Abstract
Background
Distal radius fractures (DRFs) are one of the most common major fractures. Despite their frequency, the tradeoffs in different outcomes after casting or surgery for closed extraarticular DRFs in older adults are unknown.
Questions/purposes
(1) For adults older than 60 years with closed extraarticular DRFs, what are the tradeoffs in outcomes for choosing casting versus surgery? (2) In what settings would surgery be preferred over casting?
Method
This is a secondary analysis of data from the Wrist and Radius Injury Surgical Trial (WRIST), a randomized, multicenter clinical trial that enrolled patients from April 10, 2012 to December 31, 2016. For WRIST, researchers recruited patients older than 60 years who sustained closed extraarticular distal radius fractures from 24 sites in the United States, Canada, and Singapore. We conducted a secondary analysis using data from WRIST, which had longitudinal data from a robust collection of covariates for patients who underwent surgery and casting. Among the 296 patients recruited in the WRIST study, 59% (174) of patients (mean age 71 ± 9 years) with complete sociodemographic data and 12-month follow-up for each primary outcome were included in the main analysis. More patients underwent surgery than casting (72% [126 of 174] versus 28% [48 of 174]). Most sociodemographic variables were similar between the surgery and casting groups, except for age and volar tilt. The surgical cohort was composed of patients randomized to external fixation, closed reduction percutaneous pinning, or volar locking plate internal fixation. The casting cohort consisted of patients who elected to be treated with closed reduction and casting. A tree-based reinforcement statistical learning method was used to determine the best treatment, either surgery or casting, to maximize functional and esthetic outcomes while minimizing pain. Tree-based reinforcement learning is a statistical learning method to build an unsupervised decision tree within a causal inference framework that will identify useful variables and their cutoff values to tailor treatment assignment accordingly to achieve the best health outcome desired. The primary outcome was minimization of pain (12-month Michigan Hand Outcomes Questionnaire pain subdomain score), maximization of grip strength, total ROM (supination and wrist arc of motion), and esthetics (12-month Michigan Hand Outcomes Questionnaire esthetics subdomain score).
Results
Casting was the best treatment to reduce pain and maximize esthetics, whereas surgery maximized grip strength and ROM. When the patient favored gaining ROM over pain reduction (more than 80:20), surgery was the preferred treatment. When the patient prioritized the importance of grip strength over pain reduction (more than 70:30), surgery was also the preferred treatment.
Conclusion
There are tradeoffs in outcomes after treating patients older than 60 years with closed extraarticular distal radius fractures with casting or surgery. When patients are attempting to balance minimizing pain and improving functional outcomes, unless they desire maximal functional recovery, casting may be the better treatment. Surgery may be beneficial if patients want to regain as much grip strength and ROM as possible, even with the possibility of having residual pain. These findings can be referenced for more concrete preoperative counseling and patient expectation management before treatment selection.
Level of Evidence
Level III, therapeutic study.
Introduction
Distal radius fractures (DRFs) are the most common type of fractures encountered in the emergency room, with more than 640,000 fractures yearly [9] and an incidence of 16 to 32 fractures per 10,000 person-years [5, 16, 20]. Treatment options for DRFs vary from casting to surgical fixation with external fixators, closed reduction percutaneous pinning, and volar locking plates. Randomized controlled trials of adults with DRFs suggest better long-term functional outcomes in patients who underwent surgical fixation than in patients who were treated nonoperatively [21]. However, various studies in older patients who sustained closed extraarticular DRFs indicate that casting and surgery have no clinically meaningful difference in long-term functional or patient-reported outcomes [2, 11, 31].
The contrasting evidence and lack of consensus guidelines for treating closed extraarticular DRFs in older adults produce substantial variations in surgical and nonsurgical treatment selection [6], and the tradeoffs in outcomes between treatment type for DRFs in older patients are unknown [1]. To further elucidate the best treatment for balancing different outcomes after closed extraarticular DRFs in older adults, the goal of this study was to establish personalized treatment assignment rules to assist surgeons and patients in making the best decision that magnifies the most desired outcome type. To accomplish this, we used a statistical learning method called tree-based reinforcement learning based on the principles of machine learning [14] and causal inference [15]. The most beneficial aspect of tree-based reinforcement learning is its ability to discern patient subgroups or individuals who may most benefit from a treatment or sequence of treatments to improve an outcome [26]. Statistical and machine learning technologies such as tree-based reinforcement learning can be used to create more personalized treatment algorithms to deliver precision medicine to patients [22]. Leveraging data from the Wrist and Radius Injury Surgical Trial (WRIST) [8], we applied tree-based reinforcement learning to develop clinical decision rules in order to minimize pain, and maximize grip strength, esthetics, and ROM.
We therefore asked: (1) For adults older than 60 years with closed extraarticular DRFs, what are the tradeoffs in outcomes for choosing casting versus surgery? (2) In what settings would surgery be preferred over casting?
