recurrences have occurred in the first 2 years of follow-up.
The time factor plays an important role in the combined treatment of head and neck cancer. Peters et al. [15] postulated that after surgery, residual tumor cells would repopulate to their maximum potential. Because salivary gland tumors generally grow slowly, a lesser adverse effect would be expected from the delay between surgery and postoperative RT, although our data suggest a lower LC when the delay exceeds 60 days.
Regarding irradiation doses, a dose of at least 60 Gy is recommended. The decision to electively treat neck nodes should be based on the T stage and histological type of the tumor. Postoperative cervical radiotherapy is indicated in cases of lymph node infiltration, with doses between 50–66 Gy depending on the degree of involvement. In our study, no statistically significant relationship was found between the RT dose and local control, probably because the radiotherapy treatment was quite homogeneous and with similar irradiation doses. Effectively, the mean dose was 62 Gy, and the total dose was adjusted to the state of the resection margin.
Due to the fact that the time interval of the series was long, various technical improvements have been adopted that have optimized the treatment and made it possible to administer lower doses of radiation to healthy tissues [from RT3D, intensity modulated RT (IMRT) [16] or volumetric RT (VMAT) [17], adaptive radiotherapy [18]..
Conclusion
Based on our results and the review of published studies, we recommend postoperative radiotherapy for T3–4 parotid tumors, incomplete resection (R1 or R2), perineural invasion, or high-grade tumors.
Footnotes
Conflicts of interest
None declared.
Funding
None declared.
References
- 1.Gupta A, Koochakzadeh S, Neskey DM, et al. Incidence and survival trends of parotid malignancies over 42 years. Head Neck. 2020;42(9):2308–2315. doi: 10.1002/hed.26172. [DOI] [PubMed] [Google Scholar]
- 2.Seethala RR, Stenman G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol. 2017;11(1):55–67. doi: 10.1007/s12105-017-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood U, Koshy M, Goloubeva O, et al. Adjuvant radiation therapy for high-grade and/or locally advanced major salivary gland tumors. Arch Otolaryngol Head Neck Surg. 2011;137(10):1025–1030. doi: 10.1001/archoto.2011.158. [DOI] [PubMed] [Google Scholar]
- 4.Terhaard CHJ, Lubsen H, Rasch CRN, et al. Dutch Head and Neck Oncology Cooperative Group. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 2005;61(1):103–111. doi: 10.1016/j.ijrobp.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong JG, Harrison LB, Spiro RH, et al. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg. 1990;116(3):290–293. doi: 10.1001/archotol.1990.01870030054008. [DOI] [PubMed] [Google Scholar]
- 6.Garden AS, el-Naggar AK, Morrison WH, et al. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys. 1997;37(1):79–85. doi: 10.1016/s0360-3016(96)00464-6. [DOI] [PubMed] [Google Scholar]
- 7.North CA, Lee DJ, Piantadosi S, et al. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 1990;18(6):1319–1326. doi: 10.1016/0360-3016(90)90304-3. [DOI] [PubMed] [Google Scholar]
- 8.Parsons JT, Mendenhall WM, Stringer SP, et al. Management of minor salivary gland carcinomas. Int J Radiat Oncol Biol Phys. 1996;35(3):443–454. doi: 10.1016/s0360-3016(96)80005-8. [DOI] [PubMed] [Google Scholar]
- 9.Dunn EJ, Kent T, Hines J, et al. Parotid neoplasms: a report of 250 cases and review of the literature. Ann Surg. 1976;184(4):500–506. doi: 10.1097/00000658-197610000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah K, Javed F, Alcock C, et al. Parotid cancer treatment with surgery followed by radiotherapy in Oxford over 15 years. Ann R Coll Surg Engl. 2011;93(3):218–222. doi: 10.1308/003588411X565969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borthne A, Kjellevold K, Kaalhus O, et al. Salivary gland malignant neoplasms: treatment and prognosis. Int J Radiat Oncol Biol Phys. 1986;12(5):747–754. doi: 10.1016/0360-3016(86)90032-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim YH, Chung WK, Jeong JU, et al. Evaluation of Prognostic Factors for the Parotid Cancer Treated With Surgery and Postoperative Radiotherapy. Clin Exp Otorhinolaryngol. 2020;13(1):69–76. doi: 10.21053/ceo.2019.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzen A, Buchali A, Lieder A. The rising incidence of parotid metastases: our experience from four decades of parotid gland surgery. Acta Otorhinolaryngol Ital. 2017;37(4):264–269. doi: 10.14639/0392-100X-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkbride P, Liu FF, O’Sullivan B, et al. Outcome of curative management of malignant tumours of the parotid gland. J Otolaryngol. 2001;30(5):271–279. doi: 10.2310/7070.2001.19527. [DOI] [PubMed] [Google Scholar]
- 15.Peters LJ, Withers HR. Applying radiobiological principles to combined modality treatment of head and neck cancer-the time factor. Int J Radiat Oncol Biol Phys. 1997;39(4):831–836. doi: 10.1016/s0360-3016(97)00466-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Eisbruch A. IMRT for head and neck cancer: reducing xerostomia and dysphagia. J Radiat Res. 2016;57(Suppl 1):i69–i75. doi: 10.1093/jrr/rrw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teoh M, Clark CH, Wood K, et al. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011;84(1007):967–996. doi: 10.1259/bjr/22373346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter KU, Fernandes LL, Vineberg KA, et al. Parotid glands dose-effect relationships based on their actually delivered doses: implications for adaptive replanning in radiation therapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;87(4):676–682. doi: 10.1016/j.ijrobp.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]