Patients and Methods
Study Design and Setting
This decision analytical model was a secondary retrospective analysis of the randomized clinical trial, WRIST [8]. Participants were enrolled from 24 study sites in the United States, Canada, and Singapore from April 10, 2012 to December 31, 2016 and underwent external fixation, closed reduction percutaneous pinning, volar locking plate internal fixation, or casting. WRIST included patients older than 60 years who sustained isolated displaced closed extraarticular DRFs with the following radiographic criteria: dorsal angulation > 10°, radial inclination < 15°, or radial shortening > 3 mm. Among the eligible patients, 187 were treated operatively and were randomized either to external fixation, closed reduction percutaneous pinning, or volar locking plate internal fixation. The remaining 117 patients who declined surgery were managed nonoperatively with casting [8]. Patients with bilateral fractures, open fractures, previous DRF in the same wrist, or additional severe trauma were excluded.
Participants
Among the patients randomized to the surgical arm, 17% (31 of 187) patients were lost to follow-up, and 45% (53 of 117) were lost to follow-up in the casting arm. Baseline demographic variables between the included patients and those lost to follow-up were not different aside from the modified 5-item frailty index (Supplementary Table 1; http://links.lww.com/CORR/A596). This is an expected finding because some patients developed terminal illnesses and died during the study; these individuals were part of the patients lost to follow-up (Fig. 1). For this secondary analysis, among a total of 296 patients recruited in WRIST, 174 patients (mean age 71 ± 9 years; 88% [153 patients] were women) with complete demographic characteristics and 12-month follow-up were included in the main analysis (Fig. 1). A greater proportion of the study cohort underwent surgery than casting (72% [126 of 174] versus 28% [48 of 174]). Other than age and volar tilt, there were no differences in baseline clinical characteristics between patients who underwent casting and those who underwent surgery (Table 1). We assumed that the data were missing completely at random (MCAR), enabling generalization of the complete case cohort to the original study cohort [23]. Then, missing data were imputed using random forest methods to perform a sensitivity analysis of the primary results. Detailed protocols of the study design, enrollment, and main results of the WRIST study have been described previously [7].
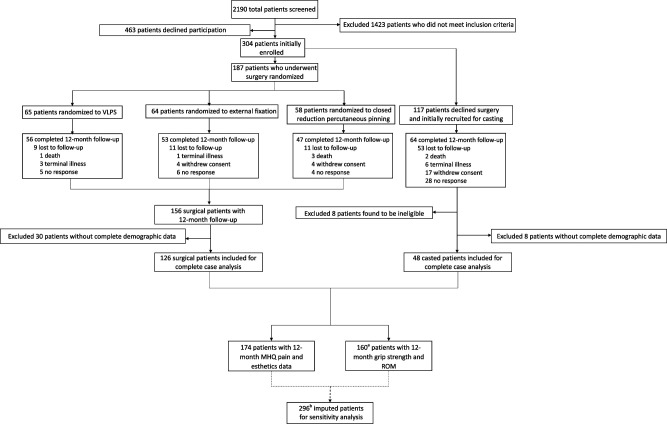
Fig. 1.
This CONSORT diagram represents the current secondary analysis based on the WRIST trial; aof the 174 patients with 12-month follow-up, 14 did not have 12-month grip strength or ROM data; b304 total patients initially enrolled subtracted by 8 patients later found ineligible.
Table 1.
Descriptive statistics of patient cohorts
| Factor | Overall (n = 174) | Casting (n = 48) | Surgery (n = 126) | p value |
| Age in years | 71 ± 9 | 76 ± 11 | 69 ± 8 | < 0.001 |
| Women | 88 (153) | 92 (44) | 87 (109) | 0.31 |
| Number of comorbidities | 4 ± 2 | 4 ± 3 | 3 ± 2 | 0.20 |
| Smoker | 44 (77) | 46 (22) | 44 (55) | 0.80 |
| mFI-5 score | 1 ± 1 | 1 ± 1 | 1 ± 1 | 0.25 |
| Dominant hand injury | 45 (78) | 42 (20) | 46 (58) | 0.61 |
| Radial height, mm | 9 ± 3 | 9 ± 3 | 8 ± 3 | 0.24 |
| Radial inclination, ° | 16 ± 5 | 17 ± 5 | 16 ± 5 | 0.35 |
| Patients with dorsal tilt | 84 (146) | 77 (37) | 87 (109) | 0.17 |
| Volar tilt, ° | 13 ± 14 | 9 ± 13 | 15 ± 14 | 0.02 |
| Ulnar variance, mm | 3 ± 3 | 2 ± 3 | 3 ± 3 | 0.42 |
| Ulnar styloid fracture | 48 (83) | 50 (24) | 47 (59) | 0.71 |
Data presented as mean ± SD or % (n); mFI-5 = modified 5-item frailty index.
Outcomes
We included four primary outcomes in the analysis: pain, esthetics, grip strength, and ROM. Pain was measured using the Michigan Hand Outcomes Questionnaire pain subdomain scale. To isolate pain secondary to distal radius fractures from that of other chronic etiologies, the difference in Michigan Hand Outcomes Questionnaire pain subdomain scores between the injured and uninjured hand was taken as the standardized pain score. The pain score ranged from 0 to 100, with higher numbers corresponding to more severe pain. Esthetics were measured using the Michigan Hand Outcomes Questionnaire esthetics subdomain score ranging from 0 to 100, with larger values indicating less deformity. Similarly, the standardized difference in esthetics scores between the injured and uninjured hand was implemented. Grip strength was defined as the ratio between the injured and uninjured hand to indicate the degree of strength recovery. The difference in wrist arc of motion and supination between the injured and uninjured hands was totaled to create a composite ROM variable, with larger values indicating greater mobility.
Primary and Secondary Study Outcomes
Our primary goal was to identify the tradeoffs in different outcomes between surgery and casting after closed extraarticular distal radius fracture in patients older than 60 years. To accomplish this, we first employed tree-based reinforcement learning statistical learning method to the WRIST data to find the treatment that maximized each of the four primary outcomes (minimized pain, maximized esthetics, maximized ROM, maximized grip strength).
Our secondary goal was to identify the degree of tradeoff between pain reduction and maximizing a functional outcome when surgery is preferred over casting, simulating a patient who desires to decrease pain while maximizing another outcome. To accomplish this, we built another model using tree-based reinforcement learning that minimized pain while concurrently maximizing another primary outcome (such as, trying to mitigate pain while maximizing ROM). Each outcome measure was given a weight, α, to simulate the degree of importance that outcome has to the patient before deciding on casting or surgery:
With this, α ranged from 0 to 1 in increments of 0.1. For example, if a patient placed 50% importance in outcome A and 50% importance in outcome B, α would be 0.5 to place equal weight on outcome A and B.
Tree-based Reinforcement Learning
Tree-based reinforcement learning is a semiparametric statistical learning method that can be applied to either data collected from clinical trials or observational data to estimate personalized treatment decision rules based on patient-level variables and other potential tailoring variables. Tailoring variables are any patient-specific variables related to a patient’s disease process or injury that help delineate whether one treatment is better than an alternative for a specific patient population or individual. Tao et al. [26] proposed a tree-based reinforcement learning method to directly estimate the best treatment decision rules, that is, how to assign treatments using patients’ individual values for the variables the algorithm identifies. The tree-based reinforcement learning model creates an unsupervised decision tree to map a patient’s covariates to recommended therapies. Several unique advantages of tree-based reinforcement learning exist over traditional regression modeling. First, the primary objective of tree-based reinforcement learning is to identify tailoring variables and their associated inflection point values at which the outcome of interest is improved with a given treatment for a specific patient population. This is inherently different from a classic regression analysis, which strives to fit a mathematical relationship between a dependent variable and one or more independent variables. Second, tree-based reinforcement learning combines semiparametric regression with flexible tree-based learning, which permits fewer model assumptions than the classic regression modeling. Lastly, tree-based reinforcement learning includes a purity measure that uses propensity scores (an augmented inverse propensity weighted [AIPW] estimator) to correct for biases and estimates the counterfactual mean outcome, which is the unobserved outcome if the patient had received the unselected treatment [26]. This feature facilitates a more robust strategy to address biases. Prior studies have used tree-based reinforcement learning to determine the best treatments for various medical and surgical problems [12, 17, 25].
We estimated the propensity score using the SuperLearner package in R (Supplementary Fig. 1; http://links.lww.com/CORR/A597), an algorithm that combines the predictions of multiple machine learning models such as random forest and lasso generalized linear models to calculate an ensemble prediction model that has a better performance than any individual model [28]. To estimate the propensity score of treatment allocation, we fitted a logistics regression model adjusted for the covariates described previously (Table 1).
Ethical Approval
We obtained approval for this study from the institutional review board at the University of Michigan Medical School, Ann Arbor, MI, USA (approval number HUM00028291). This study followed the Transparent Reporting of Multivariable Prediction Model for Individual Prognosis or Diagnosis guidelines. This trial was registered at ClinicalTrials.gov (number NCT01589692).
Statistical Analysis
We applied the tree-based reinforcement learning method to the WRIST data to conduct a complete case analysis. The improvement of four individual outcomes and three combined outcomes was the objective of the tree-based reinforcement learning model. The treatment option broadly compared casting and surgery. Because no specific surgical treatment guidelines exist for extraarticular fractures in older patients [1] and to maximize sample size, we included all three types of procedures in the surgical group. In addition, some studies suggest there are no differences in long-term functional or patient-reported outcomes after closed reduction percutaneous pinning or volar locking plates in patients with extraarticular fractures [8, 10]. Clinically relevant demographic and baseline clinical characteristics of the study population that were collected as part of the WRIST study were considered explanatory variables in the model: age, gender, smoking history, number of comorbidities, modified 5-item frailty index score [30], dominant hand injury, post-reduction radial height, radial inclination, volar tilt, ulnar variance, and presence of an ulnar styloid fracture. Then, for each outcome, a linear regression for the conditional mean outcome was established, including the explanatory variables and their interaction terms with the treatment option. To construct the decision tree, we specified the minimal node size to be 15 (10% of the study population size), and a minimum purity improvement of 2% was needed for a new split. All analyses were performed using R version 3.6.2 and R Studio version 1.3.959 (R Foundation for Statistical Computing). A two-sided t-test was used for continuous variables, and a chi-square test was used for categorical variables. An a priori significance level was set at p < 0.05.
Results
What Are the Tradeoffs in Outcomes between Surgery versus Casting?
After controlling for potentially confounding variables such as age, sex, number of comorbidities, and 5-item modified frailty index score, we found that casting resulted in the placement of greater value on pain and esthetics after DRF treatments (Table 2; Model 1 when α = 1 and 0, respectively), but surgery was preferred when attempting to maximize grip strength and ROM (Table 2; Models 2 and 3 when α = 0). This output was consistent irrespective of any covariates.
Table 2.
Estimated decision rules for various optimization goals
| Model 1: outcome = α•Pain+ (1- α)• MHQ esthetic | Model 2: outcome = α•Paina (1- α)•Combined ROM | Model 3: outcome = α•Paina+ (1- α)•Grip strength | |||
| α | Optimal treatment | α | Optimal treatment | α | Optimal treatment |
| 0 | Casting | 0 | Surgery | 0 | Surgery |
| 0.1 | Casting | 0.1 | Surgery | 0.1 | Surgery |
| 0.2 | Casting | 0.2 | Casting | 0.2 | Surgery |
| 0.3 | Casting | 0.3 | Casting | 0.3 | Casting |
| 0.4 | Casting | 0.4 | Casting | 0.4 | Casting |
| 0.5 | Casting | 0.5 | Casting | 0.5 | Casting |
| 0.6 | Casting | 0.6 | Casting | 0.6 | Casting |
| 0.7 | Casting | 0.7 | Casting | 0.7 | Casting |
| 0.8 | Casting | 0.8 | Casting | 0.8 | Casting |
| 0.9 | Casting | 0.9 | Casting | 0.9 | Casting |
| 1 | Casting | 1 | Casting | 1 | Casting |
α: Degree of importance given to each outcome where α = 1 signifies most important and α = 0 signifies not important.
From the MHQ Pain Subdomain Score; MHQ = Michigan Hand Outcomes Questionnaire.
In What Settings Would Surgery be Preferred over Casting?
When improving pain in conjunction with any of the remaining three primary outcomes, the suggested treatment varied with the weight of importance placed on each outcome. For example, when improving pain and ROM, the best estimated treatment changed from casting to surgery when the patient placed more than 80% emphasis on improving ROM (Table 2, Model 2). Similarly, when improving pain and grip strength, the best estimated treatment shifted from casting to surgery when the patient placed more than 70% emphasis on improving grip strength (Table 2, Model 3). Casting was the preferred treatment when attempting to minimize pain and restore esthetics (Table 2, Model 1). Comparing the mean observed outcomes in our patient cohort with the mean counterfactual outcomes that would have occurred after following the treatment recommended by the model consistently demonstrated higher standardized mean outcomes (outcome of 1 being the best, 0 being the worst) after following the treatment recommended by the model (Supplementary Table 2; http://links.lww.com/CORR/A598).
A sensitivity analysis with imputed data of patients with missing data provided similar estimated results to our main analysis. When improving pain and grip strength simultaneously with 80% to 90% emphasis on increasing grip strength, tailoring variables were identified by the tree-based reinforcement learning model, suggesting that patients with radial inclination larger than 9° and ulnar variance larger than 2.1 mm should undergo surgery (Table 3).
Table 3.
Sensitivity analysis of estimated decision rules for various optimization goals with imputed data
| Model 1: outcome = α•Paina+ (1- α)• MHQ esthetic | Model 2: outcome = α•Paina+ (1- α)•Combined ROM | Model 3: outcome = α•Paina+ (1- α)•Grip strength | |||
| α | Optimal treatment | α | Optimal treatment | α | Optimal treatment |
| 0 | Surgery if ulnar variance > 2.8 mm, otherwise casting | 0 | Surgery | 0 | Surgery |
| 0.1 | Casting | 0.1 | Surgery | 0.1 | Surgery if ulnar variance > 2.1 mm and radial inclination > 9°, otherwise casting |
| 0.2 | Casting | 0.2 | Surgery | 0.2 | Same as above |
| 0.3 | Casting | 0.3 | Casting | 0.3 | Casting |
| 0.4 | Casting | 0.4 | Casting | 0.4 | Casting |
| 0.5 | Casting | 0.5 | Casting | 0.5 | Casting |
| 0.6 | Casting | 0.6 | Casting | 0.6 | Casting |
| 0.7 | Casting | 0.7 | Casting | 0.7 | Casting |
| 0.8 | Casting | 0.8 | Casting | 0.8 | Casting |
| 0.9 | Casting | 0.9 | Casting | 0.9 | Casting |
| 1 | Casting | 1 | Casting | 1 | Casting |
From the MHQ pain subdomain score; MHQ = Michigan Hand Outcomes Questionnaire.
Discussion
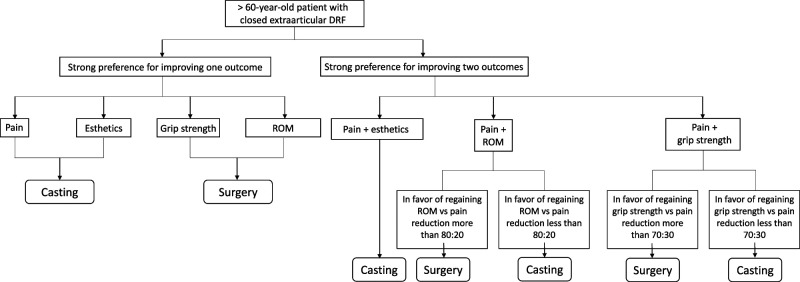
To date, there is no consensus whether extraarticular distal radius fractures in older patients should be treated operatively or nonoperatively. Some studies suggest volar locking plate fixation yields better long-term functional outcomes than casting [21, 24], while others suggest that there are no clinically meaningful differences in activities of daily living or overall quality of life [4, 11, 31]. This lack of consensus and conflicting evidence produces substantial variations in practice patterns [6] and creates ambiguity in preoperative counseling. In this secondary decision analysis of a randomized controlled trial studying closed extraarticular DRFs in older patients, we aimed to assess the tradeoffs in outcome types when older patients undergo casting versus operative management for closed extraarticular distal radius fractures using a statistical learning method, tree-based reinforcement learning. We found that surgical fixation was the preferred treatment for improving ROM or grip strength. However, for maximal reduction of pain and increase in esthetic outcome, casting was the preferred treatment. When attempting to improve both pain and functional outcomes, the most favorable treatment hinged on which outcome was more favored by the patient. Unless the patient heavily preferred maximizing functional outcomes over pain, casting remained the preferred treatment in the model. (Fig. 2) These findings may provide a framework for counseling older patients with closed extraarticular distal radius fractures and improve shared decision-making.
Fig. 2.
Flowchart algorithm summarizing the main findings of the study.
Limitations
The results of this study must be interpreted in the context of several limitations. The findings of this study cannot be generalized to all distal radius fracture types, especially intraarticular fractures, or patients younger than 60 years of age because it only included older patients with closed extraarticular distal radius fractures. The variation in the number of patients enrolled from different centers may contribute to selection bias. In addition, the main analysis only included patients for whom complete data were available, which resulted in less modeling data and may have introduced selection bias from missing data. However, an exploration of missing data confirmed that they were missing randomly; therefore, selection bias was likely minimized. Although the modeling was based on a multicenter, randomized clinical trial, other tailoring variables may influence outcomes that were not collected in WRIST. Tree-based reinforcement learning usually identifies the best treatment options for different patients based on tailoring variables. Our model did not identify such variables and suggested that one treatment is superior to another for maximizing an outcome type. This may be attributable to the relative homogeneity of the patient population in WRIST because of its strict inclusion and exclusion criteria. The AIPW estimator requires either the propensity model or the outcome model to be correctly specified, and if both are misspecified it may provide incorrect estimates. We used the AIPW estimator because of its increased efficiency and reduced variability, but our model may be limited because we only partially understood the treatment assignment mechanism from the trial design. This analysis benefited from high-quality randomized clinical trial data in addition to the robustness of a tree-based reinforcement learning model to determine treatment regimens that improve different outcomes for closed extraarticular DRFs in older adults.
What Are the Tradeoffs in Outcomes between Surgery versus Casting?
Our model selected casting to minimize pain and maximize esthetics while favoring surgery to most improve grip strength and ROM. Persistent pain after DRFs is common and has been an active area of research [13, 18, 19]. It has been speculated that pain outcomes after the treatment of extraarticular DRFs are less influenced by fracture displacement than in patients with intraarticular fractures [13]. The correlation between restored articular congruity and improved outcomes has been the principal reason for operative fixation of intraarticular fractures [27]. Because all included patients sustained extraarticular fractures, the tree-based reinforcement learning model in our study may have selected casting to minimize postoperative pain. This finding is consistent with the results of a previous study that found less pain after casting than after surgical fixation [3]. Another possible explanation for better pain control after casting than after surgery pertains to catastrophic thinking. Psychological factors such as catastrophic thinking have been implicated as individual predictors of pain after orthopaedic trauma [29]. Future decision analysis studies should incorporate validated psychological assessment tools, such as the Pain Catastrophizing Scale, to control for baseline psychological factors. By contrast, the model’s prediction of casting to maximize the esthetic outcome was an unexpected finding. This may be because older patients with this type of DRF still express overall satisfaction with their appearance, even when there is an objective deformity by measurement [4]. Surgical treatment maximizing functional outcomes is a validation of such conclusions from previous studies [21, 24]. An important consideration is that these findings were true in patients older than 60 years who sustained closed extraarticular distal radius fractures, regardless of postreduction radiological parameters, age, or other demographic factors. When a patient strongly prefers to improve one specific outcome, our findings may guide providers on how best to counsel patients.
In What Settings Would Surgery be Preferred over Casting?
When patients placed more than 70% importance on improving grip strength or 80% importance on improving ROM, our model chose surgery as the preferred treatment. This analysis was conducted to quantify how much tradeoff between improving pain and a functional outcome must occur for the model to select surgery. Recently, higher-quality randomized controlled trials have compared nonoperative treatment with surgical fixation in older patients with DRFs. However, even with robust study designs, the evidence of these studies is conflicting. Two randomized controlled trials investigated the treatment of older patients with volar locking plates with those who were treated with closed reduction and immobilization with casting [4, 24]. One randomized controlled trial found improved grip strength in the volar locking plate group compared with the casting group, but outcomes such as ROM, pain, DASH score, and Patient-Rated Wrist Evaluation score were not different between the two groups 1 year after the intervention [4]. In contrast, another randomized controlled trial observed that the volar locking plate group had better DASH, Patient-Rated Wrist Evaluation scores, wrist flexion, ulnar deviation, and grip strength than the casting group [24]. But to our knowledge, no study has investigated the tradeoffs in outcomes when treating older patients with closed extraarticular distal radius fractures with nonoperative or operative management. Furthermore, this study quantifies the approximate extent of the tradeoffs between pain and functional outcomes necessary for surgery to be preferred over casting. These quantifications are helpful when counseling patients preoperatively: If full recovery is represented as 100%, but this must be divided between pain and function, how much importance would you, he patient, place on pain and function? Depending on this answer, the surgeon may more readily recommend one treatment over the other. It was surprising to find such a significant role of pain in the overall conclusions of the model. It is unclear whether this emphasis on pain outcomes is unique to this patient population or fracture type. Future studies should investigate the effects of age and fracture type on outcome tradeoffs after distal radius fracture management.
Conclusion
In patients older than 60 years with closed extraarticular fractures, there are tradeoffs in outcomes when choosing between casting and surgery. Active older patients who desire maximal functional recovery may benefit the most from surgery, whereas more sedentary patients may be treated with casting. When reducing pain is the primary aim, casting appears to be the favored treatment regardless of radiological parameters or patient characteristics in this cohort. Future studies should investigate how different fracture patterns and patient characteristics, especially preoperative psychosocial health, affect the tradeoffs in outcomes after nonoperative and operative management of distal radius fractures.
Group Authors
Members of The WRIST Group include:
Michigan Medicine (coordinating center): Kevin C. Chung H. Myra Kim, Steven C. Haase, Jeffrey N. Lawton, John R. Lien, Adeyiza O. Momoh, Kagan Ozer, Erika D. Sears, Jennifer F. Waljee, Matthew S. Brown, Hoyune E. Cho, Brett F. Michelotti, Sunitha Malay, Melissa J. Shauver.
Beth Israel Deaconess Medical Center: Tamara D. Rozental, Paul T. Appleton, Edward K. Rodriguez, Laura N. Deschamps, Lindsay Mattfolk, Katiri Wagner.
Brigham and Women’s Hospital: Philip Blazar, Brandon E. Earp, W. Emerson Floyd, Dexter L. Louie.
Duke Health: Fraser J. Leversedge, Marc J. Richard, David S. Ruch, Suzanne Finley, Cameron Howe, Maria Manson, Janna Whitfield.
Fraser Health Authority: Bertrand H. Perey, Kelly Apostle, Dory Boyer, Farhad Moola, Trevor Stone, Darius Viskontas, Mauri Zomar, Karyn Moon, Raely Moon.
HealthPartners Institute for Education and Research: Loree K. Kalliainen, Christina M. Ward, James W. Fletcher, Cherrie A. Heinrich, Katharine S. Pico, Ashish Y. Mahajan, Brian W. Hill, Sandy Vang.
Johns Hopkins Medicine: Dawn M. Laporte, Erik A. Hasenboehler, Scott D. Lifchez, Greg M. Osgood, Babar Shafiq, Jaimie T. Shores, Vaishali Laljani.
Kettering Health Network: H. Brent Bamberger, Timothy W. Harman, David W. Martineau, Carla Robinson, Brandi Palmer.
London Health Sciences Centre: Ruby Grewal, Ken A. Faber, Joy C. MacDermid, Kate Kelly, Katrina Munro, Joshua I. Vincent.
Massachusetts General Hospital: David Ring, Jesse B. Jupiter, Abigail Finger, Jillian S. Gruber, Rajesh K. Reddy, Taylor M. Pong, Emily R. Thornton.
Mayo Clinic: David G. Dennison, Sanjeev Kakar, Marco Rizzo, Alexander Y. Shin, Tyson L. Scrabeck.
The MetroHealth System: Kyle Chepla, Kevin Malone, Harry A. Hoyen, Blaine Todd Bafus, Roderick B. Jordan, Bram Kaufman, Ali Totonchil, Dana R. Hromyak, Lisa Humbert.
National University of Singapore: Sandeep Sebastin, Sally Tay.
Northwell Health: Kate W. Nellans, Sara L. Merwin.
Norton Healthcare: Ethan W. Blackburn, Sandra J. Hanlin, Barbara Patterson.
OrthoCarolina Research Institute: R. Glenn Gaston, R. Christopher Cadderdon, Erika Gordon Gantt, John S. Gaul, Daniel R. Lewis, Bryan J. Loeffler, Lois K. Osier, Paul C. Perlik, W. Alan Ward, Benjamin Connell, Pricilla Haug, Caleb Michalek.
Pan Am Clinic/University of Manitoba: Tod A. Clark, Sheila McRae.
University of Connecticut Health: Jennifer Moriatis Wolf, Craig M. Rodner, Katy Coyle.
University of Oklahoma Medicine: Thomas P. Lehman, Yuri C. Lansinger, Gavin D. O’Mahony, Kathy Carl, Janet Wells.
University of Pennsylvania Health System: David J. Bozentka, L. Scott Levin, David P. Steinberg, Annamarie D. Horan, Denise Knox, Kara Napolitano.
University of Pittsburgh Medical Center: John Fowler, Robert Goitz, Cathy A. Naccarelli, Joelle Tighe.
University of Rochester: Warren C. Hammert, Allison W. McIntyre, Krista L. Noble, Kaili Waldrick.
University of Washington Medicine: Jeffery B. Friedrich, David Bowman, Angela Wilson.
Wake Forest Baptist Health: Zhongyu Li, L. Andrew Koman, Benjamin R. Graves, Beth P. Smith, Debra Bullard.
Supplementary Material
Footnotes
Members of the WRIST Group are listed in an appendix at the end of this article.
The institution of one or more of the authors (KCC) has received, during the study period, funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health), Grant R01AR062066.
One of the authors (KCC) certifies receipt of personal payments or benefits, during the study period, in an amount of USD less than 10,000 from Wolters Kluwer; in an amount of USD less than 10,000 from Elsevier; in an amount of USD 10,000 to 100,000 from Axogen; and in an amount of USD 10,000 to 100,000 from Integra.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the University of Michigan Medical School, Ann Arbor, MI, USA (approval number HUM00028291). Clinical Trial Registry Number: NCT01589692.
Contributor Information
Alfred P. Yoon, Email: alfredy@med.umich.edu.
Yibo Wang, Email: wangyb@umich.edu.
Lu Wang, Email: luwang@umich.edu.
Collaborators: Kevin C. Chung H. Myra Kim, Steven C. Haase, Jeffrey N. Lawton, John R. Lien, Adeyiza O. Momoh, Kagan Ozer, Erika D. Sears, Jennifer F. Waljee, Matthew S. Brown, Hoyune E. Cho, Brett F. Michelotti, Sunitha Malay, Melissa J. Shauver, Tamara D. Rozental, Paul T. Appleton, Edward K. Rodriguez, Laura N. Deschamps, Lindsay Mattfolk, Katiri Wagner. Philip Blazar, Brandon E. Earp, W. Emerson Floyd, Dexter L. Louie, raser J Leversedge, Marc J. Richard, David, S. Ruch, Suzanne Finley, Cameron Howe, Maria Manson, Janna Whitfield, Bertrand H Perey, Kelly Apostle, Dory Boyer, Farhad Moola, Trevor Stone, Darius Viskontas, Mauri Zomar, Karyn Moon, Raely Moon, Loree K. Kalliainen, Christina M. Ward, James W. Fletcher, Cherrie A. Heinrich, Katharine S. Pico, Ashish Y. Mahajan, Brian W. Hill, Sandy Vang, Dawn M. Laporte, Erik A. Hasenboehler, Scott D. Lifchez, Greg M. Osgood, Babar Shafiq, Jaimie T. Shores, Vaishali Laljani, H. Brent Bamberger, Timothy W. Harman, David W. Martineau, Carla Robinson, Brandi Palmer. Ruby Grewal, Ken A. Faber, Joy C. MacDermid, Kate Kelly, Katrina Munro, Joshua I. Vincent, David Ring, Jesse B. Jupiter, Abigail Finger, Jillian S. Gruber, Rajesh K. Reddy, Taylor M. Pong, Emily R. Thornton, David G. Dennison, Sanjeev Kakar, Marco Rizzo, Alexander Y. Shin, Tyson L. Scrabeck, Kyle Chepla, Kevin Malone, Harry A. Hoyen, Blaine Todd Bafus, Roderick B. Jordan, Bram Kaufman, Ali Totonchil, Dana R. Hromyak, Lisa Humbert, Sandeep Sebastin, Sally Tay, Kate W. Nellans, Sara L. Merwin, Ethan W. Blackburn, Sandra J. Hanlin, Barbara Patterson. R. Glenn Gaston, R. Christopher Cadderdon, Erika Gordon Gantt, John S. Gaul, Daniel R. Lewis, Bryan J. Loeffler, Lois K. Osier, Paul C. Perlik, W. Alan Ward, Benjamin Connell, Pricilla Haug, Caleb Michalek, Tod A. Clark, Sheila McRae, Jennifer Moriatis Wolf, Craig M. Rodner, Katy Coyle, Thomas P. Lehman, Yuri C. Lansinger, Gavin D. O’Mahony, Kathy Carl, Janet Wells, David J. Bozentka, L. Scott Levin, David P. Steinberg, Annamarie D. Horan, Denise Knox, Kara Napolitano. John Fowler, Robert Goitz, Cathy A. Naccarelli, Joelle Tighe, Warren C. Hammert, Allison W. McIntyre, Krista L. Noble, Kaili Waldrick, Jeffery B. Friedrich, David Bowman, Angela Wilson. Zhongyu Li, L. Andrew Koman, Benjamin R. Graves, Beth P. Smith, and Debra Bullard
References
- 1.American Academy of Orthopaedic Surgeons. The treatment of distal radius fractures: guideline and evidence report. Available at: https://www.aaos.org/globalassets/quality-and-practice-resources/distal-radius/distal-radius-fractures-clinical-practice-guideline.pdf. Accessed May 4, 2021.
- 2.Aktekin CN, Altay M, Gursoy Z, et al. Comparison between external fixation and cast treatment in the management of distal radius fractures in patients aged 65 years and older. J Hand Surg Am. 2010;35:736-742. [DOI] [PubMed] [Google Scholar]
- 3.Arora R, Gabl M, Gschwentner M, et al. A comparative study of clinical and radiologic outcomes of unstable colles type distal radius fractures in patients older than 70 years: nonoperative treatment versus volar locking plating. J Orthop Trauma. 2009;23:237-242. [DOI] [PubMed] [Google Scholar]
- 4.Arora R, Lutz M, Deml C, et al. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93:2146-2153. [DOI] [PubMed] [Google Scholar]
- 5.Bentohami A, Bosma J, Akkersdijk G, et al. Incidence and characteristics of distal radial fractures in an urban population in The Netherlands. Eur J Trauma Emerg Surg. 2014;40:357-361. [DOI] [PubMed] [Google Scholar]
- 6.Bruce KK, Merenstein DJ, Narvaez MV, et al. Lack of agreement on distal radius fracture treatment. J Am Board Fam Med. 2016;29:218-225. [DOI] [PubMed] [Google Scholar]
- 7.Chung KC, Kim HM, Haase SC, et al. Reflections 1 year into the 21-center national institutes of health-funded wrist study: a primer on conducting a multicenter clinical trial. J Hand Surg Am. 2013;38:1194-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung KC, Kim HM, Malay S, et al. The wrist and radius injury surgical trial: 12-month outcomes from a multicenter international randomized clinical trial. Plast Reconstr Surg. 2020;145:1054e-1066e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26:908-915. [DOI] [PubMed] [Google Scholar]
- 10.Dzaja I, MacDermid JC, Roth J, Grewal R. Functional outcomes and cost estimation for extra-articular and simple intra-articular distal radius fractures treated with open reduction and internal fixation versus closed reduction and percutaneous Kirschner wire fixation. Can J Surg. 2013;56:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92:1851-1857. [DOI] [PubMed] [Google Scholar]
- 12.Forsell E, Isacsson N, Blom K, et al. Predicting treatment failure in regular care internet-delivered cognitive behavior therapy for depression and anxiety using only weekly symptom measures. J Consult Clin Psychol. 2020;88:311-321. [DOI] [PubMed] [Google Scholar]
- 13.Grewal R, MacDermid JC, Pope J, Chesworth BM. Baseline predictors of pain and disability one year following extra-articular distal radius fractures. Hand. 2007;2:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hastie T, Tibshirani R, James G, Witten D. An Introduction to Statistical Learning with Applications in R. Springer; 2013. [Google Scholar]
- 15.Hernan MA, Robins JM. Causal Inference: What If . Chapman & Hall/CRC; 2020. [Google Scholar]
- 16.Karl JW, Olson PR, Rosenwasser MP. The epidemiology of upper extremity fractures in the United States, 2009. J Orthop Trauma. 2015;29:e242-e244. [DOI] [PubMed] [Google Scholar]
- 17.Laber EB, Meyer NJ, Reich BJ, et al. Optimal treatment allocations in space and time for on-line control of an emerging infectious disease. J R Stat Soc Ser C Appl Stat. 2018;67:743-770. [PMC free article] [PubMed] [Google Scholar]
- 18.MacDermid JC, Donner A, Richards RS, Roth JH. Patient versus injury factors as predictors of pain and disability six months after a distal radius fracture. J Clin Epidemiol. 2002;55:849-854. [DOI] [PubMed] [Google Scholar]
- 19.Mehta SP, MacDermid JC, Richardson J, MacIntyre NJ, Grewal R. Baseline pain intensity is a predictor of chronic pain in individuals with distal radius fracture. J Orthop Sports Phys Ther. 2015;45:119-127. [DOI] [PubMed] [Google Scholar]
- 20.Mellstrand-Navarro C, Pettersson H, Tornqvist H, Ponzer S. The operative treatment of fractures of the distal radius is increasing: results from a nationwide Swedish study. Bone Joint J. 2014;96:963-969. [DOI] [PubMed] [Google Scholar]
- 21.Mulders MA, Walenkamp MM, van Dieren S, Goslings JC, Schep NW. Volar plate fixation versus plaster immobilization in acceptably reduced extra-articular distal radial fractures: a multicenter randomized controlled trial. J Bone Joint Surg Am. 2019;101:787-796. [DOI] [PubMed] [Google Scholar]
- 22.Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347-1358. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Inference and missing data. Biometrika. 1976;63:581-592. [Google Scholar]
- 24.Saving J, Wahlgren SS, Olsson K, et al. Nonoperative treatment compared with volar locking plate fixation for dorsally displaced distal radial fractures in the elderly: a randomized controlled trial. J Bone Joint Surg Am. 2019;101:961-969. [DOI] [PubMed] [Google Scholar]
- 25.Speth KA, Yoon AP, Wang L, Chung KC. Assessment of tree-based statistical learning to estimate optimal personalized treatment decision rules for traumatic finger amputations. JAMA Netw Open. 2020;3:e1921626-e1921626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao Y, Wang L, Almirall D. Tree-based reinforcement learning for estimating optimal dynamic treatment regimes. Ann Appl Stat. 2018;12:1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trumble TE, Schmitt SR, Vedder NB. Factors affecting functional outcome of displaced intra-articular distal radius fractures. J Hand Surg Am. 1994;19:325-340. [DOI] [PubMed] [Google Scholar]
- 28.Van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol. 2007;6:Article25. [DOI] [PubMed] [Google Scholar]
- 29.Vranceanu A-M, Bachoura A, Weening A, et al. Psychological factors predict disability and pain intensity after skeletal trauma. J Bone Joint Surg Am. 2014;96:e20. [DOI] [PubMed] [Google Scholar]
- 30.Wilson JM, Holzgrefe RE, Staley CA, Schenker ML, Meals CG. Use of a 5-item modified frailty index for risk stratification in patients undergoing surgical management of distal radius fractures. J Hand Surg Am. 2018;43:701-709. [DOI] [PubMed] [Google Scholar]
- 31.Wong TC, Chiu Y, Tsang WL, et al. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur. 2010;35:202-208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.